- Department of Liver Surgery and Transplantation, Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai, China
The high mortality rate associated with hepatocellular carcinoma (HCC) is partly due to the high proportion of patients who present with advanced stage disease at diagnosis, for whom there are limited treatment options. For selected patients with initially unresectable HCC, locoregional and/or systemic treatments can result in tumor downstaging and consequently provide opportunities for surgical intervention and the potential for long-term survival. Therefore, the key aim of ‘conversion therapy’ is to reduce tumor burden so that patients become amenable to surgical resection. Various therapies have been investigated as candidates for downstaging patients with potentially resectable HCC including transarterial chemoembolization, transarterial radioembolization with yttrium-90 microspheres, radiotherapy, systemic therapies and combination or multimodality treatment approaches. However, downstaging conversion therapy remains controversial and there are several challenges such as defining the criteria used to identify the population of patients who are ‘potentially resectable’, the criteria used to define successful downstaging, and the optimum treatment approach to maximize the success of downstaging therapy. In this review article, we summarize clinical experience and evidence of downstaging conversion treatment in patients identified as having ‘potentially resectable’ HCC.
Introduction
Worldwide, liver cancer is the sixth most commonly diagnosed cancer, with an estimated 905,677 new cases and 830,180 deaths in 2020 (1). The incidence rates associated with liver cancer are 2-3 times higher in men versus women (14.1 versus 5.2 per 100,000 individuals), with an overall mortality rate of 8.7 per 100,000 individuals (1). In China, Liver Cancer is the fourth most commonly diagnosed cancer, behind lung, stomach, and breast cancers, with 410,038 new cases in 2020 and 391,152 deaths (2). Similar to the global epidemiology, age-standardized incidence rates of liver cancer in China are higher in men than women (27.6 versus 9.0 per 100,000 individuals) and associated with an overall mortality rate of 17.2 per 100,000 individuals. Thus, both incidence and mortality rates for liver cancer are approximately 2-fold higher in China than global estimates (2). In addition, the estimated 5-year survival rate for Chinese patients with HCC is 12.2% (3).
The high mortality rate associated with HCC is partly due to the high proportion of patients who present with advanced disease, for whom there are limited treatment options (4). Surgical treatment provides the best opportunity for achieving long-term survival in HCC patients and is mainly comprised of hepatectomy and liver transplantation. For unresectable HCC, the application of preoperative treatments such as transarterial chemoembolization (TACE) may result in tumor downstaging and consequently provide initially ineligible patients with opportunities for surgical intervention. Improved long-term survival may be achieved in HCC patients undergoing resection after downstaging.
Downstaging conversion therapy is an emerging treatment approach for HCC that aims to reduce tumor burden using locoregional or systemic therapy so that patients become amenable to surgical resection. This type of preoperative therapy in HCC is controversial. However, evidence is accumulating to suggest that successful downstaging therapy followed by surgical resection is achievable in a subpopulation of patients. The criteria used to identify the population of patients who are ‘potentially resectable’, the criteria used to define successful downstaging, and the optimum treatment approach to maximize the success of downstaging therapy are all factors that remain subject to ongoing debate. This article will overview clinical experience to date with downstaging conversion treatment in patients identified as having ‘potentially resectable’ HCC. We also review clinical trials conducted in patients with unresectable HCC in which notable tumor responses were achieved, even if eligibility for resection was not reported as a treatment outcome.
Conversion Therapy: Target Population and Principles
Conversion Therapy – Target Patient Population
The causes of unresectable liver cancer can be divided into surgical causes and oncological causes. Surgical causes refer to the inability to perform safe surgical excision due to a patient’s inability to withstand surgery because of their general condition, liver function or insufficient remaining liver volume. Oncological causes refer to predicted efficacy after excision failing to surpass other, non-surgical treatment methods (5). There is a consensus for the definition of surgically unresectable liver cancer, while oncologically unresectable liver cancer is less well defined. The goal of conversion therapy is to eliminate these two causes, so as to achieve the conversion from unresectable liver cancer to resectable liver cancer (5).
While the Barcelona Cancer Liver Clinic (BCLC) staging system for liver cancer is employed extensively throughout the US and Europe, in China the China Liver Cancer Staging (CNLC) system is preferred because of its relevance to local systems and practices (6). In general terms, the CNLC system divides each stage from the BCLC into two substages, with BCLC stages 0/A, B and C translating to CNLC stages Ia, Ib, IIa, IIb, IIIa and IIIb. BCLC stage 0/A is considered very early or early HCC and the equivalent CNLC stages Ia and Ib are defined as single nodules ≤5 cm or >5 cm, respectively; stage Ib also includes the presence of 2-3 nodules ≤3 cm. The intermediate BCLC B stage is represented within the CNLC system as stages IIa (2-3 nodules >3 cm) and IIb (≥4 nodules). The BCLC stage C, defined as advanced disease, is represented in the CNLC system by stages IIIa (vascular invasion) and IIIb (extrahepatic metastases) (6–8) (Figure 1).
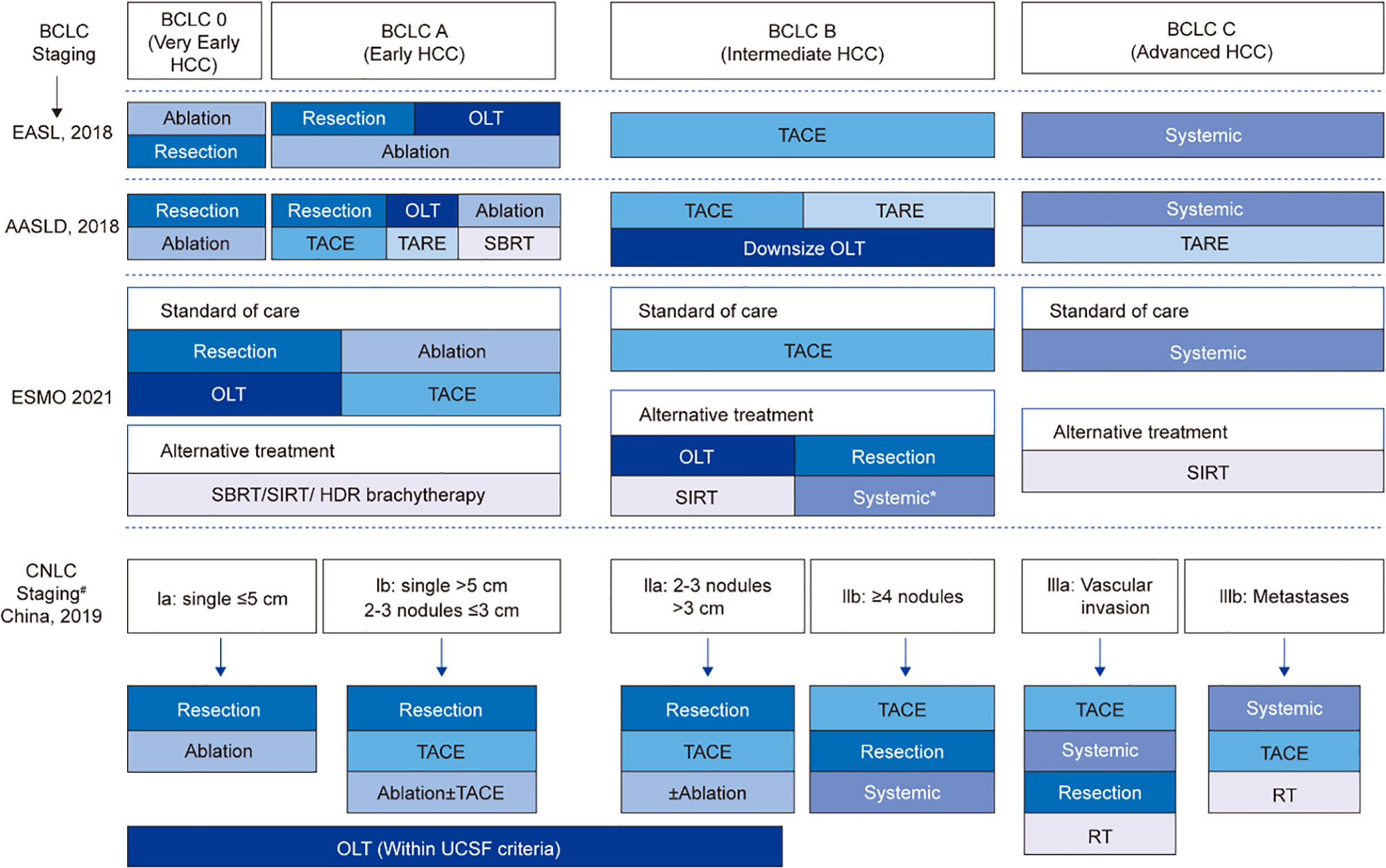
Figure 1 Comparisons of staging and treatment algorithms of HCC among 2021 ESMO, 2018 EASL, 2018 AASLD, and 2019 Chinese guidelines. *For patients who are not suitable for local therapies; #If PS = 0-2, CNLC stage I-III; if PS > 2, CNLC stage IV; BCLC, Barcelona Clinic Liver Cancer; EASL, European Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; CNLC, China liver cancer staging; OLT, orthotopic liver transplantation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; SBRT, stereotactic body radiotherapy; SIRT, selective internal radiotherapy; RT, radiation therapy; UCSF, University of California San Francisco.
Surgically unresectable CNLC-stage Ia, Ib, IIa liver cancer (considered unresectable mainly due to the patient’s general condition or liver function intolerance, insufficient remaining liver volume or insufficient resection margins) and surgically resectable CNLC-stage IIb and IIIa liver cancer (with limited tumor burden) are potentially resectable liver cancers, and multi-mode, high-intensity treatment strategies can be explored and adopted to facilitate the conversion. For surgically unresectable CNLC- stage IIb and IIIa liver cancer (for which the predicted surgical efficacy does not surpass other non-surgical treatment options), it is recommended that the current treatment norms should be followed and a gradual treatment strategy should be adopted, both the intensity and safety of treatment should be taken into account, and surgical excision should be conducted when applicable.
Conversion therapy may be considered distinct from neoadjuvant therapy. While both treatment approaches are administered as peri-surgical procedures and utilize the same modalities, they have different objectives. Neoadjuvant therapy is administered to patients with resectable disease to decrease tumor size prior to definitive surgery. In contrast, conversion therapy is administered to patients with initially unresectable disease who are considered potentially resectable after successful downstaging. However, when the treatment is applied to the patients with surgically resectable but oncologically unresectable HCC, both treatments may be overlapped in the target population (eg. surgically resectable CNLC-IIb or IIIa) or treatment objectives, eg. to change some oncological factors, such as tumor thrombi, satellite nodules, or even microvascular invasion. Basically, neo-adjuvant therapy is to decrease tumor burden or other oncological factors to improve the outcome after surgical resection, so neo-adjuvant therapy can be considered as oncological conversion therapy.
Principles of Conversion Therapy
Conversion treatment strategies should be developed under the guidance of a multidisciplinary team, including surgeons, medical oncologists, interventional radiologists and diagnostic radiologists, or other related doctors. Conversion therapy planning should take multiple factors into account including liver function, liver function reserve, the number, location and size of liver lesions, vascular invasion, comorbidities, and the specific objectives of treatment. An ideal conversion treatment should have a high objective response rate and less adverse effects on patients and following surgical operation, and strive to achieve conversion in as short a timeframe as possible. During conversion therapy, the response to treatment should be closely monitored, and the timing of surgery should be determined based on a judgment of predicted efficacy, although an objective evaluation is needed to facilitate a good judgment.
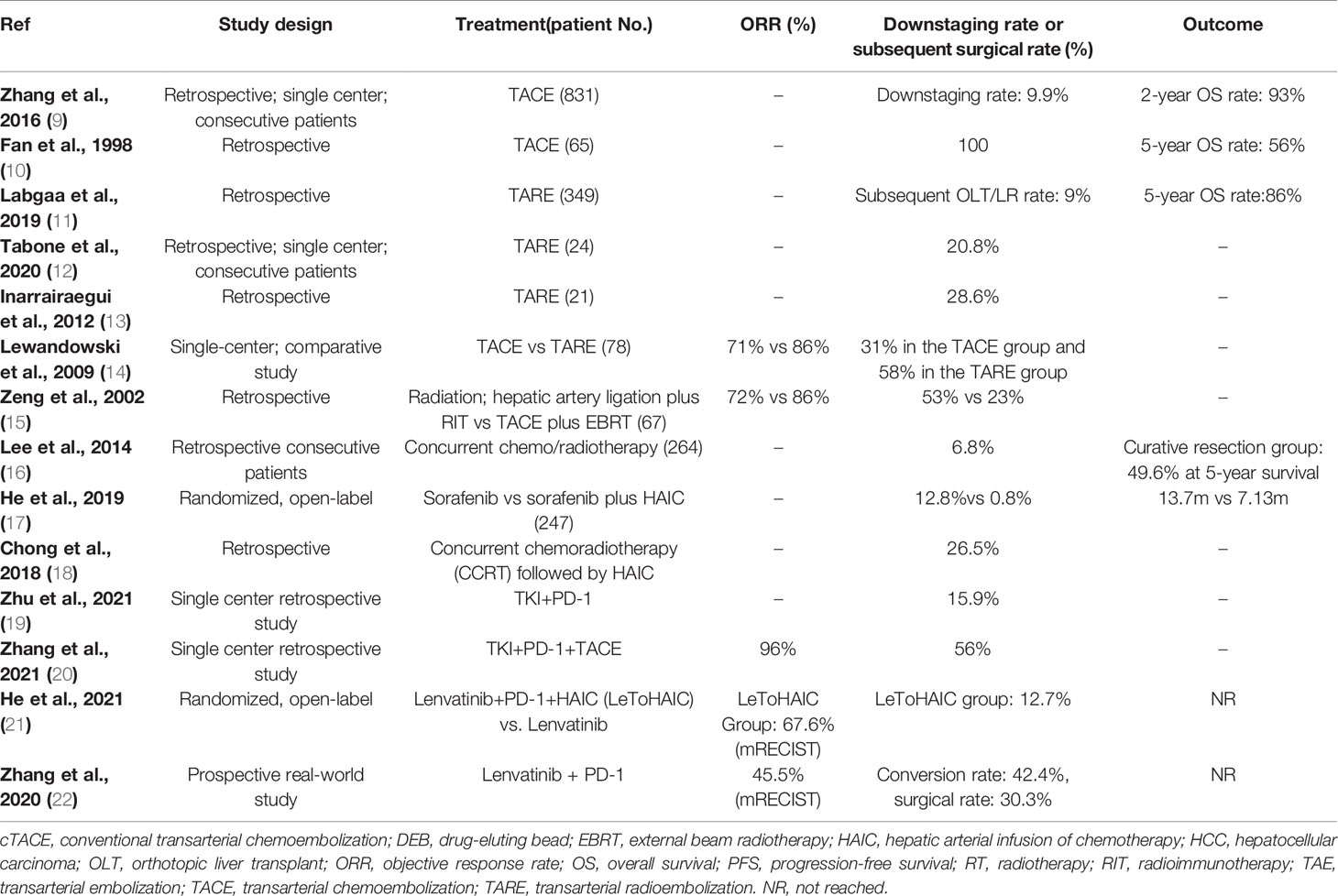
Conversion therapy for downstaging or downsizing HCC can utilize many of the treatment modalities. Various therapies that have been studied as candidates for conversion therapy include TACE, transarterial radioembolization (TARE) with yttrium-90 microspheres (Y90), systemic therapies, and combination or multimodality treatment approaches (Table 1). An early literature review suggests that 8-18% of patients presenting with unresectable HCC may be suitable for salvage surgical resection after initial palliative treatment to downstage the tumor (23).
Locoregional Treatment for Conversion Therapy
Transarterial Chemoembolization
TACE is the practice of delivering a chemotherapy agent directly to a liver tumor through the hepatic blood supply, usually the hepatic artery (24). To reduce leakage of the chemotherapy into the systemic circulation, microspheres loaded with chemotherapy have been developed (drug-eluting bead-TACE [DEB-TACE]). DEB-TACE permits sustained elution of the chemotherapy at the site of the tumor coupled with reduced systemic concentrations, allowing use of relatively high dose levels and/or frequency (24).
Data from a retrospective analysis show that patients who achieve tumor downstaging following TACE and then undergo surgical resection achieve better outcomes than patients who achieve downstaging but do not undergo surgery (9). Of the 831 patients with unresectable HCC included in this analysis who received TACE as initial treatment, 82 achieved significant downstaging and became eligible for resection. Of the patients eligible for surgery, 43 received salvage resection and 39 declined surgery. The majority of patients had an ECOG performance score 0-1 (91% and 87%) and were Child-Pugh class A (95% and 92%) in the group that received salvage resection and declined surgery respectively. Median overall survival (OS) was higher for patients who received surgery compared with those who declined (49 months versus 31 months P=0.027). A significant survival benefit favoring surgery was also achieved in subgroups of patients with macroscopic vascular invasion and with a partial response to TACE (9).
Several other studies have also indicated that TACE may be effective for downstaging tumors. In a retrospective study of patients with initially unresectable HCC who underwent hepatic resection following TACE, complete pathological tumor necrosis was achieved in 11 of 65 patients (16.9%) (10). Sixty-one patients in this series underwent resection, and 1-, 3- and 5-year survival rates were 80.0%, 65.0% and 56.0%, respectively (10). In addition, Li and colleagues reported that preoperative TACE did not impact perioperative morbidity or mortality in a multicenter, propensity matching analysis of patients with advanced HCC, 90.7% of whom had Child-Pugh class A liver function (25). Conversely, this study reported that preoperative TACE was associated with improved OS and relapse-free survival after liver resection in patients with large HCC (≥10 cm) (25).
The prospective randomized PRECISION V study compared response rates in patients receiving transarterial doxorubicin delivered via conventional TACE versus DEB-TACE (26). The ECOG performance score ratio (0/1) and Child-Pugh classification ratio (A/B) were similar (80/28 Vs. 74/19 and 89/19 Vs. 77/16) between the groups receiving TACE versus DEB-TACE, respectively. At 6 months, DEB-TACE was associated with significantly higher rates of complete response compared with conventional TACE (27% versus 22%), contributing to a higher overall response rate (ORR) for patients receiving DEB-TACE (52% versus 43%). In addition, the improved response rates among patients receiving DEB-TACE were more pronounced in the subgroup of patients with advanced disease. This study did not assess post-treatment eligibility for resection and therefore the translation of response rates into resectability is unclear. While subsequent studies have assessed DEB-TACE as a bridging therapy prior to liver transplant (27–29), the role of this treatment modality as a conversion therapy prior to surgical resection requires further confirmation.
Hepatic Artery Infusion Chemotherapy
Interestingly, interim results from a phase 3 study of neoadjuvant HAIC with FOLFOX in patients with resectable HCC (BCLC stage A or B) showed that HAIC monotherapy can reduce the incidence of microvascular tumor thrombi, and this may suggest a role for HAIC monotherapy as part of a conversion therapy strategy (30). A study of patients with HCC and portal vein invasion who received either sorafenib or sorafenib in combination with hepatic arterial infusion of chemotherapy (HAIC; oxaliplatin, fluorouracil and leucovorin) indicated that the combination modality may have potential as a downstaging approach for patients with potentially resectable disease (17). In this study, 16 of 125 patients receiving sorafenib plus HAIC subsequently underwent curative resection and three patients achieved a pathologic complete response. Furthermore, resection was also possible in one patient with initial disease progression on sorafenib alone, who then crossed over to the combination arm (17). Another retrospective study comparing lenvatinib monotherapy with lenvatinib combined with toripalimab and HAIC (LeToHAIC) for the treatment of advanced HCC, in patients with an ECOG score of 0 or 1, found that LeToHAIC combination therapy was associated with longer progression free survival, longer OS, higher ORR and more complete responses than lenvatinib monotherapy (21). In addition, 9 patients in the LeToHAIC group received curative surgical resection following tumor shrinkage. A further study by Zhang et al. of 34 patients with unresectable liver cancer who were treated with PD-1 inhibitors combined with TKI and TACE, reported a conversion resection rate of 56% (20).
Transarterial Radioembolization With Yttrium-90 Microspheres
TARE with Y90 microspheres is a form of selective internal radiotherapy (31). Several studies have successfully demonstrated tumor downstaging with TARE in patients with unresectable HCC (11, 12, 32). In a review of 349 patients with unresectable HCC who received TARE, 48% of whom were Child-Pugh class C, 10 were subsequently able to undergo liver resection, and an additional 22 patients received a liver transplant (11). In this cohort, TARE was associated with a decrease in viable nodules and led to tumor downsizing and downstaging (based on BCLC staging criteria). A single-center study reported that around 20% of patients with unresectable HCC and portal vein thrombosis achieved downstaging following TARE and became eligible for surgery (12). Of the 24 patients included in this series, five received surgical resection following TARE, four underwent right trisectionectomy and one received a liver transplant. Median survival in the five patients was 54 months (95% confidence interval [CI]: 17-92) compared to median survivals of 30 months (95% CI: 18-42) and 11 months (95% CI: 8-14) in patients who achieved partial response/stable disease (n=8) and those with progressive disease (n=11) following TARE. In this series, high tumor absorbed radiation and low pre-treatment alpha-fetoprotein (AFP) levels were significantly associated with the probability of successful downstaging. A further similar analysis of 21 patients with United Network for Organ Sharing (UNOS) stage T3 HCC indicated that 6/21 patients were downstaged and eligible for radical treatment with curative intent following TACE (13). Of these six patients, three underwent resection, two received a liver transplant, and one received ablation and then underwent resection. In this series, patients who were treated radically were significantly younger and had higher tumor volumes than those who did not achieve radical treatment. Finally, a number of studies that assessed the role of selective internal radiation therapy (SIRT) as conversion therapy for unresectable liver cancer have been summarized in a literature review by Cucchetti and colleagues (33). These authors suggest that SIRT can lead to considerable downsizing of tumors, and also promote hypertrophy of the contralateral lobe. A complete response rate of approximately 10% in patients with HCC receiving SIRT and an objective response rate of ~40% were reported, coupled with a maximum contralateral hypertrophy above 40% (33).
There is evidence to suggest that TARE may offer advantages over TACE in terms of improved response rates and safety as a candidate modality for conversion therapy. In a non-randomized trial, TARE was associated with a higher response rate than TACE (partial response rates of 61% and 37%) and resulted in more patients being downstaged from UNOS T3 to UNOS T2 and becoming eligible for transplant (14). Almost all patients in this study had a Child-Pugh class of A (56% and 53%) or B (44% and 42%) across the TARE and TACE groups respectively. A retrospective analysis of patients treated with TACE or TARE over a 9-year period also indicated that TARE offered several advantages, including an improved response rate, longer time to progression, and less toxicity compared with TACE (18). In both the TARE and TACE groups most patients had a Child-Pugh class of A (54% and 55%) or B (44% and 43%). Zori and colleagues reported that TARE is associated with improved survival and less microvascular invasion, and required fewer administrations over the same time period compared with TACE (34). Finally, another advantage of TARE may be its ability to cause hypertrophy of the contralateral future liver remnant, which may be useful in potential candidates for resection with a small liver remnant (35–37).
Radiotherapy
The potential benefit of adding external beam radiotherapy (EBRT) to TACE as a strategy for tumor downstaging has been evaluated in multiple studies. A retrospective analysis of 203 patients with unresectable HCC without tumor thrombus, lymph node involvement, or extrahepatic metastases indicated a significant improvement in objective response rate among patients who received TACE plus EBRT compared with those who received TACE alone, and a numerical increase in the number of patients who became eligible for resection (38). Patients were selected for the combination therapy according to physician preference, and in most cases (49/54) this was due to defective lipiodol uptake during the TACE procedure, assessed by follow-up computed tomography (CT) scan. Approximately 85% of the patients enrolled in this study had tumors measuring >5 cm and were considered unsuitable for curative treatment. Objective response rates were 76% (41/54) among patients receiving EBRT plus TACE and 30.9% (46/149) among those receiving TACE alone (P<0.001), and sequential resection rates were 20.4% (11/54) compared with 12.8% (19/149), respectively (P=0.177) (38). Another retrospective study from the same group compared hepatic artery ligation plus radioimmunotherapy (RIT with 131I Hepama-1) with TACE plus EBRT in patients with unresectable HCC (15). Within the RIT group 4 patients (11%) had portal vein thrombi compared to 7 patients (20%) in the EBRT group. Objective responses were achieved in 85% (30/35) of those in the TACE plus EBRT group, and sequential resection rates following treatment were 23% (8/35) in this group (15). In addition, a randomized clinical trial evaluated the efficacy and safety of TACE plus external beam radiotherapy (TACE-RT group) compared with sorafenib for patients with hepatocellular carcinoma and macroscopic vascular invasion (39). The TACE-RT group showed a significantly higher radiologic response rate than the sorafenib group at 24 weeks (15 [33.3%] vs 1 [2.2%]; P < .001), and curative surgical resection was conducted for 5 patients (11.1%) in the TACE-RT group owing to downstaging. Finally, one study evaluated the oncological outcomes and prognostic factors of surgical resection after downstaging with localized concurrent chemoradiotherapy (CCRT) followed by hepatic arterial infusion chemotherapy (HAIC) in HCC patients with portal vein tumor thrombosis (PVTT) (40). Among 98 patients in the CCRT group, 26 patients (26.5%) underwent subsequent curative resection. During the same study period, 18 patients with PVTT underwent surgical resection as the first treatment. Clinicopathological characteristics and oncological outcomes between groups were compared. The median follow-up period was 13 months (range 1-131 months). Disease-specific survival was significantly different between the resection after localized CCRT group and the resection-first group (40). Overall, these studies indicate that combination regimen based on radiotherapy may represent a useful adjunctive treatment for patients with potentially resectable HCC who are candidates for tumor downstaging.
Systemic Therapy
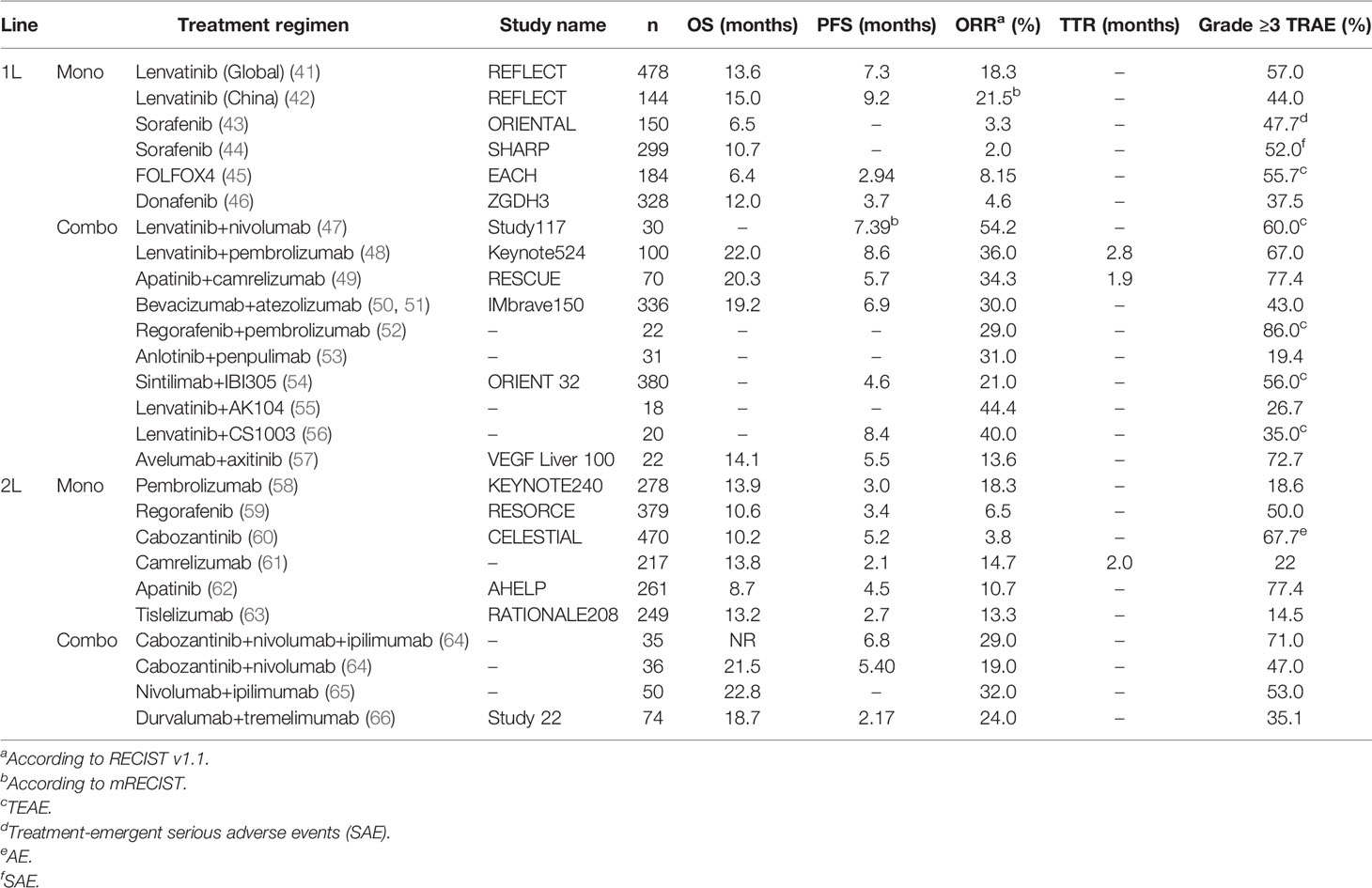
Systemic therapies including tyrosine kinase inhibitors (TKIs), immunotherapy, and chemotherapy, are widely used in the palliative treatment of HCC. Traditionally, systemic therapies for HCC have been associated with relatively low response rates and therefore neoadjuvant and downstaging therapy have not been part of standard management protocols. However, recent advances in systemic therapy have seen improved response rates, leading to a re-evaluation of the value of systemic therapy in the conversion therapy setting (Table 2).
Several studies have reported high ORRs with lenvatinib, either alone or in combination with other systemic treatments, in patients with initially unresectable HCC, making this a promising option for conversion/downstaging therapy. In the phase 3 REFLECT trial, lenvatinib was associated with a significantly higher ORR than sorafenib in patients with advanced unresectable HCC who had not received prior treatment for advanced disease and were Child-Pugh class A (41). In this non-inferiority study, the ORR was 40.6% in patients receiving lenvatinib versus 12.4% in those treated with sorafenib (odds ratio [OR], 5.01 [95% CI: 3.59-7.01]; P<0.0001) by modified RECIST (mRECIST), and the ORR by RECIST was 18.8% versus 6.5% (OR, 3.34 [95% CI: 2.17-5.14]; P<0.001). A network meta-analysis compare response rates, survival outcomes, and safety of first-line systemic therapies for advanced hepatocellular carcinoma, and the results showed that lenvatinib is associated with the best ORR of all systemic therapies included in the analysis (67). A further study from Japan included 107 consecutive patients who underwent lenvatinib treatment for advanced HCC, the majority of patients in this study had an ECOG score of 0 (87.9%) and were Child-Pugh class A (92.5%). Of the 107 patients included, 16 subsequently received surgical intervention, and R0 resection was achieved in nine (8.4%) patients. Survival analysis confirmed that successful conversion to R0 resection was associated with a longer time to treatment failure (68). Thus, data from the studies suggest that lenvatinib may have utility as a conversion therapy in patients who present with advanced, initially unresectable HCC.
Results from several small sample studies also support combination therapy with TKIs combined with immunotherapy in the conversion treatment setting in patients with unresectable HCC. For example, Zhu et al. reported 63 cases of patients with initially unresectable liver cancer treated with PD-1 inhibitors combined with a TKI, 60 of whom were classed as Child-Pugh A, and the conversion resection rate was 15.9% (19). A further study by Lu et al. of 33 patients with unresectable liver cancer with measurable PVTT (by mRECIST) and no extrahepatic metastasis who were treated with PD-1 inhibitors combined with Lenvatinib, reported a conversion rate of 42.4% (22). Huang reported that the response of intrahepatic tumor was less significant than that of tumor thrombi when treated with the combination of lenvatinib and PD-1 antibody (69), which suggested locoregional therapy can be used to improve the control of intrahepatic tumor when tumor thrombi is necrotic.
Multimodal Treatment Approaches
Multimodality treatment approaches combine two or more treatment modalities with the aim of improving clinical outcomes beyond those achieved with either modality alone. Combining multiple therapies may provide cumulative benefits in term of efficacy beyond those offered by either modality alone, but may also be limited by non-overlapping toxicity profiles inherited from both modalities. Studies on multimodality treatment approaches in the setting of HCC conversion therapy with the intent of HCC downstaging are currently limited.
Concurrent chemoradiotherapy has been assessed for downstaging patients with unresectable HCC (16). In this study, 264 patients received radiotherapy (45.0 Gy with fractional dose of 1.8 Gy) and concurrent intra-arterial chemotherapy with 5-fluorouracil (500 mg/day), 15 of whom (83.3%) were classified as Child-Pugh class A. Eighteen of these patients (6.8%) subsequently underwent hepatic resection after achieving a response,. At the time of surgery, six patients had complete response, 11 had partial remission, and one had stable disease. In this study, cases were considered resectable when tumor-free margins and sufficient remnant volumes were obtained without extrahepatic metastasis. Median time from chemoradiotherapy to resection was 6.2 months (range 1-21 months), and median OS and disease-free survival were 61.8 months and 24.1 months, respectively. Three patients remained without evidence of disease recurrence for >5 years and all three had complete or partial remission with 90-100% necrosis post surgery (16).
A network meta-analysis of different embolization treatment strategies for unresectable HCC suggested that chemoembolization combined with external radiotherapy or local liver ablation could significantly improve tumor response rates compared with embolization alone (70). The OR (odds ratio) for achieving an objective response relative to control was 142 (95% CI: 55.9-395.4) with TACE plus ablation and 13.9 (95% CI: 6.9-31.3) with TACE alone. However, all treatments assessed in this analysis also increased the risk for serious adverse events relative to control, with the largest increases seen in patients receiving two treatment modalities. For example, the OR for a serious adverse event relative to control in patients receiving TACE plus ablation was 11.7 (95% CI: 1.5-128.7), and 14.6 (95% CI: 4.7-67.7) in those receiving TACE alone (70).
Discussion
Surgery remains the only potentially curative treatment modality for patients with resectable HCC and normal liver function; however, only a minority of patients with HCC are eligible for resection at diagnosis. Conversion therapy aimed at tumor downstaging can increase the proportion of patients with HCC who are eligible for surgical resection; however, this approach is not routinely recommended in clinical practice at present due to a lack of supporting evidence. Despite this, accumulating evidence suggests that selected patients may achieve adequate downstaging following TACE or TARE to enable surgical resection. Lenvatinib-based combination therapy is also a promising option for conversion therapy in patients with potentially resectable disease with encouraging ORRs reported in clinical trials enrolling patients with initially unresectable HCC. Multimodality therapy may increase the proportion of patients eligible to undergo surgery or other curative treatments and reduce disease recurrence rates, allowing patients to experience a cancer-free, and drug-free status with long survival and good quality of life (71). It should be mentioned that there are other non-tumor focused approaches to conversion therapy such as techniques aiming to increase residual liver volume by inducing liver hypertrophy such as portal vein ligation (PVS) (72–74), artery ligation (75), and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (76, 77). However, limitations of these approaches include a relatively long time for liver hyperplasia following PVE, which increases the chance of further disease progression, and an increased risk of complications with the use of ALPPS (78).
Ultimately, data from large randomized controlled trials are still required to clarify the clinical benefit of conversion therapy in patients diagnosed with unresectable HCC and identify specific patient groups likely to benefit from this approach. There is also a need for clearer definitions regarding which patients should initiate downstaging protocols and what criteria should be met before resection can be attempted. This will become increasingly important if treatments aimed at downstaging begin to differ from those used for palliative care. For now, preliminary data support the concept of downstaging in patients with potentially resectable HCC, offering the potential to provide clinical benefit to a population of patients in urgent need of expanded treatment options.
Author Contributions
H-CS and X-DZ contributed to the conception and conduct of this review article, drafted and revised the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
H-CS received honorarium for consulting or lecture from Eisai, MSD, Bayer, Roche, Gilead, Abbott, Beigene, Hengrui, Innovent, TopAlliance.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Fact Sheet. Available at: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (Accessed 10 February, 2021).
2. China Fact Sheets. Available at: https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf (Accessed July 14th, 2021).
3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed With One of 18 Cancers From 322 Population-Based Registries in 71 Countries. Lancet (London England) (2018) 391(10125):1023–75. doi: 10.1016/S0140-6736(17)33326-3
4. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global Patterns of Hepatocellular Carcinoma Management From Diagnosis to Death: The BRIDGE Study. Liver Int (2015) 35(9):2155–66. doi: 10.1111/liv.12818
5. Sun H-C, Zhou J, Liu X, Qing X, Weidong J. Chinese Expert Consensus on Conversion Therapy in Hepatocellular Carcinoma (2021 Edition). Chin J Digest Surg (2021) 20:600–16. doi: 10.3760/cma.j.cn115610-20210512-00223
6. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. Chinese Clinical Guidelines for the Management of Hepatocellular Carcinoma: Updates and Insights. Hepatobil Surg Nutr (2020) 9(4):452–63. doi: 10.21037/hbsn-20-480
7. Updated Treatment Recommendations for Hepatocellular Carcinoma (HCC) From the ESMO Clinical Practice Guidelines . Available at: https://www.esmo.org/guidelines/gastrointestinal-cancers/hepatocellular-carcinoma/eupdate-hepatocellular-carcinoma-treatment-recommendations (Accessed 05 March 2021).
8. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
9. Zhang Y, Huang G, Wang Y, Liang L, Peng B, Fan W, et al. Is Salvage Liver Resection Necessary for Initially Unresectable Hepatocellular Carcinoma Patients Downstaged by Transarterial Chemoembolization? Ten Years of Experience. Oncologist (2016) 21(12):1442–9. doi: 10.1634/theoncologist.2016-0094
10. Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, et al. Improved Survival With Resection After Transcatheter Arterial Chemoembolization (TACE) for Unresectable Hepatocellular Carcinoma. Digest Surg (1998) 15(6):674–8. doi: 10.1159/000018676
11. Labgaa I, Tabrizian P, Titano J, Kim E, Thung SN, Florman S, et al. Feasibility and Safety of Liver Transplantation or Resection After Transarterial Radioembolization With Yttrium-90 for Unresectable Hepatocellular Carcinoma. HPB (2019) 21(11):1497–504. doi: 10.1016/j.hpb.2019.03.360
12. Tabone M, Calvo A, Russolillo N, Langella S, Carbonatto P, Tesoriere RL, et al. Downstaging Unresectable Hepatocellular Carcinoma by Radioembolization Using 90-Yttrium Resin Microspheres: A Single Center Experience. J Gastrointest Oncol (2020) 11(1):84–90. doi: 10.21037/jgo.2019.06.01
13. Iñarrairaegui M, Pardo F, Bilbao JI, Rotellar F, Benito A, D'Avola D, et al. Response to Radioembolization With Yttrium-90 Resin Microspheres may Allow Surgical Treatment With Curative Intent and Prolonged Survival in Previously Unresectable Hepatocellular Carcinoma. Eur J Surg Oncol (2012) 38(7):594–601. doi: 10.1016/j.ejso.2012.02.189
14. Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A Comparative Analysis of Transarterial Downstaging for Hepatocellular Carcinoma: Chemoembolization Versus Radioembolization. Am J Transpl (2009) 9(8):1920–8. doi: 10.1111/j.1600-6143.2009.02695.x
15. Zeng ZC, Tang ZY, Yang BH, Liu KD, Wu ZQ, Fan J, et al. Comparison Between Radioimmunotherapy and External Beam Radiation Therapy for Patients With Hepatocellular Carcinoma. Eur J Nucl Med Mol Imag (2002) 29(12):1657–68. doi: 10.1007/s00259-002-0996-x
16. Lee IJ, Kim JW, Han KH, Kim JK, Kim KS, Choi JS. Concurrent Chemoradiotherapy Shows Long-Term Survival After Conversion From Locally Advanced to Resectable Hepatocellular Carcinoma. Yonsei Med J (2014) 55:1489–97. doi: 10.3349/ymj.2014.55.6.1489
17. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol (2019) 5(7):953–60. doi: 10.1001/jamaoncol.2019.0250
18. Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology (2011) 140(2):497–507.e492. doi: 10.1053/j.gastro.2010.10.049
19. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Sun HC, et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma With Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer (2021) 10(4):320–9. doi: 10.1159/000514313
20. Zhang T, Zhang J, Zhang X, Mu H, Yu G, Xing W. Triple Combination Therapy Comprising Angiogenesis Inhibitors, Anti-PD-1 Antibodies, and Hepatic Arterial Infusion Chemotherapy in Patients With Advanced Hepatocellular Carcinoma. J Clin Oncol (2021) 39(15_suppl):e16124–4. doi: 10.1200/JCO.2021.39.15_suppl.e16124
21. He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, Toripalimab, Plus Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib Alone for Advanced Hepatocellular Carcinoma. Ther Adv Med Oncol (2021) 13:17588359211002720. doi: 10.1177/17588359211002720
22. Zhang W, Hu B, Han J, Wang H, Wang ZB, Chen MY, et al. Preliminary Report on the Study of Conversion Therapy of Advanced Hepatocellular Carcinoma Combined PD-1 Inhibitors With Multi-Target Tyrosine Kinase Inhibitors. Chin J Hepatobil Surg (2020) 26(12):947–8. doi: 10.3760/cma.j.cn113884-20201203-00611
23. Lau WY, Lai EC. Salvage Surgery Following Downstaging of Unresectable Hepatocellular Carcinoma–a Strategy to Increase Resectability. Ann Surg Oncol (2007) 14(12):3301–9. doi: 10.1245/s10434-007-9549-7
24. Kishore SA, Bajwa R, Madoff DC. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers (Basel) (2020) 12(4):791. doi: 10.3390/cancers12040791
25. Lu LC, Hsu C, Shao YY, Chao Y, Yen CJ, Shih IL, et al. Differential Organ-Specific Tumor Response to Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Liver Cancer (2019) 8(6):480–90. doi: 10.1159/000501275
26. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. Cardiovasc Intervention Radiol (2010) 33(1):41–52. doi: 10.1007/s00270-009-9711-7
27. Affonso BB, Galastri FL, da Motta Leal Filho JM, Nasser F, Falsarella PM, Cavalcante RN, et al. Long-Term Outcomes of Hepatocellular Carcinoma That Underwent Chemoembolization for Bridging or Downstaging. World J Gastroenterol (2019) 25(37):5687–701. doi: 10.3748/wjg.v25.i37.5687
28. Frenette CT, Osorio RC, Stark J, Fok B, Boktour MR, Guy J, et al. Conventional TACE and Drug-Eluting Bead TACE as Locoregional Therapy Before Orthotopic Liver Transplantation: Comparison of Explant Pathologic Response. Transplantation (2014) 98(7):781–7. doi: 10.1097/TP.0000000000000121
29. Galastri FL, Nasser F, Affonso BB, Valle LG, Odísio BC, Motta-Leal Filho JM, et al. Imaging Response Predictors Following Drug Eluting Beads Chemoembolization in the Neoadjuvant Liver Transplant Treatment of Hepatocellular Carcinoma. World J Hepatol (2020) 12(1):21–33. doi: 10.4254/wjh.v12.i1.21
30. Li S, Zhong C, Li Q, Zou J, Wang Q, Shang C, et al. Neoadjuvant Transarterial Infusion Chemotherapy With FOLFOX Could Improve Outcomes of Resectable BCLC Stage a/B Hepatocellular Carcinoma Patients Beyond Milan Criteria: An Interim Analysis of a Multi-Center, Phase 3, Randomized, Controlled Clinical Trial. J Clin Oncol (2021) 39(15_suppl):4008–8. doi: 10.1200/JCO.2021.39.15_suppl.4008
31. Bhangoo MS, Karnani DR, Hein PN, Giap H, Knowles H, Issa C, et al. Radioembolization With Yttrium-90 Microspheres for Patients With Unresectable Hepatocellular Carcinoma. J Gastrointest Oncol (2015) 6(5):469–78. doi: 10.3978/j.issn.2078-6891.2015.056
32. Titano J, Voutsinas N, Kim E. The Role of Radioembolization in Bridging and Downstaging Hepatocellular Carcinoma to Curative Therapy. Semin Nucl Med (2019) 49(3):189–96. doi: 10.1053/j.semnuclmed.2019.01.003
33. Cucchetti A, Cappelli A, Ercolani G, Mosconi C, Cescon M, Golfieri R, et al. Selective Internal Radiation Therapy (SIRT) as Conversion Therapy for Unresectable Primary Liver Malignancies. Liver Cancer (2016) 5(4):303–11. doi: 10.1159/000449341
34. Zori AG, Limaye AR, Firpi R, Morelli G, Soldevila-Pico C, Suman A, et al. Locoregional Therapy Protocols With and Without Radioembolization for Hepatocellular Carcinoma as Bridge to Liver Transplantation. Am J Clin Oncol (2020) 43(5):325–33. doi: 10.1097/COC.0000000000000678
35. Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, et al. Radiation Lobectomy: Preliminary Findings of Hepatic Volumetric Response to Lobar Yttrium-90 Radioembolization. Ann Surg Oncol (2009) 16(6):1587–96. doi: 10.1245/s10434-009-0454-0
36. Lewandowski RJ, Donahue L, Chokechanachaisakul A, Kulik L, Mouli S, Caicedo J, et al. (90) Y Radiation Lobectomy: Outcomes Following Surgical Resection in Patients With Hepatic Tumors and Small Future Liver Remnant Volumes. J Surg Oncol (2016) 114(1):99–105. doi: 10.1002/jso.24269
37. Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation Lobectomy: Time-Dependent Analysis of Future Liver Remnant Volume in Unresectable Liver Cancer as a Bridge to Resection. J Hepatol (2013) 59(5):1029–36. doi: 10.1016/j.jhep.2013.06.015
38. Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, et al. A Comparison of Chemoembolization Combination With and Without Radiotherapy for Unresectable Hepatocellular Carcinoma. Cancer J (2004) 10(5):307–16. doi: 10.1097/00130404-200409000-00008
39. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol (2018) 4(5):661–9. doi: 10.1001/jamaoncol.2017.5847
40. Chong JU, Choi GH, Kim KS, Seong J, Han KH, Choi JS. Downstaging With Localized Concurrent Chemoradiotherapy Can Identify Optimal Surgical Candidates in Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. Ann Surg Oncol (2018) 25(11):3308–15. doi: 10.1245/s10434-018-6653-9
41. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 non-Inferiority Trial. Lancet (London England) (2018) 391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1
42. Qin SK, Bai YX, Ouyang XN, Cheng Y, Chen ZD, Ren ZG, et al. REFLECT-A Phase 3 Trial Comparing Efficacy and Safety of Lenvatinib to Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma: An Analysis of Chinese Subset. Oral Presentation Presented at CSCO (2017) Xiamen.
43. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region With Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol (2009) 10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7
44. Llovet J, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857
45. Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, Multicenter, Open-Label Study of Oxaliplatin Plus Fluorouracil/Leucovorin Versus Doxorubicin as Palliative Chemotherapy in Patients With Advanced Hepatocellular Carcinoma From Asia. J Clin Oncol Off J Am Soc Clin Oncol (2013) 31(28):3501–8. doi: 10.1200/JCO.2012.44.5643
46. Qin SK, Bi F, Gu SZ, Bai YX, Chen ZD, Wang ZS, et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol (2021) 39(27):3002–11. doi: 10.1200/JCO.21.00163
47. Kudo M, Ikeda M, Motomura K, Okusaka T, Kato N, Dutcus CE, et al. A Phase Ib Study of Lenvatinib (LEN) Plus Nivolumab (NIV) in Patients (Pts) With Unresectable Hepatocellular Carcinoma (uHCC): Study 117. J Clin Oncol (2020) 38(4_suppl):513–3. doi: 10.1200/JCO.2020.38.4_suppl.513
48. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
49. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in Combination With Apatinib in Patients With Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-Label, Phase II Trial. Clin Cancer Res Off J Am Assoc Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
50. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
51. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: Updated Overall Survival (OS) Data From a Global, Randomized, Open-Label Phase III Study of Atezolizumab (Atezo) + Bevacizumab (Bev) Versus Sorafenib (Sor) in Patients (Pts) With Unresectable Hepatocellular Carcinoma (HCC). J Clin Oncol (2021) 39(3_suppl):267–7. doi: 10.1200/JCO.2021.39.3_suppl.267
52. Galle PR, Kim RD, Sung MW, Harris WP, Waldschmidt D, Cabrera R, et al. 990p Updated Results of a Phase Ib Study of Regorafenib (REG) Plus Pembrolizumab (PEMBRO) for First-Line Treatment of Advanced Hepatocellular Carcinoma (HCC). Ann Oncol (2020) 31:S691–2. doi: 10.1016/j.annonc.2020.08.1106
53. Han C, Ye S, Hu C, Shen L, Qin Q, Bai Y, et al. Clinical Activity and Safety of Penpulimab (Anti-PD-1) With Anlotinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: An Open-Label, Multicenter, Phase Ib/II Trial (AK105-203). Front Oncol (2021) 11:684867. doi: 10.3389/fonc.2021.684867
54. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab Plus a Bevacizumab Biosimilar (IBI305) Versus Sorafenib in Unresectable Hepatocellular Carcinoma (ORIENT-32): A Randomised, Open-Label, Phase 2-3 Study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
55. Bai L, Sun M, Xu A, Bai Y, Wu J, Shao G, et al. Phase 2 Study of AK104 (PD-1/CTLA-4 Bispecific Antibody) Plus Lenvatinib as First-Line Treatment of Unresectable Hepatocellular Carcinoma. J Clin Oncol (2021) 39(15_suppl):4101–1. doi: 10.1200/JCO.2021.39.15_suppl.4101
56. Shen L, Zhang Y, Guo Y, Li W, Gong JF, Ma Z, et al. 987p A Phase Ib Study of the PD-1 Antagonist CS1003 Plus Lenvatinib (LEN) in Chinese Patients (Pts) With the First-Line (1L) Unresectable Hepatocellular Carcinoma (uHCC). Ann Oncol (2020) 31:S690–1. doi: 10.1016/j.annonc.2020.08.1103
57. Kudo M, Motomura K, Wada Y, Inaba Y, Sakamoto Y, Kurosaki M, et al. Avelumab in Combination With Axitinib as First-Line Treatment in Patients With Advanced Hepatocellular Carcinoma: Results From the Phase 1b VEGF Liver 100 Trial. Liver Cancer (2021) 10(3):249–59. doi: 10.1159/000514420
58. Finn RS, Ryoo BY, Merle P, Bouattour M, Lim HY, Breder V, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307
59. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for Patients With Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2017) 389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9
60. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in Patients With Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med (2018) 379(1):54–63. doi: 10.1056/NEJMoa1717002
61. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in Patients With Previously Treated Advanced Hepatocellular Carcinoma: A Multicentre, Open-Label, Parallel-Group, Randomised, Phase 2 Trial. Lancet Oncol (2020) 21(4):571–80. doi: 10.1016/S1470-2045(20)30011-5
62. Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as Second-Line or Later Therapy in Patients With Advanced Hepatocellular Carcinoma (AHELP): A Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Gastroenterol Hepatol (2021) 6(7):559–68. doi: 10.1016/S2468-1253(21)00109-6
63. Ducreux M, Abou-Alfa G, Ren Z, Edeline J, Li Z, Assenat E, et al. O-1 Results From a Global Phase 2 Study of Tislelizumab, an Investigational PD-1 Antibody, in Patients With Unresectable Hepatocellular Carcinoma. Ann Oncol (2021) 32:S217. doi: 10.1016/j.annonc.2021.05.005
64. Yau T, Zagonel V, Santoro A, Acosta-Rivera M, Choo SP, Matilla A, et al. Nivolumab (NIVO) + Ipilimumab (IPI) + Cabozantinib (CABO) Combination Therapy in Patients (Pts) With Advanced Hepatocellular Carcinoma (aHCC): Results From CheckMate 040. J Clin Oncol (2020) 38(4_suppl):478–8. doi: 10.1200/JCO.2020.38.4_suppl.478
65. He AR, Yau T, Hsu C, Kang YK, Kim TY, Santoro A, et al. Nivolumab (NIVO) + Ipilimumab (IPI) Combination Therapy in Patients (Pts) With Advanced Hepatocellular Carcinoma (aHCC): Subgroup Analyses From CheckMate 040. J Clin Oncol (2020) 38(4_suppl):512–2. doi: 10.1200/JCO.2020.38.4_suppl.512
66. Song X, Kelley RK, Khan A, Standifer N, Krishna R, Liu L, et al. Exposure-Response (E-R) Efficacy and Safety (E-S) Analyses of Tremelimumab as Monotherapy or in Combination With Durvalumab in Patients (Pts) With Unresectable Hepatocellular Carcinoma (uHCC). J Clin Oncol (2021) 39(3_suppl):313–3. doi: 10.1200/JCO.2021.39.3_suppl.313
67. Han Y, Zhi W, Xu F, Zhang C, Huang X, Luo J. Selection of First-Line Systemic Therapies for Advanced Hepatocellular Carcinoma: A Network Meta-Analysis of Randomized Controlled Trials. World J Gastroenterol (2021) 27(19):2415–33. doi: 10.3748/wjg.v27.i19.2415
68. Shindoh J, Kawamura Y, Kobayashi Y, Kobayashi M, Akuta N, Okubo S, et al. Prognostic Impact of Surgical Intervention After Lenvatinib Treatment for Advanced Hepatocellular Carcinoma. Ann Surg Oncol (2021) 28(12):7663–72. doi: 10.1245/s10434-021-09974-0
69. Huang C, Zhu XD, Shen YH, Wu D, Ji Y, Ge NL, et al. Organ Specific Responses to First-Line Lenvatinib Plus Anti-PD-1 Antibodies in Patients With Unresectable Hepatocellular Carcinoma: A Retrospective Analysis. Biomark Res (2021) 9(1):19. doi: 10.1186/s40364-021-00274-z
70. Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative Effectiveness of Different Transarterial Embolization Therapies Alone or in Combination With Local Ablative or Adjuvant Systemic Treatments for Unresectable Hepatocellular Carcinoma: A Network Meta-Analysis of Randomized Controlled Trials. PloS One (2017) 12(9):e0184597. doi: 10.1371/journal.pone.0184597
71. Kudo M. A Novel Treatment Strategy for Patients With Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer (2021) 10:539–44. doi: 10.1159/000519749
72. Aloia TA. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy: Portal Vein Embolization Should Remain the Gold Standard. JAMA Surg (2015) 150(10):927–8. doi: 10.1001/jamasurg.2015.1646
73. Piron L, Deshayes E, Escal L, Souche R, Herrero A, Pierredon-Foulongne MA, et al. [Portal Vein Embolization: Present and Future]. Bull Cancer (2017) 104(5):407–16. doi: 10.1016/j.bulcan.2017.03.009
74. European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. Eur J Cancer (2012) 48(5):599–641. doi: 10.1016/j.ejca.2011.12.021
75. Dupre A, Hitier M, Peyrat P, Chen Y, Meeus P, Rivoire M. Associating Portal Embolization and Artery Ligation to Induce Rapid Liver Regeneration in Staged Hepatectomy. Br J Surg (2015) 102(12):1541–50. doi: 10.1002/bjs.9900
76. Glantzounis GK, Tokidis E, Basourakos SP, Ntzani EE, Lianos GD, Pentheroudakis G. The Role of Portal Vein Embolization in the Surgical Management of Primary Hepatobiliary Cancers. A Systematic Review. Eur J Surg Oncol (2017) 43(1):32–41. doi: 10.1016/j.ejso.2016.05.026
77. Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential Arterial and Portal Vein Embolizations Before Right Hepatectomy in Patients With Cirrhosis and Hepatocellular Carcinoma. Br J Surg (2006) 93(9):1091–8. doi: 10.1002/bjs.5341
78. Li P-P, Huang G, Jia NY, Pan ZY, Liu H, Yang Y, et al. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy Versus Sequential Transarterial Chemoembolization and Portal Vein Embolization in Staged Hepatectomy for HBV-Related Hepatocellular Carcinoma: A Randomized Comparative Study. Hepatobil Surg Nutr (2020). doi: 10.1200/JCO.2020.38.15_suppl.4578
Keywords: hepatocellular carcinoma, downstaging, conversion therapy, initial unresectable, systemic
Citation: Sun H-C and Zhu X-D (2021) Downstaging Conversion Therapy in Patients With Initially Unresectable Advanced Hepatocellular Carcinoma: An Overview. Front. Oncol. 11:772195. doi: 10.3389/fonc.2021.772195
Received: 07 September 2021; Accepted: 28 October 2021;
Published: 18 November 2021.
Edited by:
Alessandro Vitale, University Hospital of Padua, ItalyReviewed by:
LeDu Zhou, Central South University, ChinaXiufeng Liu, Nanjing General Hospital of Nanjing Military Command, China
Copyright © 2021 Sun and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Chuan Sun, c3VuLmh1aWNodWFuQHpzLWhvc3BpdGFsLnNoLmNu
 Hui-Chuan Sun
Hui-Chuan Sun Xiao-Dong Zhu
Xiao-Dong Zhu