- 1Peking University People’s Hospital, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Beijing, China
- 2Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China
- 3Research Unit of Key Technique for Diagnosis and Treatments of Hematologic Malignancies, Chinese Academy of Medical Sciences, Beijing, China
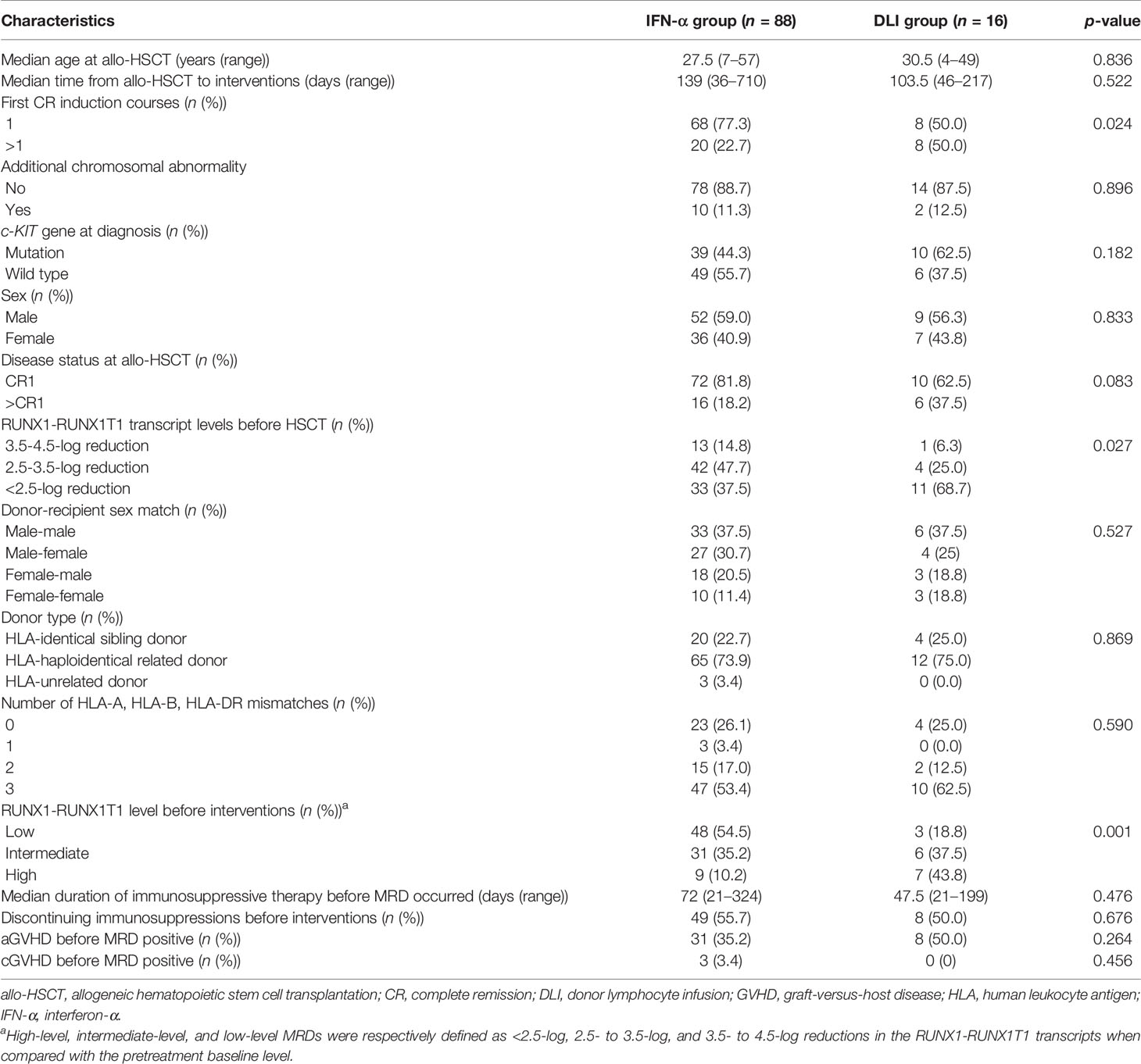
In patients with t(8;21) acute myeloid leukemia (AML), recurrent minimal residual disease (MRD) measured by RUNX1-RUNX1T1 transcript levels can predict relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT). This study aimed to compare the efficacy of preemptive interferon (IFN)-α therapy and donor lymphocyte infusion (DLI) in patients with t(8;21) AML following allo-HSCT. We also evaluated the appropriate method for patients with different levels of RUNX1-RUNX1T1 transcripts. In this retrospective study, consecutive patients who had high-risk t(8;21) AML and received allo-HSCT were enrolled. The inclusion criteria were as follows: (1) age ≤65 years; (2) regained MRD positive following allo-HSCT. MRD positive was defined as the loss of a ≥4.5-log reduction and/or <4.5-log reduction in the RUNX1-RUNX1T1 transcripts, and high-level, intermediate-level, and low-level MRDs were, respectively, defined as <2.5-log, 2.5−3.5-log, and 3.5−4.5-log reductions in the transcripts compared with the pretreatment baseline level. Patients with positive RUNX1-RUNX1T1 could receive preemptive IFN-α therapy or DLI, which was primarily based on donor availability and the intentions of physicians and patients. The patients received recombinant human IFN-α-2b therapy by subcutaneous injection twice a week every 4 weeks. IFN-α therapy was scheduled for six cycles or until the RUNX1-RUNX1T1 transcripts were negative for at least two consecutive tests. The rates of MRD turning negative for patients with low-level, intermediate-level, and high-level RUNX1-RUNX1T1 receiving IFN-α were 87.5%, 58.1%, and 22.2%, respectively; meanwhile, for patients with intermediate-level and high-level RUNX1-RUNX1T1 receiving DLI, the rates were 50.0% and 14.3%, respectively. For patients with low-level and intermediate-level RUNX1-RUNX1T1, the probability of overall survival at 2 years was higher in the IFN-α group than in the DLI group (87.6% vs. 55.6%; p = 0.003). For patients with high levels of RUNX1-RUNX1T1, the probability of overall survival was comparable between the IFN-α and DLI groups (53.3% vs. 83.3%; p = 0.780). Therefore, patients with low-level and intermediate-level RUNX1-RUNX1T1 could benefit more from preemptive IFN-α therapy compared with DLI. Clinical outcomes were comparable between preemptive IFN-α therapy and DLI in patients with high-level RUNX1-RUNX1T1; however, they should be further improved.
1 Introduction
Acute myeloid leukemia (AML) with t(8;21) is a heterogeneous disease, and relapse can occur in 40–50% of patients treated with chemotherapy alone, even if it is considered to have a good prognosis (1, 2). Minimal residual disease (MRD) after chemotherapy can predict the relapse of t(8;21) AML (3–6), and allogeneic hematopoietic stem cell transplantation (allo-HSCT) can further decrease relapse and improve survival in patients with persistent RUNX1-RUNX1T1 after chemotherapy (5, 7–9). However, relapse remains experienced by nearly 20% of patients following allo-HSCT (10).
Regular monitoring of MRD after allo-HSCT can identify patients with a higher risk of relapse (11, 12). The MRD measured by the level of RUNX1-RUNX1T1 transcript has been identified as an effective predictor of relapse in patients with t(8;21) AML after allo-HSCT (13, 14). Therefore, intervention directed by MRD (i.e., preemptive intervention) is a rational option for relapse prophylaxis. One of the most critical immunotherapies after allo-HSCT is donor lymphocyte infusion (DLI) (15–17). Wang et al. (14) reported that preemptive DLI could prevent relapse and improve survival in patients with t(8;21) AML. Interferon-α (IFN-α) is another important immunotherapy after allo-HSCT (18–22); Mo et al. (20) reported that the survival of patients with MRD positive without any intervention was significantly lower than those receiving preemptive IFN-α therapy (20). Therefore, IFN-α therapy and DLI could improve the prognosis of patients with MRD following allo-HSCT. However, which preemptive intervention is more superior for t (8;21) AML patients receiving allo-HSCT is still unclear. Mo et al. (21) reported that the prognosis of preemptive DLI and IFN-α therapy was comparable, but their study included a small sample size of patients with t(8;21) AML. To date, no studies have compared the efficacy of preemptive DLI and IFN-α therapy in patients with t(8;21) AML.
Furthermore, we observed that RUNX1-RUNX1T1 transcript levels influenced the efficacy of preemptive IFN-α therapy; however, the influence of MRD levels on IFN-α therapy could not be further evaluated due to a small sample size of patients with higher levels of RUNX1-RUNX1T1 transcripts (20). In contrast, Wang et al. (14) reported that patients with a higher level of RUNX1-RUNX1T1 transcript could still benefit from DLI. Therefore, patients with different levels of RUNX1-RUNX1T1 transcript may benefit from different interventions. However, no studies have compared the efficacy of preemptive DLI and IFN-α therapy at different levels of RUNX1-RUNX1T1 transcript and the selection of appropriate preemptive interventions according to RUNX1-RUNX1T1 transcript levels remains unknown.
Therefore, this retrospective study aimed to compare the efficacy of preemptive DLI and IFN-α therapy in patients with t(8;21) AML following allo-HSCT. Furthermore, we also evaluated the appropriate intervention methods for patients with different levels of RUNX1-RUNX1T1 transcripts.
2 Methods
2.1 Patients
Consecutive patients who had high-risk t(8;21) AML and received allo-HSCT at the Peking University Institute of Hematology (PUIH) were enrolled. The inclusion criteria were as follows: (1) were ≤65 years old (2) and regained MRD positive following allo-HSCT (5).
The exclusion criteria for IFN-α therapy were as follows: (1) active graft-versus-host disease (GVHD); (2) active and uncontrolled infections; (3) severe myelosuppression; (4) organ failure; and (5) hematologic relapse.
The exclusion criteria for DLI were as follows: (1) active GVHD; (2) active and uncontrolled infections; (3) organ failure; and (4) hematologic relapse (20).
One hundred and four patients were enrolled between October 1, 2013 and February 28, 2021 (Table 1). Forty-two patients were previously reported by Mo et al. (23), and in this study, they were followed up further. The endpoint analysis of the last follow-up was on September 1, 2021.
2.2 Transplant Regimens
Cytosine arabinoside, busulfan, cyclophosphamide (CY), and simustine were included in the preconditioning. The human leukocyte antigen (HLA)-unrelated donor (URD) and HLA-haploidentical donor (HID) groups received rabbit antithymocyte globulin (ATG, Supplementary Methods) (24–26). HID HSCT recipients received ATG and low-dose posttransplant CY (PTCY) for GVHD prophylaxis according to the protocol registered at http://clinicaltrials.gov/NCT02412423 (Supplementary Methods and Supplementary Figure S1) (24–28). Protocols for stem cell harvesting, donor selection, and HLA typing have been previously described in detail (29–32).
2.3 MRD Monitoring and Definition
The protocol for RUNX1-RUNX1T1 monitoring after allo-HSCT was performed according to the protocol of PUIH (5, 13). The definition of MRD positive was a loss of RUNX1-RUNX1T1 transcripts ≥4.5-log reduction and/or the <4.5-log reduction (20. Low-level, intermediate-level, and high-level MRD was defined as a reduction in transcripts of 3.5−4.5-log, 2.5−3.5-log, and <2.5-log, respectively, compared with the baseline level before treatment.
2.4 Protocol for Preemptive DLI and IFN-α Therapy
In this retrospective study, patients with positive RUNX1-RUNX1T1 received preemptive IFN-α therapy or DLI before hematologic relapse after allo-HSCT (12). The therapeutic option was primarily based on donor availability and the intentions of physicians and patients.
The patients received recombinant human IFN-α-2b therapy by subcutaneous injection twice a week every 4 weeks. IFN-α therapy was scheduled for six cycles or until the RUNX1-RUNX1T1 transcripts were negative for at least two consecutive tests (Supplementary Methods). IFN-α therapy could be prolonged upon the request of patients. IFN-α therapy was discontinued in patients with grade ≥3 toxicity, severe infection, severe GVHD, nonrelapse mortality (NRM), or relapse.
Granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cells were administered instead of unstimulated donor blood lymphocytes. All patients received short-term immunosuppressive drugs after DLI. Patients could receive chemotherapy 48–72 h before DLI (i.e., chemo-DLI) (Supplementary Methods) (16, 17).
MRD status was regularly monitored at 1, 2, 3, 4.5, 6, 9, and 12 months after preemptive intervention and at 6-month intervals thereafter.
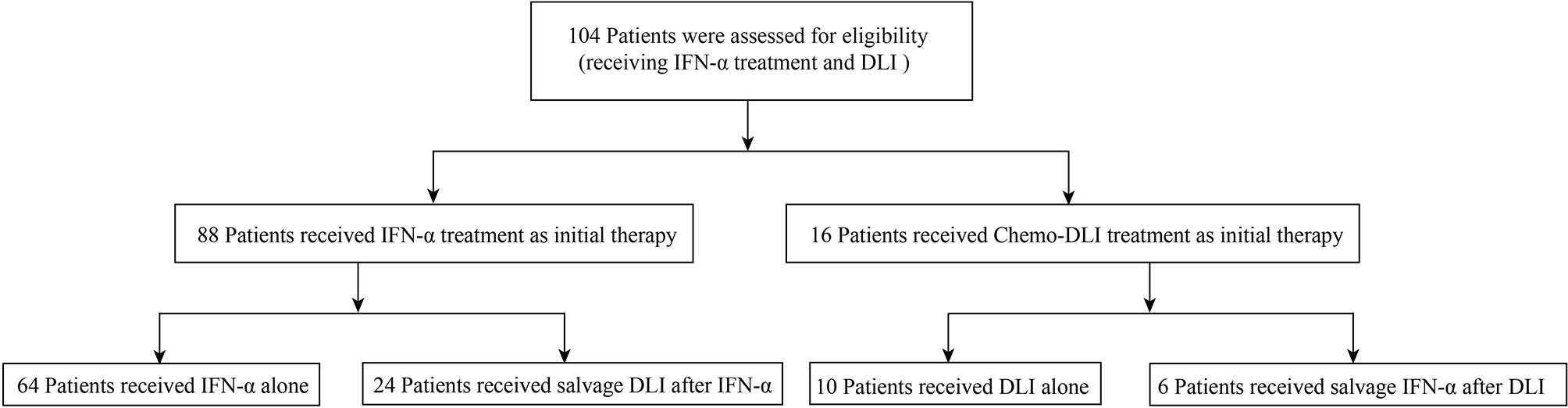
For patients with persistent and increasing levels of MRD (e.g., levels of RUNX1-RUNX1T1 transcripts increased by 1-log) or those who regained MRD positive after receiving MRD-negative status, if they were in the IFN-α group, they could be switched to the DLI group and vice versa (Figure 1).
2.5 Diagnosis and Therapy of GVHD After Preemptive Immunotherapy
GVHD diagnosis and therapy were based on common international criteria (33–38).
2.6 Definition and Assessment
Relapse was defined according to common international criteria (39). Patients who showed relapse were not considered to have MRD. NRM was defined as death without relapse or disease progression. Leukemia-free survival (LFS) was defined as a lifetime with continuous complete remission (CR). The event of overall survival (OS) was the death of any cause.
2.7 Statistical Analysis
The χ2 and Fisher’s exact tests were used to compare categorical variables. The Mann–Whitney U test was used to compare continuous variables. The cumulative incidences of relapse, NRM, and GVHD were calculated using competing risk analyses (40). The probabilities of OS and LFS were estimated using the Kaplan−Meier method. The full analysis set (FAS) included all participants who received DLI (n = 16) or IFN-α (n = 88) as initial therapy at the time of MRD positive. The per-protocol set (PPS) analysis included patients who received DLI (n = 10) or IFN-α therapy (n = 64) alone and those who received both DLI and IFN-α (n = 30) were excluded from the PPS analysis.
Cox proportional hazards regression with a backward stepwise model selection approach was used to estimate hazard ratios for clinical outcomes in a multivariate analysis. The following variables were included: sex, disease status (>CR1 vs. CR1), c-KIT gene at diagnosis (wild type vs. mutation), pre-HSCT RUNX1-RUNX1T1 level (high-level vs. intermediate-level vs. low-level), donor type (identical sibling donor vs. alternative donor), and RUNX1-RUNX1T1 level before preemptive interventions (high-level vs. intermediate-level vs. low-level). Independent variables with p < 0.05 were identified as statistically significant, and p > 0.1 was sequentially excluded from the model. Data analyses were performed primarily using SPSS software (SPSS Inc., Chicago, IL, USA) and the R software package (version 4.1.1; http://www.r-project.org).
3 Results
3.1 Patient Characteristics
Patient characteristics are shown in Table 1, Figure 2, and Supplemental Table S1. In particular, six HID HSCT recipients received ATG and low-dose PTCY for GVHD prophylaxis. A total of 51, 37, and 16 patients showed low-level, intermediate-level, and high-level RUNX1-RUNX1T1, respectively, after allo-HSCT. We observed that donor type, Kit mutation, other karyotypic abnormalities, and duration of immunosuppressive therapy before MRD were not associated with posttransplant RUNX1-RUNX1T1 levels (Supplementary Tables S2, S3 and Supplementary Figure S1); however, pre-transplant transcripts were associated with post-transplant RUNX1-RUNX1T1 levels (Supplementary Table S2 and Supplementary Figure S1C).
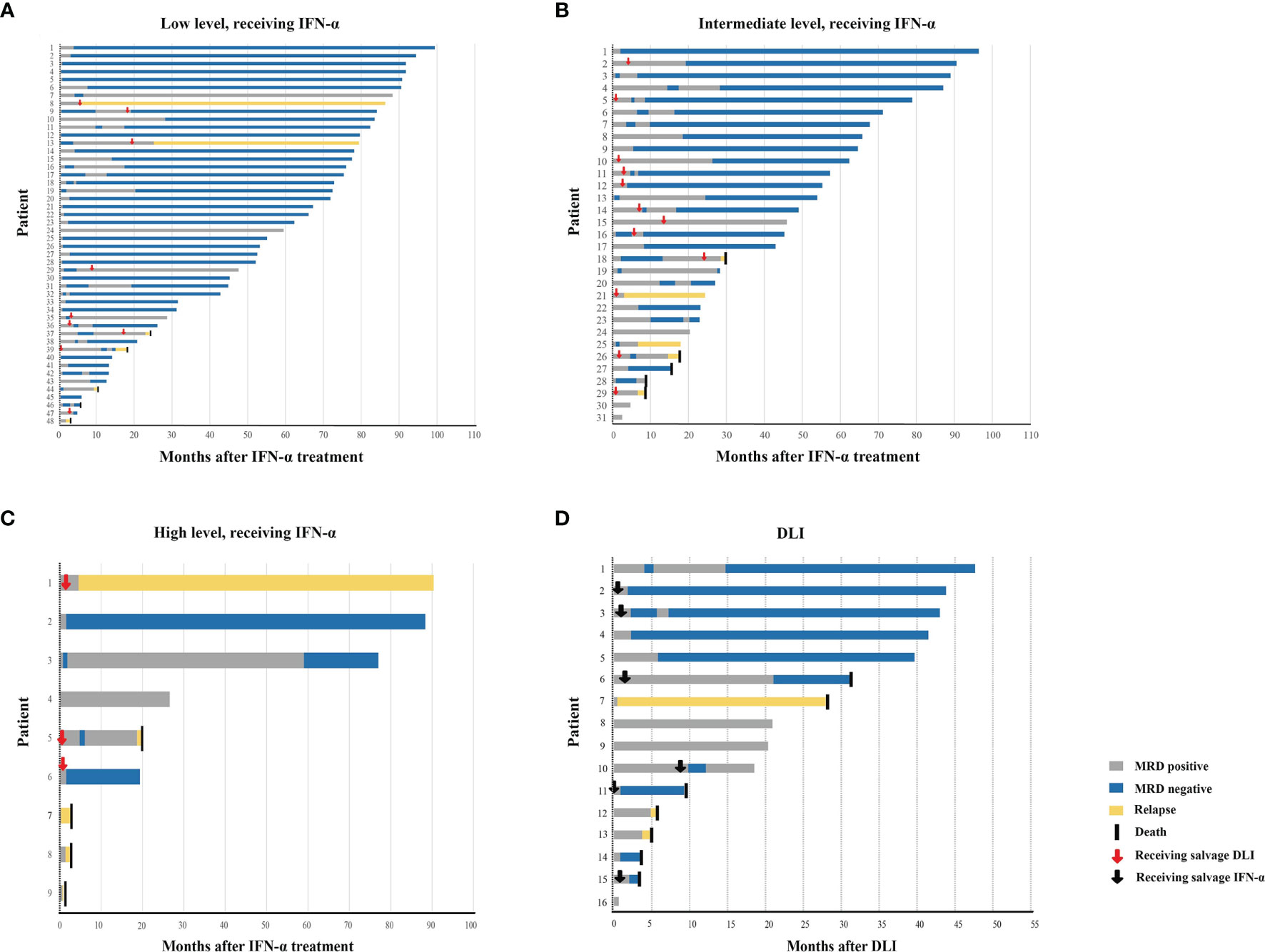
Figure 2 Response. Swimmer plot displayed patients receiving preemptive IFN-α therapy with low-level RUNX1-RUNX1T1 (A), intermediate-level RUNX1-RUNX1T1 (B), and high-level RUNX1-RUNX1T1 transcripts (C), respectively, and patients receiving preemptive DLI (D). IFN, interferon; DLI, donor lymphocyte infusion.
Eighty-eight patients received IFN-α as initial therapy. The median number of cycles of IFN-α therapy was 3 cycles (range, 1–26 cycles), and 24 of them received salvage DLI (chemo-DLI, 20; DLI alone, 4) after IFN-α therapy. Sixteen patients received DLI as initial therapy (chemo-DLI, 10; DLI alone, 6), and six of them received IFN-α as salvage therapy after DLI. The causes of NRM are infection, diffused alveolar hemorrhage, and GVHD (Supplementary Table S4). The cumulative incidences of relapse, NRM, LFS, and OS at 2 years after preemptive interventions were 16.8% [95% confidence interval (CI), 8.7%−24.8%] versus 19.6% (95% CI, 0.0%−40.5%) (p = 0.810), 3.6% (95% CI, 0.0%−7.7%) versus 20.1% (95% CI, 0.0%−41.3%) (p = 0.001), 78.2% (95% CI, 69.8%−87.7%) versus 60.3% (95% CI, 40.0%−90.9%) (p = 0.023), and 84.2% (95% CI, 76.6%−92.5%) versus 66.7% (95% CI, 46.6%−95.3%) (p = 0.004), respectively, for the IFN-α and DLI groups.
3.2 Correlation Between RUNX1-RUNX1T1 Status Following Allo-HSCT and Preemptive Interventions
3.2.1 Low-Level RUNX1-RUNX1T1 Before Immunotherapy
Of the 48 patients who received IFN-α as initial therapy, 42 of them achieved MRD negative (87.5%, Supplementary Table S5), and the median duration from intervention to MRD turning negative was 43.5 days (range, 11–846 days). Nine patients received salvage DLI (chemo-DLI, 8; DLI alone, 1) after IFN-α therapy (regained positive after achieving negative, 5; persistent positive, 4), and three patients receiving chemo-DLI (3/8, 37.5%) achieved MRD negative.
Three patients received DLI as initial therapy (chemo-DLI, 1; DLI alone, 2), but none of them achieved MRD negative (Supplementary Table S5). A patient received salvage IFN-α after chemo-DLI and achieved MRD negative thereafter.
3.2.2 Intermediate-Level RUNX1-RUNX1T1 Before Immunotherapy
Of the 31 patients who received IFN-α treatment as initial therapy, 18 of them achieved MRD negative (58.1%, Supplementary Table S5), and the median duration from intervention to MRD turning negative was 117 days (range, 16–556 days). Twelve patients received salvage DLI (chemo-DLI, 9; DLI alone, 3) after IFN-α therapy (regained positive after achieving negative, 2; persistent positive, 10), and seven of them (58.3%) achieved MRD negative (chemo-DLI, 6; DLI alone, 1).
Six patients received DLI as initial therapy (chemo-DLI, 2; DLI alone, 4), three of them (50.0%) achieved MRD negative (Supplementary Table S5), and the duration from intervention to MRD turning negative was 25, 121, and 174 days, respectively. Three patients with persistent MRD positive received salvage IFN-α therapy after DLI, and all of them achieved MRD negative thereafter.
3.2.3 High-Level RUNX1-RUNX1T1 Before Immunotherapy
Nine patients received IFN-α therapy as initial therapy; two achieved MRD negative (22.2%, Supplementary Table S5), and the duration from intervention to MRD turning negative was 23 and 48 days, respectively. Three patients with persistent MRD positive received salvage chemo-DLI after IFN-α therapy. Although two of them (66.7%) achieved a transient MRD negative after that, both experienced relapse.
Seven patients received chemo-DLI as initial therapy, 1 (14.3%) achieved MRD negative (Supplementary Table S5), and the duration from intervention to MRD turning negative was 68 days. Two patients with persistent MRD positive received salvage IFN-α therapy after chemo-DLI, and both achieved MRD-negative status afterward.
3.3 Chronic GVHD After Preemptive Immunotherapy
The cumulative incidence of total chronic GVHD (cGVHD) at 2 years after preemptive immunotherapy was 45.1% (95% CI, 32.4%−57.8%) in patients receiving IFN-α therapy alone, 57.1% (95% CI, 4.4%−100.0%) in patients receiving DLI alone, and 75.3% (95% CI, 58.6%−92.0%) in patients receiving both DLI and IFN-α therapy (p = 0.154). The cumulative incidence of severe cGVHD at 2 years after preemptive immunotherapy was 3.2% (95% CI, 0.0%−7.5%) in patients receiving IFN-α therapy alone, 0.0% in patients receiving DLI alone, and 10.1% (95% CI, 0.0%−21.2%) in patients receiving both DLI and IFN-α therapy (p = 0.288)
3.4 Relapse, NRM, and Survival After Preemptive Immunotherapy
3.4.1 FAS
For patients with low-level and intermediate-level RUNX1-RUNX1T1 (i.e., 2.5−4.5-log reduction), the 2-year cumulative incidence of relapse after the intervention was comparable between the IFN-α and DLI groups, but the IFN-α group showed a lower cumulative incidence of NRM (Table 2). The probability of survival at 2 years in the IFN-α group was also significantly better than that of the DLI group (Table 2 and Figures 3A, B). Particularly, for patients with low levels of RUNX1-RUNX1T1, the cumulative incidence of relapse, NRM, LFS, and OS at 2 years after IFN-α therapy was 11.3% (95% CI, 1.8%−20.7%; 2.1% (95% CI, 0.0%−6.3%), 86.6% (95% CI, 77.1%−97.2%), and 88.8% (95% CI, 79.8%−98.6%), respectively.
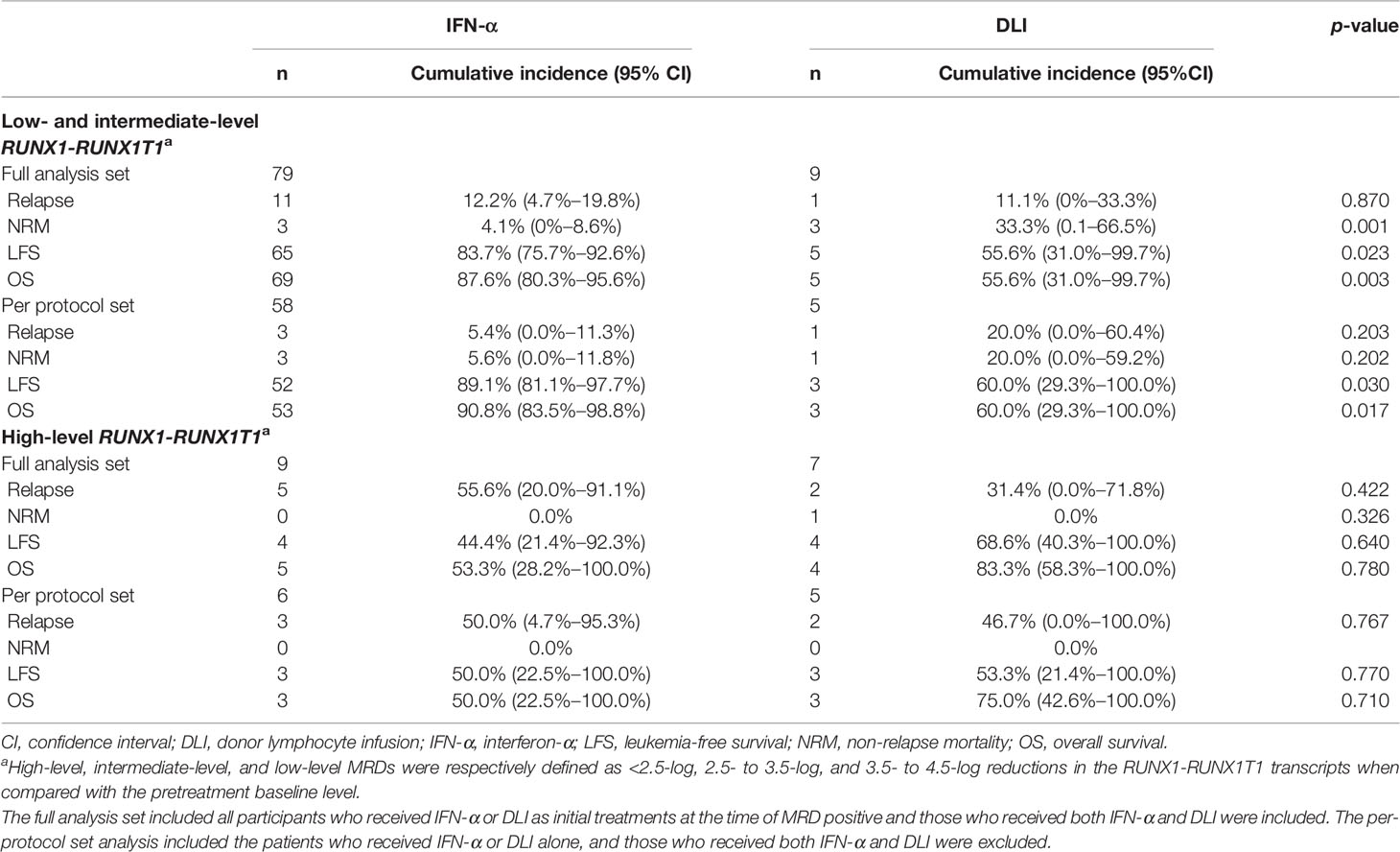
Table 2 The 2-year cumulative incidence of relapse, NRM, LFS, and OS after preemptive interventions.
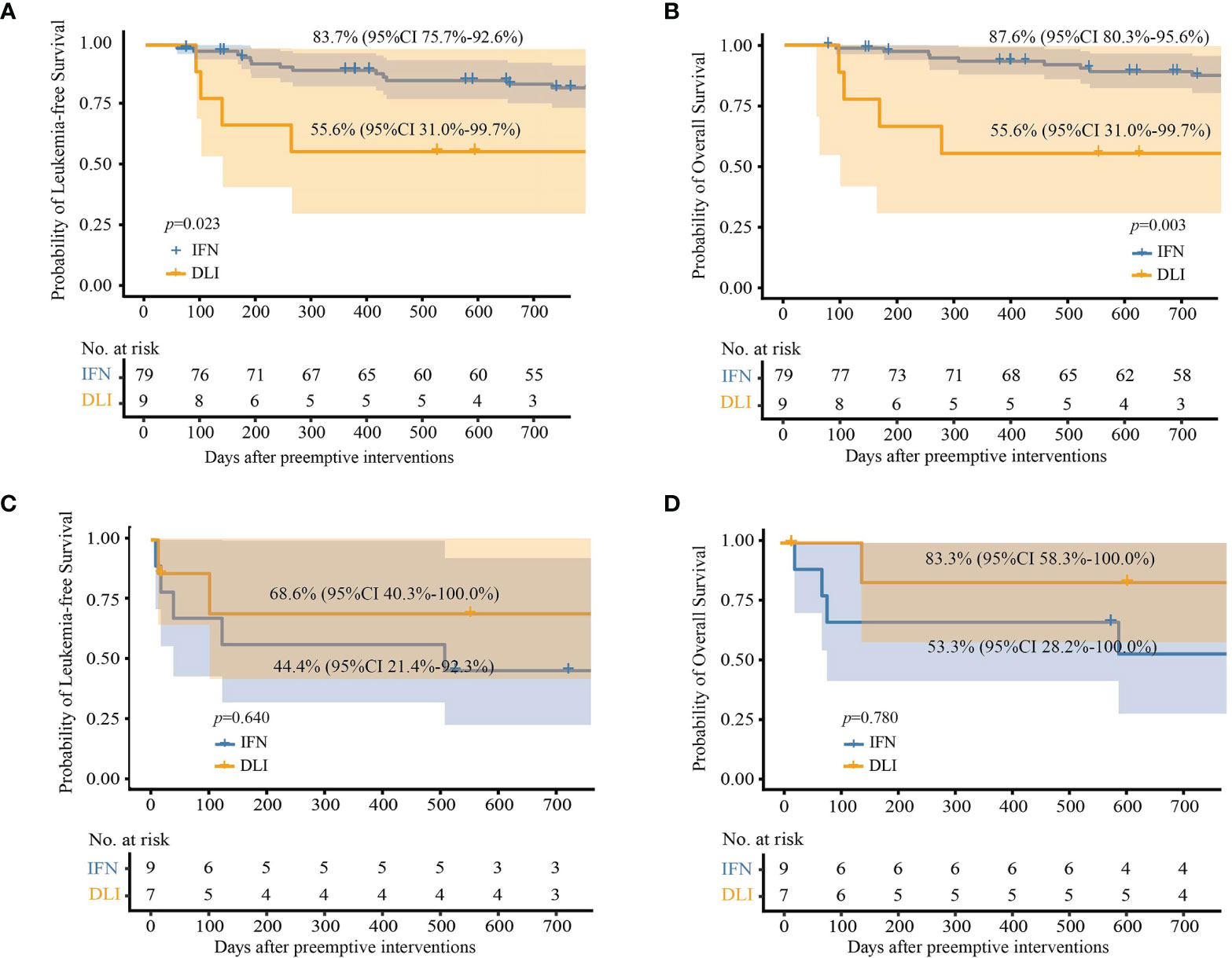
Figure 3 Probabilities of survival at 2 years after preemptive immunotherapies in full analysis set. (A) Leukemia-free survival in patients with low- and intermediate-level RUNX1-RUNX1T1; (B) overall survival in patients with low- and intermediate-level RUNX1-RUNX1T1; (C) leukemia-free survival in patients with high-level RUNX1-RUNX1T1; (D) overall survival in patients with high-level RUNX1-RUNX1T1.
For patients with high-level RUNX1-RUNX1T1 (i.e., <2.5-log reduction), the probabilities of survival at 2 years after intervention were all comparable between the IFN-α and DLI groups (Table 2 and Figures 3C, D).
In multivariate analysis, for patients receiving preemptive IFN-α therapy, the relapse and survival of the low-level and intermediate-level RUNX1-RUNX1T1 groups were superior to those of the high-level group (Table 3). In addition, identical sibling donors also predicted a high risk of relapse and poorer survival.
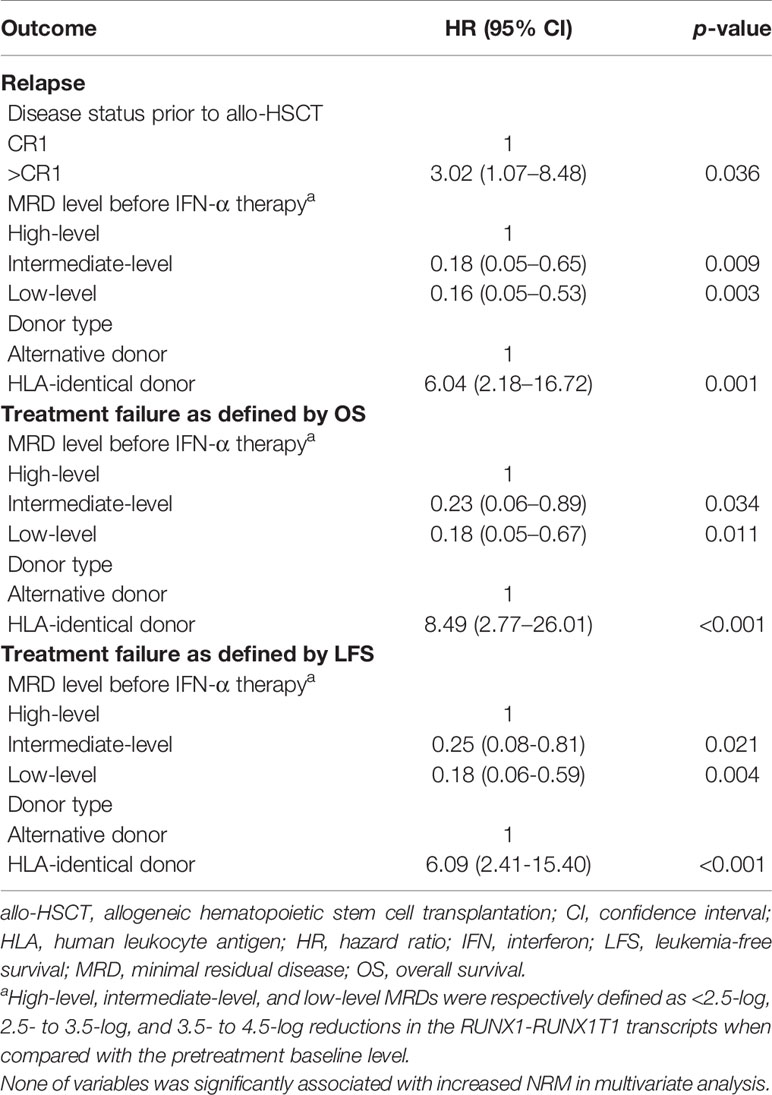
Table 3 Multivariate analysis of risk factors for the 2-year clinical outcomes after preemptive IFN-α therapy in full analysis set.
3.4.2 PPS Analysis
In this analysis, patients who received both DLI and IFN-α treatment were excluded, and 64 and 10 patients in the IFN-α and DLI groups, respectively (Table 2).
For patients with low-level and intermediate-level RUNX1-RUNX1T1, the IFN-α group also showed significantly better OS and LFS rates than those of the DLI group (Table 2 and Figures 4A, B). Particularly, for patients with low-level RUNX1-RUNX1T1, the incidence of NRM, relapse, LFS, and OS at 2 years after IFN-α therapy was 5.2% (95% CI, 0.0%−12.3%), 2.6% (95% CI, 0.0%−7.6%), 92.2% (95% CI, 84.2%−100.0%), and 92.2% (95% CI, 84.2%−100.0%), respectively.
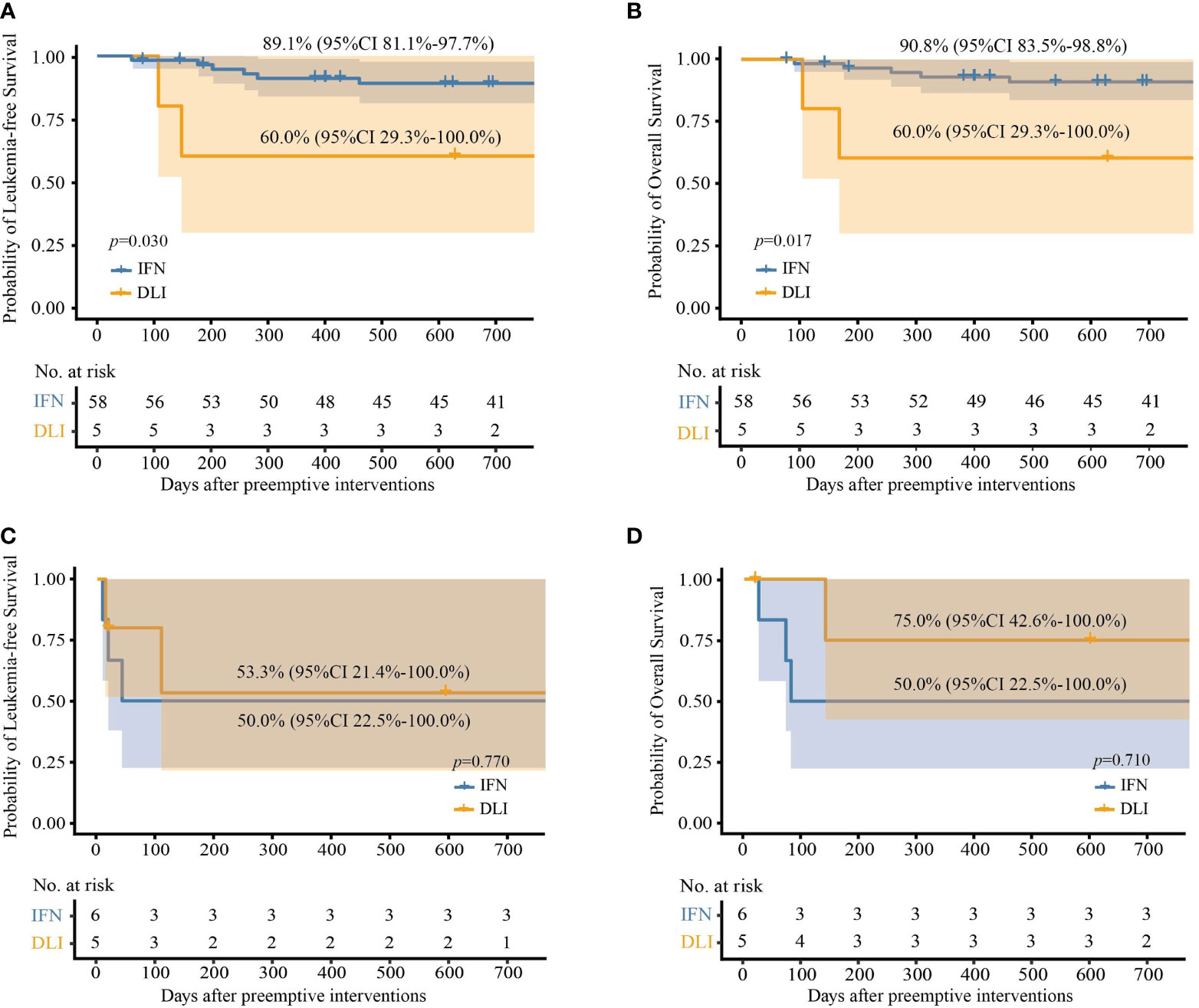
Figure 4 Probabilities of survival at 2 years after preemptive immunotherapies in per protocol set. (A) Leukemia-free survival in patients with low- and intermediate-level RUNX1-RUNX1T1; (B) overall survival in patients with low- and intermediate-level RUNX1-RUNX1T1; (C) leukemia-free survival in patients with high-level RUNX1-RUNX1T1; (D) overall survival in patients with high-level RUNX1-RUNX1T1.
For patients with high-level RUNX1-RUNX1T1, the probabilities of survival at 2 years after the intervention were all comparable between the IFN-α and DLI groups (Table 2 and Figures 4C, D).
In multivariate analysis, for patients receiving preemptive IFN-α treatment, the OS of the low-level and intermediate-level RUNX1-RUNX1T1 group was superior to that of the high-level group (Supplementary Table S6). Furthermore, identical sibling donors predicted poorer survival.
3.4.3 Analysis of Patients Who Received Both IFN-α and Chemo-DLI
A total of 30 patients receiving both IFN-α and DLI were included in this analysis (DLI followed by IFN, n = 6; IFN followed by DLI, n = 24; Figure 1).
For patients with low-level and intermediate-level RUNX1-RUNX1T1, the cumulative incidence of NRM at 2 years was lower in IFN-α followed by DLI group (0% vs. 50.0%, 95% CI, 0.0%−100.0%; p =0.001) than those in DLI followed by the IFN-α group, but the probability of relapse and survival were all comparable between the groups (Supplementary Table S7).
Three patients with high-level RUNX1-RUNX1T1 received salvage DLI after IFN-α therapy. Two of them achieved MRD negative, but one of them experienced relapse and died. The patient with persistent MRD positive also experienced a relapse. Two patients received salvage IFN-α after DLI, one of whom achieved MRD negative but died from pneumonia, and the other achieved MRD negative and persistent LFS until the last follow-up.
4 Discussion
This study showed that patients with low-level and intermediate-level RUNX1-RUNX1T1 could benefit from preemptive IFN-α therapy. The clinical outcomes of preemptive IFN-α therapy and DLI in patients with high-level RUNX1-RUNX1T1 were unsatisfactory. To our knowledge, this is the first study to compare the efficacy of preemptive IFN-α therapy and DLI in a population of patients with a specific disease [i.e., t(8;21) AML] following allo-HSCT.
This study showed that the NRM rate was <10% in patients who received preemptive IFN-α therapy, similar to our previous studies (20, 22). Klingemann et al. (41) also reported that no life-threatening complications occurred during IFN-α therapy after HSCT. And, the results showed that the rate of NRM appeared to be higher in the DLI group than in the IFN-α group for patients with low-level and intermediate-level RUNX1-RUNX1T1, suggesting that the safety of IFN-α therapy may be more satisfactory in these patients. This may be because IFN-α therapy was administered in divided doses and could be adjusted if early signs of toxicity or GVHD were observed.
The graft-versus-leukemia (GVL) effect, which is strongly associated with cGVHD (42, 43), is the main mechanism for IFN-α clearing of MRD (44). The incidence of cGVHD was comparable between the preemptive DLI and IFN-α therapy groups (51.1% vs. 62.5%, p = 0.405). Therefore, the capacity to induce GVL was comparable between IFN-α therapy and DLI (19, 21) and could contribute to the similar rate of MRD achieving negative results between these two methods. However, cGHVD, particularly severe cGVHD, can influence quality of life (45, 46) and cause mortality (47). In this study, only 3.2% of patients experienced severe cGVHD, and no patients died from GVHD after IFN-α therapy. Therefore, the intensity of IFN-α-induced cGVHD was easy to be controlled.
We observed that the relapse rate was nearly one-third (27.4%) even in patients with low-level RUNX1-RUNX1T1 after transplantation (Qin et al., data unpublished) if no preemptive interventions were administered. In patients with low-level and intermediate-level RUNX1-RUNX1T1, most of them achieved MRD negative after IFN-α therapy. The rate of relapse was low, and the rate of survival was >80%, particularly for those with low-level RUNX1-RUNX1T1. In our previous study, the relapse and survival rates were 8% and 75%, respectively, for patients who were negative for RUNX1-RUNX1T1 in the first 3 months after allo-HSCT (14). Therefore, with the help of preemptive IFN-α therapy, patients with low-level and intermediate-level RUNX1-RUNX1T1 achieved comparable outcomes with those with persistent MRD-negative status after allo-HSCT. Patients with low-level and intermediate-level RUNX1-RUNX1T1 have been suggested to benefit more from IFN-α therapy, which could preferably be started in patients with a relatively low tumor burden (48).
In patients with high-level RUNX1-RUNX1T1, neither preemptive DLI nor IFN-α showed satisfactory outcomes. The survival of the IFN-α and DLI groups was comparable due to the small sample size of the DLI recipients. Furthermore, patients with high-level RUNX1-RUNX1T1 who showed an unsatisfactory response to DLI achieved MRD negative after salvage IFN-α treatment; however, those who showed an unsatisfactory response to IFN-α did not benefit from salvage DLI. We also observed that IFN-α salvage treatment was effective for patients who did not respond satisfactorily to preemptive DLI (18, 49). The number of patients who received both IFN-α and DLI was too small to draw any conclusions in this study, but this was an interesting phenomenon that suggested that therapeutic order may influence the outcomes of preemptive immunotherapy, and it is worth identifying in the future. Meanwhile, many new drugs (e.g., BCL-2 inhibitor) could be used in the treatment of AML, which would help to further improve the clinical outcomes of patients with high-level RUNX1-RUNX1T1 (50–52).
This was not a randomized trial, which was a limitation of the present study. Many patients might be inclined to choose IFN-α therapy because it can be conveniently performed in an outpatient setting, particularly for those with low-level and intermediate-level RUNX1-RUNX1T1. Therefore, it was too early to draw strong conclusions that DLI was inferior to IFN-α therapy in these patients, which should be confirmed by a randomized trial. Secondly, the number of patients with high-level RUNX1-RUNX1T1 was small. Because most of the patients with low-level and intermediate-level RUNX1-RUNX1T1 could clear the MRD after IFN-α therapy, the evolution of MRD was stopped in the early stage and did not develop into high-level RUNX1-RUNX1T1. Thus, the efficacy of DLI and IFN-α therapy in patients with high level of RUNX1-RUNX1T1 should also be further investigated.
Conclusion
This study showed that patients with low-level and intermediate-level RUNX1-RUNX1T1 could benefit more from preemptive IFN-α therapy compared with DLI. Clinical outcomes were comparable between preemptive IFN-α therapy and DLI in patients with high-level RUNX1-RUNX1T1; however, they should be further improved. In the future, randomized trials will compare the efficacy of IFN-α therapy with that of DLI in these patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
X-DM and X-JH designed the study. M-ZS, SF, X-HZ, L-PX, YW, C-HY, HC, Y-HC, WH, F-RW, J-ZW, X-SZ, Y-ZQ, Y-JC, and K-YL collected the data. M-ZS, SF, X-DM, and X-JH analyzed the data and drafted the manuscript. All authors contributed to the data interpretation, manuscript preparation, and approval of the final version.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2017YFA0104500), CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2019-I2M-5-034), the Capital’s Funds for Health Improvement and Research (grant number 2018-4-4089), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant number 81621001), the Program of the National Natural Science Foundation of China (grant number 82170208), the Key Program of the National Natural Science Foundation of China (grant number 81930004), and the Fundamental Research Funds for the Central Universities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.773394/full#supplementary-material
References
1. Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective Evaluation of Gene Mutations and Minimal Residual Disease in Patients With Core Binding Factor Acute Myeloid Leukemia. Blood (2013) 121(12):2213–23. doi: 10.1182/blood-2012-10-462879
2. Marcucci G, Mrózek K, Ruppert AS, Maharry K, Kolitz JE, Moore JO, et al. Prognostic Factors and Outcome of Core Binding Factor Acute Myeloid Leukemia Patients With t(8;21) Differ From Those of Patients With inv(16): A Cancer and Leukemia Group B Study. J Clin Oncol (2005) 23(24):5705–17. doi: 10.1200/jco.2005.15.610
3. Weisser M, Haferlach C, Hiddemann W, Schnittger S. The Quality of Molecular Response to Chemotherapy Is Predictive for the Outcome of AML1-ETO-Positive AML and Is Independent of Pretreatment Risk Factors. Leukemia (2007) 21(6):1177–82. doi: 10.1038/sj.leu.2404659
4. Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal Residual Disease Monitoring by Quantitative RT-PCR in Core Binding Factor AML Allows Risk Stratification and Predicts Relapse: Results of the United Kingdom MRC AML-15 Trial. Blood (2012) 120(14):2826–35. doi: 10.1182/blood-2012-06-435669
5. Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, et al. MRD-Directed Risk Stratification Treatment May Improve Outcomes of t(8;21) AML in the First Complete Remission: Results From the AML05 Multicenter Trial. Blood (2013) 121(20):4056–62. doi: 10.1182/blood-2012-11-468348
6. Morschhauser F, Cayuela JM, Martini S, Baruchel A, Rousselot P, Socié G, et al. Evaluation of Minimal Residual Disease Using Reverse-Transcription Polymerase Chain Reaction in t(8;21) Acute Myeloid Leukemia: A Multicenter Study of 51 Patients. J Clin Oncol (2000) 18(4):788–94. doi: 10.1200/jco.2000.18.4.788
7. Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. The Consensus on Indications, Conditioning Regimen, and Donor Selection of Allogeneic Hematopoietic Cell Transplantation for Hematological Diseases in China-Recommendations From the Chinese Society of Hematology. J Hematol Oncol (2018) 11(1):33. doi: 10.1186/s13045-018-0564-x
8. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Disease Risk Comorbidity Index for Patients Receiving Haploidentical Allogeneic Hematopoietic Transplantation. Engineering (2021) 7(2):162–9. doi: 10.1016/j.eng.2020.12.005
9. Guo H, Chang YJ, Hong Y, Xu LP, Wang Y, Zhang XH, et al. Dynamic Immune Profiling Identifies the Stronger Graft-Versus-Leukemia (GVL) Effects With Haploidentical Allografts Compared to HLA-Matched Stem Cell Transplantation. Cell Mol Immunol (2021) 18(5):1172–85. doi: 10.1038/s41423-020-00597-1
10. Yoon JH, Kim HJ, Kim JW, Jeon YW, Shin SH, Lee SE, et al. Identification of Molecular and Cytogenetic Risk Factors for Unfavorable Core-Binding Factor-Positive Adult AML With Post-Remission Treatment Outcome Analysis Including Transplantation. Bone Marrow Transplant (2014) 49(12):1466–74. doi: 10.1038/bmt.2014.180
11. Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H, et al. The Consensus on the Monitoring, Treatment, and Prevention of Leukemia Relapse After Allogeneic Hematopoietic Stem Cell Transplantation in China. Cancer Lett (2018) 438:63–75. doi: 10.1016/j.canlet.2018.08.030
12. Mo XD, Lv M, Huang XJ. Preventing Relapse After Haematopoietic Stem Cell Transplantation for Acute Leukaemia: The Role of Post-Transplantation Minimal Residual Disease (MRD) Monitoring and MRD-Directed Intervention. Br J Haematol (2017) 179(2):184–97. doi: 10.1111/bjh.14778
13. Qin YZ, Wang Y, Xu LP, Zhang XH, Chen H, Han W, et al. The Dynamics of RUNX1-RUNX1T1 Transcript Levels After Allogeneic Hematopoietic Stem Cell Transplantation Predict Relapse in Patients With t(8;21) Acute Myeloid Leukemia. J Hematol Oncol (2017) 10(1):44. doi: 10.1186/s13045-017-0414-2
14. Wang Y, Wu DP, Liu QF, Qin YZ, Wang JB, Xu LP, et al. In Adults With t(8;21)AML, Posttransplant RUNX1/RUNX1T1-Based MRD Monitoring, Rather Than c-KIT Mutations, Allows Further Risk Stratification. Blood (2014) 124(12):1880–6. doi: 10.1182/blood-2014-03-563403
15. Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk Stratification-Directed Donor Lymphocyte Infusion Could Reduce Relapse of Standard-Risk Acute Leukemia Patients After Allogeneic Hematopoietic Stem Cell Transplantation. Blood (2012) 119(14):3256–62. doi: 10.1182/blood-2011-09-380386
16. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Salvage Chemotherapy Followed by Granulocyte Colony-Stimulating Factor-Primed Donor Leukocyte Infusion With Graft-vs.-Host Disease Control for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation: Prognostic Factors and Clinical Outcomes. Eur J Haematol (2016) 96(3):297–308. doi: 10.1111/ejh.12591
17. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Comparison of Outcomes After Donor Lymphocyte Infusion With or Without Prior Chemotherapy for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Ann Hematol (2017) 96(5):829–38. doi: 10.1007/s00277-017-2960-7
18. Mo X, Zhang X, Xu L, Wang Y, Yan C, Chen H, et al. Interferon-Alpha Salvage Treatment Is Effective for Patients With Acute Leukemia/Myelodysplastic Syndrome With Unsatisfactory Response to Minimal Residual Disease-Directed Donor Lymphocyte Infusion After Allogeneic Hematopoietic Stem Cell Transplantation. Front Med (2019) 13(2):238–49. doi: 10.1007/s11684-017-0599-3
19. Mo X, Zhang X, Xu L, Wang Y, Yan C, Chen H, et al. Minimal Residual Disease-Directed Immunotherapy for High-Risk Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Front Med (2019) 13(3):354–64. doi: 10.1007/s11684-018-0665-5
20. Mo XD, Wang Y, Zhang XH, Xu LP, Yan CH, Chen H, et al. Interferon-Alpha Is Effective for Treatment of Minimal Residual Disease in Patients With t(8;21) Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Prospective Registry Study. Oncologist (2018) 23(11):1349–57. doi: 10.1634/theoncologist.2017-0692
21. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Interferon-Alpha: A Potentially Effective Treatment for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2015) 21(11):1939–47. doi: 10.1016/j.bbmt.2015.06.014
22. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. IFN-Alpha Is Effective for Treatment of Minimal Residual Disease in Patients With Acute Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant (2017) 23(8):1303–10. doi: 10.1016/j.bbmt.2017.04.023
23. Mo XD, Wang Y, Zhang XH, Xu LP, Yan CH, Chen H, et al. Interferon-α Is Effective for Treatment of Minimal Residual Disease in Patients With t(8;21) Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Prospective Registry Study. Oncologist (2018) 23(11):1349–57. doi: 10.1634/theoncologist.2017-0692
24. Xiao-Jun H, Lan-Ping X, Kai-Yan L, Dai-Hong L, Yu W, Huan C, et al. Partially Matched Related Donor Transplantation Can Achieve Outcomes Comparable With Unrelated Donor Transplantation for Patients With Hematologic Malignancies. Clin Cancer Res (2009) 15(14):4777–83. doi: 10.1158/1078-0432.Ccr-09-0691
25. Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs.Identical-Sibling Transplant for AML in Remission: A Multicenter, Prospective Study. Blood (2015) 125(25):3956–62. doi: 10.1182/blood-2015-02-627786
26. Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Haploidentical Hematopoietic Stem Cell Transplantation Without In Vitro T-Cell Depletion for the Treatment of Hematological Malignancies. Bone Marrow Transplant (2006) 38(4):291–7. doi: 10.1038/sj.bmt.1705445
27. Chang YJ, Luznik L, Fuchs EJ, Huang XJ. How Do We Choose the Best Donor for T-Cell-Replete, HLA-Haploidentical Transplantation? J Hematol Oncol (2016) 9:35. doi: 10.1186/s13045-016-0265-2
28. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The Consensus From The Chinese Society of Hematology on Indications, Conditioning Regimens and Donor Selection for Allogeneic Hematopoietic Stem Cell Transplantation: 2021 Update. J Hematol Oncol (2021) 14(1):145. doi: 10.1186/s13045-021-01159-2
29. Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Treatment of Acute Leukemia With Unmanipulated HLA-Mismatched/Haploidentical Blood and Bone Marrow Transplantation. Biol Blood Marrow Transplant (2009) 15(2):257–65. doi: 10.1016/j.bbmt.2008.11.025
30. Mo X, Zhang Y, Zhang X, Xu L, Wang Y, Yan C, et al. The Role of Collateral Related Donors in Haploidentical Hematopoietic Stem Cell Transplantation. Sci Bull (2018) 63(20):1376–82. doi: 10.1016/j.scib.2018.08.008
31. Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the Best Donor for a Related HLA Haplotype-Mismatched Transplant? Blood (2014) 124(6):843–50. doi: 10.1182/blood-2014-03-563130
32. Wang Y, Liu Q-F, Lin R, Yang T, Xu Y-J, Mo X-D, et al. Optimizing Antithymocyte Globulin Dosing in Haploidentical Hematopoietic Cell Transplantation: Long-Term Follow-Up of a Multicenter, Randomized Controlled Trial. Sci Bull (2021) 124(6):843–50. doi: 10.1016/j.scib.2021.06.002
33. Liu SN, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Prognostic Factors and Long-Term Follow-Up of Basiliximab for Steroid-Refractory Acute Graft-Versus-Host Disease: Updated Experience From a Large-Scale Study. Am J Hematol (2020) 95(8):927–36. doi: 10.1002/ajh.25839
34. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and Management of Graft Versus Host Disease After Stem-Cell Transplantation for Haematological Malignancies: Updated Consensus Recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol (2020) 7(2):e157–67. doi: 10.1016/S2352-3026(19)30256-X
35. Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and Treatment of GVHD: EBMT-ELN Working Group Recommendations for a Standardized Practice. Bone Marrow Transplant (2014) 49(2):168–73. doi: 10.1038/bmt.2013.107
36. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant (2015) 21(3):389–401.e1. doi: 10.1016/j.bbmt.2014.12.001
37. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant (2005) 11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004
38. Dignan FL, Amrolia P, Clark A, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and Management of Chronic Graft-Versus-Host Disease. Br J Haematol (2012) 158(1):46–61. doi: 10.1111/j.1365-2141.2012.09128.x
39. Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol (2003) 21(24):4642–9. doi: 10.1200/JCO.2003.04.036
40. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of Failure Probabilities in the Presence of Competing Risks: New Representations of Old Estimators. Stat Med (1999) 18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o
41. Klingemann HG, Grigg AP, Wilkie-Boyd K, Barnett MJ, Eaves AC, Reece DE, et al. Treatment With Recombinant Interferon (Alpha-2b) Early After Bone Marrow Transplantation in Patients at High Risk for Relapse [Corrected]. Blood (1991) 78(12):3306–11. doi: 10.1182/blood.V78.12.3306.3306
42. Mo XD, Xu LP, Zhang XH, Liu DH, Wang Y, Chen H, et al. Chronic GVHD Induced GVL Effect After Unmanipulated Haploidentical Hematopoietic SCT for AML and Myelodysplastic Syndrome. Bone Marrow Transplant (2015) 50(1):127–33. doi: 10.1038/bmt.2014.223
43. Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Sensitivity of Hematological Malignancies to Graft-Versus-Host Effects: An EBMT Megafile Analysis. Leukemia (2014) 28(11):2235–40. doi: 10.1038/leu.2014.145
44. Smits EL, Anguille S, Berneman ZN. Interferon–α May Be Back on Track to Treat Acute Myeloid Leukemia. Oncoimmunology (2013) 2(4):e23619. doi: 10.4161/onci.23619
45. Mo XD, Xu LP, Liu DH, Chen YH, Zhang XH, Chen H, et al. Health Related Quality of Life Among Patients With Chronic Graft-Versus-Host Disease in China. Chin Med J (Engl) (2013) 126(16):3048–52.
46. Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-Reported Quality of Life Is Associated With Severity of Chronic Graft-Versus-Host Disease as Measured by NIH Criteria: Report on Baseline Data From the Chronic GVHD Consortium. Blood (2011) 117(17):4651–7. doi: 10.1182/blood-2010-11-319509
47. Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, et al. Global and Organ-Specific Chronic Graft-Versus-Host Disease Severity According to the 2005 NIH Consensus Criteria. Blood (2011) 118(15):4242–9. doi: 10.1182/blood-2011-03-344390
48. Anguille S, Lion E, Willemen Y, Van Tendeloo VF, Berneman ZN, Smits EL. Interferon-α in Acute Myeloid Leukemia: An Old Drug Revisited. Leukemia (2011) 25(5):739–48. doi: 10.1038/leu.2010.324
49. Mo X, Zhao X, Xu L, Liu D, Zhang X, Chen H, et al. Interferon α: The Salvage Therapy for Patients With Unsatisfactory Response to Minimal Residual Disease-Directed Modified Donor Lymphocyte Infusion. Chin Med J (Engl) (2014) 127(14):2583–7.
50. Lai C, Doucette K, Norsworthy K. Recent Drug Approvals for Acute Myeloid Leukemia. J Hematol Oncol (2019) 12(1):100. doi: 10.1186/s13045-019-0774-x
51. DeWolf SE, Tallman MS. How I Treat Relapsed or Refractory AML. Blood (2020) 136(9):1023–32. doi: 10.1182/blood.2019001982
Keywords: RUNX1-RUNX1T1, allogeneic hematopoietic stem cell transplantation, preemptive, interferon, donor lymphocyte infusion
Citation: Fan S, Shen M-Z, Zhang X-H, Xu L-P, Wang Y, Yan C-H, Chen H, Chen Y-H, Han W, Wang F-R, Wang J-Z, Zhao X-S, Qin Y-Z, Chang Y-J, Liu K-Y, Huang X-J and Mo X-D (2022) Preemptive Immunotherapy for Minimal Residual Disease in Patients With t(8;21) Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Oncol. 11:773394. doi: 10.3389/fonc.2021.773394
Received: 09 September 2021; Accepted: 10 December 2021;
Published: 06 January 2022.
Edited by:
Fabio Guolo, San Martino Hospital (IRCCS), ItalyReviewed by:
Daniela Damiani, Hematology and Stem Cell Transplant, ItalyBenjamin Kent Nagy Tomlinson, University Hospitals Cleveland Medical Center, United States
Copyright © 2022 Fan, Shen, Zhang, Xu, Wang, Yan, Chen, Chen, Han, Wang, Wang, Zhao, Qin, Chang, Liu, Huang and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Dong Mo, bXhkNDUzQDE2My5jb20=
†These authors have contributed equally to this work
 Shuang Fan
Shuang Fan Meng-Zhu Shen
Meng-Zhu Shen Xiao-Hui Zhang
Xiao-Hui Zhang Lan-Ping Xu
Lan-Ping Xu Yu Wang
Yu Wang Chen-Hua Yan1
Chen-Hua Yan1 Ya-Zhen Qin
Ya-Zhen Qin Xiao-Jun Huang
Xiao-Jun Huang Xiao-Dong Mo
Xiao-Dong Mo