
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 08 November 2021
Sec. Pediatric Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.772411
This article is part of the Research TopicCritical Complications In Pediatric Oncology and Hematopoietic Cell TransplantView all 31 articles
 Lama Elbahlawan1*
Lama Elbahlawan1* Jenny McArthur1
Jenny McArthur1 Cara E. Morin2
Cara E. Morin2 Hafeez Abdelhafeez3
Hafeez Abdelhafeez3 M. Beth McCarville2
M. Beth McCarville2 Robert E. Ruiz4
Robert E. Ruiz4 Saumini Srinivasan5
Saumini Srinivasan5 Amr Qudeimat6
Amr Qudeimat6Pulmonary complications are common in children following hematopoietic cell transplantation (HCT) and contribute to their morbidity and mortality. Early diagnosis is essential for management and prevention of progression of lung injury and damage. In many cases, diagnosis can be challenging and may require diagnostic imaging and more invasive testing such as bronchoscopy and lung biopsy. We report the case of a 12-year-old girl who developed recurrent episodes of acute respiratory failure requiring intensive care unit admission in the post-HCT phase and describe the diagnostic and multidisciplinary approach for her management. In addition, we review the diagnostic approach of pulmonary complications post-HCT and highlight the utility and risks of bronchoscopy and lung biopsy in these children.
Pulmonary complications are common in children following hematopoietic cell transplantation (HCT) and contribute to morbidity and mortality in the post-HCT phase (1–3). Early identification, diagnosis, and treatment of these complications are essential to limit morbidity and improve outcome. However, diagnosis can be quite challenging as etiologies can overlap and obscure diagnosis. In such complicated cases, laboratory testing and diagnostic imaging may not establish diagnosis and further invasive diagnostic testing such as bronchoscopy and lung biopsy might be needed. We report a 12-year-old girl who developed recurrent episodes of respiratory distress requiring intensive care unit (ICU) admission in the post-HCT phase and describe the diagnostic challenges and the multidisciplinary approach that is crucial in similar situations. Furthermore, we present a review of the diagnostic approach of pulmonary complications post-HCT and highlight the utility of bronchoscopy and lung biopsy to establish diagnosis in these children.
A 12-year-old female patient with a history of T-cell acute lymphoblastic leukemia presented to our ICU on Day +38 after her second haploidentical HCT due to a failed extubation in the OR post bronchoscopy. Our patient had previously undergone a haploidentical HCT with prep regimen consisting of fludarabine, thiotepa, melphalan, cyclophosphamide and rabbit ATG followed by a TCRα/β+ and CD19+ depleted graft infusion. GCSF was administered until engraftment and CD45RA-depleted donor lymphocyte infusion was given post engraftment for immune reconstitution. Our patient’s first haploidentical HCT was complicated by Cytomegalovirus (CMV) viremia and acute grade II gastrointestinal graft versus host disease (GVHD). CMV reactivation was treated with ganciclovir, foscarnet and cytogam administration and GVHD became quiescent with oral budesonide and beclomethasone. She had a CNS relapse on Day +150, with subsequent bone marrow relapse. She underwent remission induction chemotherapy and proceeded to have a second haploidentical HCT. During her second HCT, she received reduced intensity conditioning with fludarabine, thiotepa, melphalan, cyclophosphamide, and rabbit ATG, followed by a TCRα/β+ depleted graft and subsequent memory T cell infusion (CD45A+ depletion). Our patient’s second HCT was complicated by CMV reactivation requiring ganciclovir therapy as well as rising LDH, complement level (CH50), proteinuria, and acute kidney injury suspicious for transplant-associated thrombotic microangiopathy (TMA). She was started on eculizumab on Day +8 after her second HCT and achieved engraftment on Day +11. Engraftment was complicated by fevers and hyperferritinemia suspicious for secondary hemophagocytic lymphohistiocytosis treated briefly with Anakinra that was discontinued since she did not meet criteria for diagnosis of hemophagocytic lymphohistiocytosis. She was switched from sirolimus to ruxolitinib for GVHD prophylaxis. She developed a brief oxygen requirement on Day +20, which responded well to diuresis and she was discharged to home on Day +34. On Day +37 she was readmitted with tachypnea, a new oxygen requirement, and a diffuse bilateral airspace disease on chest x-ray (Figure 1A). A bronchoscopy was performed on day +38 to evaluate for an infectious etiology after elective intubation, and she was successfully extubated on Day +39. Broncho-alveolar lavage fluid was PCR positive for CMV, but no other infectious source was identified. Her ganciclovir was switched to foscarnet with cytogam added to improve coverage. Her condition improved and she transferred back to the HCT ward on Day +44. On Day +49, she was readmitted to the ICU with worsening hypoxia. CT scan showed diffuse ground glass appearance with air trapping in the lower lobes (Figures 1B, C). Despite serum CMV titers remaining low, her respiratory status continued to worsen. Lung biopsy by video-assisted thoracoscopic surgery (VATS) was performed on Day +55 due to uncertainty surrounding her diagnosis. Her lung biopsy demonstrated organizing pneumonia with patchy consolidation (Figure 2A), characterized by prominent fibroblastic proliferation in terminal airways and numerous macrophages occupying airspaces, compatible with cryptogenic organizing pneumonia (COP) (Figure 2B). An occlusive arterial thrombus was noted (Figure 2C), a possible sequela of TMA. Given these biopsy findings, she was started on methylprednisolone 1 mg/kg/day for COP. Steroids were well tolerated and were tapered over a period of 4 months. Her eculizumab dosing was increased to twice per week for better control of her TMA, and imatinib was added to prevent progression of fibrosis. Her respiratory status slowly improved, and she was transitioned back to the HCT unit on Day +73, with stable settings on bilevel positive airway pressure (BIPAP) support at night and minimal (1–2 liters/min) supplemental nasal cannula oxygen during the day. She was discharged to home on Day +84 with the same level of respiratory support. Imatinib was discontinued on day +169 and she was continued on Ruxolitinib. Clinical assessment on her last follow-up on day +315 showed continued improvement in her lung function with tolerance of her gradual wean of the BIPAP support. Her clinical course is summarized in Figure 3.
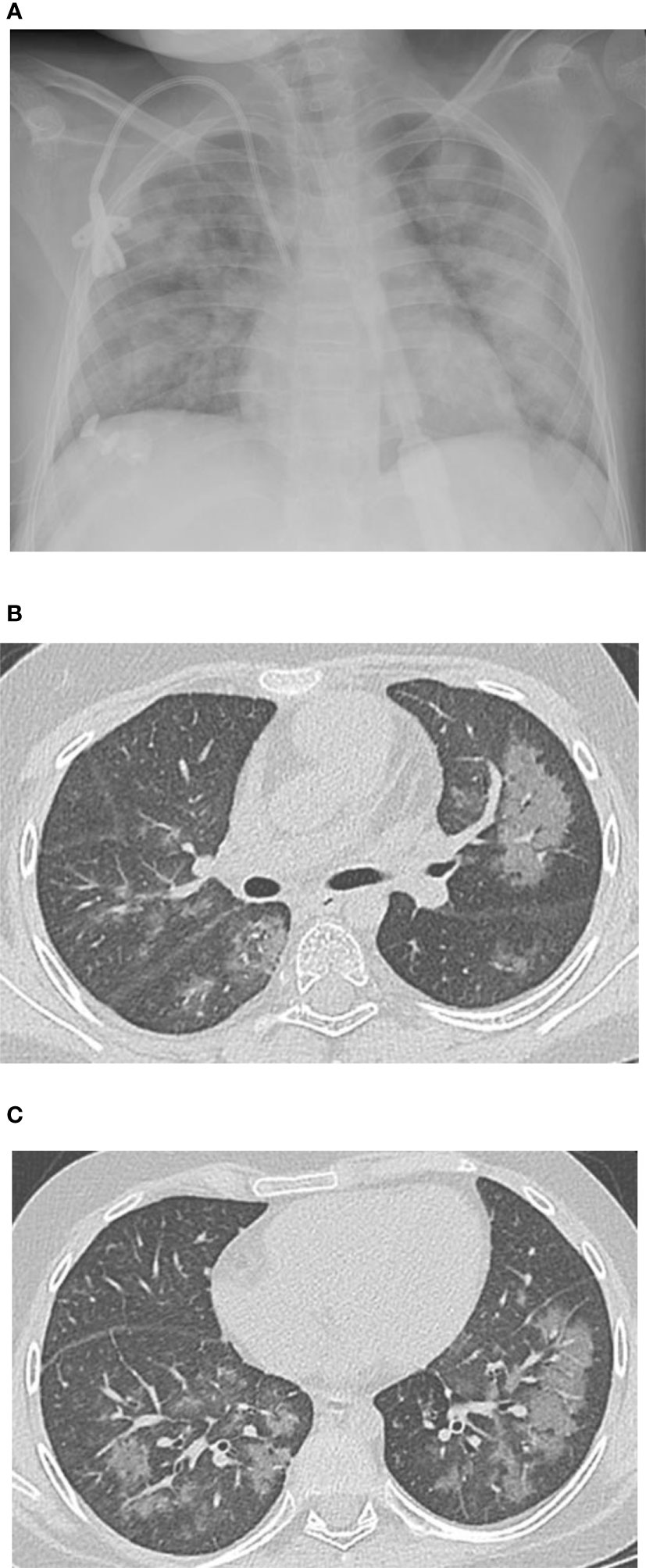
Figure 1 (A) Chest x-ray obtained Day +42 demonstrates diffuse airspace disease bilaterally with more focal areas of consolidation in the left lung. (B, C) Thin-section computed tomography (CT) obtained Day +49 (from 2nd transplant) demonstrate diffuse ground-glass opacification involving all 5 lobes.
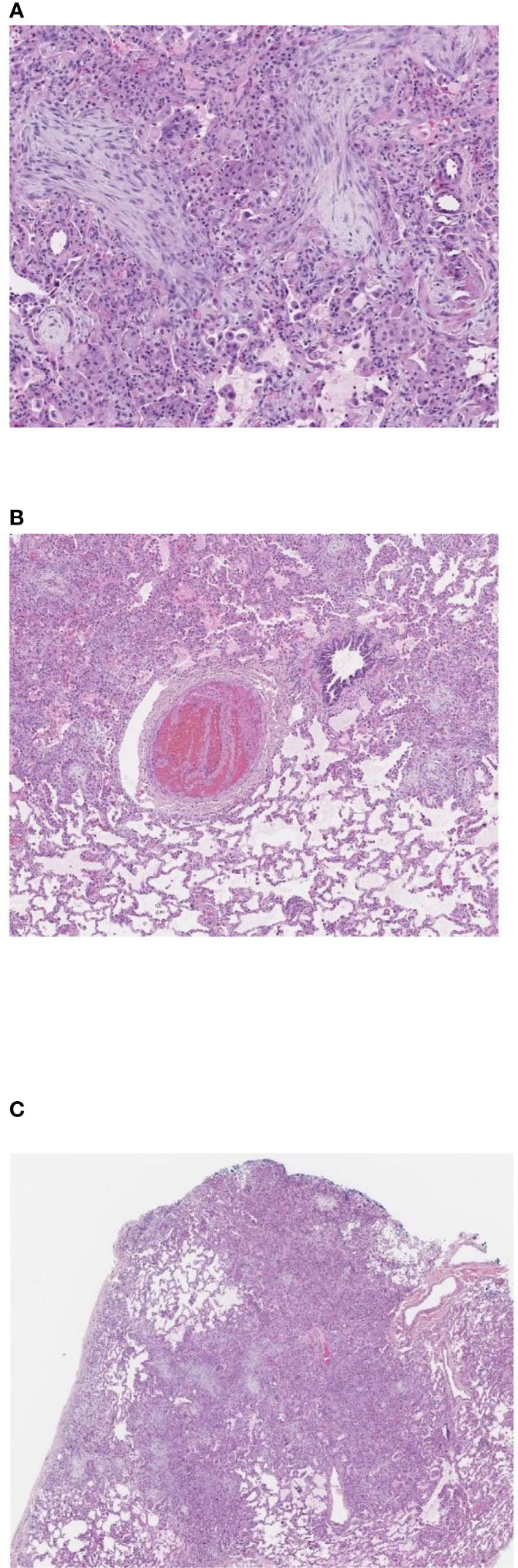
Figure 2 Pulmonary wedge biopsy, Day +55. (A) Patchy consolidation, 2x. (B) Organizing pneumonia with fibroblastic proliferation in terminal airways and numerous macrophages occupying airspaces, 10x. (C) Pulmonary arterial thrombus with alternating bands of fibrin and platelets (lines of Zahn), 4x. (A–C). Hematoxylin and eosin stain, magnifications using Leica Biosystems Aperio ImageScope.
Pulmonary complications post-HCT can be related to infectious or non-infectious etiologies. Infections causing pneumonia or ARDS can occur at any stage during the HCT course. However, infectious organisms will vary with the specific immune deficiency, whether humoral or cellular, depending on the timing of transplant. The rate of infectious complications has decreased with better strategies for pre-emptive testing and surveillance as well as use of antimicrobial prophylaxis. However, the incidence of non-infectious lung injury is still on the rise. Late onset non-infectious pulmonary complications usually follow a more predictable timeline after HCT (Table 1) (1, 4–9).
Pulmonary complications post-HCT are challenging for the radiologist due to pre-existing co-morbidities and co-existence of infectious and non-infectious etiologies, many of which have overlapping imaging findings. Chest radiographs are rarely sufficiently diagnostic and frequently falsely negative (10–17). Thus, chest computed tomography (CT) and specifically high-resolution chest CT has become commonplace in the management of pulmonary complications in post-HCT patients due to superior detection and characterization of parenchymal abnormalities (11–13, 17, 18). The term “high-resolution CT” engenders some confusion. Previously, this term referred to a technique of thin collimation (1–2 mm) coupled with a high-spatial-frequency reconstruction algorithm, performed by sampling the lung at staggered intervals (e.g., four slices evenly spaced from apices to bases or one slice every 2 cm) (19). Modern multi-detector helical CT scanners acquire “thin slices” (<1 mm), routinely allowing for contiguous imaging of the entire lung parenchyma and multiple high-resolution reconstructive algorithms to be performed following image acquisition. Imaging of the entire chest is typically performed without intravenous contrast during suspended full inspiration and repeated during suspended expiration. Examination of the lungs in expiration allows assessment of focal areas of air trapping that are not evident during inspiration. With modern-day multi-detector row CT scanners, the entire chest can be scanned in less than 1 sec for infant-sized patients and less than 2 sec for older children (20).
Imaging findings on CT must be interpreted within the appropriate clinical context. In the first 30 days, the frequency of infectious and non-infectious complications is similar (18). After 30 days, with immune system reconstitution, the frequency of infectious complications decreases and the spectrum of infectious organisms changes. In the neutropenic phase, infectious complications commonly include bacterial pneumonia, Respiratory syncytial virus (RSV), and invasive fungal pneumonia such as aspergillus. Bacterial pneumonia characteristically produces focal segmental or lobar pulmonary opacities. RSV and other viruses typically have a bilateral distribution and are often non-specific, ranging from normal CT scans to small nodules, ground-glass attenuation, and consolidation due to atelectasis or a combination of these (21, 22). Guidelines for imaging diagnosis of pulmonary aspergillosis include presence of one of the following patterns on CT: Dense, rounded lesion(s) with or without a halo sign (surrounding ground-glass hazy opacity); air crescent sign; cavity; or segmental lobar consolidation (23, 24). Criteria for other pulmonary mold diseases include prior criteria, with the addition of the reversed halo sign (central ground-glass opacity surrounded by denser crescentic consolidation). After 30 days, common pulmonary infectious agents include herpes simplex virus (HSV) or varicella zoster (VZ), CMV, pneumocystis jiroveci, and fungal pneumonia. As with RSV, findings with HSV, VZ, and CMV are non-specific and can include diffuse or multifocal areas of ground-glass attenuation, consolidations, and/or nodules (21, 25).
Non-infectious complications in the first 30 days include pulmonary edema, IPS, DAH, and PERDS. The most common complication in the first 30 days is pulmonary edema, which manifests on CT as enlargement of pulmonary vessels, diffuse ground glass opacification, septal thickening, and commonly cardiomegaly and/or pleural effusions. CT findings in IPS are non-specific and may include focal or diffuse airspace or reticular opacities in the setting of rapidly progressive respiratory failure. CT findings of DAH include diffuse ground-glass opacities and a “crazy-paving” pattern, related to intra- and inter-lobular thickening. Patients typically do not have cardiomegaly, prominent pulmonary vessels, or effusions differentiating this entity from edema. PERDS also demonstrates diffuse ground glass opacification, which can be associated with thickening of the interlobular septa, perihilar or peribronchial consolidation, and pleural effusions. Patients typically do not have cardiomegaly or other findings of pulmonary edema. Clinically, the presence of a skin rash and fever may be helpful for differentiation. In the early post-transplant period (30–100 days after HCT), IPS continues to be a common cause of respiratory symptoms. Additionally, acute GVHD can occur, although it is rare. CT findings of acute GVHD are non-specific and may include diffuse parenchymal opacities that resemble pulmonary edema (18).
Non-infectious late pulmonary complications (>100 days post HCT) include BOS, COP, or non-classifiable interstitial pneumonia (26). BOS demonstrates evidence of air trapping manifested as a mosaic attenuation pattern on CT. Mosaic attenuation is a finding of intermixed areas of low attenuation with areas of normal or increased attenuation. On expiratory images, the areas of decreased attenuation become more conspicuous. Thus, in patients >100 days post-HCT, it is recommended that CT be performed with inspiration and expiration phases (16, 27, 28). Additionally, patients with BOS demonstrate bronchiectasis. Findings of organizing pneumonia COP on CT include parenchymal consolidation with dilated bronchi, ground-glass opacities, nodular opacities, and/or the reversed-halo sign (18). Non-classifiable interstitial pneumonia is a third CT finding seen in post-HCT patients related to chronic GVHD. CT findings include ground glass opacities, reticulation, and the crazy-paving pattern in a predominant peribronchial distribution, as well as traction bronchiectasis progressing to more typical findings of fibrosis including bronchiectasis and honeycombing (18, 26).
The pulmonary function testing (PFT) is done routinely before HCT to assess baseline lung function. PFT does not usually have a role in early diagnosis of these pulmonary complications. However, PFT can be useful as a follow-up tool to assess response to therapy as well as a prognostic tool. BOS is characterized by an obstructive pattern with FEV1/FVC (forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio) <0.7 and FEV1 <75%. COP is characterized by a restrictive pattern with TLC less than the fifth percentile and normal FEV1/FVC (3).
Bronchoscopy with bronchoalveolar lavage (BAL) is usually performed with the acute onset and persistence of respiratory symptoms after antimicrobials initiation. In addition, if diffuse alveolar hemorrhage is highly suspected, BAL can confirm the diagnosis by retrieving sequential bloodier aliquots with BAL. Diagnostic yield of BAL in children post-HCT ranges from 30 to 67% and results in change of therapy in 34–68% of cases (Table 2). Viral and bacterial organisms are the most detected; the most common virus identified is CMV. Many factors can reduce the yield of this procedure, including duration of antimicrobial therapy before the procedure (30), acute GVHD GII-IV, and immunosuppressive therapy at the time of BAL (31). In a cohort of 57 post-HCT patients, shorter time between abnormal CXR and the BAL correlated with positive yield (2 days vs 6 days in patients with negative yield) (33). BAL yield is reported to be 2.5× higher when done within 4 days of presentation compared to later, and as high as 75% when performed within 24 h (38). Complications are reported in 1–31% of patients but are usually mild and short lived. Common complications include mild, transient hypoxia and bleeding. Recent introduction of metagenomic sequencing of these samples is promising and may increase the BAL yield by detecting a causative pathogen (39).
Earlier reports of surgical lung biopsy (SLB) questioned the benefits of this procedure due to higher complication rates and lack of modification of therapy with the findings (40, 41). However, advances in surgical techniques as well as better understanding and therapies of pulmonary complications post-HCT improved yield and utility of this procedure. Surgical lung biopsy can be performed either as open biopsy through thoracotomy or currently more often thoracoscopically by video-assisted thoracoscopic surgery (VATS). Compared to other lung biopsy approaches, SLB usually provides better tissue samples, as tissue sampling is done under direct visualization. Recent reports show a relatively high diagnostic yield that ranges from 71 to 100% in children post-HCT (Table 3). In particular, lung biopsy is superior to BAL in diagnosing non-infectious etiologies such as COP and BOS. Infectious etiology is identified in 15–58% of pediatric HCT patients with this procedure. In fact, many infectious etiologies can be diagnosed and identified solely by the BAL without the need for lung biopsy, which may explain the lower infectious yield. In most instances (>70% of cases), lung biopsy leads to change in therapy and often involves adding immunosuppressive therapy. Children often require chest tube placement for a brief period post-operatively. Other uncommon complications that can occur in the context of lung biopsy include prolonged air leak, hemothorax, and splenic injury.
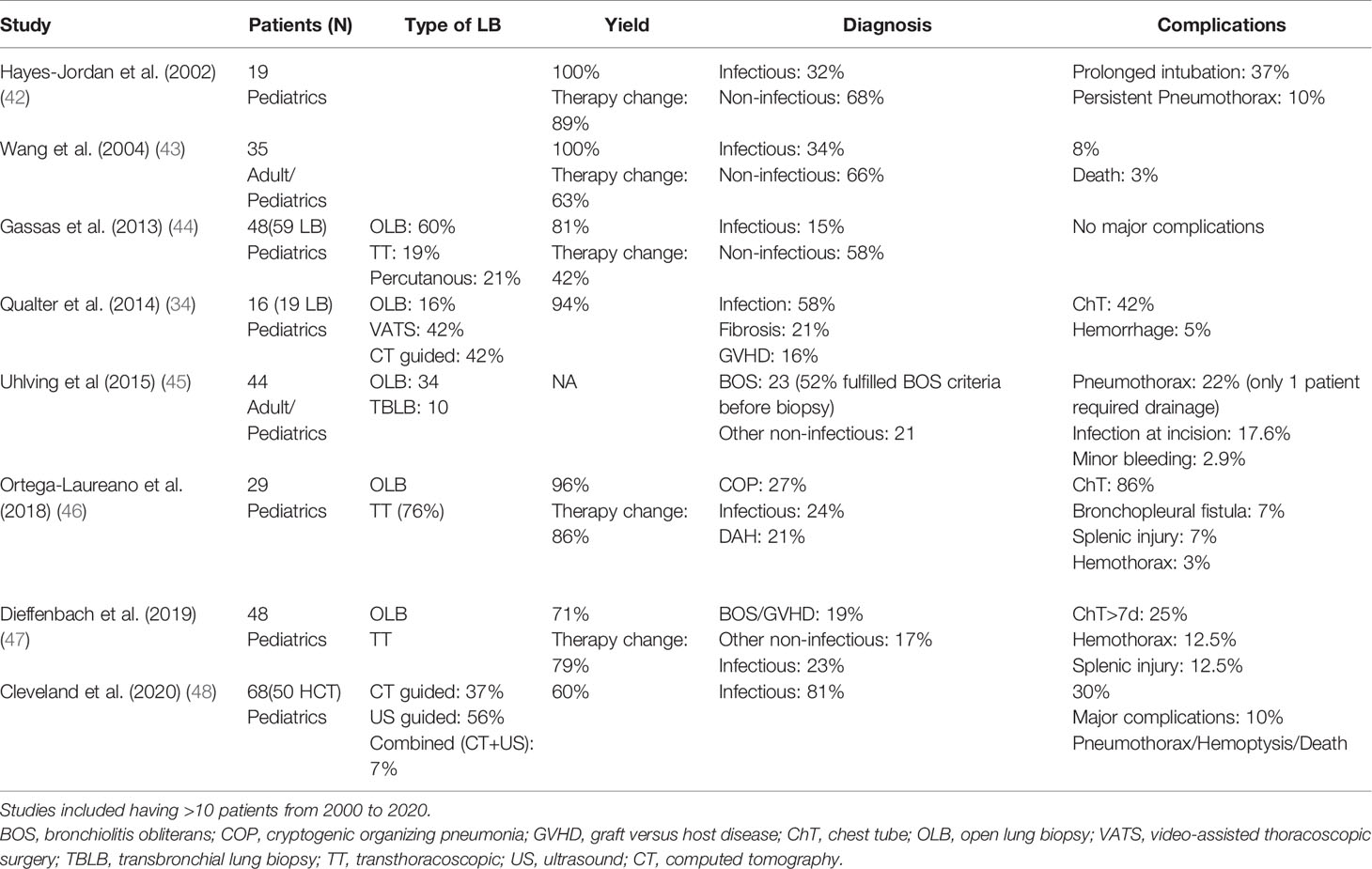
Table 3 Summary of studies evaluating lung biopsy in children post-HCT with pulmonary complications.
Percutaneous lung biopsy is another approach to retrieve a lung tissue sample for examination. It can be performed by ultrasound if the lesion or abnormality is in the lung periphery or by CT guidance. Recently, Cleveland et al. reported the largest cohort of 68 immunocompromised pediatric patients (50 HCT) who underwent 73 percutaneous lung biopsies (48). Percutaneous lung biopsy was performed under either CT guidance (37%) or US guidance (56%) or both (7%), mostly for lesions that are pleural spaced (84%). Lesions were non-malignant in 48% of cases (total diagnostic yield of 60% including malignancy diagnosis). Major complications such as pneumothorax, pulmonary hemorrhage, and death occurred in 10% of patients. Complication rates were lower in the US-guided vs CT guided biopsy approach, which can be attributed to the fact that the biopsy of deeper parenchymal lesions was done only by CT guidance.
The utility of transbronchial lung biopsy (TBLB) is questionable secondary to the low diagnostic yield and increased risk of complications compared to bronchoscopy (49). In a large cohort of 130 adults post-HCT, transbronchial biopsy yield was <50% and <5% for infectious diagnostic yield. In addition, TBLB had a 3× higher risk of complications compared to BAL alone and did not lead to therapy modification. Transbronchial biopsies are performed less often in the pediatric HCT population as they render increased risk without a clear benefit.
Approximately 35% of pediatric HCT patients undergo BAL, and less often lung biopsy (8%) (34). Which procedure gives the most helpful diagnostic information with the lowest complication rate remains to be answered. Many studies have compared both procedures in immunocompromised children but found no consensus on the superiority of one procedure (30, 50). In a study that compared BAL to lung biopsy in a pediatric cohort post-HCT, the diagnostic yield of BAL was 40% compared to 94% with lung biopsy. The median days of intubation was 8.5 days in the BAL group compared to 4 days in the lung biopsy group (34). A meta-analysis of 95 studies in cancer and HCT patients (both adult and children) compared the efficacy and complications of BAL vs lung biopsy. Non-infectious etiology was diagnosed in lung biopsy more often than BAL; however, the rate of complication was higher in the lung biopsy group compared to the BAL group (0.15 vs 0.008) (51).
The decision of which procedure to choose depends on the situation and often requires a multidisciplinary team discussion among transplant, infectious, pulmonary, surgical, radiology, and ICU teams. If an infection or DAH is suspected, BAL is often the initial diagnostic approach. However, in some instances, even with the detection of a pathogen on BAL and giving appropriate treatment, respiratory status may not normalize. In these situations, multifactorial etiologies may be present and lung biopsy can likely establish other diagnosis. In addition, SLB should be the initial diagnostic tool when COP, BOS, pulmonary fibrosis, and/or treatment toxicity are suspected.
Diagnosis of pulmonary complication post-HCT is complex and often requires a multidisciplinary approach. Initial diagnostic imaging may suggest the etiology of the pulmonary process. However, in many instances, further diagnostic testing such as BAL or lung biopsy is needed. BAL is useful for diagnosis of infectious pulmonary complications; however, lung biopsy is superior for diagnosis of non-infectious pulmonary complications. Benefits and risks of these procedures should be considered thoroughly, but an early decision aids in early identification and treatment of the pulmonary process and prevents further lung damage.
LE and AQ: conceptualization. LE, JM, CM, HA, MM, RR, SS, and AQ: writing—review and editing. All authors have reviewed and approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
This research was funded by the American Lebanese Syrian Associated Charities (ALSAC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Special thanks to Vani Shanker for scientific editing of the manuscript and Gail Deutsch, MD, for expert pathology consultation.
1. Elbahlawan L, Srinivasan A, Morrison RR. A Critical Care and Transplantation-Based Approach to Acute Respiratory Failure After Hematopoietic Stem Cell Transplantation in Children. Biol Blood Marrow Transplant (2016) 22:617–26. doi: 10.1016/j.bbmt.2015.09.015
2. Patriarca F, Skert C, Sperotto A, Damiani D, Cerno M, Geromin A, et al. Incidence, Outcome, and Risk Factors of Late-Onset Noninfectious Pulmonary Complications After Unrelated Donor Stem Cell Transplantation. Bone Marrow Transplant (2004) 33:751–8. doi: 10.1038/sj.bmt.1704426
3. Elbahlawan L, Galdo AM, Ribeiro RC. Pulmonary Manifestations of Hematologic and Oncologic Diseases in Children. Pediatr Clin North Am (2021) 68:61–80. doi: 10.1016/j.pcl.2020.09.003
4. Eikenberry M, Bartakova H, Defor T, Haddad IY, Ramsay NK, Blazar BR, et al. Natural History of Pulmonary Complications in Children After Bone Marrow Transplantation. Biol Blood Marrow Transplant (2005) 11:56–64. doi: 10.1016/j.bbmt.2004.09.008
5. Park M, Koh KN, Kim BE, Im HJ, Seo JJ. Clinical Features of Late Onset Non-Infectious Pulmonary Complications Following Pediatric Allogeneic Hematopoietic Stem Cell Transplantation. Clin Transplant (2011) 25:E168–176. doi: 10.1111/j.1399-0012.2010.01357.x
6. Lucena CM, Torres A, Rovira M, Marcos MA, de la Bellacasa JP, Sánchez M, et al. Pulmonary Complications in Hematopoietic SCT: A Prospective Study. Bone Marrow Transplant (2014) 49:1293–9. doi: 10.1038/bmt.2014.151
7. Elbahlawan L, Rains KJ, Stokes DC. Respiratory Care Considerations in the Childhood Cancer Patient. Respir Care (2017) 62:765–75. doi: 10.4187/respcare.05223
8. Khosla J, Yeh AC, Spitzer TR, Dey BR. Hematopoietic Stem Cell Transplant-Associated Thrombotic Microangiopathy: Current Paradigm and Novel Therapies. Bone Marrow Transplant (2018) 53:129–37. doi: 10.1038/bmt.2017.207
9. Montani D, Lau EM, Dorfmüller P, Girerd B, Jaïs X, Savale L, et al. Pulmonary Veno-Occlusive Disease. Eur Respir J (2016) 47:1518–34. doi: 10.1183/13993003.00026-2016
10. Harris B, Geyer AI. Diagnostic Evaluation of Pulmonary Abnormalities in Patients With Hematologic Malignancies and Hematopoietic Cell Transplantation. Clin Chest Med (2017) 38:317–31. doi: 10.1016/j.ccm.2016.12.008
11. Barloon TJ, Galvin JR, Mori M, Stanford W, Gingrich RD. High-Resolution Ultrafast Chest CT in the Clinical Management of Febrile Bone Marrow Transplant Patients With Normal or Nonspecific Chest Roentgenograms. Chest (1991) 99:928–33. doi: 10.1378/chest.99.4.928
12. Graham NJ, Müller NL, Miller RR, Shepherd JD. Intrathoracic Complications Following Allogeneic Bone Marrow Transplantation: CT Findings. Radiology (1991) 181:153–6. doi: 10.1148/radiology.181.1.1887025
13. Heussel CP, Kauczor HU, Heussel GE, Fischer B, Begrich M, Mildenberger P, et al. Pneumonia in Febrile Neutropenic Patients and in Bone Marrow and Blood Stem-Cell Transplant Recipients: Use of High-Resolution Computed Tomography. J Clin Oncol (1999) 17:796–805. doi: 10.1200/JCO.1999.17.3.796
14. Korones DN, Hussong MR, Gullace MA. Routine Chest Radiography of Children With Cancer Hospitalized for Fever and Neutropenia: Is it Really Necessary? Cancer (1997) 80:1160–4. doi: 10.1002/(SICI)1097-0142(19970915)80:6<1160::AID-CNCR20>3.0.CO;2-5
15. Maschmeyer G, Donnelly JP. How to Manage Lung Infiltrates in Adults Suffering From Haematological Malignancies Outside Allogeneic Haematopoietic Stem Cell Transplantation. Br J Haematol (2016) 173:179–89. doi: 10.1111/bjh.13934
16. Roberts SD, Wells GM, Gandhi NM, York NR, Maron G, Razzouk B, et al. Diagnostic Value of Routine Chest Radiography in Febrile, Neutropenic Children for Early Detection of Pneumonia and Mould Infections. Support Care Cancer (2012) 20:2589–94. doi: 10.1007/s00520-011-1366-7
17. Schueller G, Matzek W, Kalhs P, Schaefer-Prokop C. Pulmonary Infections in the Late Period After Allogeneic Bone Marrow Transplantation: Chest Radiography Versus Computed Tomography. Eur J Radiol (2005) 53:489–94. doi: 10.1016/j.ejrad.2004.06.009
18. Peña E, Souza CA, Escuissato DL, Gomes MM, Allan D, Tay J, et al. Noninfectious Pulmonary Complications After Hematopoietic Stem Cell Transplantation: Practical Approach to Imaging Diagnosis. Radiographics (2014) 34:663–83. doi: 10.1148/rg.343135080
19. Kazerooni EA. High-Resolution CT of the Lungs. AJR Am J Roentgenol (2001) 177:501–19. doi: 10.2214/ajr.177.3.1770501
20. Gottumukkala RV, Kalra MK, Tabari A, Otrakji A, Gee MS. Advanced CT Techniques for Decreasing Radiation Dose, Reducing Sedation Requirements, and Optimizing Image Quality in Children. Radiographics (2019) 39:709–26. doi: 10.1148/rg.2019180082
21. Escuissato DL, Gasparetto EL, Marchiori E, Rocha Gde M, Inoue C, Pasquini R, et al. Pulmonary Infections After Bone Marrow Transplantation: High-Resolution CT Findings in 111 Patients. AJR Am J Roentgenol (2005) 185:608–15. doi: 10.2214/ajr.185.3.01850608
22. Franquet T, Rodriguez S, Martino R, Giménez A, Salinas T, Hidalgo A. Thin-Section CT Findings in Hematopoietic Stem Cell Transplantation Recipients With Respiratory Virus Pneumonia. AJR Am J Roentgenol (2006) 187:1085–90. doi: 10.2214/AJR.05.0439
23. Alexander BD, Lamoth F, Heussel CP, Prokop CS, Desai SR, Morrissey CO, et al. Guidance on Imaging for Invasive Pulmonary Aspergillosis and Mucormycosis: From the Imaging Working Group for the Revision and Update of the Consensus Definitions of Fungal Disease From the EORTC/MSGERC. Clin Infect Dis (2021) 72:S79–s88. doi: 10.1093/cid/ciaa1855
24. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
25. Chong S, Kim TS, Cho EY. Herpes Simplex Virus Pneumonia: High-Resolution CT Findings. Br J Radiol (2010) 83:585–9. doi: 10.1259/bjr/51409455
26. Song I, Yi CA, Han J, Kim DH, Lee KS, Kim TS, et al. CT Findings of Late-Onset Noninfectious Pulmonary Complications in Patients With Pathologically Proven Graft-Versus-Host Disease After Allogeneic Stem Cell Transplant. AJR Am J Roentgenol (2012) 199:581–7. doi: 10.2214/AJR.11.7165
27. Franquet T, Müller NL, Lee KS, Giménez A, Flint JD. High-Resolution CT and Pathologic Findings of Noninfectious Pulmonary Complications After Hematopoietic Stem Cell Transplantation. AJR Am J Roentgenol (2005) 184:629–37. doi: 10.2214/ajr.184.2.01840629
28. Ooi GC, Peh WC, Ip M. High-Resolution Computed Tomography of Bronchiolitis Obliterans Syndrome After Bone Marrow Transplantation. Respiration (1998) 65:187–91. doi: 10.1159/000029257
29. Ben-Ari J, Yaniv I, Nahum E, Stein J, Samra Z, Schonfeld T. Yield of Bronchoalveolar Lavage in Ventilated and Non-Ventilated Children After Bone Marrow Transplantation. Bone Marrow Transplant (2001) 27:191–4. doi: 10.1038/sj.bmt.1702773
30. Armenian SH, Hoffman JA, Butturini AM, Kapoor N, Mascarenhas L. Invasive Diagnostic Procedures for Pulmonary Infiltrates in Pediatric Hematopoietic Stem Cell Transplant Recipients. Pediatr Transplant (2007) 11:736–42. doi: 10.1111/j.1399-3046.2007.00733.x
31. Kasow KA, King E, Rochester R, Tong X, Srivastava DK, Horwitz EM, et al. Diagnostic Yield of Bronchoalveolar Lavage is Low in Allogeneic Hematopoietic Stem Cell Recipients Receiving Immunosuppressive Therapy or With Acute Graft-Versus-Host Disease: The St. Jude Experience, 1990-2002. Biol Blood Marrow Transplant (2007) 13:831–7. doi: 10.1016/j.bbmt.2007.03.008
32. Efrati O, Gonik U, Bielorai B, Modan-Moses D, Neumann Y, Szeinberg A, et al. Fiberoptic Bronchoscopy and Bronchoalveolar Lavage for the Evaluation of Pulmonary Disease in Children With Primary Immunodeficiency and Cancer. Pediatr Blood Cancer (2007) 48:324–9. doi: 10.1002/pbc.20784
33. Forslöw U, Remberger M, Nordlander A, Mattsson J. The Clinical Importance of Bronchoalveolar Lavage in Allogeneic SCT Patients With Pneumonia. Bone Marrow Transplant (2010) 45:945–50. doi: 10.1038/bmt.2009.268
34. Qualter E, Satwani P, Ricci A, Jin Z, Geyer MB, Alobeid B, et al. A Comparison of Bronchoalveolar Lavage Versus Lung Biopsy in Pediatric Recipients After Stem Cell Transplantation. Biol Blood Marrow Transplant (2014) 20:1229–37. doi: 10.1016/j.bbmt.2014.04.019
35. Nadimpalli S, Foca M, Satwani P, Sulis ML, Constantinescu A, Saiman L. Diagnostic Yield of Bronchoalveolar Lavage in Immunocompromised Children With Malignant and Non-Malignant Disorders. Pediatr Pulmonol (2017) 52:820–6. doi: 10.1002/ppul.23644
36. Tang FF, Zhao XS, Xu LP, Zhang XH, Chen YH, Mo XD, et al. Utility of Flexible Bronchoscopy With Polymerase Chain Reaction in the Diagnosis and Management of Pulmonary Infiltrates in Allogeneic HSCT Patients. Clin Transplant (2018) 32(1):e13146. doi: 10.1111/ctr.13146
37. Eroglu-Ertugrul NG, Yalcin E, Oguz B, Ocal T, Kuskonmaz B, Emiralioglu N, et al. The Value of Flexible Bronchoscopy in Pulmonary Infections of Immunosuppressed Children. Clin Respir J (2020) 14:78–84. doi: 10.1111/crj.13103
38. Shannon VR, Andersson BS, Lei X, Champlin RE, Kontoyiannis DP. Utility of Early Versus Late Fiberoptic Bronchoscopy in the Evaluation of New Pulmonary Infiltrates Following Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2010) 45:647–55. doi: 10.1038/bmt.2009.203
39. Huang J, Jiang E, Yang D, Wei J, Zhao M, Feng J, et al. Metagenomic Next-Generation Sequencing Versus Traditional Pathogen Detection in the Diagnosis of Peripheral Pulmonary Infectious Lesions. Infect Drug Resist (2020) 13:567–76. doi: 10.2147/IDR.S235182
40. Dunn JC, West KW, Rescorla FJ, Tres Scherer LR, Engum SA, Rouse TM, et al. The Utility of Lung Biopsy in Recipients of Stem Cell Transplantation. J Pediatr Surg (2001) 36:1302–3. doi: 10.1053/jpsu.2001.25799
41. Shorter NA, Ross AJ 3rd, August C, Schnaufer L, Zeigler M, Templeton JM, et al. The Usefulness of Open-Lung Biopsy in the Pediatric Bone Marrow Transplant Population. J Pediatr Surg (1988) 23:533–7. doi: 10.1016/S0022-3468(88)80363-4
42. Hayes-Jordan A, Benaim E, Richardson S, Joglar J, Srivastava DK, Bowman L, et al. Open Lung Biopsy in Pediatric Bone Marrow Transplant Patients. J Pediatr Surg (2002) 37:446–52. doi: 10.1053/jpsu.2002.30854
43. Wang JY, Chang YL, Lee LN, Chen JH, Tang JL, Yang PC, et al. Diffuse Pulmonary Infiltrates After Bone Marrow Transplantation: The Role of Open Lung Biopsy. Ann Thorac Surg (2004) 78:267–72. doi: 10.1016/j.athoracsur.2004.03.002
44. Gassas A, Craig-Barnes H, Dell SD, Cox P, Schechter T, Doyle J, et al. Severe Lung Injury and Lung Biopsy in Children Post-Hematopoietic Stem Cell Transplantation: The Differences Between Allogeneic and Autologous Transplantation. Pediatr Transplant (2013) 17:278–84. doi: 10.1111/petr.12060
45. Uhlving HH, Andersen CB, Christensen IJ, Gormsen M, Pedersen KD, Buchvald F, et al. Biopsy-Verified Bronchiolitis Obliterans and Other Noninfectious Lung Pathologies After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2015) 21:531–8. doi: 10.1016/j.bbmt.2014.12.004
46. Ortega-Laureano L, Santiago T, Maron G, Davidoff AM, Fernandez-Pineda I. Surgical Lung Biopsy in Children After Hematopoietic Cell Transplantation. J Pediatr Surg (2018) 53:1129–33. doi: 10.1016/j.jpedsurg.2018.02.069
47. Dieffenbach BV, Madenci AL, Murphy AJ, Weldon CB, Weil BR, Lehmann LE. Therapeutic Impact and Complications Associated With Surgical Lung Biopsy After Allogeneic Hematopoietic Stem Cell Transplantation in Children. Biol Blood Marrow Transplant (2019) 25:2181–5. doi: 10.1016/j.bbmt.2019.06.026
48. Cleveland H, Chau A, Jeng Z, Gardner G, Yoo R, Zhang W, et al. Percutaneous Lung Biopsy in Immunocompromised Pediatric Patients. J Vasc Interv Radiol (2020) 31:93–8. doi: 10.1016/j.jvir.2019.07.016
49. O’Dwyer DN, Duvall AS, Xia M, Hoffman TC, Bloye KS, Bulte KS, et al. Transbronchial Biopsy in the Management of Pulmonary Complications of Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2018) 53:193–8. doi: 10.1038/bmt.2017.238
50. Elbahlawan LM, Avent Y, Montoya L, Wilder K, Pei D, Cheng C, et al. Safety and Benefits of Bronchoalveolar Lavage and Lung Biopsy in the Management of Pulmonary Infiltrates in Children With Leukemia. J Pediatr Hematol Oncol (2016) 38:597–601. doi: 10.1097/MPH.0000000000000644
51. Chellapandian D, Lehrnbecher T, Phillips B, Fisher BT, Zaoutis TE, Steinbach WJ, et al. Bronchoalveolar Lavage and Lung Biopsy in Patients With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: A Systematic Review and Meta-Analysis. J Clin Oncol (2015) 33:501–9. doi: 10.1200/JCO.2014.58.0480
Keywords: pulmonary complications, hematopoietic (Stem) cell transplantation (HCT), lung biopsy, broncho alveolar lavage (BAL), diagnostic approach, diagnostic imaging
Citation: Elbahlawan L, McArthur J, Morin CE, Abdelhafeez H, McCarville MB, Ruiz RE, Srinivasan S and Qudeimat A (2021) Pulmonary Complications in Children Following Hematopoietic Cell Transplantation: A Case Report and Review of the Diagnostic Approach. Front. Oncol. 11:772411. doi: 10.3389/fonc.2021.772411
Received: 08 September 2021; Accepted: 20 October 2021;
Published: 08 November 2021.
Edited by:
Kris Michael Mahadeo, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Mira Kohorst, Mayo Clinic, United StatesCopyright © 2021 Elbahlawan, McArthur, Morin, Abdelhafeez, McCarville, Ruiz, Srinivasan and Qudeimat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lama Elbahlawan, bGFtYS5lbGJhaGxhd2FuQHN0anVkZS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.