
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 05 January 2022
Sec. Cancer Molecular Targets and Therapeutics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.757456
Background: This meta-analysis was designed to explore the relationship between the level of serum potassium and the treatment effect of epidermal growth factor receptor (EGFR) antagonist in advanced non-small cell lung cancer (aNSCLC).
Methods: We searched phase II/III prospective clinical trials on treatment with EGFR antagonists for aNSCLC patients. The objective response rate (ORR) and/or the disease control rate (DCR) and the incidence of hypokalemia of high grade (equal to or greater than grade 3) were summarized from all eligible trials. Heterogeneity, which was evaluated by Cochran’s Q-test and the I2 statistics, was used to determine whether a random effects model or a fixed effects model will be used to calculate pooled proportions. Subgroup analysis was performed on different interventions, line types, phases, and drug numbers.
Results: From 666 potentially relevant articles, 36 clinical trials with a total of 9,761 participants were included in this meta-analysis. The pooled ORR was 16.25% (95%CI = 12.45–21.19) when the incidence of hypokalemia was 0%–5%, and it increased to 34.58% (95%CI = 24.09–45.07) when the incidence of hypokalemia was greater than 5%. The pooled DCR were 56.03% (95%CI = 45.03–67.03) and 64.38% (95%CI = 48.60–80.17) when the incidence rates of hypokalemia were 0%–5% and greater than 5%, respectively. The results of the subgroup analysis were consistent with the results of the whole population, except for not first-line treatment, which may have been confounded by malnutrition or poor quality of life in long-term survival.
Conclusion: The efficacy of anti-EGFR targeted therapy was positively associated with the hypokalemia incidence rate. Treatment effects on the different serum potassium strata need to be considered in future clinical trials with targeted therapy.
Lung cancer is the most common cause of death from cancer, accounting for 1.80 million deaths in 2020, and its incidence was still increasing (1, 2). Based on cell origin, non-small cell lung cancer (NSCLC) is responsible for 80%–85% of lung primary malignancies (3). As a transmembrane glycoprotein, epidermal growth factor receptor (EGFR) was the first growth factor receptor to be proposed as a target for cancer therapy (4). EGFR is a member of the ErbB family of receptors that, once activated, leads to the excitation of subsequent intracellular signaling pathways; it can regulate cellular proliferation, differentiation, migration, and apoptosis (5, 6). There are two main classes of EGFR antagonists: anti-EGFR monoclonal antibodies (e.g., cetuximab and panitumumab) and small-molecule EGFR tyrosine kinase inhibitors (TKIs) (e.g., erlotinib and gefitinib) (4). These antagonists exert their activities through binding to the extracellular domain of EGFR, competing for receptor binding by occluding the ligand-binding region, blocking the ligand-induced EGFR tyrosine kinase activation, and inhibiting EGFR autophosphorylation and downstream signaling (4, 7). EGFR antagonists are beneficial for human epithelial cancers, especially for lung carcinoma.
Potassium is an important element in the human body, amounting to about 50 mEq/kg. Ninety-eight percent of K+ is found within cells, while only 2% is in the extracellular fluid (8). There is evidence showing that elevated extracellular potassium characteristic of the extracellular space within tumors reduced the uptake and consumption of local nutrients by antitumor T cells (9). T cells in the tumor microenvironment are under metabolic constraints that dampen their activity and lead to cancer progression (10), indicating that high levels of potassium in the tumor microenvironment may suppress T-cell effector function. A cohort study also revealed that the level of fasting serum potassium in healthy men was positively associated with long-term cancer risk (11). Moreover, previous studies have claimed that hypokalemia is a major adverse event in the treatment of NSCLC that may provoke cardiac arrhythmias and/or respiratory arrest, thus requiring close monitoring and rapid correction (12, 13). In the immune system, the disorder of potassium homeostasis has been indicated as a determinant of immune dysfunction (8).
Therefore, we hypothesized that there would be an association between the level of serum potassium and the effect of targeted therapy on NSCLC patients. To verify the hypothesis, we conducted a meta-analysis to explore the relation between the efficacy of anti-EGFR therapy on NSCLC and the incidence of hypokalemia.
A literature search was conducted in electronic datasets from PubMed, Embase, and Cochrane Library in April 2019 using the following various combinations of different keywords: “EGFR”, “epidermal growth factor receptor”, “monoclonal antibodies”, “tyrosine kinase inhibitors”, “cetuximab”, “gefitinib”, “erlotinib”, “icotinib”, “dacomitinib”, “afatinib”, “osimertinib”, “necitumumab”, “panitumumab”, “non-small cell lung cancer” “NSCLC”, and “hypokalemia”. The search was restricted to clinical trials published in English. The relevant reviews and meta-analyses were also examined for inclusive trials.
Inclusion of relevant studies was based on the following criteria: 1) patients were pathologically confirmed to have stage III or IV NSCLC; 2) research studies were phase II/III prospective clinical trials; 3) all patients were administered anti-EGFR therapy alone or combined with other therapy; and 4) studies that reported the objective response rate (ORR) and/or disease control rate (DCR) and the exact number of patients with occurrences of hypokalemia of high grade (equal to or greater than grade 3).
Two reviewers independently reviewed the studies and reached consensus on all items. The following pieces of information were abstracted from the included studies: first author, publication year, country/region, phase of trial, line of treatment, intervention, number of patients, median age, sex ratio, ORR, DCR, and incidences of hypokalemia of grade ≥3. The study quality was independently assessed by the same two reviewers according to the Jadad score, which included randomization, blinding, and withdrawal, ranging from 0 to 5 points (14). Among all the included trials, the anti-EGFR monoclonal antibody or TKI treatment arms were included; otherwise, chemotherapy arms were collected for supplementary analysis. Placebo arms were excluded.
The ORR, DCR, and the incidence of hypokalemia of high grade (grade 3 or higher) were summarized from the data of all eligible trials. We calculated the proportions and 95% confidence intervals (CIs) of the ORR and DCR for each eligible trial. Heterogeneity among studies was evaluated using the Cochran’s Q-test and the I2 statistics (15). The pooled proportions were calculated using a random effects model when the p-value <0.10 for the Q-test or the I2 >50%. Otherwise, a fixed effects model was chosen. All p-values were two-tailed, and statistical significance was considered at p < 0.05. To determine whether the intervention type, line of treatment, trial phase, and drug numbers could represent potential sources of heterogeneity, subgroup analysis was performed. A sensitivity analysis was conducted with the pooled ORR/DCR re-calculated after excluding each trial at a time individually. All data analyses and the generation of forest plots were performed using R software (version 3.6.2).
Of the 666 potentially relevant articles with anti-EGFR therapy screened, 36 clinical trials were finally included in this meta-analysis (Figure 1). Of these 36 studies, 15 were single-armed trials and 21 were randomized controlled trials. Eighteen studies used anti-EGFR treatment as first-line therapy and 18 did not. Treatment with anti-EGFR TKIs was evaluated in 26 studies, while 10 were studies on treatment with monoclonal antibodies. Twenty-five were phase II and 11 were phase III trials. A total of 9,761 patients were available for analysis. The characteristics of these trials are listed in Table 1. The relationship between hypokalemia incidence and ORR/DCR is scattered and fitted in Figure 2. A positive association could be observed in both scatter plots, except for an outlier in the lower right corner of the ORR plot. Except for the outlier, the highest hypokalemia incidence rate was 11.76%, with ORR of 82.35% and DCR of 94.12%. The lowest hypokalemia incidence rate was 0.00% in nine arms, with ORR ranging from 2.86% to 55.97% and DCR from 18.18% to 87.42%.
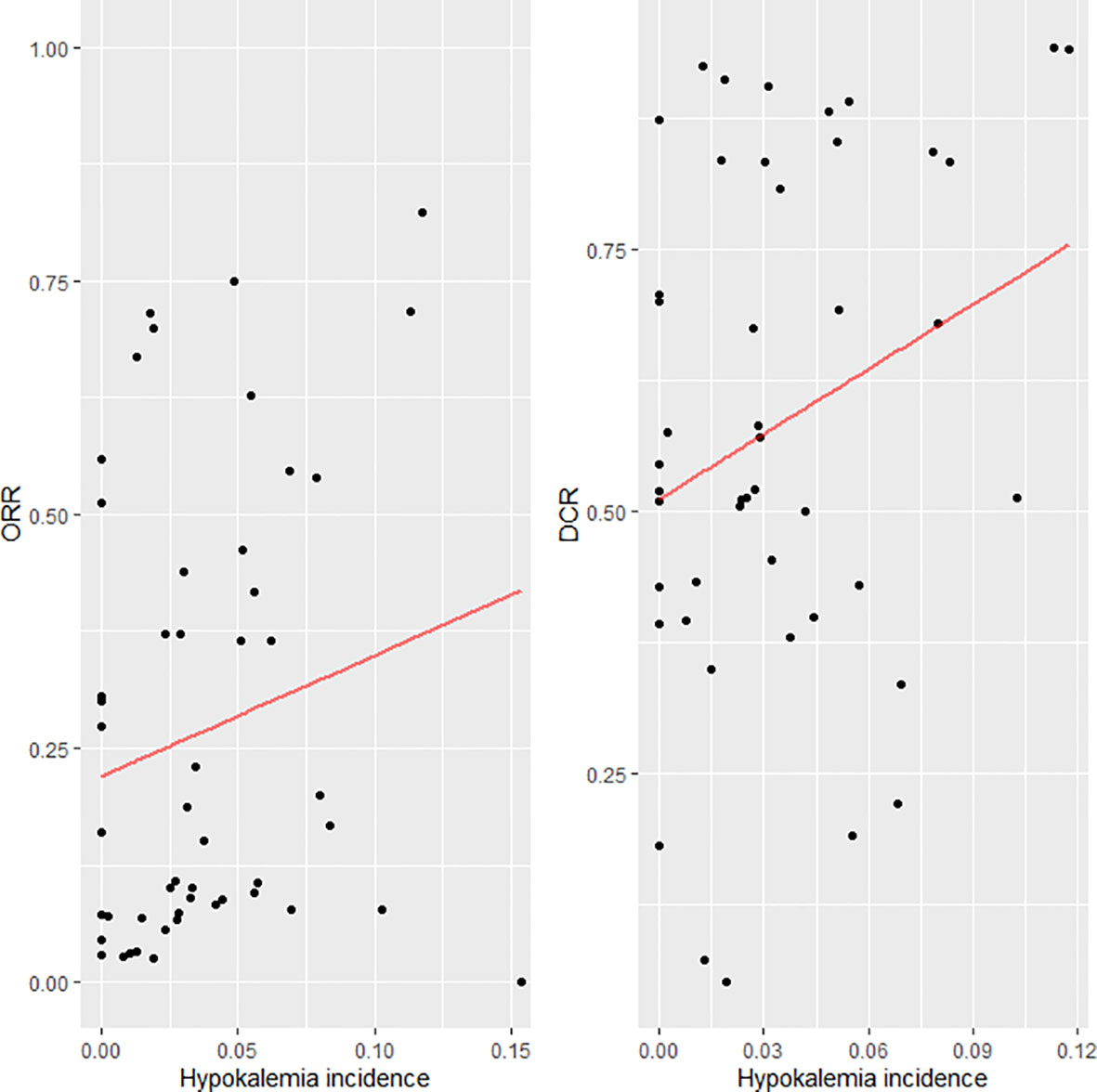
Figure 2 Scatter plot and fitted line for the incidence of hypokalemia and objective response rate (ORR)/disease control rate (DCR).
We observed that the pooled ORR was positively associated with the incidence of hypokalemia. The pooled ORR was 16.25% (95%CI = 12.45–21.19) when the incidence of hypokalemia was 0%–5%, while it increased to 34.58% (95%CI = 24.09–45.07) when the incidence of hypokalemia was greater than 5% (Figure 3). In the subgroup analysis on intervention type, the association was consistent. For TKI therapy, the pooled ORRs were 18.10% (95%CI = 13.73–23.86%) and 25.24% (95%CI = 10.29–40.19) when the hypokalemia incidence rates were ≤5% and >5%, respectively. Similar better ORRs with higher hypokalemia incidence rates could be observed in the monoclonal antibody treatment arms. As for the line of treatment, the pooled ORRs related to first-line treatment were 36.19% (95%CI = 19.59–52.80) and 53.01% (95%CI = 44.43–61.59) when the hypokalemia incidence rates were 0%–5% and >5%, respectively. However, for the other treatment types that were not first line, the ORRs were 11.58% (95%CI = 7.58–17.70) and 9.40% (95%CI = 7.31–11.49) when the hypokalemia incidence rates were 0%–5% and >5%, respectively. For the subgroup analysis on the different phases and drug numbers, the results were consistent with those of the whole population (Figure 3).
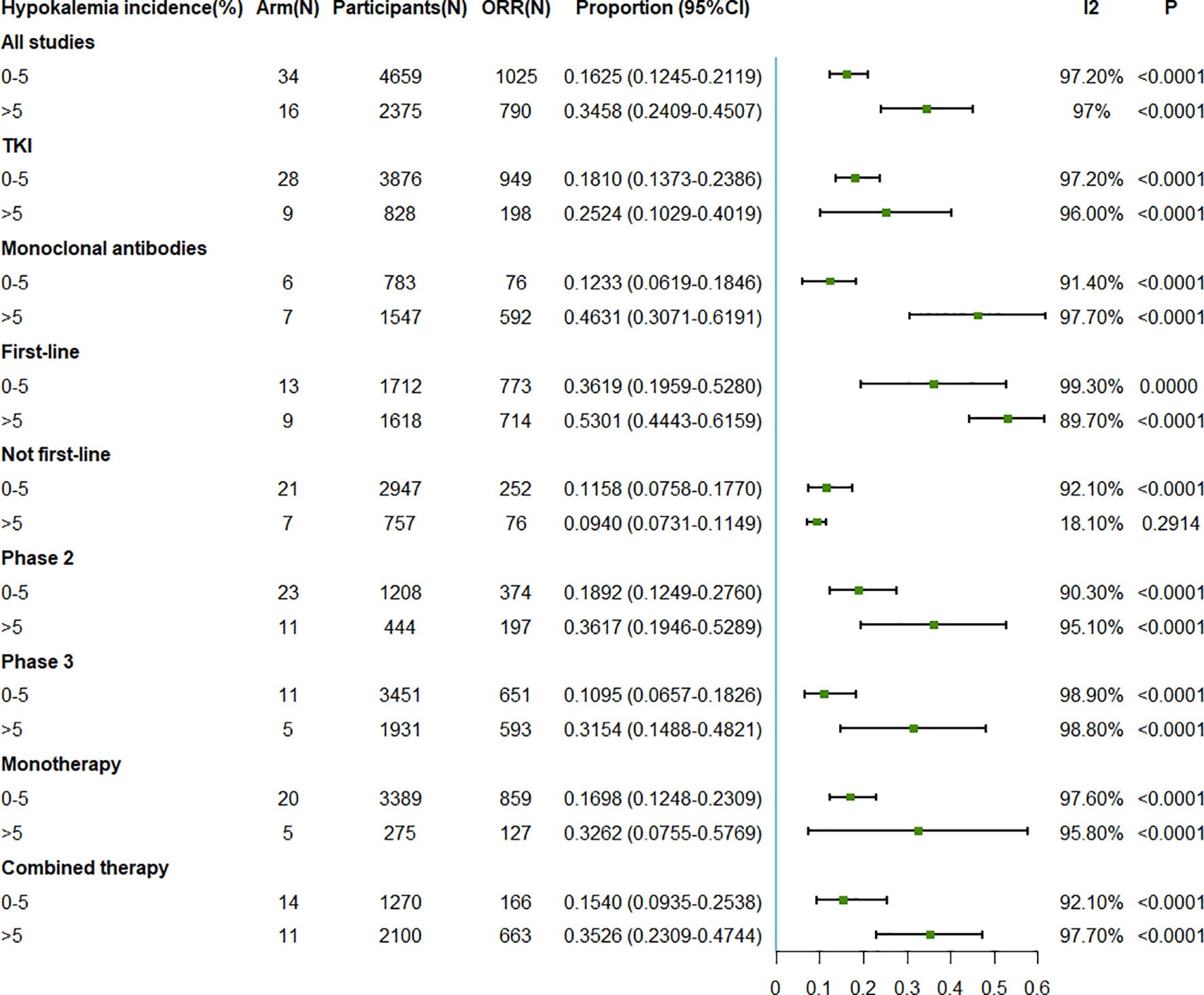
Figure 3 Forest plot for the meta-analysis of the ORR of anti-epidermal growth factor receptor (EGFR) targeted therapy for different incidence rates of grade 3–5 hypokalemia. ORR, objective response rate; TKI, tyrosine kinase inhibitor.
The pooled DCRs associated with EGFR antagonist were 56.03% (95%CI = 45.03–67.03) when the incidence of hypokalemia was 0%–5% and 64.38% (95%CI = 48.60–80.17) when the incidence of hypokalemia was >5% (Figure 4). In the subgroup analysis on the different intervention types, first-line treatment, different phases, and different drug numbers, the results were consistent with those observed in the whole population. However, similar to the ORR for the not first-line treatment, a higher DCR was observed with a lower hypokalemia incidence rate (Figure 4).
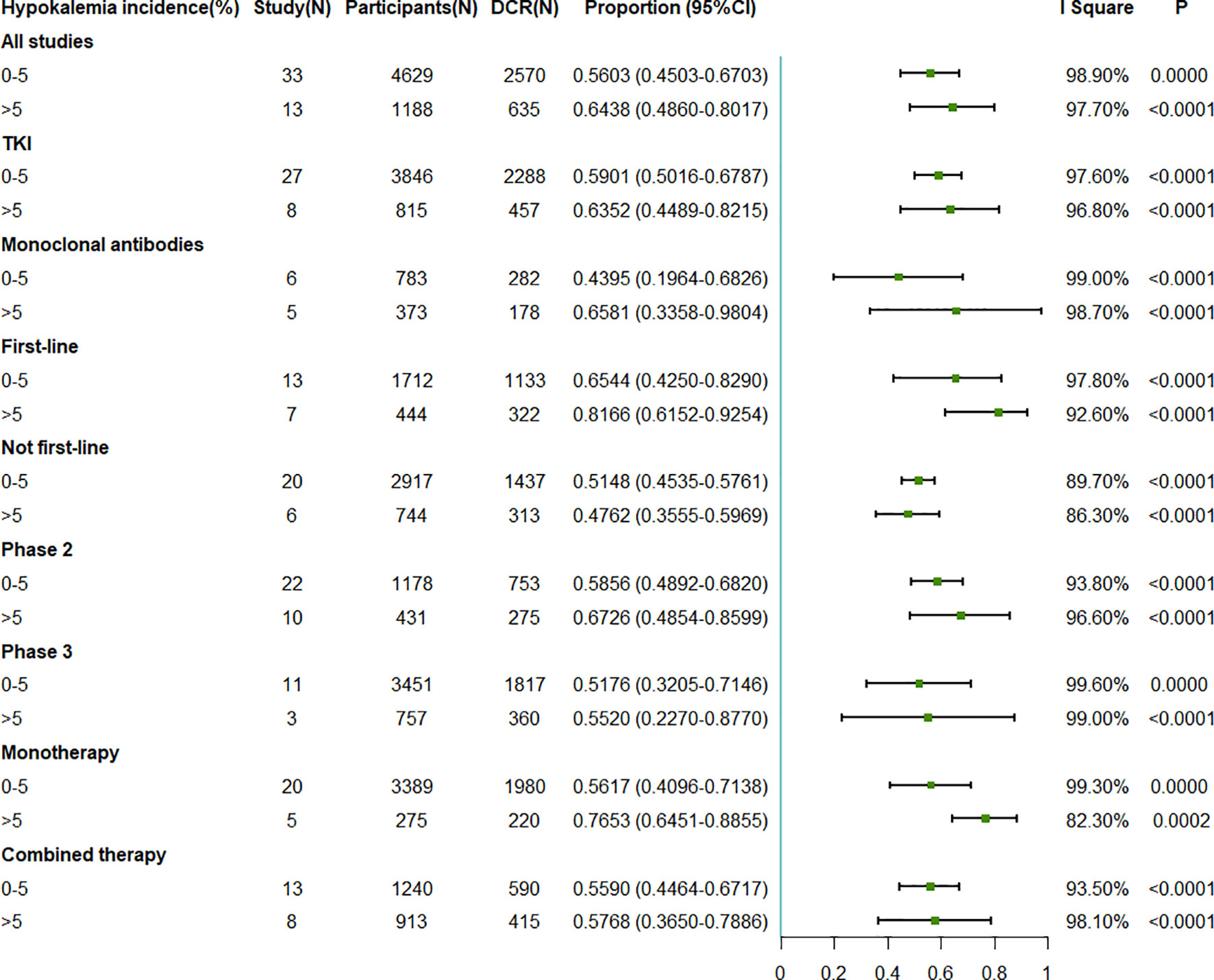
Figure 4 Forest plot for the meta-analysis of the DCR of anti-epidermal growth factor receptor (EGFR) targeted therapy for different incidence rates of grade 3–5 hypokalemia. DCR, disease control rate; TKI, tyrosine kinase inhibitor.
Sensitivity analysis showed non-obvious pooled ORR/DCR changes observed when excluding each trial at a time (Supplementary Tables S1, S2).
To the best of our knowledge, this is the first meta-analysis suggesting an association of an elevated incidence of hypokalemia with an increase in anti-EGFR treatment efficacy. The pooled ORRs were 16.25% and 34.58% and the pooled DCRs were 56.03% and 64.38% when the hypokalemia incidence rate ranges from ≤5% to >5%. These results indicated that the response to cancer therapy was associated with the serum potassium level.
In the carcinoma microenvironment, the concentrations of ions would be affected by high local levels of cellular apoptosis and necrosis. Potassium, as the most abundant intracellular ion, was significantly elevated 5–10 times in the tumor interstitial fluid compared with that in normal serum and benign tissue (52). Similarly, specific experimental apoptosis or necrosis was observed with the release of potassium into the extracellular microenvironment (52, 53). The elevated K+ acutely inhibited the T-cell receptor-induced production of effector cytokines, which resulted in subsequent immunosuppression (52). The elevated serum potassium limited the activity of antitumor T cells with metabolic constraints, eventually contributing to cancer progression (10). From this point of view, the hypokalemic microenvironment may strengthen the function of the immune system against tumor cells. The results were contradictory to the ORR and DCR (Supplementary Tables S3, S4) when considering the association between the effect of chemotherapy and the incidence of hypokalemia in our meta-analysis. Although a higher hypokalemia incidence was associated with a higher DCR, we observed inconsistent results for ORR. Thus, the association between cancer therapy and serum potassium level may be limited to targeted therapy. However, the exact mechanism between targeted therapy and hypokalemia is still unknown, and some researchers conjecture that this phenomenon may be due to the direct nephrotoxicity of targeted therapy (54, 55).
Overall, a higher incidence of hypokalemia was associated with better ORR and DCR. However, we observed an inverse association for the not first-line studies. In antitumor clinical trials, the possible causes of hypokalemia included drug nephrotoxicity and poor quality of life induced by the side effects of drugs, such as diarrhea, anorexia, and vomiting. A low serum potassium level enhanced the function of the immune system, making targeted therapies more effective. On the other hand, the better treatment effect of an anti-EGFR regimen with higher ORR and DCR may indicate longer survival of carcinoma patients, but possibly with worse quality of life. It is possible that the positive effect of hypokalemia on cancer treatment may be confounded by malnutrition with high loss and/or low potassium intake. From this viewpoint, the benefit from hypokalemia was offset by patients’ poor living conditions. To further explore our hypothesis on quality of life, the toxicity data and adverse event records of the 36 studies were collected. A higher incidence (6.7%) of diarrhea (grade ≥3) could be observed in the EGFR antagonist arm compared with that (1.3%) in other treatment arms (including chemotherapy, placebo, etc., data not shown). In the EGFR antagonist arm, there was a difference between the line of treatment and the incidence of diarrhea (grade ≥3), 3.94% and 9.17% for first line and not first line, respectively. Thus, it is possible that the change of serum potassium caused by diarrhea confounded the relationship between serum potassium level and treatment efficacy in the not first-line intervention. As for anorexia/decreased appetite (grade ≥3), weight loss/decreased weight (grade ≥3), and nausea/vomiting (grade ≥3), there were no obvious differences between the anti-EGFR arm and other treatment arms (data not shown). Also, in the EGFR antagonist arm, there was little difference between the different lines of treatment. Future mechanism research works and clinical trials are warranted to explore the effects of targeted therapy on serum potassium.
Previous studies have supported the association between lower serum potassium concentration and better outcomes in carcinoma, and more hypokalemia could be observed in targeted therapy. A Swedish perspective prostate cancer study, conducted with 11,492 participants, claimed that a weak positive association was observed between higher pre-diagnostic serum potassium (>5 mEq/L) and overall death (56). The Food and Drug Administration (FDA) review of panitumumab (Vectibix) for first-line use in metastatic colorectal cancer found that all grades of hypokalemia were observed with a 34% incidence rate and grades 3–5 with approximately 10% incidence rate. However, the incidence rates of hypokalemia in the non-panitumumab group were 14% and 4% for all grades and grades 3–5, respectively (54, 57). In a meta-analysis with a total of 2,254 participants, a higher incidence of grade 3 and 4 hypokalemia was positively associated with cetuximab-based therapy for advanced cancer (55). Similarly, when compared with non-cetuximab therapy, a higher risk of grade 3 and 4 hypokalemia with an odds ratio of 1.81 (95%CI = 1.12–2.93) was observed in the cetuximab arm (55). These studies, combined with our analysis, support a low serum potassium level as possibly beneficial for cancer patients in targeted therapy. Future studies are warranted to focus on how to maintain lower serum potassium levels to achieve better clinical outcomes.
However, hypokalemia, as an adverse event in cancer therapy, should be given sufficient attention for safety. Fluid and electrolyte imbalances were thought to be associated with increased mortality among hospitalized critically ill patients (56). In hospitalized cancer patients, hypokalemia is a common and important phenomenon, which may cause serious consequences such as cardiac arrhythmias and/or respiratory arrest. For outpatients, whose serum potassium levels were monitored even less closely than those of hospitalized ones, hypokalemia is also a dangerous adverse event (12, 55). Thus, monitoring of the serum potassium level in targeted therapy, even in cancer therapy, should be emphasized in this setting (13). Timely correction of modifiable clinical factors and management of electrolytes should not be ignored during the overall regimen period (58). The management of hypokalemia is based on strategies minimizing persistent losses and replacing serum potassium (54). Some research studies have revealed that the cause of hypokalemia is the compensation of serum magnesium deficiency (55, 59). Brief clinical check of blood magnesium ion concentrations is always warranted (60). Potassium replacement of a large amount should be gradually carried out, avoiding rebound hyperkalemia, until the clinical status of the cancer patient remains stable (54, 60). Thus, it is worth exploring how to keep a trade-off serum potassium level for both treatment effect and safety consideration to optimize prognosis.
Some limitations of our research are worth considering. Firstly, as a meta-analysis, the results were affected by the quality of each clinical trial. These included trials had different populations, follow-up durations, with or without chemotherapies, and different EGFR antagonists. The usage frequency of targeted therapy also varied among the trials, and some drugs were even only involved in a single clinical trial, e.g., “poziotinib”. Thus, detailed subgroup analysis for each anti-EGFR therapy was not possible. Moreover, only ORR and DCR were considered as the efficacy outcomes with different hypokalemia incidence levels, while time-to-event outcomes, such as overall survival and progression-free survival, are more important efficacy indexes in cancer therapy. Finally, it is impossible to obtain individual data for more detailed analysis to control for potential confounders.
In conclusion, our analysis has shown that the efficacy of anti-EGFR targeted therapy was associated with the incidence rate of hypokalemia. Compared with a hypokalemia incidence of 0%–5%, higher ORR and DCR could be observed with a hypokalemia incidence rate greater than 5%. Close monitoring and timely management of electrolytes should be emphasized in a carcinoma regimen, especially in targeted treatment. Different treatment effects should be considered for different serum potassium strata in future clinical trials with anti-EGFR therapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YZ, DY, TL, and JB were responsible for concept and design. JZ, YY, and XC acquired, analyzed, interpreted the data. JZ, YY, XC, and DY drafted the manuscript. YZ, JB, and DY critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.757456/full#supplementary-material
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
2. Organization WH. Cancer. Available at: https://www.who.int/news-room/fact-sheets/detail/cancer2021 (Accessed June 15, 2021).
3. Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The Emerging Treatment Landscape of Targeted Therapy in Non-Small-Cell Lung Cancer. Signal Transduct Target Ther (2019) 4:61. doi: 10.1038/s41392-019-0099-9
4. Ciardiello F, Tortora G. EGFR Antagonists in Cancer Treatment. N Engl J Med (2008) 358(11):1160–74. doi: 10.1056/NEJMra0707704
5. Singh D, Attri BK, Gill RK, Bariwal J. Review on EGFR Inhibitors: Critical Updates. Mini Rev Med Chem (2016) 16(14):1134–66. doi: 10.2174/1389557516666160321114917
6. Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs Resistance via EGFR-Independent Signaling Pathways. Mol Cancer (2018) 17(1):53. doi: 10.1186/s12943-018-0793-1
7. Dancey J, Sausville EA. Issues and Progress With Protein Kinase Inhibitors for Cancer Treatment. Nat Rev Drug Discov (2003) 2(4):296–313. doi: 10.1038/nrd1066
8. Wang C, Zhang R, Wei X, Lv M, Jiang Z. Metalloimmunology: The Metal Ion-Controlled Immunity. Adv Immunol (2020) 145:187–241. doi: 10.1016/bs.ai.2019.11.007
9. Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, et al. T Cell Stemness and Dysfunction in Tumors Are Triggered by a Common Mechanism. Science (2019) 363(6434):eaau0135. doi: 10.1126/science.aau0135
10. Baixauli F, Villa M, Pearce EL. Potassium Shapes Antitumor Immunity. Science (2019) 363(6434):1395–6. doi: 10.1126/science.aaw8800
11. Falk RS, Heir T, Robsahm TE, Tretli S, Sandvik L, Erikssen JE, et al. Fasting Serum Levels of Potassium and Sodium in Relation to Long-Term Risk of Cancer in Healthy Men. Clin Epidemiol (2020) 12:1–8. doi: 10.2147/CLEP.S216438
12. Lin SH, Halperin ML. Hypokalemia: A Practical Approach to Diagnosis and Its Genetic Basis. Curr Med Chem (2007) 14(14):1551–65. doi: 10.2174/092986707780831050
13. Cantini L, Merloni F, Rinaldi S, Lenci E, Marcantognini G, Meletani T, et al. Electrolyte Disorders in Advanced Non-Small Cell Lung Cancer Patients Treated With Immune Check-Point Inhibitors: A Systematic Review and Meta-Analysis. Crit Rev Oncol Hematol (2020) 151:102974. doi: 10.1016/j.critrevonc.2020.102974
14. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
16. Niho S, Kubota K, Goto K, Yoh K, Ohmatsu H, Kakinuma R, et al. First-Line Single Agent Treatment With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer: A Phase II Study. J Clin Oncol (2006) 24(1):64–9. doi: 10.1200/JCO.2005.02.5825
17. Jackman DM, Yeap BY, Lindeman NI, Fidias P, Rabin MS, Temel J, et al. Phase II Clinical Trial of Chemotherapy-Naive Patients > or = 70 Years of Age Treated With Erlotinib for Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2007) 25(7):760–6. doi: 10.1200/JCO.2006.07.5754
18. Belani CP, Schreeder MT, Steis RG, Guidice RA, Marsland TA, Butler EH, et al. Cetuximab in Combination With Carboplatin and Docetaxel for Patients With Metastatic or Advanced-Stage Nonsmall Cell Lung Cancer: A Multicenter Phase 2 Study. Cancer (2008) 113(9):2512–7. doi: 10.1002/cncr.23902
19. Crino L, Cappuzzo F, Zatloukal P, Reck M, Pesek M, Thompson JC, et al. Gefitinib Versus Vinorelbine in Chemotherapy-Naive Elderly Patients With Advanced Non-Small-Cell Lung Cancer (INVITE): A Randomized, Phase II Study. J Clin Oncol (2008) 26(26):4253–60. doi: 10.1200/JCO.2007.15.0672
20. Lynch TJ, Fenton D, Hirsh V, Bodkin D, Middleman EL, Chiappori A, et al. A Randomized Phase 2 Study of Erlotinib Alone and in Combination With Bortezomib in Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol (2009) 4(8):1002–9. doi: 10.1097/JTO.0b013e3181aba89f
21. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab Plus Chemotherapy in Patients With Advanced Non-Small-Cell Lung Cancer (FLEX): An Open-Label Randomised Phase III Trial. Lancet (2009) 373(9674):1525–31. doi: 10.1016/S0140-6736(09)60569-9
22. Govindan R, Bogart J, Stinchcombe T, Wang X, Hodgson L, Kratzke R, et al. Randomized Phase II Study of Pemetrexed, Carboplatin, and Thoracic Radiation With or Without Cetuximab in Patients With Locally Advanced Unresectable Non-Small-Cell Lung Cancer: Cancer and Leukemia Group B Trial 30407. J Clin Oncol (2011) 29(23):3120–5. doi: 10.1200/JCO.2010.33.4979
23. Ahn MJ, Yang JC, Liang J, Kang JH, Xiu Q, Chen YM, et al. Randomized Phase II Trial of First-Line Treatment With Pemetrexed-Cisplatin, Followed Sequentially by Gefitinib or Pemetrexed, in East Asian, Never-Smoker Patients With Advanced Non-Small Cell Lung Cancer. Lung Cancer (2012) 77(2):346–52. doi: 10.1016/j.lungcan.2012.03.011
24. Blumenschein GR Jr., Ciuleanu T, Robert F, Groen HJ, Usari T, Ruiz-Garcia A, et al. Sunitinib Plus Erlotinib for the Treatment of Advanced/Metastatic Non-Small-Cell Lung Cancer: A Lead-in Study. J Thorac Oncol (2012) 7(9):1406–16. doi: 10.1097/JTO.0b013e31825cca1c
25. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib Versus Placebo for Patients With Advanced, Metastatic Non-Small-Cell Lung Cancer After Failure of Erlotinib, Gefitinib, or Both, and One or Two Lines of Chemotherapy (LUX-Lung 1): A Phase 2b/3 Randomised Trial. Lancet Oncol (2012) 13(5):528–38. doi: 10.1016/S1470-2045(12)70087-6
26. Scagliotti GV, Krzakowski M, Szczesna A, Strausz J, Makhson A, Reck M, et al. Sunitinib Plus Erlotinib Versus Placebo Plus Erlotinib in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer: A Phase III Trial. J Clin Oncol (2012) 30(17):2070–8. doi: 10.1200/JCO.2011.39.2993
27. Belani CP, Nemunaitis JJ, Chachoua A, Eisenberg PD, Raez LE, Cuevas JD, et al. Phase 2 Trial of Erlotinib With or Without PF-3512676 (CPG 7909, A Toll-Like Receptor 9 Agonist) in Patients With Advanced Recurrent EGFR-Positive Non-Small Cell Lung Cancer. Cancer Biol Ther (2013) 14(7):557–63. doi: 10.4161/cbt.24598
28. Kim ES, Moon J, Herbst RS, Redman MW, Dakhil SR, Velasco MR Jr., et al. Phase II Trial of Carboplatin, Paclitaxel, Cetuximab, and Bevacizumab Followed by Cetuximab and Bevacizumab in Advanced Nonsquamous Non-Small-Cell Lung Cancer: SWOG S0536. J Thorac Oncol (2013) 8(12):1519–28. doi: 10.1097/JTO.0000000000000009
29. Kim ES, Neubauer M, Cohn A, Schwartzberg L, Garbo L, Caton J, et al. Docetaxel or Pemetrexed With or Without Cetuximab in Recurrent or Progressive Non-Small-Cell Lung Cancer After Platinum-Based Therapy: A Phase 3, Open-Label, Randomised Trial. Lancet Oncol (2013) 14(13):1326–36. doi: 10.1016/S1470-2045(13)70473-X
30. Ellis PM, Shepherd FA, Millward M, Perrone F, Seymour L, Liu G, et al. Dacomitinib Compared With Placebo in Pretreated Patients With Advanced or Metastatic Non-Small-Cell Lung Cancer (NCIC CTG BR.26): A Double-Blind, Randomised, Phase 3 Trial. Lancet Oncol (2014) 15(12):1379–88. doi: 10.1016/S1470-2045(14)70472-3
31. Janne PA, Ou SI, Kim DW, Oxnard GR, Martins R, Kris MG, et al. Dacomitinib as First-Line Treatment in Patients With Clinically or Molecularly Selected Advanced Non-Small-Cell Lung Cancer: A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol (2014) 15(13):1433–41. doi: 10.1016/S1470-2045(14)70461-9
32. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib Versus Cisplatin Plus Gemcitabine for First-Line Treatment of Asian Patients With Advanced Non-Small-Cell Lung Cancer Harbouring EGFR Mutations (LUX-Lung 6): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2014) 15(2):213–22. doi: 10.1016/S1470-2045(13)70604-1
33. Han JY, Lee SH, Lee GK, Yun T, Lee YJ, Hwang KH, et al. Phase I/II Study of Gefitinib (Iressa((R))) and Vorinostat (IVORI) in Previously Treated Patients With Advanced Non-Small Cell Lung Cancer. Cancer Chemother Pharmacol (2015) 75(3):475–83. doi: 10.1007/s00280-014-2664-9
34. Heigener DF, Pereira JR, Felip E, Mazal J, Manzyuk L, Tan EH, et al. Weekly and Every 2 Weeks Cetuximab Maintenance Therapy After Platinum-Based Chemotherapy Plus Cetuximab as First-Line Treatment for Non-Small Cell Lung Cancer: Randomized Non-Comparative Phase IIIb NEXT Trial. Target Oncol (2015) 10(2):255–65. doi: 10.1007/s11523-014-0336-7
35. Lara PN Jr., Longmate J, Mack PC, Kelly K, Socinski MA, Salgia R, et al. Phase II Study of the AKT Inhibitor MK-2206 Plus Erlotinib in Patients With Advanced Non-Small Cell Lung Cancer Who Previously Progressed on Erlotinib. Clin Cancer Res (2015) 21(19):4321–6. doi: 10.1158/1078-0432.CCR-14-3281
36. Lee DH, Lee JS, Wang J, Hsia TC, Wang X, Kim J, et al. Pemetrexed-Erlotinib, Pemetrexed Alone, or Erlotinib Alone as Second-Line Treatment for East Asian and Non-East Asian Never-Smokers With Locally Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer: Exploratory Subgroup Analysis of a Phase II Trial. Cancer Res Treat (2015) 47(4):616–29. doi: 10.4143/crt.2014.051
37. Liu D, Shen YX, Zhao WX, Jiang GL, Chen JY, Fan M. Feasibility of Cetuximab and Chemoradiotherapy Combination in Chinese Patients With Unresectable Stage III Non-Small Cell Lung Cancer: A Preliminary Report. Chin J Cancer Res (2015) 27(2):172–80. doi: 10.3978/j.issn.1000-9604.2014.11.05
38. Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-Line Erlotinib Versus Gemcitabine/Cisplatin in Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Analyses From the Phase III, Randomized, Open-Label, ENSURE Study. Ann Oncol (2015) 26(9):1883–9. doi: 10.1093/annonc/mdv270
39. Lee VH, Leung DK, Choy TS, Lam KO, Lam PM, Leung TW, et al. Efficacy and Safety of Afatinib in Chinese Patients With EGFR-Mutated Metastatic Non-Small-Cell Lung Cancer (NSCLC) Previously Responsive to First-Generation Tyrosine-Kinase Inhibitors (TKI) and Chemotherapy: Comparison With Historical Cohort Using Erlotinib. BMC Cancer (2016) 16:147. doi: 10.1186/s12885-016-2201-9
40. Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib Versus Gefitinib as First-Line Treatment of Patients With EGFR Mutation-Positive Non-Small-Cell Lung Cancer (LUX-Lung 7): A Phase 2B, Open-Label, Randomised Controlled Trial. Lancet Oncol (2016) 17(5):577–89. doi: 10.1016/S1470-2045(16)30033-X
41. Han JY, Lee KH, Kim SW, Min YJ, Cho E, Lee Y, et al. A Phase II Study of Poziotinib in Patients With Epidermal Growth Factor Receptor (EGFR)-Mutant Lung Adenocarcinoma Who Have Acquired Resistance to EGFR-Tyrosine Kinase Inhibitors. Cancer Res Treat (2017) 49(1):10–9. doi: 10.4143/crt.2016.058
42. Spigel DR, Mekhail TM, Waterhouse D, Hadley T, Webb C, Burris HA 3rd, et al. First-Line Carboplatin, Pemetrexed, and Panitumumab in Patients With Advanced Non-Squamous KRAS Wild Type (WT) Non-Small-Cell Lung Cancer (NSCLC). Cancer Invest (2017) 35(8):541–6. doi: 10.1080/07357907.2017.1344698
43. Spigel DR, Rubin MS, Gian VG, Shipley DL, Burris HA 3rd, Kosloff RA, et al. Sorafenib and Continued Erlotinib or Sorafenib Alone in Patients With Advanced Non-Small Cell Lung Cancer Progressing on Erlotinib: A Randomized Phase II Study of the Sarah Cannon Research Institute (SCRI). Lung Cancer (2017) 113:79–84. doi: 10.1016/j.lungcan.2017.09.007
44. Thomas M, Sadjadian P, Kollmeier J, Lowe J, Mattson P, Trout JR, et al. A Randomized, Open-Label, Multicenter, Phase II Study Evaluating the Efficacy and Safety of BTH1677 (1,3-1,6 Beta Glucan; Imprime PGG) in Combination With Cetuximab and Chemotherapy in Patients With Advanced Non-Small Cell Lung Cancer. Invest New Drugs (2017) 35(3):345–58. doi: 10.1007/s10637-017-0450-3
45. Wakelee HA, Gettinger S, Engelman J, Janne PA, West H, Subramaniam DS, et al. A Phase Ib/II Study of Cabozantinib (XL184) With or Without Erlotinib in Patients With non-Small Cell Lung Cancer. Cancer Chemother Pharmacol (2017) 79(5):923–32. doi: 10.1007/s00280-017-3283-z
46. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib Versus Gefitinib as First-Line Treatment for Patients With EGFR-Mutation-Positive Non-Small-Cell Lung Cancer (ARCHER 1050): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(11):1454–66. doi: 10.1016/S1470-2045(17)30608-3
47. Hata A, Katakami N, Kaji R, Yokoyama T, Kaneda T, Tamiya M, et al. Afatinib Plus Bevacizumab Combination After Acquired Resistance to EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Non-Small Cell Lung Cancer: Multicenter, Single-Arm, Phase 2 Trial (ABC Study). Cancer (2018) 124(19):3830–8. doi: 10.1002/cncr.31678
48. Herbst RS, Redman MW, Kim ES, Semrad TJ, Bazhenova L, Masters G, et al. Cetuximab Plus Carboplatin and Paclitaxel With or Without Bevacizumab Versus Carboplatin and Paclitaxel With or Without Bevacizumab in Advanced NSCLC (SWOG S0819): A Randomised, Phase 3 Study. Lancet Oncol (2018) 19(1):101–14. doi: 10.1016/S1470-2045(17)30694-0
49. Lu S, Li W, Zhou C, Hu CP, Qin S, Cheng G, et al. Afatinib vs Erlotinib for Second-Line Treatment of Chinese Patients With Advanced Squamous Cell Carcinoma of the Lung. Onco Targets Ther (2018) 11:8565–73. doi: 10.2147/OTT.S161506
50. Oda N, Hotta K, Ninomiya K, Minami D, Ichihara E, Murakami T, et al. A Phase II Trial of EGFR-TKI Readministration With Afatinib in Advanced Non-Small-Cell Lung Cancer Harboring a Sensitive Non-T790M EGFR Mutation: Okayama Lung Cancer Study Group Trial 1403. Cancer Chemother Pharmacol (2018) 82(6):1031–8. doi: 10.1007/s00280-018-3694-5
51. Reckamp KL, Frankel PH, Ruel N, Mack PC, Gitlitz BJ, Li T, et al. Phase II Trial of Cabozantinib Plus Erlotinib in Patients With Advanced Epidermal Growth Factor Receptor (EGFR)-Mutant Non-Small Cell Lung Cancer With Progressive Disease on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy: A California Cancer Consortium Phase II Trial (NCI 9303). Front Oncol (2019) 9:132. doi: 10.3389/fonc.2019.00132
52. Gurusamy D, Clever D, Eil R, Restifo NP. Novel “Elements” of Immune Suppression Within the Tumor Microenvironment. Cancer Immunol Res (2017) 5(6):426–33. doi: 10.1158/2326-6066.CIR-17-0117
53. Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, et al. Ionic Immune Suppression Within the Tumour Microenvironment Limits T Cell Effector Function. Nature (2016) 537(7621):539–43. doi: 10.1038/nature19364
54. Abbas A, Mirza MM, Ganti AK, Tendulkar K. Renal Toxicities of Targeted Therapies. Target Oncol (2015) 10(4):487–99. doi: 10.1007/s11523-015-0368-7
55. Cao Y, Liu L, Liao C, Tan A, Gao F. Meta-Analysis of Incidence and Risk of Hypokalemia With Cetuximab-Based Therapy for Advanced Cancer. Cancer Chemother Pharmacol (2010) 66(1):37–42. doi: 10.1007/s00280-009-1131-5
56. Ghoshal A, Garmo H, Hammar N, Jungner I, Malmstrom H, Walldius G, et al. Can Pre-Diagnostic Serum Levels of Sodium and Potassium Predict Prostate Cancer Survival? BMC Cancer (2018) 18(1):1169. doi: 10.1186/s12885-018-5098-7
57. Giusti RM, Cohen MH, Keegan P, Pazdur R. FDA Review of a Panitumumab (Vectibix) Clinical Trial for First-Line Treatment of Metastatic Colorectal Cancer. Oncologist (2009) 14(3):284–90. doi: 10.1634/theoncologist.2008-0254
58. Li Y, Chen X, Shen Z, Wang Y, Hu J, Xu J, et al. Electrolyte and Acid-Base Disorders in Cancer Patients and its Impact on Clinical Outcomes: Evidence From a Real-World Study in China. Ren Fail (2020) 42(1):234–43. doi: 10.1080/0886022X.2020.1735417
59. Whang R, Welt LG. Observations in Experimental Magnesium Depletion. J Clin Invest (1963) 42:305–13. doi: 10.1172/JCI104717
Keywords: hypokalemia, targeted therapy, EGFR antagonist, NSCLC, meta-analysis
Citation: Zhou J, Bai J, Yue Y, Chen X, Lange T, You D and Zhao Y (2022) Association of Hypokalemia Incidence and Better Treatment Response in NSCLC Patients: A Meta-Analysis and Systematic Review on Anti-EGFR Targeted Therapy Clinical Trials. Front. Oncol. 11:757456. doi: 10.3389/fonc.2021.757456
Received: 12 August 2021; Accepted: 02 December 2021;
Published: 05 January 2022.
Edited by:
Wen-Qing Li, Peking University Cancer Hospital, ChinaReviewed by:
Tao Zhang, Shandong University, ChinaCopyright © 2022 Zhou, Bai, Yue, Chen, Lange, You and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Zhao, eXpoYW9AbmptdS5lZHUuY24=; Dongfang You, eW91ZG9uZ2ZhbmdAbmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.