- 1Laboratory of Molecular Pharmacology, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, China
- 2South Sichuan Institute of Translational Medicine, Luzhou, China
- 3Laboratory of Personalised Cell Therapy & Cell Medicines, School of Pharmacy, Southwest Medical University, Luzhou, China
- 4School of Basic Medicine, Southwest Medical University, Luzhou, China
- 5Department of Pathology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 6Department of Oncology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 7School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, Hong Kong SAR, China
Gastrointestinal cancer is by far the most common malignancy and the most common cause of cancer-related deaths worldwide. Recent studies have shown that long non-coding RNAs (lncRNAs) play an important role in the epigenetic regulation of cancer cells and regulate tumor progression by affecting chromatin modifications, gene transcription, translation, and sponge to miRNAs. In particular, lncRNA has recently been found to possess open reading frame (ORF), which can encode functional small peptides or proteins. These peptides interact with its targets to regulate transcription or the signal axis, thus promoting or inhibiting the occurrence and development of tumors. In this review, we summarize the involvement of lncRNAs and the function of lncRNAs encoded small peptides in gastrointestinal cancer.
1 Introduction
From data obtained during the ENCODE project, it is estimated that the human genome contains both protein-coding and non-coding regions, with less than 2% annotated as protein-coding (1). Most of the non-protein-coding parts of the human genome have long been considered as junk DNA. With the development of deep sequencing technology, a large number of previously unknown transcripts have been identified. The vast majority of these transcripts (>99%) are thought to be long non-coding RNAs (lncRNAs) due to the lack of obvious long protein-coding open reading frame (ORF) and clear homologs in other organisms (2). LncRNAs are a class of important non-coding RNAs, whose transcripts are longer than 200 nucleotides (nt), and have no obvious protein-coding function and no sequence similarity. LncRNAs can be further classified as antisense, intronic, intergenic, and enhancer lncRNAs (3). LncRNAs can perform numerous molecular functions, including regulating transcription patterns, regulating protein activity, performing structural or organizational functions, altering RNA processing events, and acting as precursors for small RNAs (4). In cancer, many lncRNAs are dysregulated, and some of them has been proved to be very specific and sensitive tumor markers, like PCA3 in prostate tumors (5, 6). In previous studies, due to the non-coding nature of lncRNAs, a large number of lncRNAs were thought to function in cancer through their own transcripts. However, recent studies have revealed that lncRNA can encode small peptides through transcripts which can bind ribosomes to performs biological functions, which is a new breakthrough in the study of lncRNA (7).
Gastrointestinal cancers comprise the esophagus, stomach, liver, bile duct, gallbladder, pancreas, colon, appendix or rectum cancer, which together constitute the leading cause of cancer-related deaths worldwide (8). Obesity, smoking, Helicobacter Pylori, hepatitis C virus (HCV) and hepatitis B virus (HBV) are known environmental risk factors for the development of gastrointestinal cancer (9). Despite the advances of chemotherapy in the treatment of some gastrointestinal cancers, the prognosis for many patients remains poor, with a 5-year survival rate of less than 10% (10, 11). Current treatment strategies also include radiotherapy, surgery and targeted therapy. Immunotherapy like checkpoint inhibitors blocking PD-1 and cytotoxic T lymphocyte associated antigen 4(CTLA-4) immunotherapy may benefit patients, but further clinical trials are needed (12).
Given the paucity of treatment options for gastrointestinal cancers, new therapeutic target is urgently needed. The clinical implication of the bifunctional lncRNAs awaits further exploration. In this review, we provide a brief overview of the role of lncRNA and especially summarize the function of small peptides encoded by lncRNAs in gastrointestinal cancers.
2 Function of lncRNAs in Gastrointestinal Cancer
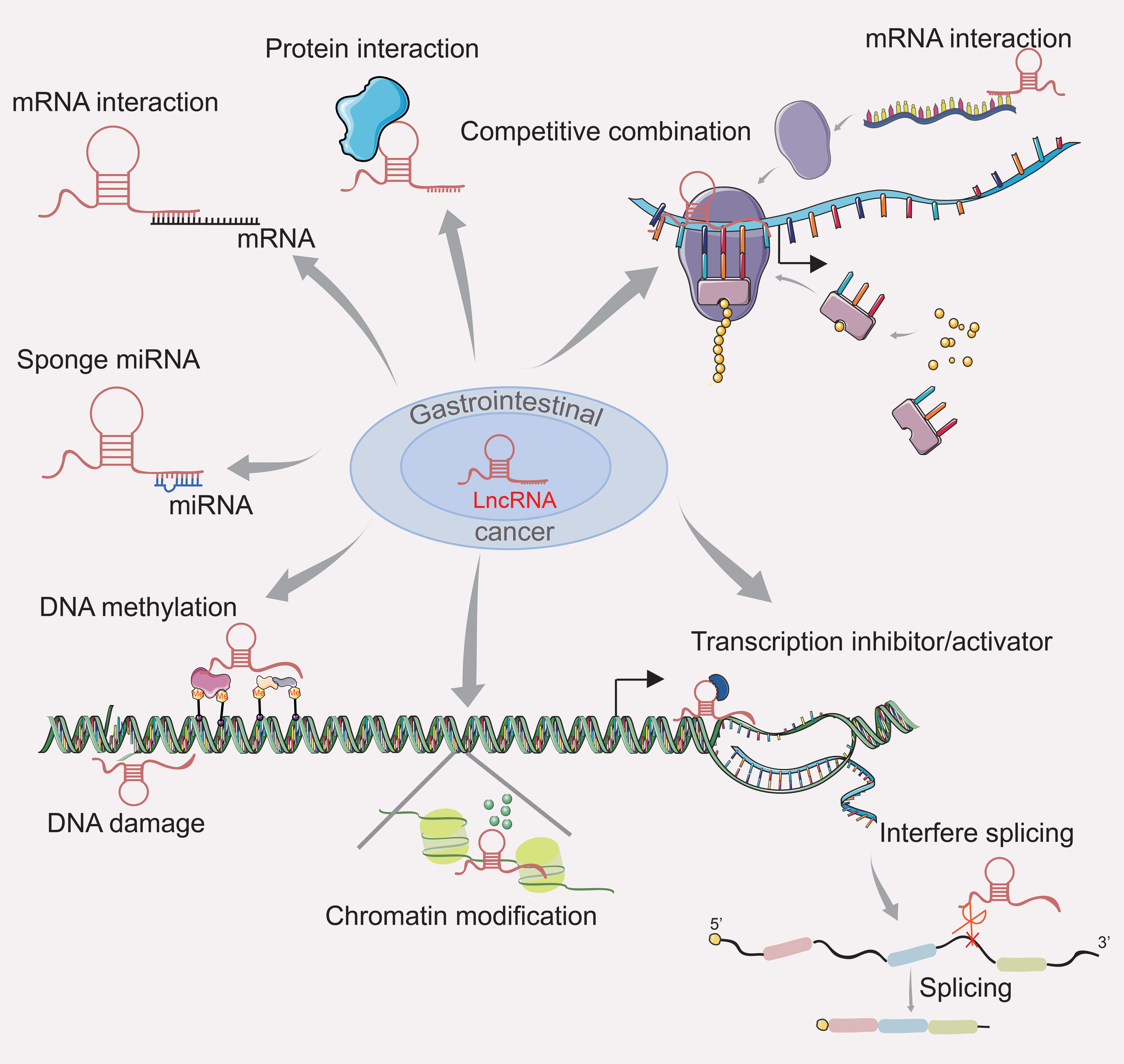
According to global cancer statistics, cancer of the digestive tract is the leading cause of cancer and cancer-related deaths (8). Recent studies have shown that abnormal lncRNA expression is related to tumorigenesis, progression, invasion, and overall survival in patients with gastrointestinal cancer (13). Studies of lncRNA dysregulation in gastrointestinal cancer are shown in Supplementary Table 1. LncRNAs have been identified as important players in gene regulation. LncRNA regulates gene expression mainly by participating in transcriptional regulation, such as inhibited RNA polymerase II, chromatin remodeling and histone modification, interfered mRNA splicing, and complementary double chains formed with the transcripts of the protein coding genes and endogenous siRNA produced in response to the Dicer enzyme to interfere the expression (4). In addition, LncRNAs can affect post-transcriptional processing, act as a structural component to form nucleic acid protein complex with the protein, regulate the activity of the protein or change the cellular localization of the protein and even as a “sponge small molecule competitive effect on the role of endogenous RNA, etc. (14, 15). The role of lncRNAs in gastrointestinal cancer is shown in Figure 1.
2.1 Transcriptional Regulation
2.1.1 DNA Methylation and DNA Repair
DNA methylation occurs in gene promoters, genomes, and intergenic regions of the genome, and plays a key role in both chromatin organization and gene expression (16). Differential genes affected by lncRNA lincDUSP were mainly enriched in DNA damage response and cell cycle control pathways. In addition, the identification of the locus of lincDUSP chromatin by ChIRP-Seq showed that it was associated with genes involved in replication-related DNA damage responses and cell cycle control. Further studies showed that deletion of the lincDUSP gene in colon cancer cell lines increased the accumulation of early s-phase cells and the formation of γH2AX lesions, suggesting increased induction of DNA damage response (17). LncRNA DACOR1 induces down regulation of cystathionine synthetase, resulting in elevated levels of s-adenosylmethionine, a key methyl donor for DNA methylation, suggesting that DACOR1 leads to abnormal DNA methylation and the occurrence of colon cancer (18). LncRNA HOTAIR may enhance 5-fluorouracil-induced apoptosis by reducing the methylation of the MTHFR promoter in esophageal cancer cells (19).
2.1.2 Chromatin Modification
Some lncRNAs can also bind to nucleosome mobilization complexes to mediate chromatin structure remodeling, thus modulating gene expression. Most lncRNAs work with DNA-binding proteins, such as chromatin-modifying complexes, and regulate the expression of multiple genes through epigenetic regulation (20–22). The expression of lncRNA HOTAIR is associated with PRC2 -functional genome-wide reprogramming not only in breast cancer but also in colorectal cancer. It regulates polycomb-dependent chromatin modification, and its upregulation may be a key factor in metastasis progression (23). LncRNA NMR is a novel NSUN2 methylated lncRNA, upregulated in esophageal squamous cell carcinoma (ESCC), functioned as a key factor of ESCC progress. NMR can directly bind to BPTF, which is a chromatin regulator that contributes to ATP-dependent chromatin remodeling (24), and promote the expression of MMP3 and MMP10 by ERK1/2 pathway through recruiting BPTF to chromatin (25).
2.1.3 Alternative Splicing
Alternative splicing (AS) can selectively remove introns, retain exons, and produce two or more mRNA splicing isomers, thus increasing the diversity of gene phenotypes and protein functions in eukaryotes (26, 27). In recent years, many AS changes have been found to play an important role in the development of cancer. With the development of high-throughput sequencing and RNA-seq techniques, thousands of non-coding RNAs have been identified as critical for regulating AS at multiple levels in cancer (28). LncRNA DGCR5 binds directly to serine-and arginine-rich splicing factor 1(SRSF1) to increase its stability, thereby stimulating the Mcl-1 alternative splicing event to promoting the occurrence of esophageal squamous-cell carcinoma in vivo (29). LncRNA MALAT1 promotes cell proliferation, migration and invasion by increasing AKAP-9 expression in colorectal cancer cells through SRPK1-catalyzed phosphorylation of SRSF1 (30). Furthermore, SRPK1 phosphorylation of SRSF1 was found to modulate the alternative splicing of Rac1b in colorectal cells (31).
2.1.4 Transcription
Transcription is the transfer of genetic information from the DNA level to the RNA level, it is the key step for genes to perform their functions. Currently, lncRNA has been found to be involved in regulating gene transcription (32). Furthermore, lncRNA has also been found to be involved in transcriptional regulation that affects the development of gastrointestinal cancer. In colorectal cancer, lncRNA HITT, in cooperation with Ezh2, inhibits HIF-1α transcription and inhibits hypoxia adaptation in colorectal cancer cells (33). Besides, lncRNA SATB2-AS1 directly binds to WDR5 and GADD45A, cis-activating SATB2 transcription via mediating histone H3 lysine 4 tri-methylation deposition and DNA demethylation of the promoter region of SATB2, thus inhibiting CRC transfer and modulating CRC immune response (34). In addition, lncRNA has also been found to regulate cancer by silencing gene expression. AS1DHRS4 is a natural antisense head to head transcript, which can silence the DHRS4 gene cluster in cis and trans form (35). LncRNA CASC2 silences BCL2 expression through EZH2-dependent binding to the promoter region of BCL2, thus enhancing the cytotoxicity of berberine-induced colorectal cancer cells (36). Similarly, lncRNA HOXA transcript at the distal tip promotes colorectal cancer growth partially via silencing of p21 expression (37).
2.2 Translational Regulation
The control of translation is often considered a major factor in determining protein levels in cells. LncRNAs can affect tumor progression by regulating the translation of coding RNAs. LncRNA GMAN, overexpressed in gastric cancer, regulates ephrin A1 mRNA translation through competitive binding with GMAN-As, thus promoting proliferation and metastasis in gastric cancer (38). In addition, lncRNA can also act by binding to ribosomal mRNAs. Ribosomes, organelles that mediate protein synthesis, are often typically seen as a key factor in the translation process and ribosome recruitment is seen as a regulatory endpoint (39). Till now, there are few studies on the mechanism of lncRNA affecting the translation process through the interaction with ribosomal proteins in gastrointestinal cancer. In colorectal cancer, lncRNA LUCAT1 binds to UBA52, which encodes ubiquitin and 60s ribosomal protein L40(RPL40), and affects its stability in a proteasome dependent manner (40). Equally, ribosomal protein S24 mRNA binds to lncRNA MVIH to enhance their stability, thereby activating angiogenesis in colorectal cancer by inhibiting the secretion of PGK1 (41).
2.3 miRNA Sponge
In addition to binding to coding RNA and proteins, lncRNAs have also been found to act as sponge by binding to miRNA molecules and reducing their ability to target mRNAs in gastrointestinal cancers. In colorectal cancer, lncRNA MALAT1 can sponge miR-106b-5p and enhanced microtubule migration via SLAIN2 to promote invasion and metastasis (42). LncRNA 00152 acts as competitive endogenous RNA and modulates NRP1 expression by sponging with miRNA-206, thus promoting cancer progression in colorectal cancer (43). In gastric cancer, lncRNA LINC01133 is down-regulated in gastric cancer tissues and cell lines, and acts as competitive endogenous RNA (ceRNA) via sponging miR-106a-3p to regulate APC expression and Wnt/β-catenin pathway to inhibit gastric cancer progression and metastasis (44). Similarly, lncRNA XIST acts as ceRNA to inhibit the miR-497/MACC1 axis and promote the growth and invasion of gastric cancer cells (45). In addition, there are also studies on the biological function of lncRNAs through “sponging” miRNA in liver cancer (46), esophageal cancer (47), pancreatic cancer (48), cholangiocarcinoma (49) and gallbladder cancer (50), indicating that lncRNAs’ role in “sponging” miRNAs has significant significance in gastrointestinal cancer.
3 LncRNA Encoded Small Peptides
Although lncRNAs are defined as transcripts without coding ability, many studies in recent years have found open reading frame (ORF) in lncRNAs by bioinformatics analysis (51). Recent studies have also found that some lncRNAs appear to be highly correlated with the ribosome, suggesting that lncRNA may contain coding regions for translation that can encode short-chain small peptides (52). Analysis of ribosomal map data showed that 40% of lncRNAs and pseudogene RNA expressed in human cells were translated, and mass spectrometry confirmed that lncRNAs could indeed be translated into small peptides (53, 54). Sebastian et al. analyzed 354 translatable open reading frames from lncRNAs and obtained 22 small peptides from mass spectrometry (55). The translated lncRNAs are preferentially located in the cytoplasm, while the untranslated lncRNAs are preferentially located in the nucleus. The translation efficiency of cytoplasmic lncRNAs is almost equal to mRNAs, indicating that cytoplasmic lncRNAs are bound by ribosomes and translated, but most of the small peptides produced by lncRNAs may be highly unstable by-products and have no function (53).
Although many small peptides encoded by lncRNA ORF have been found so far, only part of their biological functions have been revealed. These small peptides are usually conserved and involved in a wide range of biological processes (7). LncRNA-encoded small peptides play important roles in inflammation, signal transduction and metabolism etc. (56, 57). LncRNA lncVLDLR can encode a 44-aa inflammation-modulating micropeptide (IMP) that can participate in inflammatory responses by interacting with transcriptional coactivators (56). LncRNA linc00116 encodes a 56-aa peptide named Mtln that can connect respiration and lipid metabolism (58). Micropeptide encoded by lncRNA Toddler can accelerate gastrula formation by activating APJ/Apelin receptor signals (59). Recent studies have also found that these small peptides encoded by lncRNAs can play a vital role in the development of cancer (Table 1).
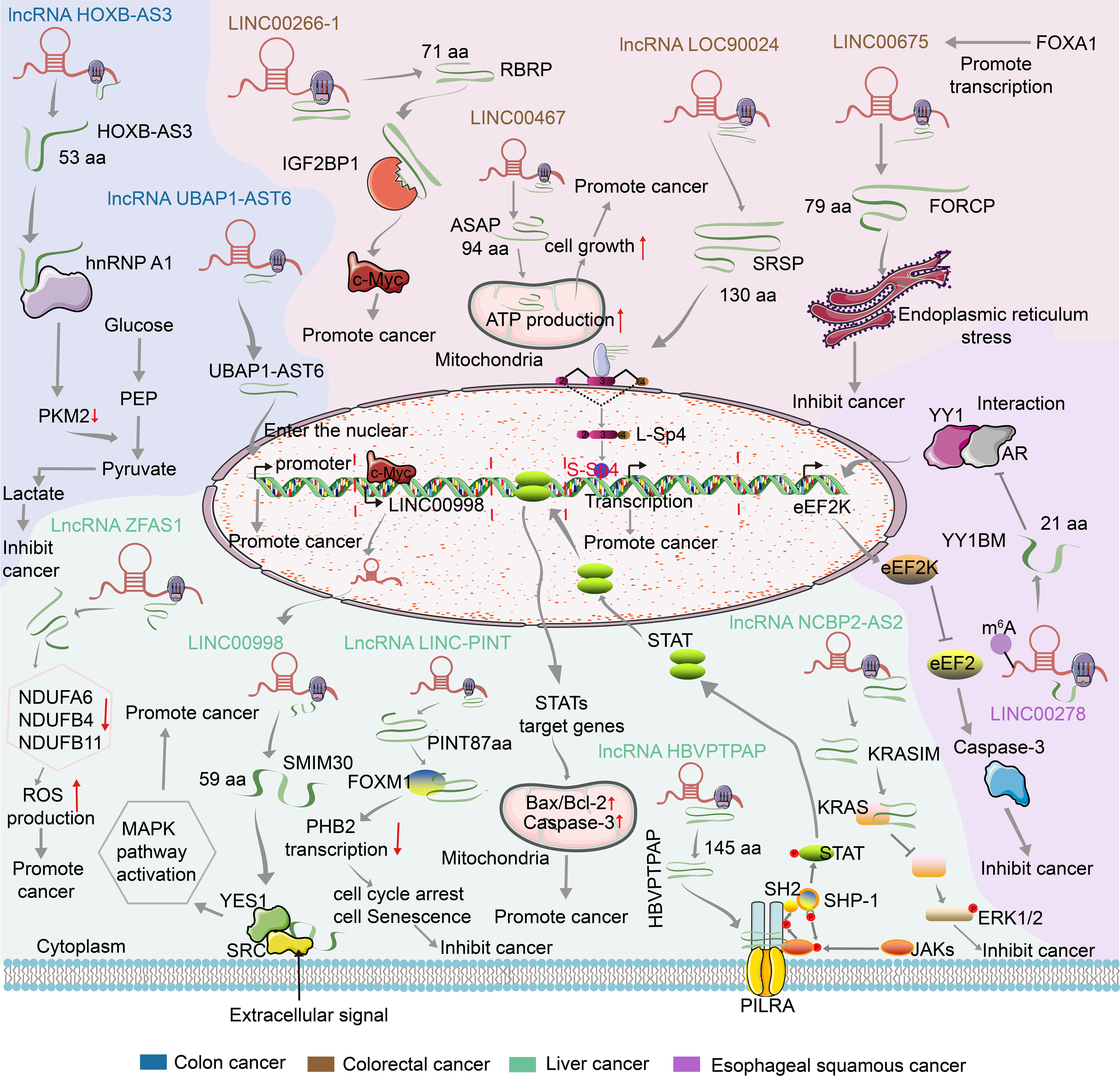
At present, several functional peptides encoded by lncRNA have been found in gastrointestinal cancer. Their RNA level studies are shown in Table 2 and their mechanism of action by encoding small peptides is summarized in Figure 2.
3.1 LncRNA HOXB-AS3
LncRNA HOXB-AS3 was found to be associated with tumorigenesis and development since its discovery. First reported by J Huang and colleagues, lncRNA HOXB-AS3 can encode a conserved 53-aa peptide to inhibit the growth of colon cancer (60). In the following studies, although the effects of lncRNA HOXB-AS3 on other tumors were also reported, it is interesting to note that the studies were only at the RNA level and mostly showed cancer-promoting properties. For example, low expression of HOXB-AS3 can reduce the expression of lactate dehydrogenase A and extracellular acidification rate through the sponge miR-378a-3p, thus promoting the growth, invasion and migration of epithelial ovarian cancer (81). The expression of HOXB-AS3 was significantly increased in non-small-cell lung carcinoma tissues and cells, and the activation of PI3K-AKT increased the proliferation, migration and invasion of lung cancer (82). In acute myeloid leukemia, HOXB-AS3 is a poor prognostic marker that interacts with ErbB3-binding protein 1 and leads ErbB3-binding protein 1 to ribosomal DNA sites to regulate ribosomal RNA transcription and protein synthesis from scratch to promote tumor (84, 85). HOXB-AS3 can also promote the tumorigenesis of epithelial ovarian cancer through Wnt/β-catenin signaling pathway (87), and promote endometrial cancer cell proliferation and inhibit apoptosis by targeting miR-498-5p to regulate ADAM9 expression (86). In addition to its effect on tumors, HOXB-AS3 has been reported to inhibit cardiomyocyte proliferation induced by targeting and down regulation of miRNA-875-3p protection (112).
In gastrointestinal cancers, studies of HOXB-AS3 at RNA levels have shown a promoting effect on liver cancer. HOXB-AS3 is highly expressed in liver cancer tissues, and can inhibit cell apoptosis, promote proliferation and induce cell cycle stagnation in G0/G1 phase by binding with DNMT1 locus to inhibit the expression of p53 (83). In colon cancer, HOXB-AS3 can competitively bind to arginine residues in the RGG motif of hnRNP A1 by encoding a conserved 53-aa peptide, thus blocking hnRNP A1 dependent pyruvate kinase M(PKM) splicing, PKM2 formation and subsequent colon cancer cell metabolic reprogramming to inhibit tumorigenesis. The mean overall survival time of colon cancer patients with high HOXB-AS3 peptide expression was 1.6 times that of those with low HOXB-AS3 peptide expression, suggesting that high HOXB-AS3 peptide expression may reduce the risk of death. Further studies showed that HOXB-AS3 peptide, not lncRNA HOXB-AS3, inhibits colon cancer cell growth, invasion, migration, colony formation, and tumor growth in vitro and in vivo (60).
3.2 LncRNA UBAP1-AST6
Sequencing of full-length translated mRNA by different studies from human colorectal cancer (113), liver cancer (114), and cervical cancer (115) showed that 1028 ~ 3330 lncRNAs were bound to ribosomes. Among them, 2969 lncRNAs had canonical open reading frames (ORFs) beginning with AUG, which could encode proteins of at least 50 amino acids in length. Mass spectrometry and western blot analysis by Shaohua L et al. confirmed that lncRNA UBAP1-AST6 could encode a peptide. The function-related translation ratio (TR), defined as the ratio of mRNA to total mRNA of a gene, was found to be significantly higher in the average TR of lncRNA than in known coding genes, indicating that the translation of new proteins was more active. Localization of EGFP and mCherry fusion protein revealed that UBAP1-AST6 was localized in the nucleus. UBAP1-AST6 acts as a possible tumor promoter in its protein form, and its overexpression can significantly promote cell proliferation and clone formation, but not in its UBAP1-AST6 RNA form (63).
3.3 LncRNA LINC00675
LINC00675 was found to have both anti-cancer and pro-cancer effects. However, in gastrointestinal carcinoma, LINC00675 mainly shows tumor suppressor properties. Shuo Z et al. found that LINC00675 enhanced the phosphorylation of vimentin on Ser83 to inhibit the progression of gastric cancer (99). LINC00675 inhibits cell proliferation and migration in colorectal cancer by acting on miR-942 and Wnt/β-catenin signaling (96). LINC00675 can also inhibit tumorigenesis and epithelial-mesenchymal transition by inhibiting the Wnt/β-catenin signaling pathway in esophageal squamous cell carcinoma (95).
A recent study shows that LINC00675 is regulated by the precursor transcription factor FOXA1 and can encode a small conserved 79-amino acid protein called FORCP in colorectal cancer. The FORCP protein is mainly located in the endoplasmic reticulum. The FORCP transcript was not detected in most cell types, but was abundant in well-differentiated colorectal cancer cells, suggesting that FORCP is a novel small conserved protein encoded by misannotated lncRNA. FORCP can inhibit cell proliferation, clone formation and tumorigenesis (69).
3.4 LncRNA LOC90024
Nan M et al. used RNA sequencing to purify ribosomal-bound RNA and found that lncRNA LOC90024 could bind to the ribosome, suggesting that LOC90024 might be translated into a protein/peptide. LncRNA LOC90024 was confirmed to encode a small protein SRSP of 130 amino acids by GFP fusion protein, mass spectrometry and western blot assay. SRSP is endogenous and produced naturally in human cells and tissues. Increased LOC90024 and SRSP levels are associated with poor prognosis in CRC patients. However, SRSP rather than LOC90024 lncRNA itself promoted CRC tumorigenesis. Mechanistically, SRSP acts on RNA splicing regulator SRSF3 to regulate mRNA splicing. SRSP increased the binding of SRSF3 to exon 3 of transcription factor Sp4, which promoted the formation of “ oncogene” long Sp4 subtype (L-Sp4 protein) and inhibited the formation of “tumor suppressor” short Sp4 isoform (S-Sp4 peptide). Overall, the results reveal that small lncRNA-encoded protein SRSP induces “carcinogenesis” (70).
3.5 LncRNA LINC00266-1
In RNA level studies, LINC00266-1 has been found to promote the development of osteosarcoma by stimulating the LINC00266-1/mir-548c-3p/SMAD2 feedback loop (102). Song Z et al. found that LINC00266 could encode a 71 aa polypeptide, which was named “RBRP” as it mainly interacts with RNA-binding proteins, including m6A reader IGF2BP1. RBRP is expressed naturally and internally. Compared with the control group, RBRP was up-regulated in CRC tissues and cells, and the high expression of RBRP was an independent prognostic factor in CRC patients. LINC00266-1 lncRNA and RBRP expression levels were increased in primary cancer cells and highly metastatic cancer cells, but RBRP peptide, not lncRNA LinC00266-1 itself, promoted tumorigenesis. RBRP enhances mRNA stability and expression of c-Myc by binding to IGF2BP1 and enhancing m6A recognition by IGF2BP1 on RNAs, like c-Myc mRNA, thus promoting tumorigenesis. RBRP is a regulatory subunit of m6A reader and enhances m6A recognition of target RNA by m6A reader to play its carcinogenic role (71).
3.6 LINC00467
In colorectal cancer, up-regulation of LINC00467 reduces the expression of cyclin A1, cyclin D1, CDK2, CDK4 and Twist1, and enhances the expression of E-cadherin, thus promoting the development of colorectal cancer (105). Increased expression of LINC00467 promotes cell proliferation and metastasis and inhibits apoptosis by targeting miR-451a (106). Li et al. found that LINC00467 regulates metastasis and chemical resistance of colorectal cancer by competing with Ferritin Light Chain (FTL) for the miR-133b binding site (107). Recent studies have found that lncRNA LINC00467 can encode a 94-aa micropeptide, which is located in mitochondria, to enhance the construction of ATP synthase by interacting with subunits α and γ (ATP5A and ATP5C), thus increasing ATP synthase activity and mitochondrial oxygen consumption rate to promote colorectal cancer cell proliferation (72).
3.7 LncRNA LINC00998
Through the RIP-seq analysis of Ribosomal protein S6, Yanan P et al. found that LINC00998 has the ability to encode a 59aa endogenous small peptide, named SMIM30. SMIM30 is a conserved and hydrophobic membrane peptide and highly expressed in HCC. High level of SMIM30 is predictive of poor prognosis. The SMIM30 Peptide, rather than LINC00998, promotes proliferation, invasion and migration of hepatoma cells in vitro and in vivo. In addition, SMIM30 is transcribed by c-Myc and interacts with non-receptor tyrosine kinases SRC/YES1 to drive membrane anchoring, thus activating MAPK signaling pathways (73).
3.8 LncRNA HBVPTPAP
LCRNA HBVPTPAP, which was classified as long non-coding RNA according to the structural characteristics of the full-length sequence, is a new gene screened from HepG2 cell line by Yongzhi L et al. using suppression subtractive hybridization. With the discovery of a unique and complete open reading frame and related coding capability validation, lncRNA HBVPTPAP was found to encode a small 145aa peptide. The subcellular localization of lncRNA HBVPTPAP is mainly in the cytoplasm, which may activate the downstream JAK/STAT signaling pathway through the interaction between the encoded peptide and the PILRA intracellular domain to induce apoptosis of hepatoma cells (74).
3.9 lncRNA NCBP2-AS2
In RNA level studies, lncRNA NCBP2-AS2 was found to be abnormally expressed in osteoblasts of osteoporosis patients and may be involved in inflammatory responses (116). Dongbo Zhou et al. found that the differential expression of NCBP2-AS2 is related to the occurrence and development of lung cancer and may regulate cancer-related Wnt signaling pathway (104). In further studies, Wenli Xu et al. found through ribosomal profiling analysis that lncRNA NCBP2-AS2 had the ability to encode a 90-aa small peptide named KRASIM. KRASIM is highly conserved and significantly down-regulated in hepatocellular carcinoma. The high expression of KRASIM can inhibit proliferation, clone formation and cell cycle progression of hepatocellular carcinoma cells. KRASIM interacts with KRAS protein to inhibit ERK signaling, leading to the suppression of hepatocellular carcinoma cell growth (75).
3.10 LncRNA ZFAS1
Studies have shown that up-regulation of lncRNA ZFAS1 can promote metastasis in liver cancer (117). LncRNA ZFAS1 has also been reported to be a biomarker for liver cancer (108, 109). H-L Zhou et al. found that lncRNA ZFAS1 enhanced the proliferation of liver cancer by inhibiting miR-193a-3p (110). Recent research evidence has revealed that lncRNA ZFAS1 can encode a small peptide to down-regulate NADH dehydrogenase expression (NDUFA6, NDUFB11 and NDUFB4) to enhance ROS production, thus promoting cancer development (76).
3.11 LncRNA LINC-PINT
Xiaohong X et al. used a computer aging model to identify senescence related lncRNAs and detected 6 differentially expressed lncRNAs, in which LINC-PINT was significantly differentially expressed in senescent cells, indicating that it was involved in cellular aging of liver cancer. Subsequent studies found that lncRNA LINC-PINT can encode an 87-aa small peptide named PINT87aa to perform biological functions. PINT87aa can induces cell cycle arrest and cell senescence by directly binding to FOXM1 to block PHB2 transcription (77).
3.12 LncRNA LINC00278
By screening differentially expressed lncRNAs in males, Siqi W et al. found that LINC00278 was significantly down-regulated in esophageal squamous cell carcinoma tissues compared with adjacent normal tissues. LINC00278 can encode a 21-amino acid Yin Yang 1 (YY1)-binding micropeptide, named YY1BM. YY1BM inhibits eEF2K transcription by blocking the interaction between YY1 and androgen receptor (AR), thus promoting eEF2 activity and leading to ESCC apoptosis. LINC00278 has a classic m6A modified motif near the termination codon of YY1BM, which can interact with YTHDF1 and in turn promote the translation of YY1BM. However, smoking increased ALKBH5 expression and decreased the m6A modification of LINC00278, thus inhibiting the translation of YY1BM and inducing ESCC progression (79).
4 Conclusions and Perspectives
Only about 1.5% of the human genome contains protein-coding genes, and the rest of the genome contains non-coding sequences. Most of these non-coding DNA sequences can be transcribed into RNA, including tens of thousands of lncRNAs and thousands of miRNAs (118). LncRNA has been found to play an important role in gastrointestinal cancer, including through regulating chromatin structure, regulating transcription, translation, and acting as a sponge-miRNA interaction to inhibit or promote cancer (119). But as lncRNA has been known to be non-coding RNA for a long time since it was recognized, these studies are only based on RNA levels. Interestingly, some of these lncRNAs have recently been found to have open reading frame and can regulate the development of tumors by encoding small peptides or proteins. This finding is also reported in gastrointestinal cancers, such as lncRNA HOXB-AS3 and lncRNA UBAP1-AST6 in colon cancer, LINC00675, lncRNA LOC90024, LINC00266-1 and LINC00467 in colorectal cancer, LINC00998, lncRNA HBVPTPAP, lncRNA NCBP2-AS2, lncRNA ZFAS1 and lncRNA LINC-PINT in liver cancer, LINC00278 in asophageal squamous cancer.
Although there have been a large number of studies on lncRNA in gastrointestinal cancer, several aspects are still lacking. First of all, although there are studies on lncRNA regulation of translation, there is still a lack of in-depth research on the regulation of transcription by lncRNA interacting with ribosomal proteins. Second, it is still unclear whether the binding of lncRNA to proteins can affect tumor development by changing protein localization and protein properties in gastrointestinal cancer. Finally, the fact that lncRNA encodes peptides breaks the previous understanding of lncRNA. The mechanisms of lncRNA to perform functions become more complicated. Current studies have shown that the small peptides encoded by lncRNA are involved in the regulation of gastrointestinal cancer. However, more comprehensive and in-depth studies of lncRNA encoded small peptides and their mechanisms are needed before potential application of it in the prevention and treatment of gastrointestinal cancer.
Author Contributions
YaC and WL searched and reviewed published articles and wrote the manuscript. LY, YZ, XW, ML, FD, YuC, ZY, QW, and TY conducted online data searches and critically reviewed the article. JS and ZX made substantial contributions to the conception, design of the study and made revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (No. 81972643, No. 82172962), Sichuan Science and Technology Project (2021YJ0201) and Luxian People’s Government and Southwest Medical University Scientific and Technological Achievements Transfer and Transformation Strategic Cooperation Project (2019LXXNYKD-07).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.777374/full#supplementary-material
References
1. Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of Transcription in Human Cells. Nature (2012) 7414:101–8. doi: 10.1038/nature11233
2. Banfai B, Jia H, Khatun J, Wood E, Risk B, Gundling WE Jr., et al. Long Noncoding RNAs Are Rarely Translated in Two Human Cell Lines. Genome Res (2012) 9:1646–57. doi: 10.1101/gr.134767.111
3. Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE V7 Catalog of Human Long Noncoding RNAs: Analysis of Their Gene Structure, Evolution, and Expression. Genome Res (2012) 9:1775–89. doi: 10.1101/gr.132159.111
4. Wilusz JE, Sunwoo H, Spector DL. Long Noncoding RNAs: Functional Surprises From the RNA World. Genes Dev (2009) 13:1494–504. doi: 10.1101/gad.1800909
5. de Kok JB, Verhaegh GW, Roelofs RW, Hessels D, Kiemeney LA, Aalders TW, et al. DD3(PCA3), a Very Sensitive and Specific Marker to Detect Prostate Tumors. Cancer Res (2002) 9:2695–8.
6. Wieczorek E, Reszka E. mRNA, microRNA and lncRNA as Novel Bladder Tumor Markers. Clin Chim Acta (2018) 477:141–53. doi: 10.1016/j.cca.2017.12.009
7. Choi SW, Kim HW, Nam JW. The Small Peptide World in Long Noncoding RNAs. Brief Bioinform (2019) 5:1853–64. doi: 10.1093/bib/bby055
8. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 1:7–30. doi: 10.3322/caac.21590
9. Pourhoseingholi MA, Vahedi M, Baghestani AR. Burden of Gastrointestinal Cancer in Asia; an Overview. Gastroenterol Hepatol Bed Bench (2015) 1:19–27.
10. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric Cancer: Epidemiology, Prevention, Classification, and Treatment. Cancer Manag Res (2018) 10:239–48. doi: 10.2147/CMAR.S149619
11. Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of Gastric Cancer. World J Gastroenterol (2014) 7:1635–49. doi: 10.3748/wjg.v20.i7.1635
12. Abdul-Latif M, Townsend K, Dearman C, Shiu KK, Khan K. Immunotherapy in Gastrointestinal Cancer: The Current Scenario and Future Perspectives. Cancer Treat Rev (2020) 88:102030. doi: 10.1016/j.ctrv.2020.102030
13. Khajehdehi M, Khalaj-Kondori M, Ghasemi T, Jahanghiri B, Damaghi M. Long Noncoding RNAs in Gastrointestinal Cancer: Tumor Suppression Versus Tumor Promotion. Dig Dis Sci (2021) 2:381–97. doi: 10.1007/s10620-020-06200-x
14. Wang X, Song X, Glass CK, Rosenfeld MG. The Long Arm of Long Noncoding RNAs: Roles as Sensors Regulating Gene Transcriptional Programs. Cold Spring Harb Perspect Biol (2011) 1:a003756. doi: 10.1101/cshperspect.a003756
15. Yang G, Lu X, Yuan L. LncRNA: A Link Between RNA and Cancer. Biochim Biophys Acta (2014) 11:1097–109. doi: 10.1016/j.bbagrm.2014.08.012
16. Jones PA. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat Rev Genet (2012) 7:484–92. doi: 10.1038/nrg3230
17. Forrest ME, Saiakhova A, Beard L, Buchner DA, Scacheri PC, LaFramboise T, et al. Colon Cancer-Upregulated Long Non-Coding RNA lincDUSP Regulates Cell Cycle Genes and Potentiates Resistance to Apoptosis. Sci Rep (2018) 1:7324. doi: 10.1038/s41598-018-25530-5
18. Merry CR, Forrest ME, Sabers JN, Beard L, Gao XH, Hatzoglou M, et al. DNMT1-Associated Long Non-Coding RNAs Regulate Global Gene Expression and DNA Methylation in Colon Cancer. Hum Mol Genet (2015) 21:6240–53. doi: 10.1093/hmg/ddv343
19. Zhang S, Zheng F, Zhang L, Huang Z, Huang X, Pan Z, et al. LncRNA HOTAIR-Mediated MTHFR Methylation Inhibits 5-Fluorouracil Sensitivity in Esophageal Cancer Cells. J Exp Clin Cancer Res (2020) 1:131. doi: 10.1186/s13046-020-01610-1
20. Ponting CP, Oliver PL, Reik W. Evolution and Functions of Long Noncoding RNAs. Cell (2009) 4:629–41. doi: 10.1016/j.cell.2009.02.006
21. Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell (2007) 7:1311–23. doi: 10.1016/j.cell.2007.05.022
22. Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many Human Large Intergenic Noncoding RNAs Associate With Chromatin-Modifying Complexes and Affect Gene Expression. Proc Natl Acad Sci U S A (2009) 28:11667–72. doi: 10.1073/pnas.0904715106
23. Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, et al. Long Noncoding RNA HOTAIR Regulates Polycomb-Dependent Chromatin Modification and Is Associated With Poor Prognosis in Colorectal Cancers. Cancer Res (2011) 20:6320–6. doi: 10.1158/0008-5472.CAN-11-1021
24. Dar AA, Nosrati M, Bezrookove V, de Semir D, Majid S, Thummala S, et al. The Role of BPTF in Melanoma Progression and in Response to BRAF-Targeted Therapy. J Natl Cancer Inst (2015) 107(5):djv034. doi: 10.1093/jnci/djv034
25. Li Y, Li J, Luo M, Zhou C, Shi X, Yang W, et al. Novel Long Noncoding RNA NMR Promotes Tumor Progression via NSUN2 and BPTF in Esophageal Squamous Cell Carcinoma. Cancer Lett (2018) 430:57–66. doi: 10.1016/j.canlet.2018.05.013
26. Keren H, Lev-Maor G, Ast G. Alternative Splicing and Evolution: Diversification, Exon Definition and Function. Nat Rev Genet (2010) 5:345–55. doi: 10.1038/nrg2776
27. Yang X, Coulombe-Huntington J, Kang S, Sheynkman GM, Hao T, Richardson A, et al. Widespread Expansion of Protein Interaction Capabilities by Alternative Splicing. Cell (2016) 4:805–17. doi: 10.1016/j.cell.2016.01.029
28. Liu Y, Liu X, Lin C, Jia X, Zhu H, Song J, et al. Noncoding RNAs Regulate Alternative Splicing in Cancer. J Exp Clin Cancer Res (2021) 1:11. doi: 10.1186/s13046-020-01798-2
29. Duan Y, Jia Y, Wang J, Liu T, Cheng Z, Sang M, et al. Long Noncoding RNA DGCR5 Involves in Tumorigenesis of Esophageal Squamous Cell Carcinoma via SRSF1-Mediated Alternative Splicing of Mcl-1. Cell Death Dis (2021) 6:587. doi: 10.1038/s41419-021-03858-7
30. Hu ZY, Wang XY, Guo WB, Xie LY, Huang YQ, Liu YP, et al. Long Non-Coding RNA MALAT1 Increases AKAP-9 Expression by Promoting SRPK1-Catalyzed SRSF1 Phosphorylation in Colorectal Cancer Cells. Oncotarget (2016) 10:11733–43. doi: 10.18632/oncotarget.7367
31. Goncalves V, Henriques AF, Pereira JF, Neves Costa A, Moyer MP, Moita LF, et al. Phosphorylation of SRSF1 by SRPK1 Regulates Alternative Splicing of Tumor-Related Rac1b in Colorectal Cells. RNA (2014) 4:474–82. doi: 10.1261/rna.041376.113
32. Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, et al. Local Regulation of Gene Expression by lncRNA Promoters, Transcription and Splicing. Nature (2016) 7629:452–5. doi: 10.1038/nature20149
33. Wang X, Wang Y, Li L, Xue X, Xie H, Shi H, et al. A lncRNA Coordinates With Ezh2 to Inhibit HIF-1alpha Transcription and Suppress Cancer Cell Adaption to Hypoxia. Oncogene (2020) 9:1860–74. doi: 10.1038/s41388-019-1123-9
34. Xu M, Xu X, Pan B, Chen X, Lin K, Zeng K, et al. LncRNA SATB2-AS1 Inhibits Tumor Metastasis and Affects the Tumor Immune Cell Microenvironment in Colorectal Cancer by Regulating SATB2. Mol Cancer (2019) 1:135. doi: 10.1186/s12943-019-1063-6
35. Li Q, Su Z, Xu X, Liu G, Song X, Wang R, et al. AS1DHRS4, a Head-to-Head Natural Antisense Transcript, Silences the DHRS4 Gene Cluster in Cis and Trans. Proc Natl Acad Sci U S A (2012) 35:14110–5. doi: 10.1073/pnas.1116597109
36. Dai W, Mu L, Cui Y, Li Y, Chen P, Xie H, et al. Long Noncoding RNA CASC2 Enhances Berberineinduced Cytotoxicity in Colorectal Cancer Cells by Silencing BCL2. Mol Med Rep (2019) 2:995–1006. doi: 10.3892/mmr.2019.10326
37. Lian Y, Ding J, Zhang Z, Shi Y, Zhu Y, Li J, et al. The Long Noncoding RNA HOXA Transcript at the Distal Tip Promotes Colorectal Cancer Growth Partially via Silencing of P21 Expression. Tumour Biol (2016) 6:7431–40. doi: 10.1007/s13277-015-4617-2
38. Zhuo W, Liu Y, Li S, Guo D, Sun Q, Jin J, et al. Long Noncoding RNA GMAN, Up-Regulated in Gastric Cancer Tissues, Is Associated With Metastasis in Patients and Promotes Translation of Ephrin A1 by Competitively Binding GMAN-As. Gastroenterology (2019) 3:676–91 e11. doi: 10.1053/j.gastro.2018.10.054
39. Guo H. Specialized Ribosomes and the Control of Translation. Biochem Soc Trans (2018) 4:855–69. doi: 10.1042/BST20160426
40. Zhou Q, Hou Z, Zuo S, Zhou X, Feng Y, Sun Y, et al. LUCAT1 Promotes Colorectal Cancer Tumorigenesis by Targeting the Ribosomal Protein L40-MDM2-P53 Pathway Through Binding With UBA52. Cancer Sci (2019) 4:1194–207. doi: 10.1111/cas.13951
41. Wang Y, Wu Y, Xiao K, Zhao Y, Lv G, Xu S, et al. RPS24c Isoform Facilitates Tumor Angiogenesis Via Promoting the Stability of MVIH in Colorectal Cancer. Curr Mol Med (2020) 5:388–95. doi: 10.2174/1566524019666191203123943
42. Zhuang M, Zhao S, Jiang Z, Wang S, Sun P, Quan J, et al. MALAT1 Sponges miR-106b-5p to Promote the Invasion and Metastasis of Colorectal Cancer via SLAIN2 Enhanced Microtubules Mobility. EBioMedicine (2019) 41:286–98. doi: 10.1016/j.ebiom.2018.12.049
43. Chen ZP, Wei JC, Wang Q, Yang P, Li WL, He F, et al. Long Noncoding RNA 00152 Functions as a Competing Endogenous RNA to Regulate NRP1 Expression by Sponging With Mirna206 in Colorectal Cancer. Int J Oncol (2018) 3:1227–36. doi: 10.3892/ijo.2018.4451
44. Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, et al. LINC01133 as ceRNA Inhibits Gastric Cancer Progression by Sponging miR-106a-3p to Regulate APC Expression and the Wnt/beta-Catenin Pathway. Mol Cancer (2018) 1:126. doi: 10.1186/s12943-018-0874-1
45. Ma L, Zhou Y, Luo X, Gao H, Deng X, Jiang Y. Long Non-Coding RNA XIST Promotes Cell Growth and Invasion Through Regulating miR-497/MACC1 Axis in Gastric Cancer. Oncotarget (2017) 3:4125–35. doi: 10.18632/oncotarget.13670
46. Li D, Zhang J, Li J. Role of miRNA Sponges in Hepatocellular Carcinoma. Clin Chim Acta (2020) 500:10–9. doi: 10.1016/j.cca.2019.09.013
47. Li Z, Wu X, Gu L, Shen Q, Luo W, Deng C, et al. Long Non-Coding RNA ATB Promotes Malignancy of Esophageal Squamous Cell Carcinoma by Regulating miR-200b/Kindlin-2 Axis. Cell Death Dis (2017) 6:e2888. doi: 10.1038/cddis.2017.245
48. Lou W, Ding B, Fu P. Pseudogene-Derived lncRNAs and Their miRNA Sponging Mechanism in Human Cancer. Front Cell Dev Biol (2020) 8:85. doi: 10.3389/fcell.2020.00085
49. Zhu H, Zhai B, He C, Li Z, Gao H, Niu Z, et al. LncRNA TTN-AS1 Promotes the Progression of Cholangiocarcinoma via the miR-320a/Neuropilin-1 Axis. Cell Death Dis (2020) 8:637. doi: 10.1038/s41419-020-02896-x
50. Wu XS, Wang F, Li HF, Hu YP, Jiang L, Zhang F, et al. LncRNA-PAGBC Acts as a microRNA Sponge and Promotes Gallbladder Tumorigenesis. EMBO Rep (2017) 10:1837–53. doi: 10.15252/embr.201744147
51. Chugunova A, Navalayeu T, Dontsova O, Sergiev P. Mining for Small Translated ORFs. J Proteome Res (2018) 1:1–11. doi: 10.1021/acs.jproteome.7b00707
52. Nam JW, Choi SW, You BH. Incredible RNA: Dual Functions of Coding and Noncoding. Mol Cells (2016) 5:367–74. doi: 10.14348/molcells.2016.0039
53. Ji Z, Song R, Regev A, Struhl K. Many lncRNAs, 5'utrs, and Pseudogenes Are Translated and Some Are Likely to Express Functional Proteins. Elife (2015) 4:e08890. doi: 10.7554/eLife.08890
54. Sun H, Chen C, Shi M, Wang D, Liu M, Li D, et al. Integration of Mass Spectrometry and RNA-Seq Data to Confirm Human Ab Initio Predicted Genes and lncRNAs. Proteomics (2014) 23-24:2760–8. doi: 10.1002/pmic.201400174
55. Mackowiak SD, Zauber H, Bielow C, Thiel D, Kutz K, Calviello L, et al. Extensive Identification and Analysis of Conserved Small ORFs in Animals. Genome Biol (2015) 16:179. doi: 10.1186/s13059-015-0742-x
56. Hartford CCR, Lal A. When Long Noncoding Becomes Protein Coding. Mol Cell Biol (2020) 40(6):e00528–19. doi: 10.1128/MCB.00528-19
57. Xing J, Liu H, Jiang W, Wang L. LncRNA-Encoded Peptide: Functions and Predicting Methods. Front Oncol (2020) 10:622294. doi: 10.3389/fonc.2020.622294
58. Chugunova A, Loseva E, Mazin P, Mitina A, Navalayeu T, Bilan D, et al. LINC00116 Codes for a Mitochondrial Peptide Linking Respiration and Lipid Metabolism. Proc Natl Acad Sci USA (2019) 11:4940–5. doi: 10.1073/pnas.1809105116
59. Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, et al. Toddler: An Embryonic Signal That Promotes Cell Movement via Apelin Receptors. Science (2014) 6172:1248636. doi: 10.1126/science.1248636
60. Huang JZ, Chen M, Chen D, Gao XC, Zhu S, Huang H, et al. A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth. Mol Cell (2017) 1:171–84.e6. doi: 10.1016/j.molcel.2017.09.015
61. Leng F, Miu YY, Zhang Y, Luo H, Lu XL, Cheng H, et al. A Micro-Peptide Encoded by HOXB-AS3 Promotes the Proliferation and Viability of Oral Squamous Cell Carcinoma Cell Lines by Directly Binding With IGF2BP2 to Stabilize C-Myc. Oncol Lett (2021) 4:697. doi: 10.3892/ol.2021.12958
62. D'Lima NG, Ma J, Winkler L, Chu Q, Loh KH, Corpuz EO, et al. A Human Microprotein That Interacts With the mRNA Decapping Complex. Nat Chem Biol (2017) 2:174–80. doi: 10.1038/nchembio.2249
63. Lu S, Zhang J, Lian X, Sun L, Meng K, Chen Y, et al. A Hidden Human Proteome Encoded by ‘Non-Coding’ Genes. Nucleic Acids Res (2019) 15:8111–25. doi: 10.1093/nar/gkz646
64. Wang Y, Wu S, Zhu X, Zhang L, Deng J, Li F, et al. LncRNA-Encoded Polypeptide ASRPS Inhibits Triple-Negative Breast Cancer Angiogenesis. J Exp Med (2020) 217(3):jem.20190950. doi: 10.1084/jem.20190950
65. Guo B, Wu S, Zhu X, Zhang L, Deng J, Li F, et al. Micropeptide CIP2A-BP Encoded by LINC00665 Inhibits Triple-Negative Breast Cancer Progression. EMBO J (2020) 1:e102190. doi: 10.15252/embj.2019102190
66. Polycarpou-Schwarz M, Gross M, Mestdagh P, Schott J, Grund SE, Hildenbrand C, et al. The Cancer-Associated Microprotein CASIMO1 Controls Cell Proliferation and Interacts With Squalene Epoxidase Modulating Lipid Droplet Formation. Oncogene (2018) 34:4750–68. doi: 10.1038/s41388-018-0281-5
67. Charpentier M, Croyal M, Carbonnelle D, Fortun A, Florenceau L, Rabu C, et al. IRES-Dependent Translation of the Long non Coding RNA Meloe in Melanoma Cells Produces the Most Immunogenic MELOE Antigens. Oncotarget (2016) 37:59704–13. doi: 10.18632/oncotarget.10923
68. Szafron LM, Balcerak A, Grzybowska EA, Pienkowska-Grela B, Felisiak-Golabek A, Podgorska A, et al. The Novel Gene CRNDE Encodes a Nuclear Peptide (CRNDEP) Which Is Overexpressed in Highly Proliferating Tissues. PloS One (2015) 5:e0127475. doi: 10.1371/journal.pone.0127475
69. Li XL, Pongor L, Tang W, Das S, Muys BR, Jones MF, et al. A Small Protein Encoded by a Putative lncRNA Regulates Apoptosis and Tumorigenicity in Human Colorectal Cancer Cells. Elife (2020) 9:e53734. doi: 10.7554/eLife.53734
70. Meng N, Chen M, Chen, Chen XH, Wang JZ, Zhu S, et al. Small Protein Hidden in lncRNA LOC90024 Promotes “Cancerous” RNA Splicing and Tumorigenesis. Adv Sci (Weinh) (2020) 10:1903233. doi: 10.1002/advs.201903233
71. Zhu S, Wang JZ, Chen, He YT, Meng N, Chen M, et al. An Oncopeptide Regulates M(6)A Recognition by the M(6)A Reader IGF2BP1 and Tumorigenesis. Nat Commun (2020) 1:1685. doi: 10.1038/s41467-020-15403-9
72. Ge Q, Jia D, Cen D, Qi Y, Shi C, Li J, et al. Micropeptide ASAP Encoded by LINC00467 Promotes Colorectal Cancer Progression by Directly Modulating ATP Synthase Activity. J Clin Invest (2021) e152911. doi: 10.1172/JCI152911
73. Pang Y, Liu Z, Han H, Wang B, Li W, Mao C, et al. Peptide SMIM30 Promotes HCC Development by Inducing SRC/YES1 Membrane Anchoring and MAPK Pathway Activation. J Hepatol (2020) 5:1155–69. doi: 10.1016/j.jhep.2020.05.028
74. Lun YZ, Pan ZP, Liu SA, Sun J, Han M, Liu B, et al. The Peptide Encoded by a Novel Putative lncRNA HBVPTPAP Inducing the Apoptosis of Hepatocellular Carcinoma Cells by Modulating JAK/STAT Signaling Pathways. Virus Res (2020) 287:198104. doi: 10.1016/j.virusres.2020.198104
75. Xu W, Deng B, Lin P, Liu C, Li B, Huang Q, et al. Ribosome Profiling Analysis Identified a KRAS-Interacting Microprotein That Represses Oncogenic Signaling in Hepatocellular Carcinoma Cells. Sci China Life Sci (2020) 4:529–42. doi: 10.1007/s11427-019-9580-5
76. Guo ZW, Meng Y, Zhai XM, Xie C, Zhao N, Li M, et al. Translated Long Non-Coding Ribonucleic Acid ZFAS1 Promotes Cancer Cell Migration by Elevating Reactive Oxygen Species Production in Hepatocellular Carcinoma. Front Genet (2019) 10:1111. doi: 10.3389/fgene.2019.01111
77. Xiang X, Fu Y, Zhao K, Miao R, Zhang X, Ma X, et al. Cellular Senescence in Hepatocellular Carcinoma Induced by a Long Non-Coding RNA-Encoded Peptide PINT87aa by Blocking FOXM1-Mediated PHB2. Theranostics (2021) 10:4929–44. doi: 10.7150/thno.55672
78. Li M, Li X, Zhang Y, Wu H, Zhou H, Ding X, et al. Micropeptide MIAC Inhibits HNSCC Progression by Interacting With Aquaporin 2. J Am Chem Soc (2020) 14:6708–16. doi: 10.1021/jacs.0c00706
79. Wu S, Zhang L, Deng J, Guo B, Li F, Wang Y, et al. A Novel Micropeptide Encoded by Y-Linked LINC00278 Links Cigarette Smoking and AR Signaling in Male Esophageal Squamous Cell Carcinoma. Cancer Res (2020) 13:2790–803. doi: 10.1158/0008-5472.CAN-19-3440
80. Cao Y, Yang R, Lee I, Zhang W, Sun J, Meng X, et al. Prediction of LncRNA-Encoded Small Peptides in Glioma and Oligomer Channel Functional Analysis Using in Silico Approaches. PloS One (2021) 3:e0248634. doi: 10.1371/journal.pone.0248634
81. Xu S, Jia G, Zhang H, Wang L, Cong Y, Lv M, et al. LncRNA HOXB-AS3 Promotes Growth, Invasion and Migration of Epithelial Ovarian Cancer by Altering Glycolysis. Life Sci (2021) 264:118636. doi: 10.1016/j.lfs.2020.118636
82. Jiang W, Kai J, Li D, Wei Z, Wang Y, Wang W. lncRNA HOXB-AS3 Exacerbates Proliferation, Migration, and Invasion of Lung Cancer via Activating the PI3K-AKT Pathway. J Cell Physiol (2020) 10:7194–203. doi: 10.1002/jcp.29618
83. Zhang XM, Chen H, Zhou B, Zhang QY, Liao Y, Wang JS, et al. lncRNA HOXB-AS3 Promotes Hepatoma by Inhibiting P53 Expression. Eur Rev Med Pharmacol Sci (2018) 20:6784–92. doi: 10.26355/eurrev_201810_16145
84. Papaioannou D, Petri A, Dovey OM, Terreri S, Wang E, Collins FA, et al. The Long Non-Coding RNA HOXB-AS3 Regulates Ribosomal RNA Transcription in NPM1-Mutated Acute Myeloid Leukemia. Nat Commun (2019) 1:5351. doi: 10.1038/s41467-019-13259-2
85. Huang HH, Chen FY, Chou WC, Hou HA, Ko BS, Lin CT, et al. Long Non-Coding RNA HOXB-AS3 Promotes Myeloid Cell Proliferation and Its Higher Expression Is an Adverse Prognostic Marker in Patients With Acute Myeloid Leukemia and Myelodysplastic Syndrome. BMC Cancer (2019) 1:617. doi: 10.1186/s12885-019-5822-y
86. Xing Y, Sun X, Li F, Jiang X, Jiang A, Li X, et al. Long Non-Coding RNA (lncRNA) HOXB-AS3 Promotes Cell Proliferation and Inhibits Apoptosis by Regulating ADAM9 Expression Through Targeting miR-498-5p in Endometrial Carcinoma. J Int Med Res (2021) 6:3000605211013548. doi: 10.1177/03000605211013548
87. Zhuang XH, Liu Y, Li JL. Overexpression of Long Noncoding RNA HOXB-AS3 Indicates an Unfavorable Prognosis and Promotes Tumorigenesis in Epithelial Ovarian Cancer via Wnt/beta-Catenin Signaling Pathway. Biosci Rep (2019) 39(8):BSR20190906. doi: 10.1042/BSR20190906
88. Zhu L, Liu XL, Fu ZQ, Qiu P, Feng T, Yan B, et al. LINC00675 Suppresses Proliferative, Migration and Invasion of Clear Cell Renal Cell Carcinoma via the Wnt/beta-Catenin Pathway. Eur Rev Med Pharmacol Sci (2020) 5:2313–20. doi: 10.26355/eurrev_202003_20497
89. Yao M, Shi X, Li Y, Xiao Y, Butler W, Huang Y, et al. LINC00675 Activates Androgen Receptor Axis Signaling Pathway to Promote Castration-Resistant Prostate Cancer Progression. Cell Death Dis (2020) 8:638. doi: 10.1038/s41419-020-02856-5
90. Pan Y, Fang Y, Xie M, Liu Y, Yu T, Wu X, et al. LINC00675 Suppresses Cell Proliferation and Migration via Downregulating the H3K4me2 Level at the SPRY4 Promoter in Gastric Cancer. Mol Ther Nucleic Acids (2020) 22:766–78. doi: 10.1016/j.omtn.2020.09.038
91. Li Z, Li Y, Wang Q. LINC00675 Is a Prognostic Factor and Regulates Cell Proliferation, Migration and Invasion in Glioma. Biosci Rep (2018) 38(5):BSR20181039. doi: 10.1042/BSR20181039
92. Liu X, Ke J, Gu L, Tang H, Luo X. Long Non-Coding RNA LINC00675 Is Associated With Bladder Cancer Metastasis and Patient Survival. J Gene Med (2020) 9:e3210. doi: 10.1002/jgm.3210
93. Lu L, Li S, Zhang Y, Luo Z, Chen Y, Ma J, et al. GFI1-Mediated Upregulation of LINC00675 as a ceRNA Restrains Hepatocellular Carcinoma Metastasis by Sponging miR-942-5p. Front Oncol (2020) 10:607593. doi: 10.3389/fonc.2020.607593
94. Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol (2018) 11:1543–52. doi: 10.1001/jamaoncol.2018.3676
95. Zhong YB, Shan AJ, Lv W, Wang J, Xu JZ. Long Non-Coding RNA LINC00675 Inhibits Tumorigenesis and EMT via Repressing Wnt/beta-Catenin Signaling in Esophageal Squamous Cell Carcinoma. Eur Rev Med Pharmacol Sci (2018) 23:8288–97. doi: 10.26355/eurrev_201812_16526
96. Shan Z, An N, Qin J, Yang J, Sun H, Yang W. Long Non-Coding RNA Linc00675 Suppresses Cell Proliferation and Metastasis in Colorectal Cancer via Acting on miR-942 and Wnt/Beta-Catenin Signaling. BioMed Pharmacother (2018) 101:769–76. doi: 10.1016/j.biopha.2018.02.123
97. Li DD, Fu ZQ, Lin Q, Zhou Y, Zhou QB, Li ZH, et al. Linc00675 Is a Novel Marker of Short Survival and Recurrence in Patients With Pancreatic Ductal Adenocarcinoma. World J Gastroenterol (2015) 31:9348–57. doi: 10.3748/wjg.v21.i31.9348
98. Giulietti M, Righetti A, Principato G, Piva F. LncRNA Co-Expression Network Analysis Reveals Novel Biomarkers for Pancreatic Cancer. Carcinogenesis (2018) 8:1016–25. doi: 10.1093/carcin/bgy069
99. Zeng S, Xie X, Xiao YF, Tang B, Hu CJ, Wang SM, et al. Long Noncoding RNA LINC00675 Enhances Phosphorylation of Vimentin on Ser83 to Suppress Gastric Cancer Progression. Cancer Lett (2018) 412:179–87. doi: 10.1016/j.canlet.2017.10.026
100. Zhang G, Chen Z, Zhang Y, Li T, Bao Y, Zhang S. Inhibition of miR-103a-3p Suppresses the Proliferation in Oral Squamous Cell Carcinoma Cells via Targeting RCAN1. Neoplasma (2020) 3:461–72. doi: 10.4149/neo_2020_190430N382
101. Liu H, Gu X, Wang G, Huang Y, Ju S, Huang J, et al. Copy Number Variations Primed lncRNAs Deregulation Contribute to Poor Prognosis in Colorectal Cancer. Aging (Albany NY) (2019) 16:6089–108. doi: 10.18632/aging.102168
102. Zheng S, Wan L, Ge D, Jiang F, Qian Z, Tang J, et al. LINC00266-1/miR-548c-3p/SMAD2 Feedback Loop Stimulates the Development of Osteosarcoma. Cell Death Dis (2020) 7:576. doi: 10.1038/s41419-020-02764-8
103. Cai H, Yu Y, Ni X, Li C, Hu Y, Wang J, et al. LncRNA LINC00998 Inhibits the Malignant Glioma Phenotype via the CBX3-Mediated C-Met/Akt/mTOR Axis. Cell Death Dis (2020) 12:1032. doi: 10.1038/s41419-020-03247-6
104. Zhou D, Xie M, He B, Gao Y, Yu Q, He B, et al. Microarray Data Re-Annotation Reveals Specific lncRNAs and Their Potential Functions in Non-Small Cell Lung Cancer Subtypes. Mol Med Rep (2017) 4:5129–36. doi: 10.3892/mmr.2017.7244
105. He X, Li S, Yu B, Kuang G, Wu Y, Zhang M, et al. Up-Regulation of LINC00467 Promotes the Tumourigenesis in Colorectal Cancer. J Cancer (2019) 25:6405–13. doi: 10.7150/jca.32216
106. Bai Y, Wu H, Han B, Xu K, Liu Y, Liu Y, et al. Long Intergenic Non-Protein Coding RNA-467 Targets microRNA-451a in Human Colorectal Cancer. Oncol Lett (2020) 5:124. doi: 10.3892/ol.2020.11987
107. Li Z, Liu J, Chen H, Zhang Y, Shi H, Huang L, et al. Ferritin Light Chain (FTL) Competes With Long Noncoding RNA Linc00467 for miR-133b Binding Site to Regulate Chemoresistance and Metastasis of Colorectal Cancer. Carcinogenesis (2020) 4:467–77. doi: 10.1093/carcin/bgz181
108. Luo P, Liang C, Zhang X, Liu X, Wang Y, Wu M, et al. Identification of Long Non-Coding RNA ZFAS1 as a Novel Biomarker for Diagnosis of HCC. Biosci Rep (2018) 38(4):BSR20171359. doi: 10.1042/BSR20171359
109. Tan C, Cao J, Chen L, Xi X, Wang S, Zhu Y, et al. Noncoding RNAs Serve as Diagnosis and Prognosis Biomarkers for Hepatocellular Carcinoma. Clin Chem (2019) 7:905–15. doi: 10.1373/clinchem.2018.301150
110. Zhou HL, Zhou YF, Feng ZT. Long Noncoding RNA ZFAS1 Promotes Hepatocellular Carcinoma Proliferation by Epigenetically Repressing miR-193a-3p. Eur Rev Med Pharmacol Sci (2019) 22:9840–7. doi: 10.26355/eurrev_201911_19547
111. Lin JC, Yang PM, Liu TP. PERK/ATF4-Dependent ZFAS1 Upregulation Is Associated With Sorafenib Resistance in Hepatocellular Carcinoma Cells. Int J Mol Sci (2021) 22(11):5848. doi: 10.3390/ijms22115848
112. Lu Q, Huo J, Liu P, Bai L, Ma A. lncRNA HOXB-AS3 Protects Doxorubicin-Induced Cardiotoxicity by Targeting miRNA-875-3p. Exp Ther Med (2020) 2:1388–92. doi: 10.3892/etm.2019.8335
113. Zhong J, Cui Y, Guo J, Chen Z, Yang L, He QY, et al. Resolving Chromosome-Centric Human Proteome With Translating mRNA Analysis: A Strategic Demonstration. J Proteome Res (2014) 1:50–9. doi: 10.1021/pr4007409
114. Chang C, Li L, Zhang C, Wu S, Guo K, Zi J, et al. Systematic Analyses of the Transcriptome, Translatome, and Proteome Provide a Global View and Potential Strategy for the C-HPP. J Proteome Res (2014) 1:38–49. doi: 10.1021/pr4009018
115. Lian X, Guo J, Gu W, Cui Y, Zhong J, Jin J, et al. Genome-Wide and Experimental Resolution of Relative Translation Elongation Speed at Individual Gene Level in Human Cells. PloS Genet (2016) 2:e1005901. doi: 10.1371/journal.pgen.1005901
116. Centofanti F, Santoro M, Marini M, Visconti VV, Rinaldi AM, Celi M, et al. Identification of Aberrantly-Expressed Long Non-Coding RNAs in Osteoblastic Cells From Osteoporotic Patients. Biomedicines (2020) 8(3):65. doi: 10.3390/biomedicines8030065
117. Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z, et al. Amplification of Long Noncoding RNA ZFAS1 Promotes Metastasis in Hepatocellular Carcinoma. Cancer Res (2015) 15:3181–91. doi: 10.1158/0008-5472.CAN-14-3721
118. Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int J Mol Sci (2019) 20(22):5758. doi: 10.3390/ijms20225758
Keywords: long non-coding RNA (lncRNA), ORF, small peptide, gastrointestinal cancer, function
Citation: Chen Y, Long W, Yang L, Zhao Y, Wu X, Li M, Du F, Chen Y, Yang Z, Wen Q, Yi T, Xiao Z and Shen J (2021) Functional Peptides Encoded by Long Non-Coding RNAs in Gastrointestinal Cancer. Front. Oncol. 11:777374. doi: 10.3389/fonc.2021.777374
Received: 15 September 2021; Accepted: 28 October 2021;
Published: 23 November 2021.
Edited by:
Peng Qu, National Institutes of Health (NIH), United StatesReviewed by:
Yafeng He, National Heart, Lung, and Blood Institute (NHLBI), United StatesLikui Feng, The Rockefeller University, United States
Copyright © 2021 Chen, Long, Yang, Zhao, Wu, Li, Du, Chen, Yang, Wen, Yi, Xiao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Shen, amluZ3NoZW5Ac3dtdS5lZHUuY24=; Zhangang Xiao, emhhbmdhbmd4aWFvQHN3bXUuZWR1LmNu
†These authors have contributed equally to this work
 Yao Chen1,2,3†
Yao Chen1,2,3† Weili Long
Weili Long Liqiong Yang
Liqiong Yang Yueshui Zhao
Yueshui Zhao Xu Wu
Xu Wu Zhihui Yang
Zhihui Yang Tao Yi
Tao Yi Zhangang Xiao
Zhangang Xiao Jing Shen
Jing Shen