- 1Department of Gastroenterology, Yancheng Third People’s Hospital, Yancheng, China
- 2School of Medicine, Zhejiang University, Hangzhou, China
- 3Chu Kochen Honors College, Zhejiang University, Hangzhou, China
- 4Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Liver cirrhosis tends to increase the risk in the management of gastrointestinal tumors. Patients with gastrointestinal cancers and liver cirrhosis often have serious postoperative complications and poor prognosis after surgery. Multiple studies have shown that the stage of gastrointestinal cancers and the grade of cirrhosis can influence surgical options and postoperative complications. The higher the stage of cancer and the poorer the degree of cirrhosis, the less the surgical options and the higher the risk of postoperative complications. Therefore, in the treatment of patients with gastrointestinal cancer and liver cirrhosis, clinicians should comprehensively consider the cancer stage, cirrhosis grade, and possible postoperative complications. This review summarizes the treatment methods of patients with different gastrointestinal cancer complicated with liver cirrhosis.
Introduction
Gastrointestinal cancers and liver cirrhosis are common diseases worldwide. A significant number of patients with gastrointestinal cancer also suffer from liver cirrhosis. Gastrointestinal tumors mainly include esophageal cancer, liver cancer, gastric cancer, pancreatic cancer, and colorectal cancer (1, 2). Gastrointestinal cancer is the third most common cause of cancer-related death worldwide, and its incidence is on the rise globally. More than 15% of newly diagnosed cancer cases and 17% of cancer deaths are associated with gastrointestinal cancers (3, 4). The treatment of gastrointestinal cancers has been a focus of research due to their high morbidity and mortality (5). As a common complication of gastrointestinal cancer, liver cirrhosis makes its treatment more complicated.
It is well known that liver cirrhosis is not only a risk factor of primary liver cancer but also increases the risk of extrahepatic malignancies. Compared with non-cirrhotic patients, patients with cirrhosis had an increased risk of poor prognosis for non-hepatic abdominal surgery (6). More and more studies have confirmed that the presence of cirrhosis has a great impact on the surgical outcome and prognosis of patients with gastrointestinal cancers. In this review, we summarize the treatment for patients with different gastrointestinal cancers complicated with liver cirrhosis and focus on the analysis on the influence of different grades of liver cirrhosis on the treatment and postoperative complications, aiming to provide guidance for treatment options for patients with gastrointestinal cancers and liver cirrhosis.
General Introduction of Liver Cirrhosis
Liver cirrhosis (LC) is the final stage of liver fibrosis and a wound-healing reaction of chronic liver injury. The main causes of liver cirrhosis include alcoholic hepatitis, hepatitis B, hepatitis C, and non-alcoholic fatty hepatitis (7). LC is characterized by abnormal liver structure and function, accompanied by fibrous septum and nodule formation and changes in blood flow (8). The process of LC has two stages. The LC compensated period is a long-term asymptomatic phase of fibrosis, while LC decompensated period is a rapidly progressive phase with complications of portal hypertension and liver function impairment, including ascites, varicose vein bleeding, encephalopathy, jaundice, and more (9–11). LC is usually accompanied by complex alterations in the hemostatic system. Patients suffering from LC have few platelets and prolonged prothrombin time, resulting in a high rate of bleeding during surgery (12, 13). Therefore, the presence of liver cirrhosis increases the risk of treatment for gastrointestinal cancers.
Prognostic models and staging systems are instructive for the appropriate treatment of patients with liver diseases (14–16). Patients with gastrointestinal cancer and cirrhosis were mainly involved in two broad staging systems, namely, the Child–Turcotte–Pugh (CTP) score and Model for End-Stage Liver Disease (MELD) score.
The CTP score was first proposed by Child and Turcotte to predict the outcome of patients receiving portal shunt for variceal bleeding. The grades of encephalopathy and ascites and serum bilirubin, albumin, and prothrombin time were integrated into the scoring model for comprehensive consideration (17). It is also believed that the prothrombin index or international normalized ratio (INR) can be used instead of prothrombin time (14). Each variable is assigned 1–3 points. According to the specific conditions of LC, patients can be divided into three prognostic subgroups: CTP A grade (5–6 points), B grade (7–9 points), and C grade (10–15 points) (14, 17). The main limitations of the CTP score are the cutoff value of different variables and the clinical variables that need to be included in the subjective assessment (14).
The MELD score was originally designed to predict the prognosis of transjugular intrahepatic portal system shunt and was later found to be an accurate predictor of mortality in patients with end-stage liver diseases (18). The three objective variables of serum bilirubin, serum creatinine, and INR were integrated into the MELD scoring model. Compared with the CTP score, the main advantage of the MELD score model is that it is more finely layered. In addition, it includes serum creatinine, which is an important factor predicting the survival of patients with liver disease (19–21). Its limitations include the need for calculation, which makes it less convenient than the CTP score to be used in daily clinical practice and the lack of well-defined subcategories to assess the risk of personal death (14).
The staging of cirrhosis plays an important role in the treatment of patients with gastrointestinal cancers and cirrhosis. Patients with mild cirrhosis can receive most cancer treatments without serious postoperative complications, while patients with severe cirrhosis can only be treated conservatively.
Treatment of Gastric Cancer With Cirrhosis and Its Influencing Factors
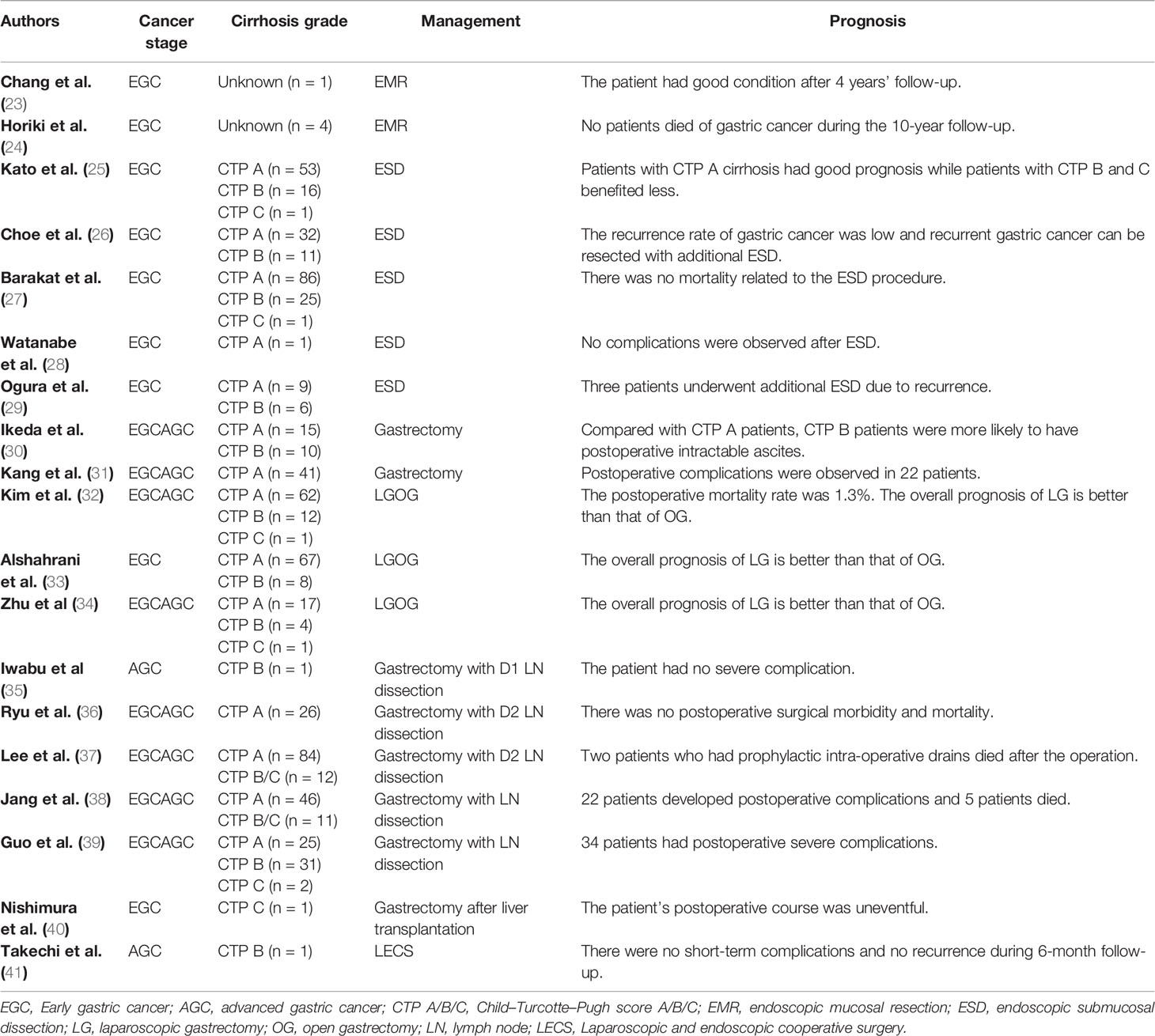
The presence of LC has a great influence on the surgical effect and prognosis of patients with gastric cancer. In a central cohort study, Zhou et al. explored the independent risk factors of gastrectomy postoperative complications in patients with gastric cancer and cirrhosis, and they found that cirrhosis is the largest independent risk factor for postoperative complications (22). This is consistent with the conclusion drawn by Jeong et al. (23). Similarly, Zullo et al. confirmed that cirrhosis appears to be a risk factor for the development of gastric cancer (24). The existence of LC increases the difficulty in the treatment of gastric cancer patients. The cirrhosis grade, gastric cancer stage, and the choice of different treatments have been discussed in several studies (Table 1). So far, a variety of treatment modalities have been reported for the treatment of gastric cancer, including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), gastrectomy, lymph node dissection, and other treatment methods.
EMR and ESD
EMR and ESD are commonly used for the treatment of early gastric cancer (EGC). EMR is suitable for lesions smaller than 10–15 mm with a very low probability of advanced histology (25). Chang et al. reported a 58-year-old male patient with gastric cancer and cirrhosis who had a good prognosis after receiving EMR. Although focal submucosal invasion occurred, there was no evidence of gastric cancer recurrence (26). In addition, Horiki et al. reported in a retrospective cohort study that four patients with gastric cancer and cirrhosis received EMR, with no patients died of the cancer and no postoperative complications occurred in the 10-year follow-up period. Therefore, it is believed that EMR is safe for patients with gastric cancer and severe comorbidities (27).
ESD enables the overall excision of large or ulcerative lesions based on EMR. A multicenter retrospective study from Japan showed that patients with CTP A cirrhosis and no hepatic cell carcinoma history are good candidates for ESD, while CTP B or C patients or with histories of HCC would benefit less from receiving ESD. In the study, LC patients had complications of bleeding and perforation (28). Choe et al. mentioned that ESD could be employed in patients with EGC and LC and in CTP B cirrhosis patients. Postoperative complications, such as bleeding and perforation, may occur (29). Barakat et al. evaluated the safety and effectiveness of ESD, and they confirmed that it is safe for EGC patients with LC to receive ESD, and ESD can effectively control the bleeding caused by CTP B or C cirrhosis during surgery (42). In addition, many studies have also proved that ESD is effective and can achieve a high overall resection rate (30, 31).
According to the above studies, EMR and ESD are suitable for EGC, and the main postoperative complications are bleeding and perforation. ESD is safe and feasible for patients with gastric cancer and CTP A cirrhosis. Due to the increased risk of bleeding, there are different opinions on whether patients with CTP B and C cirrhosis should receive ESD. More studies are needed to explore whether patients with CTP B and C cirrhosis would be beneficial to receive ESD.
Gastrectomy
Radical gastrectomy is indicated for patients with stage IB–III gastric cancer (32). Ikeda et al. compared the prognosis of gastric cancer patients with CTP A cirrhosis and those with CTP B cirrhosis after gastrectomy. They found that radical gastrectomy is safe and feasible for patients with CTP A and CTP B cirrhosis, and the most common postoperative complication is refractory ascites (33).
Radical gastrectomy mainly includes laparoscopic gastrectomy (LG) and open gastrectomy (OG). Kang et al. confirmed that LG is a feasible surgical method for patients with moderate liver dysfunction (34). Kim et al. found that patients had complications of ascites, gastric stasis, and wound infection after radical gastrectomy. After comparing the surgical outcomes of LG and OG in patients, they determined that LG combined with lymph node (LN) dissection is safer than OG in gastric cancer patients with CTP A and B cirrhosis (35). In addition, a retrospective study from Korea concluded that LG is superior to OG in terms of long-term survival and postoperative liver function recovery, and patients with cirrhosis experienced postoperative complications such as bleeding and wound infection (36). Zhu et al. showed that the surgical effect of LG is better than that of OG, and the involved patients developed ascites, wound infection, and other complications (37). Many studies revealed that LG is better than OG by comparing the efficacy and safety of LG and OG.
The surgical complications of gastrectomy are ascites, wound infection, and postoperative bleeding. Compared with OG, LG has a shorter operation time, less surgical blood loss, and shorter hospital stay (35, 36). Therefore, both LG and OG can be employed for the treatment of gastric cancer patients complicated with CTP A and CTP B cirrhosis. The effect of LG is better than that of OG. However, due to the small number of samples of gastric cancer patients with CTP C cirrhosis, the therapeutic effect of gastrectomy warrants further explore.
Lymph Node Dissection
Gastric cancer often metastasizes to the lymph nodes, and doctors perform LN dissection at the same time as gastrectomy for radical excision. The extent of gastrectomy with lymph node dissection has been widely debated. According to the extent of lymph node dissection, LN dissection can be divided into D1 and D2 LN dissection. D1 LN dissection refers to the removal of perigastric lymph nodes, and D2 LN dissection refers to the removal of perigastric lymph nodes plus lymph nodes around the left stomach, common hepatic and splenic arteries, and coeliac axis (32).
Iwabu et al. reported that a 58-year-old man with gastric cancer and CTP B cirrhosis had a good prognosis without serious complications after undergoing gastrectomy with D1 LN dissection (38). Several multiple retrospective analyses confirmed that D2 LN dissection is safe for patients with gastric cancer and CTP A cirrhosis, and some patients in the study developed complications of ascites and wound infection (39, 40). Jang et al. demonstrated the feasibility of receiving D2 LN dissection in gastric cancer patients with CTP A cirrhosis by retrospective analysis. For patients with moderate or severe liver dysfunction, D1 or smaller ranges of LN dissection appears to be a more reasonable surgical option. The frequency of complications in patients with CTP B and C cirrhosis is higher than that of patients with CTP A cirrhosis. The involved complications mainly include ascites, wound infection, and hepatic encephalopathy (41). Guo et al. suggested that gastric cancer patients with CTP A cirrhosis could receive D1 or D2 LN dissection, while patients with CTP B cirrhosis could only receive D1 LN dissection. Moreover, it is very dangerous to perform LN dissection in patients with CTP C cirrhosis. In this study, 34 patients (58.6%) developed complications of ascites, postoperative bleeding, anastomosis leakage, pneumonia, and hepatic failure (43).
Complications of ascites, postoperative bleeding, and wound infection may occur in patients during LN dissection. How to determine the extent of LN dissection has been a difficulty in treatment, mainly because excessive LN dissection could lead to increased risk of postoperative bleeding and ascites, and small LN dissection may cause incurable treatment, affecting the prognosis of patients. The grade of liver cirrhosis should be considered when determining the extent of LN dissection. In general, gastric cancer patients with CTP A cirrhosis can receive D1 or D2 dissection, and patients with CTP B cirrhosis should receive D1 or less lymph node dissection. There is currently no good dissection treatment for CTP C cirrhosis patients. However, Lee et al. proposed that it might be feasible to improve the cirrhosis and then perform LN dissection for patients with CTP C cirrhosis (40).
Other Treatment Methods
In addition to the treatments mentioned above for gastric cancer patients with cirrhosis, there are other cases reported with different treatment methods. Nishimura et al. reported that a 64-year-old woman with gastric cancer and CTP C cirrhosis first received liver transplantation, which improved liver and coagulation function. Nineteen days after liver transplantation, she underwent gastrectomy with no significant postoperative complications. This case provides new ideas for the treatment of gastric cancer patients with CTP C cirrhosis (44). A 68-year-old woman with high risk of advanced gastric cancer (AGC) and cirrhosis was reported to successfully receive laparoscopic and endoscopic cooperative surgery (LECS) without short-term postoperative complications nor recurrence after 6 months of follow-up. Therefore, LECS is a feasible palliative treatment for patients with AGC complicated with cirrhosis (45).
The surgical effects and prognosis of patients with EGC and AGC are different. The cirrhosis grade and postoperative complications also have a great influence on the treatment effect of patients. In the treatment of patients with gastric cancer and cirrhosis, cancer stage, cirrhosis grade, and postoperative complications should be taken into comprehensive consideration, so as to select the appropriate surgical plan for patients (Figure 1).
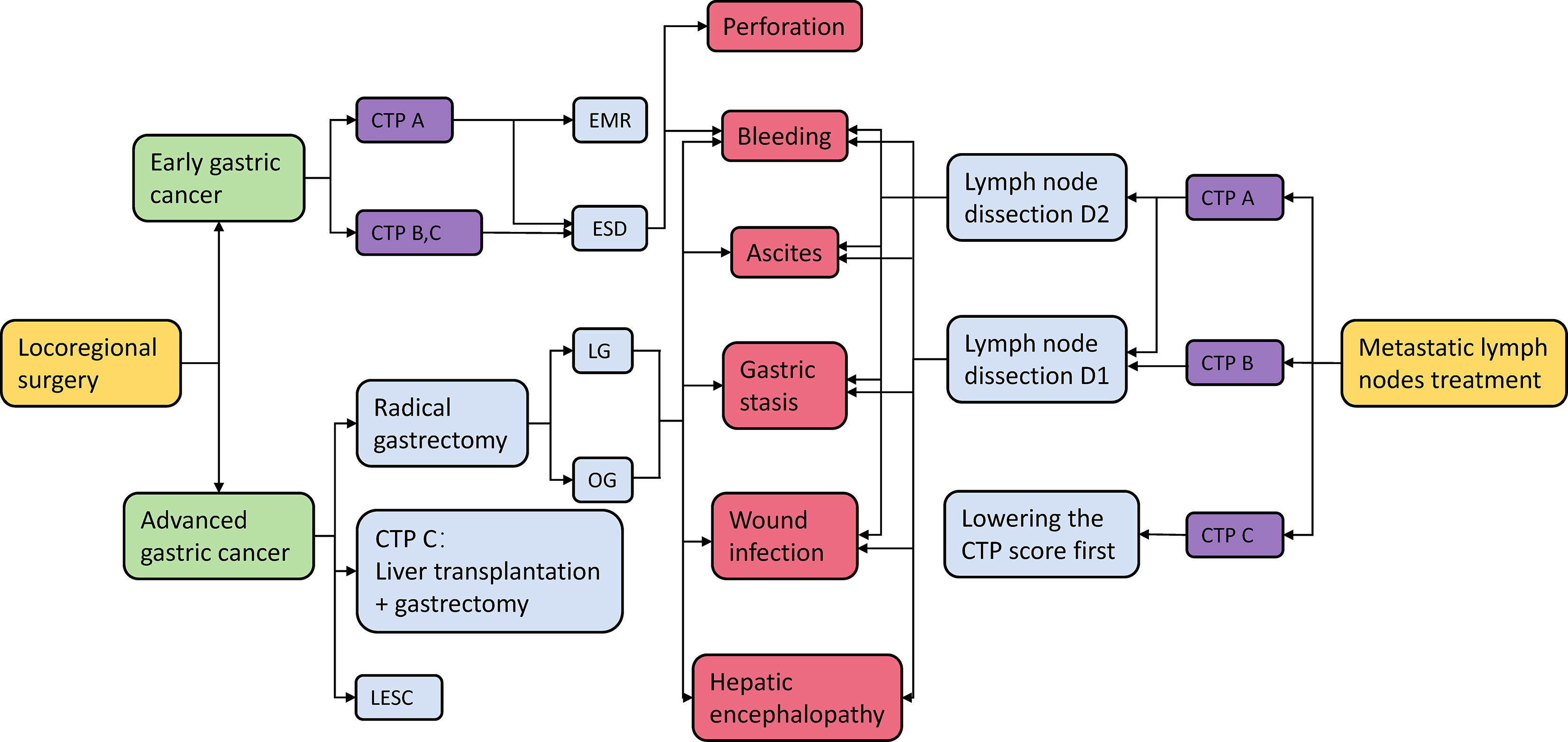
Figure 1 Gastric cancer treatment options and their possible complications. CTP A/B/C, Child–Turcotte–Pugh score A/B/C; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; LG, laparoscopic gastrectomy; OG, ipen gastrectomy; LECS, laparoscopic and endoscopic cooperative surgery.
Treatment of Esophageal Cancer With Cirrhosis and Its Influencing Factors
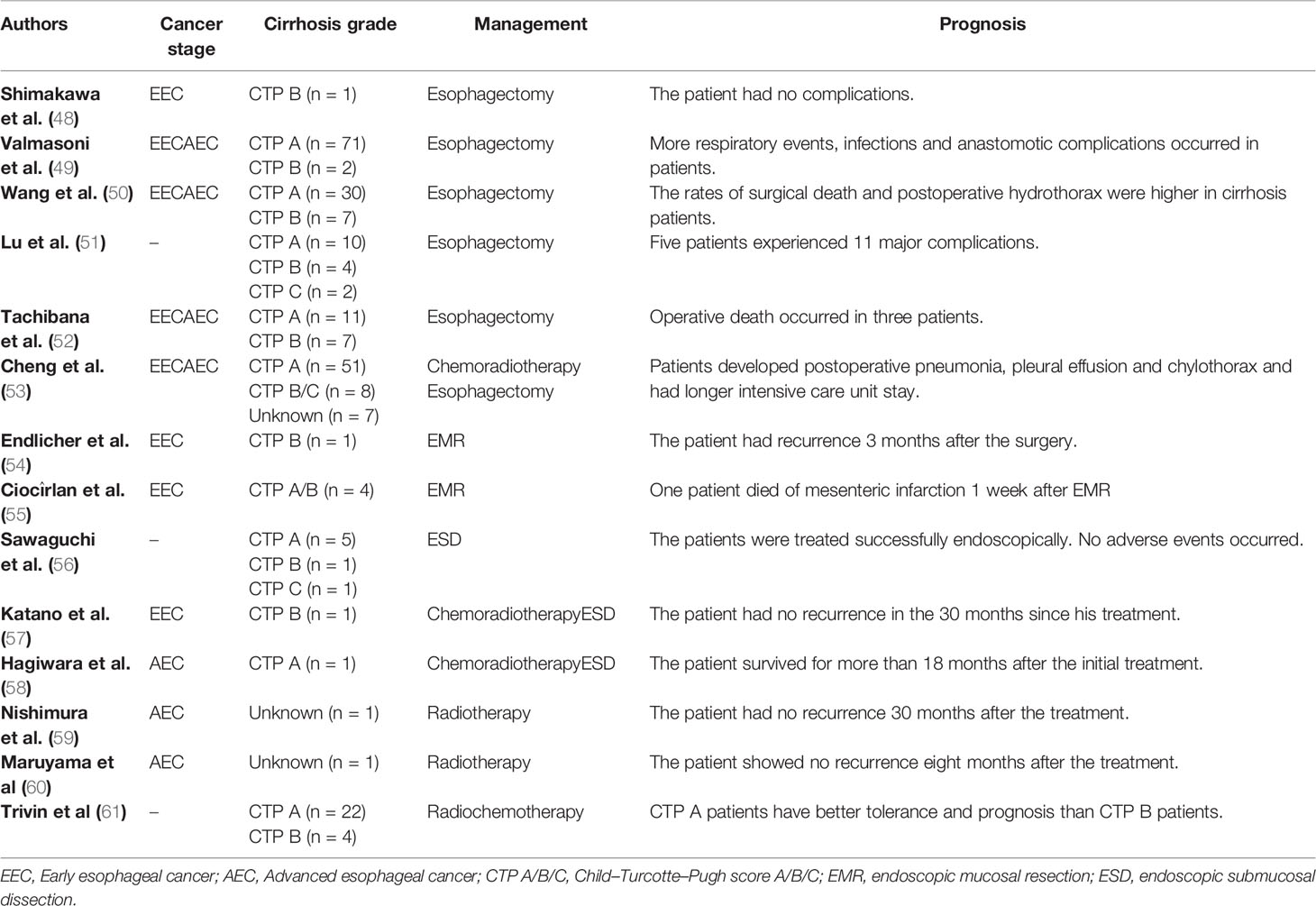
As the sixth most common cancer in the world, esophageal cancer is estimated to cause 450,000 deaths per year (46). There are two subtypes of esophageal carcinoma: squamous esophageal cell carcinoma and esophageal gland cancer. Since increased alcohol consumption is a common risk factor of esophageal cancer and cirrhosis, patients diagnosed with esophageal cancer and cirrhosis are not rare (47, 48). By analyzing the factors that affect postoperative mortality after esophagectomy, Sanz et al. found that cirrhosis is significantly associated with postoperative complications and mortality (49). Similarly, González-González et al. suggested that the presence of cirrhosis increases the incidence of complications (50). The presence of cirrhosis has a great impact on the surgery and prognosis of patients with esophageal cancer, so it is necessary to discuss the treatment methods and prognosis of esophageal cancer patients complicated with cirrhosis (Table 2).
Esophagectomy
Esophagectomy is a treatment for resectable early esophageal cancer (EEC). A 52-year-old man with esophageal cancer and with alcoholic CTP B cirrhosis was reported to successfully receive esophagectomy with a good prognosis and no significant complications (62). Valmasoni et al. demonstrated that esophagectomy can be performed in esophageal cancer patients with cirrhosis in a cohort study (63). A retrospective study from China also confirmed that esophagectomy is a feasible and beneficial treatment option for patients with esophageal cancer and cirrhosis (51).
The cirrhosis grade can affect the safety and postoperative complications of esophagectomy. Schizas et al. confirmed that compared with patients with CTP B cirrhosis, there is a significant reduction in mortality from esophagectomy in those with CTP A cirrhosis (52). In evaluating the effectiveness of esophagectomy in patients with cirrhosis and non-cirrhosis, Deng et al. found that the incidence of pulmonary complications, pleural effusion, and anastomosis leakage in patients with cirrhosis is higher than that in patients without cirrhosis. Moreover, they proposed that patients with CTP A cirrhosis could receive esophagectomy, while patients with CTP B and C cirrhosis were unsuitable for esophagectomy because of the high risk of complications (53). This conclusion is consistent with that of Lu et al. (64). A retrospective analysis from Japan suggested that patients with CTP A or CTP B cirrhosis could be treated with esophagectomy despite the high morbidity and mortality. Postoperative complications in patients mainly include pleural effusion, recurrent laryngeal nerve paralysis (RLNP), and pneumonia (54). A retrospective study from China reported that esophageal cancer patients with CTP A cirrhosis can receive esophagectomy for treatment. Patients are prone to postoperative complications of pneumonia, pleural effusion, and chylothorax (55).
Generally, esophagectomy can be performed in esophageal cancer patients with CTP A cirrhosis, but it is not clear whether patients with CTP B and C cirrhosis can receive esophagectomy. Therefore, preoperative stratification and prevention of postoperative complications can effectively reduce the risk of surgery.
EMR and ESD
Both endoscopic mucosal resection and endoscopic submucosal dissection are effective endoscopic resection methods. Endoscopic resection has a similar cure rate in some specialized centers compared to esophagectomy (56). Endlicher et al. reported that a 71-year-old woman was diagnosed with esophageal squamous cell carcinoma and alcoholic cirrhosis, and she successfully underwent EMR after ligation of esophageal varices without major bleeding complications (57). Ciocîrlan et al. reported four patients with early esophageal squamous cell carcinoma and cirrhosis, and they believed that EMR was feasible for their treatment (58). By evaluating the effectiveness and safety of ESD in the treatment of superficial esophageal carcinoma with cirrhosis and esophageal varices, a study from Japan concluded that ESD is a feasible modality for the treatment of esophageal squamous cell carcinoma in patients with cirrhosis (59).
Treatments of esophageal cancer with cirrhosis are not limited to single endoscopic resection. Katano et al. reported a case of esophageal submucosal invasive carcinoma that could not be surgically treated due to CTP B cirrhosis. They successfully treated patients with salvage ESD after chemoradiotherapy, and the patient had no obvious complications after surgery (60). A patient with advanced esophageal cancer (AEC) and CTP A alcoholic cirrhosis was reported to successfully undergo ESD after chemotherapy, and the patient had no significant postoperative complications (61).
According to the above studies, patients with cirrhosis complicated with esophageal cancer can accept ESD and EMR treatment with no obvious postoperative complications. However, the influence of cirrhosis grade on the effect of EMR and ESD surgery is not clear, and further study is needed.
Chemoradiotherapy
Nishimura et al. reported a case of AEC complicated with cirrhosis in a 69-year-old man who was successfully treated with radiotherapy. The patient did not relapse within 30 months of treatment, which suggested that radiotherapy is an effective treatment for AGC with poor general condition (65). Moreover, a male patient with multiple superficial esophageal cancer and cirrhosis was reported to be successfully treated with radiotherapy, and he had no recurrence within 8 months (66). A retrospective study from France showed that patients with CTP A cirrhosis are tolerant to chemoradiotherapy, while patients with CTP B cirrhosis should be treated with weaker rather than conventional chemoradiotherapy regimens (67).
Radiotherapy is an effective way to treat esophageal cancer complicated with cirrhosis. The strategy of chemoradiotherapy should be based on the grade of cirrhosis of patients, and doctors should formulate an appropriate chemotherapy regime according to the general condition of patients.
Due to the lack of studies, the influence of cirrhosis grade on the treatment choice of esophageal cancer patients is not completely clear, and more efforts are needed to explore the relationship. When choosing an appropriate surgical plan for patients with esophageal cancer and cirrhosis, the stage of esophageal cancer, grade of cirrhosis, and postoperative complications should be comprehensively considered (Figure 2).
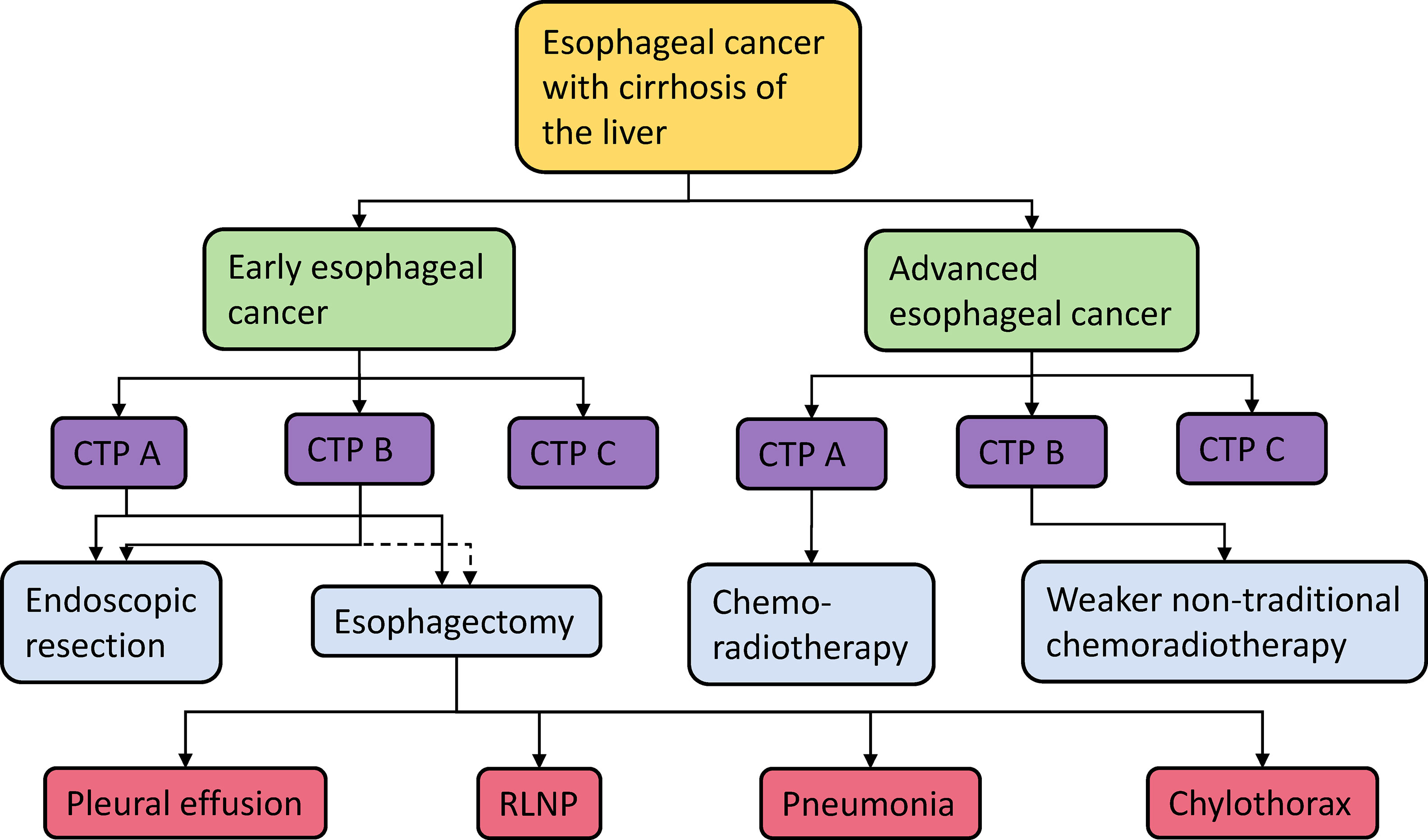
Figure 2 Esophageal cancer treatment options and their possible complications (The dotted line indicates inconsistent conclusions about whether patients can accept this treatment method). CTP A/B/C, Child–Turcotte-Pugh score A/B/C; RLNP, recurrent laryngeal nerve palsy.
Treatment of Colorectal Cancer With Cirrhosis and Its Influencing Factors
CRC is the third most common cancer worldwide and the second leading cause of cancer-related death (68). Most colorectal cancers are adenocarcinomas, and a few are squamous epithelial carcinoma and mucinous carcinoma. LC is a known risk factor of CRC (69, 70). CRC patients with cirrhosis are more likely to have complications than patients without cirrhosis. A cohort study from Danish population showed that the 30-day postoperative mortality was 24.1% in patients with cirrhosis and only 8.7% in patients without cirrhosis, indicating a significant increase in postoperative mortality (71). This is consistent with the results of other studies (72–76). Han et al. demonstrated that CRC patients with cirrhosis had a higher rate of intraoperative blood loss, higher likelihood of postoperative complications, and longer hospital stay (75). Shin et al. found that LC determines the prognosis of patients with CRC, regardless of cancer stage (73). CRC is a common disease, and surgery is its main treatment. LC is unfavorable for CRC surgery (73, 76).
Preoperative assessment of liver function is required to determine its severity before patients with CRC and cirrhosis undergo treatment, which could be evaluated by CTP score or MELD score (77, 78). By comparing the surgical outcomes of CRC patients with different cirrhosis grades, previous studies found that patients with CTP B cirrhosis had a higher incidence of complications and needed more intervention and longer hospital stay (76, 79). Meunier et al. reported that the leakage rate of CRC surgery in patients with cirrhosis was 18%, and nearly 60% of the patients suffered from CTP B or C cirrhosis (80). Severe cirrhosis of the liver, such as CTP B and C cirrhosis, may prolong wound healing and increase the risk of anastomosis leakage (81). The MELD score is also a good prognostic model for patients with cirrhosis (82, 83). CRC patients with cirrhosis could receive surgical treatment when the MELD score is <10. However, therapy to improve liver function should be performed until the MELD score is <10 when the MELD score is >10, in order to achieve better survival outcomes (75).
Radical Resection
Radical resection aims to eradicate the primary tumor, such as partial and total removal of the colon and rectum, which can be further divided into laparotomy and open radical resection. In a retrospective study from China, the average blood loss in the CRC patients with cirrhosis who underwent open radical resection was high. These patients showed high recurrence and mortality rates in follow-ups (76). Bleeding during surgery is a common and worrying complication of cirrhosis (73, 84). It is reported that estimated blood loss in patients with cirrhosis who have undergone CRC surgery is between 148 and 245 ml, which is higher than normal surgical blood loss (75, 84). Due to increased bleeding caused by cirrhosis, the difficulty of performing open radical resection and the postoperative morbidity and mortality increase significantly.
Laparotomy has high feasibility, safety, and effectiveness in managing CRC patients with cirrhosis. Compared with open radical resection, laparotomy has great advantages in reducing blood loss, shortening hospital stay, and reducing complications (85, 86). Zhou et al. suggested that laparotomy can reduce postoperative complications in patients with CRC and cirrhosis to a certain extent. In some patients with cirrhosis, laparotomy appears to be a safe, less invasive surgical alternative that can reduce bleeding and improve early recovery without additional harm to patients (81).
Interestingly, there are some controversial conclusions regarding the treatment of CRC patients with LC. Montomoli et al. examined risk factors for 30-day mortality after surgery in patients with CRC complicated with cirrhosis. They found that the relative risk of laparotomy was 6.82, while that of open radical resection was 3.01, indicating that laparoscopic resection may have a higher risk of mortality than laparotomy (71). In addition, Sabbagh et al. suggested that open radical resection should be preferred in patients with cirrhosis for colon surgery (87).
Adjuvant Therapy
Anticancer drugs must be carefully selected when chemoradiotherapy is performed on CRC patients with cirrhosis (88). In particular, the anticancer drug oxaliplatin can lead to increased risk of varicose veins, digestive hemorrhage, ascites, and portal hypertension in CRC patients, leading to poor prognosis of patients (89). Portal hypertension caused by cirrhosis must be taken into account. Madbouly et al. showed that chemoradiotherapy based on oxaliplatin does not significantly reduce cancer-specific mortality and may increase overall mortality and morbidity (90).
The use of vitamin K and the administration of fresh-frozen plasma for coagulation are alternative ideas for treatment. Careful control of bleeding may reduce postoperative bleeding (80, 91).
Treatment of Pancreatic Cancer With Cirrhosis and Its Influencing Factors
The presence of cirrhosis can affect the outcomes of pancreatic cancer treatment and the risk of postoperative complications. In a study to verify the safety of pancreatic surgery in patients with cirrhosis, Warnick et al. demonstrated that cirrhosis increases the risk of postoperative complications in patients undergoing the surgery (92). Therefore, it is very important to investigate the treatment methods and postoperative complications of patients with pancreatic cancer combined with cirrhosis.
Pancreaticoduodenectomy is the most common treatment for pancreatic cancer. Sahaab et al. reported a 71-year-old patient with pancreatic cancer and cirrhosis, and he had a good prognosis after pancreaticoduodenectomy with minor chyle leak (93). A retrospective study from Egypt confirmed an increased risk of postoperative complications in patients with cirrhosis who underwent pancreaticoduodenectomy. There was a significant increase in wound complications, internal organ bleeding, pancreatic fistula, and hospital mortality in patients with cirrhosis (94). Schizas et al. proposed that wound infection, ascites, and anastomosis leakage are the most common postoperative complications in patients with cirrhosis who undergo pancreatic cancer surgery, while non-cirrhosis patients are less likely to develop these complications (95). It is of great significance to consider postoperative complications in the treatment of patients with pancreatic cancer and cirrhosis.
The cirrhosis grade has an impact on treatment options. Fuks et al. reported successful pancreaticoduodenectomy in four patients with CTP A cirrhosis, and they confirmed that pancreaticoduodenectomy is feasible in patients with CTP A cirrhosis and pancreatic cancer. Similarly, a retrospective study from France also suggested that pancreaticoduodenectomy is feasible for patients with CTP A cirrhosis, whereas CTP B cirrhosis remains a contraindication for pancreaticoduodenectomy (96). Therefore, patients with CTP A cirrhosis and pancreatic cancer can receive pancreaticoduodenectomy, and doctors should take a conservative attitude towards patients with CTP B and C cirrhosis for pancreaticoduodenectomy.
At present, pancreaticoduodenectomy is the main method for the treatment of pancreatic cancer complicated with LC. In the course of treatment, doctors should consider the grade of cirrhosis and the risk of postoperative complications in patients.
Conclusion and Perspectives
Gastrointestinal cancers mainly include gastric cancer, esophageal cancer, colorectal cancer, and pancreatic cancer. Compared with non-cirrhosis patients with gastrointestinal cancer, patients with cirrhosis have poor treatment outcomes and prognosis. The grade of cirrhosis might limit the treatment choice of patients. Patients with mild cirrhosis can usually receive surgical treatment, while patients with severe cirrhosis should be treated conservatively. The consideration of postoperative complications plays an important role in choosing treatment modality in patients with gastrointestinal cancer and cirrhosis. Patients with gastrointestinal cancer and cirrhosis have an increased risk of postoperative complications after surgery. Different treatment methods may cause different postoperative complications, and the severity of postoperative complications is also different. More studies are needed to investigate the proper treatment options for patients with gastrointestinal cancer and different grade of LC.
In conclusion, the treatment of gastrointestinal cancer complicated with cirrhosis is not limited to the radical treatment of cancer. Doctors should consider the actual situation of gastrointestinal cancer, cirrhosis grade, and possible postoperative complications before treating patients.
Author Contributions
ZX, YL, and CZ conceived and designed the article. TH and XH performed the literature search and data analysis. HZ and DJ drafted and critically revised the work. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Beijing Bethune Charitable Foundation (No. B19296ES).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XS declared a shared parent affiliation with several of the authors ZX, YL, CZ, TH, and XH to the handling editor at the time of the review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful for grants received from the Beijing Bethune Charitable Foundation.
Abbreviations
LC, liver cirrhosis; CTP, Child–Turcotte–Pugh; MELD, Model For End-Stage Liver Disease; INR, international normalized ratio; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; EGC, early gastric cancer; LG, laparoscopic gastrectomy; OG, open gastrectomy; LN, lymph node; AGC, advanced gastric cancer; LECS, laparoscopic and endoscopic cooperative surgery; EEC, early esophageal cancer; RLNP, recurrent laryngeal nerve palsy; AEC, advanced esophageal cancer; CRC, colorectal cancer.
References
1. Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol (2015) 110(2):223–62. doi: 10.1038/ajg.2014.435 quiz 63.
2. Thomas RM, Sobin LH. Gastrointestinal Cancer. Cancer (1995) 75(S1):154–70. doi: 10.1002/1097-0142(19950101)75:1+<154::AID-CNCR2820751305>3.0.CO;2-Z
3. Aoyama T, Nakazono M, Nagasawa S, Segami K. Clinical Impact of Perioperative Oral Nutritional Treatment for Body Composition Changes in Gastrointestinal Cancer Treatment. Anticancer Res (2021) 41(4):1727–32. doi: 10.21873/anticanres.14937
4. Sharma KL, Bhatia V, Agarwal P, Kumar A. Gastrointestinal Cancers: Molecular Genetics and Biomarkers. Can J Gastroenterol Hepatol (2018) 2018:4513860. doi: 10.1155/2018/4513860
5. Wang Y, Wu J, Xu J, Lin S. Clinical Significance of High Expression of Stanniocalcin-2 in Hepatocellular Carcinoma. Biosci Rep (2019) 39(4):BSR20182057. doi: 10.1042/bsr20182057
6. Lopez-Delgado JC, Ballus J, Esteve F, Betancur-Zambrano NL, Corral-Velez V, Manez R, et al. Outcomes of Abdominal Surgery in Patients With Liver Cirrhosis. World J Gastroenterol (2016) 22(9):2657–67. doi: 10.3748/wjg.v22.i9.2657
7. GBD 2013 Mortality and Causes of Death CollaboratorsGlobal, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet (London England) (2015) 385(9963):117–71. doi: 10.1016/s0140-6736(14)61682-2
8. J J, F SL, A C. Hepatic Fibrosis. Curr Opin Gastroenterol (2009) 25:223–9. doi: 10.1097/MOG.0b013e3283279668
9. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural History and Prognostic Indicators of Survival in Cirrhosis: A Systematic Review of 118 Studies. J Hepatol (2006) 44(1):217–31. doi: 10.1016/j.jhep.2005.10.013
10. de Franchis R. Updating Consensus in Portal Hypertension: Report of the Baveno III Consensus Workshop on Definitions, Methodology and Therapeutic Strategies in Portal Hypertension. J Hepatol (2000) 33(5):846–52. doi: 10.1016/s0168-8278(00)80320-7
11. Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, et al. Compensated Cirrhosis: Natural History and Prognostic Factors. Hepatol (Baltimore Md) (1987) 7(1):122–8. doi: 10.1002/hep.1840070124
12. Aytac S, Turkay C, Bavbek N, Kosar A. Hemostasis and Global Fibrinolytic Capacity in Chronic Liver Disease. Blood Coagulation Fibrinolysis (2007) 18(7):623–6. doi: 10.1097/MBC.0b013e328285d80e
13. Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, et al. Elevated Levels of Von Willebrand Factor in Cirrhosis Support Platelet Adhesion Despite Reduced Functional Capacity. Hepatol (Baltimore Md) (2006) 44(1):53–61. doi: 10.1002/hep.21231
14. Durand F, Valla D. Assessment of Prognosis of Cirrhosis. Semin Liver Dis (2008) 28:110–22. doi: 10.1055/s-2008-1040325
15. Wu J, Guo N, Zhang X, Xiong C, Liu J, Xu Y, et al. HEV-LF(S): A Novel Scoring Model for Patients With Hepatitis E Virus-Related Liver Failure. J Viral Hepatitis (2019) 26(11):1334–43. doi: 10.1111/jvh.13174
16. Wu J, Shi C, Sheng X, Xu Y, Zhang J, Zhao X, et al. Prognostic Nomogram for Patients With Hepatitis E Virus-Related Acute Liver Failure: A Multicenter Study in China. J Clin Trans Hepatol (2021). doi: 10.14218/JCTH.2020.00117
17. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the Oesophagus for Bleeding Oesophageal Varices. Br J Surg (1973) 60(8):646–9. doi: 10.1002/bjs.1800600817
18. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol (2013) 3(1):50–60. doi: 10.1016/j.jceh.2012.11.002
19. Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS. Renal Outcomes After Liver Transplantation in the Model for End-Stage Liver Disease Era. Liver Transpl (2009) 15(9):1142–8. doi: 10.1002/lt.21821
20. Charlton MR, Wall WJ, Ojo AO, Ginès P, Textor S, Shihab FS, et al. Report of the First International Liver Transplantation Society Expert Panel Consensus Conference on Renal Insufficiency in Liver Transplantation. Liver Transpl (2009) 15(11):S1–34. doi: 10.1002/lt.21877
21. Nair S, Verma S, Thuluvath PJ. Pretransplant Renal Function Predicts Survival in Patients Undergoing Orthotopic Liver Transplantation. Hepatol (Baltimore Md) (2002) 35(5):1179–85. doi: 10.1053/jhep.2002.33160
22. Zhou J, Zhou Y, Cao S, Li S, Wang H, Niu Z, et al. Multivariate Logistic Regression Analysis of Postoperative Complications and Risk Model Establishment of Gastrectomy for Gastric Cancer: A Single-Center Cohort Report. Scand J Gastroenterol (2016) 51(1):8–15. doi: 10.3109/00365521.2015.1063153
23. Jeong O, Kyu Park Y, Ran Jung M, Yeop Ryu S. Analysis of 30-Day Postdischarge Morbidity and Readmission After Radical Gastrectomy for Gastric Carcinoma: A Single-Center Study of 2107 Patients With Prospective Data. Med (Baltimore) (2015) 94(11):e259. doi: 10.1097/MD.0000000000000259
24. Zullo A, Romiti A, Tomao S, Hassan C, Rinaldi V, Giustini M, et al. Gastric Cancer Prevalence in Patients With Liver Cirrhosis. Eur J Cancer Prev (2003) 12(3):179–82. doi: 10.1097/00008469-200306000-00002
25. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic Submucosal Dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy (2015) 47(9):829–54. doi: 10.1055/s-0034-1392882
26. Chang CC, Chen SH, Pan S, Fang CL, Lien GS. Endoscopic Mucosal Resection With a Cap-Fitted Endoscope for Early Gastric Carcinoma With Focal Submucosal Invasion in a Patient With Decompensated Liver Cirrhosis. J Formosan Med Assoc = Taiwan yi zhi (2001) 100(12):841–3.
27. Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Fukuda K, et al. Risk for Local Recurrence of Early Gastric Cancer Treated With Piecemeal Endoscopic Mucosal Resection During a 10-Year Follow-Up Period. Surg Endosc (2012) 26(1):72–8. doi: 10.1007/s00464-011-1830-y
28. Kato M, Nishida T, Hamasaki T, Kawai N, Yoshio T, Egawa S, et al. Outcomes of ESD for Patients With Early Gastric Cancer and Comorbid Liver Cirrhosis: A Propensity Score Analysis. Surg Endosc (2015) 29(6):1560–6. doi: 10.1007/s00464-014-3841-y
29. Choe WH, Kim JH, Park JH, Kim HU, Cho DH, Lee SP, et al. Endoscopic Submucosal Dissection of Early Gastric Cancer in Patients With Liver Cirrhosis. Dig Dis Sci (2018) 63(2):466–73. doi: 10.1007/s10620-017-4814-5
30. Watanabe K, Hikichi T, Nakamura J, Takagi T, Suzuki R, Sugimoto M, et al. Successful Endoscopic Submucosal Dissection for Early Gastric Cancer Adjacent to Gastric Cardia Varix. Fukushima J Med Sci (2016) 62(2):101–7. doi: 10.5387/fms.2016-2
31. Ogura K, Okamoto M, Sugimoto T, Yahagi N, Fujishiro M, Kakushima N, et al. Efficacy and Safety of Endoscopic Submucosal Dissection for Gastric Cancer in Patients With Liver Cirrhosis. Endoscopy (2008) 40(5):443–5. doi: 10.1055/s-2007-995650
32. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2016) 27(suppl 5):v38–49. doi: 10.1093/annonc/mdw350
33. Ikeda Y, Kanda T, Kosugi S, Yajima K, Matsuki A, Suzuki T, et al. Gastric Cancer Surgery for Patients With Liver Cirrhosis. World J Gastrointest Surg (2009) 1(1):49–55. doi: 10.4240/wjgs.v1.i1.49
34. Kang SJ, Jung MR, Cheong O, Park YK, Kim HG, Kim DY, et al. Is Laparoscopy-Assisted Radical Gastrectomy Safe in Patients With Child-Pugh Class A Cirrhosis? J Gastric Cancer (2013) 13(4):207–13. doi: 10.5230/jgc.2013.13.4.207
35. Kim DJ, Park CH, Kim W, Jin HM, Kim JJ, Lee HH, et al. Safety of Laparoscopic Radical Gastrectomy in Gastric Cancer Patients With Liver Cirrhosis. Surg Endosc (2017) 31(10):3898–904. doi: 10.1007/s00464-017-5420-5
36. Alshahrani AS, Gong GS, Yoo MW. Comparison of Long-Term Survival and Immediate Postoperative Liver Function After Laparoscopic and Open Distal Gastrectomy for Early Gastric Cancer Patients With Liver Cirrhosis. Gastric Cancer (2017) 20(4):744–51. doi: 10.1007/s10120-016-0675-4
37. Zhu HP, Chen MY, Pan JH, Zhong X, Li XL, Wang XF. Safety and Feasibility of Laparoscopic Radical Gastrectomy in Gastric Cancer Patients With Liver Cirrhosis. Zhonghua wei chang wai ke za zhi = Chin J gastrointest Surg (2020) 23(10):998–1002. doi: 10.3760/cma.j.cn.441530-20190809-00300
38. Iwabu J, Namikawa T, Tsuda S, Kitagawa H, Kobayashi M, Hanazaki K. Successful Distal Gastrectomy for Gastric Cancer With Child-Pugh Class B Alcoholic Liver Cirrhosis. Anticancer Res (2018) 38(5):3085–7. doi: 10.21873/anticanres.12566
39. Ryu KW, Lee JH, Kim YW, Park JW, Bae JM. Management of Ascites After Radical Surgery in Gastric Cancer Patients With Liver Cirrhosis and Minimal Hepatic Dysfunction. World J Surg (2005) 29(5):653–6. doi: 10.1007/s00268-005-7715-2
40. Lee JH, Kim J, Cheong JH, Hyung WJ, Choi SH, Noh SH. Gastric Cancer Surgery in Cirrhotic Patients: Result of Gastrectomy With D2 Lymph Node Dissection. World J Gastroenterol (2005) 11(30):4623–7. doi: 10.3748/wjg.v11.i30.4623
41. Jang HJ, Kim JH, Song HH, Woo KH, Kim M, Kae SH, et al. Clinical Outcomes of Patients With Liver Cirrhosis Who Underwent Curative Surgery for Gastric Cancer: A Retrospective Multi-Center Study. Dig Dis Sci (2008) 53(2):399–404. doi: 10.1007/s10620-007-9884-3
42. Barakat M, Singh B, Salafia C, Eskaros S. The Safety and Efficacy of Endoscopic Submucosal Dissection for Early Gastric Cancer With Concomitant Liver Cirrhosis. Eur J Gastroenterol Hepatol (2018) 30(1):118. doi: 10.1097/MEG.0000000000000996
43. Guo F, Ma S, Yang S, Dong Y, Luo F, Wang Z. Surgical Strategy for Gastric Cancer Patients With Liver Cirrhosis: A Retrospective Cohort Study. Int J Surg (2014) 12(8):810–4. doi: 10.1016/j.ijsu.2014.06.011
44. Nishimura S, Saeki H, Ikegami T, Ando K, Yamashita Y, Oki E, et al. Living Donor Liver Transplantation Followed by Total Gastrectomy–A Two-Stage Planed Operative Strategy for Early Gastric Cancer Concomitant With Decompensated Liver Cirrhosis. Anticancer Res (2014) 34(8):4307–10.
45. Takechi H, Fujikuni N, Takemoto Y, Tanabe K, Amano H, Noriyuki T, et al. Palliative Surgery for Advanced Gastric Cancer: Partial Gastrectomy Using the Inverted Laparoscopic and Endoscopic Cooperative Surgery Method. Int J Surg Case Rep (2018) 50:42–5. doi: 10.1016/j.ijscr.2018.06.042
46. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J Clin Oncol (2020) 38(23):2677–94. doi: 10.1200/jco.20.00866
47. Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. Alcohol as a Risk Factor for Liver Cirrhosis: A Systematic Review and Meta-Analysis. Drug Alcohol Rev (2010) 29(4):437–45. doi: 10.1111/j.1465-3362.2009.00153.x
48. Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer Risk Associated With Alcohol and Tobacco Use: Focus on Upper Aero-Digestive Tract and Liver. Alcohol Res Health (2006) 29(3):193–8.
49. Sanz L, Ovejero VJ, González JJ, Laso CA, Azcano E, Navarrete F, et al. Mortality Risk Scales in Esophagectomy for Cancer: Their Usefulness in Preoperative Patient Selection. Hepato-gastroenterology (2006) 53(72):869–73.
50. González-González JJ, Sanz-Alvarez L, Marqués-Alvarez L, Navarrete-Guijosa F, Martínez-Rodríguez E. Complications of Surgical Resection of Esophageal Cancer. Cirugia Espanola (2006) 80(6):349–60. doi: 10.1016/s0009-739x(06)70987-3
51. Wang ZQ, Deng HY, Yang YS, Wang Y, Hu Y, Yuan Y, et al. Can Oesophagectomy be Performed for Patients With Oesophageal Carcinoma and Concomitant Liver Cirrhosis? A Retrospective Study Based on a Propensity-Matched Cohort. Interact Cardiovasc Thorac Surg (2017) 25(3):442–7. doi: 10.1093/icvts/ivx132
52. Schizas D, Giannopoulos S, Vailas M, Mylonas KS, Giannopoulos S, Moris D, et al. The Impact of Cirrhosis on Esophageal Cancer Surgery: An Up-to-Date Meta-Analysis. Am J Surg (2020) 220(4):865–72. doi: 10.1016/j.amjsurg.2020.02.035
53. Deng HY, Zheng X, Zha P, Liang H, Huang KL, Peng L. Can We Perform Esophagectomy for Esophageal Cancer Patients With Concomitant Liver Cirrhosis? A Comprehensive Systematic Review and Meta-Analysis. Dis Esophagus (2019) 32(6):doz003. doi: 10.1093/dote/doz003
54. Tachibana M, Kotoh T, Kinugasa S, Dhar DK, Shibakita M, Ohno S, et al. Esophageal Cancer With Cirrhosis of the Liver: Results of Esophagectomy in 18 Consecutive Patients. Ann Surg Oncol (2000) 7(10):758–63. doi: 10.1007/s10434-000-0758-6
55. Cheng C, Wen YW, Tsai CY, Chao YK. Impact of Child-Pugh Class A Liver Cirrhosis on Perioperative Outcomes of Patients With Oesophageal Cancer: A Propensity Score-Matched Analysis. Eur J Cardiothorac Surg (2020). doi: 10.1093/ejcts/ezaa334
56. Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Hölscher AH. Comparison Between Endoscopic and Surgical Resection of Mucosal Esophageal Adenocarcinoma in Barrett’s Esophagus at Two High-Volume Centers. Ann Surg (2011) 254(1):67–72. doi: 10.1097/SLA.0b013e31821d4bf6
57. Endlicher E, Gelbmann C, Schlottmann K, Herfarth H, Rummele P, Friedrich A, et al. Endoscopic Mucosal Resection for Early Esophageal Cancer With Esophageal Varices. Z Gastroenterol (2004) 42(7):609–13. doi: 10.1055/s-2004-813221
58. Ciocirlan M, Chemali M, Lapalus MG, Lefort C, Souquet JC, Napoleon B, et al. Esophageal Varices and Early Esophageal Cancer: Can We Perform Endoscopic Mucosal Resection (EMR)? Endoscopy (2008) 40:E91. doi: 10.1055/s-2007-995571
59. Sawaguchi M, Jin M, Matsuhashi T, Ohba R, Hatakeyama N, Koizumi S, et al. The Feasibility of Endoscopic Submucosal Dissection for Superficial Esophageal Cancer in Patients With Cirrhosis (With Video). Gastrointest Endosc (2014) 79(4):681–5. doi: 10.1016/j.gie.2013.11.004
60. Katano A, Yamashita H, Nakagawa K. Successful Definitive Concurrent Chemoradiotherapy in a Patient With Esophageal Cancer and Child-Pugh B Cirrhosis of the Liver. J Cancer Res Ther (2019) 15(1):255–7. doi: 10.4103/jcrt.JCRT_338_17
61. Hagiwara N, Matsutani T, Haruna T, Nomura T, Yoshida H. Pedunculated Esophageal Carcinoma Endoscopically Removed Using SB Knife Jr With Detachable Snare After Neoadjuvant Chemotherapy. Clin J Gastroenterol (2020) 13(6):1036–40. doi: 10.1007/s12328-020-01214-4
62. Shimakawa T, Naritaka Y, Asaka S, Isohata N, Murayama M, Konno S, et al. Surgical Treatment for Superficial Esophageal Cancer With Liver Cirrhosis and Esophageal Varices: Report of a Case. Anticancer Res (2007) 27(5b):3507–11.
63. Valmasoni M, Pierobon ES, De Pasqual CA, Zanchettin G, Moletta L, Salvador R, et al. Esophageal Cancer Surgery for Patients With Concomitant Liver Cirrhosis: A Single-Center Matched-Cohort Study. Ann Surg Oncol (2017) 24(3):763–9. doi: 10.1245/s10434-016-5610-8
64. Lu MS, Liu YH, Wu YC, Kao CL, Liu HP, Hsieh MJ. Is it Safe to Perform Esophagectomy in Esophageal Cancer Patients Combined With Liver Cirrhosis? Interact Cardiovasc Thorac Surg (2005) 4(5):423–5. doi: 10.1510/icvts.2005.110387
65. Nishimura T, Takeda S, Yoshino S, Tokunou K, Oka M. A Case of Advanced Cervical Esophageal Cancer With Liver Cirrhosis and Diabetes Mellitus Showing a Complete Response by Radiation Therapy After the Operation for Multiple Esophageal Cancers. Gan to kagaku ryoho Cancer Chemother (2008) 35(12):2033–5.
66. Maruyama K, Nagai K, Maruyama N, Tanaka J, Katsumoto Y, Yokouchi H, et al. A Case of Multiple Superficial Esophageal Cancers With Liver Cirrhosis Successfully Treated by Radiotherapy. Gan to kagaku ryoho Cancer Chemother (2003) 30(11):1706–9.
67. Trivin F, Boucher E, Vauleon E, Cumin I, Le Prise E, Audrain O, et al. Management of Esophageal Carcinoma Associated With Cirrhosis: A Retrospective Case-Control Analysis. J Oncol (2009) 2009:173421. doi: 10.1155/2009/173421
68. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
69. Sørensen HT, Mellemkjaer L, Jepsen P, Thulstrup AM, Baron J, Olsen JH, et al. Risk of Cancer in Patients Hospitalized With Fatty Liver: A Danish Cohort Study. J Clin Gastroenterol (2003) 36(4):356–9. doi: 10.1097/00004836-200304000-00015
70. Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, et al. Risk of Liver and Other Types of Cancer in Patients With Cirrhosis: A Nationwide Cohort Study in Denmark. Hepatol (Baltimore Md) (1998) 28(4):921–5. doi: 10.1002/hep.510280404
71. Montomoli J, Erichsen R, Christiansen CF, Ulrichsen SP, Pedersen L, Nilsson T, et al. Liver Disease and 30-Day Mortality After Colorectal Cancer Surgery: A Danish Population-Based Cohort Study. BMC Gastroenterol (2013) 13:66. doi: 10.1186/1471-230x-13-66
72. Cheng YX, Tao W, Zhang H, Peng D, Wei ZQ. Does Liver Cirrhosis Affect the Surgical Outcome of Primary Colorectal Cancer Surgery? A Meta-Analysis. World J Surg Oncol (2021) 19(1):167. doi: 10.1186/s12957-021-02267-6
73. Shin N, Han EC, Won S, Ryoo SB, Choe EK, Park BK, et al. The Prognoses and Postoperative Outcomes of Patients With Both Colorectal Cancer and Liver Cirrhosis Based on a Nationwide Cohort in Korea. Ann Surg Treat Res (2020) 99(2):82–9. doi: 10.4174/astr.2020.99.2.82
74. Kim MJ, Kim DW, Cho JY, Son IT, Kang SI, Oh HK, et al. Postoperative Portomesenteric Venous Thrombosis After Colorectal Cancer Surgery. J Gastrointest Surg (2020) 24(2):396–404. doi: 10.1007/s11605-018-04085-w
75. Han EC, Ryoo SB, Park JW, Yi JW, Oh HK, Choe EK, et al. Oncologic and Surgical Outcomes in Colorectal Cancer Patients With Liver Cirrhosis: A Propensity-Matched Study. PloS One (2017) 12(6):e0178920. doi: 10.1371/journal.pone.0178920
76. Sabbagh C, Chatelain D, Nguyen-Khac E, Rebibo L, Joly JP, Regimbeau JM. Management of Colorectal Cancer in Patients With Cirrhosis: A Retrospective, Case-Matched Study of Short- and Long-Term Outcomes. Dig Liver Dis (2016) 48(4):429–34. doi: 10.1016/j.dld.2015.12.004
77. O’Leary JG, Friedman LS. Predicting Surgical Risk in Patients With Cirrhosis: From Art to Science. Gastroenterology (2007) 132(4):1609–11. doi: 10.1053/j.gastro.2007.03.016
78. Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The Safety of Intra-Abdominal Surgery in Patients With Cirrhosis: Model for End-Stage Liver Disease Score Is Superior to Child-Turcotte-Pugh Classification in Predicting Outcome. Arch Surg (Chicago Ill: 1960) (2005) 140(7):650–4. doi: 10.1001/archsurg.140.7.650
79. Lacatus M, Costin L, Bodean V, Manuc M, Vasilescu C. The Outcome of Colorectal Surgery in Cirrhotic Patients: A Case Match Report. Chirurgia (Bucur) (2018) 113(2):210–7. doi: 10.21614/chirurgia.113.2.210
80. Meunier K, Mucci S, Quentin V, Azoulay R, Arnaud JP, Hamy A. Colorectal Surgery in Cirrhotic Patients: Assessment of Operative Morbidity and Mortality. Dis Colon Rectum (2008) 51(8):1225–31. doi: 10.1007/s10350-008-9336-y
81. Zhou S, Zhu H, Li Z, Ying X, Xu M. Safety of Laparoscopic Resection for Colorectal Cancer in Patients With Liver Cirrhosis: A Retrospective Cohort Study. Int J Surg (2018) 55:110–6. doi: 10.1016/j.ijsu.2018.05.730
82. Hsu CY, Lin HC, Huang YH, Su CW, Lee FY, Huo TI, et al. Comparison of the Model for End-Stage Liver Disease (MELD), MELD-Na and MELDNa for Outcome Prediction in Patients With Acute Decompensated Hepatitis. Dig Liver Dis (2010) 42(2):137–42. doi: 10.1016/j.dld.2009.06.004
83. Huo TI, Hsia CY, Huang YH, Lin HC, Lee PC, Lui WY, et al. Selecting a Short-Term Prognostic Model for Hepatocellular Carcinoma: Comparison Between the Model for End-Stage Liver Disease (MELD), MELD-Sodium, and Five Cancer Staging Systems. J Clin Gastroenterol (2009) 43(8):773–81. doi: 10.1097/MCG.0b013e31818dd962
84. Martinez JL, Rivas H, Delgado S, Castells A, Pique JM, Lacy AM. Laparoscopic-Assisted Colectomy in Patients With Liver Cirrhosis. Surg Endosc (2004) 18(7):1071–4. doi: 10.1007/s00464-003-9222-6
85. Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, et al. Impact of Laparoscopic Liver Resection for Hepatocellular Carcinoma With F4-Liver Cirrhosis. Surg Endosc (2013) 27(7):2592–7. doi: 10.1007/s00464-013-2795-9
86. Cai YQ, Zhou J, Chen XD, Wang YC, Wu Z, Peng B. Laparoscopic Splenectomy Is an Effective and Safe Intervention for Hypersplenism Secondary to Liver Cirrhosis. Surg Endosc (2011) 25(12):3791–7. doi: 10.1007/s00464-011-1790-2
87. Sabbagh C, Cosse C, Chauffert B, Nguyen-Khac E, Joly JP, Yzet T, et al. Management of Colon Cancer in Patients With Cirrhosis: A Review. Surg Oncol (2015) 24(3):187–93. doi: 10.1016/j.suronc.2015.06.010
88. Cabibbo G, Palmeri L, Palmeri S, Craxi A. Should Cirrhosis Change Our Attitude Towards Treating Non-Hepatic Cancer? Liver Int (2012) 32(1):21–7. doi: 10.1111/j.1478-3231.2011.02629.x
89. Slade JH, Alattar ML, Fogelman DR, Overman MJ, Agarwal A, Maru DM, et al. Portal Hypertension Associated With Oxaliplatin Administration: Clinical Manifestations of Hepatic Sinusoidal Injury. Clin Colorectal Cancer (2009) 8(4):225–30. doi: 10.3816/CCC.2009.n.038
90. Madbouly KM, Hussein AM, Zeid A. Colorectal Cancer Surgery in Portal Hypertensive Patients: Does Adjuvant Oxaliplatin Affect Prognosis? Dis Colon Rectum (2013) 56(5):577–85. doi: 10.1097/DCR.0b013e318286f8fc
91. Bhangui P, Laurent A, Amathieu R, Azoulay D. Assessment of Risk for Non-Hepatic Surgery in Cirrhotic Patients. J Hepatol (2012) 57(4):874–84. doi: 10.1016/j.jhep.2012.03.037
92. Warnick P, Mai I, Klein F, Andreou A, Bahra M, Neuhaus P, et al. Safety of Pancreatic Surgery in Patients With Simultaneous Liver Cirrhosis: A Single Center Experience. Pancreatology (2011) 11(1):24–9. doi: 10.1159/000323961
93. Sahaab E, Iqbal N, Bhatti ABH. Successful Outcome After Pancreaticodoudenectomy in an Elderly Cirrhotic Patient: A Case Report. Int J Surg Case Rep (2019) 58:18–20. doi: 10.1016/j.ijscr.2019.03.034
94. El Nakeeb A, Sultan AM, Salah T, El Hemaly M, Hamdy E, Salem A, et al. Impact of Cirrhosis on Surgical Outcome After Pancreaticoduodenectomy. World J Gastroenterol (2013) 19(41):7129–37. doi: 10.3748/wjg.v19.i41.7129
95. Schizas D, Peppas S, Giannopoulos S, Lagopoulou V, Mylonas KS, Giannopoulos S, et al. The Impact of Cirrhosis on Pancreatic Cancer Surgery: A Systematic Review and Meta-Analysis. World J Surg (2021) 45(2):562–70. doi: 10.1007/s00268-020-05821-7
96. Regimbeau JM, Rebibo L, Dokmak S, Boher JM, Sauvanet A, Chopin-Laly X, et al. The Short- and Long-Term Outcomes of Pancreaticoduodenectomy for Cancer in Child A Patients Are Acceptable: A Patient-Control Study From the Surgical French Association Report for Pancreatic Surgery. J Surg Oncol (2015) 111(6):776–83. doi: 10.1002/jso.23856
Keywords: gastrointestinal cancer, liver cirrhosis, postoperative complications, cancer treatment, prognosis
Citation: Xiang Z, Li Y, Zhu C, Hong T, He X, Zhu H and Jiang D (2021) Gastrointestinal Cancers and Liver Cirrhosis: Implications on Treatments and Prognosis. Front. Oncol. 11:766069. doi: 10.3389/fonc.2021.766069
Received: 28 August 2021; Accepted: 04 October 2021;
Published: 21 October 2021.
Edited by:
Zhe-Sheng Chen, St. John’s University, United StatesCopyright © 2021 Xiang, Li, Zhu, Hong, He, Zhu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Zhu, aHVhNzgzMTRAMTYzLmNvbQ==; Danbin Jiang, ZGFuYmluamlhbmcxQDE2My5jb20=
†These authors have contributed equally to this work
 Ze Xiang
Ze Xiang Yiqi Li2,3†
Yiqi Li2,3† Tu Hong
Tu Hong Danbin Jiang
Danbin Jiang