
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 November 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.768035
Objectives: A new patient assistance program (PAP) for pembrolizumab was started in China in 2021. The researchers aimed to evaluate the economic outcomes of pembrolizumab plus pemetrexed and platinum versus chemotherapy alone in the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, based on the pricing mechanism of PAP.
Material and Methods: Survival analysis and partitioned survival model were performed to evaluate the incremental cost-effectiveness ratio (ICER) in the pembrolizumab group compared with the chemotherapy group. Survival probabilities were extracted from the data of the KEYNOTE-189 trial. Cost and utility data were gathered from published literature. The pricing mechanism of PAP was set in each cycle in the partitioned survival model, according to the progression-free survival (PFS) data of the KEYNOTE-189 trial, which included PFS-1 and PFS-2. Deterministic sensitivity analysis and probabilistic sensitivity analysis were conducted.
Results: The ICER of the pembrolizumab group versus chemotherapy group was $65,272/quality-adjusted life year (QALY), which still exceeded the willingness-to-pay threshold of three times per capita gross domestic product (GDP) of China ($33,581.22), although PAP was calculated. Sensitivity analysis implied that the price of chemotherapeutic drugs combined with pembrolizumab was one of the main influencing factors of ICER.
Conclusions: Due to various prices set by PAP and the payment for combined chemotherapy, the economic advantage of pembrolizumab plus chemotherapy in the first-line treatment of non-small cell lung cancer (NSCLC) is still not achieved in China.
Lung cancer is currently one of the most malignant tumors with the highest incidence and mortality worldwide. In 2018, the number of new cases of lung cancer was 2.094 million, accounting for 11.58% of new cases of tumors. The number of deaths due to lung cancer was 1.761 million, accounting for 18.43% of global tumor deaths (1). In China, there were 0.787 million new cases of lung cancer in 2015 with 0.52 million males and 0.267 million females, and 0.631 million deaths, based on the newly updated data of National Cancer Center in 2019 (2).
Lung cancer includes small-cell lung cancer (SCLC) and non-small cell lung cancer. Majority of lung cancers are non-small cell lung cancer, which accounts for approximately 85%–90% of all lung cancers (3). In recent years, the emergence of immunotherapy has given hope of longer survival to advanced patients, and has also increased the possibility of cure inpatients at an early stage. For non-small cell lung cancer (NSCLC), PD-1 and PD-L1 inhibitors are mainly used in clinical practice, which block the interaction of PD-1 and PD-L1, activate the immune system, and prevent tumor immune escape. In China, pembrolizumab, a representative PD-1 inhibitor, is gradually being approved for more indications. On April 2, 2019, the National Medical Products Administration (NMPA) of the People’s Republic of China approved pembrolizumab combined with pemetrexed and cisplatin chemotherapy as first-line treatment of metastatic non-squamous NSCLC, without sensitizing EGFR or ALK mutations. On September 30, 2019, NMPA approved pembrolizumab as a single agent for the first-line treatment of locally advanced or metastatic NSCLC with PD-L1 TPS ≥ 1%, without sensitizing EGFR or ALK mutations. On November 25, 2019, NMPA approved pembrolizumab combined with paclitaxel or paclitaxel (albumin-bound) and carboplatin chemotherapy for the first-line treatment of metastatic squamous NSCLC (4). With the approval of more indications of pembrolizumab, an increasing number of patients are receiving pembrolizumab treatment. Although patients’ lives are prolonged, the expensive price of immunotherapy also places a heavy economic burden. The pharmacoeconomic analysis of pembrolizumab and chemotherapy first-line combination treatment in Chinese patients conducted by Wan and Jiang showed that the incremental cost-effectiveness ratio (ICER) values were $92,533/quality-adjusted life-year (QALY) and $96,644/QALY, respectively (5, 6), which exceeded three times the per capita gross domestic product (GDP) of $33,581.22 in China in 2020 (7).
In December 2020, the National Healthcare Security Administration and the Ministry of Human Resources and Social Security of the People’s Republic of China started a new round of price negotiations on the access to innovative medicines. Although pembrolizumab sustained its original price, a new patient assistance program (PAP) started under the circumstance of fierce price competition from other PD-1 inhibitors. The purpose of the present study was to explore the construction of a pharmacoeconomic model, considering the cost parameters influenced by PAP, and evaluate the change in ICER for patients.
This study was based on a global, double-blind, placebo-controlled phase 3 clinical trial (KEYNOTE-189). The trial aimed to compare the combination of pemetrexed and a platinum-based drug plus either pembrolizumab or placebo as first-line treatment in patients with non-squamous NSCLC (8).
WebPlotDigitizer (https://apps.automeris.io/wpd/index.zh_CN.html) was used to extract the data of the overall survival (OS) and progression-free survival (PFS) curves in the KEYNOTE-189 trial. Based on the OS and PFS data, the survHE package of R (V4.0.3) was used to fit and extrapolate the PFS and OS curves. According to the Akaike information criterion (AIC) and Bayesian information criterion (BIC), combined with visual inspection, log-logistic distribution for OS curve (shape=1.351,7, scale=22.160,0, AIC=1,925.219, BIC=1,933.251) and PFS curve (shape=1.532,8, scale=9.378,1, AIC=2,248.140, BIC=2,256.173) in the pembrolizumab group; log-logistic distribution for OS curve (shape=1.422,9, scale=11.754,6, AIC=1,142.539, BIC=1,149.194), and the gengamma distribution for PFS curve (mu=1.738,0, sigma=0.875,6, Q=0.325,3, AIC= 1,088.212, BIC=1,098.196) in the placebo group were selected (Figure 1) (see Supplementary Material) (9).
The Heemod package of R was used to construct the partitioned survival model. Three states were included: progression-free survival, progressive disease (PD), and death (Figure 2). Each cycle was set to 3 weeks in this model, according to the medication plan of the KEYNOTE-189 trial. A total of 10 years was simulated, which nearly reached to a lifetime, considering the poor prognosis of patients with metastatic NSCLC. The main outcomes were cost and QALYs, which were discounted at a rate of 5% (10), and three times per capita GDP of 2020 in China was selected as the threshold for ICER. Meanwhile, cost and health outcome data were subjected to half-cycle correction.
This analysis adopted a health care perspective in China, which only analyzed direct medical costs. It included the cost of the drug, management of serious adverse events, medical services, and follow-up. The drug prices were derived from the median value of winning bid prices in Chinese provinces, and the cost of medical service and follow-up were derived from the median value of cost in tertiary medical institutions in sample areas (11), and other costs were derived from published literature (Table 1).
All the costs were converted into United States dollars using the exchange rate: 1 US dollar = 6.4721 Chinese yuan renminbi.
Two phases of the medication plans were involved on the KEYNOTE-189 trial (see Supplementary Material). Thus, time-dependent cost parameters were considered in the R program. In the first phase, patients were assigned to receive 200 mg pembrolizumab plus pemetrexed (870mg) and carboplatin (511.87mg) every 3 weeks for 4 cycles, followed by pembrolizumab and pemetrexed for up to 35 cycles, followed by pemetrexed maintenance therapy until disease progression in the pembrolizumab group. The original pembrolizumab price was $2,768.50/100mg (11). Patients may participate in the patient assistance program in China. According to the newest PAP (program name: KEY TO LIFE) of pembrolizumab in 2021, patients who bought the first two cycles of pembrolizumab would receive the next two cycles for free, continuing to buy the fifth and sixth cycles of pembrolizumab; next cycles remaining for up to 2 years (35 cycles) would also be free. Considering PAP, the price of pembrolizumab was different due to patient survival time. If pembrolizumab was more effective for patients, the price would be lower in each cycle. In the KEYNOTE-189 trial, the median PFS was 9.0months, which was equivalent to 12 cycles. After PAP, the average price of patients with PFS in the first cycle was set as follows:
(1)
(2)
(3)
(4)
(5)
If the patient used pembrolizumab for two years, which was equivalent to 35 cycles. After PAP, the average price of patients with PFS in the 35th cycle was set as follows:
(6)
(7)
(8)
(9)
(10)
Thus, during the 35 cycles, the price of pembrolizumab in each cycle was time-dependent and was set as follows:
(11)
Markov_cycle was internal cycle counter of R language.
Patients were assigned to receive saline placebo plus pemetrexed and carboplatin for 4 cycles, followed by saline placebo and pemetrexed maintenance therapy until disease progression in the placebo group.
After disease progression, 44.6% of patients received ≥1 subsequent therapy in the pembrolizumab group. Among these patients, 13.4% received ≥1 subsequent PD-1 or PD-L1 inhibitor, according to the KEYNOTE-189 trial. Based on this information, 13.4% of patients were assumed to choose nivolumab (185.58mg every 2 weeks), and 31.2% of patients were assumed to choose docetaxel (104.4mg every 3 weeks) (13). Meanwhile, in the placebo group, 59.2% of patients received ≥1 subsequent therapy. Among these patients, 53.9% received ≥1 subsequent PD-1 or PD-L1 inhibitors. In patients receiving PD-1 or PD-L1 inhibitors, 40.8% of the patients were crossed over to the trial group. Thus, 5.3% of patients were assumed to choose docetaxel, 13.1% were assumed to choose nivolumab, and 40.8% patients were crossed over to use pembrolizumab in this study. PFS-2, which was defined as “the time from randomization to objective tumor progression on next-line treatment or death from any cause” in the KEYNOTE-189 trial, showed that the median PFS was 4.9 months in the placebo group. Thus, after about 4.1 months, patients underwent another progression (8), and the lower limiting value - 2.9 months, about 4 cycles were included in the analysis. Considering PAP, the average price of patients in the first cycle from PFS-1 to PFS-2 in the placebo group was set as follows:
(12)
(13)
(14)
(15)
(16)
Due to the short period from PFS-1 to PFS-2 in the placebo group, the price of pembrolizumab in the following cycles was assumed to maintain the price of the first cycle.
(17)
Adverse events that were ≥3 grade and occurred in ≥3% of patients were analyzed, including nausea, anemia, fatigue, diarrhea, neutropenia, vomiting, dyspnea, asthenia, and thrombocytopenia. These adverse events were assumed to occur in the first cycle, because oncologists might change the anticancer drug if ≥3 grade adverse events frequently occur (11). Thus, adverse events were summed to the total cost only once in this study (Table 2).
The costs of medical services included diagnostic fees, intravenous injection fees, nursing fees, and hospitalization fees. Follow-up items included CT, urine tests, blood tests, and blood biochemistry (11).
The utility of PFS was set to 0.804, and the utility of PD was set to 0.321, according to a health utility study in Chinese NSCLC patients by Nafees (14).
To evaluate the stability of the results, deterministic sensitivity analyses and probabilistic sensitivity analyses were performed. DSA was conducted for all the parameters. A 25% change range was assumed for the parameters of drug price, medical service cost, and follow-up cost (18). A 15% change range was assumed for the parameters of PFS and PD utility (11), and a 0%–8% was assumed for the discount rate (10). In PSA, a Monte Carlo simulation of 10,000 iterations was performed for more stable result. The log-normal distribution was selected for the cost parameters, and the beta distribution was selected for utility parameters (19).
In the base-case analysis, the average cost-effectiveness ratios (ACER) of patients in the pembrolizumab group and placebo group were $71,494.32/QALY, $76,734.17/QALY, respectively. Patients in the pembrolizumab group obtained an additional 0.64 QALYs, but needed to pay an extra $41,774.08. The incremental cost-effectiveness ratio was $65,272/QALY. The value of GDP per capita was $11,193.74 in China in 2020 (7). Even though PAP was considered, the ICER remained more than the threshold of three times the GDP per capita ($33,581.22).Considering net benefit, the net monetary benefits (NMB) of patients in the pembrolizumab group and placebo group were $ -53,078.3 and $ -32,796.2, respectively (Table 3).
The results of the deterministic sensitivity analyses were presented in a Tornado diagram, which indicated that the price of pemetrexed, utility of PFS, and discount rate were the main influencing factors of ICER. Cost-effectiveness acceptability curve was generated to show the probabilities of cost-effectiveness, which revealed that the pembrolizumab strategy was not cost-effective compared with the placebo strategy at the threshold of $33,581.22/QALY (Figure 3).
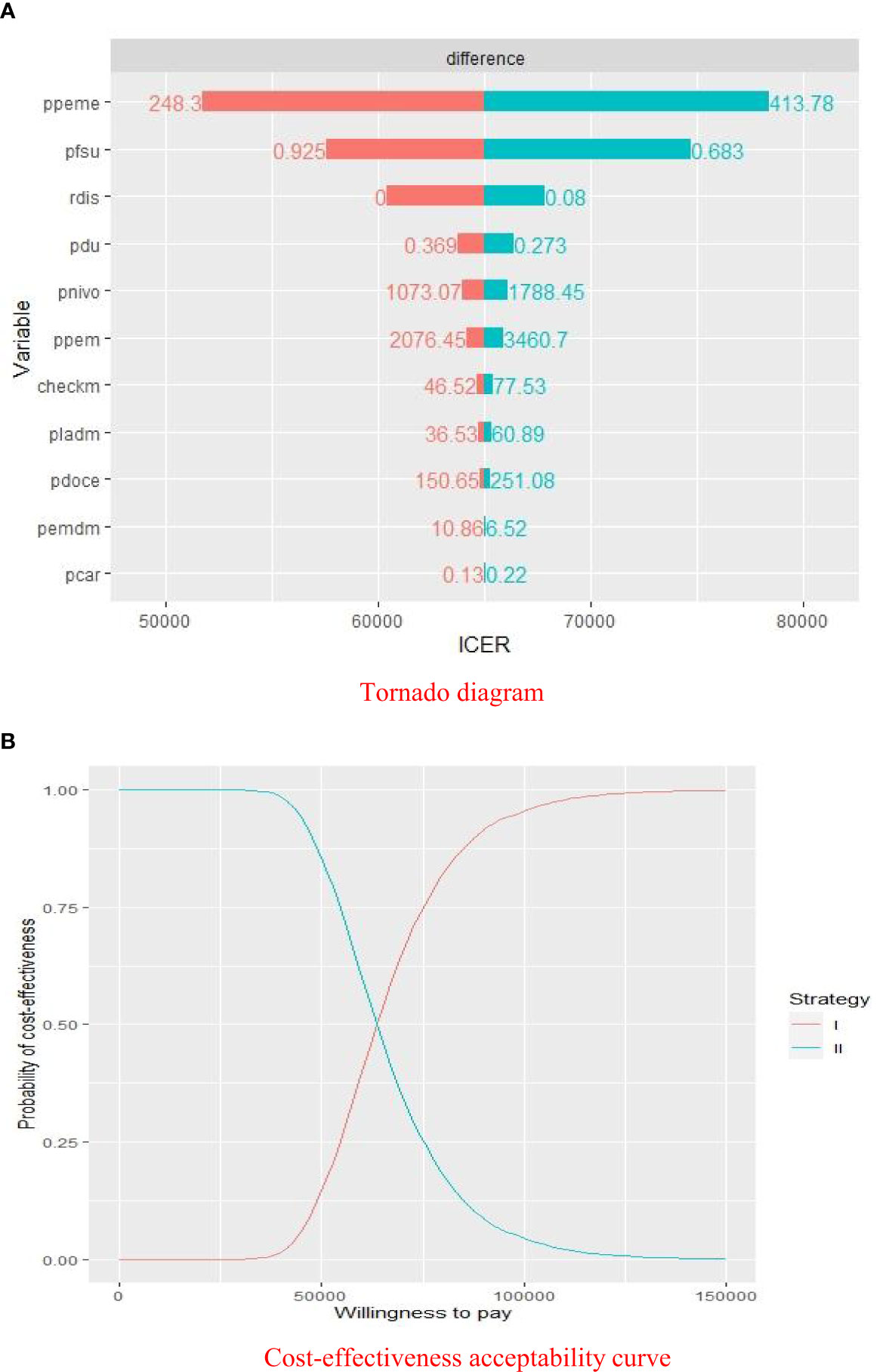
Figure 3 Sensitivity analyses of pembrolizumab group versus placebo group. (A) Deterministic sensitivity analysis of two groups. (B) Probabilistic sensitivity analysis of two groups. ICER, $/QALY; ppeme, price of pemetrexed; pfsu, utility of PFS; rdis, discount rate; pdu, utility of PD; pnivo, price of nivolumab; ppem, price of pembrolizumab; checkm, cost of imaging examination and laboratory test per cycle; pladm, cost of medical service of chemotherapy per cycle; pdoce, price of docetaxel; pemdm, cost of medical service of pembrolizumab therapy per cycle; pcar, price of carboplatin; Willingness to pay, $/QALY; strategy I, pembrolizumab plus pemetrexed and platinum; strategy II, chemotherapy alone.
In December 2020, a new round of adjustment of the National Basic Medical Care Insurance Medicine Catalog was carried out in China. Pharmacoeconomics was applied to the price negotiation of innovative medicines during the adjustment of the medical insurance catalog. In the negotiation process, main factors such as adverse events, international prices, and competitiveness were considered. Finally, three types of PD-1 inhibitors, including Tislelizumab, Toripalimab, and camrelizumab, entered the new medical insurance catalog through price cuts. The prices of these three PD-1 inhibitors were reduced to $336.83 (100mg), $324.62 (240mg), and $452.40 (200mg), respectively, lower than 30% of their original price (20). The price-cut strategy was not used for pembrolizumab to maintain the global pricing system, which resulted in the failure to enter the medical insurance catalog. Instead, KEY TO LIFE, a competitive patient assistance program, started in 2021, which reduced the patients’ payments to some extent. However, the new PAP could not guarantee every patient to obtain equal price concessions, which led to the persistence of the economic burden. Our results revealed that the ICER was $65,272/QALY, which was significantly lower than the value reported by Wan and Jiang (5, 6), but still exceeded three times per capita GDP.
To the researchers’ knowledge, this study was the first to discuss the pricing mechanism of PAP in a partitioned survival model of NSCLC. If patients obtained a better treatment effect, the price of pembrolizumab would be much lower. In this study, according to the PFS-1 and PFS-2 in the KEYNOTE-189 trial, the pricing mechanism of PAP was coded in the R language, and a time-dependent pricing system for pembrolizumab in the partitioned survival model was established, which made the cost in each cycle more precise.
Although transnational pharmaceutical companies achieved a delicate balance between maintaining global pricing strategies and a competitive price in China through PAP, the pricing mechanism of PAP sets a threshold cost for patients, which would affect the risk averters’ initial choice of pembrolizumab. An alternative incentive method should be canceling the threshold cost (the first four cycles), and let patients pay the “effective part”. This new strategy would enable the company to obtain a balance between public welfare and profit, which also depends on the exploration and discovery of more precise prediction markers for treatment efficacy. A new PAP should be established for the following cycles to reduce the economic burden. In addition, installment or return of medical expenses to patients owing to ineffective treatment are other strategies employed for pharmaceutical companies, which were already adopted such as GlaxoSmithKline’ Strimvelis and Spark Therapeutics’ Luxturna.
In the long run, price cuts remain an important pathway to gain more market share from developing countries, especially China, which could reduce the transaction costs for transnational pharmaceutical companies, such as finding transaction partners (hospital, etc.), promoting, establishing contract relationships, and fulfilling contracts. Meanwhile, this could also be a trend to occupy the market through a price cut strategy, with the emergence of an increasing number of competitors in the global market, and increasing pressure on medical insurance payment control from an increasing number of countries.
Deterministic sensitivity analyses showed that the prices of other chemotherapeutic drugs used in combination with pembrolizumab were the important influencing factors of ICER, which suggested the possibility of further reducing the economic burden on patients. In addition, with the success of the KEYNOTE-042 trial, pembrolizumab as first-line monotherapy has been an important choice for patients with NSCLC (21). Recommendations have been made in the latest “Clinical practice guideline for stage IV primary lung cancer in China (2021 version)”: pembrolizumab plus platinum-containing chemotherapy is recommended; pembrolizumab as the monotherapy can also be chosen for patients with PD-L1 scores of 1-49% (4).”However, in the latest 2021 ASCO Annual Meeting, a research by Akinboro showed that patients with PD-L1 scores 1-49% receiving chemotherapy and immunotherapy had longer PFS and OS compared to patients treated with immunotherapy alone, with median PFS 7.7 vs 4.2 months and median OS 21.4 vs 14.5 months (22). Thus, for oncologists, decision making between monotherapy and combination therapy requires further supportive evidence from clinical trials.
This study has two limitations. First, in the KEYNOTE-189 trial, the patient’s overall survival time and progression-free survival time were the results of following the trial protocol. Therefore, the trial design was followed as much as possible in the analysis of cost in our study, especially at the time of disease progression, which might be different from oncologists’ real clinical decisions. Second, since pembrolizumab has been approved in the Chinese market for a short time, there remains a lack of evidence from a real-world study to further verify the long-term cost and effectiveness of pembrolizumab in patients in this study. Recently, a retrospective cohort study of nearly 20,000 medical insurance patients found that the survival benefit of using immune checkpoint inhibitors in the first-line treatment of NSCLC in the real world was much lower than that of clinical trials (23).
Considering the background of fierce competition in the Chinese immunotherapy market, a new PAP of pembrolizumab started in 2021, which reduced patients’ economic burden. However, due to various prices set by PAP and the payment allocated for combined chemotherapy, the economic advantage of pembrolizumab plus chemotherapy in the first-line treatment of NSCLC was not achieved.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YC and HW studied the concept and made the whole design. YC, MZ, and MYZ collected the data from the literature. ZG gave the specific explanation about the use of anticancer drug. YC and WH established the model and conducted the analysis of data. YC were the main drafter of the manuscript. All authors contributed to the article and approved the submitted version.
The Educational Department of Liaoning Province Funding Project (No. QNRW2020007). The funding source had no role in the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.768035 /full#supplementary-material
PAP, Patient assistance program; ICER, Incremental cost-effectiveness ratio; PFS, Progression-free survival; QALY, Quality-adjusted life year; GDP, Gross domestic product; NSCLC, Non-small-cell lung cancer; SCLC, Small-cell lung cancer; NMPA, National Medical Products Administration; OS, Overall-survival; AIC, Akaike information criterion; BIC, Bayesian information criterion; PD, Progressive disease; DSA, Deterministic sensitivity analyses; PSA, Probabilistic sensitivity analyses; ACER, Average cost-effectiveness ratio; NMB, Net monetary benefit.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of Cancer Epidemiology in China, 2015. Chin J Oncol (2019) 41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005
3. Wu B, Gu XH, Zhang Q. Cost-Effectiveness of Osimertinib for EGFR Mutation-Positive Non-Small Cell Lung Cancer After Progression Following First-Line EGFR TKI Therapy. J Thorac Oncol (2018) 13:184–93. doi: 10.1016/j.jtho.2017.10.012
4. Chinese Association for Clinical Oncologists and Medical Oncology Branch of Chinese International Exchange and Promotion Association for Medical and Healthcare. Clinical Practice Guideline for Stage IV Primary Lung Cancer in China (2021 Version). Chin J Oncol (2021) 43:39–59. doi: 10.3760/cma.j.cn112152-20201009-00884
5. Wan N, Zhang TT, Hua SH, Lu ZL, Ji B, Li LX, et al. Cost-Effectiveness Analysis of Pembrolizumab Plus Chemotherapy With PD-L1 Test for the First-Line Treatment of NSCLC. Cancer Med (2020) 9:1683–93. doi: 10.1002/cam4.2793
6. Jiang Y, Wang XW. Cost-Effectiveness Analysis of Pembrolizumab Plus Standard Chemotherapy Versus Chemotherapy Alone for First-Line Treatment of Metastatic Non-Squamous Non–Small-Cell Lung Cancer in China. Eur J Hosp Pharm (2020) 0:1–6. doi: 10.1136/ejhpharm-2020-002208
7. National Bureau of Statistics of the People’s Republic of China. Statistical Bulletin of the National Economy and Social Development in 2020 . Available at: http://www.stats.gov.cn/tjsj/zxfb/202102 /t20210227_1814154.html (Accessed April 8, 2021).
8. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38:1505–17. doi: 10.1200/JCO.19.03136
9. Guyot P, Ades AE, Ouwens MJ, Welton N. Enhanced Secondary Analysis of Survival Data: Reconstructing the Data From Published Kaplan-Meier Survival Curves. BMC Med Res Methodol (2012) 12:9. doi: 10.1186/1471-2288-12-9
10. Liu GE, Hu SL, Wu JH, Wu J, Dong ZH, Li HC, et al. China Guidelines for Pharmacoeconomic Evaluations (2020 Version). Beijing: China Market Press (2020). p. 27.
11. Xu H, Ma AX. Cost-Effectiveness Analysis of Pembrolizumab Versus Chemotherapy as First-Line Treatment in Non-Small Cell Lung Cancer With Different PD-L1 Expression Levels Based on Partitioned Survival Model. Chin J Hosp Pharm (2020) 40:1–8. doi: 10.13286/j.1001-5213.2020.23.14
12. Zheng DN, Zhou HF, Zheng HR. Cost-Effectiveness of Nivolumab for Recurrent NSCLC Progressing During or After Previous Platinum Failure, and Tumor PD-L1 Expression ( > 1%). Cent South Pharm (2021) 19:153–7. doi: 10.7539/j.issn.1672-2981.2021.01.030
13. Xu H, Ma AX. Cost-Effectiveness Analysis of Pembrolizumab Versus Chemotherapy as First-Line Treatment in non-Small Cell Lung Cancer in China Based on Markov Model. Chin J Mod Appl Pharm (2021) 38:473–9. doi: 10.13748/j.cnki.issn1007-7693.2021.04.016
14. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health State Utilities in Non-Small Cell Lung Cancer: An International Study. Asia Pac J Clin Oncol (2017) 13:e195–203. doi: 10.1111/ajco.12477
15. Shi X, Zhu QY. Cost-Utility Analysis of Afatinib and Gefitinib in First-Line Treatment of EGFR Mutation-Positive Non-Small Cell Lung Cancer. Chin J Mod Appl Pharm (2019) 36:2701–6. doi: 10.13748/j.cnki.issn1007-7693.2019.21.013
16. Guan HJ, Liu G, Xie F, Sheng YN, Shi LW. Cost-Effectiveness of Osimertinib as a Second-Line Treatment in Patients With EGFR-Mutated Advanced Non-Small Cell Lung Cancer in China. Clin Ther (2019) 41:2308–2320.e11. doi: 10.1016/j.clinthera.2019.09.008
17. Zhang SZ, Yu JJ, Liu JH, Li YP, Zhang WD, Xu T, et al. Drug Usage and Costs of Pneumonia Inpatients in Karamay Central Hospital in 2014. Chin J Evid-Based Med (2017) 17:880–3. doi: 10.7507/1672-2531.201608043
18. Wu B, Zhang Q, Sun J. Cost-Effectiveness of Nivolumab Plus Ipilimumab as First-Line Therapy in Advanced Renal-Cell Carcinoma. J Immunother Cancer (2018) 6:124. doi: 10.1186/s40425-018-0440-9
19. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD, et al. Model Parameter Estimation and Uncertainty Analysis: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making (2012) 32:722–32. doi: 10.1177/0272989X12458348
20. Li XX. The Prices of Three Anticancer Drugs Decreased in the New National Basic Medical Care Insurance Medicine Catalogue, and the Largest Drop was Up to 85%. Available at: https://www.thepaper.cn/newsDetail_forward_11505337 (Accessed April 22, 2021).
21. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
22. Akinboro O, Vallejo JJ, Mishra-Kalyani PS, Larkins EA, Drezner NL, Tang SH, et al. Outcomes of Anti-PD-(L1) Therapy in Combination With Chemotherapy Versus Immunotherapy (IO) Alone for First-Line (1L) Treatment of Advanced non-Small Cell Lung Cancer (NSCLC) With PD-L1 Score 1-49%: FDA Pooled Analysis. Available at: https://meetings.asco.org/abstracts-presentations/196990 (Accessed June 29, 2021).
Keywords: non-small cell lung cancer, pembrolizumab, cost-effectiveness analysis, partitioned survival model, patient assistance program
Citation: Cai Y, Hui W, Zhu M, Zhang M, Gao Z and Wu H (2021) Cost-Effectiveness Analysis of Pembrolizumab Plus Pemetrexed and Platinum Versus Chemotherapy Alone as First-Line Treatment in Metastatic Non-Squamous Non–Small Cell Lung Cancer: A Reconstruction of Partitioned Survival Model Based on Time Dependent Pricing Mechanism of Patient Assistance Program. Front. Oncol. 11:768035. doi: 10.3389/fonc.2021.768035
Received: 31 August 2021; Accepted: 08 November 2021;
Published: 26 November 2021.
Edited by:
Mohamed Rahouma, Weill Cornell Medical Center, United StatesReviewed by:
Longfeng Zhang, Fujian Provincial Cancer Hospital, ChinaCopyright © 2021 Cai, Hui, Zhu, Zhang, Gao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huazhang Wu, aHp3dUBjbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.