- 1Department of Gynecology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Obstetrics and Gynecology, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
Background: Myometrial invasion has been demonstrated to correlate to clinicopathological characteristics and prognosis in endometrial cancer. However, not all the studies have the consistent results and no meta-analysis has investigated the association of myometrial invasion with lymphovascular space invasion (LVSI), lymph node metastasis (LNM), recurrence, and overall survival (OS). Therefore, a meta-analysis was performed to evaluate the relationship between myometrial invasion and clinicopathological characteristics or overall survival in endometrial cancer.
Materials and Methods: A search of Pubmed, Embase, and Web of Science was carried out to collect relevant studies from their inception until June 30, 2021. The quality of each included study was evaluated using Newcastle–Ottawa scale (NOS) scale. Review Manager version 5.4 was employed to conduct the meta-analysis.
Results: A total of 79 articles with 68,870 endometrial cancer patients were eligible including 9 articles for LVSI, 29 articles for LNM, 8 for recurrence, and 37 for OS in this meta-analysis. Myometrial invasion was associated with LVSI (RR 3.07; 95% CI 2.17–4.35; p < 0.00001), lymph node metastasis (LNM) (RR 4.45; 95% CI 3.29–6.01; p < 0.00001), and recurrence (RR 2.06; 95% CI 1.58–2.69; p < 0.00001). Deep myometrial invasion was also significantly related with poor OS via meta-synthesis of HRs in both univariate survival (HR 3.36, 95% CI 2.35–4.79, p < 0.00001) and multivariate survival (HR 2.00, 95% CI 1.59–2.53, p < 0.00001). Funnel plot suggested that there was no significant publication bias in this study.
Conclusion: Deep myometrial invasion correlated to positive LVSI, positive LNM, cancer recurrence, and poor OS for endometrial cancer patients, indicating that myometrial invasion was a useful evaluation criterion to associate with clinical outcomes and prognosis of endometrial cancer since depth of myometrial invasion can be assessed before surgery. The large scale and comprehensive meta-analysis suggested that we should pay more attention to myometrial invasion in clinical practice, and its underlying mechanism also deserves further investigation.
Introduction
Endometrial cancer is the most prevalent gynecological malignancy in developed countries (1) and the sixth most common cancer in women with continuously increasing incidence and associated mortality (2). Myometrial invasion, lymphovascular space invasion (LVSI), lymph node metastasis (LNM), and recurrence are the important molecular events and clinical behaviors for endometrial cancer. Among them, myometrial invasion is the quietly early action of cancer cells. In addition, three-dimensional ultrasound and magnetic resonance imaging are applied for preoperative assessment of the depth of myometrial invasion (3), and frozen sections are used for intraoperative estimation (4). It is meaningful to classify patients with initial stages as low-risk or high-risk patients for surgical planning when the above diagnostic methods are becoming more accurate with a better specificity and sensitivity. Therefore, it deserves more attention on myometrial invasion in endometrial cancer.
Myometrial invasion is defined as the invasion of endometrial cancer cells into myometrium. The depth of invasion is critical to the evaluation of surgical-pathological staging. According to the International Federation of Gynecology and Obstetrics (FIGO) staging system, stage IA includes those tumors with myometrial invasion of less than 50% or without myometrial invasion, and stage IB refers to more than 50% of invasion into myometrium. Although the underlying mechanism of myometrial invasion is still unclear, it is one of critical considerations for the surgery types and therapeutic methods. This is because accumulated evidences show that myometrial invasion is related to LVSI, LNM, recurrence, and OS of endometrial cancer in different reports. However, there are some different sounds, and not all the studies share the similar results. Specifically, there were more patients with positive LVSI in superficial myometrial invasion group when compared to deep myometrial invasion group (5); there was no statistically significant difference for positive LNM between superficial and deep myometrial invasion groups (6). In addition, there was no statistically significant difference for recurrence between superficial and deep myometrial invasion groups (7, 8). Therefore, a further study is valuable.
Currently, it is still a mystery whether the above non-uniform results change our previous conclusions and consensus. Until now, there is no aggregated estimate about the relationship between myometrial invasion and LVSI, LNM, recurrence, and OS. This meta-analysis is aiming to further elucidate whether myometrial invasion correlates to LVSI, LNM, recurrence, and OS based on the data available so far. The meta-analysis of multiple clinical studies will provide comprehensive descriptions about myometrial invasion not only from the past to the present but also from clinicopathological characteristics to prognostic value. We hope that the study also could provide us more certainty and confidence in mention and further investigation of myometrial invasion of endometrial cancer.
Materials and Methods
Literature Search Strategy
Literature was searched from Pubmed, Embase, and Web of Science from their inception until June 2021. The study published only in English was further considered. The main search terms were formulated as follows: “endometrial cancer”, “endometrial carcinoma”, “endometrial tumor”, “uterine carcinoma”, “uterine cancer”, “endometrial neoplasms”, “myometrial invasion”, “myometrial infiltration”, “clinicopathological factors”, “lymphovascular space invasion”, “lymph node metastasis”, “prognostic marker”, “prognosis”, “overall survival”, “recurrence”, and “relapse”.
Inclusion and Exclusion Criteria
The study had to meet the following inclusion criteria: (1) the patients only had endometrial cancer; (2) enough data about clinicopathological factors (myometrial invasion, LVSI, LNM, or recurrence) and/or related information to extract hazard ratio (HR) and standard error (SE) of lnHR for OS; (3) article was published in English. The exclusion criteria included the following terms: (1) reviews, meta-analysis, animal experiments, and case reports; (2) republished articles; (3) incomplete and unpublished studies; (4) the study did not meet the design. Two reviewers independently reviewed the literatures according to the predefined strategy and criteria. The articles were screened with two researchers independently (JW and PX). The disagreements were further settled through discussion and resolved by a third investigator when necessary.
Data Extraction and Quality Assessment
Two investigators (JW and PX) were assigned to assess the eligibility of all studies. Moreover, a third investigator (XZ) resolved the disagreements when necessary. The following information from each study was extracted: first author, publication year, the region of the study population, the number of participants, design type. For LVSI, LNM and recurrence, and the numbers in case and control groups were extracted respectively. For OS, HR estimate with 95% confidence interval (CI) for OS was extracted. The quality of included studies was assessed using Newcastle–Ottawa scale (NOS) scale, and the score of the quality ranged from 0 to 9.
Data Analysis
Version 5.4 software of Review Manager was applied for this meta-analysis. Risk ratio (RR) with 95% CI was pooled to investigate the association between myometrial invasion and clinicopathological features (LVSI, LNM, and recurrence). HR with 95% CI was combined to study the effect of myometrial invasion on OS. The HR was extracted directly when the HR with 95% CI was reported. SE was calculated using the equation: SElnHR = (lnUpperCI − lnLowerCI)/3.92 (9). If the article did not provide direct HR while Kaplan–Meier survival curve was shown, Engauge Digitizer software was performed to acquire HR with SE (10). A random-effects model was conducted if significant heterogeneity (p ≤ 0.1, I2 > 50%) was shown. Publication bias was evaluated by the shape of funnel plot. Statistically significant difference was pointed out when a p value was less than 0.05.
Results
Study Search Results
The predefined search strategy identified 1,385 records. After screening of titles and abstracts, 1,058 records were excluded including 35 non-English papers, 18 duplicated records, 208 review/meta/letter/abstract, 19 animal studies, and 778 irrelated literature. Full text of 327 articles was assessed, and 248 records were excluded including 8 studies with the same included patients, 11 basic research, and 229 articles without adequate data. Finally, 79 studies of total 68,870 patients were eligible (5–8, 11–85), including 9 articles for LVSI, 29 articles for LNM, 8 for recurrence, and 37 for OS. The included studies and Newcastle-Ottawa scores are presented in Table 1.
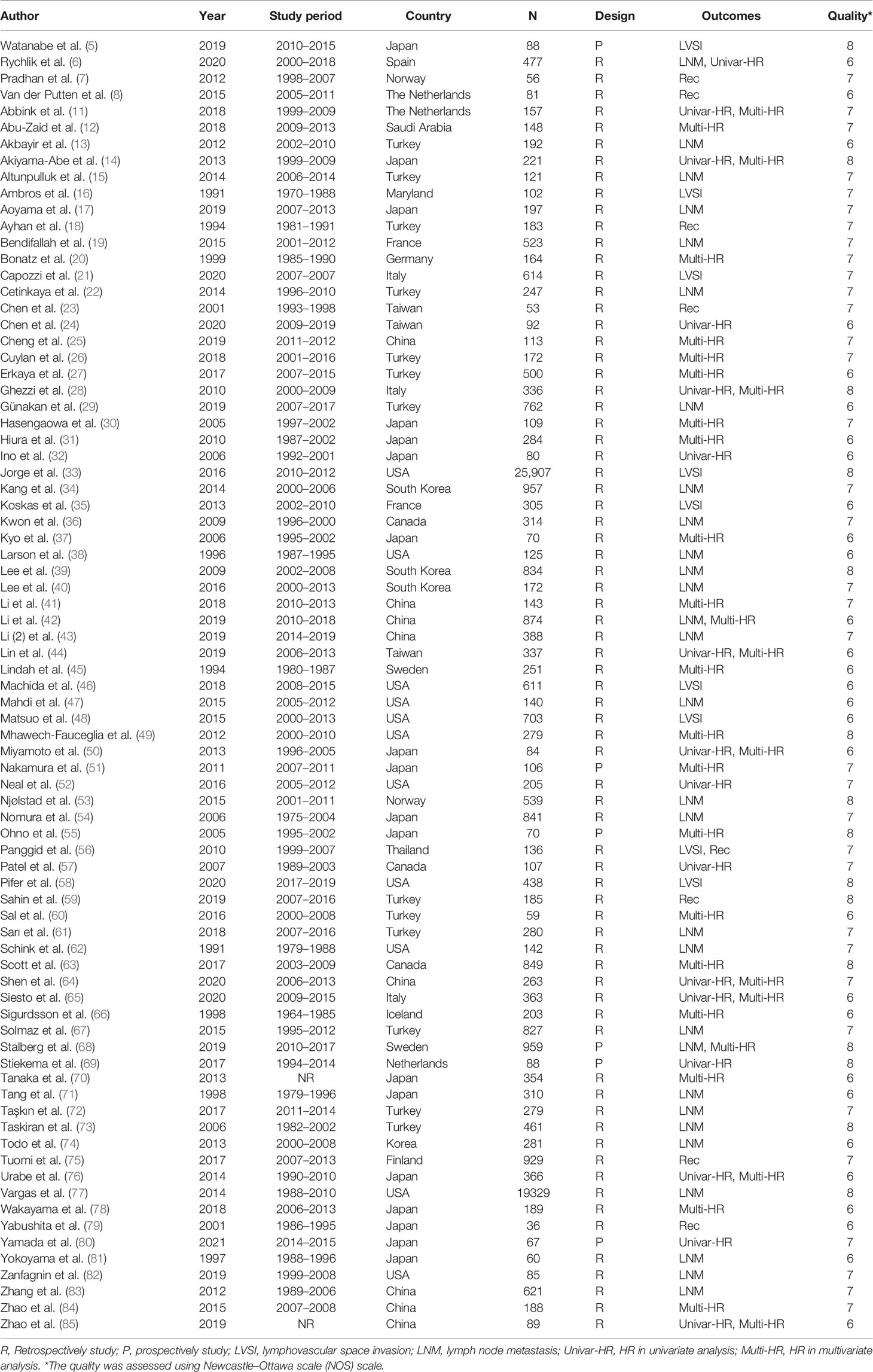
Myometrial Invasion Is Associated With LVSI in Endometrial Cancer
Nine studies with a total of 28,904 endometrial cancer patients were finally included for this analysis. The random-effects model was applied due to the significant between-study heterogeneity (I2 = 91%, p < 0.00001). The risk ratio, which was expressed as >1/2 group versus <1/2 group, was 3.07 (CI 95% 2.17–4.35, p < 0.00001) (Figure 1). The pooled result showed that there was a link between the depth of myometrial invasion and the risk of LVSI. Combined with the clinical information from the included studies, the result indicated that patients with deeper myometrial invasion of endometrial cancer into myometrium (>1/2) were more prone to LVSI.
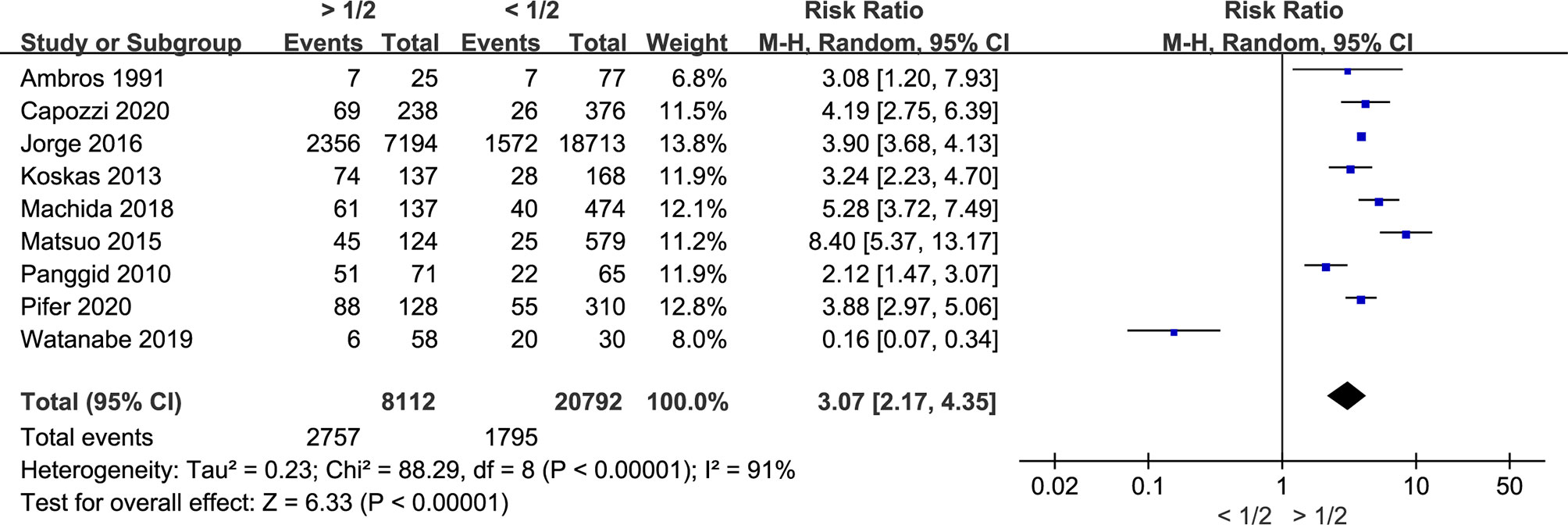
Figure 1 Meta-analysis of the association between myometrial invasion and LVSI in endometrial cancer.
Myometrial Invasion Is Associated With LNM in Endometrial Cancer
Twenty-nine studies including 31,262 endometrial cancer patients were eligible for analysis. The random-effects model was conducted for the significant between-study heterogeneity (I2 = 92%, p < 0.00001). The risk ratio was 4.45 (CI 95% 3.29–6.01, p < 0.00001) (Figure 2). The aggregated estimate of myometrial invasion was significantly associated with LVSI. According to the included studies, the result showed that deeper myometrial invasion is associated with the tendency of LNM in endometrial cancer.
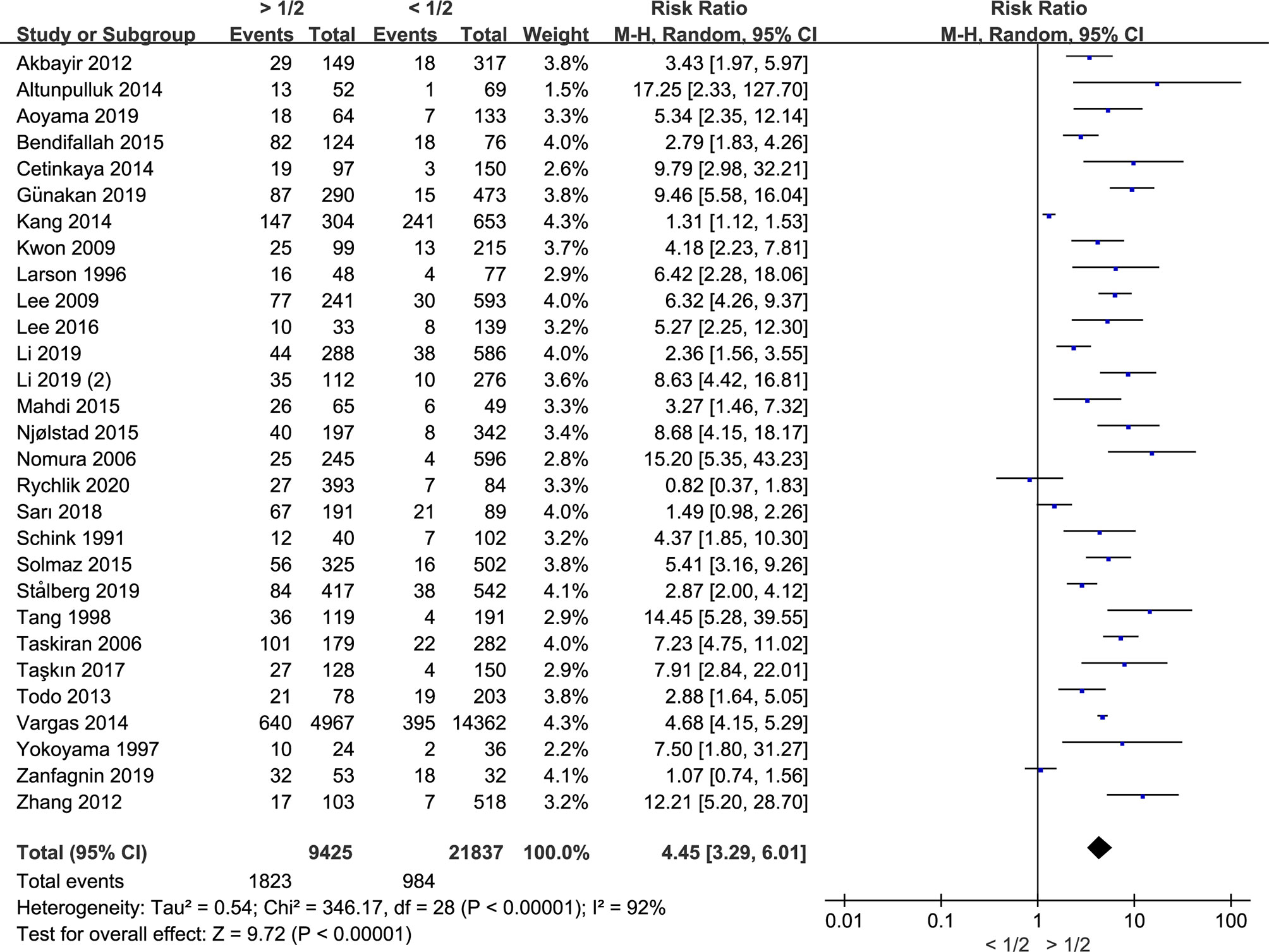
Figure 2 Meta-analysis of the association between myometrial invasion and LNM in endometrial cancer.
Myometrial Invasion Is Associated With the Recurrence of Endometrial Cancer
Since recurrence is the leading cause of death in cancers, further investigation is conducted by us on the association between myometrial invasion and the recurrence of endometrial cancer. Eight studies including 1,649 patients were included. During the analysis, we found that there was no significant between-study heterogeneity (I2 = 16%; p = 0.30), and fixed-effects model was used. Myometrial invasion was significantly associated with the recurrence of endometrial cancer since the risk ratio was 2.06 (CI 95% 1.58–2.69, p < 0.00001) (Figure 3). Therefore, deep myometrial invasion is associated with higher risk of endometrial cancer recurrence.
Figure 3 Meta-analysis of the association between myometrial invasion and recurrence in patients with endometrial cancer.
Myometrial Invasion Is Associated With OS in Endometrial Cancer
Thirty-seven studies including 9,416 patients examined the association between myometrial invasion and OS in endometrial cancer. The pooled HRs of all-cause mortality with >1/2 myometrial invasion compared to <1/2 myometrial invasion were evaluated using random-effects model, and the results are presented in Figure 4. Pooled HRs of OS for univariate and multivariate analyses (HR 3.36, 95% CI 2.35–4.79, P < 0.00001, and HR 2.00, 95% CI 1.59–2.53, P < 0.00001, respectively) showed that the group with deep myometrial invasion was related with a higher risk of OS than the group with less than 1/2 myometrial invasion. Therefore, deep myometrial invasion is associated with poor survival in endometrial cancer.

Figure 4 Meta-analysis of the association between myometrial invasion and overall survival in endometrial cancer patients according to HR from univariate or multivariate survival analyses.
Publication Bias of Included Studies
Funnel plot was applied for the assessment of publication bias in the literature, and tests for funnel plot asymmetry were applied only when there were at least 10 studies included in a meta-analysis. The shape of the funnel plot for the included 29 studies on the association between myometrial invasion and LNM was not significantly asymmetrical, indicating that there was no significant publication bias (Figure 5A). The results also show that no obvious publication bias was indicated in all included studies investigating myometrial invasion on OS in both univariate and multivariate analyses (Figures 5B, C).
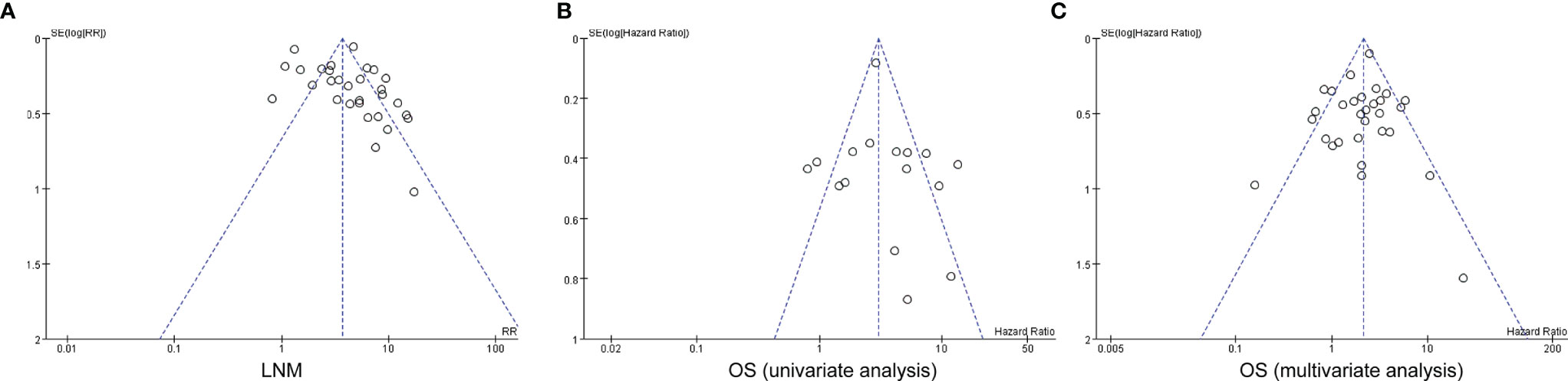
Figure 5 Funnel plots for potential publication bias. Funnel plot analysis of lymph node metastasis (A), and univariate and multivariate survival analyses (B, C). LNM, lymph node metastasis; OS, overall survival.
Discussion
Although there are many studies showing that myometrial invasion is definitely correlated to LVSI, LNM, recurrence, and OS, and we have reached an agreement that myometrial invasion is absolutely critical in the development of endometrial cancer, there are some inequable results, which makes us more or less feel lack confidence about that. Therefore, we searched all the studies about the relationship between myometrial invasion and clinicopathological characteristics (LVSI, LNM, and recurrence) or OS and conducted this meta-analysis.
The presence of LVSI is significantly associated with pelvic and paraaortic lymph node metastasis, recurrence, and poor prognosis (86, 87). As for lymph node metastasis, it is one of the evaluation criteria for the surgical-pathological staging and therapeutic schedule and is an extremely important determinant of the outcome. We paid extra attention to recurrence because it is uniformly associated with poor survival. Compared to LVSI, LNM, and recurrence, myometrial invasion is a much earlier molecular event and could be the initial driving force for the further progress of cancer cells. In addition, the depth of myometrial invasion before surgery can be accessed. Therefore, we should not only dig deeper into the underlying molecular mechanism but also pay more attention to the relevant clinical study. Since there are many studies about the relationship between myometrial invasion and clinicopathological characteristics (LVSI, LNM, and recurrence) or OS while not all the reports are consistent, we thereby pooled all the eligible studies and performed this meta-analysis.
Seventy-nine studies with a total of 68,870 endometrial cancer patients were finally included for this meta-analysis. Among them, nine studies with a total of 28,904 endometrial cancer patients were for LVSI. The pooled result showed patients with deeper myometrial invasion of endometrial cancer into myometrium (>1/2) were more prone to LVSI. As for LNM, 29 studies including 31,262 endometrial cancer patients were eligible for analysis, and the results demonstrated that deeper myometrial invasion is associated with the tendency of LNM in endometrial cancer. Furthermore, myometrial invasion was significantly associated with the recurrence of endometrial cancer according to the meta-analysis of eight studies including 1,649 patients. Since LVSI, LNM, and recurrence are independent prognostic factors for endometrial cancer patients, myometrial invasion would also be a prognostic factor. As it turned out, the group with deep myometrial invasion was related with a greater risk of OS than the group with less than 1/2 myometrial invasion based on not only univariate survival analysis but also multivariate survival analysis. Therefore, the results indicate that myometrial invasion is associated with LVSI, LNM, recurrence, and OS with much more confidence. Combined with preoperative assessment of the depth of myometrial invasion, now we know more information in regard to LVSI, LNM, recurrence, and OS of these patients before surgery, which suggests that we should especially pay more attention to myometrial invasion in clinical practice, and its underlying mechanism also deserves further investigation.
Potential limitations exist in this study, and meta-analysis without the classification of endometrial cancer is the obvious one. In the past, dualistic classification is the leading theory for the classification, which divides endometrial cancer into type I and type II tumors (88). According to histology, WHO classified endometrial cancer into the following subtypes: endometrioid, serous, mucinous, clear-cell, mixed, squamous-cell, transitional-cell, small-cell, and undifferentiated carcinomas (89). Among them, endometrioid carcinoma and serous carcinoma account for the majority. In this study, we check all the included 79 articles and found that histologic type was not only confined to endometrioid subtype although endometrioid carcinoma is the most common one. And 53 articles of the included 79 studies did not exclude other histologic types, so we did not further conduct the analysis based on histological classification. Recently, endometrial cancer is categorized into four genomic types: DNA polymerase epsilon (POLE) (ultramutated), microsatellite-instable (MSI) (hypermutated), copy-number low (endometrioid), and copy-number high (serous-like) tumors as the quick development of next-generation sequencing (90). The above genomic classification can facilitate the treatment tailored to specific subgroups and potentially enable the delivery of precision medicine to endometrial cancer patients. However, most included studies in the present meta-analysis did not perform the molecular classification, which may be because modern classification in molecular subtypes is relatively new and expensive, and thereby it is still not widely carried out in clinical practice. Given that, we appeal researchers to conduct more studies about the molecular classification and hope that more endometrial cancer patients benefit from the development of precision medicine.
Apart from classification, other potential limitations still exist in this study: (1) The data from the included studies were from the published articles instead of the original information of individual patient; (2) most included articles are the retrospective studies, and the evidence level is lower than that of prospective randomized clinical trial; (3) one of inclusion criteria is that article was published in English and negative results not being reported, which increase the risk of publication bias; (4) the number of included studies is relatively small, especially for LVSI and recurrence, which may cause biased results; (5) the heterogeneity of aggregated results was significant, and the random-effects model was applied.
Conclusion
In summary, a large scale and comprehensive meta-analysis of the association between myometrial invasion and other clinicopathological characteristics and prognosis is provided in the present study. Our results show that myometrial invasion is associated with LVSI, LNM, recurrence, and OS, indicating that deep myometrial invasion is a useful evaluation criterion to associate with poor clinical outcomes and prognosis in endometrial cancer patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
JW, PX, and XZ: conceptualization. JW, PX, and XY: data curation and original draft writing. QY, XX, and GZ: statistical analysis. JW and XZ: manuscript review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (Grant numbers: 81802591 and 81974225) and National Key R&D Program of China (Grant number: 2017YFC1001202).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Christensen JW, Dueholm M, Hansen ES, Marinovskij E, Lundorf E, Ortoft G. Assessment of Myometrial Invasion in Endometrial Cancer Using Three-Dimensional Ultrasound and Magnetic Resonance Imaging. Acta Obstet Gynecol Scand (2016) 95(1):55–64. doi: 10.1111/aogs.12806
4. Karatasli V, Cakir I, Sahin H, Ayaz D, Sanci M. Can Preoperative Magnetic Resonance Imaging Replace Intraoperative Frozen Sectioning in the Evaluation of Myometrial Invasion for Early-Stage Endometrial Carcinoma? Ginekol Pol (2019) 90(3):128–33. doi: 10.5603/GP.2019.0023
5. Watanabe T, Honma R, Kojima M, Nomura S, Furukawa S, Soeda S, et al. Prediction of Lymphovascular Space Invasion in Endometrial Cancer Using the 55-Gene Signature Selected by DNA Microarray Analysis. PloS One (2019) 14(9):e0223178. doi: 10.1371/journal.pone.0223178
6. Rychlik A, Zapardiel I, Baquedano L, Martinez Maestre MA, Querleu D, Coronado Martin PJ. Clinical Relevance of High-Intermediate Risk Endometrial Cancer According to European Risk Classification. Int J Gynecol Cancer (2020) 30(10):1528–34. doi: 10.1136/ijgc-2020-001693
7. Pradhan M, Davidson B, Abeler VM, Danielsen HE, Trope CG, Kristensen GB, et al. DNA Ploidy May Be a Prognostic Marker in Stage I and II Serous Adenocarcinoma of the Endometrium. Virchows Arch (2012) 461(3):291–8. doi: 10.1007/s00428-012-1275-2
8. van der Putten LJ, Geels YP, Ezendam NP, van der Putten HW, Snijders MP, van de Poll-Franse LV, et al. Lymphovascular Space Invasion and the Treatment of Stage I Endometrioid Endometrial Cancer. Int J Gynecol Cancer (2015) 25(1):75–80. doi: 10.1097/IGC.0000000000000306
9. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
10. Ioannidis JP, Panagiotou OA. Comparison of Effect Sizes Associated With Biomarkers Reported in Highly Cited Individual Articles and in Subsequent Meta-Analyses. JAMA (2011) 305(21):2200–10. doi: 10.1001/jama.2011.713
11. Abbink K, Zusterzeel PL, Geurts-Moespot AJ, Herwaarden AEV, Pijnenborg JM, Sweep FC, et al. HE4 Is Superior to CA125 in the Detection of Recurrent Disease in High-Risk Endometrial Cancer Patients. Tumour Biol (2018) 40(2):1010428318757103. doi: 10.1177/1010428318757103
12. Abu-Zaid A, Alsabban M, Abuzaid M, Alomar O, Salem H, Al-Badawi IA. Preoperative Anemia as a Prognostic Factor in Endometrioid-Type Endometrial Carcinoma. J Obstet Gynaecol Can (2018) 40(11):1393–400. doi: 10.1016/j.jogc.2018.05.005
13. Akbayir O, Corbacioglu A, Goksedef BP, Numanoglu C, Akca A, Guraslan H, et al. The Novel Criteria for Predicting Pelvic Lymph Node Metastasis in Endometrioid Adenocarcinoma of Endometrium. Gynecol Oncol (2012) 125(2):400–3. doi: 10.1016/j.ygyno.2012.01.051
14. Akiyama-Abe A, Minaguchi T, Nakamura Y, Michikami H, Shikama A, Nakao S, et al. Loss of PTEN Expression is an Independent Predictor of Favourable Survival in Endometrial Carcinomas. Br J Cancer (2013) 109(6):1703–10. doi: 10.1038/bjc.2013.455
15. Dogan Altunpulluk M, Kir G, Topal CS, Cetiner H, Gocmen A. The Association of the Microcystic, Elongated and Fragmented (MELF) Invasion Pattern in Endometrial Carcinomas With Deep Myometrial Invasion, Lymphovascular Space Invasion and Lymph Node Metastasis. J Obstet Gynaecol (2015) 35(4):397–402. doi: 10.3109/01443615.2014.960827
16. Ambros RA, Kurman RJ. Combined Assessment of Vascular and Myometrial Invasion as a Model to Predict Prognosis in Stage I Endometrioid Adenocarcinoma of the Uterine Corpus. Cancer (1992) 69(6):1424–31. doi: 10.1002/1097-0142(19920315)69:6<1424::aid-cncr2820690620>3.0.co;2-5
17. Aoyama T, Takano M, Miyamoto M, Yoshikawa T, Kato K, Sakamoto T, et al. Pretreatment Neutrophil-To-Lymphocyte Ratio Was a Predictor of Lymph Node Metastasis in Endometrial Cancer Patients. Oncology (2019) 96(5):259–67. doi: 10.1159/000497184
18. Ayhan A, Tuncer ZS, Tuncer R, Yuce K, Kucukali T. Risk Factors for Recurrence in Clinically Early Endometrial Carcinoma: An Analysis of 183 Consecutive Cases. Eur J Obstet Gynecol Reprod Biol (1994) 57(3):167–70. doi: 10.1016/0028-2243(94)90294-1
19. Bendifallah S, Canlorbe G, Laas E, Huguet F, Coutant C, Hudry D, et al. A Predictive Model Using Histopathologic Characteristics of Early-Stage Type 1 Endometrial Cancer to Identify Patients at High Risk for Lymph Node Metastasis. Ann Surg Oncol (2015) 22(13):4224–32. doi: 10.1245/s10434-015-4548-6
20. Bonatz G, Luttes J, Hamann S, Mettler L, Jonat W, Parwaresch R. Immunohistochemical Assessment of P170 Provides Prognostic Information in Endometrial Carcinoma. Histopathology (1999) 34(1):43–50. doi: 10.1046/j.1365-2559.1999.00564.x
21. Capozzi VA, Sozzi G, Uccella S, Ceni V, Cianciolo A, Gambino G, et al. Novel Preoperative Predictive Score to Evaluate Lymphovascular Space Involvement in Endometrial Cancer: An Aid to the Sentinel Lymph Node Algorithm. Int J Gynecol Cancer (2020) 30(6):806–12. doi: 10.1136/ijgc-2019-001016
22. Cetinkaya K, Atalay F, Bacinoglu A. Risk Factors of Lymph Node Metastases With Endometrial Carcinoma. Asian Pac J Cancer Prev (2014) 15(15):6353–6. doi: 10.7314/apjcp.2014.15.15.6353
23. Chen CA, Cheng WF, Lee CN, Wei LH, Chu JS, Hsieh FJ, et al. Cytosol Vascular Endothelial Growth Factor in Endometrial Carcinoma: Correlation With Disease-Free Survival. Gynecol Oncol (2001) 80(2):207–12. doi: 10.1006/gyno.2000.6048
24. Chen HH, Ting WH, Sun HD, Wei MC, Lin HH, Hsiao SM. Predictors of Survival in Women With High-Risk Endometrial Cancer and Comparisons of Sandwich Versus Concurrent Adjuvant Chemotherapy and Radiotherapy. Int J Environ Res Public Health (2020) 17(16):5941. doi: 10.3390/ijerph17165941
25. Cheng L, Zhao T, Li S, Wang Y, Fei H, Meng F. Overexpression of HPIP as a Biomarker for Metastasis and Prognosis Prediction in Endometrial Cancer Patients. J Clin Lab Anal (2019) 33(8):e22959. doi: 10.1002/jcla.22959
26. Cuylan ZF, Oz M, Ozkan NT, Comert GK, Sahin H, Turan T, et al. Prognostic Factors and Patterns of Recurrence in Lymphovascular Space Invasion Positive Women With Stage IIIC Endometriod Endometrial Cancer. J Obstet Gynaecol Res (2018) 44(6):1140–9. doi: 10.1111/jog.13615
27. Erkaya S, Oz M, Topcu HO, Sirvan AL, Gungor T, Meydanli MM. Is Lower Uterine Segment Involvement a Prognostic Factor in Endometrial Cancer? Turk J Med Sci (2017) 47(1):300–6. doi: 10.3906/sag-1602-137
28. Ghezzi F, Cromi A, Siesto G, Giudici S, Serati M, Formenti G, et al. Prognostic Significance of Preoperative Plasma Fibrinogen in Endometrial Cancer. Gynecol Oncol (2010) 119(2):309–13. doi: 10.1016/j.ygyno.2010.07.014
29. Gunakan E, Atan S, Haberal AN, Kucukyildiz IA, Gokce E, Ayhan A. A Novel Prediction Method for Lymph Node Involvement in Endometrial Cancer: Machine Learning. Int J Gynecol Cancer (2019) 29(2):320–4. doi: 10.1136/ijgc-2018-000033
30. Hasengaowa, Kodama J, Kusumoto T, Shinyo Y, Seki N, Hiramatsu Y. Prognostic Significance of Syndecan-1 Expression in Human Endometrial Cancer. Ann Oncol (2005) 16(7):1109–15. doi: 10.1093/annonc/mdi224
31. Hiura M, Nogawa T, Matsumoto T, Yokoyama T, Shiroyama Y, Wroblewski J. Long-Term Survival in Patients With Para-Aortic Lymph Node Metastasis With Systematic Retroperitoneal Lymphadenectomy Followed by Adjuvant Chemotherapy in Endometrial Carcinoma. Int J Gynecol Cancer (2010) 20(6):1000–5. doi: 10.1111/IGC.0b013e3181d80aff
32. Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, et al. Indoleamine 2,3-Dioxygenase Is a Novel Prognostic Indicator for Endometrial Cancer. Br J Cancer (2006) 95(11):1555–61. doi: 10.1038/sj.bjc.6603477
33. Jorge S, Hou JY, Tergas AI, Burke WM, Huang Y, Hu JC, et al. Magnitude of Risk for Nodal Metastasis Associated With Lymphvascular Space Invasion for Endometrial Cancer. Gynecol Oncol (2016) 140(3):387–93. doi: 10.1016/j.ygyno.2016.01.002
34. Kang S, Lee JM, Lee JK, Kim JW, Cho CH, Kim SM, et al. A Web-Based Nomogram Predicting Para-Aortic Nodal Metastasis in Incompletely Staged Patients With Endometrial Cancer: A Korean Multicenter Study. Int J Gynecol Cancer (2014) 24(3):513–9. doi: 10.1097/IGC.0000000000000090
35. Koskas M, Bassot K, Graesslin O, Aristizabal P, Barranger E, Clavel-Chapelon F, et al. Impact of Lymphovascular Space Invasion on a Nomogram for Predicting Lymph Node Metastasis in Endometrial Cancer. Gynecol Oncol (2013) 129(2):292–7. doi: 10.1016/j.ygyno.2013.02.027
36. Kwon JS, Qiu F, Saskin R, Carey MS. Are Uterine Risk Factors More Important Than Nodal Status in Predicting Survival in Endometrial Cancer? Obstet Gynecol (2009) 114(4):736–43. doi: 10.1097/AOG.0b013e3181b96ec6
37. Kyo S, Sakaguchi J, Ohno S, Mizumoto Y, Maida Y, Hashimoto M, et al. High Twist Expression Is Involved in Infiltrative Endometrial Cancer and Affects Patient Survival. Hum Pathol (2006) 37(4):431–8. doi: 10.1016/j.humpath.2005.12.021
38. Larson DM, Connor GP, Broste SK, Krawisz BR, Johnson KK. Prognostic Significance of Gross Myometrial Invasion With Endometrial Cancer. Obstet Gynecol (1996) 88(3):394–8. doi: 10.1016/0029-7844(96)00161-5
39. Lee KB, Ki KD, Lee JM, Lee JK, Kim JW, Cho CH, et al. The Risk of Lymph Node Metastasis Based on Myometrial Invasion and Tumor Grade in Endometrioid Uterine Cancers: A Multicenter, Retrospective Korean Study. Ann Surg Oncol (2009) 16(10):2882–7. doi: 10.1245/s10434-009-0535-0
40. Lee J, Kong TW, Paek J, Chang SJ, Ryu HS. Predicting Model of Lymph Node Metastasis Using Preoperative Tumor Grade, Transvaginal Ultrasound, and Serum CA-125 Level in Patients With Endometrial Cancer. Int J Gynecol Cancer (2016) 26(9):1630–5. doi: 10.1097/IGC.0000000000000820
41. Li P, Yin H, Meng F, Liu S, Liu H, Ma R. High TRIM44 Expression in Endometrial Carcinoma Is Associated With a Poorer Patient Outcome. Pathol Res Pract (2018) 214(5):727–31. doi: 10.1016/j.prp.2018.03.007
42. Li M, Wu S, Xie Y, Zhang X, Wang Z, Zhu Y, et al. Cervical Invasion, Lymphovascular Space Invasion, and Ovarian Metastasis as Predictors of Lymph Node Metastasis and Poor Outcome on Stages I to III Endometrial Cancers: A Single-Center Retrospective Study. World J Surg Oncol (2019) 17(1):193. doi: 10.1186/s12957-019-1733-2
43. Li Y, Cong P, Wang P, Peng C, Liu M, Sun G. Risk Factors for Pelvic Lymph Node Metastasis in Endometrial Cancer. Arch Gynecol Obstet (2019) 300(4):1007–13. doi: 10.1007/s00404-019-05276-9
44. Lin YJ, Hu YW, Twu NF, Liu YM. The Role of Adjuvant Radiotherapy in Stage I Endometrial Cancer: A Single-Institution Outcome. Taiwan J Obstet Gynecol (2019) 58(5):604–9. doi: 10.1016/j.tjog.2019.07.005
45. Lindahl B, Ranstam J, Willen R. Five Year Survival Rate in Endometrial Carcinoma Stages I–II: Influence of Degree of Tumour Differentiation, Age, Myometrial Invasion and DNA Content. Br J Obstet Gynaecol (1994) 101(7):621–5. doi: 10.1111/j.1471-0528.1994.tb13654.x
46. Machida H, Hom MS, Adams CL, Eckhardt SE, Garcia-Sayre J, Mikami M, et al. Intrauterine Manipulator Use During Minimally Invasive Hysterectomy and Risk of Lymphovascular Space Invasion in Endometrial Cancer. Int J Gynecol Cancer (2018) 28(2):208–19. doi: 10.1097/IGC.0000000000001181
47. Mahdi H, Jernigan A, Nutter B, Michener C, Rose PG. Lymph Node Metastasis and Pattern of Recurrence in Clinically Early Stage Endometrial Cancer With Positive Lymphovascular Space Invasion. J Gynecol Oncol (2015) 26(3):208–13. doi: 10.3802/jgo.2015.26.3.208
48. Matsuo K, Garcia-Sayre J, Medeiros F, Casabar JK, Machida H, Moeini A, et al. Impact of Depth and Extent of Lymphovascular Space Invasion on Lymph Node Metastasis and Recurrence Patterns in Endometrial Cancer. J Surg Oncol (2015) 112(6):669–76. doi: 10.1002/jso.24049
49. Mhawech-Fauceglia P, Wang D, Syriac S, Godoy H, Dupont N, Liu S, et al. Synuclein-Gamma (SNCG) Protein Expression Is Associated With Poor Outcome in Endometrial Adenocarcinoma. Gynecol Oncol (2012) 124(1):148–52. doi: 10.1016/j.ygyno.2011.09.037
50. Miyamoto T, Suzuki A, Asaka R, Ishikawa K, Yamada Y, Kobara H, et al. Immunohistochemical Expression of Core 2 Beta1,6-N-Acetylglucosaminyl Transferase 1 (C2GnT1) in Endometrioid-Type Endometrial Carcinoma: A Novel Potential Prognostic Factor. Histopathology (2013) 62(7):986–93. doi: 10.1111/his.12107
51. Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The Measurement of SUVmax of the Primary Tumor Is Predictive of Prognosis for Patients With Endometrial Cancer. Gynecol Oncol (2011) 123(1):82–7. doi: 10.1016/j.ygyno.2011.06.026
52. Neal SA, Graybill WS, Garrett-Mayer E, McDowell ML, McLean VE, Watson CH, et al. Lymphovascular Space Invasion in Uterine Corpus Cancer: What Is its Prognostic Significance in the Absence of Lymph Node Metastases? Gynecol Oncol (2016) 142(2):278–82. doi: 10.1016/j.ygyno.2016.05.037
53. Njolstad TS, Trovik J, Hveem TS, Kjaereng ML, Kildal W, Pradhan M, et al. DNA Ploidy in Curettage Specimens Identifies High-Risk Patients and Lymph Node Metastasis in Endometrial Cancer. Br J Cancer (2015) 112(10):1656–64. doi: 10.1038/bjc.2015.123
54. Nomura H, Aoki D, Suzuki N, Susumu N, Suzuki A, Tamada Y, et al. Analysis of Clinicopathologic Factors Predicting Para-Aortic Lymph Node Metastasis in Endometrial Cancer. Int J Gynecol Cancer (2006) 16(2):799–804. doi: 10.1111/j.1525-1438.2006.00529.x
55. Ohno Y, Ohno S, Suzuki N, Kamei T, Inagawa H, Soma G, et al. Role of Cyclooxygenase-2 in Immunomodulation and Prognosis of Endometrial Carcinoma. Int J Cancer (2005) 114(5):696–701. doi: 10.1002/ijc.20777
56. Panggid K, Cheewakriangkrai C, Khunamornpong S, Siriaunkgul S. Factors Related to Recurrence in Non-Obese Women With Endometrial Endometrioid Adenocarcinoma. J Obstet Gynaecol Res (2010) 36(5):1044–8. doi: 10.1111/j.1447-0756.2010.01289.x
57. Patel S, Portelance L, Gilbert L, Tan L, Stanimir G, Duclos M, et al. Analysis of Prognostic Factors and Patterns of Recurrence in Patients With Pathologic Stage III Endometrial Cancer. Int J Radiat Oncol Biol Phys (2007) 68(5):1438–45. doi: 10.1016/j.ijrobp.2007.02.003
58. Pifer PM, Bhargava R, Patel AK, Ling DC, Vargo JA, Orr BC, et al. Is the Risk of Substantial LVSI in Stage I Endometrial Cancer Similar to PORTEC in the North American Population? - A Single-Institution Study. Gynecol Oncol (2020) 159(1):23–9. doi: 10.1016/j.ygyno.2020.07.024
59. Sahin H, Meydanli MM, Sari ME, Kocaman E, Cuylan ZF, Yalcin I, et al. Recurrence Patterns and Prognostic Factors in Lymphovascular Space Invasion-Positive Endometrioid Endometrial Cancer Surgically Confined to the Uterus. Taiwan J Obstet Gynecol (2019) 58(1):82–9. doi: 10.1016/j.tjog.2018.11.016
60. Sal V, Demirkiran F, Erenel H, Tokgozoglu N, Kahramanoglu I, Bese T, et al. Expression of PTEN and Beta-Catenin and Their Relationship With Clinicopathological and Prognostic Factors in Endometrioid Type Endometrial Cancer. Int J Gynecol Cancer (2016) 26(3):512–20. doi: 10.1097/IGC.0000000000000626
61. Sari ME, Meydanli MM, Yalcin I, Sahin H, Coban G, Celik H, et al. Risk Factors for Lymph Node Metastasis Among Lymphovascular Space Invasion-Positive Women With Endometrioid Endometrial Cancer Clinically Confined to the Uterus. Oncol Res Treat (2018) 41(12):750–4. doi: 10.1159/000492585
62. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor Size in Endometrial Cancer. Cancer (1991) 67(11):2791–4. doi: 10.1002/1097-0142(19910601)67:11<2791::aid-cncr2820671113>3.0.co;2-s
63. Scott SA, van der Zanden C, Cai E, McGahan CE, Kwon JS. Prognostic Significance of Peritoneal Cytology in Low-Intermediate Risk Endometrial Cancer. Gynecol Oncol (2017) 145(2):262–8. doi: 10.1016/j.ygyno.2017.03.011
64. Shen J, Chen Q, Li N, Bai X, Wang F, Li B. TWIST1 Expression and Clinical Significance in Type I Endometrial Cancer and Premalignant Lesions: A Retrospective Clinical Study. Med (Baltimore) (2020) 99(48):e23397. doi: 10.1097/MD.0000000000023397
65. Siesto G, Romano F, Ieda NP, Vitobello D. Survival Outcomes After Surgical Management of Endometrial Cancer: Analysis After the First 10-Year Experience of Robotic Surgery in a Single Center. Int J Med Robot (2020) 16(6):1–9. doi: 10.1002/rcs.2157
66. Sigurdsson K, Sigurdardottir B, Steinsson S, Benediktsdottir K, Sigurvinsson T, Sigvaldason H. Survival and Prognostic Factors of Endometrial Cancer Patients in Iceland 1964-1985: Can Attendance at Population-Based Pap-Smear Screening Affect Survival? Int J Cancer (1998) 79(2):166–74. doi: 10.1002/(sici)1097-0215(19980417)79:2<166::aid-ijc12>3.0.co;2-8
67. Solmaz U, Mat E, Dereli M, Turan V, Gungorduk K, Hasdemir P, et al. Lymphovascular Space Invasion and Cervical Stromal Invasion Are Independent Risk Factors for Nodal Metastasis in Endometrioid Endometrial Cancer. Aust N Z J Obstet Gynaecol (2015) 55(1):81–6. doi: 10.1111/ajo.12321
68. Stalberg K, Bjurberg M, Borgfeldt C, Carlson J, Dahm-Kahler P, Floter-Radestad A, et al. Lymphovascular Space Invasion as a Predictive Factor for Lymph Node Metastases and Survival in Endometrioid Endometrial Cancer - a Swedish Gynecologic Cancer Group (SweGCG) Study. Acta Oncol (2019) 58(11):1628–33. doi: 10.1080/0284186X.2019.1643036
69. Stiekema A, Lok C, Korse CM, van Driel WJ, van der Noort V, Kenter GG, et al. Serum HE4 Is Correlated to Prognostic Factors and Survival in Patients With Endometrial Cancer. Virchows Arch (2017) 470(6):655–64. doi: 10.1007/s00428-017-2115-1
70. Tanaka Y, Terai Y, Kawaguchi H, Fujiwara S, Yoo S, Tsunetoh S, et al. Prognostic Impact of EMT (Epithelial-Mesenchymal-Transition)-Related Protein Expression in Endometrial Cancer. Cancer Biol Ther (2013) 14(1):13–9. doi: 10.4161/cbt.22625
71. Tang X, Tanemura K, Ye W, Ohmi K, Tsunematsu R, Yamada T, et al. Clinicopathological Factors Predicting Retroperitoneal Lymph Node Metastasis and Survival in Endometrial Cancer. Jpn J Clin Oncol (1998) 28(11):673–8. doi: 10.1093/jjco/28.11.673
72. Taskin S, Sukur YE, Varli B, Koyuncu K, Seval MM, Ates C, et al. Nomogram With Potential Clinical Use to Predict Lymph Node Metastasis in Endometrial Cancer Patients Diagnosed Incidentally by Postoperative Pathological Assessment. Arch Gynecol Obstet (2017) 296(4):803–9. doi: 10.1007/s00404-017-4477-7
73. Taskiran C, Yuce K, Geyik PO, Kucukali T, Ayhan A. Predictability of Retroperitoneal Lymph Node Metastasis by Using Clinicopathologic Variables in Surgically Staged Endometrial Cancer. Int J Gynecol Cancer (2006) 16(3):1342–7. doi: 10.1111/j.1525-1438.2006.00534.x
74. Todo Y, Choi HJ, Kang S, Kim JW, Nam JH, Watari H, et al. Clinical Significance of Tumor Volume in Endometrial Cancer: A Japan-Korea Cooperative Study. Gynecol Oncol (2013) 131(2):294–8. doi: 10.1016/j.ygyno.2013.08.008
75. Tuomi T, Pasanen A, Leminen A, Butzow R, Loukovaara M. Prediction of Site-Specific Tumor Relapses in Patients With Stage I-II Endometrioid Endometrial Cancer. Int J Gynecol Cancer (2017) 27(5):923–30. doi: 10.1097/IGC.0000000000000970
76. Urabe R, Hachisuga T, Kurita T, Kagami S, Kawagoe T, Matsuura Y, et al. Prognostic Significance of Overexpression of P53 in Uterine Endometrioid Adenocarcinomas With an Analysis of Nuclear Grade. J Obstet Gynaecol Res (2014) 40(3):812–9. doi: 10.1111/jog.12215
77. Vargas R, Rauh-Hain JA, Clemmer J, Clark RM, Goodman A, Growdon WB, et al. Tumor Size, Depth of Invasion, and Histologic Grade as Prognostic Factors of Lymph Node Involvement in Endometrial Cancer: A SEER Analysis. Gynecol Oncol (2014) 133(2):216–20. doi: 10.1016/j.ygyno.2014.02.011
78. Wakayama A, Kudaka W, Matsumoto H, Aoyama H, Ooyama T, Taira Y, et al. Lymphatic Vessel Involvement Is Predictive for Lymph Node Metastasis and an Important Prognostic Factor in Endometrial Cancer. Int J Clin Oncol (2018) 23(3):532–8. doi: 10.1007/s10147-017-1227-6
79. Yabushita H, Shimazu M, Yamada H, Sawaguchi K, Noguchi M, Nakanishi M, et al. Occult Lymph Node Metastases Detected by Cytokeratin Immunohistochemistry Predict Recurrence in Node-Negative Endometrial Cancer. Gynecol Oncol (2001) 80(2):139–44. doi: 10.1006/gyno.2000.6067
80. Yamada S, Tsuyoshi H, Yamamoto M, Tsujikawa T, Kiyono Y, Okazawa H, et al. Prognostic Value of 16α-18F-Fluoro-17β-Estradiol Positron Emission Tomography as a Predictor of Disease Outcome in Endometrial Cancer: A Prospective Study. J Nucl Med (2021) 62(5):636–42. doi: 10.2967/jnumed.120.244319
81. Yokoyama Y, Maruyama H, Sato S, Saito Y. Risk Factors Predictive of Para-Aortic Lymph Node Metastasis in Endometrial Carcinomas. J Obstet Gynaecol Res (1997) 23(2):179–87. doi: 10.1111/j.1447-0756.1997.tb00829.x
82. Zanfagnin V, Huang Y, Mc Gree ME, Weaver AL, Casarin J, Multinu F, et al. Predictors of Extensive Lymphatic Dissemination and Recurrences in Node-Positive Endometrial Cancer. Gynecol Oncol (2019) 154(3):480–6. doi: 10.1016/j.ygyno.2019.07.006
83. Zhang C, Wang C, Feng W. Clinicopathological Risk Factors for Pelvic Lymph Node Metastasis in Clinical Early-Stage Endometrioid Endometrial Adenocarcinoma. Int J Gynecol Cancer (2012) 22(8):1373–7. doi: 10.1097/IGC.0b013e318269f68e
84. Zhao J, Liu T, Yu G, Wang J. Overexpression of HABP1 Correlated With Clinicopathological Characteristics and Unfavorable Prognosis in Endometrial Cancer. Tumour Biol (2015) 36(2):1299–306. doi: 10.1007/s13277-014-2761-8
85. Zhao X, Fan Y, Lu C, Li H, Zhou N, Sun G, et al. PCAT1 Is a Poor Prognostic Factor in Endometrial Carcinoma and Associated With Cancer Cell Proliferation, Migration and Invasion. Bosn J Basic Med Sci (2019) 19(3):274–81. doi: 10.17305/bjbms.2019.4096
86. Dong Y, Cheng Y, Tian W, Zhang H, Wang Z, Li X, et al. An Externally Validated Nomogram for Predicting Lymph Node Metastasis of Presumed Stage I and II Endometrial Cancer. Front Oncol (2019) 9:1218. doi: 10.3389/fonc.2019.01218
87. Sadozye AH, Harrand RL, Reed NS. Lymphovascular Space Invasion as a Risk Factor in Early Endometrial Cancer. Curr Oncol Rep (2016) 18(4):24. doi: 10.1007/s11912-016-0505-1
88. Bokhman JV. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol Oncol (1983) 15(1):10–7. doi: 10.1016/0090-8258(83)90111-7
89. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial Cancer. Lancet (2005) 366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8
Keywords: endometrial cancer, myometrial invasion, lymphovascular space invasion, lymph node metastasis, recurrence, overall survival, meta-analysis
Citation: Wang J, Xu P, Yang X, Yu Q, Xu X, Zou G and Zhang X (2021) Association of Myometrial Invasion With Lymphovascular Space Invasion, Lymph Node Metastasis, Recurrence, and Overall Survival in Endometrial Cancer: A Meta-Analysis of 79 Studies With 68,870 Patients. Front. Oncol. 11:762329. doi: 10.3389/fonc.2021.762329
Received: 21 August 2021; Accepted: 30 September 2021;
Published: 21 October 2021.
Edited by:
Alberto Farolfi, Istituto Scientifico Romagnolo per lo Studio e il Trattamento dei Tumori (IRCCS), ItalyReviewed by:
Eliane T. Taube, Charité, GermanyAizhen Cai, The First Medical Center of Chinese PLA General Hospital, China
Copyright © 2021 Wang, Xu, Yang, Yu, Xu, Zou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinmei Zhang, emhhbmd4aW5tQHpqdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Jianzhang Wang1†
Jianzhang Wang1† Xueying Yang
Xueying Yang Xinmei Zhang
Xinmei Zhang