
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 28 January 2022
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.766148
Background: Lung adenocarcinoma can transform into small-cell lung cancer (SCLC) when resistance to tyrosine kinase inhibitors (TKIs) develops. Approximately 3% to 10% of epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) could transform to SCLC. This phenomenon has been described in several case reports and small patient series. However, the characteristics and treatment outcomes of this population have not been comprehensively reported, and their clinical course is poorly characterized.
Methods: We performed a systematic review of the published literature to summarize the clinical and pathological features and prognosis of the reported cases and analyzed the demographics, disease features, and outcomes.
Results: A total of 72 patients (50 females and 22 males) initially diagnosed with lung adenocarcinoma were included. EGFR mutations included 19-deletion (75%), L858R (22%), and G719X (3%). All patients received EGFR-TKIs before SCLC transformation. The median time from diagnosis to transformation was 20.5 months (95% CI, 15.45 to 26.55 months). Of the 67 patients with post-translational gene test results, 58 maintained their EGFR mutation, and only 1 of 18 with prior T790M positivity retained T790M mutation. After the pathological transformation, both conventional chemotherapy regimen and chemotherapy combined targeted therapy yielded high response rates. The disease control rate of first-line therapy after transformation was 76%, while the objective response rate was 48%. The median overall survival (OS) since diagnosis was 27 months (95% CI, 22.90 to 31.10 months), whereas median OS since SCLC transformation was 8.5 months (95% CI, 5.50 to 11.60 months).
Conclusion: The prognosis of transformed SCLC is worse than primary SCLC. The response rate to conventional chemotherapy was high. However, the progression-free survival and OS after transformation were short and the prognosis was poor with first-line therapies. New therapies are needed in the management of transformed SCLC.
Lung cancer (LC) is the leading cause of cancer-related death. The 2020 Globocan project (1) reported an estimated 2.2 million new cases of LC globally. In the past decades, two broad histological subtypes of LC have been recognized, non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). These two subtypes are commonly reckoned as different diseases due to their distinct biology and genomic characteristics. NSCLC represents about 85% of all cases, arising from the respiratory epithelium, and is further divided into adenocarcinoma and squamous-cell carcinoma. SCLC, accounting for the remaining 15% of cases, is a highly aggressive tumor of neuroendocrine origin, which is characterized by rapid disease progression and early development of metastases (2–4). However, the concept of small cell transformation (SCT) links them together. Histological transformation of epidermal growth factor receptor (EGFR) mutant lung adenocarcinoma (LADC) into SCLC was first reported in 2006 (5).
With the discovery of a series of LC-driven genes, many studies in China and worldwide have shown that targeted therapeutic drugs greatly improve and prolong the prognosis and survival of patients with NSCLC carrying corresponding driven genes (6–10). Forty percent to 50% of patients with LADC have EGFR-sensitive mutations (3). The only two common activating and sensitizing EGFR mutations included in prospective clinical trials are 19-deletion (19-del) and exon 21-L858R (21-L858R) point mutations. Ten percent to 20% of patients with NSCLC harbor uncommon EGFR mutations that have variable sensitivity to different EGFR TKIs. However, progression of the disease is inevitable after a median time of approximately 9.0–12.0 months (11, 12). The main resistance mechanism of EGFR-TKIs is the emergence of secondary EGFR-T790M mutations. Among the first-/second-generation EGFR-TKIs-resistant patients, 50% of patients have secondary EGFR-T790M mutations (13–15). Repeated biopsy cohort studies have shown that approximately 3%–10% of acquired EGFR-TKIs resistance is related to histological transformation to SCLC (16, 17).
The mechanism of SCLC transformation remains unclear. Several studies have shown that alveolar type II cells may be common precursors of both LADC and SCLC (4, 18, 19). LADC arising from alveolar type II cells and harboring EGFR mutations might trans-differentiate to SCLC under the selective pressure of TKI therapy. Genomic sequencing of tumor samples from repeated biopsies of LADC progressing on TKIs revealed that most SCLC retained the same EGFR mutation type of the LADC counterpart (20). Only a few case reports and small series have been published due to the rarity of this phenomenon. In 2017, Roca et al. (21) reviewed the literature of reported cases for SCLC transformation. However, the study did not strictly select the criteria for inclusion, and the number of transformation cases was only 39.
In this study, we once again reviewed the literature of all reported cases of SCLC diagnosed in patients treated with TKIs for EGFR-mutated LADC. Excluded cases initially mixed with SCLC components and ineffective TKIs treatment. The aim was to obtain explorative information on the clinical and pathological characteristics and the prognosis of the identified patients with a transformed SCLC phenotype.
JX and LX respectively systematically searched the literature using PubMed/Medline (US National Library of Medicine National Institutes of Health) and EMBASE (2006 to present) with the following keywords: “transformation from NSCLC to SCLC”, “NSCLC transformation in SCLC”, “resistance to TKIs”, and “TKIs treatment”. Search results were limited to human studies in English. A manual review of reference lists in relevant publications was also carried out to identify additional articles. Conversely, abstracts from scientific meetings were not included. For duplicated publications, we selected the most recent version. The PRISMA flow diagram showing the selection process for this systematic review is depicted in Figure 1.
Inclusion criteria: (1) patients with LADC diagnosed with EGFR mutation; (2) a prior history of using EGFR-TKIs treatment before the transformation of LADC, including the first/second/third generation; (3) EGFR-TKIs was used for at least 3 months and the efficacy evaluation was at least stable disease (SD); and (4) SCLC should be diagnosed in high-quality tumor biopsies or well-preserved cytological samples according to 2015 WHO classification (22).
Data that were collected included demographic information, tumor histology, molecular pathology, clinical treatments, and outcomes of all published patients that were extracted from the full-length articles by JX and LX. Data were included in a specific database and analyzed as a case series. We defined the time to SCLC transformation (ttSCLC) as the time from the initial pathological diagnosis of LADC to the additional biopsy revealing the metachronous SCLC phenotype. We defined the T-ttSCLC as the time from the initial TKIs usage to the additional biopsy revealing the metachronous SCLC phenotype. Disease control rate (DCR) was defined as the percentage of patients with complete response or partial response or stable disease.
Survival curves were illustrated using the Kaplan–Meier method and compared with the log-rank test. Single-factor exploratory analyses were performed using Cox proportional hazards regression model to test the prognostic value of patient and tumor characteristics [hazard ratios (HR) and 95% confidence intervals (CIs)] for overall survival (OS). p-values <0.05 (two-sided) were considered statistically significant. Kaplan–Meier survival curve chart was used to assess the validity of the proportional hazard assumptions. The primary end point of first-line treatment after transfer was objective response rate (ORR) by independent radiology assessment. ORR was defined as the percentage of patients with at least one visit response of complete response or partial response confirmed. All statistical analyses were performed using Statistica software, version 8 (StatSoft Inc., Tulsa, OK) and SPSS, version 25.0 (SPSS, Chicago, IL). GraphPad Prism version 8.0 is used for graphing.
Our search strategy identified a total of 601 articles (Figure 1). Among these, 42 articles were relevant reports of patients with the histopathological transformation from LADC to SCLC after TKIs therapy (4, 16, 18, 23–61). Of these 42 articles, 32 were case reports (4, 23–53), and 10 were small patient series (16, 18, 54–61). A total of 72 patients with transformed SCLC from a prior LADC were identified (Appendix Table).
Table 1 summarized the characteristics of the 72 identified patients. The median age of patients was 56.5 years (range 31 to 76 years). Twenty two were males (30.6%) and fifty were females (69.4%); 70.8% of the patients were Asian and 29.2% were white. The smoking status, available in 64 patients, was positive in nineteen patients (29.7%), including former smokers. Forty-five patients (70.3%) were never smokers; 95% of the cases were diagnosed with advanced LADC (TNM stage III or IV). All cases had positive EGFR mutation. The EGFR mutation profile was as follows: exon 19-deletion in 54 patients (75%), exon 21-L858R in 16 patients (22.2%), and exon 18-G719X in 2 patients (2.8%). In one case, the EGFR status was wild type at diagnosis but became mutated on exon 19 at disease progression, suggesting a false-negative result at first diagnosis.
Among the 72 patients, 64 patients (88.8%) received TKIs as first-line therapy, 7 patients (9.7%) as second-line therapy, and 1 patient (1.3%) as third-line therapy. Sixty-one patients (84.7%) received 1st-generation TKIs (Erlotinib, Gefitinib, Icontinib), nine patients (12.5%) received 2nd-generation TKI (Afatinib), while only 2 patients (2.7%) were treated with a 3rd-generation TKI (Osimertinib).
The estimated median time to ttSCLC was 20.5 months (95% CI, 15.45 to 26.55 months), and the estimated median time to T-ttSCLC was 18.5 months (95% CI, 13.86 to 23.39 months) (Figure 2). The transformation time of SCLC in most cases is between 6 and 36 months, and 50% of them occur within 1 to 2 years (Figure 3).
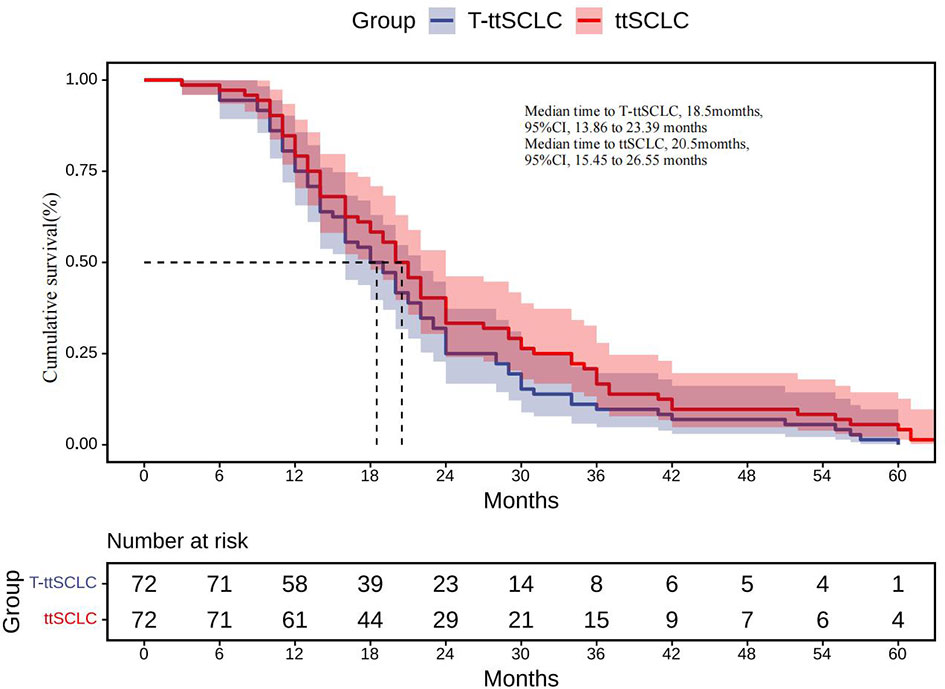
Figure 2 ttSCLC, Kaplan–Meier estimates of time from initial diagnosis of LADC to development of SCLC phenotype; T-ttSCLC, Kaplan–Meier estimates of time from start of TKI treatment to development of SCLC phenotype. LADC, Lung adenocarcinoma; SCLC, Small cell lung cancer; TKI, Tyrosine kinase inhibitor.
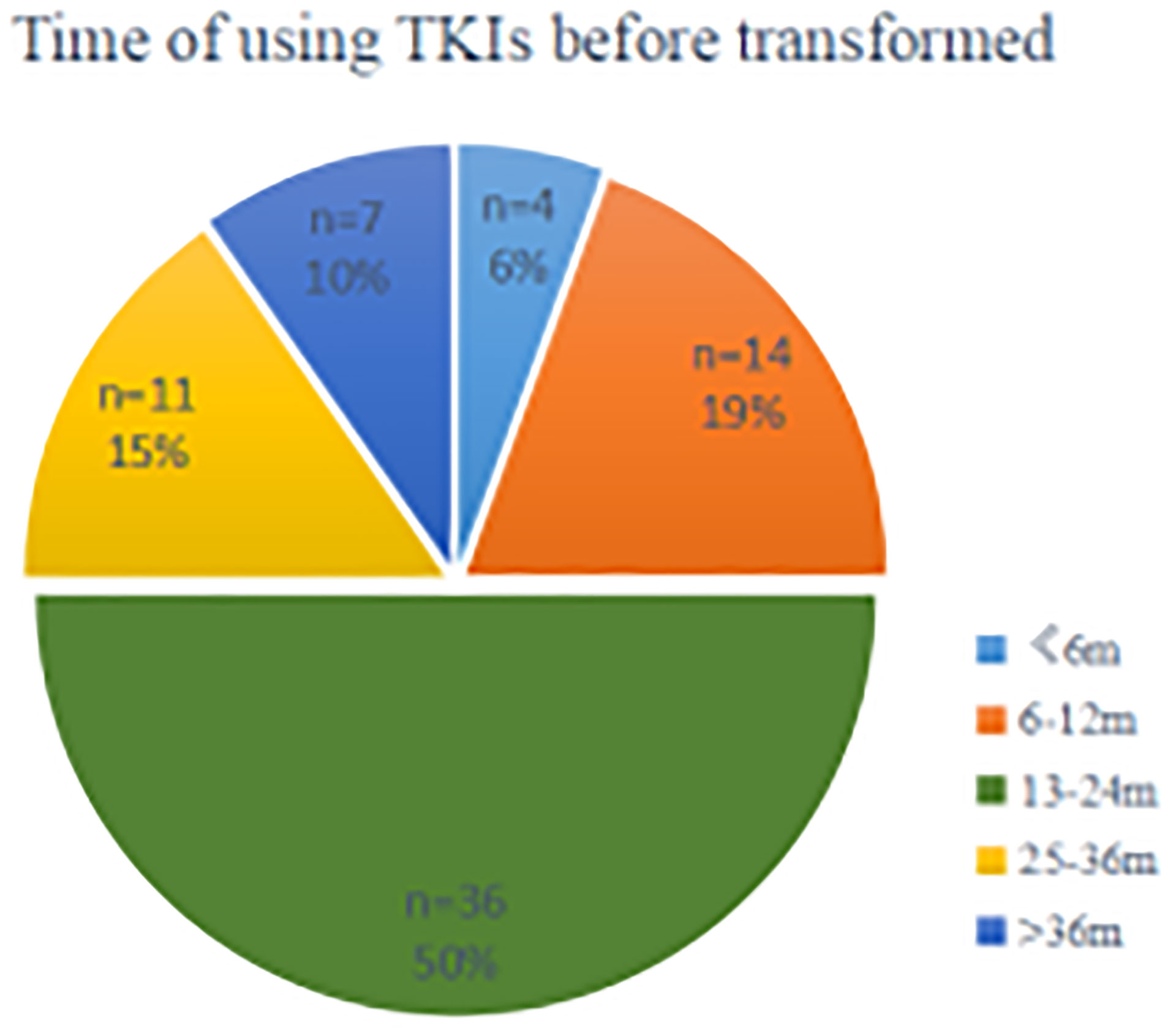
Figure 3 A segmented time scale diagram from the beginning of TKIs using the occurrence of pathological transformation. TKIs, Tyrosine kinase inhibitors; n, number; m, months.
In the univariate analysis (Figure 4), female gender was associated with a predictor of longer ttSCLC of borderline significance (HR, 0.65; 95% CI, 0.391–1.083; p = 0.098), while tobacco smoking (either former or current) was also associated with an earlier occurrence of ttSCLC phenotype of borderline significance (HR, 0.61; 95% CI, 0.346–1.072; p = 0.085). The initial diagnosis of stage IV cases may have a longer transformation time of SCLC than early and medium-term cases (HR, 0.57; 95% CI, 0.298–1.094; p = 0.091). Furthermore, age, race, first-line TKIs type, and initial TKIs line had no significant correlation with ttSCLC and T-ttSCLC.
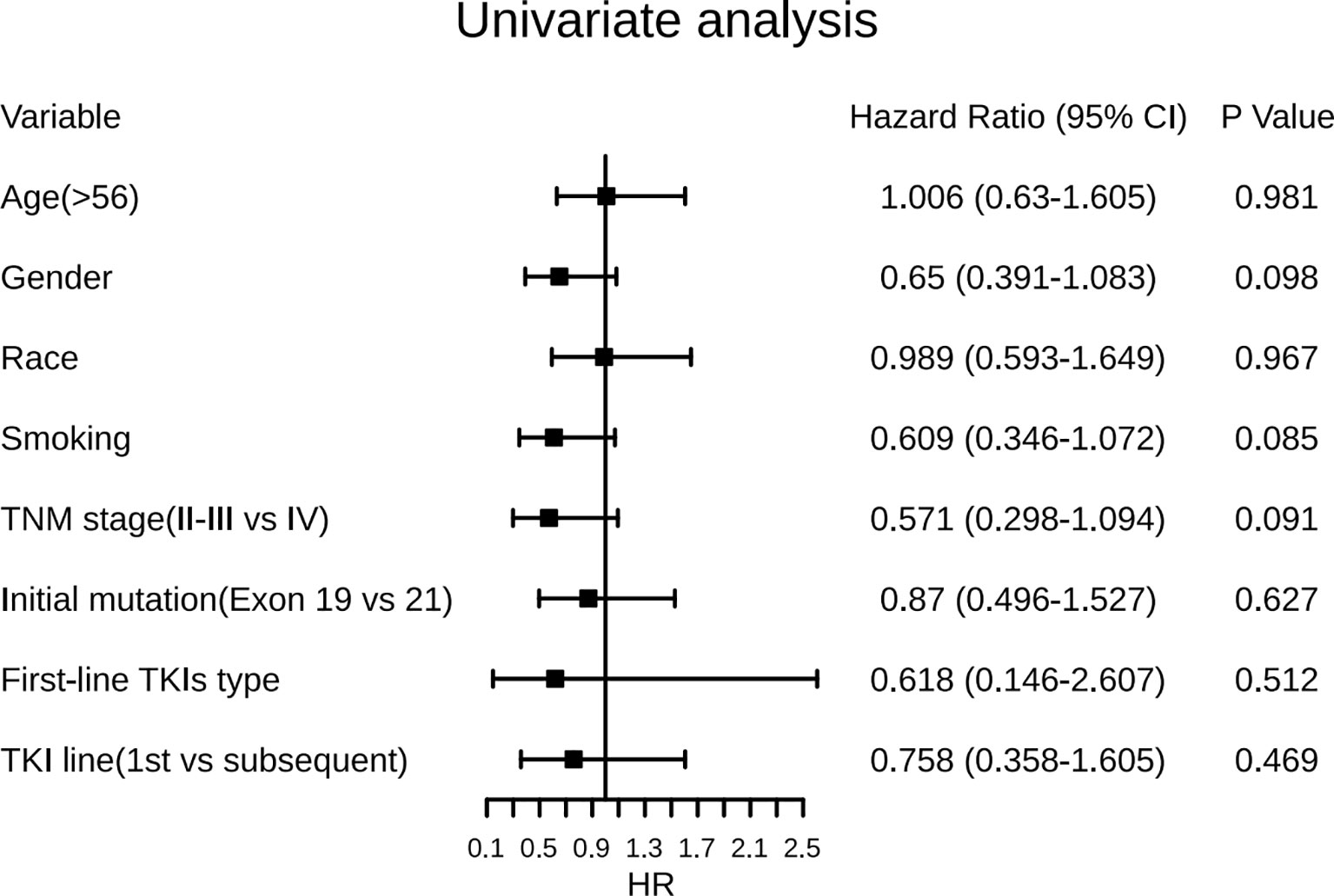
Figure 4 Forest plots of the effect of several factors on time from diagnosis of LADC to development of SCLC phenotype. LADC, Lung adenocarcinoma; SCLC, Small cell lung cancer; TKIs, Tyrosine kinase inhibitors; Exon 19, EGFR exon 19-deletion; Exon 21, EGFR exon 21-L858R.
The therapy before transformation includes targeted therapy (1st-/2nd-/3rd-generation TKIs), chemotherapy, radiotherapy, and combined treatment. All cases have a history of TKIs therapy and have response efficacy before transformation, and TKIs used in first-line treatment account for 90.3% (Appendix Table). The median PFS for TKIs first-line therapy was 15 months (95% CI, 12.07 to 17.93 months), while the total median PFS for first-line therapy was 13 months (95% CI, 11.04 to 14.96months) (Figure 5).
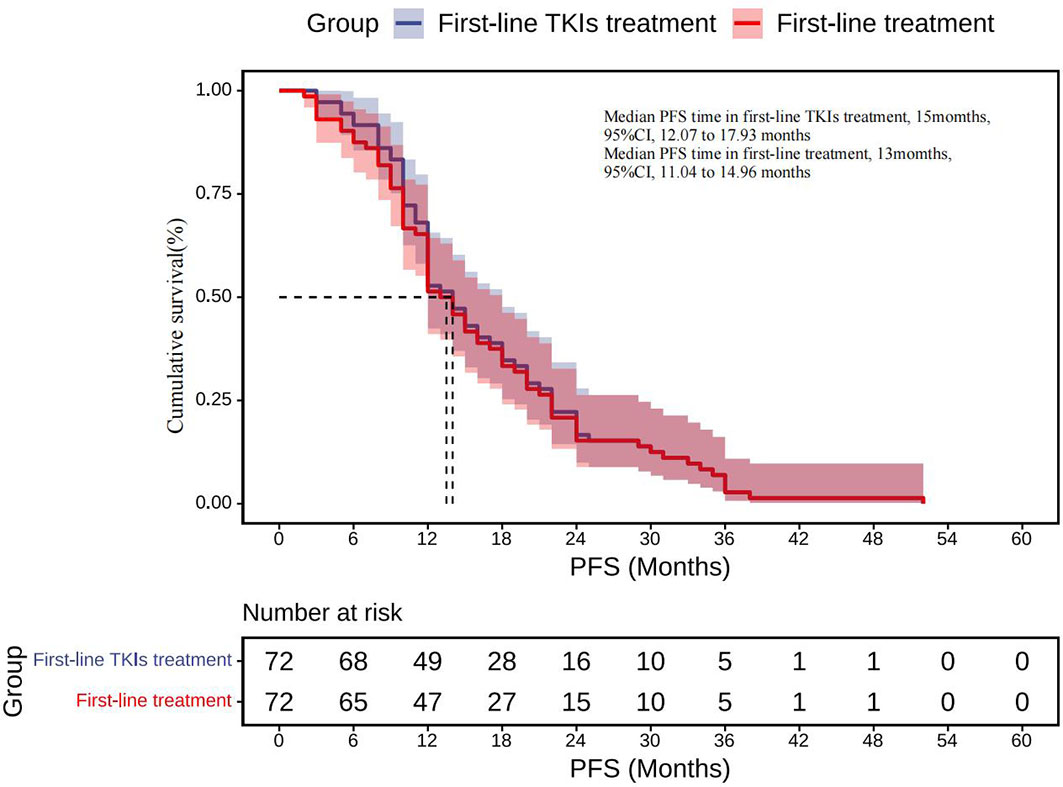
Figure 5 First-line TKIs treatment: Kaplan–Meier estimates of PFS for TKIs first-line therapy; First-line treatment: Kaplan–Meier estimates of total PFS for first-line therapy. SCLC, Small cell lung cancer; TKIs, Tyrosine kinase inhibitors; PFS, Progression-free survival.
A total of 64 cases had first-line therapy data after transformation (Table 2), of which 42 cases (66%) were classic chemotherapy regimens containing platinum, 9 cases received chemotherapy combined with targeted therapy, 4 cases received chemotherapy combined with radiotherapy, 3 cases continued single-targeted therapy, 2 cases received chemotherapy combined with radiotherapy and targeted therapy, and 4 cases did not receive further treatment. The disease control rate (DCR) of first-line therapy after transformation was 76%, while the objective response rate (ORR) was 48% (Figure 6). Thirty-seven patients had specific first-line progression-free survival (PFS) after transformation (Figure 7), of which 15 cases have PFS for no more than 3 months, 10 cases for 3 to 6 months, and 12 cases for more than 6 months, including 2 cases for more than 1 year. The median PFS was 4.0 months (95% CI, 3.16 to 7.50 months). However, the median PFS of the combined treatment group was over 6 months. Compared with conventional chemotherapy, combination therapy (including chemotherapy combined with radiotherapy, chemotherapy combined with targeted therapy, and chemotherapy combined with chemotherapy and targeted therapy) has a longer median PFS time (4 months vs. 7 months).
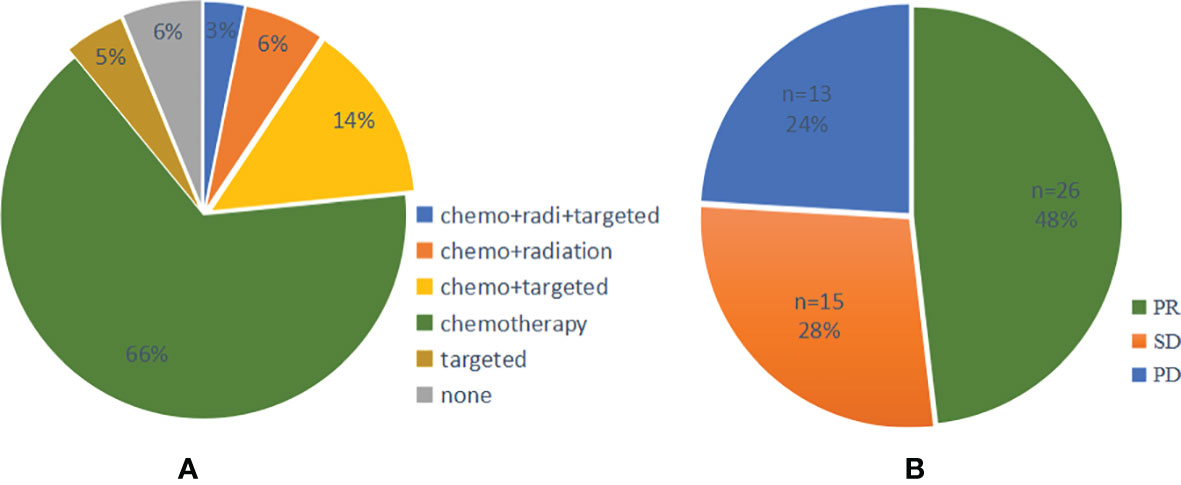
Figure 6 (A) Pie chart of first-line treatment and (B) efficacy after SCLC transformation. PR, Partial response; SD, Stable disease; PD, Progressive disease; SCLC, Small cell lung cancer.
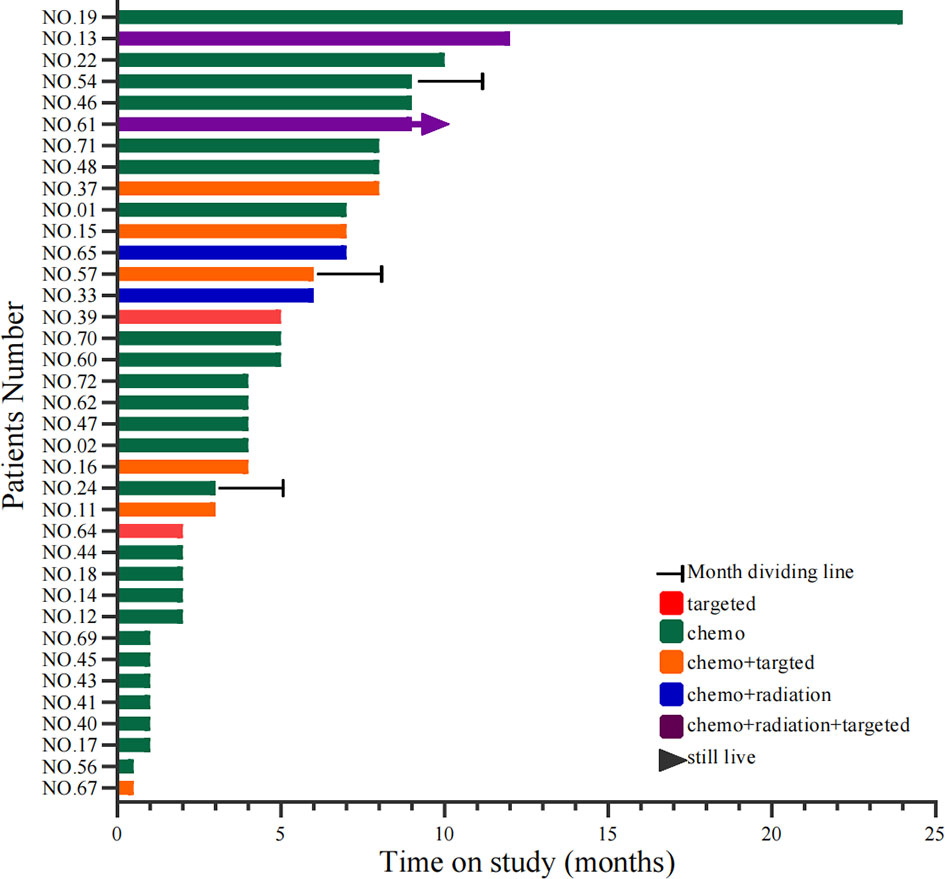
Figure 7 Swimming lane diagram of PFS in first-line treatment after SCLC transformation. PFS, Progression-free survival; SCLC, Small cell lung cancer. Target type: No. 39, osimertinib; No. 64, afatinib; No. 11/15/37/67, erlotinib; No. 16, osimertinib; No. 57, gefitinib.
Information on survival status after SCLC diagnosis was available in 33 patients (45.8%) (Figure 8). The calculated median OS after SCLC diagnosis was 8.5 months (95% CI, 5.50 to 11.50 months) (Figure 9). Unlike the median PFS of first-line treatment after transformation, there was no significant difference in the median OS between the conventional chemotherapy group and the combined treatment group (8 months vs. 9 months). The median OS since diagnosis of LADC was 27 months (95% CI, 22.90 to 31.10 months) (Figure 9). The initial TNM stage was medium-term, which was the only factor significantly associated with shorter OS after diagnosis of SCLC (HR, 0.92; 95% CI, 0.043–0.853; p = 0.030) (Figure 10). Gender, age, race, smoking status, initial mutation type, and line of TKIs therapy did not predict the patients’ outcome. Moreover, the ttSCLC (dichotomized at the median value) failed to impact patient survival from the time of SCLC transformation.
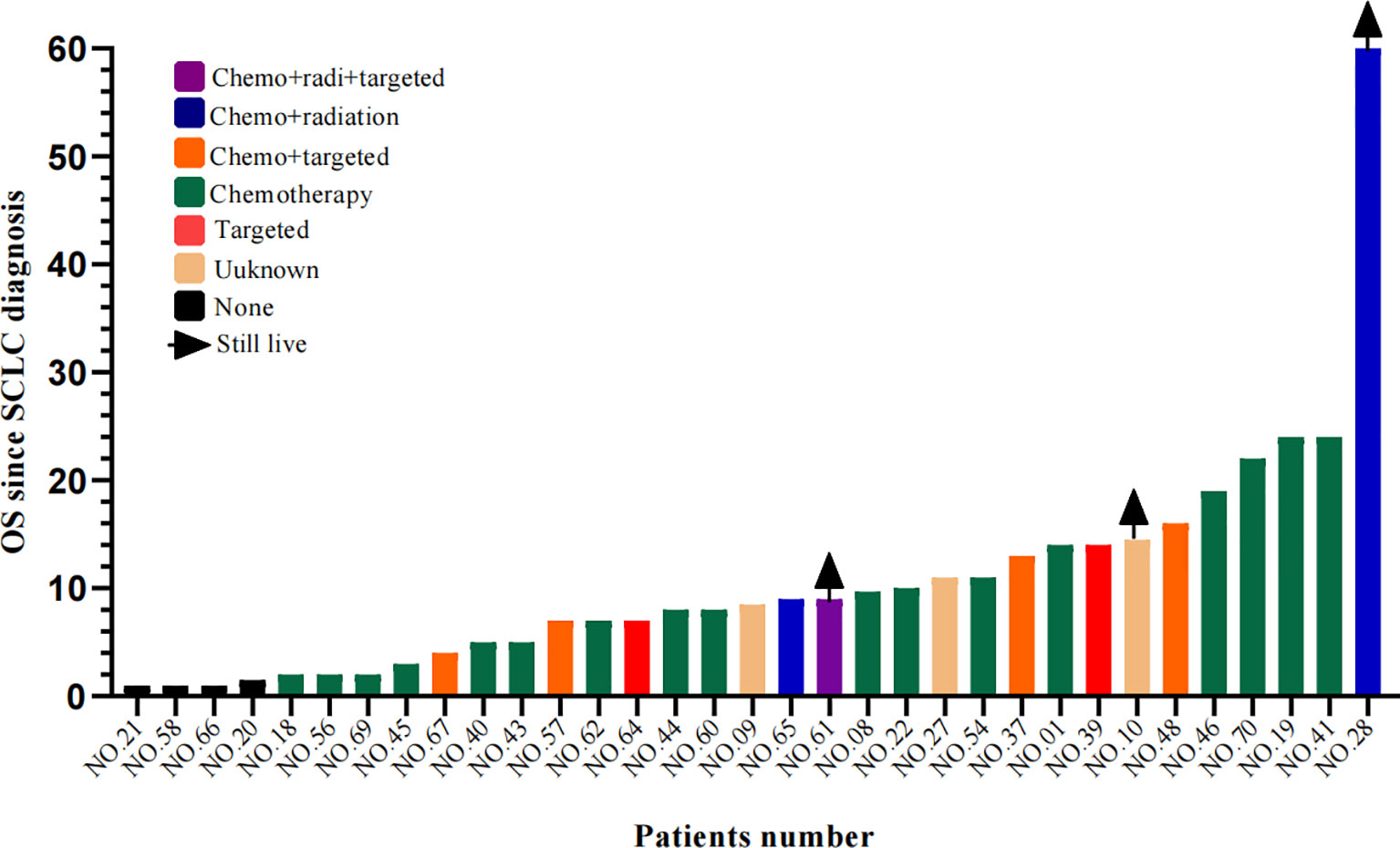
Figure 8 Swimming lane diagram of OS in different therapies after SCLC transformation. OS, Overall survival.
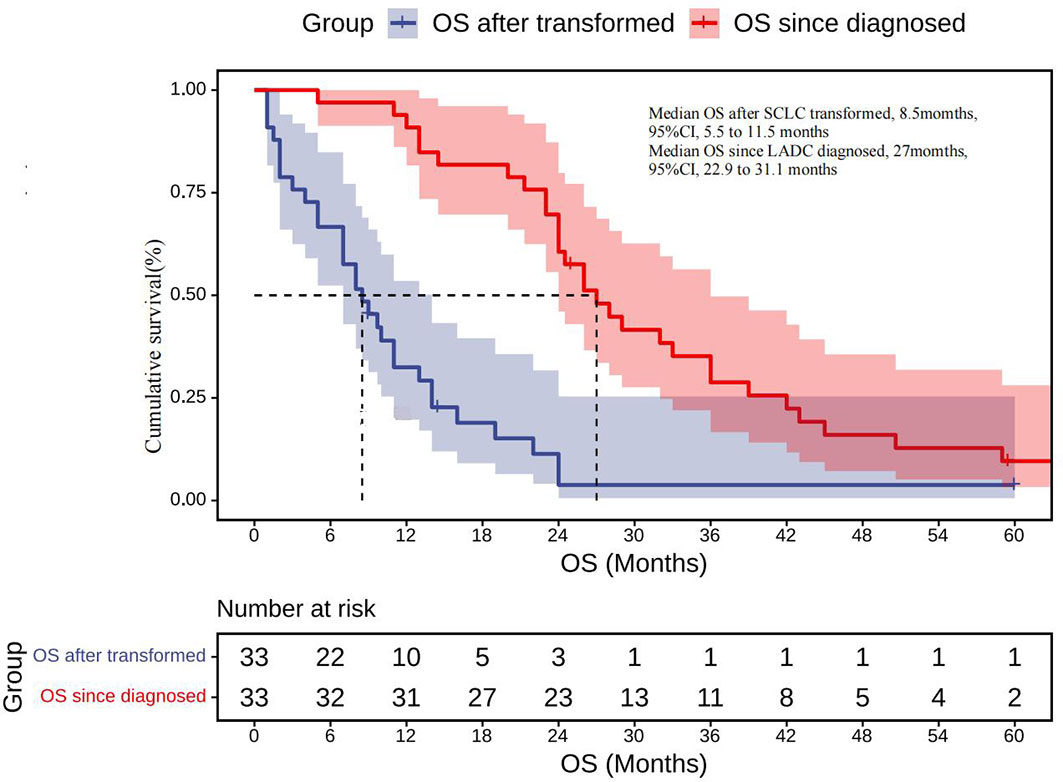
Figure 9 OS after being transformed: Kaplan–Meier estimates of OS since the time of SCLC transformation; OS since diagnosed: Kaplan–Meier estimates of OS since the time of diagnosis. OS, overall survival.
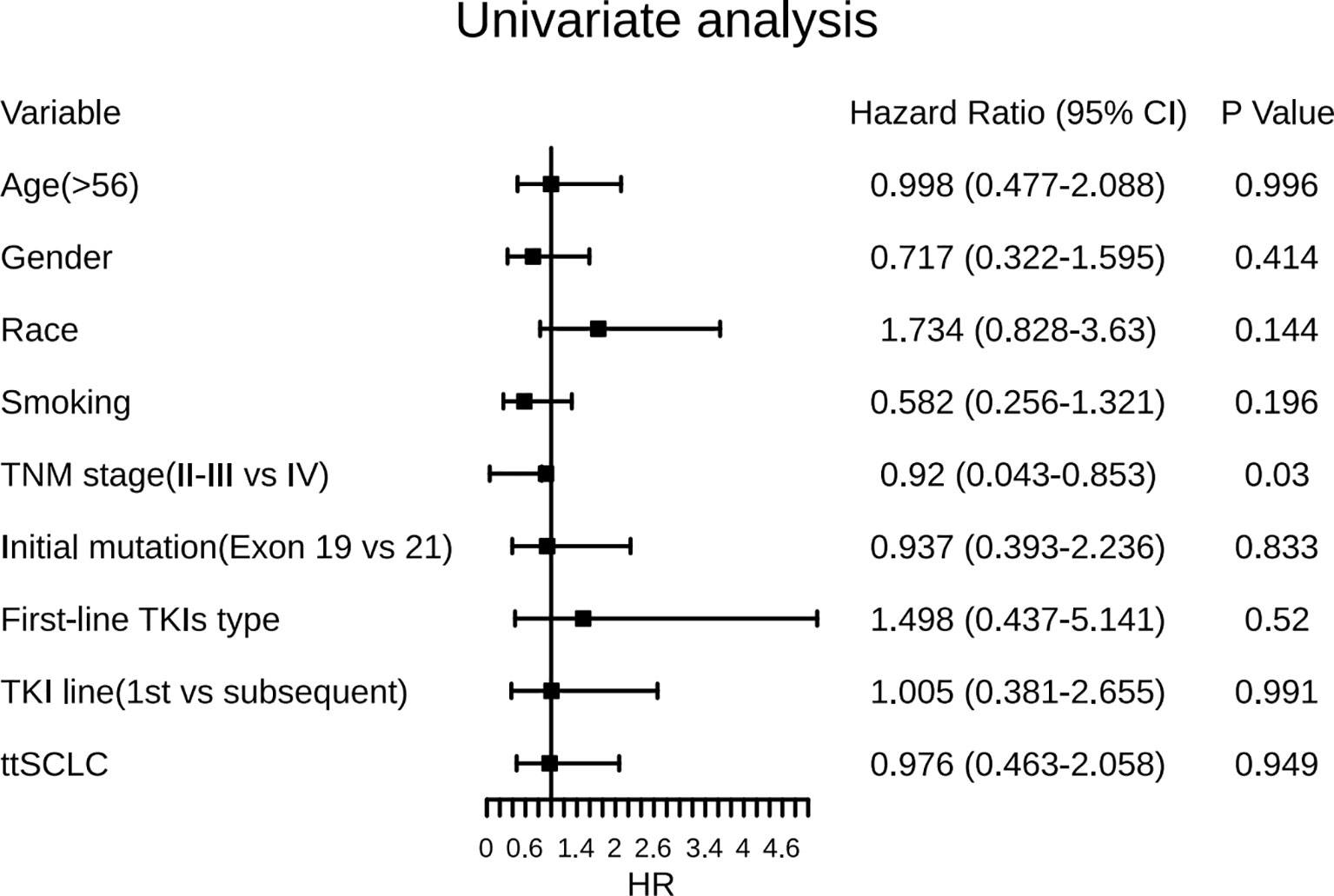
Figure 10 Forest plots of the effect of several factors on OS after development of SCLC phenotype. ttSCLC, the time to small cell lung cancer transformation; TKIs, Tyrosine kinase inhibitors; Exon 19, EGFR Exon 19-deletion; Exon 21, EGFR Exon 21-L858R; OS, overall survival.
Transformation to SCLC is a resistance mechanism to EGFR-TKIs that develops in LADC. A systematic analysis on the transformation of SCLC was published in 2017 (21), including 39 patients. Since then, the literature on this aspect were only case reports or small series of retrospective analyses. There is a lack of research with a large sample size. We systematically searched and summarized the relevant English publications on the transformation of SCLC after EGFR-TKIs resistance in LADC since 2006.
There are generally two hypotheses about the transformation of LADC into SCLC. One hypothesis for the SCT phenomenon is that there is a small amount of SCLC component in LADC patients when they are initially diagnosed. The SCLC component will become dominant if the LADC component was killed by the EGFR-TKIs (4). This is a pseudo-SCT; although this hypothesis existed for a long time, strong evidence is still lacking. On the contrary, the second hypothesis, that both LADC and SCLC may arise from identical cell clones, is widely accepted (16). Previous studies have found that most transformed SCLC samples have their original EGFR mutation (4). It was reported that targeted disruption of TP53 and RB1 in alveolar type II cells led to the development of SCLC (62). In our study, 67 cases had gene detection results after SCLC transformation, of which 87% retained EGFR 19-del or 21-L858R mutation. However, it is noteworthy that 17 cases had combined T790M mutation before transformation, but only 1 case retained T790M mutation after transformation (Appendix A). The relative absence of T790M is consistent with the loss of EGFR dependence in transformed SCLC, as reported by Offin (63), and whether the loss of T790M mutation is related to SCLC transformation needs further study.
To determine that TKIs resistance is caused by SCLC transformation, we formulated a stricter inclusion criteria than that in the 2017 article (4), which requires each case to receive TKIs treatment for at least 3 months. The efficacy evaluation is at least SD. We excluded cases where the initial pathology contained components of small cell lung cancer. A total of 72 cases were included in our study. To our knowledge, this cohort represents the largest report to date of clinical outcomes for patients with EGFR-sensitive mutations LADC who developed SCLC transformation after TKIs resistance.
We found that the baseline demographic characteristics of people who have undergone SCLC transformation are similar to those of people who have not undergone transformation, which is different from the findings of previous studies (4, 64). As mentioned above, apart from the T790M mutation, most transformed SCLC cases maintained the same EGFR mutation present in the former LADC, including 18-G719X, 19-deletion, and 21-L858R mutations. EGFR mutations are extremely rare in primary SCLC (65). The persistence of the same EGFR mutation suggests that the SCLC phenotype is clonally derived from the primary LADC, providing further evidence that the transformed SCLC component originated from the LADC component, not the original one.
We observed that SCLC transformation occurs over a wide time span, seen as early as 3 months and as late as more than 6 years after the diagnosis of LADC. However, the median time to transformation was 20.5 months. Seventy-five percent of cases received TKIs therapy for more than 12 months before SCLC transformation. The median PFS of first-line TKIs treatment was 14 months, which appears to be longer than the PFS reported in patients treated with TKIs in prospective clinical trials (8–11 months). This observation suggests that patients obtaining a long-term benefit with these drugs are at a higher risk of trans-differentiating to an SCLC phenotype at progression. We also analyzed factors potentially predicting SCLC transformation; however, no significant factors were found to be associated with SCLC transformation. Differently from previous studies, our study shows that the female gender is only borderline related to a longer ttSCLC, while smoking status is also borderline related to the trend of early SCLC phenotype when progressing to TKIs treatment, while other factors are not significantly related to the length of ttSCLC.
After transformation, clinical behavior mimics classic (EGFR wild-type) SCLC on many levels, with frequent but transient responses to platinum etoposide. The median PFS was 4 months after first-line treatment and the median OS was 8.5 months. Compared with primary SCLC, the clinical prognosis of transformed SCLC is worse (66). We found that combination therapy (including chemotherapy combined with radiotherapy, chemotherapy combined with targeted therapy, and chemotherapy combined with chemotherapy and targeted therapy) had a longer median PFS than conventional chemotherapy (4 months vs. 7 months). However, there was no significant benefit in the combined treatment group in the transformed median OS.
The overall prognosis of transformed SCLC is poor, so it is urgent to explore more treatment methods. Currently, immunocombination therapy has become a new standard for the first-line treatment of extensive SCLC with the median OS reaching 15 months (67). Whether transformed SCLC can also benefit from immune checkpoint inhibitors needs further clinical exploration and research.
The limitations of our study must be noted, i.e., that the patient data were retrospectively collected from published articles. The retrospective nature of this study may have influenced some results, such as treatments and response assessments. Due to the limitations of this retrospective study, some cases lack the data to be analyzed, and it is impossible to obtain samples for further detection and analysis. Our center has been conducting a prospective case inclusion study and will further explore the differences in the expression of the whole gene spectrum before and after SCLC transformation to find the relevant driver genes of SCLC transformation.
Our pooled analysis shows that the realization of SCLC phenotype mostly takes more than 1 year, and the prognosis of patients after SCLC diagnosis is worse than primary SCLC. Chemotherapy combined with targeting and radiotherapy can improve PFS of transformed SCLC but cannot benefit OS. In the future, novel therapies must be explored.
The original contributions presented in the study are included in the article/Supplementary Material. further inquiries can be directed to the corresponding author.
Concept and design: JX and LX. Article writing: JX. Extraction and collection of data: JX and LX. Statistical analysis: JX and LX. Drafting and revision of manuscript: JX and ZY. Supervision: ZY. Final approval of the manuscript: JX, XL, BW, WK, YC, and ZY. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Clinical Key Specialty Construction Project of Fujian Province (No. 2015-593 and No. 2017YZ0001-2) and the 900th Hospital of the Joint Logistic Support Force of China: International Cooperative Research Program (Grant/Award Number: 2017L03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.766148/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Ettinger DS, Aisner J. Changing Face of Small-Cell Lung Cancer: Real and Artifact. J Clin Oncol: Off J Am Soc Clin Oncol (2006) 24:4526–7. doi: 10.1200/jco.2006.07.3841
3. Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clinics Chest Med (2020) 41:1–24. doi: 10.1016/j.ccm.2019.10.001
4. Oser MG, Niederst MJ, Sequist LV, Sequist LV, Engelman JA. Transformation From Non-Small-Cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol (2015) 16:e165–172. doi: 10.1016/s1470-2045(14)71180-5
5. Zakowski MF, Ladanyi M, Kris MG. EGFR Mutations in Small-Cell Lung Cancers in Patients Who Have Never Smoked. N Eng J (2007) 13, 213–5. doi: 10.1056/NEJMc053610
6. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. New Engl J Med (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
7. Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol: Off J Am Soc Clin Oncol (2020) 38:115–23. doi: 10.1200/jco.19.01488
8. Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-Line Erlotinib Versus Gemcitabine/Cisplatin in Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Analyses From the Phase III, Randomized, Open-Label, ENSURE Study. Ann Oncol: Off J Eur Soc Med Oncol (2015) 26:1883–9. doi: 10.1093/annonc/mdv270
9. Wu YL, Sequist LV, Tan EH, Geater SL, Orlov S, Zhang L, et al. Afatinib as First-Line Treatment of Older Patients With EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Subgroup Analyses of the LUX-Lung 3, LUX-Lung 6, and LUX-Lung 7 Trials. Clin Lung Cancer (2018) 19:e465–79. doi: 10.1016/j.cllc.2018.03.009
10. Zhang YQ, Jiang LJ, Jiang SX, Xu YF, Zhou BB, Huang GH, et al. Gefitinib With or Without Transarterial Infusion Chemotherapy (Cisplatin) for Large Nonsmall Cell Lung Cancer With Epidermal Growth Factor Receptor Mutations. J Vasc Interventional Radiol: JVIR (2019) 30:1004–12. doi: 10.1016/j.jvir.2018.12.705
11. Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol: Off J Am Soc Clin Oncol (2020) 38:124–36. doi: 10.1200/jco.19.01154
12. Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol: Off J Am Soc Clin Oncol (2020) 38:538–47. doi: 10.1200/jco.19.00457
13. Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of Acquired Resistance to First- and Second-Generation EGFR Tyrosine Kinase Inhibitors. Ann Oncol: Off J Eur Soc Med Oncol (2018) 29:i10–9. doi: 10.1093/annonc/mdx703
14. Lim SM, Syn NL, Cho BC, Soo RA. Acquired Resistance to EGFR Targeted Therapy in Non-Small Cell Lung Cancer: Mechanisms and Therapeutic Strategies. Cancer Treat Rev (2018) 65:1–10. doi: 10.1016/j.ctrv.2018.02.006
15. Wagener-Ryczek S, Heydt C, Süptitz J, Michels S, Falk M, Alidousty C, et al. Mutational Spectrum of Acquired Resistance to Reversible Versus Irreversible EGFR Tyrosine Kinase Inhibitors. BMC Cancer (2020) 20:408. doi: 10.1186/s12885-020-06920-3
16. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci Transl Med (2011) 3:75ra26. doi: 10.1126/scitranslmed.3002003
17. Shao Y, Zhong DS. Histological Transformation After Acquired Resistance to Epidermal Growth Factor Tyrosine Kinase Inhibitors. Int J Clin Oncol (2018) 23:235–42. doi: 10.1007/s10147-017-1211-1
18. Xiaohua Shi HD, Liu X, Zhou L, Zhou L, Liang Z. Genetic Alterations and Protein Expression in Combined Small Cell Lung Cancers and Small Cell Lung Cancers Arising From Lung Adenocarcinomas After Therapy With Tyrosine Kinase Inhibitors. Oncotarget (2016) 7:34240–9. doi: 10.18632/oncotarget.9083
19. Ferone G, Lee MC, Sage J, Berns A. Cells of Origin of Lung Cancers: Lessons From Mouse Studies. Genes Dev (2020) 34:1017–32. doi: 10.1101/gad.338228.120
20. Marcoux N, Gettinger SN, O'Kane G, Arbour KC, Neal JW, Husain H, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas Clinical Outcomes. J Clin Oncol (2019) 37:278–85. doi: 10.1200/JCO.18
21. Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of Patients With Lung Adenocarcinoma With Transformation to Small-Cell Lung Cancer Following Tyrosine Kinase Inhibitors Treatment: A Systematic Review and Pooled Analysis. Cancer Treat Rev (2017) 59:117–22. doi: 10.1016/j.ctrv.2017.07.007
22. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2015) 10:1243–60. doi: 10.1097/jto.0000000000000630
23. Ren X, Cai X, Li J, Zhang X, Yu J, Song X, et al. Histological Transformation of Lung Adenocarcinoma to Small Cell Lung Cancer With Mutant C797S Conferring Acquired Resistance to Osimertinib. J Int Med Res (2020) 48:300060520927918. doi: 10.1177/0300060520927918
24. Ma S, He Z, Fu H, Wang L, Wu X, Zhang Z, et al. Dynamic Changes of Acquired T790M Mutation and Small Cell Lung Cancer Transformation in a Patient With EGFR-Mutant Adenocarcinoma After First- and Third-Generation EGFR-Tkis: A Case Report. Transl Lung Cancer Res (2020) 9:139–43. doi: 10.21037/tlcr.2020.01.07
25. Xie Z, Gu Y, Lin X, Ouyang M, Qin Y, Zhang J, et al. Unexpected Favorable Outcome to Etoposide and Cisplatin in a Small Cell Lung Cancer Transformed Patient: A Case Report. Cancer Biol Ther (2019) 20:1172–5. doi: 10.1080/15384047.2019.1617561
26. Tenjin Y, Nakamura K, Ishizuka S, Saruwatari K, Sato R, Tomita Y, et al. Small Cell Lung Cancer Derived From Adenocarcinoma With Mutant Epidermal Growth Factor Receptor Provides a Signature of Transcriptional Alteration in Tumor Cells. Internal Med (Tokyo Japan) (2019) 58:3261–5. doi: 10.2169/internalmedicine.2988-19
27. Tang K, Jiang N, Kuang Y, He Q, Li S, Luo J, et al. Overcoming T790M Mutant Small Cell Lung Cancer With the Third-Generation EGFR-TKI Osimertinib. Thorac Cancer (2019) 10:359–64. doi: 10.1111/1759-7714.12927
28. Fiore M, Trecca P, Perrone G, Amato M, Righi D, Trodella L, et al. Histologic Transformation to Small-Cell Lung Cancer Following Gefitinib and Radiotherapy in a Patient With Pulmonary Adenocarcinoma. Tumori (2019) 105:Np12–np16. doi: 10.1177/0300891619832261
29. D’Haene N, Le Mercier M, Salmon I, Mekinda Z, Remmelink M, Berghmans T, et al. SMAD4 Mutation in Small Cell Transformation of Epidermal Growth Factor Receptor Mutated Lung Adenocarcinoma. Oncologist (2019) 24:9–13. doi: 10.1634/theoncologist.2018-0016
30. Yao Y, Zhu Z, Wu Y, Chai Y. Histologic Transformation From Adenocarcinoma to Both Small Cell Lung Cancer and Squamous Cell Carcinoma After Treatment With Gefitinib: A Case Report. Medicine (2018) 97:e0650. doi: 10.1097/md.0000000000010650
31. Sonoda T, Nishikawa S, Sakakibara R, Saiki M, Ariyasu R, Koyama J, et al. EGFR T790M Mutation After Chemotherapy for Small Cell Lung Cancer Transformation of EGFR-Positive Non-Small Cell Lung Cancer. Respir Med Case Rep (2018) 24:19–21. doi: 10.1016/j.rmcr.2018.03.009
32. Iijima Y, Hirotsu Y, Mochizuki H, Amemiya K, Oyama T, Uchida Y, et al. Dynamic Changes and Drug-Induced Selection of Resistant Clones in a Patient With EGFR-Mutated Adenocarcinoma That Acquired T790M Mutation and Transformed to Small-Cell Lung Cancer. Clin Lung Cancer (2018) 19:e843–7. doi: 10.1016/j.cllc.2018.07.002
33. Xu Y, Huang Z, Gong L, Fan Y. A Case of Resistance to Tyrosine Kinase Inhibitor Therapy: Small Cell Carcinoma Transformation Concomitant With Plasma-Genotyped T790M Positivity. Anticancer Drugs (2017) 28:1056–61. doi: 10.1097/CAD.0000000000000540
34. Xia LM, Luo MH, Jiang YL, Zheng Q, Cui LL, Zhou YY. A Case Report of Secondary Acute Promyelocytic Leukemia After Treatment of Translating to Small Cell Lung Cancer in Lung Adenocarcinoma. Chin J Hematol (2017) 38:347. doi: 10.3760/cma.j.issn.0253-2727.2017.04.018
35. Wahab A, Kesari K, Chaudhary S, Khan M, Khan H, Smith S, et al. Sequential Occurrence of Small Cell and Non-Small Lung Cancer in a Male Patient: Is it a Transformation? Cancer Biol Ther (2017) 18:940–3. doi: 10.1080/15384047.2017.1394546
36. Tokaca N, Wotherspoon A, Nicholson AG, Fotiadis N, Thompson L, Popat S, et al. Lack of Response to Nivolumab in a Patient With EGFR-Mutant Non-Small Cell Lung Cancer Adenocarcinoma Sub-Type Transformed to Small Cell Lung Cancer. Lung Cancer (Amsterdam Netherlands) (2017) 111:65–8. doi: 10.1016/j.lungcan.2017.07.012
37. Tambo Y, Sone T, Kasahara K. Combination Treatment Using an EGFR Tyrosine Kinase Inhibitor Plus Platinum-Based Chemotherapy for a Patient With Transformed Small Cell Carcinoma and EGFR-Mutated Lung Adenocarcinoma. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2017) 12:e199–200. doi: 10.1016/j.jtho.2017.07.034
38. Nishikawa S, Tambo Y, Ninomiya H, Oguri T, Kawashima Y, Takano N, et al. A Case Treated With Nivolumab After Small Cell Lung Cancer Transformation of Mutant EGFR Non-Small Cell Lung Cancer. Ann Oncol: Off J Eur Soc Med Oncol (2016) 27:2300–2. doi: 10.1093/annonc/mdw431
39. Lin Q, Cai GP, Yang KY, Yang L, Chen CS, Li YP, et al. Case Report: Small Cell Transformation and Metastasis to the Breast in a Patient With Lung Adenocarcinoma Following Maintenance Treatment With Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. BMC Cancer (2016) 16:593. doi: 10.1186/s12885-016-2623-4
40. Ham JS, Kim S, Kim HK, Byeon S, Sun JM, Lee SH, et al. Two Cases of Small Cell Lung Cancer Transformation From EGFR Mutant Adenocarcinoma During AZD9291 Treatment. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2016) 11:e1–4. doi: 10.1016/j.jtho.2015.09.013
41. Shuping Xue TY, Zhang Y, Shan L. Clinical Observation of Translating to Small Cell Lung Cancer Following Treatment With EGFR-Tyrosine Kinase Inhibitors in Lung Adenocarcinoma. Zhongguo Fei Ai Za Zhi (2015) 18:656–60. doi: 10.3779/j.issn.1009-3419.2015.10.10
42. Hwang KE, Jung JW, Oh SJ, Park MJ, Shon YJ, Choi KH, et al. Transformation to Small Cell Lung Cancer as an Acquired Resistance Mechanism in EGFR-Mutant Lung Adenocarcinoma: A Case Report of Complete Response to Etoposide and Cisplatin. Tumori (2015) 101:e96–98. doi: 10.5301/tj.5000276
43. Watanabe S, Sone T, Matsui T, Yamamura K, Tani M, Okazaki A, et al. Transformation to Small-Cell Lung Cancer Following Treatment With EGFR Tyrosine Kinase Inhibitors in a Patient With Lung Adenocarcinoma. Lung Cancer (Amsterdam Netherlands) (2013) 82:370–2. doi: 10.1016/j.lungcan.2013.06.003
44. Popat S, Wotherspoon A, Nutting CM, Gonzalez D, Nicholson AG, O'Brien M. Transformation to “High Grade” Neuroendocrine Carcinoma as an Acquired Drug Resistance Mechanism in EGFR-Mutant Lung Adenocarcinoma. Lung Cancer (Amsterdam Netherlands) (2013) 80:1–4. doi: 10.1016/j.lungcan.2012.12.019
45. van Rie S, Thunnissen E, Heideman D, Smit EF, Biesma B. A Patient With Simultaneously Appearing Adenocarcinoma and Small-Cell Lung Carcinoma Harbouring an Identical EGFR Exon 19 Mutation. Ann Oncol: Off J Eur Soc Med Oncol (2012) 23:3188–9. doi: 10.1093/annonc/mds525
46. Leonetti A, Minari R, Mazzaschi G, Gnetti L, La Monica S, Alfieri R, et al. Small Cell Lung Cancer Transformation as a Resistance Mechanism to Osimertinib in Epidermal Growth Factor Receptor-Mutated Lung Adenocarcinoma: Case Report and Literature Review. Front Oncol (2021) 11:642190. doi: 10.3389/fonc.2021.642190
47. Jiang SY, Zhao J, Wang MZ, Huo Z, Zhang J, Zhong W, et al. Small-Cell Lung Cancer Transformation in Patients With Pulmonary Adenocarcinoma: A Case Report and Review of Literature. Medicine (2016) 95:e2752. doi: 10.1097/md.0000000000002752
48. Furugen M, Uechi K, Hirai J, Aoyama H, Saio M, Yoshimi N, et al. An Autopsy Case of Two Distinct, Acquired Drug Resistance Mechanisms in Epidermal Growth Factor Receptor-Mutant Lung Adenocarcinoma: Small Cell Carcinoma Transformation and Epidermal Growth Factor Receptor T790M Mutation. Internal Med (Tokyo Japan) (2015) 54:2491–6. doi: 10.2169/internalmedicine.54.5481
49. Fallet V, Ruppert AM, Poulot V, Lacave R, Belmont L, Antoine M, et al. Secondary Resistance to Erlotinib: Acquired T790M Mutation and Small-Cell Lung Cancer Transformation in the Same Patient. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2012) 7:1061–3. doi: 10.1097/JTO.0b013e31824fea45
50. Alam N, Gustafson KS, Ladanyi M, Zakowski MF, Kapoor A, Truskinovsky AM, et al. Small-Cell Carcinoma With an Epidermal Growth Factor Receptor Mutation in a Never-Smoker With Gefitinib-Responsive Adenocarcinoma of the Lung. Clin Lung Cancer (2010) 11:E1–4. doi: 10.3816/CLC.2010.n.046
51. Morinaga R, Okamoto I, Furuta K, Kawano Y, Sekijima M, Dote K, et al. Sequential Occurrence of Non-Small Cell and Small Cell Lung Cancer With the Same EGFR Mutation. Lung Cancer (Amsterdam Netherlands) (2007) 58:411–3. doi: 10.1016/j.lungcan.2007.05.014
52. Piotrowska Z, Niederst MJ, Karlovich CA, Wakelee HA, Neal JW, Mino-Kenudson M, et al. Heterogeneity Underlies the Emergence of EGFRT790 Wild-Type Clones Following Treatment of T790M-Positive Cancers With a Third-Generation EGFR Inhibitor. Cancer Discovery (2015) 5:713–22. doi: 10.1158/2159-8290.CD-15-0399
53. Kim WJ, Kim S, Choi H, Chang J, Shin HJ, Park CK, et al. Histological Transformation From Non-Small Cell to Small Cell Lung Carcinoma After Treatment With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor. Thorac Cancer (2015) 6:800–4. doi: 10.1111/1759-7714.12217
54. Jin CB, Yang L. Histological Transformation of Non-Small Cell Lung Cancer: Clinical Analysis of Nine Cases. World J Clin cases (2021) 9:4617–26. doi: 10.12998/wjcc.v9.i18.4617
55. Xie T, Li Y, Ying J, Cai W, Li J, Lee KY, et al. Whole Exome Sequencing (WES) Analysis of Transformed Small Cell Lung Cancer (SCLC) From Lung Adenocarcinoma (LUAD). Transl Lung Cancer Res (2020) 9:2428–39. doi: 10.21037/tlcr-20-1278
56. Yang H, Liu L, Zhou C, Xiong Y, Hu Y, Yang N, et al. The Clinicopathologic of Pulmonary Adenocarcinoma Transformation to Small Cell Lung Cancer. Medicine (2019) 98:e14893. doi: 10.1097/MD.0000000000014893
57. Lin MW, Su KY, Su TJ, Chang CC, Lin JW, Lee YH, et al. Clinicopathological and Genomic Comparisons Between Different Histologic Components in Combined Small Cell Lung Cancer and Non-Small Cell Lung Cancer. Lung Cancer (Amsterdam Netherlands) (2018) 125:282–90. doi: 10.1016/j.lungcan.2018.10.006
58. Minari R, Bordi P, Del Re M, Facchinetti F, Mazzoni F, Barbieri F, et al. Primary Resistance to Osimertinib Due to SCLC Transformation: Issue of T790M Determination on Liquid Re-Biopsy. Lung Cancer (Amsterdam Netherlands) (2018) 115:21–7. doi: 10.1016/j.lungcan.2017.11.011
59. June-Koo Lee JL, Kim S, Kim S, Kim S, Youk J, Park S, et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol (2017) 35:26. doi: 10.1200/JCO10.1200/JCO.2016
60. Ahn S, Hwang SH, Han J, Choi YL, Lee SH, Ahn JS, et al. Transformation to Small Cell Lung Cancer of Pulmonary Adenocarcinoma: Clinicopathologic Analysis of Six Cases. J Pathol Trans Med (2016) 50:258–63. doi: 10.4132/jptm.2016.04.19
61. Norkowski E, Ghigna MR, Lacroix L, Le Chevalier T, Fadel É, Dartevelle P, et al. Small-Cell Carcinoma in the Setting of Pulmonary Adenocarcinoma: New Insights in the Era of Molecular Pathology. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2013) 8:1265–71. doi: 10.1097/JTO.0b013e3182a407fa
62. Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of Origin of Small Cell Lung Cancer: Inactivation of Trp53 and Rb1 in Distinct Cell Types of Adult Mouse Lung. Cancer Cell (2011) 19:754–64. doi: 10.1016/j.ccr.2011.04.019
63. Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at Risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2019) 14:1784–93. doi: 10.1016/j.jtho.2019.06.002
64. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients With Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2014) 9:154–62. doi: 10.1097/jto.0000000000000033
65. Le X, Desai NV, Majid A, Karp RS, Huberman MS, Rangachari D, et al. De Novo Pulmonary Small Cell Carcinomas and Large Cell Neuroendocrine Carcinomas Harboring EGFR Mutations: Lack of Response to EGFR Inhibitors. Lung Cancer (Amsterdam Netherlands) (2015) 88:70–3. doi: 10.1016/j.lungcan.2015.02.003
66. Jones GS, Elimian K, Baldwin DR, Hubbard RB, McKeever TM, et al. A Systematic Review of Survival Following Anti-Cancer Treatment for Small Cell Lung Cancer. Lung Cancer (Amsterdam Netherlands) (2020) 141:44–55. doi: 10.1016/j.lungcan.2019.12.015
67. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (Impower133). J Clin Oncol: Off J Am Soc Clin Oncol (2021) 39:619–30. doi: 10.1200/jco.20.01055
Keywords: lung adenocarcinoma, epidermal growth factor receptor (EGFR), transformation, small-cell lung cancer (SCLC), systematic review
Citation: Xu J, Xu L, Wang B, Kong W, Chen Y and Yu Z (2022) Outcomes in Patients With Lung Adenocarcinoma With Transformation to Small Cell Lung Cancer After EGFR Tyrosine Kinase Inhibitors Resistance: A Systematic Review and Pooled Analysis. Front. Oncol. 11:766148. doi: 10.3389/fonc.2021.766148
Received: 28 August 2021; Accepted: 31 December 2021;
Published: 28 January 2022.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian Weill Cornell Medical Center, United StatesReviewed by:
Maria Lucia Reale, San Luigi Gonzaga University Hospital, ItalyCopyright © 2022 Xu, Xu, Wang, Kong, Chen and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongyang Yu, eXV6eTUyN0BzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.