- 1Department of Radiation Oncology, the First Affiliated Hospital of the USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
- 2Hefei Cancer Hospital, Chinese Academy of Sciences, Hefei, China
- 3Department of Radiotherapy and Oncology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, China
Numerous clinical studies investigated how low expression of CD9 predicts poor prognosis of solid tumor. However, the results were inconclusive. This present meta-analysis was therefore performed to determine the prognostic value of CD9 expression in solid tumors. In this meta-analysis, 25 studies involving 5,555 participants were included; the result showed strong significant associations between declined expression of CD9 and all endpoints: overall survival (OS) (hazard ratio (HR) = 1.88, 95% CI = 1.45–2.43, p < 0.000) and time to progression (TTP) (HR = 2.0, 95% CI = 1.38–2.88, p < 0.000). The subgroup analysis was also performed, which revealed that the associations between CD9 downregulated expression related to poor OS in lung cancer and head and neck cancer. Also, low expression of CD9 was significantly connected with poor TTP in patients with head and neck cancer. The adverse prognostic impact of decreased expression of CD9 was observed in patients of different ethnicities. In conclusion, these results showed that declined expression of CD9 was associated with poor survival in human solid tumors. CD9 may be a valuable prognostic predictive biomarker and a potential therapeutic target in human solid tumors.
Introduction
CD9, a 24- to 27-kDa cell surface glycoprotein, also known as motility-related protein-1 (MRP-1), leukemia-associated cell surface antigen p24, and TSPAN29, belongs to the transmembrane-4 superfamily (TM4SF), which consists of four transmembrane domains, a small intracellular loop, and two extracellular loops (1, 2). CD9 was initially acknowledged as an antigen for a monoclonal antibody, acquired via immunization of mice with pre-B cells, and was subsequently revealed to be ubiquitously expressed on hematopoietic and non-hematopoietic cells (3–5), as well as malignant tumor (1, 2). Through interacting with a variety of cell-surface molecules, CD9 participates in numbers of biological activities including cell adhesion, motility, metastasis (6–8), sperm–egg fusion (9), growth, survival, signal transduction (10, 11), apoptosis (12, 13), and differentiation (14–16).
Many researches indicated that the expression of CD9 was declined in most solid tumors, including lung cancer (17–20), breast carcinoma (21–23), colorectal cancer (24–26), clear cell renal cell carcinoma (27), malignant pleural mesothelioma (28), laryngeal cancer (29), and gastrointestinal stromal tumor (GIST) (30), and that decreased expression of CD9 strongly correlated with the progression, increased risk of recurrence, angiogenesis, and metastasis of some malignant tumors (26, 30, 31), as well as a significantly increased risk of malignancy (32). The study of Lewitowicz has shown that above 98% of GISTs have moderate-to-strong expression of CD9 (33). This proof implied that MRP-1 would be further reckoned as a promising indicator of prognosis of cancer.
A great deal of reports manifested that reducing expressed MRP-1 was associated with dismal prognosis of different cancers. However, in locally advanced gastric cancer, high expression of CD9 was negatively associated with the prognosis (33). The outcome of these individual researches was inconsistent. Consequentially, this meta-analysis was deliberately calculated to illuminate the outcome value of CD9, and this glycoprotein may be a promising therapeutic target in solid tumors.
Materials and Methods
Publication Search
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (34). A complete network research was done by electronic databases PubMed, Embase, and Web of Science (up to July 20, 2021) through different combinations of the search items: “CD9,” “MRP1,” and “cancer”/”tumor”/”neoplasm”/”carcinoma” and the following limits: human subjects and reports in the English language. All probably appropriate articles were selected, and their references were prudently browsed to recognize else applicable essay. While several researches of the same patient population existed, we involved the available data with the largest sample size.
Inclusion Criteria
Reports that meet the following criteria were considered eligible: a) calculated the expression of CD9 for predicting outcome (overall survival (OS) or time to progression (TTP)) in human cancer, b) provided hazard ratios (HRs) with 95% CIs or sufficient information that could estimate it, and c) classified CD9 expression as “high” and “low” or “positive” and “negative.”
Exclusion Criteria
The literatures were excluded based upon the subsequent standard: a) researches were published as laboratory paper, case reports, letters, editorials, and abstracts, as well as reviews and proficient opinions; b) experiments were done in vitro or in vivo and not based upon patients; c) literatures without information on HRs, 95% CI of OS and/or disease-free survival (DFS), or even the Kaplan–Meier (K-M) survival curves; d) researches that described the survival outcome of other indicators; and e) unpublished studies.
Data Extraction
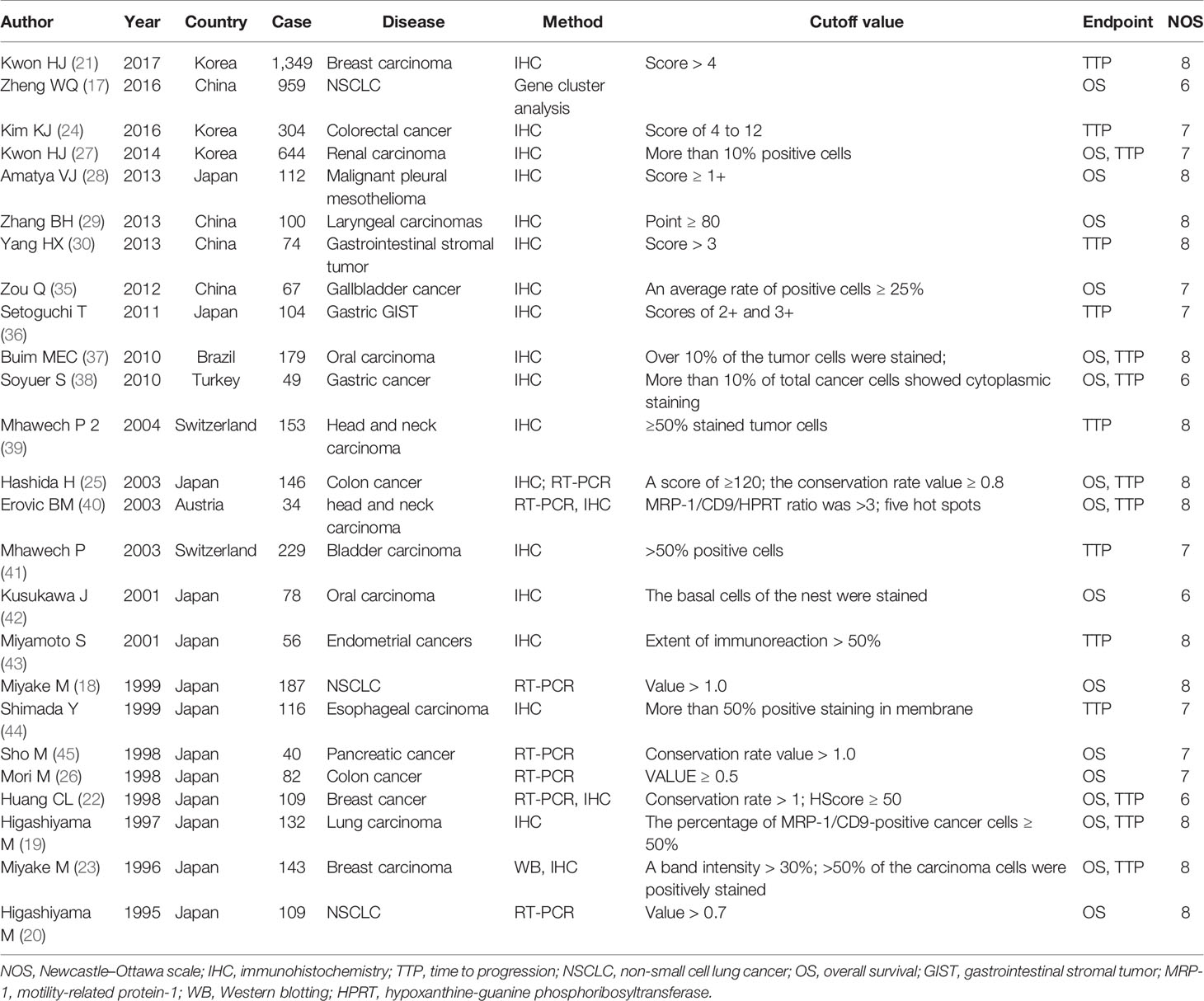
Two reviewers extracted data from the articles independently and carefully using a standardized form. The third author would independently extract data from the original article. If disagreements exist, consensus would be reached through debate. This article was founded on OS and TTP. DFS and time to recurrence (TTR) that had similar definition were merged into TTP of tumor. The following information was extracted from selected papers: the first author, publication year, country of participants, number of patients considered, kinds of cancer, cutoff values, OS, TTP, Newcastle–Ottawa scale (NOS) score. The chief characteristics of these researches are shown in Table 1. For some articles, HR can be directly obtained; for the researches in which survival data are presented only with K-M curves, Tierney’s method was used to calculate the HR and 95% CI (46). NOS was employed to evaluate the eligible literature. The scores of eligible articles vary from 6 to 9, which mean that the methodological quality of these papers was high.
Statistical Analysis
Stata14.0 (StataCorp, College Station, TX) was used to perform the statistical analysis. Initially, we calculated the connection between MRP-1 and endpoints (OS and TTP) though pooled HRs and 95% CIs. Cochran’s Q test and Higgins I-squared statistic were applied to evaluate whether heterogeneity was present between selected articles. Heterogeneity was considered significant when p < 0.1 or I2 > 50% (47), and then a random-effects model was employed to pool the HRs and 95% CIs; otherwise, a fixed-effects model was used (35). Moreover, subgroup analysis was used to explore the source of heterogeneity. Begg’s and Egger’s tests were employed to discover the potential publication bias. When publication bias indeed exists, the Duval and Tweedie trim and fill method (37) was exploited. For the robust estimation of the results of statistical analysis, sensitivity analysis was performed by removing the original study one by one. The p-value for all tests was two-tailed, and p < 0.05 was defined as statistically significant, except for heterogeneity.
Results
Demographic Characteristics
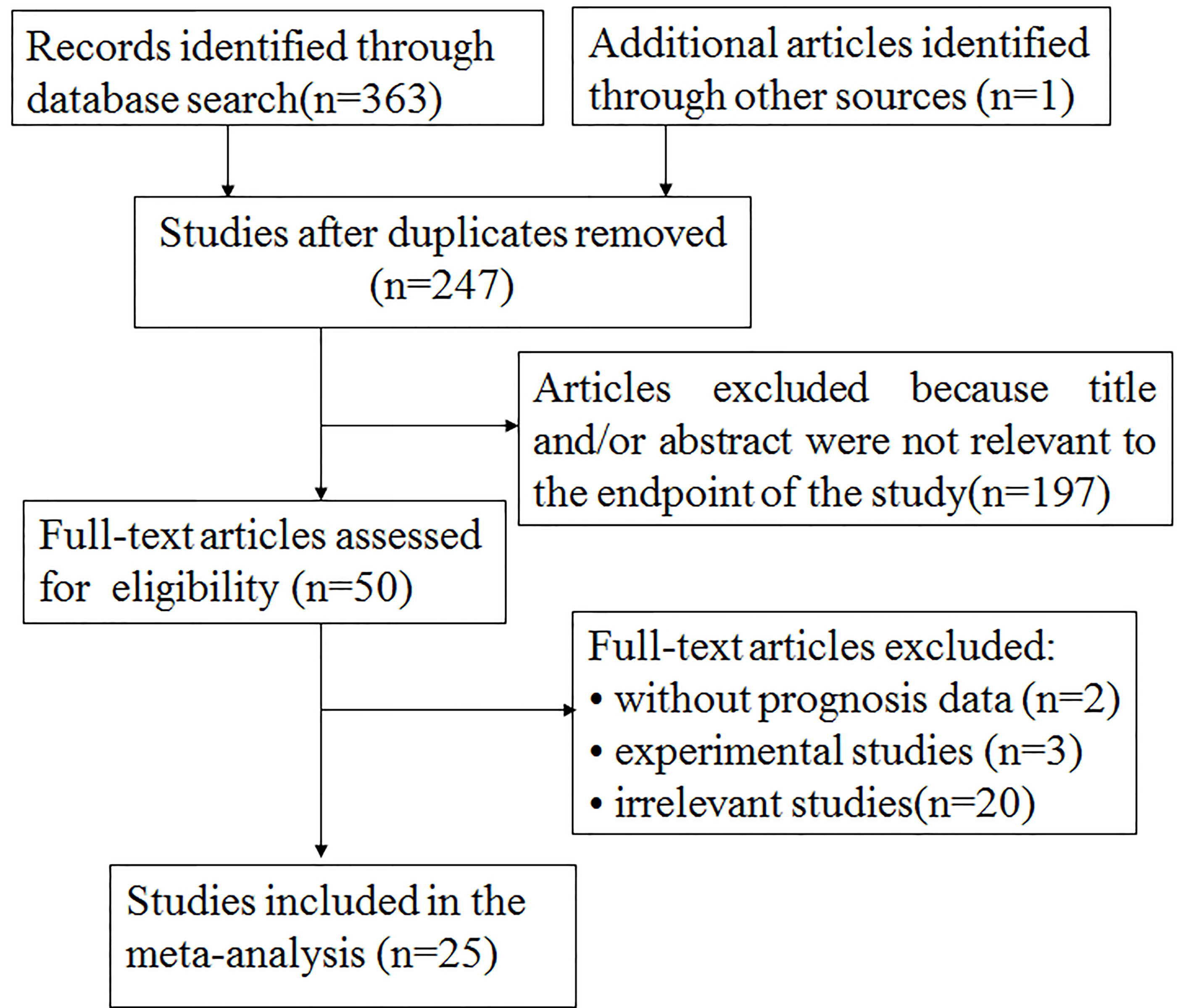
The particular process of searching and filtering is displayed in Figure 1. We preferentially retrieved 364 records from PubMed, Embase, and Web of Science in accordance with the criteria mentioned previously. Among them, 117 duplicate reports were removed. After the abstracts or full text was scanned, 222 records were excluded by reason of no relevant information provided (n = 197); experimental research (n = 3); without prognosis data (n = 2); and irrelevance (n = 20). Ultimately, 25 records including 5,555 participants were involved in the meta-analysis. The median specimen size was 222, ranging from 34 to 1,349. Among all cohorts, three studies estimated breast carcinoma (21–23), four studies estimated lung cancer (17–20), three studies evaluated colon cancer (24–26), one study estimated clear cell renal cell carcinoma (27), one study estimated malignant pleural mesothelioma (28), one study assessed laryngeal squamous cell carcinomas (29), one study assessed GIST (30), one study evaluated gallbladder cancer (38), one study evaluated gastric GIST (39), two studies investigated oral squamous cell carcinoma (40, 41), one study investigated gastric cancer (42), two studies investigated squamous cell carcinoma of the head and neck (43, 44), one study investigated urothelial carcinoma of the bladder (45), one study investigated endometrial cancers (48), one study investigated esophageal squamous cell carcinoma (49), and one study investigated pancreatic cancer (50). Five studies (25%) were focused on Asians and 20 (75%) on Caucasians. Overall, 17 papers reported on OS and 16 papers on TTP.
Evidence Synthesis
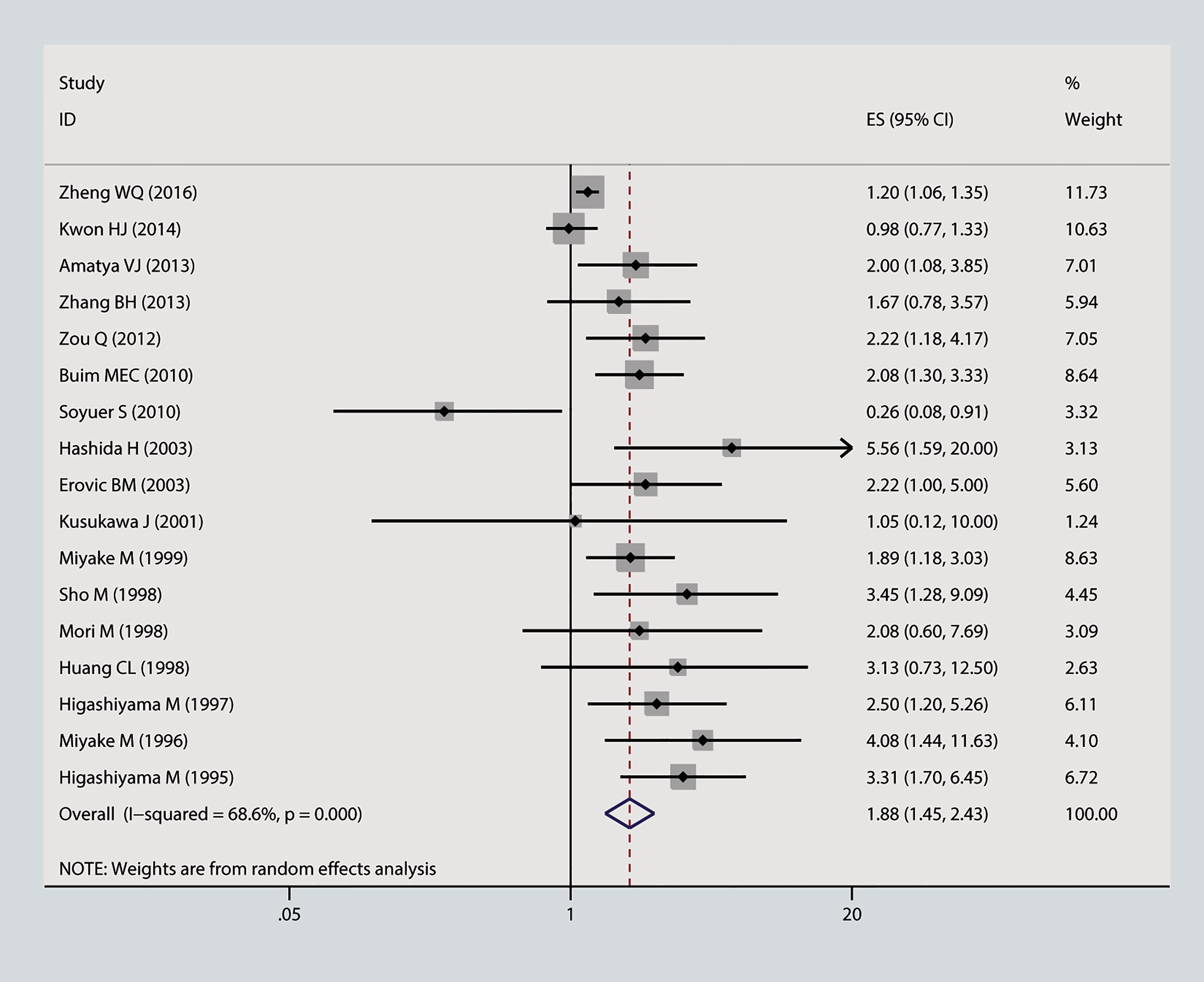
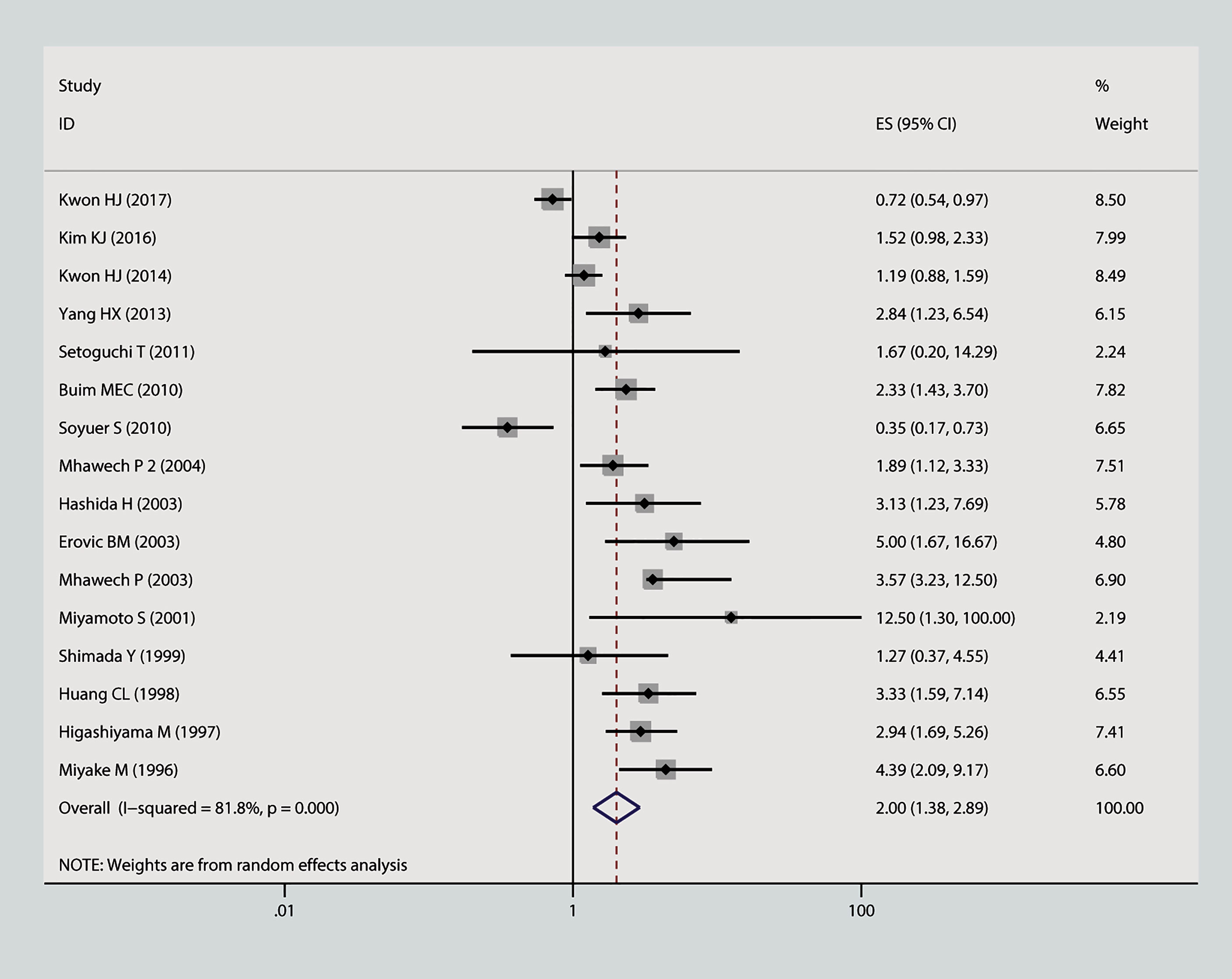
In this meta-analysis, we estimated the correlation between CD9 and prognosis of tumor. As is shown in Figure 2, the result of heterogeneity test illustrated that p < 0.001 and I2 = 68.2%; therefore, a random-effects model was applied to evaluate the pooled HR and 95% CI of OS. The outcome implied that declined expression of CD9 was positively correlated with poor OS in solid tumor (pooled HR = 1.88, 95% CI = 1.45–2.43, p = 0.000). As is displayed in Figure 3, a random-effects model was employed to calculate the pooled HR and 95% CI of TTP as well; the heterogeneity test reported p < 0.001 and I2 = 81.7%. The results indicated that low expression of CD9 was significantly associated with shorter TTP (pooled HR = 2, 95% CI = 1.38–2.88, p = 0.000). To explore the source of heterogeneity, subgroup study was therefore executed. We discovered that decreased expression of CD9 was connected to shorter OS in Asians participants (HR = 1.96, 95% CI = 1.49–2.58, p < 0.001; random effects: I2 = 66.5%, p < 0.001), as well as in lung cancer (HR = 1.93, 95% CI = 1.18–3.17, p < 0.001; random effects: I2 = 79.3%, p = 0.002) and head and neck cancer (HR = 1.98, 95% CI = 1.39–2.82, p < 0.001; fixed effects: I2 = 0%, p = 0.89). The correlation was also detected between decreased expression of CD9 and poor TTP in either Asians patients (HR = 1.38, 95% CI = 1.17–1.62, p < 0.001; random effects: I2 = 80.4%, p < 0.001) or Caucasian patients (HR = 1.87, 95% CI = 1.41–2.48, p < 0.001; random effects: I2 = 85.4%, p < 0.001), and in patients with head and neck cancer (HR = 2.31, 95% CI = 1.59–3.36, p < 0.001; fixed effects: I2 = 11.5%, p = 0.323). No other significant connection between CD9 and the two endpoints was detected in the other subgroup analyses.
Publication Bias and Sensitivity Analysis
Begg’s funnel plot and Egger’s test were applied to measure the publication bias of the literature. As is shown in Figure 4A, Begg’s and Egger’s test scores of OS were correspondingly p = 0.869 and p = 0.008. The funnel plot for the OS showed asymmetry. At the same time, the calculation of the TTP yielded publication biases (Begg’s test, p = 0.86 and Egger’s test, p = 0.024) (Figure 4B). Consequently, the trim and fill method was employed to make the pooled HR more dependable, and the result showed that the pooled p-value was also less than 0.01 (figure not shown). Furthermore, sensitivity analysis was performed by removing one study in turn, which revealed that no single study would significantly affect the pooled HRs of OS and TTP (Figure 5).
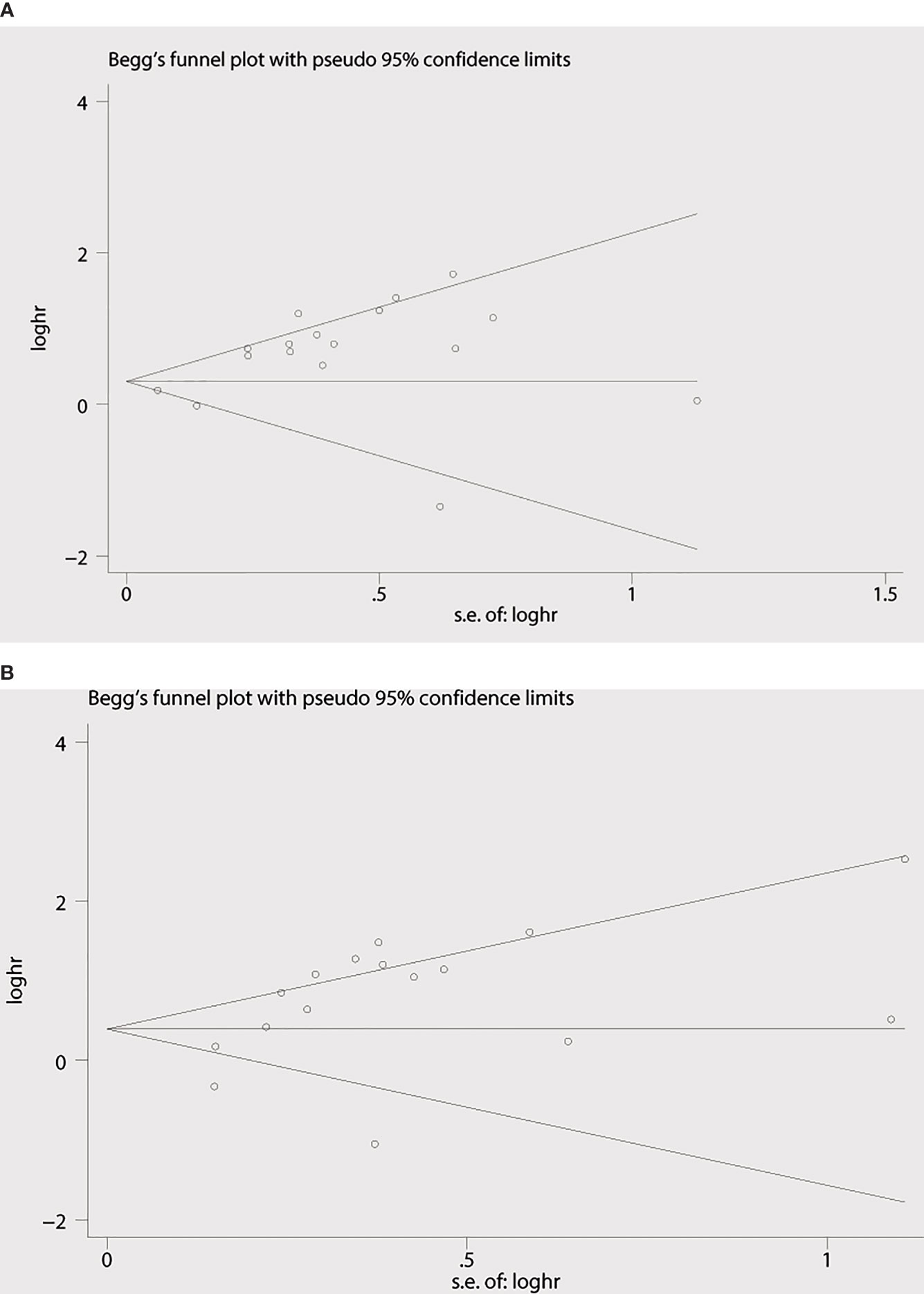
Figure 4 Begg’s funnel plots for the studies involved in the meta-analysis. (A) Overall survival. (B) Time to progression (TTP). loghr, logarithm of hazard ratios; s.e., standard error.
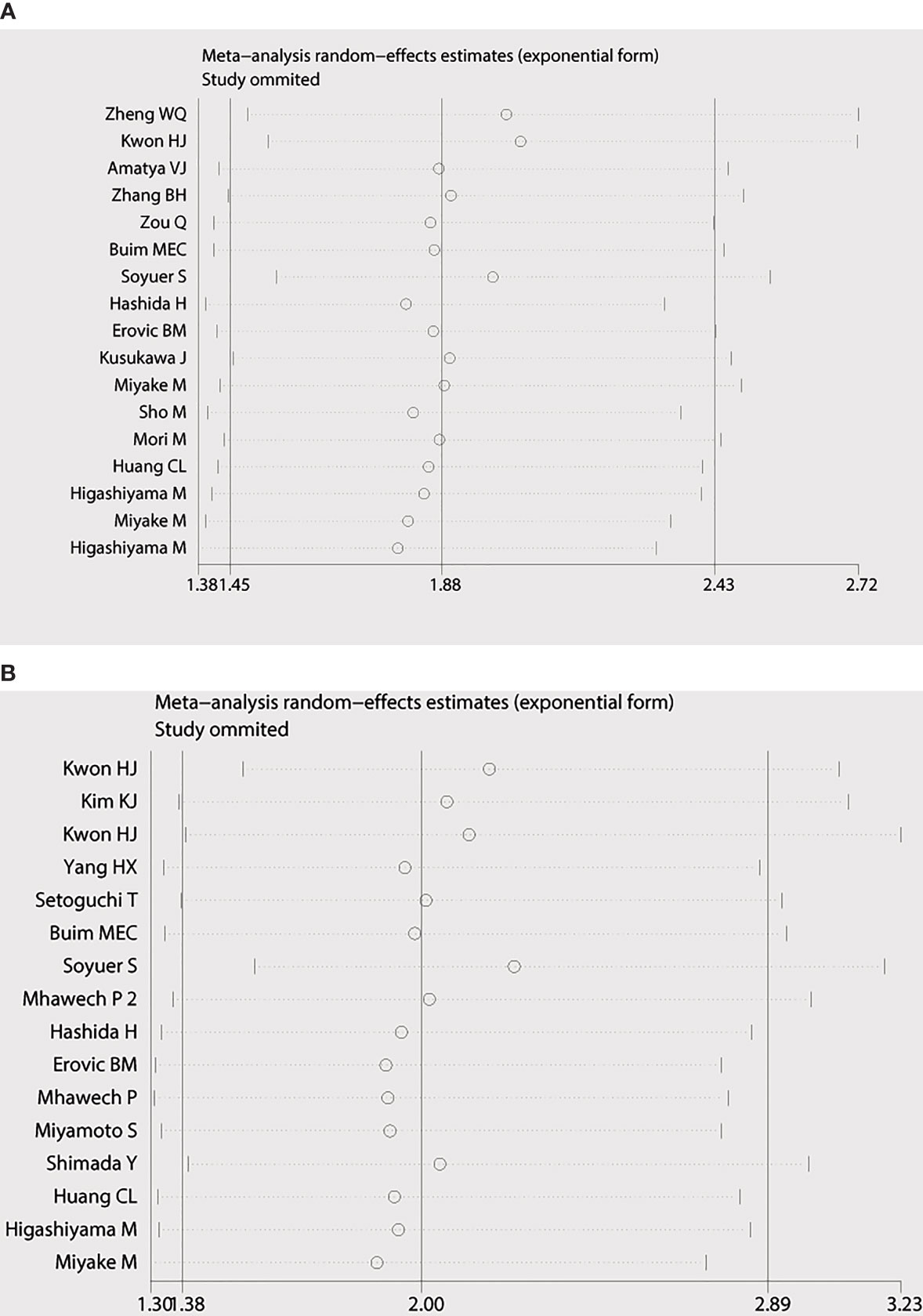
Figure 5 Sensitivity analysis of the meta-analysis. (A) Overall survival. (B) Time to progression (TTP).
Discussions
Cancer is the most deadly disease threatening human life. With the increasing incidence of cancer, it is very urgent to explore a therapeutic target. Many clinical studies investigated that declined expression of CD9 was associated with poor prognosis. It is implied that CD9 may be a promising therapeutic target for solid tumors. However, the major individual study had limited participants, and the conclusion was inconsistent. Therefore, we performed this meta-analysis to explore the correlation between CD9 expression and prognosis of solid tumor.
A total of 25 different studies that included 5,555 participants were involved in this current meta-analysis. The results definitely demonstrated that declined expression of MRP-1 was an adverse effect factor to prognosis, with both shorter OS (pooled HR = 1.88, 95% CI = 1.45–2.43, p < 0.001) and TTP (pooled HR = 2.0, 95% CI = 1.38–2.88, p = 0.000). Likewise, the subgroup analysis confirmed that decreased CD9 expression was positively correlated with shorter TTP in Asians and Caucasian patients and shorter OS in Asians patients. When data were sorted in terms of cancer types, the outcome revealed that low expression of CD9 was a poor prognosis indicator for OS in participants with lung cancer and head and neck cancer, as well TTP in patients with head and neck cancer.
This study is so far the first and most comprehensive meta-analysis to systematically explore the possible prognostic role of CD9 downregulation in solid tumors. Our measurable results are intensely in favor of the view that low CD9 expression is associated with poor OS and TTP. In addition, the meta-analysis also reflects the following important implications. First, declined CD9 expression may be a poor outcome indicator in solid tumors. In this article, we involved various cancer types. The aggregate results indicate that reduced CD9 expression is associated with shorter OS and TTP, which can be extended to all solid tumors. Furthermore, it emphasizes that CD9 may be a promising therapeutic target and prognostic indicator for solid tumors.
In addition to the encouraging results, this calculable meta-analysis still has limitations. First, most of the included studies are shown to be retrospective, and positive results are more likely to be published. Moreover, the methods for assessing CD9 expression and cutoff values are inconsistent. Therefore, our results may be overestimated.
Significant heterogeneity also existed in this analysis. Sensitivity and subgroup analyses based on ethnicity and cancer type were conducted to detect the source of the heterogeneities. Heterogeneity was observed in subgroups of race and tumor type included except for head and neck cancer. This suggests that many different factors caused the heterogeneity. First, the method of detection and the year of publication were different. Second, both IHC and semi-quantitative evaluation methods were affected by many factors such as antibody quality, concentration and incubation time, and personal operation methods. Third, the sample source of each study was various. This may be an implicit factor for the existence of heterogeneity.
In conclusion, this meta-analysis clearly demonstrates that decreased CD9 expression in solid tumor tissues is associated with low survival rate. We believe that CD9 may be a helpful prognostic indicator and a promising therapeutic target for solid tumor. However, further studies related to specific tumor types and opinions are needed to confirm the clinical value of CD9 expression in solid tumor.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
M-bC and X-yL designed this study. PZ and MS contributed to the literature search, review, and data extraction. PZ and XC conducted the statistical analyses. PZ, MS, and R-xS contributed to the manuscript drafting. PZ and X-yL contributed to the manuscript revision. All authors contributed to the article and approved the submitted version
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (WK9110000177); National Natural Science Foundation (81773192, 82072712); Natural Science Foundation of Jiangsu Province (BK20171248); Jiangsu Youth Medical Talents Project (QNRC2016527).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci (2001) 58:1189–205. doi: 10.1007/PL00000933
2. Berditchevski F, Odintsova E. Tetraspanins as Regulators of Protein Trafficking. Traffic (2007) 8:89–96. doi: 10.1111/j.1600-0854.2006.00515.x
3. Fernvik E, Hallden G, Hed J, Lundahl J. Intracellular and Surface Distribution of CD9 in Human Eosinophils. APMIS (1995) 103:699–706. doi: 10.1111/j.1699-0463.1995.tb01426.x
4. Horvath G, Serru V, Clay D, Billard M, Boucheix C, Rubinstein E. CD19 is Linked to the Integrin-Associated Tetraspans CD9, CD81, and CD82. J Biol Chem (1998) 273:30537–43. doi: 10.1074/jbc.273.46.30537
5. Tai XG, Yashiro Y, Abe R, Toyooka K, Wood CR, Morris J. A Role for CD9 Molecules in T Cell Activation. J Exp Med (1996) 184:753–8. doi: 10.1084/jem.184.2.753
6. Berditchevski F. Complexes of Tetraspanins With Integrins: More Than Meets the Eye. J Cell Sci (2001) 114:4143–51. doi: 10.1242/jcs.114.23.4143
7. Jones PH, Bishop LA, Watt FM. Functional Significance of CD9 Association With Beta 1 Integrins in Human Epidermal Keratinocytes. Cell Adhes Commun (1996) 4:297–305. doi: 10.3109/15419069609010773
8. Baudoux B, Castanares-Zapatero D, Leclercq-Smekens M, Berna N, Poumay Y. The Tetraspanin CD9 Associates With the Integrin Alpha6beta4 in Cultured Human Epidermal Keratinocytes and is Involved in Cell Motility. Eur J Cell Biol (2000) 79:41–51. doi: 10.1078/s0171-9335(04)70006-0
9. Tachibana I, Hemler ME. Role of Transmembrane 4 Superfamily (TM4SF) Proteins CD9 and CD81 in Muscle Cell Fusion and Myotube Maintenance. J Cell Biol (1999) 146:893–904. doi: 10.1083/jcb.146.4.893
10. Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 Superfamily Proteins Associate With Activated Protein Kinase C (PKC) and Link PKC to Specific Beta(1) Integrins. J Biol Chem (2001) 276:25005–13. doi: 10.1074/jbc.M102156200
11. Huang CL, Liu D, Masuya D, Kameyama K, Nakashima T, Yokomise H, et al. MRP-1/CD9 Gene Transduction Downregulates Wnt Signal Pathways. Oncogene (2004) 23:7475–83. doi: 10.1038/sj.onc.1208063
12. Murayama Y, Miyagawa J, Oritani K, Yoshida H, Yamamoto K, Kishida O, et al. CD9-Mediated Activation of the P46 Shc Isoform Leads to Apoptosis in Cancer Cells. J Cell Sci (2004) 117:3379–88. doi: 10.1242/jcs.01201
13. Ono M, Handa K, Withers DA, Hakomori S. Motility Inhibition and Apoptosis are Induced by Metastasis-Suppressing Gene Product CD82 and its Analogue CD9, With Concurrent Glycosylation. Cancer Res (1999) 59:2335–9.
14. Boucheix C, Duc GH, Jasmin C, Rubinstein E. Tetraspanins and Malignancy. Expert Rev Mol Med (2001) 2001:1–17. doi: 10.1017/S1462399401002381
15. Levy S, Shoham T. The Tetraspanin Web Modulates Immune-Signalling Complexes. Nat Rev Immunol (2005) 5:136–48. doi: 10.1038/nri1548
16. Seipold L, Saftig P. The Emerging Role of Tetraspanins in the Proteolytic Processing of the Amyloid Precursor Protein. Front Mol Neurosci (2016) 9:149. doi: 10.3389/fnmol.2016.00149
17. Zheng W, Jiang C, Li R. Integrin and Gene Network Analysis Reveals That ITGA5 and ITGB1 are Prognostic in non-Small-Cell Lung Cancer. Onco Targets Ther (2016) . 9:2317–27. doi: 10.2147/OTT.S91796
18. Miyake M, Adachi M, Huang C, Higashiyama M, Kodama K, Taki T. A Novel Molecular Staging Protocol for non-Small Cell Lung Cancer. Oncogene (1999) 18:2397–404. doi: 10.1038/sj.onc.1202556
19. Higashiyama M, Doi O, Kodama K, Yokouchi H, Adachi M, Huang CL, et al. Immunohistochemically Detected Expression of Motility-Related Protein-1 (MRP-1/CD9) in Lung Adenocarcinoma and its Relation to Prognosis. Int J Cancer (1997) 74:205–11. doi: 10.1002/(sici)1097-0215(19970422)74:2<205::aid-ijc12>3.0.co;2-c
20. Higashiyama M, Taki T, Ieki Y, Adachi M, Huang CL, Koh T, et al. Reduced Motility Related Protein-1 (MRP-1/CD9) Gene Expression as a Factor of Poor Prognosis in non-Small Cell Lung Cancer. Cancer Res (1995) 55:6040–4.
21. Kwon HJ, Choi JE, Kang SH, Son Y, Bae YK. Prognostic Significance of CD9 Expression Differs Between Tumour Cells and Stromal Immune Cells, and Depends on the Molecular Subtype of the Invasive Breast Carcinoma. Histopathology (2017) 70:1155–65. doi: 10.1111/his.13184
22. Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake ML. Correlation of Reduction in MRP-1/CD9 and KAI1/CD82 Expression With Recurrences in Breast Cancer Patients. Am J Pathol (1998) 153:973–83. doi: 10.1016/s0002-9440(10)65639-8
23. Miyake M, Nakano K, Itoi SI, Koh T, Taki T. Motility-Related Protein-1 (MRP-1/CD9) Reduction as a Factor of Poor Prognosis in Breast Cancer. Cancer Res (1996) 56:1244–9.
24. Kim KJ, Kwon HJ, Kim MC, Bae YK. CD9 Expression in Colorectal Carcinomas and Its Prognostic Significance. J Pathol Transl Med (2016) 50:459–68. doi: 10.4132/jptm.2016.10.02
25. Hashida H, Takabayashi A, Tokuhara T, Hattori N, Taki T, Hasegawa H, et al. Clinical Significance of Transmembrane 4 Superfamily in Colon Cancer. Br J Cancer (2003) 89:158–67. doi: 10.1038/sj.bjc.6601015
26. Mori M, Mimori K, Shiraishi T, Haraguchi M, Ueo H, Barnard GF, et al. Motility Related Protein 1 (MRP1/CD9) Expression in Colon Cancer. Clin Cancer Res (1998) 4:1507–10.
27. Kwon HJ, Min SY, Park MJ, Lee C, Park JH, Chae JY, et al. Expression of CD9 and CD82 in Clear Cell Renal Cell Carcinoma and its Clinical Significance. Pathol Res Pract (2014) 210:285–90. doi: 10.1016/j.prp.2014.01.004
28. Amatya VJ, Takeshima Y, Aoe K, Fujimoto N, Okamoto T, Yamada T, et al. CD9 Expression as a Favorable Prognostic Marker for Patients With Malignant Mesothelioma. Oncol Rep (2013) 29:21–8. doi: 10.3892/or.2012.2116
29. Zhang BH, Liu W, Li L, Lu JG, Sun YN, Jin DJ, et al. KAI1/CD82 and MRP1/CD9 Serve as Markers of Infiltration, Metastasis, and Prognosis in Laryngeal Squamous Cell Carcinomas. Asian Pac J Cancer Prev (2013) 14:3521–6. doi: 10.7314/apjcp.2013.14.6.3521
30. Yang H, Shen C, Zhang B, Chen H, Chen Z, Chen J. Expression and Clinicopathological Significance of CD9 in Gastrointestinal Stromal Tumor. J Korean Med Sci (2013) 28:1443–8. doi: 10.3346/jkms.2013.28.10.1443
31. Cajot JF, Sordat I, Silvestre T, Sordat B. Differential Display Cloning Identifies Motility-Related Protein (MRP1/CD9) as Highly Expressed in Primary Compared to Metastatic Human Colon Carcinoma Cells. Cancer Res (1997) 57:2593–7.
32. Nankivell P, Williams H, McConkey C, Webster K, High A, MacLennan K, et al. Tetraspanins CD9 and CD151, Epidermal Growth Factor Receptor and Cyclooxygenase-2 Expression Predict Malignant Progression in Oral Epithelial Dysplasia. Br J Cancer (2013) 109:864–74. doi: 10.1038/bjc.2013.600
33. Lewitowicz P, Matykiewicz J, Koziel D, Chrapek M, Horecka-Lewitowicz A, Gluszek S. CD63 and GLUT-1 Overexpression Could Predict a Poor Clinical Outcome in GIST: A Study of 54 Cases With Follow-Up. Gastroenterol Res Pract (2016) 2016:6478374. doi: 10.1155/2016/6478374
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Prisma Group, Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int J Surg (2010) 8:336–41. doi: 10.1371/journal.pmed.1000097
35. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials. Control Clin Trials (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
36. Egger M, Davey SG, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) . 315:629–34. doi: 10.1136/bmj.315.7109.629
37. Duval S, Tweedie R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
38. Zou Q, Xiong L, Yang Z, Lv F, Yang L, Miao X. Expression Levels of HMGA2 and CD9 and its Clinicopathological Significances in the Benign and Malignant Lesions of the Gallbladder. World J Surg Oncol (2012) 10:92. doi: 10.1186/1477-7819-10-92
39. Setoguchi T, Kikuchi H, Yamamoto M, Baba M, Ohta M, Kamiya K, et al. Microarray Analysis Identifies Versican and CD9 as Potent Prognostic Markers in Gastric Gastrointestinal Stromal Tumors. Cancer Sci (2011) 102:883–9. doi: 10.1111/j.1349-7006.2011.01872.x
40. Buim ME, Lourenco SV, Carvalho KC, Cardim R, Pereira C, Carvalho AL, et al. Downregulation of CD9 Protein Expression is Associated With Aggressive Behavior of Oral Squamous Cell Carcinoma. Oral Oncol (2010) 46:166–71. doi: 10.1016/j.oraloncology.2009.11.009
41. Kusukawa J, Ryu F, Kameyama T, Mekada E. Reduced Expression of CD9 in Oral Squamous Cell Carcinoma: CD9 Expression Inversely Related to High Prevalence of Lymph Node Metastasis. J Oral Pathol Med (2001) 30:73–9. doi: 10.1034/j.1600-0714.2001.300202.x
42. Soyuer S, Soyuer I, Unal D, Ucar K, Yildiz OG, Orhan O. Prognostic Significance of CD9 Expression in Locally Advanced Gastric Cancer Treated With Surgery and Adjuvant Chemoradiotherapy. Pathol Res Pract (2010) 206:607–10. doi: 10.1016/j.prp.2010.04.004
43. Mhawech P, Dulguerov P, Tschanz E, Verdan C, Ares C, Allal AS. Motility-Related Protein-1 (MRP-1/CD9) Expression can Predict Disease-Free Survival in Patients With Squamous Cell Carcinoma of the Head and Neck. Br J Cancer (2004) 90:471–5. doi: 10.1038/sj.bjc.6601542
44. Erovic BM, Pammer J, Hollemann D, Woegerbauer M, Geleff S, Fischer MB, et al. Motility-Related Protein-1/CD9 Expression in Head and Neck Squamous Cell Carcinoma. Head Neck (2003) 25:848–57. doi: 10.1002/hed.10306
45. Mhawech P, Herrmann F, Coassin M, Guillou L, Iselin CE. Motility-Related Protein 1 (MRP-1/CD9) Expression in Urothelial Bladder Carcinoma and its Relation to Tumor Recurrence and Progression. Cancer (2003) 98:1649–57. doi: 10.1002/cncr.11698
46. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
47. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
48. Miyamoto S, Maruyama A, Okugawa K, Akazawa K, Baba H, Maehara Y, et al. Loss of Motility-Related Protein 1 (MRP1/CD9) and Integrin Alpha3 Expression in Endometrial Cancers. Cancer (2001) 92(3):542–8. doi: 10.1002/1097-0142(20010801)92:3<542::AID-CNCR1353>3.0.CO;2-8
49. Miyamoto S, Maruyama A, Okugawa K, Akazawa K, Baba H, Maehara Y, et al. Prognostic Factors of Oesophageal Squamous Cell Carcinoma From the Perspective of Molecular Biology. Br J Cancer (1999) 80:1281–8. doi: 10.1002/1097-0142(20010801)92:3<542::aid-cncr1353>3.0.co;2-8
Keywords: CD9, solid tumors, prognosis, overall survival, time to progression
Citation: Zeng P, Si M, Sun R-x, Cheng X, Li X-y and Chen M-b (2021) Prognostic Value of CD9 in Solid Tumor: A Systematic Review and Meta-Analysis. Front. Oncol. 11:764630. doi: 10.3389/fonc.2021.764630
Received: 01 September 2021; Accepted: 28 October 2021;
Published: 19 November 2021.
Edited by:
Giuseppe Palma, Istituto Nazionale Tumori Fondazione G. Pascale (IRCCS), ItalyReviewed by:
Sitong Liu, Sichuan University, ChinaJuliano Andreoli Miyake, Federal University of Santa Catarina, Brazil
Copyright © 2021 Zeng, Si, Sun, Cheng, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-yang Li, ZHJ4eWxAdXN0Yy5lZHUuY24=; Min-bin Chen, Y21iMTk4MUAxNjMuY29t
 Ping Zeng
Ping Zeng Meng Si
Meng Si Rui-xia Sun
Rui-xia Sun Xu Cheng
Xu Cheng Xiao-yang Li
Xiao-yang Li Min-bin Chen
Min-bin Chen