- 1Department of Neurosurgery, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Eight-Year Program of Clinical Medicine, Peking Union Medical College Hospital (PUMCH), Chinese Academe of Medical Sciences & Peking Union Medical College (CAMS & PUMC), Beijing, China
Most pituitary tumors are considered benign adenomas, and only 0.1%–0.2% of them present metastasis and are defined as pituitary carcinomas (PCs). Refractory pituitary adenomas (PAs) lie between benign adenomas and true malignant PCs and are defined as aggressive-invasive PAs, characterized by a high Ki-67 index, rapid growth, frequent recurrence, and resistance to conventional treatments. Refractory PAs and PCs are notoriously difficult to manage because of limited therapeutic options. Vascular endothelial growth factor (VEGF) plays a crucial role in angiogenesis not only during development but also during pathological processes in pituitary tumors. Recently, increasing numbers of preclinical studies and clinical research have demonstrated that anti-VEGF therapy plays an important role in pituitary tumors. The purpose of this review is to report the role of VEGF in the development and pathology of pituitary tumors and the progress of anti-VEGF therapy in pituitary tumors, including refractory PAs and PCs. Previous preclinical studies indicated that cyclin-dependent kinase 5 (CDK5)-mediated VEGF expression might play a crucial role in the development of PAs. Vascular endothelial growth inhibitors have been reported as independent predictors of invasion in human PAs and have been indicated as markers for poor outcome. Furthermore, several studies have reported that angiogenesis decreases tumor sizes in experimental animal models of pituitary tumors. The expression of VEGF is relatively high in PAs; therefore, anti-VEGF therapy has been used in some refractory PAs and PCs. To date, anti-VEGF has been reported as monotherapy, in combination with temozolomide (TMZ), TMZ and radiotherapy, and with pasireotide, which might be a promising alternative therapy for refractory PAs and PCs resistant to conventional treatments. However, the role of anti-VEGF therapy in pituitary tumors is still controversial due to a lack of large-scale clinical trials. In summary, the results from preclinical studies and clinical trials indicated that anti-VEGF therapy monotherapy or in combination with other treatments may be a promising alternative therapy for refractory PAs and PCs resistant to conventional treatments. More preclinical studies and clinical trials are needed to further evaluate the exact efficacy of anti-VEGF in refractory PAs and PCs.
Introduction
Pituitary adenomas (PAs) are common tumors arising in the anterior pituitary gland with the second highest incidence, representing approximately 10%–15% of intracranial primary tumors (1–3). Most PAs are considered benign tumors that can be cured by surgery and medication. However, a subset of invasive PAs with a high Ki-67, rapid growth, and early recurrences is refractory to conventional treatments such as surgery, medication, and radiotherapy and are referred to as refractory PAs (4). Rarely, 0.1%–0.2% of pituitary tumors can present with either craniospinal dissemination or systemic metastases, which are true malignant tumors and defined as pituitary carcinomas (PCs) (5). Refractory PAs and PCs are notoriously difficult to manage because of limited availability of therapeutic approaches. Recently, temozolomide (TMZ) has been recommended as a first-line treatment for refractory PAs and PCs by the European Society of Endocrinology due to its promising efficacy. However, only approximately 60% of patients show a response to TMZ, and some patients develop resistance during treatment (6, 7). Therefore, the discovery of new therapeutic targets is of particular importance for the management of refractory PAs and PCs. Recent studies have shown that vascular endothelial growth factor (VEGF) and its receptor (VEGFR) play crucial roles in angiogenesis not only in its development but also during pathological processes in pituitary tumors (8). Moreover, an increasing number of clinical case reports have demonstrated that anti-VEGF therapy is beneficial in treating refractory PAs and PCs. Here, this review presents the role of the VEGF/VEGFR pathway in angiogenesis of pituitary tumors and the progress of anti-VEGF therapy in pituitary tumors, including refractory PAs and PCs.
Angiogenesis in Pituitary Tumors
Angiogenesis, the process of blood vessel growth, is essential for tumor progression and metastasis (9). During angiogenesis, an organized vascular network develops from a primitive vascular network (10). Angiogenesis correlates with the development of metastasis (11–13), recurrence (14), and poor prognosis (15, 16) in many human tumors, including breast, bladder, prostate, and stomach tumors. Contrary to most solid tumors, PA tissue contains fewer blood vessels than normal pituitary glands (17). In particular, not only was the number of vessels much lower but also the size of each vessel was much smaller in PAs than in normal pituitary glands (17–22). The angiogenesis between different PA subtypes is divergent among studies. Jugenburg et al. (22) reported that PAs have significantly lower vascular densities than non-tumorous adenohypophyses. Pituitary prolactin (PRL)-secreting adenomas have the highest vascular densities, and growth hormone (GH)-producing adenomas have the lowest vascular densities. However, no differences were observed between noninvasive and invasive PAs. Primary PCs show no significant increase in vascular densities, but some metastatic tumors exhibit high vascularity. These results indicated that PAs have a limited capacity to induce angiogenesis. Another study demonstrated that the highest counts of immunopositive vascular profiles were noted in follicle-stimulating hormone (FSH)-expressing adenomas, whereas the lowest vascular density was observed in GH-expressing tumors (22, 23). Angiogenesis has been shown to be related to clinical behavior, prognosis, and response to treatment in many different types of PAs. Turner et al. (17, 24) reported that invasive macroprolactinomas were significantly more vascular than noninvasive tumors; however, medical therapy with metyrapone or bromocriptine did not influence angiogenesis in adenomas. Vidal et al. (25) also reported a tendency of invasive PAs to be more highly vascularized than noninvasive PAs; the highest level of microvessel density was found in PCs, while the lowest was found in GH-producing adenomas. Moreover, they demonstrated that the microvessel density of macroadenomas in older patients was significantly higher than that in patients younger than 40 years (25). In summary, PAs are usually less vascularized than normal pituitary glands, while PCs are more vascular than PAs. Although the vascular densities may be related to tumor size, proliferation, hemorrhage, and the treatment response of PAs (19–22, 25), it is still unclear what specific role they play in the tumorigenesis and progression of PAs.
Vascular Endothelial Growth Factor Expression in Pituitary Tumors
VEGFs are key mediators of endothelial cell proliferation, angiogenesis, and vascular permeability. VEGFs are a family of angiogenic and lymphangiogenic growth factors. VEGF pathways comprise multiple VEGF glycoproteins (VEGFA, VEGFB, VEGFC, VEGFD, and VEGFE) and multiple transmembrane receptors (VEGFR1, VEGFR2, and VEGFR3) (26). VEGFA, commonly referred to as VEGF, has multiple isoforms as a result of alternative exon splicing (27). Although they have various affinities, these isoforms are all capable of binding to VEGFR1 or VEGFR2. VEGFR has intracellular tyrosine kinase activity, which is considered to be the major mediator of the angiogenic properties of VEGF. VEGF binds to the external membrane domain of VEGFR and causes intracellular signaling in endothelial cells, resulting in proliferation and migration (28). VEGF and VEGFR contribute to a potential therapeutic target in a variety of tumors (29–31). VEGF and its receptors are regularly overexpressed in a wide variety of human cancers, including PAs and PCs. Although the concordance of VEGF expression between studies may be poor, in general, VEGF immunoreactivity is moderate to strong in most cases (32). Lloyd et al. (33) analyzed VEGF expression in 148 cases and found positive staining in all subtypes, with a mild to moderate degree in 92.3% (131/142) of PAs and a strong degree in 100% (6/6) of PCs. Fukui et al. (34) also found that VEGF expression was weak in 12.5% (6/48), moderate in 54.2% (26/48), and strong in 33.3% (16/48) in a total of 48 PAs. Wang et al. (35) reported that 58.9% of 197 PAs had strong VEGF expression. VEGF mRNA was detected in more than 85% of PAs and had a significant correlation with VEGF protein expression (32, 36). VEGF expression varies in different subtypes of PAs (33, 35, 37). High VEGF expression was found in nonfunctioning (19, 21, 33, 35, 38) and pituitary adrenocorticotropic hormone (ACTH) (19, 33, 35)-, GH (19, 33, 38)-, PRL (35, 37, 38)-, and FSH (35, 37)-secreting PAs. In tumor tissues, pituitary GH- and PRL-secreting adenomas had diffuse VEGF distribution, while ACTH-, TSH-, and luteinizing hormone (LH)-secreting adenomas showed focal VEGF expression (32, 36, 39). In addition to tumor cells, VEGF mRNA and VEGF expression were mainly present in endothelial cells and folliculostellate cells (36, 40, 41). PCs had significantly higher VEGF mRNA amplification and stronger VEGF immunostaining than those of PAs (33). Therefore, different subtypes of PAs have different levels of VEGF, indicating that anti-VEGF therapy has distinct therapeutic effects on different subtypes of PAs.
VEGF has significant roles in the development of tumor neovascularity and peritumoral edema. Anti-VEGF antibodies removed 75%–99% of the permeability activity (42). Evidence has shown that VEGF is correlated with the pathogenesis of cystic formation in PAs (34). Other features affected by VEGF expression remain controversial. Overexpression of VEGF was associated with intratumoral hemorrhage (43), extrasellar invasion (37, 44), and rapid recurrence (37), although these findings were not significant in other studies (19, 21, 34, 35, 37, 38, 45, 46). Moreover, as shown in several studies, VEGF expression had no relation with tumor size (19, 34, 35, 45) or Ki-67 index (21, 38, 43). Moreover, no clear association was found between microvessel density and VEGF expression (19, 21). The low microvessel density despite VEGF overexpression has caused researchers to ask if inhibitory factors related to VEGF exist in PAs (36). The role of VEGF in the development and progression of PAs is still controversial; however, the expression of VEGF has not yet been used as a conclusive marker of the aggressive behavior of PAs. Current studies indicate that VEGF might play a role in tumoral vascular growth, not by increasing the number of vessels, but by other mechanisms, such as an increase in vascular permeability that favors the abundant diffusion of nutrients.
Preclinical Studies of Angiogenesis in Pituitary Tumors
Preclinical data indicated that VEGF is a potential therapeutic target in PAs. A previous study demonstrated that VEGF plays a crucial role in tumor angiogenesis during the development of a rat prolactinoma animal model (40). Estrogen-induced prolactinoma expresses a high level of VEGF associated with marked angiogenesis (47). Anti-VEGF resulted in a significant shrinkage in tumor volume, a decrease in the Ki-67 index, and the repair of pituitary vessels (48). Additionally, the characteristic “blood lakes” in prolactinoma were replaced by repaired microvascular structures on three-dimensional (3D) observation under a confocal laser scanning microscope. The current first-line therapy for prolactinomas is dopamine (DA) agonists (Das). Dopamine D2 receptors (D2Rs), which are widely localized in the anterior and intermediate lobes of pituitary glands, can combine with DA to activate signaling cascades (49). DA therapy targeting D2R yields an excellent response in prolactinomas and some clinical benefits in non-prolactinoma pituitary tumors (50). The decrease in D2R expression may explain the resistance to DA. Previous studies have identified the association between VEGF and D2R. In D2R knockout mice, Cristina et al. (51) reported increases in VEGF mRNA transcription, VEGF expression, and highly vascular adenomas. When treating D2R-deficient mice with anti-VEGF, Luque et al. (52, 53) noticed a substantial decrease in serum prolactin, a reduction in tumor size, and a significant decrease in vascularity. Furthermore, anti-VEGF might have additive effects in combination with drugs targeting complementary pathways related to angiogenesis. In mice with hemorrhagic prolactinoma, monotherapy with anti-VEGF or DA can restrain tumor growth and improve vascular remodeling. Only the combination of anti-VEGF and DA can suppress intratumoral hemorrhage (54). In concurrence, prolonged DA treatment enhanced pituitary VEGF expression in wild-type mice (51). These findings provide a provocative possibility of combination therapy with anti-VEGF and DA.
Therapeutic Targeting of Vascular Endothelial Growth Factors in Pituitary Tumors
Bevacizumab
PAs and PCs highly express VEGF, which is one of the justifications for targeting VEGF and its receptors in this disease. Anti-VEGF has demonstrated significant activity as a single agent in murine studies. The recombinant humanized monoclonal antibody bevacizumab is the first approved agent directed against VEGF (Figure 1 and Table 1). The common side effects of bevacizumab are fatigue, hoarseness, and hypertension. The rare side effects of this agent include clotting, hemorrhage, wound-healing disorders, gastrointestinal perforation, reversible posterior leukoencephalopathy syndrome, and proteinuria (55). Bevacizumab needs to be administered only every 2 or 3 weeks due to its prolonged half-life. This agent can be readily combined with chemotherapy agents, and preclinical evidence indicates synergy for some combinations of chemotherapeutic compounds when used alongside bevacizumab. Bevacizumab has thus far been the drug most tried for targeting the VEGF pathway in pituitary tumors.
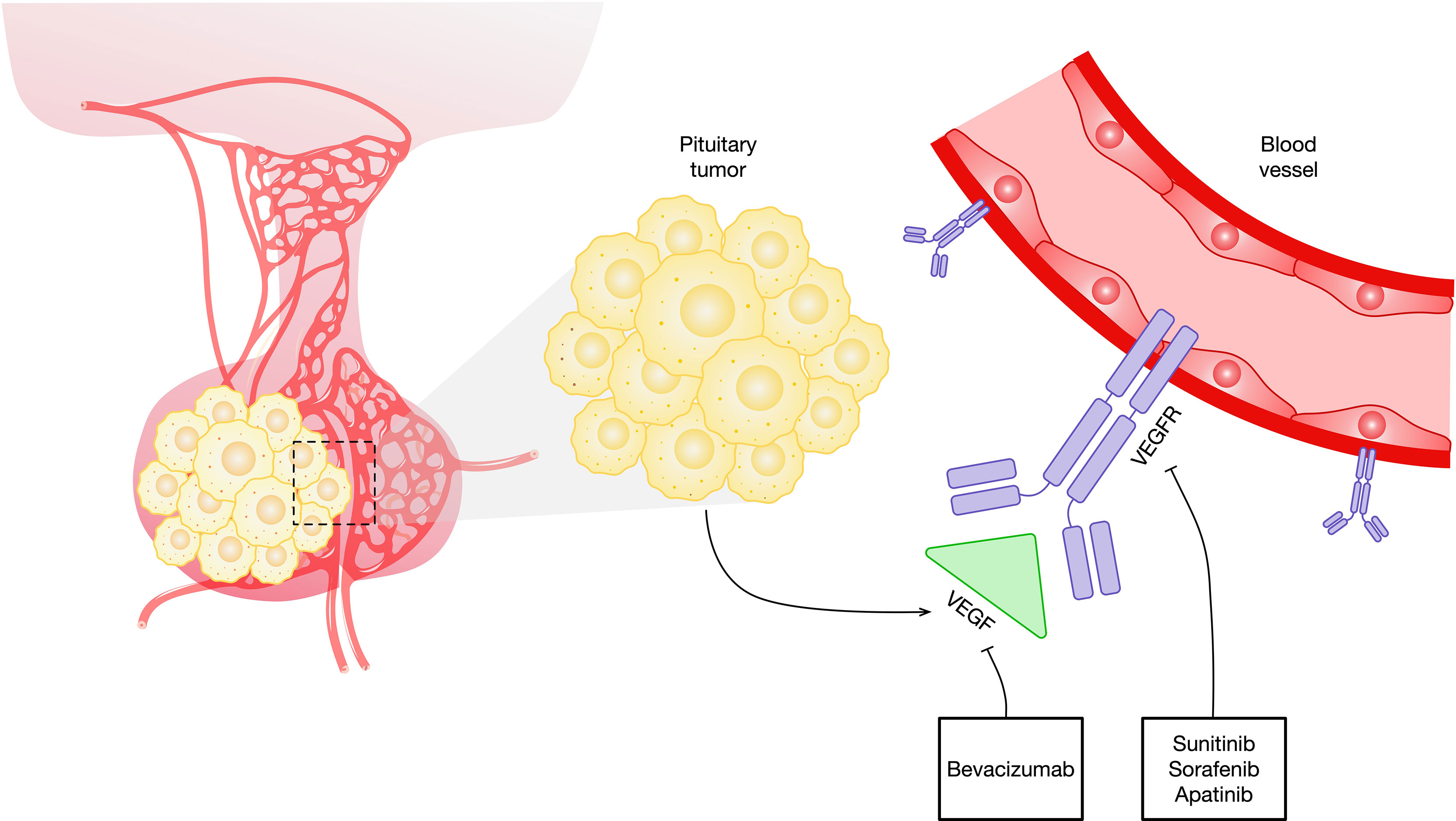
Figure 1 Schematic representation of antiangiogenic agents that target the VEGF and VEGF signaling pathways in pituitary tumors. VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
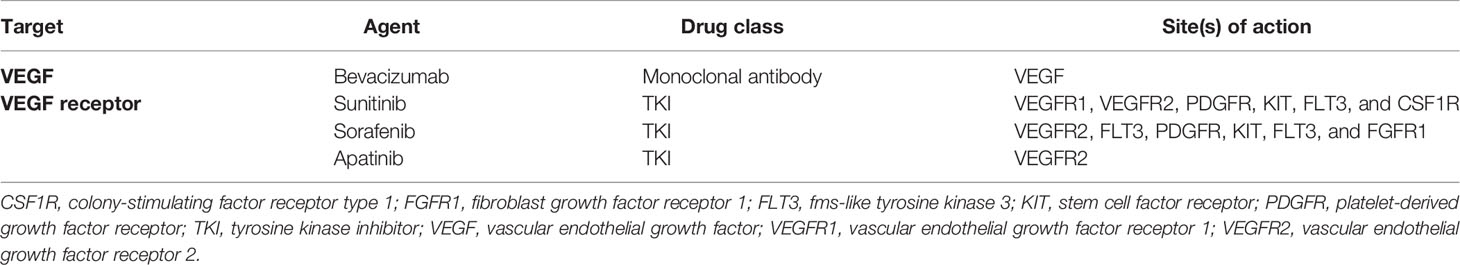
Table 1 Targets and sites of action of the VEGF angiogenesis receptor and ligand in pituitary tumors.
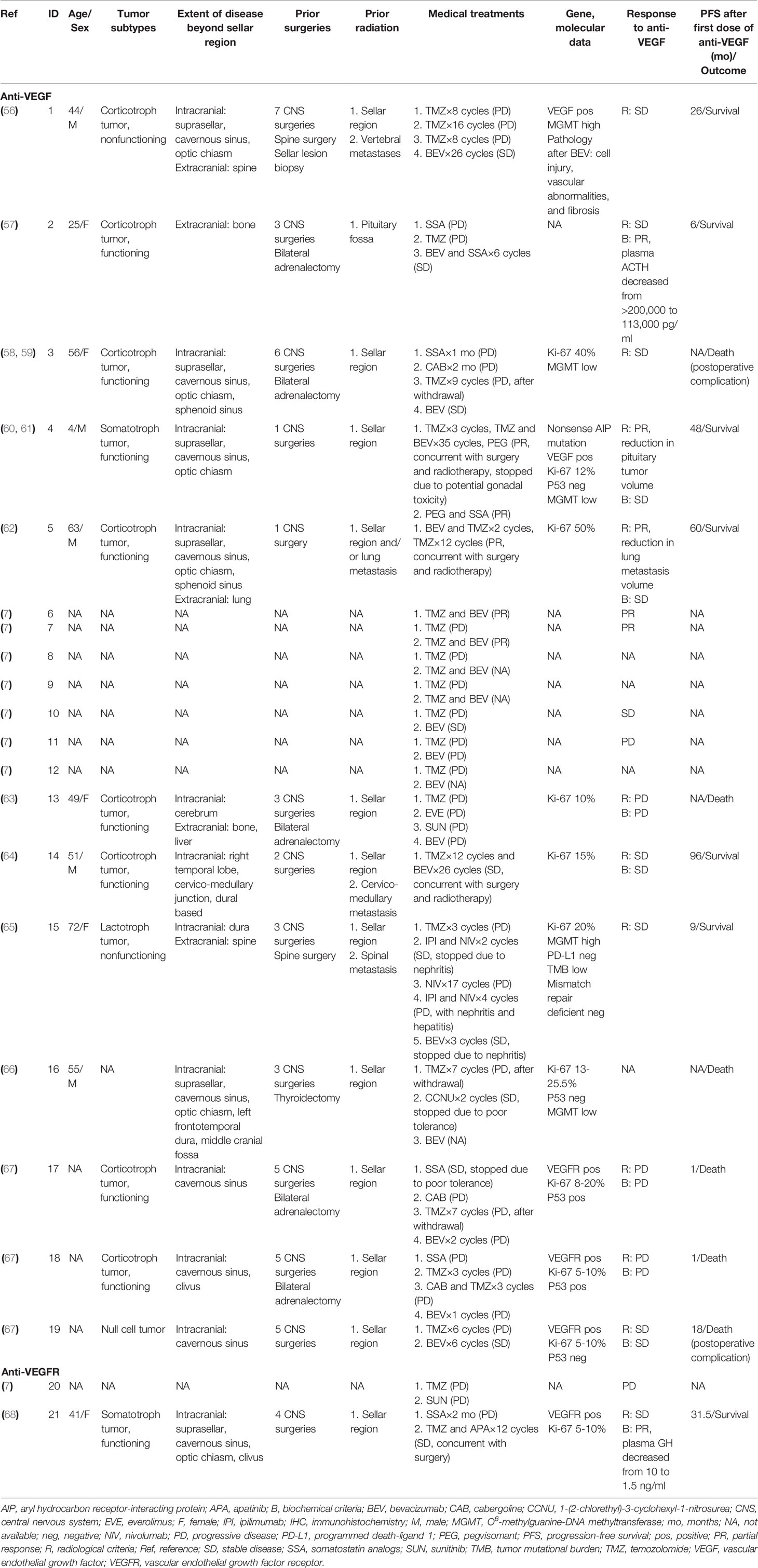
The published clinical cases (7, 56–68) are presented in Table 2. In this review, we used the same criteria used in the recent European Society of Endocrinology survey (7). A complete radiological response was defined as no visible tumor, partial response (PR) as at least 30% tumor regression, stable disease (SD) as less than 30% regression but no more than a 10% increase, and progressive disease (PD) as more than a 10% increase in tumor size or presentation of new metastasis. For functioning tumors, complete biochemical response was defined as normalization of hormone concentration, PR as more than a 20% reduction in hormone, SD as less than but no more than a 20% change in hormone, and PD as more than a 20% increase in hormone levels. To date, 19 cases treated with bevacizumab have been reported. Among these cases, eight are corticotroph tumors. Three were other subtypes (one somatotroph, one lactotroph, and one null cell), and the subtypes of other cases were not available. The majority of the PAs (8/11) were clinically functioning when the cases were reported; five in 12 cases presented with extracranial metastases, and seven in 12 were diagnosed with PC at the time of data collection. Most of the patients (9/12) underwent more than two surgeries in the sella. All patients received radiotherapy. One hundred percent (10/10) of tumors showed a Ki-67 index ≥10% at the last pathological examination.
Of the 12 patients to whom TMZ was administered prior to bevacizumab, all yielded PD. A second course of TMZ was administered to two patients (one on monotherapy, one on TMZ combined with cabergoline), which resulted in further progress. Notably, O6-methylguanine-DNA methyltransferase (MGMT) immunohistochemistry was observed to be low in two and high in two. None of the four cases responded to TMZ. Bevacizumab was chosen as the second- or third-line therapy after TMZ failed. Six patients achieved SD [five on monotherapy, one on somatostatin analog (SSA) + bevacizumab], and four had disease progression. Ortiz et al. (56) reported an aggressive silent corticotroph cell PA that progressed to carcinoma despite TMZ administration and was subsequently treated with bevacizumab, achieving 26 months of SD, as documented on serial MRI and positron emission tomography scans. Bevacizumab therapy resulted in severe cell injury, vascular abnormalities, and fibrosis in tumors. This case first revealed the effectiveness of targeting VEGF in blocking angiogenesis, thus inhibiting tumor growth. VEGF immunoreactivity was positive in this case. However, VEGF/VEGFR immunoreactivity may not directly demonstrate efficacy. In another three patients with VEGFR expression in PC, two showed poor responses to bevacizumab (67).
In the other seven cases, bevacizumab was administered in parallel with TMZ as the first-line therapy. Although the outcomes were not available in two cases, PR or SD was reported in five patients, including one who failed to receive TMZ as a first-line therapy. Preclinical studies showed that most PAs exhibited low expression of MGMT and high expression of VEGF, while the expression of VEGF was positively associated with MGMT (35). TMZ and bevacizumab might be considered a combination therapy under the premise of indications. Touma et al. (62) reported a patient with ACTH-secreting PC who received adenomectomy in combination with radiation, TMZ, and bevacizumab and was kept in remission over 5 years of follow-up after therapy. Rotman et al. (64) reported a comparable result in another case with a corticotroph PC. The patient underwent surgery and radiotherapy for metastasis, followed by combined, overlapping chemotherapy with TMZ and bevacizumab, leading to a progression-free survival of 8 years. In the ESE survey (7) on 166 patients with aggressive PAs or PCs, seven were administered bevacizumab once, as shown in Table 2. Three patients were treated with bevacizumab monotherapy, resulting in SD in one patient and PD in one patient. Four patients took bevacizumab combined with TMZ, and 50% (2/2) had PR. These observations are consistent with other studies that have shown complementary effects of anti-VEGF combined with drugs targeting alternative pathways implicated in angiogenesis and further underline the importance of combination therapies when choosing bevacizumab.
Importantly, bevacizumab is a new option in the treatment of aryl hydrocarbon receptor-interacting protein (AIP)-related PA. Inactivating germline mutations in the AIP gene are linked to PA predisposition. Korbonits et al. (60) and Dutta et al. (61) treated a 4-year-old child diagnosed with AIP-mutated somatotroph PA with combination therapy of TMZ and bevacizumab concomitantly with radiation and pegvisomant, which stabilized tumor growth and hormone secretion over 4 years. This case revealed that bevacizumab could play a role in controlling genetically driven refractory PAs.
Tyrosine Kinase Inhibitors
Although bevacizumab has been the most studied VEGF inhibitor in pituitary tumors, various other agents are in development (Table 1). The majority of these agents are tyrosine kinase (TK) inhibitors. Sunitinib and sorafenib are small molecules that inhibit multiple TK receptors, some of which are implicated in angiogenesis, tumor growth, and metastatic progression (Figure 1) (69–71). Sunitinib and sorafenib have been approved in different clinical scenarios such as advanced renal cell carcinoma (72) and local or metastatic thyroid carcinoma refractory to radioactive iodine treatment (73) and hence are used in the treatment of pituitary metastasis from renal cell carcinoma (74–81) and thyroid carcinoma (82, 83). Apatinib, also known as rivoceranib, is a TK inhibitor that selectively targets VEGFR (Figure 1) (84). The toxicity and side-effect profile of TK inhibitors varies as a function of their target TKs, including hematological events (anemia, neutropenia, and thrombocytopenia), diarrhea, nausea, fatigue, hypertension, skin rash, elevation of liver enzymes, and proteinuria.
Sunitinib has been reported in the treatment of PAs and PC in two cases thus far. Both cases had observed PD (Table 2). Apatinib was administered in a 41-year-old female in combination with TMZ as a second-line treatment (68). This patient was diagnosed with GH-secreting recurrent PA that resisted surgeries, radiation, and SSA. As VEGFR was expressed in the tumor, apatinib and TMZ were recommended. She achieved stabilization in the tumor and a decrease in serum GH levels over a period of 31.5 months of follow-up.
TK inhibitors might represent a therapeutic target in PAs associated with somatic genetic defects. Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder characterized by tumors of the pituitary gland, parathyroid gland, endocrine-gastrointestinal tract, and pancreas. In patients with MEN1, PAs are usually diagnosed at an earlier age, have higher degrees of aggressiveness and invasiveness, are more often resistant to treatment, and have higher risks of recurrence than sporadic PAs (85). Murine studies support that targeted angiogenesis in MEN1 leads to an obvious inhibition of pituitary tumor growth and hormone secretion and a significantly increased tumor-free survival time. Additionally, the vascular density in pancreatic islet tumors was significantly reduced by the treatment (86). Sunitinib was approved to treat locally advanced or metastatic pancreatic neuroendocrine tumors and refractory gastrointestinal stromal tumors (87, 88). Sunitinib has also been studied in MEN1 syndrome (89–92). However, data are still limited to drive any conclusion on the treatment of MEN1-related PAs.
To date, although attempts at bevacizumab and TK inhibitors in pituitary tumors have not gone beyond case studies, the anti-VEGF/VEGFR pathway has shown promise as an alternative therapy for patients with refractory PAs and PCs resistant to conventional treatments. Furthermore, the anti-VEGF/VEGFR pathway in combination with TMZ, TMZ and/or radiotherapy with SSA might have a synergistic therapeutic effect. However, the specific efficacy of the anti-VEGF/VEGFR pathway in patients with refractory PAs and PCs still needs further large-scale prospective clinical trials for confirmation.
Conclusion
In summary, the results from preclinical studies and clinical trials indicated that anti-VEGF monotherapy or in combination with other treatments may be promising alternative therapies for patients with refractory PAs and PCs resistant to conventional treatments. However, more preclinical studies and large-scale prospective clinical trials are needed to further evaluate the exact efficacy of anti-VEGF in pituitary tumors.
Author Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
Financial support for this study was provided by the Scientific Research Project of Capital Health Development in 2018 (grant number: 2018-4-4018), the CAMS Innovation fund for Medical Science (grant number: CIFMS, 2017-12M-2-005), and the Beijing Natural Science Foundation (grant number: 7182137). The funding institutions had no role in the design of the study, data collection and analysis, the decision to publish, or the preparation of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aflorei ED, Korbonits M. Epidemiology and Etiopathogenesis of Pituitary Adenomas. J Neurooncol (2014) 117(3):379–94. doi: 10.1007/s11060-013-1354-5
2. Melmed S. Pathogenesis of Pituitary Tumors. Nat Rev Endocrinol (2011) 7(5):257–66. doi: 10.1038/nrendo.2011.40
3. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The Prevalence of Pituitary Adenomas: A Systematic Review. Cancer (2004) 101(3):613–9. doi: 10.1002/cncr.20412
4. Dai C, Liu X, Ma W, Wang R. The Treatment of Refractory Pituitary Adenomas. Front Endocrinol (Lausanne) (2019) 10:334. doi: 10.3389/fendo.2019.00334
5. Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. 4th Edition Ed. Lyons: IARC (2017).
6. Elbelt U, Schlaffer SM, Buchfelder M, Knappe UJ, Vila G, Micko A, et al. Efficacy of Temozolomide Therapy in Patients With Aggressive Pituitary Adenomas and Carcinomas-a German Survey. J Clin Endocrinol Metab (2020) 105(3):e660–75. doi: 10.1210/clinem/dgz211
7. McCormack A, Dekkers OM, Petersenn S, Popovic V, Trouillas J, Raverot G, et al. Treatment of Aggressive Pituitary Tumours and Carcinomas: Results of a European Society of Endocrinology (ESE) Survey 2016. Eur J Endocrinol (2018) 178(3):265–76. doi: 10.1530/eje-17-0933
8. Yang Q, Li X. Molecular Network Basis of Invasive Pituitary Adenoma: A Review. Front Endocrinol (Lausanne) (2019) 10:7. doi: 10.3389/fendo.2019.00007
10. Carmeliet P. Angiogenesis in Life, Disease and Medicine. Nature (2005) 438(7070):932–6. doi: 10.1038/nature04478
11. Weidner N, Semple JP, Welch WR, Folkman J. Tumor Angiogenesis and Metastasis–Correlation in Invasive Breast Carcinoma. N Engl J Med (1991) 324(1):1–8. doi: 10.1056/NEJM199101033240101
12. Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor Angiogenesis Correlates With Metastasis in Invasive Prostate Carcinoma. Am J Pathol (1993) 143(2):401–9.
13. Folkman J. Role of Angiogenesis in Tumor Growth and Metastasis. Semin Oncol (2002) 29(6 Suppl 16):15–8. doi: 10.1053/sonc.2002.37263
14. Maeda K, Chung YS, Takatsuka S, Ogawa Y, Sawada T, Yamashita Y, et al. Tumor Angiogenesis as a Predictor of Recurrence in Gastric Carcinoma. J Clin Oncol (1995) 13(2):477–81. doi: 10.1200/JCO.1995.13.2.477
15. Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, et al. Tumor Angiogenesis: A New Significant and Independent Prognostic Indicator in Early-Stage Breast Carcinoma. J Natl Cancer Inst (1992) 84(24):1875–87. doi: 10.1093/jnci/84.24.1875
16. Bochner BH, Cote RJ, Weidner N, Groshen S, Chen SC, Skinner DG, et al. Angiogenesis in Bladder Cancer: Relationship Between Microvessel Density and Tumor Prognosis. J Natl Cancer Inst (1995) 87(21):1603–12. doi: 10.1093/jnci/87.21.1603
17. Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in Pituitary Adenomas and the Normal Pituitary Gland. J Clin Endocrinol Metab (2000) 85(3):1159–62. doi: 10.1210/jcem.85.3.6485
18. Schechter J. Ultrastructural Changes in the Capillary Bed of Human Pituitary Tumors. Am J Pathol (1972) 67(1):109–26.
19. Viacava P, Gasperi M, Acerbi G, Manetti L, Cecconi E, Bonadio AG, et al. Microvascular Density and Vascular Endothelial Growth Factor Expression in Normal Pituitary Tissue and Pituitary Adenomas. J Endocrinol Invest (2003) 26(1):23–8. doi: 10.1007/BF03345118
20. Takada K, Yamada S, Teramoto A. Correlation Between Tumor Vascularity and Clinical Findings in Patients With Pituitary Adenomas. Endocr Pathol (2004) 15(2):131–9. doi: 10.1385/ep:15:2:131
21. Niveiro M, Aranda FI, Peiro G, Alenda C, Pico A. Immunohistochemical Analysis of Tumor Angiogenic Factors in Human Pituitary Adenomas. Hum Pathol (2005) 36(10):1090–5. doi: 10.1016/j.humpath.2005.07.015
22. Jugenburg M, Kovacs K, Stefaneanu L, Scheithauer BW. Vasculature in Nontumorous Hypophyses, Pituitary Adenomas, and Carcinomas: A Quantitative Morphologic Study. Endocr Pathol (1995) 6(2):115–24. doi: 10.1007/BF02739874
23. Pawlikowski M, Pisarek H, Jaranowska M. Immunocytochemical Investigations on the Vascularization of Pituitary Adenomas. Endocr Pathol (1997) 8(3):189–93. doi: 10.1007/BF02738785
24. Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in Pituitary Adenomas - Relationship to Endocrine Function, Treatment and Outcome. J Endocrinol (2000) 165(2):475–81. doi: 10.1677/joe.0.1650475
25. Vidal S, Kovacs K, Horvath E, Scheithauer BW, Kuroki T, Lloyd RV. Microvessel Density in Pituitary Adenomas and Carcinomas. Virchows Arch (2001) 438(6):595–602. doi: 10.1007/s004280000373
26. Ferrara N, Gerber HP, LeCouter J. The Biology of VEGF and Its Receptors. Nat Med (2003) 9(6):669–76. doi: 10.1038/nm0603-669
27. Harper SJ, Bates DO. VEGF-a Splicing: The Key to Anti-Angiogenic Therapeutics? Nat Rev Cancer (2008) 8(11):880–7. doi: 10.1038/nrc2505
28. Claesson-Welsh L, Welsh M. VEGFA and Tumour Angiogenesis. J Intern Med (2013) 273(2):114–27. doi: 10.1111/joim.12019
29. Frezzetti D, Gallo M, Maiello MR, D’Alessio A, Esposito C, Chicchinelli N, et al. VEGF as a Potential Target in Lung Cancer. Expert Opin Ther Targets (2017) 21(10):959–66. doi: 10.1080/14728222.2017.1371137
30. Schneider BP, Sledge GW Jr. Drug Insight: VEGF as a Therapeutic Target for Breast Cancer. Nat Clin Pract Oncol (2007) 4(3):181–9. doi: 10.1038/ncponc0740
31. Choueiri TK, Kaelin WG Jr. Targeting the HIF2-VEGF Axis in Renal Cell Carcinoma. Nat Med (2020) 26(10):1519–30. doi: 10.1038/s41591-020-1093-z
32. Melnic E, Cimpean AM, Gaje PN, Raica M. Influence of Hormone Profile on Vascular Endothelial Growth Factor (VEGF a) Expression in Human Pituitary Adenomas. Anticancer Res (2014) 34(10):5867.
33. Lloyd RV, Scheithauer BW, Kuroki T, Vidal S, Kovacs K, Stefaneanu L. Vascular Endothelial Growth Factor (VEGF) Expression in Human Pituitary Adenomas and Carcinomas. Endocr Pathol (1999) 10(3):229–35. doi: 10.1007/BF02738884
34. Fukui S, Nawashiro H, Otani N, Ooigawa H, Yano A, Nomura N, et al. Vascular Endothelial Growth Factor Expression in Pituitary Adenomas. Acta Neurochir Suppl (2003) 86:519–21. doi: 10.1007/978-3-7091-0651-8_106
35. Wang Y, Li J, Tohti M, Hu Y, Wang S, Li W, et al. The Expression Profile of Dopamine D2 Receptor, MGMT and VEGF in Different Histological Subtypes of Pituitary Adenomas: A Study of 197 Cases and Indications for the Medical Therapy. J Exp Clin Cancer Res (2014) 33(1):56. doi: 10.1186/s13046-014-0056-y
36. Corlan AS, Cimpean AM, Melnic E, Raica M, Sarb S. VEGF, VEGF165b and EG-VEGF Expression Is Specifically Related With Hormone Profile in Pituitary Adenomas. Eur J Histochem (2019) 63(1):3010. doi: 10.4081/ejh.2019.3010
37. Sanchez-Ortiga R, Sanchez-Tejada L, Moreno-Perez O, Riesgo P, Niveiro M, Pico Alfonso AM. Over-Expression of Vascular Endothelial Growth Factor in Pituitary Adenomas is Associated With Extrasellar Growth and Recurrence. Pituitary (2013) 16(3):370–7. doi: 10.1007/s11102-012-0434-4
38. Cristina C, Perez-Millan MI, Luque G, Dulce RA, Sevlever G, Berner SI, et al. VEGF and CD31 Association in Pituitary Adenomas. Endocr Pathol (2010) 21(3):154–60. doi: 10.1007/s12022-010-9119-6
39. Kurosaki M, Saegert W, Abe T, Ludecke DK. Expression of Vascular Endothelial Growth Factor in Growth Hormone-Secreting Pituitary Adenomas: Special Reference to the Octreotide Treatment. Neurol Res (2008) 30(5):518–22. doi: 10.1179/174313208X289499
40. Banerjee SK, Zoubine MN, Tran TM, Weston AP, Campbell DR. Overexpression of Vascular Endothelial Growth Factor164 and its Co-Receptor Neuropilin-1 in Estrogen-Induced Rat Pituitary Tumors and GH3 Rat Pituitary Tumor Cells. Int J Oncol (2000) 16(2):253–60. doi: 10.3892/ijo.16.2.253
41. Alfer J, Neulen J, Gaumann A. Lactotrophs: The New and Major Source for VEGF Secretion and the Influence of ECM on Rat Pituitary Function In Vitro. Oncol Rep (2015) 33(5):2129–34. doi: 10.3892/or.2015.3851
42. Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, et al. Expression of the Vascular Permeability Factor/Vascular Endothelial Growth Factor Gene in Central Nervous System Neoplasms. J Clin Invest (1993) 91(1):153–9. doi: 10.1172/jci116165
43. Arita K, Kurisu K, Tominaga A, Sugiyama K, Eguchi K, Hama S, et al. Relationship Between Intratumoral Hemorrhage and Overexpression of Vascular Endothelial Growth Factor (VEGF) in Pituitary Adenoma. Hiroshima J Med Sci (2004) 53(2):23–7.
44. Yarman S, Kurtulmus N, Canbolat A, Bayindir C, Bilgic B, Ince N. Expression of Ki-67, P53 and Vascular Endothelial Growth Factor (VEGF) Concomitantly in Growth Hormone-Secreting Pituitary Adenomas; Which One has a Role in Tumor Behavior? Neuro Endocrinol Lett (2010) 31(6):823–8.
45. Iuchi T, Saeki N, Osato K, Yamaura A. Proliferation, Vascular Endothelial Growth Factor Expression and Cavernous Sinus Invasion in Growth Hormone Secreting Pituitary Adenomas. Acta Neurochir (Wien) (2000) 142(12):1345–51. doi: 10.1007/s007010070003
46. Borg SA, Kerry KE, Royds JA, Battersby RD, Jones TH. Correlation of VEGF Production With IL1 Alpha and IL6 Secretion by Human Pituitary Adenoma Cells. Eur J Endocrinol (2005) 152(2):293–300. doi: 10.1530/eje.1.01843
47. Banerjee SK, Sarkar DK, Weston AP, De A, Campbell DR. Over Expression of Vascular Endothelial Growth Factor and its Receptor During the Development of Estrogen-Induced Rat Pituitary Tumors may Mediate Estrogen-Initiated Tumor Angiogenesis. Carcinogenesis (1997) 18(6):1155–61. doi: 10.1093/carcin/18.6.1155
48. Miyajima K, Takekoshi S, Itoh J, Kakimoto K, Miyakoshi T, Osamura RY. Inhibitory Effects of Anti-VEGF Antibody on the Growth and Angiogenesis of Estrogen-Induced Pituitary Prolactinoma in Fischer 344 Rats: Animal Model of VEGF-Targeted Therapy for Human Endocrine Tumors. Acta Histochem Cytochem (2010) 43(2):33–44. doi: 10.1267/ahc.09034
49. Liu X, Tang C, Wen G, Zhong C, Yang J, Zhu J, et al. The Mechanism and Pathways of Dopamine and Dopamine Agonists in Prolactinomas. Front Endocrinol (Lausanne) (2018) 9:768. doi: 10.3389/fendo.2018.00768
50. Cooper O, Greenman Y. Dopamine Agonists for Pituitary Adenomas. Front Endocrinol (Lausanne) (2018) 9:469. doi: 10.3389/fendo.2018.00469
51. Cristina C, Díaz-Torga G, Baldi A, Góngora A, Rubinstein M, Low MJ, et al. Increased Pituitary Vascular Endothelial Growth Factor-a in Dopaminergic D2 Receptor Knockout Female Mice. Endocrinology (2005) 146(7):2952–62. doi: 10.1210/en.2004-1445
52. Luque GM, Perez-Millan MI, Ornstein AM, Cristina C, Becu-Villalobos D. Inhibitory Effects of Anti-VEGF Strategies in Experimental Dopamine-Resistant Prolactinomas. J Pharmacol Exp Ther (2011) 337(3):766–74. doi: 10.1124/jpet.110.177790
53. Luque GM, Perez-Millán MI, Ornstein AM, Cristina C, Becu-Villalobos D. Inhibitory Effects of Antivascular Endothelial Growth Factor Strategies in Experimental Dopamine-Resistant Prolactinomas. J Pharmacol Exp Ther (2011) 337(3):766–74. doi: 10.1124/jpet.110.177790
54. Chauvet N, Romanò N, Lafont C, Guillou A, Galibert E, Bonnefont X, et al. Complementary Actions of Dopamine D2 Receptor Agonist and Anti-VEGF Therapy on Tumoral Vessel Normalization in a Transgenic Mouse Model. Int J Cancer (2017) 140(9):2150–61. doi: 10.1002/ijc.30628
55. Kamba T, McDonald DM. Mechanisms of Adverse Effects of Anti-VEGF Therapy for Cancer. Br J Cancer (2007) 96(12):1788–95. doi: 10.1038/sj.bjc.6603813
56. Ortiz LD, Syro LV, Scheithauer BW, Ersen A, Uribe H, Fadul CE, et al. Anti-VEGF Therapy in Pituitary Carcinoma. Pituitary (2012) 15(3):445–9. doi: 10.1007/s11102-011-0346-8
57. O’Riordan LM, Greally M, Coleman N, Breathnach OS, Hennessy B, Thompson CJ, et al. Metastatic ACTH-Producing Pituitary Carcinoma Managed With Combination Pasireotide and Bevacizumab Following Failure of Temozolamide Therapy: A Case Report. J Clin Oncol (2013) 31(15):e13022. doi: 10.1200/jco.2013.31.15_suppl.e13022
58. Kurowska M, Malicka J, Tarach JS. Are the “Classic” and the “Modern” Forms of Nelson’s Syndrome the Same or Different Disorders? Endokrynologia Polska (2014) 65(5):425–6.
59. Kurowska M, Nowakowski A, Zielinski G, Malicka J, Tarach JS, Maksymowicz M, et al. Temozolomide-Induced Shrinkage of Invasive Pituitary Adenoma in Patient With Nelson’s Syndrome: A Case Report and Review of the Literature. Case Rep Endocrinol (2015) 2015:623092. doi: 10.1155/2015/623092
60. Korbonits M, Dutta P, Reddy KS, Bhansali A, Gupta P, Rai A, et al. Exome Sequencing Reveals Double Hit by AIP Gene Mutation and Copy Loss of Chromosome 11 But Negative Xlag in a Pituitary Adenoma of a 4 Yrs Child With Gigantism Treated With Multimodal Therapy. 98th Annual Meeting and Expo of the Endocrine Society, Boston, MA, United States. (2016).
61. Dutta P, Reddy KS, Rai A, Madugundu AK, Solanki HS, Bhansali A, et al. Surgery, Octreotide, Temozolomide, Bevacizumab, Radiotherapy, and Pegvisomant Treatment of an AIP Mutation-Positive Child. J Clin Endocrinol Metab (2019) 104(8):3539–44. doi: 10.1210/jc.2019-00432
62. Touma W, Hoostal S, Peterson RA, Wiernik A, SantaCruz KS, Lou E. Successful Treatment of Pituitary Carcinoma With Concurrent Radiation, Temozolomide, and Bevacizumab After Resection. J Clin Neurosci (2017) 41:75–7. doi: 10.1016/j.jocn.2017.02.052
63. Alshaikh OM, Asa SL, Mete O, Ezzat S. An Institutional Experience of Tumor Progression to Pituitary Carcinoma in a 15-Year Cohort of 1055 Consecutive Pituitary Neuroendocrine Tumors. Endocrine Pathol (2019) 30(2):118–27. doi: 10.1007/s12022-019-9568-5
64. Rotman LE, Vaughan TB, Hackney JR, Riley KO. Long-Term Survival After Transformation of an Adrenocorticotropic Hormone–Secreting Pituitary Macroadenoma to a Silent Corticotroph Pituitary Carcinoma. World Neurosurg (2019) 122:417–23. doi: 10.1016/j.wneu.2018.11.011
65. Lamb LS, Sim HW, McCormack AI. Case Report: A Case of Pituitary Carcinoma Treated With Sequential Dual Immunotherapy and Vascular Endothelial Growth Factor Inhibition Therapy. Front Endocrinol (Lausanne) (2020) 11:576027. doi: 10.3389/fendo.2020.576027
66. Xu L, Khaddour K, Chen J, Rich KM, Perrin RJ, Campian JL. Pituitary Carcinoma: Two Case Reports and Review of Literature. World J Clin Oncol (2020) 11(2):91–102. doi: 10.5306/wjco.v11.i2.91
67. Osterhage K, Rotermund R, Droste M, Dierlamm J, Saeger W, Petersenn S, et al. Bevacizumab in Aggressive Pituitary Adenomas - Experience With 3 Patients. Exp Clin Endocrinol Diabetes (2021) 129(3):178–85. doi: 10.1055/a-1260-3975
68. Wang Y, He Q, Meng X, Zhou S, Zhu Y, Xu J, et al. Apatinib (YN968D1) and Temozolomide in Recurrent Invasive Pituitary Adenoma: Case Report and Literature Review. World Neurosurg (2019) 124:319–22. doi: 10.1016/j.wneu.2018.12.174
69. Chow LQ, Eckhardt SG. Sunitinib: From Rational Design to Clinical Efficacy. J Clin Oncol (2007) 25(7):884–96. doi: 10.1200/JCO.2006.06.3602
70. Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical Overview of Sorafenib, a Multikinase Inhibitor That Targets Both Raf and VEGF and PDGF Receptor Tyrosine Kinase Signaling. Mol Cancer Ther (2008) 7(10):3129–40. doi: 10.1158/1535-7163.MCT-08-0013
71. Christensen JG. A Preclinical Review of Sunitinib, a Multitargeted Receptor Tyrosine Kinase Inhibitor With Anti-Angiogenic and Antitumour Activities. Ann Oncol (2007) 18(Suppl 10):x3–10. doi: 10.1093/annonc/mdm408
72. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib Versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N Engl J Med (2007) 356(2):115–24. doi: 10.1056/NEJMoa065044
73. Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-Term Analysis of the Efficacy and Tolerability of Sorafenib in Advanced Radio-Iodine Refractory Differentiated Thyroid Carcinoma: Final Results of a Phase II Trial. Eur J Endocrinol (2012) 167(5):643–50. doi: 10.1530/EJE-12-0405
74. Srikugan L, Powrie J. Effects of Sunitinib, a Protein Tyrosine Kinase Inhibitor, on a Pituitary Lesion in a Patient With Renal Carcinoma. 93rd Annual Meeting and Expo of the Endocrine Society, Boston, MA, United States. (2011). Available at: https://endo.confex.com/endo/2016endo/webprogram/Paper28082.html.
75. Yang L, Yu SY, Hu GY. Pituitary Metastasis From a Renal Cell Carcinoma Progressed After Sorafenib Treatment. Chin J Cancer (2013) 32(6):353–6. doi: 10.5732/cjc.012.10184
76. Upton TJ, Hunt PJ. Renal Cell Pituitary Metastasis Masquerading as a Prolactinoma. 96th Annual Meeting and Expo of the Endocrine Society, Chicago, IL, United States. (2014).
77. Payandeh M, Sadeghi M, Sadeghi E. The Complete Response to Targeted Drugs Without Surgery or Radiotherapy: A Case of Pituitary Metastasis From Renal Cell Carcinoma. Acta Med Iranica (2016) 54(9):617–9.
78. Wendel C, Campitiello M, Plastino F, Eid N, Hennequin L, Quétin P, et al. Pituitary Metastasis From Renal Cell Carcinoma: Description of a Case Report. Am J Case Rep (2017) 18:7–11. doi: 10.12659/ajcr.901032
79. Liu W, Varlamov E, Woltjer R, Cetas J, Fleseriu M. Metastatic Renal Cell Carcinoma to the Pituitary-a Clinical Conundrum. 100th Annual Meeting of the Endocrine Society, Chicago, IL, United States. (2018).
80. Selby LD, Stiefel HC, Skalet AH, Cardenal MS, Bhavsar KV, Winges KM. Vision Loss From Choroidal and Pituitary Metastases Secondary to Renal Cell Carcinoma: A Case Report. Neuro-Ophthalmology (2018) 42(6):391–8. doi: 10.1080/01658107.2018.1454479
81. Di Nunno V, Mollica V, Corcioni B, Fiorentino M, Nobili E, Schiavina R, et al. Clinical Management of a Pituitary Gland Metastasis From Clear Cell Renal Cell Carcinoma. Anti-Cancer Drugs (2018) 29(7):710–5. doi: 10.1097/CAD.0000000000000644
82. Hammami MM, Duaiji N, Mutairi G, Aklabi S, Qattan N, Abouzied Mel D, et al. Case Report of Severe Cushing’s Syndrome in Medullary Thyroid Cancer Complicated by Functional Diabetes Insipidus, Aortic Dissection, Jejunal Intussusception, and Paraneoplastic Dysautonomia: Remission With Sorafenib Without Reduction in Cortisol Concentration. BMC Cancer (2015) 15:624. doi: 10.1186/s12885-015-1620-3
83. Souza Mota J, Caldas AS, Nascimento AGPAC, Faria MS, Sobral CSP. Pituitary Metastasis of Thyroid Carcinoma: A Case Report. Am J Case Rep (2018) 19:896–902. doi: 10.12659/AJCR.909523
84. Scott LJ. Apatinib: A Review in Advanced Gastric Cancer and Other Advanced Cancers. Drugs (2018) 78(7):747–58. doi: 10.1007/s40265-018-0903-9
85. Syro LV, Scheithauer BW, Kovacs K, Toledo RA, Londono FJ, Ortiz LD, et al. Pituitary Tumors in Patients With MEN1 Syndrome. Clinics (Sao Paulo) (2012) 67 Suppl 1:43–8. doi: 10.6061/clinics/2012(sup01)09
86. Korsisaari N, Ross J, Wu X, Kowanetz M, Pal N, Hall L, et al. Blocking Vascular Endothelial Growth Factor-a Inhibits the Growth of Pituitary Adenomas and Lowers Serum Prolactin Level in a Mouse Model of Multiple Endocrine Neoplasia Type 1. Clin Cancer Res (2008) 14(1):249–58. doi: 10.1158/1078-0432.CCR-07-1552
87. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and Safety of Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumour After Failure of Imatinib: A Randomised Controlled Trial. Lancet (2006) 368(9544):1329–38. doi: 10.1016/S0140-6736(06)69446-4
88. Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N Engl J Med (2011) 364(6):501–13. doi: 10.1056/NEJMoa1003825
89. Palmieri G, Buonerba C, Formisano L, Damiano V, Nappi L, Federico P, et al. Sunitinib in a Men-1 Patient With Small Cell Neuroendocrine Tumor of the Thymus. Neuroendocrinology (2012) 96:11–2. doi: 10.1159/000340053
90. Li Y, Su X, Tan H. Type 2 Gastric Neuroendocrine Tumor: Report of One Case. Trans Gastroenterol Hepatol (2016) 30(1):88. doi: 10.21037/tgh.2016.11.05
91. Shell J, Patel D, Powers A, Quezado M, Killian K, Meltzer P, et al. Somatic VHL Mutation in a Patient With MEN1-Associated Metastatic Pancreatic Neuroendocrine Tumor Responding to Sunitinib Treatment: A Case Report. J Endocr Soc (2017) 1(9):1124–34. doi: 10.1210/js.2017-00156
Keywords: refractory pituitary adenomas, pituitary carcinomas, VEGF, anti-VEGF, vascular endothelial growth inhibitor
Citation: Dai C, Liang S, Sun B, Li Y and Kang J (2021) Anti-VEGF Therapy in Refractory Pituitary Adenomas and Pituitary Carcinomas: A Review. Front. Oncol. 11:773905. doi: 10.3389/fonc.2021.773905
Received: 10 September 2021; Accepted: 27 October 2021;
Published: 17 November 2021.
Edited by:
Qun Wu, Zhejiang University, ChinaReviewed by:
Yubo Wang, First Affiliated Hospital of Jilin University, ChinaRun Yu, UCLA David Geffen School of Medicine, United States
Copyright © 2021 Dai, Liang, Sun, Li and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Kang, anVua2FuZzIwMTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Congxin Dai
Congxin Dai Siyu Liang
Siyu Liang Bowen Sun
Bowen Sun Yong Li
Yong Li Jun Kang
Jun Kang