- 1Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Pathology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Langerhans cell sarcoma (LCS) is an extremely rare, malignant neoplasm that originates from Langerhans cells (LCs). Fewer than 70 cases have been reported in the English-language literature. LCS typically involves multiple organs, including the skin, lymph nodes, lungs, bone, bone marrow, liver, spleen, and soft tissues. Several etiological factors for LCS have been proposed, including immunosuppression, virus infection, and prior hematological disease. We report a rare case of LCS with Epstein–Barr virus (EBV) infection; bilateral cervical giant cysts were the initial manifestation. To our knowledge, this is the first report of LCS with EBV infection. The case information was complete, and the relevant literature was reviewed to gain insight into LCS. The case raises new questions on the oncogenic character of EBV.
Introduction
Langerhans cell sarcoma (LCS) is an extremely rare, malignant neoplasm that originates from Langerhans cells (LCs). Fewer than 70 cases have been reported in the English-language literature. LCS typically involves multiple organs, including the skin, lymph nodes, lungs, bone, bone marrow, liver, spleen, and soft tissues. Several etiological factors for LCS have been proposed, including immunosuppression, virus infection, and prior hematological disease (1). We report a rare case of LCS with Epstein–Barr virus (EBV) infection; bilateral cervical giant cysts were the initial manifestation. To our knowledge, this is the first report of LCS with EBV infection. The case information was complete, and the relevant literature was reviewed to gain insight into LCS. The case raises new questions on the oncogenic character of EBV.
Case Presentation
A 24-year-old male patient presented with a 6-month history of bilateral neck masses and a sensation of distension when swallowing, and complained that the mass had rapidly enlarged recently. The patient had a medical history that included surgery for fixation of a fracture of the right leg 16 years ago and pulmonary bullae resection 3 years ago. The patient also had a history of smoking (one pack of cigarettes per day for 7 years). Physical examination revealed two tender, fixed, and painless masses with a clear boundary (a ~7- × 6-cm mass in the left submandibular area and an ~8- × 7-cm mass in the right submandibular area; Figure 1A). Magnetic resonance imaging (MRI) (Figures 1D, E) revealed bilateral cervical cystic lesions and multiple enlarged bilateral cervical lymph nodes.
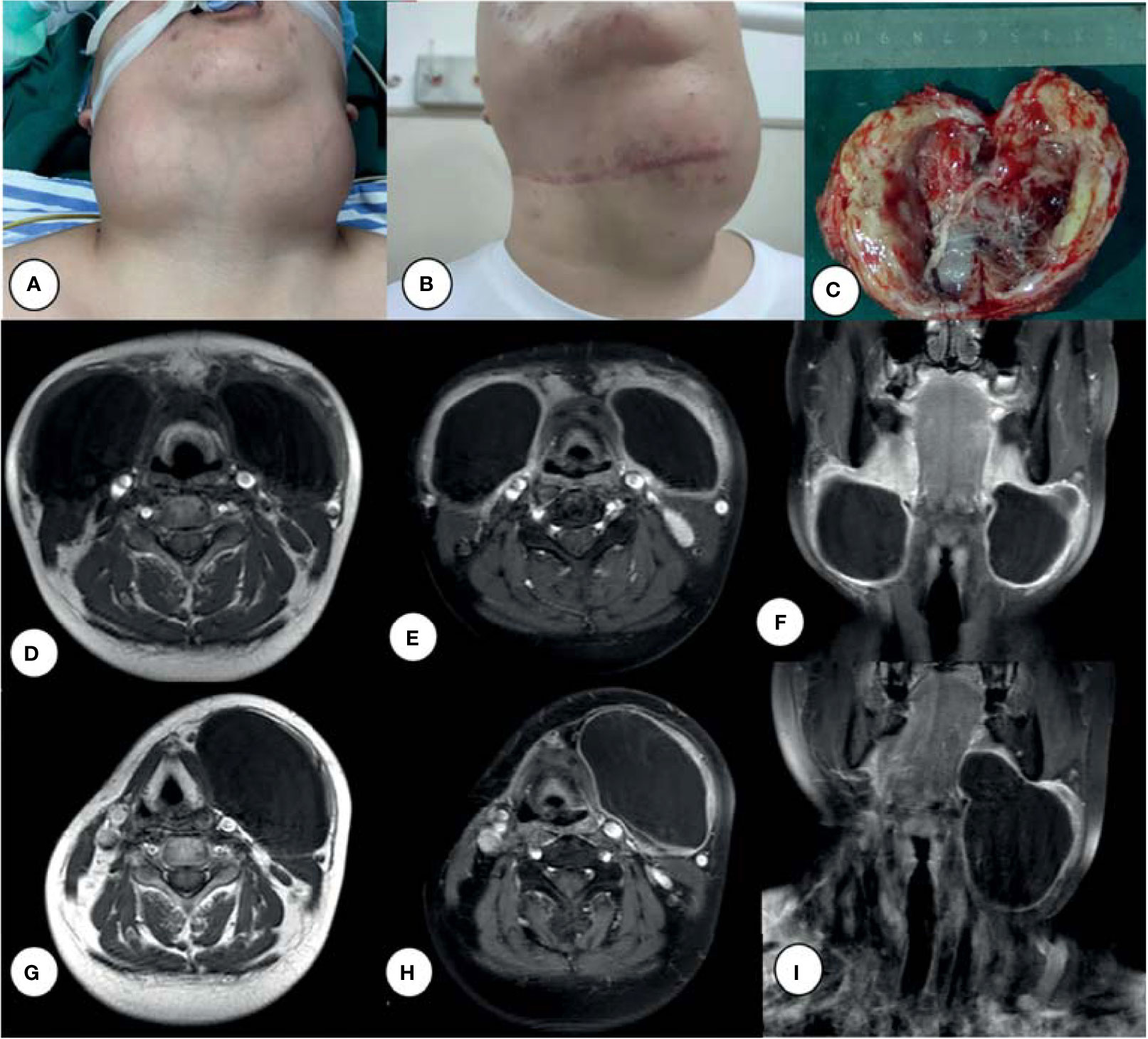
Figure 1 Bilateral cervical giant cysts (A), left cyst enlarged after chemotherapy (B), and surgical excision of the right neck mass (C). MRI of a tumor before surgery (D–F) and after (G–I) chemotherapy: T1-weighted images (D, G), Ga-enhanced T1-weighted images (E, F, H, I).
The patient underwent surgery for excision of a neck mass on April 23, 2020. Grossly, the 8- × 7-cm, pale-yellow mass was identified in the right neck and excised completely along its border up to the skull base. The mass was cystic and filled with thick, dirty yellow fluid, with a ~1-cm-thick cystic wall (Figure 1C). Based on the pathological results of proliferative lesions in lymphohematopoietic tissue, the left mass was treated after routine pathological diagnosis. The final pathology report supported a diagnosis of LCS. Following surgery, the patient received two courses of chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOP). The patient underwent a second surgery on August 1, 2021 because the left lateral cervical mass (Figure 1B) was not significantly reduced and pain was experienced when swallowing.
Pathological Findings
The neoplastic cells exhibited cytological atypia, hyperchromatic nuclei, and prominent nucleoli, and nuclear grooving was observed in some of them (Figure 2A). Immunohistochemical studies revealed that the malignant tumor cells were positive for CD1a (Figure 2B), S-100 protein (Figure 2C), and Langerin (Figure 2D). There was variable expression of CD56 (Figure 2E), cyclin D1, CD4, CD68, and CD163. The proportion of p53 was ~3%. Mitoses were frequently identified, and the Ki-67 proliferative index (Figure 2F) was ~60%. Electron microscopy demonstrated the presence of a large kidney‐shaped nucleus (Figure 2G) and typical Birbeck granules (Figure 2H), with unique striated cytoplasmic organelles characteristic of neoplastic cells.
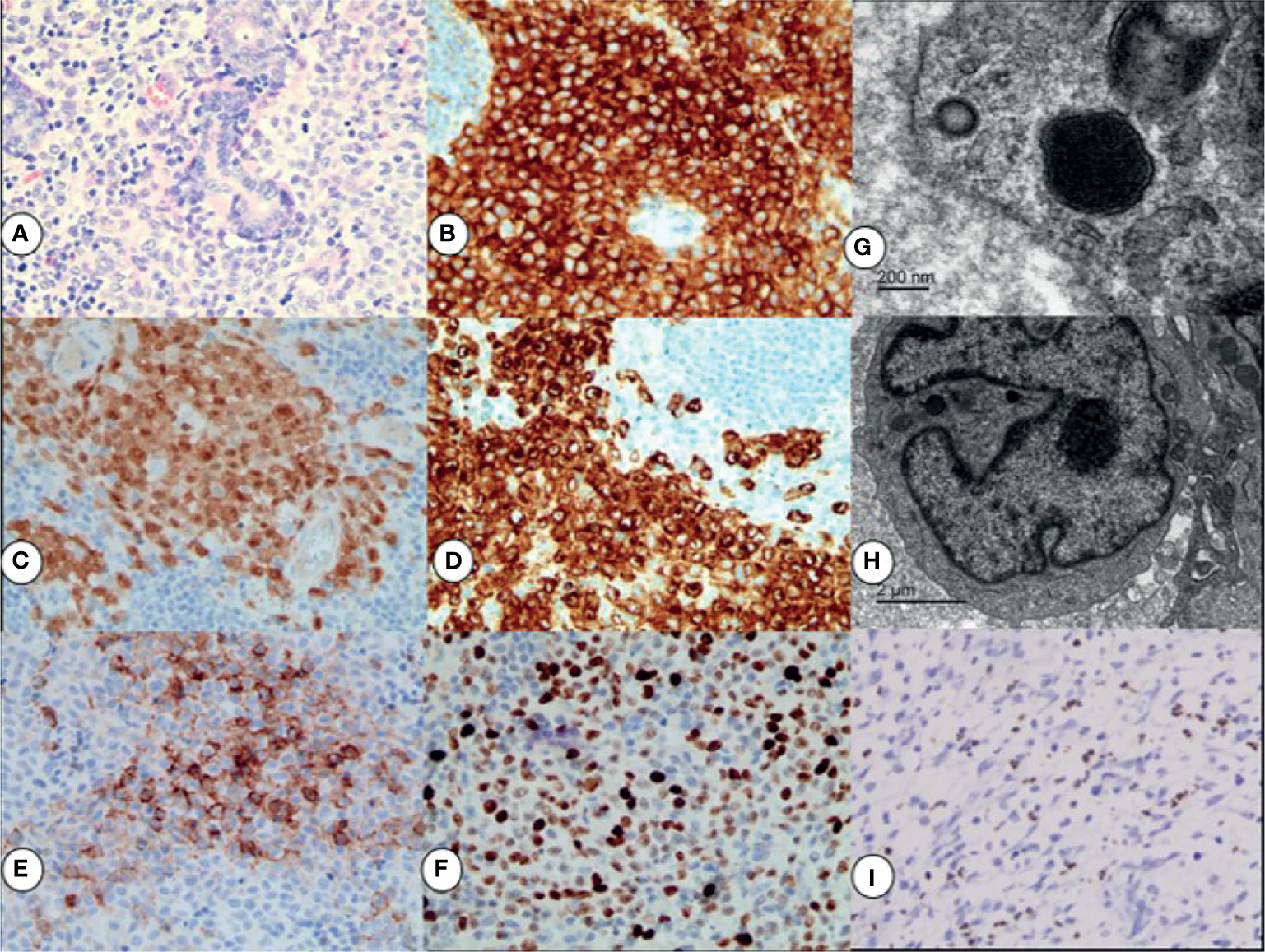
Figure 2 By H&E staining results: the neoplastic cells exhibited cytological atypia, hyperchromatic nuclei, prominent nucleoli, and a high mitotic rate (A). Immunohistochemical staining for CD1a + (B), S-100 + (C), Langerin+ (D), and CD56+ (E); the Ki67 proliferation index was ∼60% (F). All magnifications ×40. Electron micrograph showing a large kidney‐shaped nucleus (G) and typical Birbeck granules (H) in neoplastic cells. EBER in situ hybridization indicating positive signals in the nuclei of background lymphocytes (I), ×20.
Pathological findings of the second surgical specimen after chemotherapy showed that CD56, CD68, and CD163 expression became negative in neoplastic cells. Chromogen in situ hybridization for the Epstein–Barr encoding region (EBER) of background lymphocytes (Figure 2I) was positive. Molecular testing showed that the tumor was negative for BRAF V600E mutations.
Laboratory Findings
The laboratory findings revealed an EBV immunoglobulin (Ig) G of 6.4, human cytomegalovirus (HCMV) IgG of 5.6, and EBV-DNA of 1.05 × 103 copies/ml. The CD3/4/8/16/19/45/56 lymphocyte count was 559 cells/μl (range, 800–4,000 cells/μl), the T-cell count was 276 cells/μl (range, 797–2,370 cells/μl), the helper T-cell count was 138 cells/μl (range, 432–1,341 cells/μl), the killer T-cell count was 125 cells/μl (range, 238–1,075 cells/μl), the natural killer (NK) (CD16+ and CD56+) cell count was 160 cells/μl (range, 127–987 cells/μl), and the B-cell count was 115 cells/μl (range, 86–594 cells/μl). After chemotherapy, the CD3/4/8/16/19/45/56 lymphocyte count was 618 cells/μl, the T-cell count was 278 cells/μl, the helper T-cell count was 98 cells/μl, the killer T-cell count was 155 cells/μl, the NK (CD16+, CD56+) cell count was 305 cells/μl, and the B-cell count was 29 cells/μl. EBV and HCMV capsid antigen IgG, but not IgM, was positive, indicating historic rather than recent EBV and HCMV infection. The EBV DNA load was 1,050 copies/ml. Lymphatic subgroup analysis showed that the patient was in an immunosuppressed state, with reduced T cells and a low CD4/CD8 ratio. After chemotherapy, the CD4/CD8 ratio and B-cell count were lower. High-resolution computed tomography (HRCT) of the chest before chemotherapy (Figures 3A–C) showed numerous variably sized pulmonary cysts that were confluent in some places, and HRCT of the chest after chemotherapy (Figures 3D, E) showed that the pulmonary cysts were enlarged and thin-walled. MRI of the neck after chemotherapy (Figures 1G–I) showed that the cyst had again increased in size and the cystic wall was thinner than before (Figures 1D–F). The patient’s general condition was assessed by positron emission tomography-computed tomography (PET-CT) (Figures 3F–I). A 90 × 85-mm mass was detected in the left neck with a maximal standardized uptake value (SUVmax) of ~5.7 (Figure 3F); the multiple small lymph nodes located close to the mass had an SUVmax of ~5.5. The SUVmax of pulmonary cystic lesions was ~1.6 (Figure 3G). Fluorine-18 fluorodeoxyglucose (FDG) uptake in the LCS lesions was lower than in prior reports (cases 19, 24, and 25 in Table 1). The decreased FDG uptake may be related to chemotherapy.
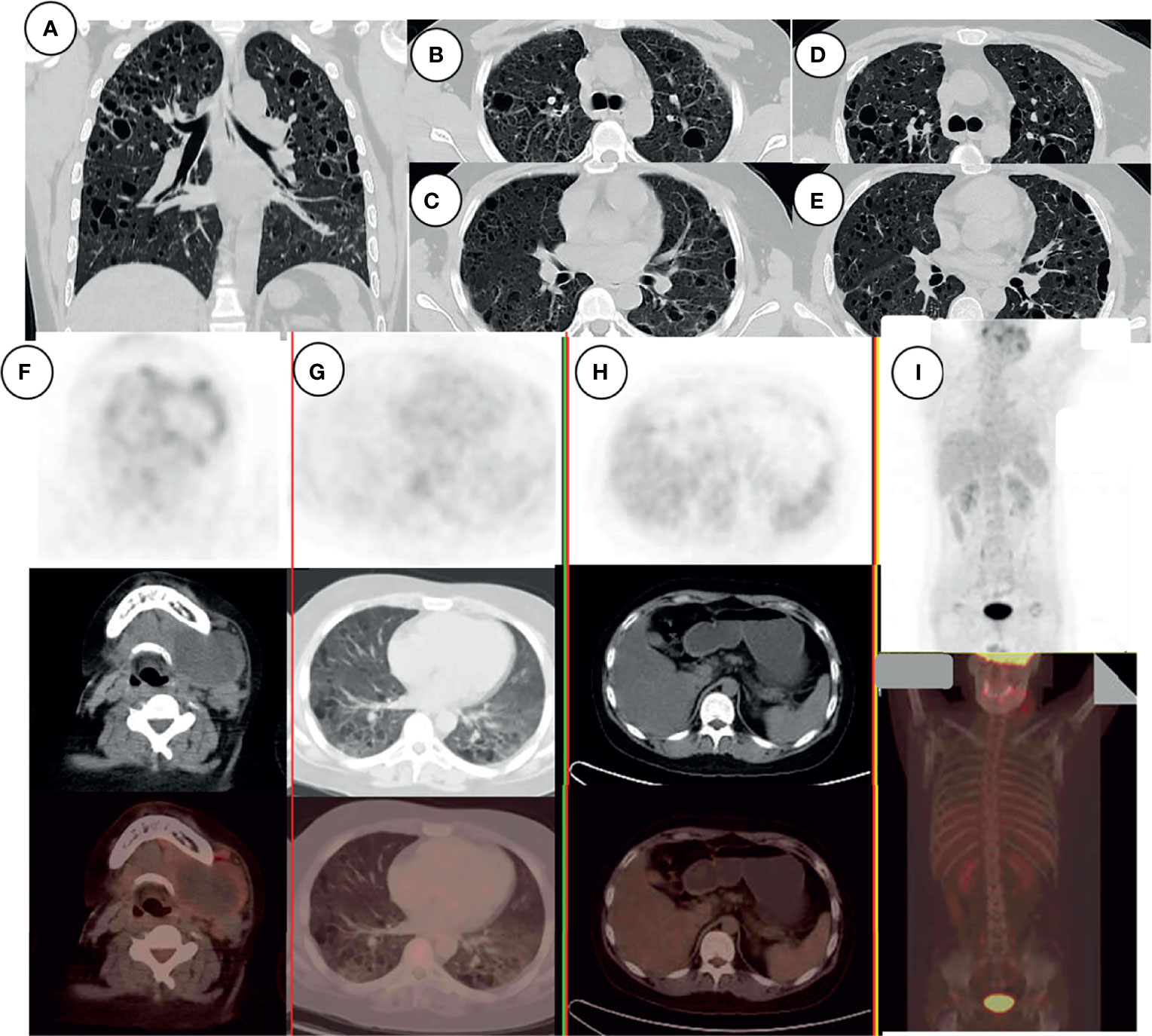
Figure 3 HRCT of the chest, showing numerous variably sized pulmonary cysts that were confluent in some places (A–C); the cysts became larger and thin-walled after chemotherapy (D, E). PET-CT examination of the patient’s general condition. Signal from the left neck mass and multiple small lymph nodes (F), pulmonary cystic lesions (G), and liver (H). There was no signal from bone (I).
Discussion
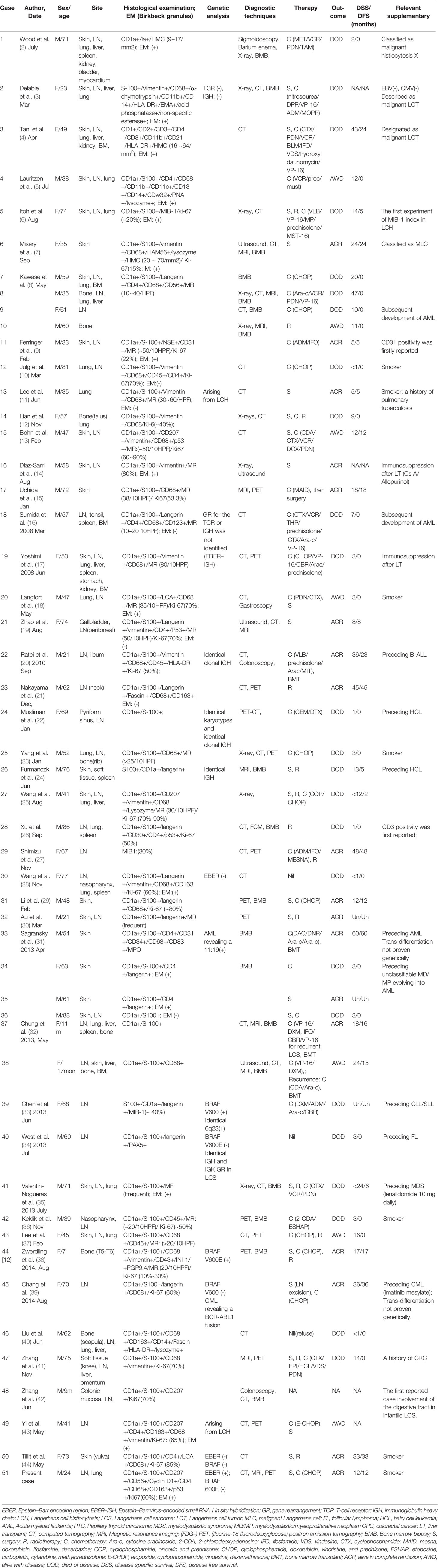
LCS is an extremely rare neoplastic proliferation of LCs with overtly malignant cytological features and unusually aggressive behavior. In the classification of tumors of the hematopoietic and lymphoid systems of the World Health Organization (WHO) (WHO-2016) (1), LCS is defined as a neoplastic disorder of LC with apparent malignant cytological features, possibly including both LCS progressed from Langerhans cell histiocytosis (LCH) and de novo LCS. LCS is distinguished from LCH, which is also involves the neoplastic proliferation of cells, in terms of its immunophenotypic and electron microscopic features of LC, cytologic atypia, and clinical aggressiveness (45). However, it can be difficult to classify a lesion as LCH or LCS. LCS displays typical features of malignant tumors and usually involves multiple organs, including the skin, lymph nodes, lungs, liver, spleen, kidneys, bone, bone marrow, and other soft tissues.
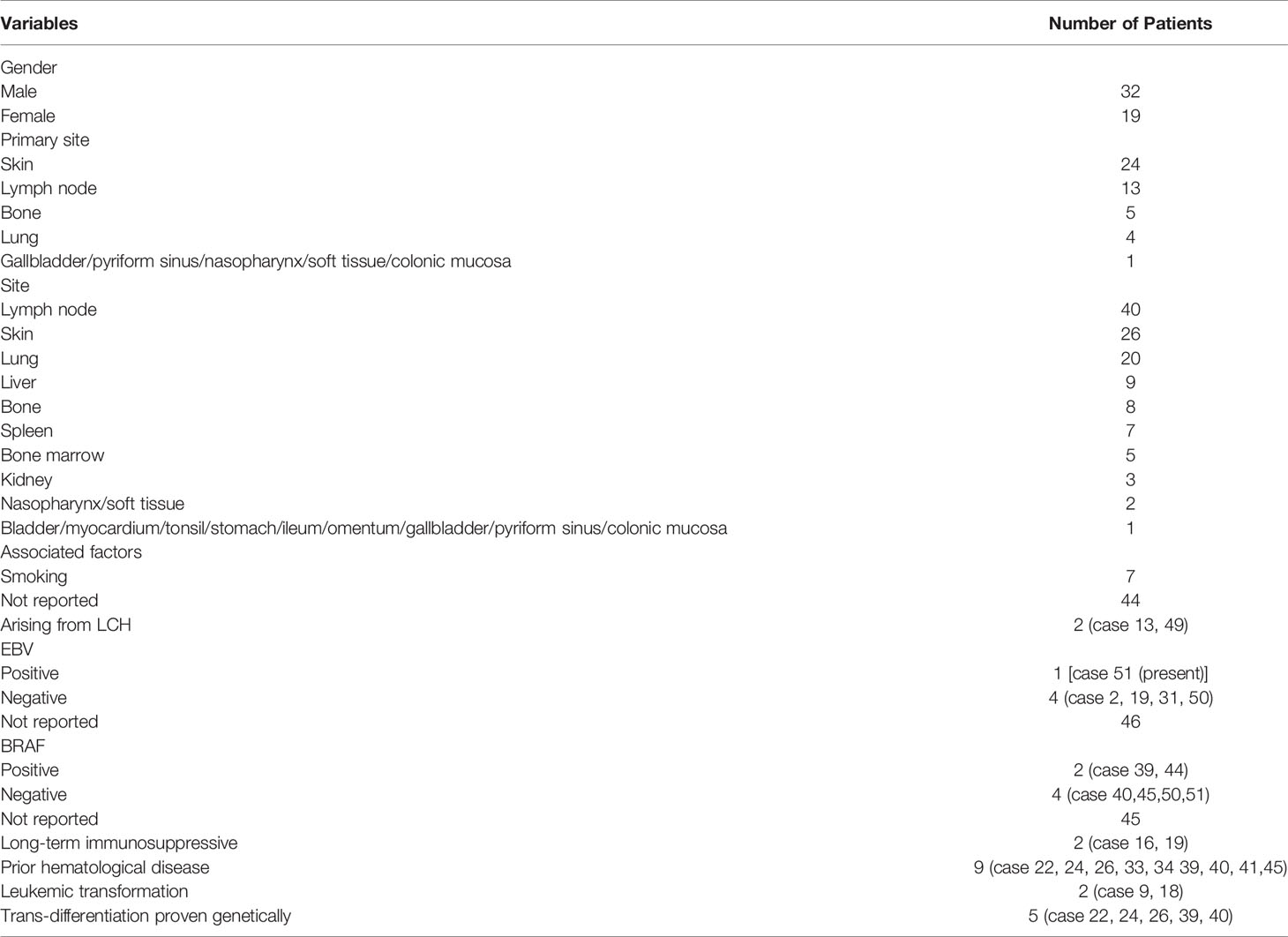
We conducted a systematic literature review on LCS from 1984 to December 2020 (keywords: Langerhans cell sarcoma), focusing on studies describing the etiology and pathology of LCS. The available reports are summarized, together with the present case, in Table 1. These cases (Cases 14, 17, 19, 22, 24, 25, 29, 33, 37, 38, 42, 43, 44, 46, 47) having no CD207 (langerin) or electron microscopic features of LCs, might not meet all the current criteria in WHO-2016 for LCS sarcoma diagnosis. LCS can occur at any age, with patients ranging from 9 months to 88 years old. As shown in Table 2, the male-to-female ratio was 1.68. In 7.9% of cases (n = 4), the primary site at diagnosis of LCS was the lungs; all four of those patients were smokers. Interestingly, pulmonary LCH is almost always associated with smoking (46). Liu (47) provided mechanistic insight into the role of tobacco smoke in the development of pulmonary Langerhans cell histiocytosis (PLCH) using a smoking mouse model. However, only a few of the reported cases of primary lung LCS considered the smoking history. The most common primary site at diagnosis was the skin among the cases reviewed herein (24 cases, 47.1%), followed by the lymph nodes (13 cases, 25.5%), bone (5 cases, 9.8%), and lung (4 cases, 7.9%; 1 case each in the gallbladder, pyriform sinus, nasopharynx, colonic mucosa, and soft tissue). At diagnosis, 25.5% of cases had local disease, 23.5% had locoregional disease, and 51.0% disseminated disease. The most common sites were the lymph nodes (40 cases, 78.4%) and skin (26 cases, 51.0%), followed by the lung (20 cases, 29.2%) and the other organs listed in Table 2.
The rarity of LCS hampers investigation of its pathogenesis. Several etiological factors have been proposed, including immunosuppression, prior hematological disease, and virus infection. Immunosuppression has been linked to increased rates of malignancy (2.7- to 13.7-fold increase post-transplant) with the risk increasing with the intensity and duration of the immunosuppression. Our review revealed two LCS cases (17, 48) occurring against a background of immunosuppression for previous liver transplants (cases 16 and 19). Furthermore, Rate’s LCS (20) case was controlled only by stopping the immunosuppression (case 22). Long-term immunosuppressive treatment after organ transplantation may promote the development of LCS.
Some LCS cases have been linked to prior hematological disease. LCS may be preceded by acute B-lymphoblastic leukemia (B-ALL) (20) (case 22), hairy cell leukemia (HCL) (49, 50) (cases 24 and 26), acute myeloid leukemia (AML) (31) (case 33), unclassifiable myelodysplastic/myeloproliferative neoplasm (MD/MP) evolving into AML (38) (case 44), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (33) (case 39), follicular lymphoma (FL) (34) (case 40), myelodysplastic syndrome (MDS) (35) (case 41), and chronic myelogenous leukemia (CML) (39) (case 45). Two cases showed leukemic transformation (8, 16) (cases 9 and 18). In summary, LCS can occur in association with other hematological disorders (20, 33, 34, 49, 50) (cases 22, 24, 26, 39, and 40) to which it is clonally related. These cases carry the same T-cell receptor (TCR) or Ig heavy chain (IGH) gene rearrangements and chromosomal aberrations as the associated lymphoid neoplasms, suggesting a process of transdifferentiation [WHO-2016 (1)]. LCS can exhibit acute leukemic transformation, and a wide variety of clonal malignancies can transdifferentiate into LCS.
Viral infections are associated with approximately 12% of all cancers worldwide. LCs are present beyond the middle of the spinous epidermal layer and function as sentinel or antigen-presenting cells that can capture invading viruses (51). The interaction between LCs and viruses results in highly variable responses. The inflammatory nature of LCH lesions raises the possibility that infection and immune dysregulation may be the mechanisms of pathogenesis (52). Several viruses have been studied as potential etiological factors of LCH, including EBV (53), human herpesvirus 6 (HHV-6) (54), cytomegalovirus (CMV) (55), herpes simplex virus (HSV) (56), and Merkel-cell polyoma virus (MCV or MCPyV) (57). Murakami reported that MCV-related molecules are present in more than half of LCH cases, and in some dermatopathy lymphadenopathy cases (58), and that three LCS cases were positive for viral DNA sequences (59). It was postulated that a high MCV load in LCS lesions is an important oncogenic factor in LCS cells. EBV is the etiological agent in several malignancies and may play a role in the pathogenesis of LCH (60, 61). The main reservoir of latent EBV infection in vivo is the B-lymphocyte population. EBV latently infects a unique subset of blood-borne mononuclear cells that are direct precursors of LCs derived from B lymphocytes and could be reactivated and replicated in LCs (62, 63). Daniel revealed that Hodgkin lymphoma (HL) with excess Langerhans cell shows greater LMP1/EBV expression, which may increase cytokine production by activating nuclear factor kappa B (NF-κB), and thus explain the abundance of LCs (64). Therefore, evidence of EBV infection in LCS would be interesting, and the association of EBV infection with LCS should be investigated.
In our case, the patient had an EBV-DNA level of 1.05 × 103, and chromogen in situ hybridization for EBER of background lymphocytes was positive. This is the first reported LCS case positive for EBV markers. Lymphatic subgroup analysis (CD3/4/8/16/19/45/56) showed that the patient was in an immunosuppressive state with reduced T cells and a low CD4/CD8 ratio. Allograft recipients given T-cell-suppressive drugs to prevent graft rejection and HIV-infected individuals who progress to profound T-lymphopenia and late-stage acquired immunodeficiency syndrome (AIDS) provide the clearest evidence of a key role for T cells in the control of EBV-induced disease (65). Profound T-cell depletion of the allograft represents a major risk factor for EBV-induced post-transplantation lymphoproliferative disorder (EBV-PTLD), which is a life-threatening complication of allogeneic hematopoietic cell transplantation (66). In the absence of T-cell control, the lymphoproliferative disease seen in late-stage AIDS is the equivalent of classical PTLD, which is characterized by the growth of EBV-transformed lymphoblastoid cell line (LCL)-like cells, often in the central nervous system (65). EBV-associated smooth muscle tumors (SMTs) are rare malignancies that occur exclusively in immunocompromised patients, typically due to posttransplant immunosuppression or HIV infection (67). Moreover, immunosuppressants, including methotrexate (MTX) and tacrolimus (TAC), are widely used to treat patients with rheumatoid arthritis (RA), and their adverse effects have been known to cause other iatrogenic immunodeficiency-associated lymphoproliferative disorders (OIIA-LPDs). Seiji reported that the tumor cells were positive for EBV in 8 (17%) of 48 patients; background cells were positive in 32 (82%) of 39 patients with available data in the literature review of MTX-associated T-LPDs (MTX T-LPDs) (68). The presence of EBV reflects a profound immunodeficiency and may drive the development or a rapid progression of the tumor.
We postulated that our patient likely developed LCS due to EBV infection under conditions of congenital or acquired immunosuppression via a mechanism similar to EBV-PTLD, EBV-positive SMTs (EBV + SMTs), or MTX T-LPDs. The case raises new questions regarding the oncogenic nature of EBV.
The pathological results were consistent with LCS. Immunohistochemistry was performed on samples obtained before and after chemotherapy. The expression of CD56, CD68, and CD163 became negative in the neoplastic cells after chemotherapy, possibly attributable to the effectiveness of chemotherapy. Some experts have recommended using CD56 as a marker for differential diagnosis of LCS and LCH. Kawase found that tumor cells in all four cases of LCS in their study were positive for CD56 (8), whereas the tumor cells in all eight cases of LCH were negative. Our findings indicated that CD56 may be a clinically relevant predictor of an intractable course of LCS. The present case was negative for the BRAF V600E mutation, which involves a molecular change underlying the pathogenesis of many malignancies. Almost half of all cases of LCH reportedly harbor the BRAF V600E mutation (69), while only two of the cases of LCS reviewed herein (33, 38) (cases 39 and 44) had the BRAF V600E mutation. Given the poor outcomes of LCS, we suggest that immunohistochemical testing for the BRAF mutation should be performed. Vemurafenib, a BRAF inhibitor, may have therapeutic potential, especially in older individuals in whom combined therapy is expected to be poorly tolerated (38) (case 44).
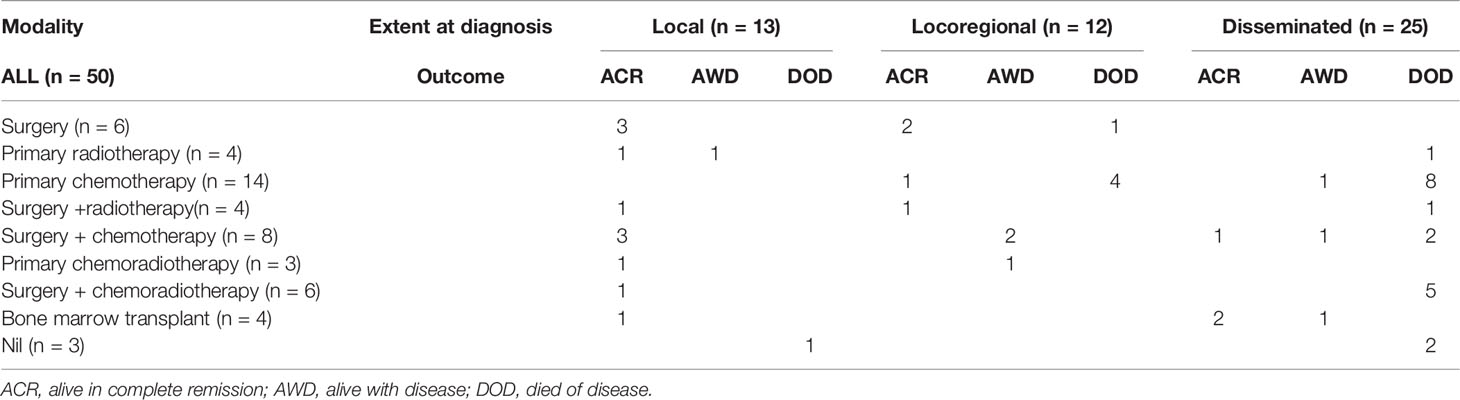
Because of its rarity, the optimal treatment strategy for LCS has not been established, and treatment depends on the affected site and scope (Table 3). For localized nodular disease, one patient (case 40) with a history of FL received no therapy due to severe disease progression, 84.6% (11/13) achieved complete remission with monotherapy (only surgery was used in cases 6, 13, and 35; only radiotherapy was performed in case 23) or multimodal therapy, and 7.7% (1/13) receiving only radiotherapy were alive with disease at the last follow-up. For locoregional disease, 33.3% (4/12) achieved complete remission with monotherapy (only surgery was used in cases 16 and 21; only chemotherapy was performed in case 11) and multimodal therapy (case 32 received surgery with adjuvant chemotherapy), 25% (3/12) were alive with disease at the last follow-up (cases 15 and 20 received surgery with adjuvant chemotherapy, case 49 received chemoradiotherapy), and 41.7% (5/12) died from their disease (only chemotherapy was performed in cases 24, 25, 34, and 42; only surgery was performed in case 36). Among the patients with disseminated disease, 12% (3/25) achieved complete remission [cases 22 and 37 received a bone marrow transplant (BMT) after chemotherapy and case 51 received surgery with adjuvant chemotherapy], 12% (3/25) were alive with disease at the last follow-up (only chemotherapy was performed in case 11; case 43 received surgery with adjuvant chemotherapy; case 13 received a BMT after chemotherapy), and 76% (19/25) died from their disease. For local or locoregional disease restricted to the skin and lymph nodes, there were good outcomes with all treatment modalities. Of the 25 cases of disseminated LCS, only 3 achieved complete remission at the last follow-up. One patient with disease restricted to the lung and cervical lymph nodes underwent surgery with adjuvant chemotherapy, achieving complete remission. The remaining two patients were treated with BMT; one developed recurrence at 15 months but was alive at 24 months, and the other was cured after 18 months (case 37). Despite receiving conventional combination chemotherapy, surgery, and radiotherapy, 76% (19/25) of disseminated LCS patients showed a poor prognosis and a short survival period because these patients typically have multiple organ involvement and distant metastasis. A patient with disease in more than two organs at diagnosis was reported by Chung (32) (case 37) and achieved complete remission, demonstrating that BMT is the only effective treatment for disseminated LCS.
For LCS with BRAF V600E mutations, vemurafenib, a BRAF V600E mutant inhibitor, has shown efficacy as a targeted, alternative treatment (69). Although the association of EBV and LCS is unclear, patients with high EBV loads may be candidates for antiviral therapy. A reduction in viral load may prevent the development of diseases such as PTLD during primary infection, in addition to other malignant diseases associated with latency (70). Smoking cessation is an important recommendation for smokers with LCS, given that it leads to partial regression in around half of patients with isolated PLCH (46). The present case is particularly uncommon in that the patient developed LCS with EBV infection of the bilateral cervical giant cysts and lung lesions and was treated by a combination of surgery, an anthracycline-containing regimen (ACR) and CHOP chemotherapy, after declining antiviral therapy.
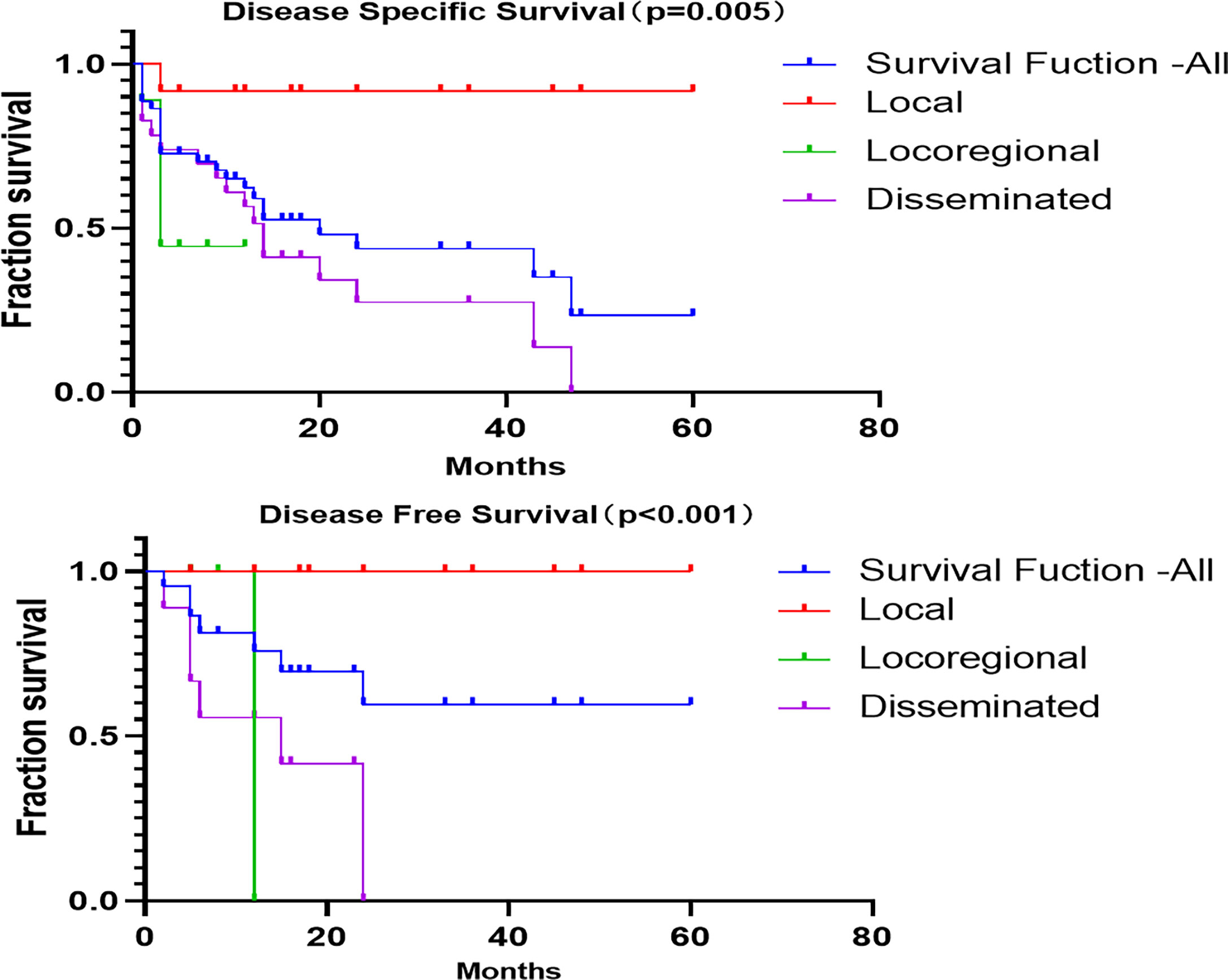
The 1-, 3-, and 5-year disease-specific survival (DSS) for all of the patients with LCS was 54.6%, 15.9%, and 2.3%, respectively. Unfortunately, none of the patients with locoregional disease survived to 3 years, and none with disseminated disease survived to 5 years (Figure 4). The 1-, 3-, and 5-year disease-free survival (DFS) for all patients with LCS was 68.2%, 18.2%, and 2.3%, respectively. Unfortunately, none of the patients with locoregional and disseminated disease survived to 3 years. The overall DSS was 28.06 months, with a DFS of 21.22 months. There were significant differences in DSS and DFS among the local, locoregional, and disseminated disease cohorts (p = 0.005 and p < 0.001 respectively; Table 4).
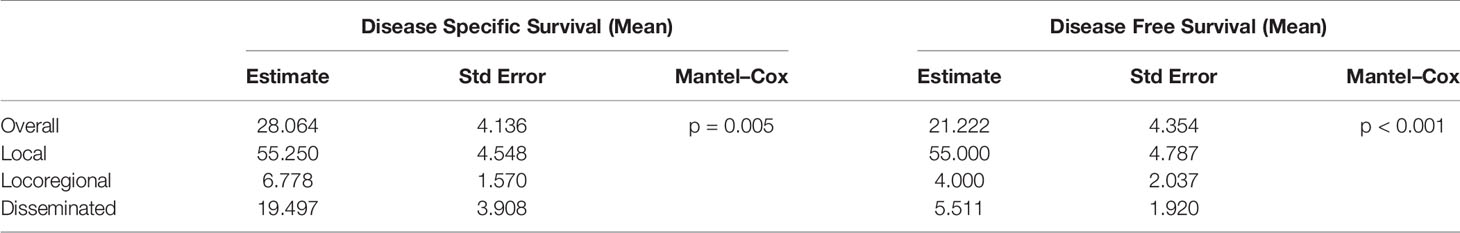
Table 4 Disease-specific and disease-free survival calculations from Kaplan–Meier survival analysis.
In summary, LCS has a poor prognosis and requires pathological diagnosis because of its non-specific clinical manifestations and imaging findings. We reported a rare EBV-positive LCS with bilateral lateral cervical giant cysts as the initial manifestation. The case information was complete, and relevant literature was reviewed to gain insight into LCS. The case raises new questions regarding the oncogenic nature of EBV.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the First Affiliated Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YG and S-HZ designed and wrote the manuscript. Z-ZC, Y-YB, and L-FS reviewed the references and made the tables. H-TY made the immunohistochemical pictures. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the Center of Electron Microscopy of Zhejiang University and State Key Laboratory for Diagnosis and Treatment of Infectious Diseases of Zhejiang University. We obtain the patient’s appropriate consents, permissions, and releases about personal disease details and images for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
2. Wood C, Wood GS, Deneau DG, Oseroff A, Beckstead JH, Malin J. Malignant Histiocytosis X. Report of a Rapidly Fatal Case in an Elderly Man. Cancer (1984) 54:347–52.
3. Delabie J, De Wolf-Peeters C, De Vos R, Vandenberghe E, Kennes K, De Jonge I, et al. True Histiocytic Neoplasm of Langerhans' Cell Type. J Pathol (1991) 163:217–23.
4. Tani M, Ishii N, Kumagai M, Ban M, Sasase A, Mishima Y. Malignant Langerhans Cell Tumour. Br J Dermatol (1992) 126:398–403.
5. Lauritzen AF, Delsol G, Hansen NE, Horn T, Ersbøll J, Hou-Jensen K, et al. Histiocytic Sarcomas and Monoblastic Leukemias. A Clinical, Histologic, and Immunophenotypical Study. Am J Clin Pathol (1994) 102:45–54.
6. Itoh H, Miyaguni H, Kataoka H, Akiyama Y, Tateyama S, Marutsuka K, et al. Primary Cutaneous Langerhans Cell Histiocytosis Showing Malignant Phenotype in an Elderly Woman: Report of a Fatal Case. J Cutaneous Pathol (2001) 28:371–8.
7. Misery L, Godard W, Hamzeh H, Lévigne V, Vincent C, Perrot J-L, et al. Malignant Langerhans Cell Tumor: A Case With a Favorable Outcome Associated With the Absence of Blood Dendritic Cell Proliferation. J Am Acad Dermatol (2003) 49:527–9.
8. Kawase T, Hamazaki M, Ogura M, Kawase Y, Murayama T, Mori Y, et al. CD56/NCAM-Positive Langerhans Cell Sarcoma: A Clinicopathologic Study of 4 Cases. Int J Hematol (2005) 81:323–9. doi: 10.1532/IJH97.04142
10. Jülg BD, Weidner S, Mayr D. Pulmonary Manifestation of a Langerhans Cell Sarcoma: Case Report and Review of the Literature. Virchows Archiv Int J Pathol (2006) 448:369–74.
11. Lee J-S, Ko GH, Kim HC, Jang IS, Jeon K-N, Lee J-H. Langerhans Cell Sarcoma Arising From Langerhans Cell Histiocytosis: A Case Report. J Kor Med Sci (2006) 21:577–80.
12. Lian Y-l, He H-y, Liao S-l, Yin L-j, Han Z-h, Zheng J. Langerhans Cell Sarcoma of Talus: Report of a Case. ZhonghuaBing Li Xue Za Zhi (2006) 35:697–8.
13. Bohn OL, Ruiz-Argüelles G, Navarro L, Saldivar J, Sanchez-Sosa S. Cutaneous Langerhans Cell Sarcoma: A Case Report and Review of the Literature. Int J Hematol (2007) 85:116–20.
14. Diaz-Sarrio C, Salvatella-Danés N, Castro-Forns M, Nadal A. Langerhans Cell Sarcoma in a Patient Who Underwent Transplantation. J Eur Acad Dermatol Venereol JEADV (2007) 21:973–6.
15. Uchida K, Kobayashi S, Inukai T, Noriki S, Imamura Y, Nakajima H, et al. Langerhans Cell Sarcoma Emanating From the Upper Arm Skin: Successful Treatment by MAID Regimen. J Orthopaed Sci Off J Japn Orthopaed Assoc (2008) 13:89–93.
16. Sumida K, Yoshidomi Y, Koga H, Kuwahara N, Matsuishi E, Karube K, et al. Leukemic Transformation of Langerhans Cell Sarcoma. Int J Hematol (2008) 87:527–31. doi: 10.1007/s12185-008-0063-x
17. Yoshimi A, Kumano K, Motokura T, Takazawa Y, Oota S, Chiba S, et al. ESHAP Therapy Effective in a Patient With Langerhans Cell Sarcoma. Int J Hematol (2008) 87:532–7. doi: 10.1007/s12185-008-0075-6
18. Langfort R, Radzikowska E, Czarnowska E, Wiatr E, Grajkowska W, Błasińska-Przerwa K, et al. Langerhans Cell Sarcoma With Pulmonary Manifestation, Mediastinum Involvement and Bronchoesophageal Fistula. A Rare Location and Difficulties in Histopathological Diagnosis. Pneumonol Alergol Pol (2009) 77:327–34.
19. Zhao G, Luo M, Wu Z-Y, Liu Q, Zhang B, Gao R-L, et al. Langerhans Cell Sarcoma Involving Gallbladder and Peritoneal Lymph Nodes: A Case Report. Int J Surg Pathol (2009) 17:347–53.
20. Ratei R, Hummel M, Anagnostopoulos I, Jahne D, Arnold R, Dorken B, et al. Common Clonal Origin of an Acute B-Lymphoblastic Leukemia and a Langerhans’ Cell Sarcoma: Evidence for Hematopoietic Plasticity. Haematologica (2010) 95:1461–6. doi: 10.3324/haematol.2009.021212
21. Nakayama M, Takahashi K, Hori M, Okumura T, Saito M, Yamakawa M, et al. Langerhans Cell Sarcoma of the Cervical Lymph Node: A Case Report and Literature Review. Auris Nasus Larynx (2010) 37:750–3.
22. Muslimani A, Chisti MM, Blenc AM, Boxwala I, Micale MA, Jaiyesimi I. Langerhans/dendritic Cell Sarcoma Arising From Hairy Cell Leukemia: A Rare Phenomenon. Ann Hematol (2012) 91:1485–7.
23. Yang C-J, Lee J-Y, Wu C-C, Yin H-L, Lien C-T, Liu Y-C. An Unusual Pulmonary Mass With Mediastinal Invasion and Multiple Intrapulmonary Nodules in a 52-Year-Old Man. Chest (2012) 141:253–8.
24. Furmanczyk PS, Lisle AE, Caldwell RB, Kraemer KG, Mercer SE, George E, et al. Langerhans Cell Sarcoma in a Patient With Hairy Cell Leukemia: Common Clonal Origin Indicated by Identical Immunoglobulin Gene Rearrangements. J Cutaneous Pathol (2012) 39:644–50.
25. Wang C, Chen Y, Gao C, Yin J, Li H. Multifocal Langerhans Cell Sarcoma Involving Epidermis: A Case Report and Review. Diagn Pathol (2012) 7:99.
26. Xu Z, Padmore R, Faught C, Duffet L, Burns BF. Langerhans Cell Sarcoma With an Aberrant Cytoplasmic CD3 Expression. Diagn Pathol (2012) 7:128.
27. Shimizu I, Takeda W, Kirihara T, Sato K, Fujikawa Y, Ueki T, et al. Long-Term Remission of Langerhans Cell Sarcoma by AIM Regimen Combined With Involved-Field Irradiation. [Rinsho ketsueki] Jpn J Clin Hematol (2012) 53:1911–5.
28. Wang Y-n, Zhou X-g, Wang Z. Langerhans Cell Sarcoma in the Cervical Lymph Node: A Case Report and Literature Review. Acta Haematol (2013) 129:114–20.
29. Li Y, Li B, Tian X-y, Li Z. Unusual Cutaneous Langerhans Cell Sarcoma Without Extracutaneous Involvement. Diagn Pathol (2013) 8:20.
30. Au WY, Lai C, Trendell-Smith NJ, Ng W-M, Chow DLSN. Paraneoplastic Disseminated Lentigines Heralding Aggressive Langerhans Cell Sarcoma. Ann Hematol (2013) 92:419–20.
31. Sagransky MJ, Deng AC, Magro CM. Primary Cutaneous Langerhans Cell Sarcoma: A Report of Four Cases and Review of the Literature. Am J Dermatopathol (2013) 35:196–204. doi: 10.1097/DAD.0b013e3182661c0b
32. Chung WD, Im SA, Chung NG, Park GS. Langerhans Cell Sarcoma in Two Young Children: Imaging Findings on Initial Presentation and Recurrence. Korean J Radiol (2013) 14:520–4. doi: 10.3348/kjr.2013.14.3.520
33. Chen W, Jaffe R, Zhang L, Hill C, Block AM, Sait S, et al. Langerhans Cell Sarcoma Arising From Chronic Lymphocytic Lymphoma/Small Lymphocytic Leukemia: Lineage Analysis and BRAF V600E Mutation Study. N Am J Med Sci (2013) 5:386–91. doi: 10.4103/1947-2714.114172
34. West DS, Dogan A, Quint PS, Tricker-Klar ML, Porcher JC, Ketterling RP, et al. Clonally Related Follicular Lymphomas and Langerhans Cell Neoplasms: Expanding the Spectrum of Transdifferentiation. Am J Surg Pathol (2013) 37:978–86. doi: 10.1097/PAS.0b013e318283099f
35. Valentin-Nogueras SM, Seijo-Montes R, Montalvan-Miro E, Sanchez JL. Langerhans Cell Sarcoma: A Case Report. J Cutan Pathol (2013) 40:670–5. doi: 10.1111/cup.12113
36. Keklik M, Sivgin S, Kontas O, Abdulrezzak U, Kaynar L, Cetin M. Langerhans Cell Sarcoma of the Nasopharynx: A Rare Case. Scottish Med J (2013) 58:e17–20.
37. Lee JY, Jung KE, Kim HS, Lee JY, Kim HO, Park YM. Langerhans Cell Sarcoma: A Case Report and Review of the Literature. Int J Dermatol (2014) 53:e84–7.
38. Zwerdling T, Won E, Shane L, Javahara R, Jaffe R. Langerhans Cell Sarcoma: Case Report and Review of World Literature. J Pediatr Hematol Oncol (2014) 36:419–25. doi: 10.1097/MPH.0000000000000196
39. Chang NY, Wang J, Wen MC, Lee FY. Langerhans Cell Sarcoma in a Chronic Myelogenous Leukemia Patient Undergoing Imatinib Mesylate Therapy: A Case Study and Review of the Literature. Int J Surg Pathol (2014) 22:456–63. doi: 10.1177/1066896913501382
40. Liu DT, Friesenbichler J, Holzer LA, Liegl-Atzwanger B, Beham-Schmid C, Leithner A. Langerhans Cell Sarcoma: A Case Report and Review of the Literature. Polish J Pathol Off J Polish Soc Pathologists (2016) 67:172–8.
41. Zhang Y, Qu Z, Fang F. Langerhans Cell Sarcoma Originating From Left Knee Subcutaneous Tissue: A Case Report and Literature Review. Oncol Lett (2016) 12:3687–94.
42. Zhang L, Li X, Jiang M. Unusual Fever and Diarrhea in an Infant. Gastroenterology (2019) 156:e1–4.
43. Yi W, Chen W-Y, Yang T-X, Lan J-P, Liang W-N. Langerhans Cell Sarcoma Arising From Antecedent Langerhans Cell Histiocytosis: A Case Report. Medicine (2019) 98:e14531.
44. Tillit S, Carbajal-Mamani S, Zlotecki R, Yang L-J, Esnakula A, Jacqueline C, et al. Langerhans Cell Sarcoma of the Vulva: Case Report and Review of the Literature. Gynecol Oncol Rep (2020) 32:100570.
45. Nakamine H, Yamakawa M, Yoshino T, Fukumoto T, Enomoto Y, Matsumura I. Langerhans Cell Histiocytosis and Langerhans Cell Sarcoma: Current Understanding and Differential Diagnosis. J Clin Exp Hematop (2016) 56:109–18. doi: 10.3960/jslrt.56.109
46. Radzikowska E. Update on Pulmonary Langerhans Cell Histiocytosis. Front Med (Lausanne) (2020) 7:582581. doi: 10.3389/fmed.2020.582581
47. Liu H, Osterburg AR, Flury J, Swank Z, McGraw DW, Gupta N, et al. MAPK Mutations and Cigarette Smoke Promote the Pathogenesis of Pulmonary Langerhans Cell Histiocytosis. JCI Insight (2020) 5(4):e132048. doi: 10.1172/jci.insight.132048
48. Diaz-Sarrio C, Salvatella-Danes N, Castro-Forns M, Nadal A. Langerhans Cell Sarcoma in a Patient Who Underwent Transplantation. J Eur Acad Dermatol Venereol (2007) 21:973–6. doi: 10.1111/j.1468-3083.2007.02147.x
49. Muslimani A, Chisti MM, Blenc AM, Boxwala I, Micale MA, Jaiyesimi I. Langerhans/dendritic Cell Sarcoma Arising From Hairy Cell Leukemia: A Rare Phenomenon. Ann Hematol (2012) 91:1485–7. doi: 10.1007/s00277-011-1399-5
50. Furmanczyk PS, Lisle AE, Caldwell RB, Kraemer KG, Mercer SE, George E, et al. Langerhans Cell Sarcoma in a Patient With Hairy Cell Leukemia: Common Clonal Origin Indicated by Identical Immunoglobulin Gene Rearrangements. J Cutan Pathol (2012) 39:644–50. doi: 10.1111/j.1600-0560.2012.01873.x
51. Cunningham AL, Abendroth A, Jones C, Nasr N, Turville S. Viruses and Langerhans Cells. Immunol Cell Biol (2010) 88:416–23. doi: 10.1038/icb.2010.42
52. Berres ML, Merad M, Allen CE. Progress in Understanding the Pathogenesis of Langerhans Cell Histiocytosis: Back to Histiocytosis X? Br J Haematol (2015) 169:3–13. doi: 10.1111/bjh.13247
53. Khoddami M, Nadji SA, Dehghanian P, Vahdatinia M, Shamshiri AR. Detection of Epstein-Barr Virus DNA in Langerhans Cell Histiocytosis. Jundishapur J Microbiol (2015) 8:e27219. doi: 10.5812/jjm.27219
54. Csire M, Mikala G, Jákó J, Masszi T, Jánosi J, Dolgos J, et al. Persistent Long-Term Human Herpesvirus 6 (HHV-6) Infection in a Patient With Langerhans Cell Histiocytosis. Pathol Oncol Res (2007) 13:157–60. doi: 10.1007/BF02893493
55. Khoddami M, Nadji SA, Dehghanian P, Vahdatinia M, Shamshiri AR. Cytomegalovirus and Langerhans Cell Histiocytosis: Is There a Link? Iran J Pediatr (2016) 26:e673. doi: 10.5812/ijp.673
56. Khoddami M, Nadji SA, Dehghanian P. Herpes Simplex Virus and Langerhans Cell Histiocytosis. Iran J Pathol (2017) 12:323–8. doi: 10.30699/ijp.2017.27988
57. Murakami I, Wada N, Nakashima J, Iguchi M, Toi M, Hashida Y, et al. Merkel Cell Polyomavirus and Langerhans Cell Neoplasm. Cell Commun Signal (2018) 16:49. doi: 10.1186/s12964-018-0261-y
58. Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, Horie Y, et al. Merkel Cell Polyomavirus DNA Sequences in Peripheral Blood and Tissues From Patients With Langerhans Cell Histiocytosis. Hum Pathol (2014) 45:119–26. doi: 10.1016/j.humpath.2013.05.028
59. Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, Horie Y, et al. High Viral Load of Merkel Cell Polyomavirus DNA Sequences in Langerhans Cell Sarcoma Tissues. Infect Agent Cancer (2014) 9:15. doi: 10.1186/1750-9378-9-15
60. Sakata N, Toguchi N, Kimura M, Nakayama M, Kawa K, Takemura T. Development of Langerhans Cell Histiocytosis Associated With Chronic Active Epstein-Barr Virus Infection. Pediatr Blood Cancer (2008) 50:924–7. doi: 10.1002/pbc.21249
61. Munkhdelger J, Vatanasapt P, Pientong C, Keelawat S, Bychkov A. Epstein-Barr Virus-Associated Langerhans Cell Histiocytosis of the Thyroid Gland. Head Neck Pathol (2020) 15:1054–58. doi: 10.1007/s12105-020-01247-8
62. Walling DM, Ray AJ, Nichols JE, Flaitz CM, Nichols CM. Epstein-Barr Virus Infection of Langerhans Cell Precursors as a Mechanism of Oral Epithelial Entry, Persistence, and Reactivation. J Virol (2007) 81:7249–68. doi: 10.1128/JVI.02754-06
63. Tugizov S, Herrera R, Veluppillai P, Greenspan J, Greenspan D, Palefsky JM. Epstein-Barr Virus (EBV)-Infected Monocytes Facilitate Dissemination of EBV Within the Oral Mucosal Epithelium. J Virol (2007) 81:5484–96. doi: 10.1128/JVI.00171-07
64. Benharroch D, Guterman G, Levy I, Shaco-Levy R. High Content of Langerhans Cells in Malignant Lymphoma–Incidence and Significance. Virchows Arch (2010) 457:63–7. doi: 10.1007/s00428-010-0931-7
65. Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The Immunology of Epstein-Barr Virus-Induced Disease. Annu Rev Immunol (2015) 33:787–821. doi: 10.1146/annurev-immunol-032414-112326
66. Rasche L, Kapp M, Einsele H, Mielke S. EBV-Induced Post Transplant Lymphoproliferative Disorders: A Persisting Challenge in Allogeneic Hematopoetic SCT. Bone Marrow Transplant (2014) 49:163–7. doi: 10.1038/bmt.2013.96
67. Ong KW, Teo M, Lee V, Ong D, Lee A, Tan CS, et al. Expression of EBV Latent Antigens, Mammalian Target of Rapamycin, and Tumor Suppression Genes in EBV-Positive Smooth Muscle Tumors: Clinical and Therapeutic Implications. Clin Cancer Res (2009) 15:5350–8. doi: 10.1158/1078-0432.CCR-08-2979
68. Kakiuchi S, Yakushijin K, Takagi I, Rikitake J, Akiyama H, Matsuba H, et al. Case Report: Composite Angioimmunoblastic T-Cell Lymphoma and Epstein-Barr Virus-Positive B-Cell Lymphoproliferative Disorder as Other Iatrogenic Immunodeficiency-Associated Lymphoproliferative Disorders. Front Med (Lausanne) (2020) 7:625442. doi: 10.3389/fmed.2020.625442
69. Sahm F, Capper D, Preusser M, Meyer J, Stenzinger A, Lasitschka F, et al. BRAFV600E Mutant Protein Is Expressed in Cells of Variable Maturation in Langerhans Cell Histiocytosis. Blood (2012) 120:e28–34. doi: 10.1182/blood-2012-06-429597
Keywords: Langerhans cell sarcoma, EBV, immunosuppression, cervical giant cyst, CD56
Citation: Guo Y, Zhou S-H, Cao Z-Z, Bao Y-Y, Shen L-F and Yao H-T (2022) Epstein–Barr Virus-Positive Langerhans Cell Sarcoma: Is There a Link? A Case Report. Front. Oncol. 11:769310. doi: 10.3389/fonc.2021.769310
Received: 01 September 2021; Accepted: 20 December 2021;
Published: 18 January 2022.
Edited by:
Mohamed A. Yassin, Hamad Medical Corporation, QatarReviewed by:
Marco Lucioni, University of Pavia, ItalyMaria Helena Ornellas, Universidade Estadual do Rio de Janeiro, Brazil
Copyright © 2022 Guo, Zhou, Cao, Bao, Shen and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shui-Hong Zhou, MTE5MDA1MUB6anUuZWR1LmNu
 Yu Guo
Yu Guo Shui-Hong Zhou
Shui-Hong Zhou Zai-Zai Cao1
Zai-Zai Cao1