- 1Center of Gastrointestinal Surgery, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Center of Gastric Cancer, Sun Yat-Sen University, Guangzhou, China
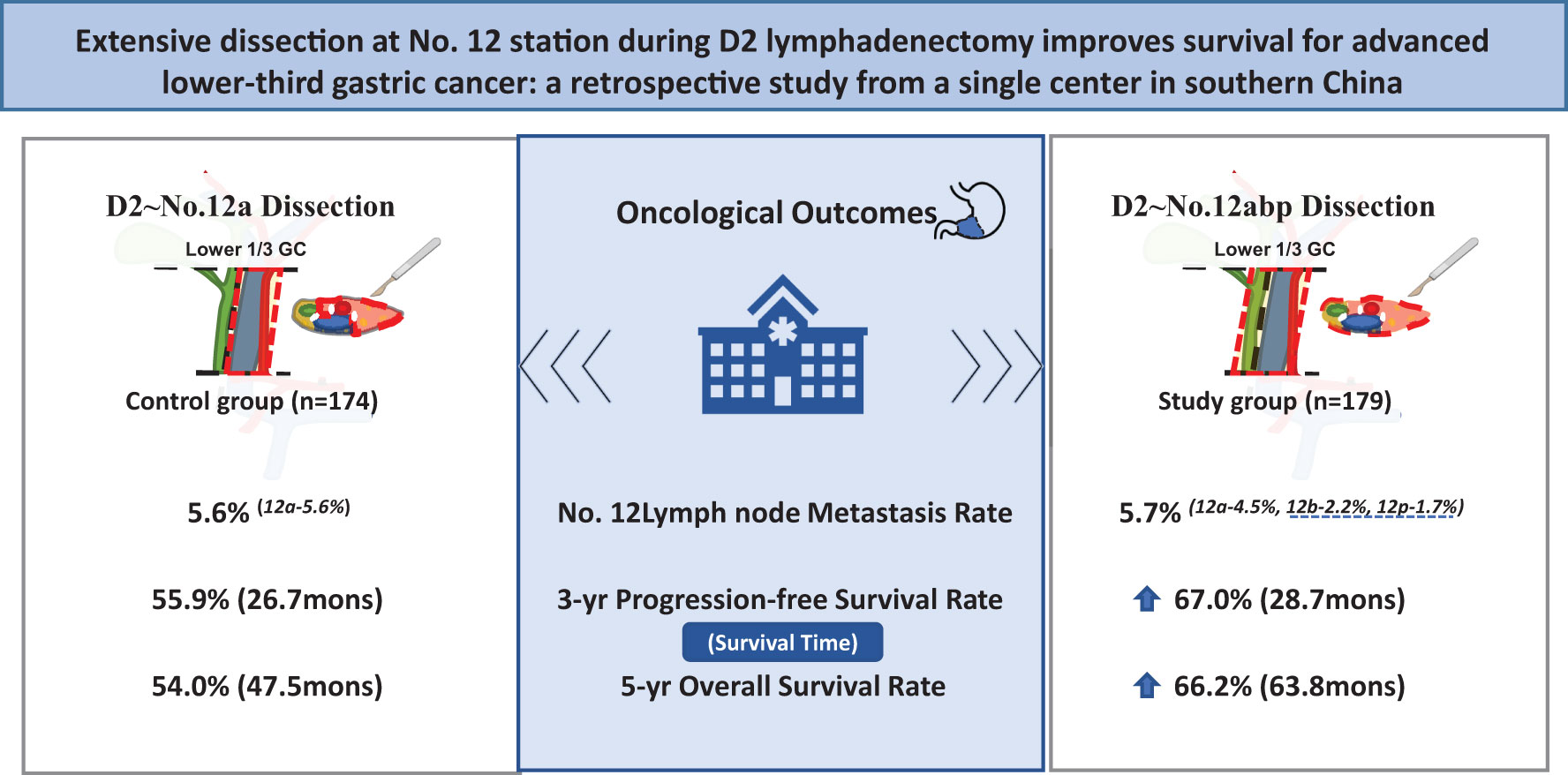
Background: D2 lymphadenectomy including No. 12a dissection has been accepted as a standard surgical management of advanced lower-third gastric cancer (GC). The necessity of extensive No. 12 nodes (No. 12a, 12b, and 12p) dissection remains controversial. This study aims to explore its impact on long-term survival for resectable GC.
Methods: From 2009 to 2016, 353 advanced lower-third GC patients undergoing at least D2 lymphadenectomy during a radical surgery were included, with 179 patients receiving No. 12a, 12b, and 12p dissection as study group. A total of 174 patients with No. 12a dissection were employed as control group. Surgical and long-term outcomes including 90-day complications incidence, therapeutic value index (TVI), 3-year progression-free survival (PFS), and 5-year overall survival (OS) were compared between both groups.
Results: No. 12 lymph node metastasis was observed in 20 (5.7%) patients, with 10 cases in each group (5.6% vs. 5.7%, p = 0.948). The metastatic rates at No. 12a, 12b, and 12p were 5.7%, 2.2%, and 1.7%, respectively. The incidence of 90-day complications was identical between both groups. Extensive No. 12 dissection was associated with increased TVI at No. 12 station (3.9 vs. 0.6), prolonged 3-year PFS rate (67.0% vs. 55.9%, p = 0.045) and 5-year OS rate (66.2% vs. 54.0%, p = 0.027). The further Cox-regression analysis showed that the 12abp dissection was an independent prognostic factor of improved survival (p = 0.026).
Conclusion: Adding No. 12b and 12p lymph nodes to D2 lymphadenectomy might be effective in surgical treatment of advanced lower-third GC and improve oncological outcomes compared with No. 12a-based D2 lymphadenectomy.
Introduction
Gastric cancer (GC) is the second most common malignance disease worldwide, and gastric adenocarcinoma accounts for approximately 95% of gastric cancer (1). Despite of recent progress in the treatment of gastric adenocarcinoma, the outcome is still poor with a 5-year overall survival (OS) rate of about 20% (2). Surgical resection with adequate lymph node dissection remains the curable therapy for such disease. Nevertheless, over 50% of patients present with locally advanced or metastatic gastric adenocarcinoma at first diagnosis, with chemotherapy or immunotherapy as the main therapeutic regimen for those patients (1, 3). Lymph node metastasis is one of the most important determinants of long-term outcomes in patients with advanced GC. The GC treatment guideline from Japanese Gastric Cancer Association recommends dissection of all first- and second-tier lymph nodes (D2) with gastrectomy as standard therapy for advanced GC in Japan (4).
The hepatoduodenal lymph nodes, known as the No. 12 lymph nodes, can be divided into three groups of nodes along the hepatic artery (No. 12a), the bile duct (No. 12b), and behind the portal vein (No. 12p). Previously, the No. 12b and 12p lymph nodes were regarded as third-tier (N3) lymph nodes. Unfortunately, the data of No. 12b and 12p lymph nodes were limited and mostly available from Japan and South Korea. Feng et al. (5) reported that the metastatic rates of No. 12b and 12p lymph nodes were 3.1% and 9.2%, respectively. The necessity of adding No. 12b and 12p dissection into D2 lymphadenectomy remains an unsolved problem in GC surgery. In China, most of GC patients were diagnosed at advanced stage, with no more than 10% early stage observed (6, 7). To fully investigate the oncological outcomes of No. 12 lymph node dissection, we retrospectively compared the short- and long-term outcomes of an extensive No. 12 dissection (No. 12abp) with No. 12a dissection alone for patients with resectable GC.
Methods
Patients
This is a single-center, retrospective study for oncological outcomes of advanced GC. From December 2009 to March 2016, patients with resectable GC who underwent a radical gastrectomy at our unit were first enrolled. The including criteria were as follows (1): confirmed diagnosis of gastric adenocarcinoma, which was restricted to the lower third of stomach and resectable based on a multidisciplinary team (MDT) discussion (8) and (2) underwent D2 lymphadenectomy with at least No. 12a lymph nodes dissected. The flow chart and exclusion criteria of the current study are shown in Figure 1. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University. All patients had signed an informed consent form for either surgery or chemotherapy. All processes involved in this study were in accordance with the ethics statements presented in the 1964 Declaration of Helsinki and its later amendments.
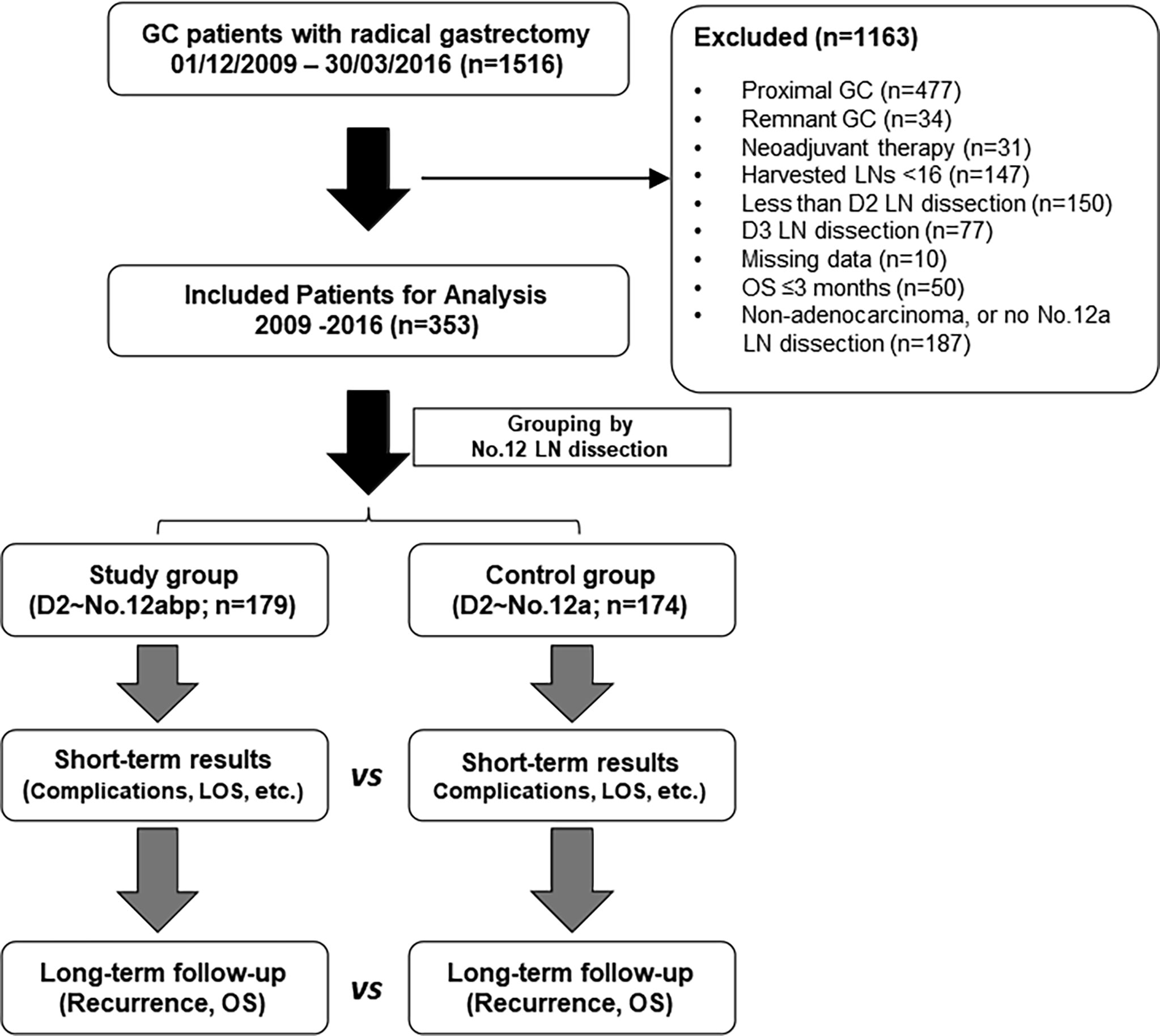
Figure 1 The flowchart of inclusion and exclusion criteria in this study. Both short- and long-term outcomes were compared between the two groups. GC, gastric cancer; LN, lymph node; LOS, length of stay; vs, versus; OS, overall survival.
Preoperative tumor staging was performed by means of contrast-enhanced computed tomography (CT) scans or endoscopic ultrasonography, and surgical plan for each patient was mainly decided through a routine MDT meeting at fixed time (9). In brief, clinical TNM stages from T2N0M0 to T4N2M0 were allowed a radical tumor resection through open or laparoscopic surgery in our unit. Patients were divided into two groups based on whether an extended lymphadenectomy at No. 12 station performed or not: control group, including those undergoing standard D2 lymphadenectomy with No. 12a dissection alone; and study group, composed of additional 12b and 12p node dissection to D2 procedure (Supplementary Figure S1). In our center, we treated No. 12a LN metastasis as a type of regional metastasis from a primary gastric cancer; therefore, we routinely dissected No. 12a LNs during the D2 lymphadenectomy.
Surgical Approaches for No. 12 Lymph Node Dissection
Given that the scope definition of No. 12a, 12b, and 12p LNs is not clearly defined according to the guidelines of American Joint Committee on Cancer (AJCC)/Union for International Cancer Control or Japanese treatment guidelines, we utilized a peer consensus which was capable to describe No. 12 LN dissection during operation (10). The modified illustration of such scope definition of No. 12a, 12b, and 12p is presented as Figure 2, similar to published demonstration (10, 11).
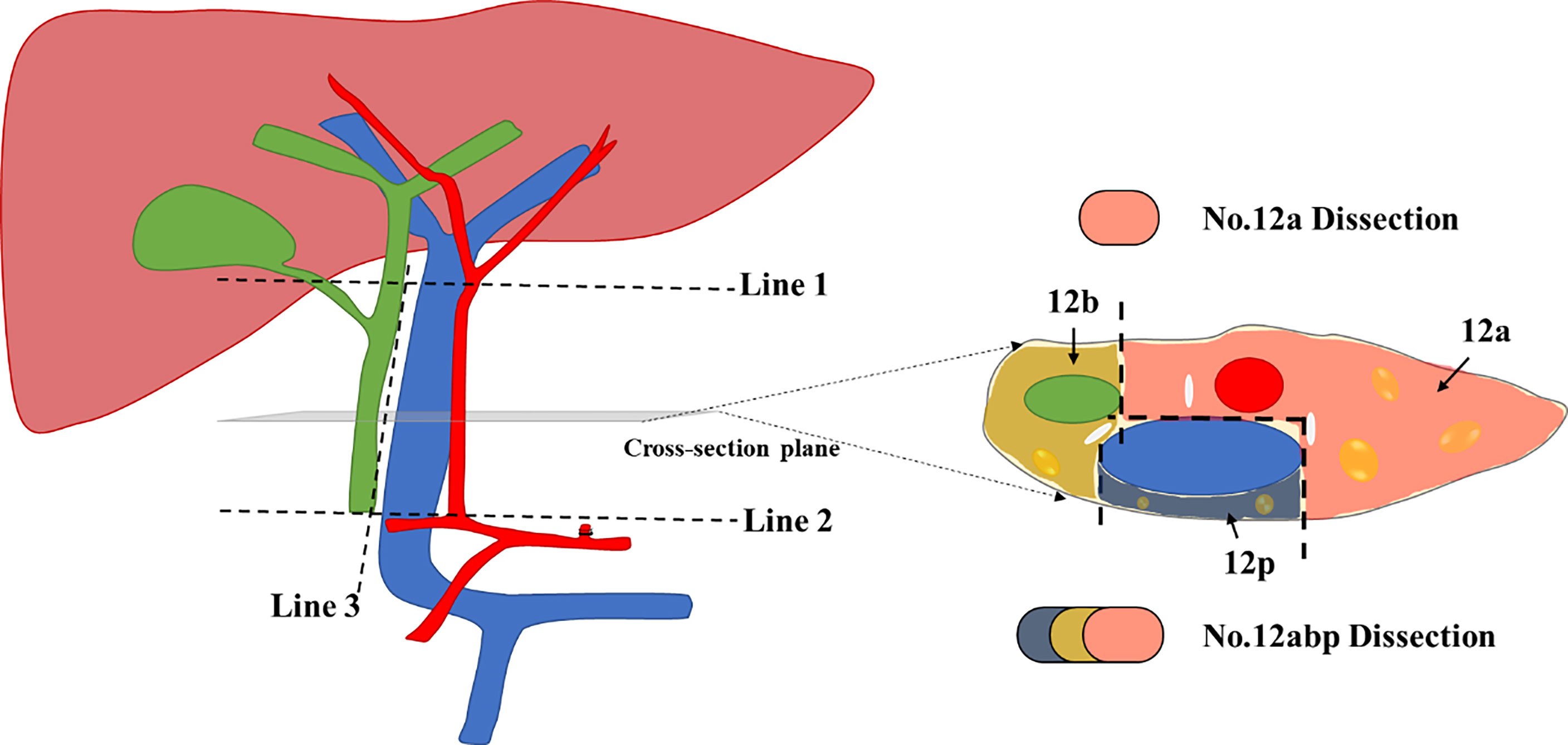
Figure 2 The modified scope definition of No. 12 Lymph nodes during lymphadenectomy for GC. Line 1 indicates the upper border of No. 12 lymph nodes (LNs), which is level-up the confluence of the left and right hepatic artery. Line 2 indicates the lower border of No. 12 LNs, which is level-up the origin of the proper hepatic artery. Line 3 indicates the left offside border of the common bile duct; The cross-section diagram of hepatoduodenal ligament reveals the separated zones for 12a, 12b, and 12p LNs, respectively. We used three distinct colors to mark each area for harvested LNs during No. 12 lymphadenectomy.
The surgical procedures of No. 12 lymph node dissection were performed as previously described (10). In short, hepatoduodenal ligament was fully exposed from the first part of duodenum to the lower border of the right liver. Afterward, the ligament was opened from the left borderline of common bile duct (Line 3, Figure 2) until the bifurcation of the left and right hepatic arteries (Line 1, Figure 2). The perivascular sheathes along the proper hepatic artery and portal vein were fully opened, with retraction bands utilized to protect vital vessels during the No. 12 lymphadenectomy. All tissues were then harvested from three different cross-section zones (Figure 2), with isolated LNs immediately stored in a station-marked container to avoid confusion with the other harvested tissues during the surgery.
Also, the detailed gastrectomy and reconstruction was mainly dictated by surgeon’s preference, with open or laparoscopic fashion employed across the study period. All surgeries were completed by over 10-year experienced gastrointestinal surgeons in our center. As for adjuvant chemotherapy, fluorouracil (5-FU)-based chemotherapy was scheduled for patients with stage II or higher, with combining S-1 (60 mg/m2) plus oxaliplatin (85 mg/m2) as a main regimen for at least eight cycles in planning. Neoadjuvant therapy was not included in the current study.
Follow-Up
The postoperative follow-up of the patients was recorded by the research nurse of our center, as previously described (12). Typically, follow-up visits at outpatient clinics were scheduled every 3 months for the first 2 years after surgery, every 6 months for the next 3 years, and then once a year or until death. Physical examination and laboratory tests (including tumor markers) were required at each visit, while chest and abdominal CT scans with enhanced contrast were performed every 6 months or annually. Gastric endoscopy was performed every year. Tumor recurrence was mainly confirmed based on clinical, radiological, or endoscopic signs of disease. Laparoscopic exploration and biopsy were applied only in cases enrolling in clinical trials. Recurrence and survival status was last updated on March 31, 2021.
Study Outcomes
The incidence of 90-day complications after radical surgery was utilized to evaluate the safety of No. 12 lymphadenectomy. The severity of complications was evaluated with the Clavien-Dindo classification (13, 14). The 3-year progression-free survival (PFS) and 5-year overall survival (OS) were employed to explore oncologic outcomes for included patients. Briefly, PFS was calculated from the time of operation until evidence of disease progression or recurrence, a new primary cancer, or cancer-related death, while OS was calculated from the time of operation to the time of death or final follow-up.
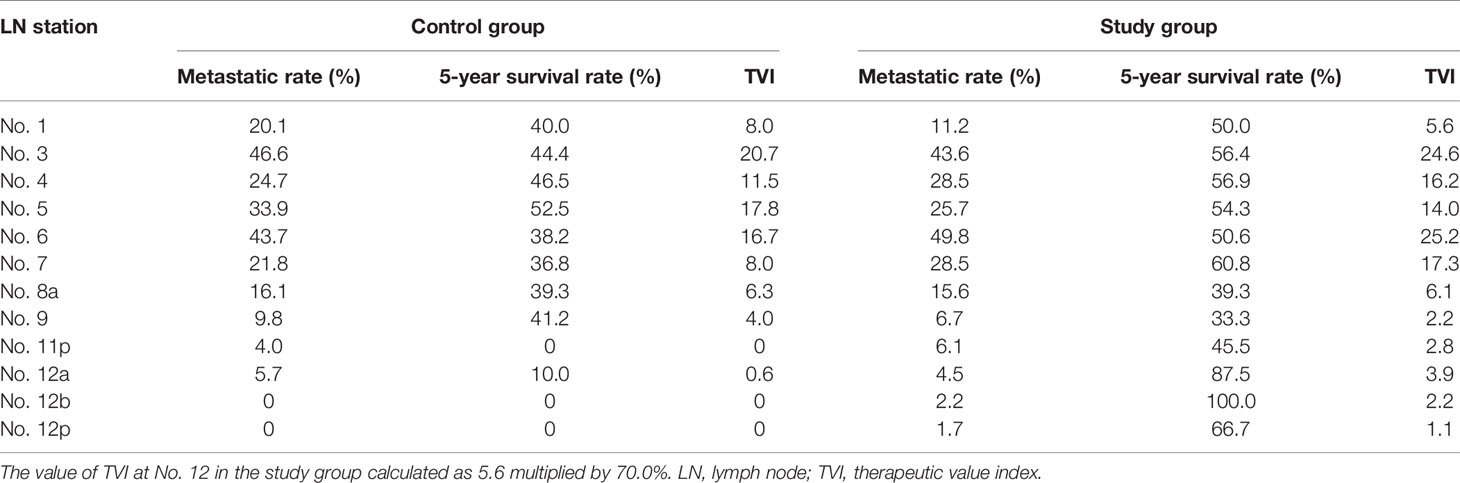
As previously mentioned (15, 16), the therapeutic value index (TVI) was employed to evaluate the impact of lymphadenectomy on long-term survival, which was calculated by multiplying the metastatic rate of lymph node by the 5-year OS rate of the patients with metastasis to that station. The incidence of lymph node metastasis, also noticed as frequency of lymph node metastasis, was calculated by dividing the number of patients with metastasis at the given lymph node station by the number of patients in whom that station was dissected.
TVI (N) = Lymph node metastatic rate (N) × 5-year OS rate (N) × 100 (N for node station)
Additionally, the ratio of lymph node metastasis, calculated by the number of positive nodes divided by the total harvested number of nodes at the given station, was employed to describe the severity of lymph node metastasis in enrolled patients.
Statistical Analysis
Descriptive statistics were used to present demographic and oncologic features of included patients. Quantitative variables were expressed as means ± standard deviation (SD) or median with range, with categorical variables described as frequency plus percentages. The continuous variables were analyzed by means of the Student’s t-test, while the categorical variables were analyzed using the Chi-square or Fisher exact test. The OS curves were calculated using the Kaplan-Meier method based on the duration of time between the primary surgical treatment and the final follow-up or death. The Log-rank test was used to evaluate the significant differences between curves. The Cox proportional hazards regression model was implemented to determine the independent prognostic factors of long-term survival. The statistical analysis was accomplished by using the statistical analysis program package for Social Science software (SPSS, version 23, IBM®, Chicago, IL, USA). Two-tailed tests were applied, with p < 0.05 considered statistically significant.
Results
Patient Characteristics
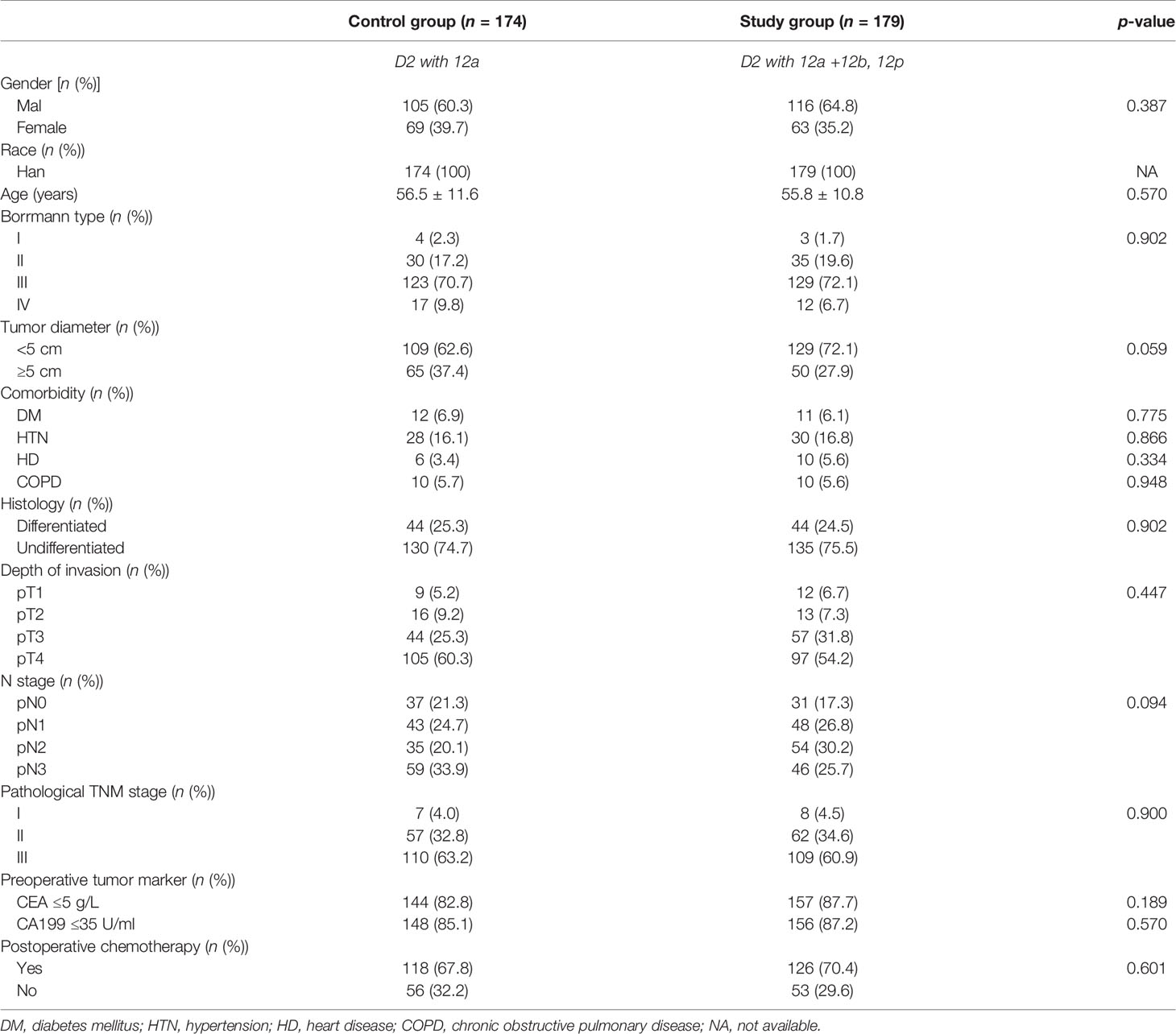
Within the study period (2009–2016), 1,516 GC patients underwent radical gastrectomy for GC at our center. Of these, 353 patients were recruited for final analysis: 174 patients had received No. 12a node dissection (control group; n = 174) and 179 patients had undergone No. 12abp node dissection (study group; n = 179). The baseline and demographic features of included patients are summarized in Table 1, without significant difference observed between both groups. Of note, 15 patients were classified into pathological IB stage according to the 7th edition of the TNM classification of GC.
Surgical and Short-Term Outcomes
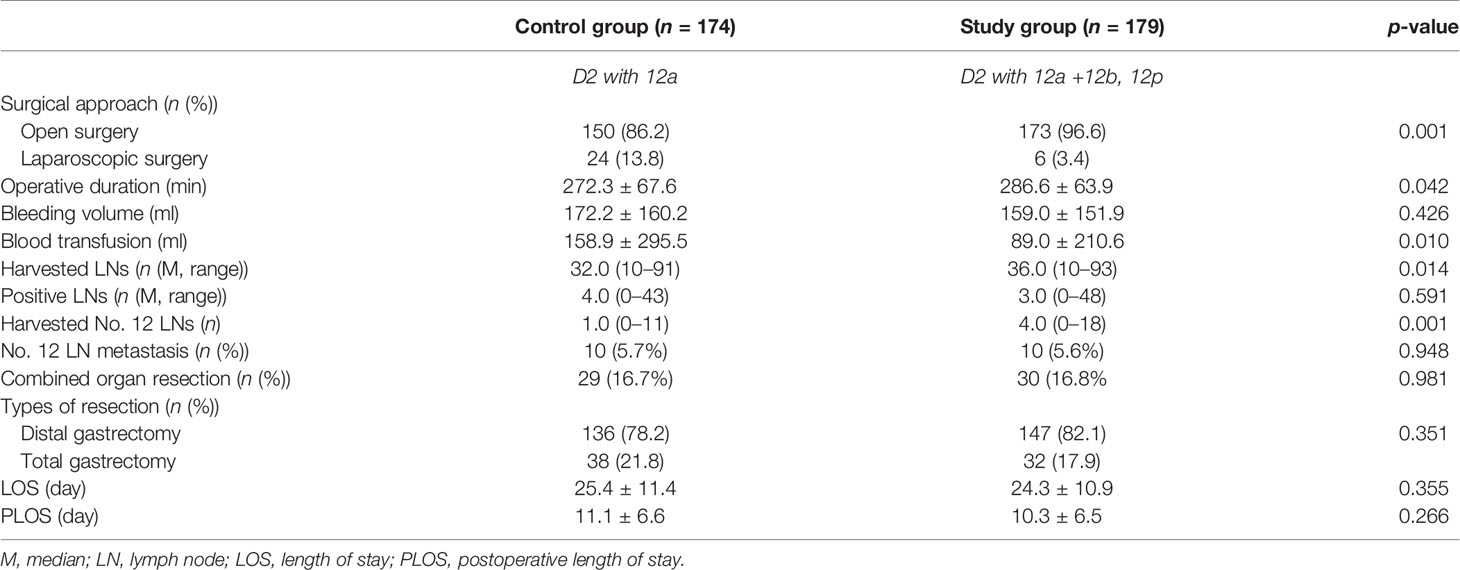
The profile of operative procedure and perioperative outcomes are summarized in Table 2. Comparing with the control group, laparoscopic operation was less frequently performed, operative duration was markedly extended, and harvested number of lymph nodes in total and No. 12 station were significantly increased in the study group (p < 0.05). Other parameters, such as intraoperative blood loss, lymph node metastasis rate for No. 12, and length of stay (LOS) in hospital or after surgery, were comparable between both groups (p > 0.05).
To evaluate the safety of aggressive lymph node dissection at No. 12 station, we explored the incidence of 90-day complications after surgery (Table 3). There was no significant difference in postoperative complications between the two groups (p > 0.05). Specifically, the incidence of biliary leakage or portal vein injury was not recorded in both groups. Of note, the excluded patients (n = 50, Figure 1) due to survival of less than 3 months did not die from severe complications after surgery. Three patients suffered from unplanned readmission, with one case for delayed intra-abdominal hemorrhage and two cases for wound infections.
In this study, both harvested and metastatic lymph nodes at N1 and N2 stations for GC were compared between the two groups (Figure 3). The overall lymph node metastatic ratios for the control and study groups were 20.4% and 16.0%, respectively. The detailed numbers of harvested and metastatic lymph nodes at each station are summarized in Supplementary Table S1. Of note, the metastatic rates at No. 12a, 12b, and 12p stations were 5.7%, 2.2%, and 1.7%, respectively.
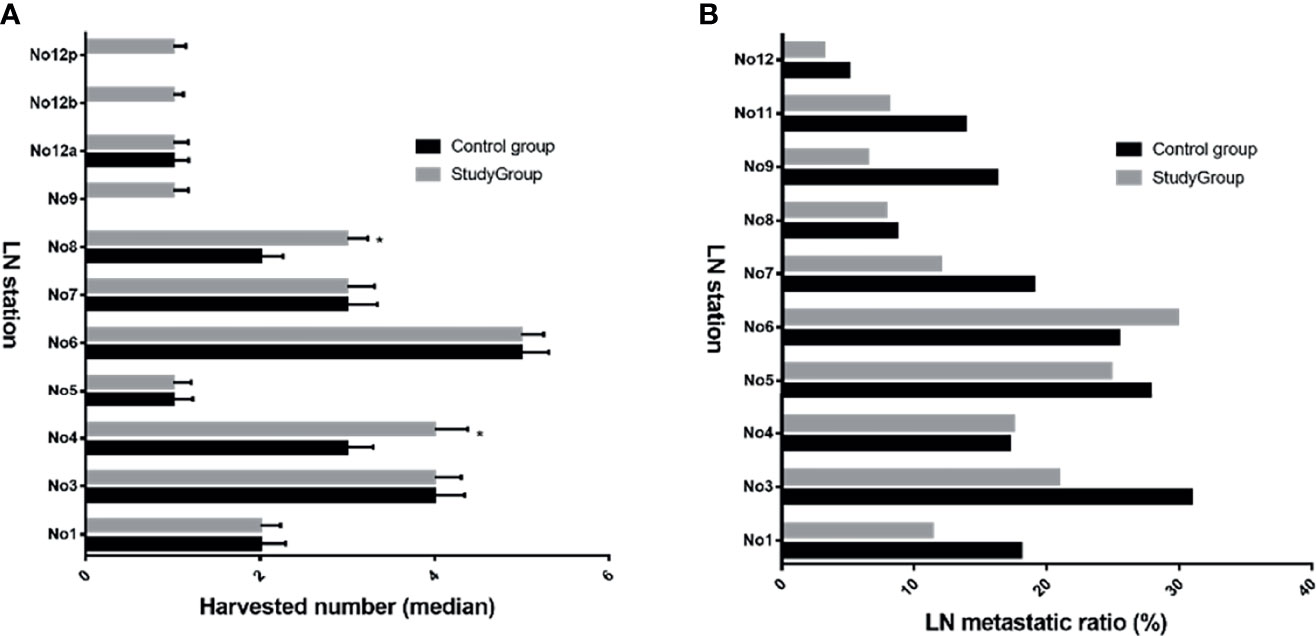
Figure 3 Harvested LNs and metastatic ratio at N1 and N2 stations between both groups. The median harvested number in study group was increased for No. 4, 8, 9, 12b, and 12p (A), *p < 0.05 vs. control group; However, most ratios of metastatic lymph nodes in study group were reduced compared with those in the control group (B). LN, lymph node.
Long-Term Outcomes
We compared TVI values at each station between the two groups and found that most of TVI values in the study group were higher than those in the control group, especially for No. 6 (25.2 vs. 16.7), No. 7 (17.3 vs. 8.0), and No. 12 (3.9 vs. 0.6) values (Table 4). Moreover, the cumulative TVI value at all dissected stations was markedly increased in the study group compared with the control group (121.2 vs. 93.6).
Within a median follow-up of 65.0 months, we have detected recurrence after curative surgery in 156 patients. Among those patients, 88 (55.1%) patients underwent D2 with No. 12a lymphadenectomy during surgery, with 68 (44.9%) patients receiving D2 with No. 12abp procedures. We compared the difference in recurrence patterns between both groups through the Venn diagram (Figure 4). The diagram shows that aggressive lymph nodes dissection at No. 12 station has a limited role in reducing distant lymph node metastasis for GC. However, the detailed comparisons (Supplementary Table S2) indicate that D2 with No. 12abp node dissection markedly improved hematogenous (p = 0.029) and peritoneal (p = 0.039) recurrence in such GC patients.
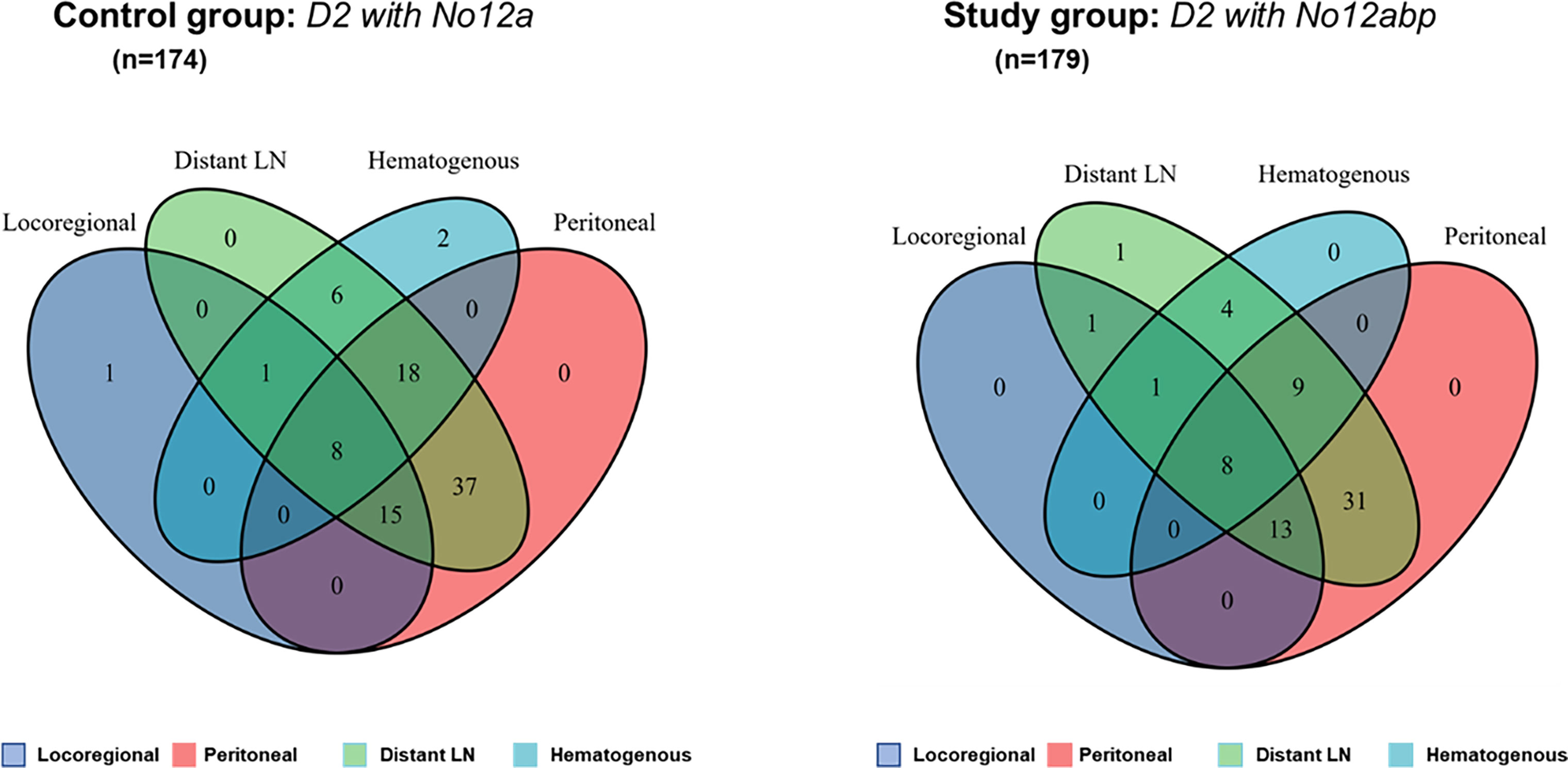
Figure 4 The Venn diagram comparison of recurrence patterns after curative surgery for GC. Numbers in such areas of diagram indicate the number of patients with four types of tumor recurrence. Both diagrams (left and right) were produced by using a public shared tool from Sangerbox.com.
As compared with the control group, the 3-year PFS rate was significantly increased in the study group (67.0% vs. 55.9%, p = 0.045; Figure 5A). The average survival time for PFS was significantly longer in the study group (28.7 months; 95% CI, 26.9–30.4) than in the control group (26.7 months; 95% CI, 24.8–28.6). Also, the 5-year OS rate was markedly improved in the study group (66.2% vs. 54.0%, p = 0.027; Figure 5B). The average overall survival time for OS was also prolonged in the study group (63.8 months; 95% CI, 59.5–68.2) than in the control group (47.5 months; 95% CI, 44.2–50.8). Of note, metastasis of No. 12 lymph nodes was associated with poor 5-year OS compared with nonmetastasis at such station (Figure 5C). Furthermore, the Cox-regression analysis showed that pathological TNM stage (p = 0.028) and No. 12 LN dissection (p = 0.026) were independent factors of predicting prognosis of gastric cancer in such populations (Figure 5D).
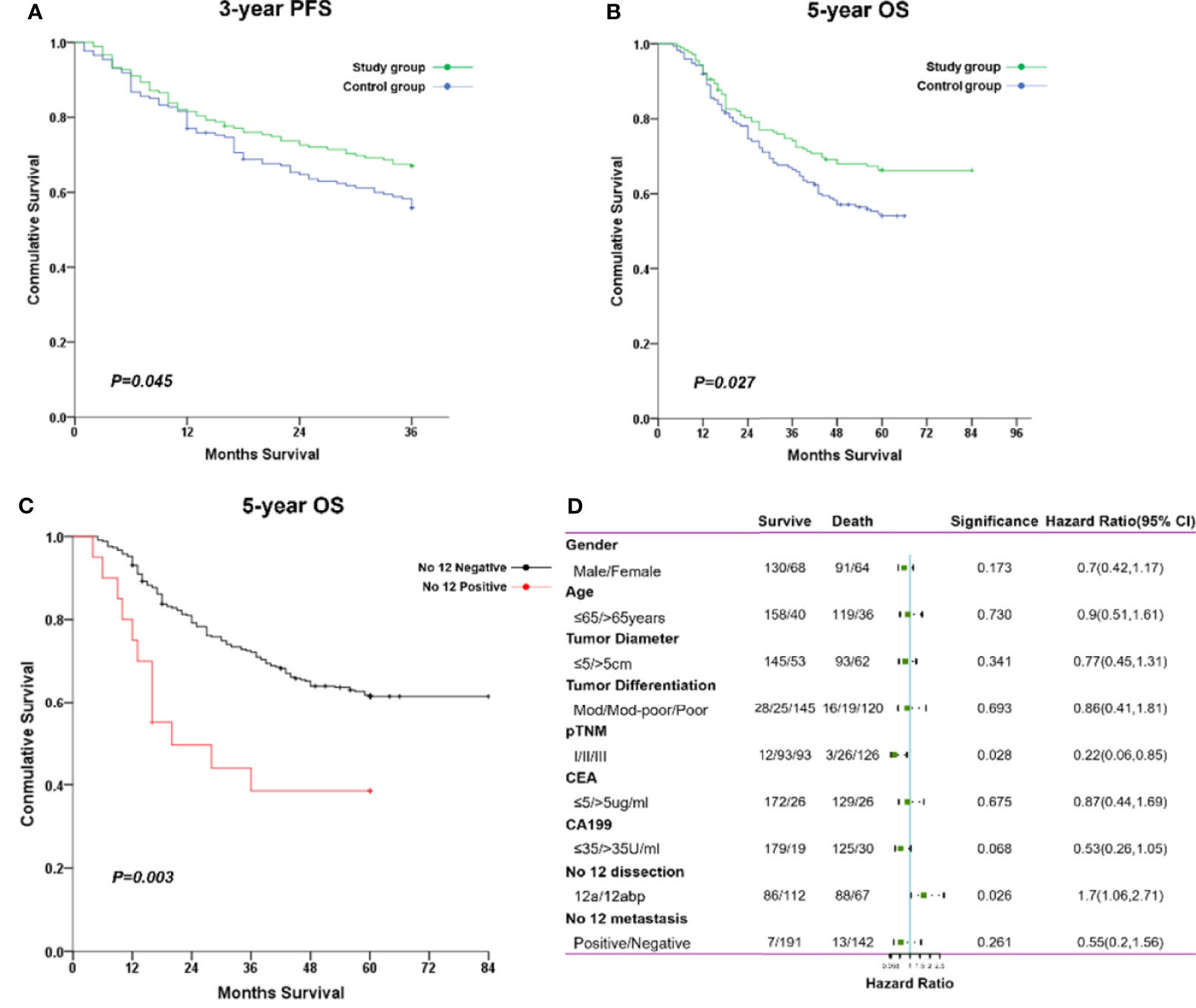
Figure 5 The comparisons of long-term survival between both groups. (A) The cumulative rate for 3-year PFS. (B) The cumulative rate for 5-year OS. (C) The cumulative rate for 5-year OS stratified with No. 12 nodal status. (D) The forest plot of factors impacting on overall survival through a multivariate cox regression analysis.
Discussion
In this study, patients with lower-third GC were included to explore the impact of adding No. 12b and 12p dissection to D2 lymphadenectomy on long-term outcomes in a single cancer center. This extended lymphadenectomy at No. 12 station revealed the metastatic rates of 2.2% and 1.7% for No. 12b and 12p, respectively. Importantly, it was associated with increased TVI at No. 12 station (3.9 vs. 0.6), prolonged both 3-year PFS rate (67.0% vs. 55.9%, p = 0.045) and 5-year OS rate (66.2% vs. 54.0%, p = 0.027). Moreover, it was regarded as an independent prognostic factor of improved survival (p = 0.026) by further Cox-regression analysis. All findings suggested that lymph nodes at No. 12b and 12p stations should be dissected during D2 lymphadenectomy for resectable lower-third GC patients.
Until now, the therapeutic efficacy of lymphadenectomy at each station was rarely evaluated, with limited data available (16). The therapeutic value index was a practical tool to deduce the actual benefit of node dissection without considering the stage migration of GC (15). We employed this novel tool to compare the efficacy of extensive No. 12 nodes dissection to standard D2 procedure. The TVI values at various tier lymph nodes were comparable with similar studies, ranging from 0 to 30.9 (16, 17). Furthermore, the value for extensive No. 12 station dissection was increased compared with No. 12a dissection alone (3.9 vs. 0.6), which suggested that additional No. 12b and 12p nodes dissection yields survival benefits for lower-third GC patients.
In anatomy, the No. 12 lymph nodes are located in the hepatoduodenal ligament, which has been regarded as the free border or the root of ventral mesogastrium (18). Therefore, it is reasonable to regard the No. 12b and 12p lymph nodes as N3 lymph nodes. In clinical practice, the No. 12 lymph nodes were minor stations but often became enlarged leading to bile duct obstruction in advanced GC, especially for lower third GC. As for lower 1/3 GC, the reported rate for No. 12 lymph node metastasis ranged from 4.7% to 11.6% (5, 19–21). Interestingly, the metastasis of No. 12b and 12p lymph nodes were often secondary to No. 5 and No. 12a lymph node metastasis. In our study, the metastatic rates of No. 12b and 12p nodes were 2.2% and 1.7%, respectively. Dissection of No. 12b and 12p lymph nodes had additional improvements in both the 3-year PFS rate and the 5-year OS rate. Multivariate regression analysis confirmed that adding such nodes to D2 lymphadenectomy could be an independent prognostic factor, which was not identical to previous study (5).
Even so, the shortcomings of this study should be mentioned here. First, the nature of a single-center retrospective study design determined the limitation of generating our findings to a more generalized population. Second, the relatively small sample size definitely compromised the power of our findings and brought certain uncertainty to the conclusion. Third, the data about the No. 14v lymph nodes were limited and failed to summarize here. Until now, its dissection depends on the preference of surgeons, with no consensus achieved yet. Several studies found that dissection of No. 14v is related to improved survival in lower-third GC, especially for patients with No. 14v metastasis (22, 23). Last, it could not overcome selecting bias and surgeon’s preference due to the nature of retrospective analysis. The LN metastatic rate was higher in the control group than in the study group, although most values of TVI were improved through an extensive No. 12 LN dissection. Therefore, multicenter randomized controlled trials are indispensable to further validate the current findings.
In conclusion, an extensive dissection of hepatoduodenal ligament lymph nodes, including No. 12a, 12b, and 12p, was safe to apply in the management of resectable lower-third GC. As compared with standardized D2 dissection with No. 12a alone, the extensive lymphadenectomy was associated with increased therapeutic value index and improved long-term outcomes. All findings supported its clinical usage for surgical treatment of advanced lower-third gastric cancer, particularly when curative operation is possible, to improve survival in such populations.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SC, HW, and YY conceived and designed the study. YY, WD, and E-TZ collected all included cases and drafted the paper. JC, ZC, and RZ performed data analyses and necessary revision of final manuscript. HW, YH, CC, and SC were responsible for the interpretation of all data and critical revision. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81702878, No. 82003112, and No. 81372341), Guangdong Basic and Applied Basic Research Foundation (2020A1515010214 and 2021A1515010473), Project 5010 of Sun Yat-Sen University (2018004), and Young Teacher Training Project of First Affiliated Hospital of Sun Yat-Sen University (19ykpy58).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Donglian Chen, Ze-E Xu, Yun Xiao, and Qiongyun Zhao for their dedicated work in the follow-up data collection and management for all patients. We also thank Shenyan Wu for her kind help in revising the English of this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.760963/full#supplementary-material
Supplementary Figure 1 | The typical demonstration of No. 12 lymph node dissection during the D2 lymphadenectomy. (A) The complete of No. 12a lymph node dissection during a laparoscopic radical distal gastrectomy. (B) The complete of No. 12a, 12b, and 12p lymph nodes dissection during an open radical distal gastrectomy. Both surgical procedures were performed under the principle of D2 lymphadenectomy for lower-third GC.
Abbreviations
GC, gastric cancer; TVI, therapeutic value index; PFS, progression-free survival; OS, overall survival; AJCC, American Joint Committee on Cancer; CT, computed tomography; SSI, surgical site infection, LOS, length of stay.
References
1. Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric Adenocarcinoma. Nat Rev Dis Primers (2017) 3:17036. doi: 10.1038/nrdp.2017.36
2. Kim Y, Spolverato G, Ejaz A, Squires MH, Poultsides G, Fields RC, et al. A Nomogram to Predict Overall Survival and Disease-Free Survival After Curative Resection of Gastric Adenocarcinoma. Ann Surg Oncol (2015) 22(6):1828–35. doi: 10.1245/s10434-014-4230-4
3. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric Cancer: Epidemiology, Prevention, Classification, and Treatment. Cancer Manag Res (2018) 10:239–48. doi: 10.2147/CMAR.S149619
4. Nakajima T. Gastric Cancer Treatment Guidelines in Japan. Gastric Cancer (2002) 5(1):1–5. doi: 10.1007/s101200200000
5. Feng JF, Huang Y, Liu J, Liu H, Sheng HY, Wei WT, et al. Risk Factors for No. 12p and No. 12b Lymph Node Metastases in Advanced Gastric Cancer in China. Ups J Med Sci (2013) 118(1):9–15. doi: 10.3109/03009734.2012.729103
6. Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, et al. Annual Report on Status of Cancer in China, 2010. Chin J Cancer Res (2014) 26(1):48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08
7. Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, et al. Cancer Survival in China, 2003-2005: A Population-Based Study. Int J Cancer (2015) 136(8):1921–30. doi: 10.1002/ijc.29227
8. Yuan Y, Ye J, Ren Y, Dai W, Peng J, Cai S, et al. The Efficiency of Electronic List-Based Multidisciplinary Team Meetings in Management of Gastrointestinal Malignancy: A Single-Center Experience in Southern China. World J Surg Oncol (2018) 16(1):146. doi: 10.1186/s12957-018-1443-1
9. Ye J, Ren Y, Dai W, Chen J, Cai S, Tan M, et al. Does Lymphadenectomy With at Least 15 Perigastric Lymph Nodes Retrieval Promise an Improved Survival for Gastric Cancer: A Retrospective Cohort Study in Southern China. J Cancer (2019) 10(6):1444–52. doi: 10.7150/jca.28413
10. Huang Y, Zhu G, Zheng W, Hua J, Yang S, Zhuang J, et al. Scope Definition and Resection Significance of No. 12a Group Lymph Nodes in Gastric Cancer. Mol Clin Oncol (2016) 5(2):257–62. doi: 10.3892/mco.2016.911
11. Kumagai K, Sano T, Hiki N, Nunobe S, Tsujiura M, Ida S, et al. Survival Benefit of "D2-Plus" Gastrectomy in Gastric Cancer Patients With Duodenal Invasion. Gastric Cancer (2018) 21(2):296–302. doi: 10.1007/s10120-017-0733-6
12. Song W, Yuan Y, Peng J, Chen J, Han F, Cai S, et al. The Delayed Massive Hemorrhage After Gastrectomy in Patients With Gastric Cancer: Characteristics, Management Opinions and Risk Factors. Eur J Surg Oncol (2014) 40(10):1299–306. doi: 10.1016/j.ejso.2014.03.020
13. Jiang X, Hiki N, Nunobe S, Fukunaga T, Kumagai K, Nohara K, et al. Postoperative Outcomes and Complications After Laparoscopy-Assisted Pylorus-Preserving Gastrectomy for Early Gastric Cancer. Ann Surg (2011) 253(5):928–33. doi: 10.1097/SLA.0b013e3182117b24
14. Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol Off J Am Soc Clin Oncol (2016) 34(12):1350–7. doi: 10.1200/jco.2015.63.7215
15. Sasako M, Mcculloch P, Kinoshita T, Maruyama K. New Method to Evaluate the Therapeutic Value of Lymph Node Dissection for Gastric Cancer. Br J Surg (1995) 82(3):346–51. doi: 10.1002/bjs.1800820321
16. Tokunaga M, Ohyama S, Hiki N, Fukunaga T, Inoue H, Yamada K, et al. Therapeutic Value of Lymph Node Dissection in Advanced Gastric Cancer With Macroscopic Duodenum Invasion: Is the Posterior Pancreatic Head Lymph Node Dissection Beneficial? Ann Surg Oncol (2009) 16(5):1241–6. doi: 10.1245/s10434-009-0345-4
17. Xu ZY, Hu C, Chen S, Du YA, Huang L, Yu PF, et al. Evaluation of D2-Plus Radical Resection for Gastric Cancer With Pyloric Invasion. BMC Surg (2019) 19(1):172. doi: 10.1186/s12893-019-0605-6
18. Shinohara H, Kurahashi Y, Ishida Y. Gastric Equivalent of the 'Holy Plane' to Standardize the Surgical Concept of Stomach Cancer to Mesogastric Excision: Updating Jamieson and Dobson's Historic Schema. Gastric Cancer (2021) 24(2):273–82. doi: 10.1007/s10120-020-01142-9
19. Maruyama K, Okabayashi K, Kinoshita T. : Progress in Gastric Cancer Surgery in Japan and Its Limits of Radicality. World J Surg (1987) 11(4):418–25. doi: 10.1007/BF01655804
20. Bollschweiler E, Boettcher K, Hoelscher AH, Sasako M, Kinoshita T, Maruyama K, et al. Preoperative Assessment of Lymph Node Metastases in Patients With Gastric Cancer: Evaluation of the Maruyama Computer Program. Br J Surg (1992) 79(2):156–60. doi: 10.1002/bjs.1800790221
21. Wu CW, Hsieh MJ, Lo SS, Tsay SH, Lui WY, P'eng FK. Lymph Node Metastasis From Carcinoma of the Distal One-Third of the Stomach. Cancer (1994) 73(12):3109–14. doi: 10.1002/1097-0142(19940615)73:12<3109::aid-cncr2820731234>3.0.co;2-x
22. Masuda TA, Sakaguchi Y, Toh Y, Aoki Y, Harimoto N, Taomoto J, et al. Clinical Characteristics of Gastric Cancer With Metastasis to the Lymph Node Along the Superior Mesenteric Vein (14v). Dig Surg (2008) 25(5):351–8. doi: 10.1159/000165382
Keywords: lymphadectomy, advanced gastric cancer, hepatoduodenal ligament, oncological outcomes, complications
Citation: Dai W, Zhai E-T, Chen J, Chen Z, Zhao R, Chen C, Yuan Y, Wu H, Cai S and He Y (2022) Extensive Dissection at No. 12 Station During D2 Lymphadenectomy Improves Survival for Advanced Lower-Third Gastric Cancer: A Retrospective Study From a Single Center in Southern China. Front. Oncol. 11:760963. doi: 10.3389/fonc.2021.760963
Received: 19 August 2021; Accepted: 14 December 2021;
Published: 11 January 2022.
Edited by:
Chun-Dong Zhang, The University of Tokyo, JapanReviewed by:
Paolo Morgagni, Morgagni-Pierantoni Hospital, ItalyLiang Zong, Changzhi People’s Hospital, China
Copyright © 2022 Dai, Zhai, Chen, Chen, Zhao, Chen, Yuan, Wu, Cai and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wu, d3VodWkzQG1haWwuc3lzdS5lZHUuY24=; Shirong Cai, Y2Fpc2hpcm9uZ0B2aXAuMTI2LmNvbQ==
†These authors have contributed equally to this work
 Weigang Dai
Weigang Dai Er-Tao Zhai
Er-Tao Zhai Jianhui Chen
Jianhui Chen Zhihui Chen
Zhihui Chen Risheng Zhao
Risheng Zhao Chuangqi Chen
Chuangqi Chen Yujie Yuan
Yujie Yuan Hui Wu
Hui Wu Shirong Cai1,2*
Shirong Cai1,2* Yulong He
Yulong He