- 1Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Hubei Center for Adverse Drug Reaction Monitoring, Wuhan, China
Background: Oxaliplatin (OXA), a third-generation platinum derivative, has become one of the main chemotherapeutic drugs for colorectal cancer and other cancers, but reports of adverse reactions are also increasing with the extensive application of OXA. In this study, post-marketing surveillance was carried out to investigate the safety profile of OXA in a real-world setting in Chinese cancer patients to provide a reference for the rational application of OXA.
Methods: All patients with cancer who received OXA-based chemotherapy in 10 tertiary hospitals in Hubei Province, China, between May 2016 and November 2016 were enrolled. A central registration method was used to document patients’ demographics, clinical use, and any incidence of adverse reactions to OXA. All adverse drug reactions (ADRs) were collected and analyzed to assess causality, severity, treatment, and outcome.
Results: In total, 3687 patients were enrolled in this study. Approximately 64.6% of the patients were male, and 68.8% were aged 50-70 years, with a mean age of 55.3 years. The proportions of patients diagnosed with colorectal and gastric cancers were 59.3% and 31.6%, respectively. In this study, the overall incidence of ADRs and serious ADRs was 42.7% and 1.3%, respectively. The most common ADRs were gastrointestinal disorders (25.7%), blood disorders (21.1%), and peripheral nervous system disorders (8.0%). The serious ADRs identified were hypersensitivity reactions, thrombocytopenia, abnormal hepatic function, and leukopenia/neutropenia. The median onset of gastrointestinal toxicity, myelosuppression, peripheral neurotoxicity, and abnormal hepatic function was 1 d, 5 d, 1 d, and 14 d, respectively. The majority (84.7%) of hypersensitivity reactions were mild to moderate, and the median time to onset of these reactions was within the first 20 min of OXA infusion. Almost 88.0% of patients who experienced ADRs recovered or improved with treatment.
Conclusion: Our data suggest that OXA-induced ADRs are very common in Chinese patients with cancer; however, more attention should be paid to hypersensitivity reactions caused by OXA. This study provides a valuable reference regarding the safe application of OXA in a real-world setting.
Introduction
Oxaliplatin (OXA) is a third-generation platinum-based anti-tumor drug that inhibits DNA and protein synthesis by forming intra- and inter-strand DNA platinum adducts, leading to tumor growth inhibition and apoptosis (1). OXA has better efficacy and lower toxicity than cisplatin and carboplatin (2) and is extensively used in various tumors, including colorectal, gastroesophageal, pancreatic, biliary, gynecologic malignancies, lung cancer and head and neck cancers (3–5). Studies have demonstrated that OXA combined with 5-fluorouracil (5-FU) and leucovorin increases survival and reduces the risk of recurrence in patients with colorectal cancer (CRC) (6, 7). Nowadays, OXA combined with 5-FU and leucovorin (FOLFOX) or with capecitabine (XELOX) has emerged as the standard regimen of adjuvant chemotherapy for stage III CRC and stage II CRC with high-risk factors and as the first-line regimen for metastatic CRC (8, 9).
With the extensive clinical application of OXA, adverse reactions towards OXA have also been reported in recent years, mainly including gastrointestinal side effects, hematologic toxicities, and dose-limiting peripheral neurotoxicity (10–12). Moreover, the reports on OXA-related hypersensitivity reactions (HSRs) are increasing gradually (13). Although OXA has been on the market for over 20 years, and there have been a few reports on its side effects, comprehensive safety profiles of OXA in large-scale populations in the real world have rarely been produced. The MOSAIC trial, a large randomized multi-institution randomized trial, only included over 1,100 patients who were receiving adjuvant FOLFOX chemotherapy for colorectal cancer (14). Thus, it is necessary to evaluate the post-marketing safety of OXA further to strengthen pharmacovigilance. In this article, a multicenter prospective study was carried out in 10 tertiary hospitals in Hubei province, China, to evaluate the post-marketing safety profile of OXA. We aimed to investigate the clinical use of OXA, the incidence of adverse reactions, time to onset, clinical manifestations, treatments, and outcomes to provide a reference for medical decision-making and the rational application of OXA.
Methods
Study Design and Patients
This multicenter observational study was conducted in 10 tertiary hospitals in Hubei Province, China, between May 2016 and November 2016. All patients with malignant tumors treated with OXA were enrolled in this study. All patients were prospectively registered upon initiation of OXA treatment by documenting the patients’ demographics, clinical application, and adverse reactions of OXA using a central monitor method.
The investigators received unified and standardized project training according to research work manual and case report forms (CRF) (Supplementary Table 1) before the study to ensure complete registration and quality data. Meanwhile, each subcenter designated special personnel (including one oncologist and one clinical pharmacist) responsible for the collection and filing of CRF. This study was conducted in accordance with the Drug Reevaluation Regulations and Guiding Principles from the Drug Evaluation Center of the State Food and Drug Administration and was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (No.TJIRB20160504). All participants were briefed and have provided written informed consent before completing the survey.
Safety Assessment
OXA-induced ADRs were collected through patients’ self-reports (especially some symptoms such as rash, cough, nausea, vomiting, abdominal pain, numbness and dizziness) during ward round and patient education as well as lab results (such as vital signs, blood routine, liver and kidney function) from HIS system, and the frequency was usually 3 times a week. The investigators needed to report all adverse reactions that occurred after OXA treatment, concomitant medications used, time to onset, symptoms, administered treatments, and outcomes. All reported ADRs were recorded according to the Provisions of Adverse Drug Reaction Reporting and Monitoring with the clinical pharmacists’ assessment of causality. This adverse reaction can be submitted only when the ADR correlation evaluation with OXA is possible or above. The severity of OXA-induced ADRs was classified according to the National Cancer Institute Common Criteria (NCI-CTCAE v5.0), and grade 3 and above adverse reactions were defined as serious ADRs. ADRs were classified using the preferred terms and system organ classes from the World Health Organization (WHO) Glossary of Adverse Drug Reactions. Additional information, such as the results of laboratory tests, was also recorded for serious ADRs.
Statistical Analysis
More than 3000 patients receiving OXA were enrolled to detect an ADR in one out of every 1000 patients with a probability of ≥95%. The collected data were verified and encoded by special personnel. All data analyses were performed using SPSS version 24.0 (SPSS Inc. Chicago, IL, USA). Descriptive analysis was used to evaluate patients’ demographics (such as sex and age), clinical characteristics such as Karnofsky performance status (KPS), diagnosis, clinical use of OXA, and the incidence of adverse reactions, time to onset, symptoms, administered treatments, and outcomes.
Results
Patient Demographics
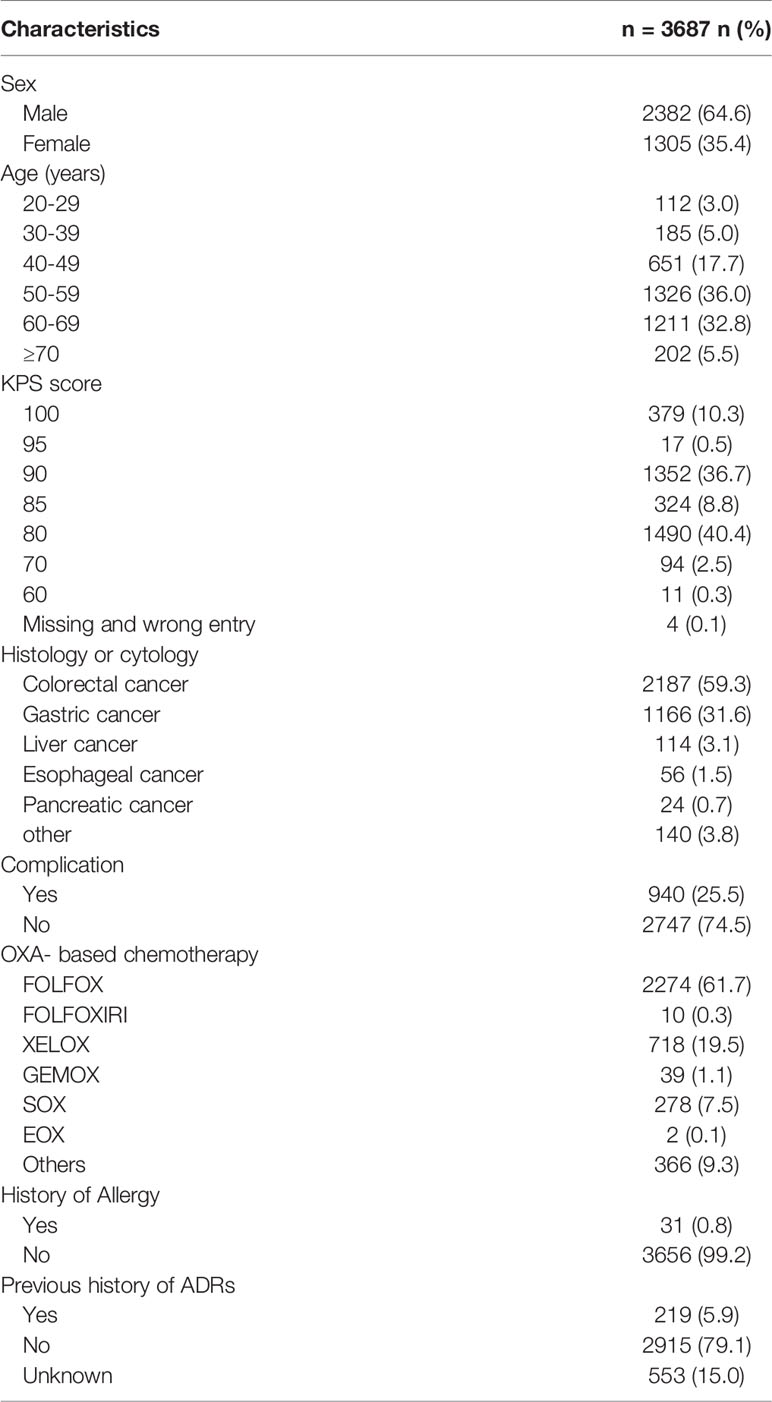
A total of 3775 case report forms were enrolled between May 1, 2016, and November 31, 2016. Eighty-eight reports were excluded due to duplication; thus, 3687 patients were enrolled for this safety study. Table 1 describes the patient demographics and clinical characteristics. Nearly 64.6% of patients were male, and most patients were between 40 and 70 years old (86.5%), with a mean (SD) age of 55.3 ( ± 10.6) years. Most patients had KPS scores of 80 (40.4%) and 90 (36.7%). Almost all (96.2%) patients were diagnosed with gastrointestinal cancer, with 59.3% and 31.6% of these patients diagnosed with colorectal and gastric cancer, respectively. Nearly two-thirds (61.7%) of the patients used the FOLFOX regimen, followed by the XELOX (19.5%) and SOX (S-1 plus OXA) (7.5%) regimens. About 25.5% of the patients had other diseases such as hypertension, hyperlipidemia, diabetes, coronary heart disease, kidney stones, and tuberculosis. Only 0.8% of the patients had a history of allergies, and 5.9% had a previous history of ADRs, mainly caused by antibiotics.
Incidence of ADRs and Serious ADRs
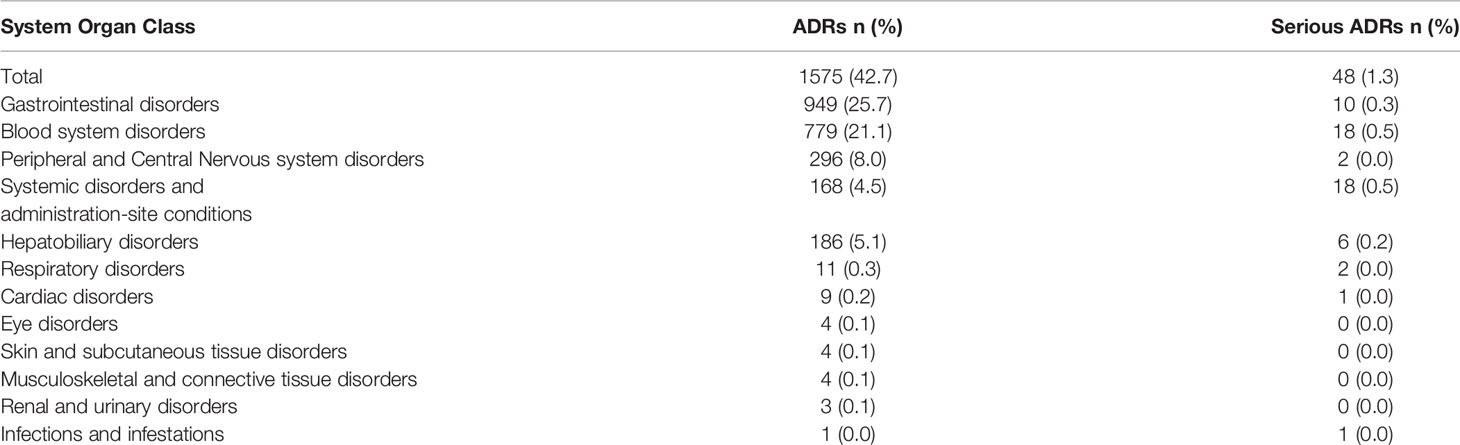
Of the 3687 patients, ADRs were reported in 1575 patients, giving an incidence rate of 42.7%. Most ADRs were grade 1 and 2, with grade 1 ADR reported in 562 patients (15.2%) and grade 2 ADR reported in 965 patients (26.2%). Serious ADRs (grade≥3) were reported in 48 patients (1.3%), showed in Figure 1. The majority (88.0%) of patients who developed ADR were healed and improved, and about 11.0% were unknown.
The most commonly reported ADRs fell into the general categories of gastrointestinal disorders (25.7%), blood disorders (21.1%), and peripheral nervous system disorders (8.0%) (Table 2). Other ADRs, such as respiratory, cardiac and eye disorders, had a low incidence rate and low severity (Table 2). However, systemic disorders such as hypersensitivity reactions occurred in 118 of the patients (3.2%), with related serious ADRs accounting for 0.5% of all cases. In addition, hepatic function abnormalities were reported in 186 patients (5.1%), with serious ADRs of this nature accounting for 0.2% of all cases (Table 2).
Most Frequently-Occurring ADRs
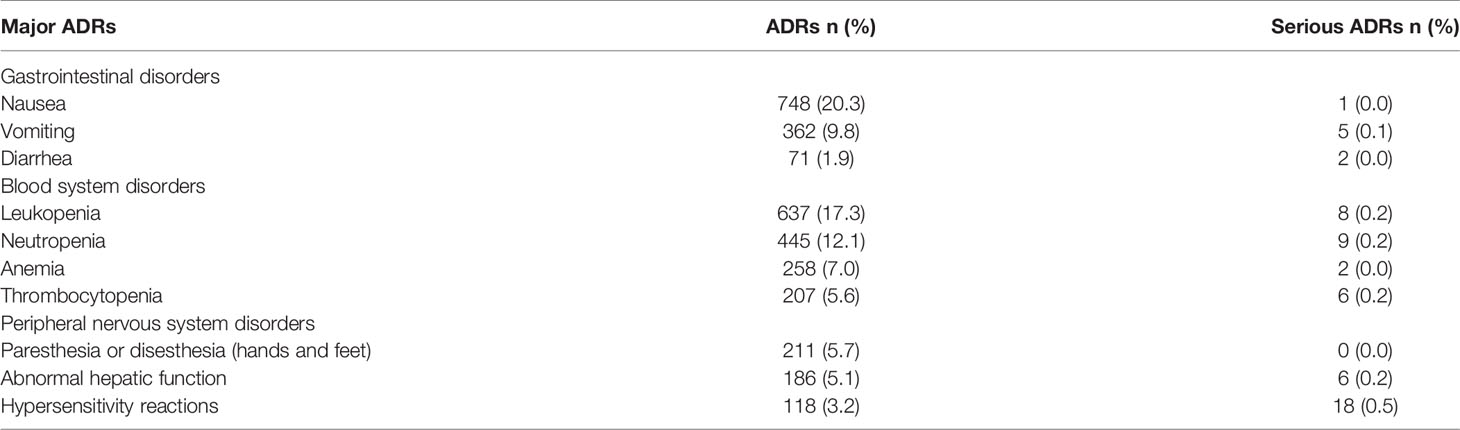
The OXA-linked ADRs with the highest frequency were nausea (20.3%), leukopenia (17.3%), neutropenia (12.1%), vomiting (9.8%), anemia (7.0%), thrombocytopenia (5.6%), and peripheral paresthesia or dysesthesia of hands and feet (5.7%). Meanwhile, the most frequent serious ADRs recorded were hypersensitivity reactions, leukopenia, neutropenia, thrombocytopenia, abnormal hepatic function, and vomiting (0.5%, 0.2%, 0.2%, 0.2%, 0.2%, 0.1%, respectively) (Table 3). Thus, gastrointestinal disorders, blood disorders, peripheral nervous system disorders, hypersensitivity reactions, and hepatic function abnormalities were regarded as the major ADRs caused by OXA.
Time to Onset, Management, and Outcome of Major ADRs
The median time from the start of OXA administration to the occurrence of gastrointestinal disorders, blood system disorders, peripheral nervous system disorders, and hepatic function abnormalities were 1 d, 5 d, 1 d, and 14 d, respectively. For hypersensitivity reactions, the median time from start to occurrence was much shorter, at only 20 min. In addition, the above 5 ADRs occurred in a median cycle of OXA chemotherapy of 3, 4, 4, 4 and 6, respectively. More than 90% of those who experienced gastrointestinal toxicity, myelosuppression, and peripheral neurotoxicity continued OXA therapy. Of those that experienced hypersensitivity reactions, approximately 85% suspended OXA administration and received the corresponding treatment (Table 4). Overall, most of these ADRs recovered and improved, and ADRs that had sequelae occurred in 11 patients who experienced hepatic dysfunction and 12 patients with myelosuppression.
OXA-Related Hypersensitivity Reactions
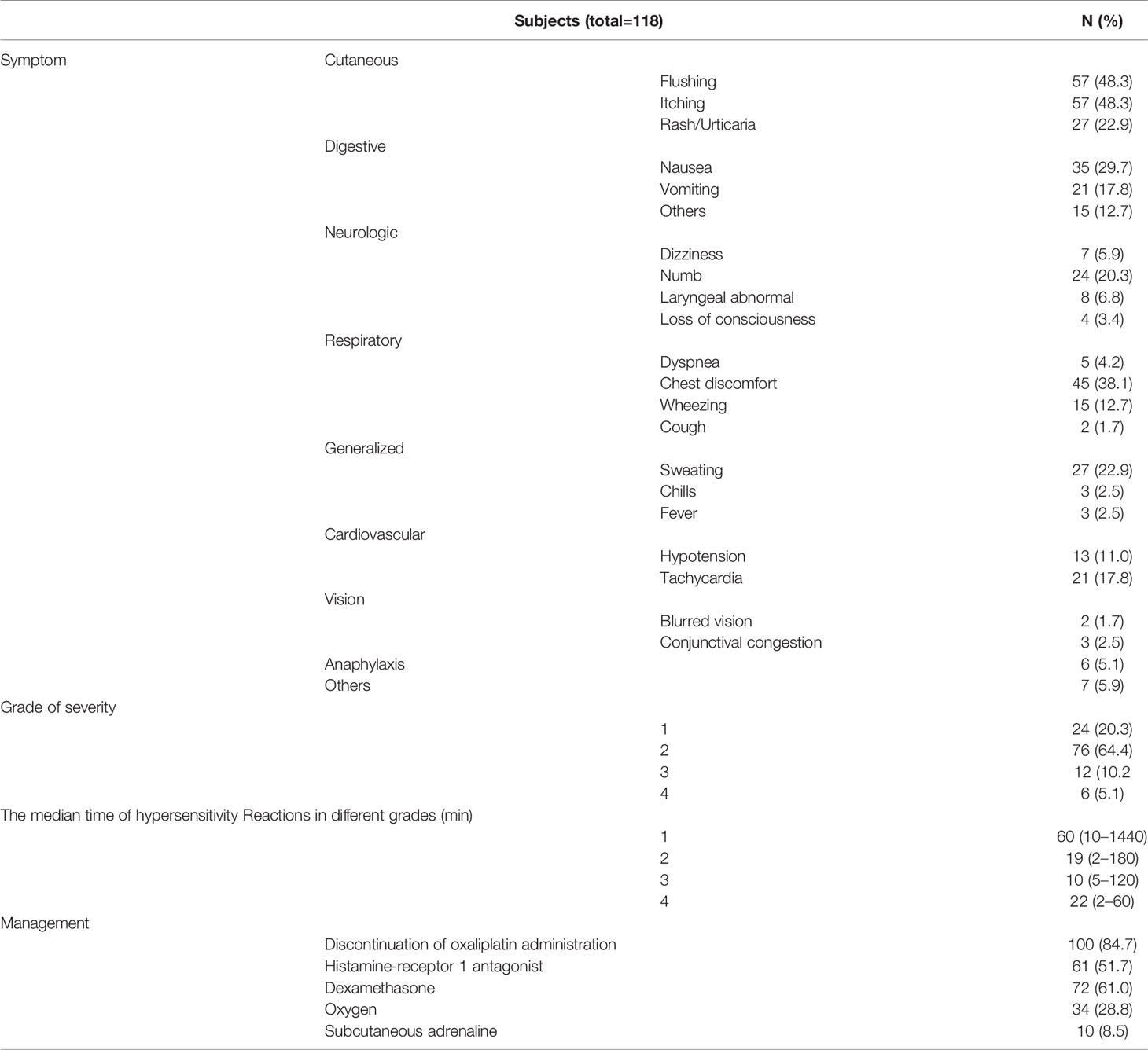
The manifestation of OXA-related HSRs is shown in Table 5. The most common events were cutaneous symptoms such as flushing (48.3%), itching (48.3%), and rashes (22.9%). For most patients (84.7%), the symptoms were grade 1 or 2, while hypersensitivity symptoms with grade ≥ 3 were reported in 18 patients (15.2%). Of these 18 patients, six experienced anaphylactic shock, characterized by wheezing, dizziness, abdominal pain, or loss of consciousness with hypotension.
The time between OXA infusion to the appearance of HSRs is shown in Figure 2, with most reactions occurring within the first hour. The time to onset varied between mild and severe cases: the median time to onset of grade 1 HSRs was 60 min, while grade 2, 3, and 4 events occurred mainly within the first 20 min after OXA infusion (Table 5). Most patients who experienced hypersensitivity (84.7%) were managed via discontinuation of treatment with OXA and the administration of hypersensitivity treatments, including dexamethasone (61.0%), histamine-receptor 1 antagonist (51.7%), oxygen (28.8%), and epinephrine (8.5%). All patients recovered or improved after the corresponding symptomatic treatment (Table 4).
Discussion
According to the 2018 Global Cancer Statistics, colorectal and gastric cancer incidence rates rank third and fourth among malignancies in China, and their mortality rate is placing fifth and second respectively (15). OXA, a third-generation platinum derivative, has become one of the mainstay chemotherapeutic drugs in gastrointestinal malignancies. The most common adverse reactions reported with this drug are gastrointestinal tract reactions, myelosuppression, peripheral neurotoxicity, and hypersensitivity reactions (14, 16). Hence, a multicenter observational study was carried out to investigate the safety profile of OXA in a real-world setting to provide a reference for the rational use of OXA.
In this study, 3687 patients who received OXA were enrolled. As far as we can confirm, at present, this is the largest real-world post-marketing safety evaluation of OXA in China. The majority (64.6%) of enrolled patients were male, within the age bracket of 50 to 69 years old. Epidemiology shows that the incidence rate of colorectal and gastric cancer in males is higher than in females, and most of these occur in middle-aged and older people (17). Thus, the characteristics of patients enrolled were consistent with previous epidemiological studies of colorectal and gastric cancer in China.
Moreover, this study showed that patients receiving OXA were mainly diagnosed with colorectal and gastric cancer (59.3% and 31.6%, respectively), and FOLFOX was the most commonly used chemotherapy regimen (61.7%), followed by XELOX (19.5%) and SOX (7.5%). According to NCCN guidelines of colorectal and gastric cancer (18, 19), FOLFOX and XELOX are the most popular chemotherapy regimens in colorectal cancer, which are widely used in neoadjuvant chemotherapy, adjuvant chemotherapy, and advanced palliative chemotherapy; XELOX and SOX are also frequently used chemotherapy regimens in gastric cancer. These suggest that the clinical application of OXA is in line with the recommendations described in these above guidelines.
Regarding the safety results, the overall incidence rate of ADRs was 42.7%, and that of serious ADRs was 1.3%. The most common reported ADRs of OXA in this study were gastrointestinal disorders (25.7%), blood disorders (21.1%), and peripheral nervous system disorders (8.0%), which were consistent with the results of other studies (14, 16, 20, 21).
Nausea, vomiting, and diarrhea were the most common gastrointestinal side effects of OXA, with a median time of onset of 1.6 d, and most patients recovered or improved quickly. Nausea and vomiting are usually mild to moderate and are readily controlled with the prophylactic administration of standard antiemetics such as dexamethasone or 5-HT3 receptor antagonists (22). Grade 1 and 2 diarrhea has been reported in OXA-treated patients with advanced colorectal cancer. The incidence of this ADR is usually higher with a protracted continuous infusion or with very high infusion doses (23). In practice, prophylaxis is not required, and the OXA dose should only be reduced in subsequent cycles if diarrhea becomes severe.
In general, hematological side effects caused by OXA include leukopenia, neutropenia, thrombocytopenia, and anemia (24). In our study, the prevalence of OXA-induced myelosuppression was 21.1%. Leukopenia (17.3%), neutropenia (12.1%), anemia (7.0%), and thrombocytopenia (5.6%) were also common hematological side effects of OXA treatment. Therefore, any patient treated with OXA should closely monitor their WBC count, platelet levels, hemoglobin levels, and absolute neutrophil count (ANC). Moreover, this study showed that thrombocytopenia was a common serious ADR, with an incidence of 0.2%, and the median time of onset from OXA treatment to this event was 4.7 d. Studies have demonstrated that thrombocytopenia was a prominent side effect of OXA-related myelosuppression (25, 26). Although thrombocytopenia of grades 3 and 4 was noted in only 3%-4% of patients exposed to OXA, its toxicity tends to increase with repeated exposures and may limit the benefits of OXA (25, 27). Recently, mechanisms of OXA-induced thrombocytopenia have emerged, including bone marrow suppression, immune-dependent mechanism, and splenic sequestration of platelets due to portal hypertension related to liver sinusoidal injury (11). However, in our study, it was difficult to determine the specific mechanism involved in OXA-induced thrombocytopenia; thus, this condition needs further study. Therefore, medical practitioners should be vigilant about thrombocytopenia caused by splenomegaly after long-term exposure to OXA and be aware of allergy-induced acute thrombocytopenia (28).
A major dose-limiting side-effect of OXA treatment is its peripheral neurotoxicity. Our study showed that OXA-induced peripheral neurotoxicity (OIPN) was reported in 266 patients (8.0%), mainly including paresthesias or dysesthesias of the hands and feet. Most reported OIPN cases were mild, and 96.6% of patients recovered or improved, with the median time to onset of 1 d. OXA induces two clinically distinct forms of peripheral neuropathy. The first is acute OIPN, which is transient, appearing only during or shortly after infusion of OXA, and can be triggered by cold stimulation. The other form, chronic OIPN, is associated with a cumulative dose of OXA and appears after administration of OXA at a total dose of 540-850 mg/m2 (12). Acute OIPN consists mainly of sensory symptoms in the form of paresthesias or dysesthesias in the distal or perioral regions and are related to the dosage and infusion rate of OXA. These symptoms are generally mild, short-lived, and completely reversible within a few hours or days (29). Thus, oncologists can prolong the duration of OXA infusion up to 6 h and request that their patients avoid cold liquids for several days after OXA therapy, which may prevent the development of acute OIPN.
While not as common as the previously discussed ADRs, hepatotoxicity also represents a serious ADR that warrants further investigation. There is evidence that the most common type of hepatotoxicity associated with OXA administration is hepatic sinusoidal injury. It is histologically characterized by sinusoidal dilatation, hepatocyte necrosis, and obliteration of hepatic venules due to sinusoidal endothelial cell damage (30–32). OXA-induced hepatic sinusoidal obstruction syndrome (HSOS) has been demonstrated in up to 77% of colorectal cancer patients with liver metastasis following OXA-based chemotherapy (33). Studies have reported that preoperative OXA was associated with HSOS and that this increased postoperative morbidity after partial hepatectomy with colorectal cancer liver metastasisn (34, 35). OXA-induced HSOS frequently presents with ascites, jaundice, right upper quadrant pain, splenomegaly with subsequent thrombocytopenia, and portal hypertension. Systemic elevation of liver enzymes is often not significant, especially in the early stage (30). We found that the incidence of abnormal hepatic function was only 5.1%; however, our study only measured hepatic biochemical parameters such as the levels of transaminases, aspartate aminotransferase, and bilirubin. Without the results of histopathological examinations, it is difficult to give a definitive diagnosis of HSOS, leading to an underestimation of the hepatotoxicity of OXA. Therefore, OXA-induced HSOS should be specially studied and evaluated in patients with metastatic colorectal cancer who received OXA-based neoadjuvant chemotherapy after hepatectomy.
OXA-related HSRs are another major problem associated with the extensive use of the drug, the occurrence of which may lead to therapy delay, discontinuation of treatment, and even death (36). The reported frequency of HSR in patients undergoing OXA-based chemotherapy ranges from less than 2% to 25% (37–42), while the prevalence of severe HSRs is 0.5-2% (38, 43). Our study reported OXA-related HSRs in 118 out of 3687 Chinese patients, giving an incidence rate of 3.2% and a severe hypersensitivity rate of 0.5%. The clinical manifestations of HSRs involve multiple systems, such as cutaneous, digestive, neurologic, and respiratory systems. This study found that the most common events were cutaneous symptoms with severities of grades 1 and 2, which corroborates the findings of our previous study (44). Events of grade 3 and above are less common, but six patients developed life-threatening cases of severe anaphylactic shock. These results align with other studies that reported OXA-induced HSRs being potentially severe and life-threatening (45). In this study, however, patients who developed HSRs were managed with the corresponding treatments, and eventually, all patients recovered or improved. This suggests that OXA-induced HSRs are controllable through close monitoring, comprehensive evaluation, and the provision of timely and effective treatment. Hence, medical staff should pay close attention to the signs of potential HSRs and inform patients to closely monitor any symptoms that may arise.
Previous studies have noted that OXA-induced hypersensitivity reactions usually occur within the first 30 min of infusion (46, 47). In our study, the median time to onset of HSRs was 20 min, but the time to onset of different grades of HSRs varied. The median time to onset of grade 1 HSRs was 60 min, while grade 2 to 4 events occurred mainly within the first 20 min of OXA infusion. Our previous retrospective analysis also demonstrated that HSRs caused by OXA might occur at any time within a cycle of therapy but were mainly observed at the first 20 min of OXA infusion (44). Thus, patients should be closely monitored for HSRs, but especially within the first 20 min after the start of an OXA infusion.
However, this study can still be further expanded. First, this was a non-interventional observational study that aimed to observe the safety profile of OXA with no control group, and there was a short observation period of only 6 months and lack of follow-up. Hence, the OXA efficacy in these patients was unknown, and some delayed ADRs such as chronic OIPN may not be immediately reported and were underestimated. Second, patients treated with OXA was the only inclusion criteria implemented, and a comparison of safety accounting for the effects of different diseases and treatment regimens was not performed. In addition, although the researchers have identified OXA-related ADRs, the effects of other drugs cannot be completely excluded because OXA is often used in combination with other chemotherapeutic drugs. Further investigation is also required to identify the risk factors that affect the incidence of OXA-induced ADRs to provide a reference for the rational application of OXA.
Conclusions
In conclusion, this large post-marketing surveillance study conducted in more than 3000 Chinese patients preliminarily explored the incidence, characteristics, cycle, occurrence time and outcome of OXA induced ADRs. Overall, OXA induced adverse reactions were very prevalent. Our results showed that gastrointestinal toxicity, hematotoxicity, peripheral neurotoxicity, HSRs and abnormal liver function were the main common ADRs of OXA, in which the latter two had unique characteristics, need more attention, and warrant close monitoring during OXA infusion. Although further studies are still required, this study provides valuable reference for the rational use of OXA and has great guidance for the management of OXA-induced ADRs in routine clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (No.TJIRB20160504). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZY and RH, first authors, contributed to the data analysis and writing of manuscript. LZ and XY contributed to organization, management, and supervision of the project. XW and XS contributed to data collection, data curation, analysis, and validation. WL and ML contributed to contributed to the editing and submission of the article. CZ and DL, the corresponding authors, involved in conception of the project, performing data analysis, review of manuscript and provision of feedback and comments to first author. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No: 7187040708), Hubei Center for Adverse drug reaction Monitoring (No: 20160422), Funding for research-oriented clinician plan of Tongji Medical College, Huazhong University of Science and Technology (No: 5001540076); Clinical toxicology foundation of Chinese Society of Toxicology (CST2020CT107).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all investigators and collaborators for the contributions to the conduct of this study, and we also thank Hubei Center for adverse drug reaction monitoring for assistance in the development of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.757196/full#supplementary-material
References
1. Grothey A, Goldberg RM. A Review of Oxaliplatin and its Clinical Use in Colorectal Cancer. Expert Opin Pharmacother (2004) 5:2159–70. doi: 10.1517/14656566.5.10.2159
2. Huang J, Zhao Y, Xu Y, Zhu Y, Huang J, Liu Y, et al. Comparative Effectiveness and Safety Between Oxaliplatin-Based and Cisplatin-Based Therapy in Advanced Gastric Cancer: A Meta-Analysis of Randomized Controlled Trials. Oncotarget (2016) 7(23):34824–31. doi: 10.18632/oncotarget.9189
3. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
4. Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw (2019) 17(5.5):603–5. doi: 10.6004/jnccn.2019.5007
5. Taylor SE, Beck TL, Krivak TC, Zorn KK, Kelley JL, Edwards RP. Oxaliplatin Salvage for Recurrent Ovarian Cancer: A Single Institution's Experience in Patient Populations With Platinum Resistant Disease or a History of Platinum Hypersensitivity[J]. Gynecol Oncol (2014) 134(1):68–72. doi: 10.1016/j.ygyno.2014.04.039
6. André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, Fluorouracil and Leukovorin as Adjuvant Treatment for Colon Cancer. N Engl J Med (2004) 350(23):2343–51. doi: 10.1056/NEJMoa032709
7. Nielsen DL, Palshof JA, Larsen FO, Jensen BV, Pfeiffer P. A Systematic Review of Salvage Therapy to Patients With Metastatic Colorectal Cancer Previously Treated With Fuorouracil, Oxaliplatin and Irinotecan +/- Targeted Therapy. Cancer Treat Rev (2014) 40(6):701–15. doi: 10.1016/j.ctrv.2014.02.006
8. Volovat SR, Volovat R, Negru SM, Danciu M, Scripcariu V. The Efficacy and Safety of Hepatic Arterial Infusion of Oxaliplatin Plus Intravenous Irinotecan, Leucovorin and Fuorouracil in Colorectal Cancer With Inoperable Hepatic Metastasis. J Chemother (2016) 28(3):235–41. doi: 10.1179/1973947815Y.0000000042
9. Ohta H, Hayashi T, Murai S, Shiouchi H, Ando Y, Kumazawa S, et al. Comparison Between Hypersensitivity Reactions to Cycles of Modified FOLFOX6 and XELOX Therapies in Patients With Colorectal Cancer. Cancer Chemoth Pharm (2017) 79(5):1021–9. doi: 10.1007/s00280-017-3294-9
10. Abu-Sbeih H, Mallepally N, Goldstein R, Chen E, Tang T, Dike UK, et al. Gastrointestinal Toxic Effects in Patients With Cancer Receiving Platinum-Based Therapy. J Cancer (2020) 11(11):3144–50. doi: 10.7150/jca.37777
11. Erdem GU, Dogan M, Demirci NS, Zengin N. Oxaliplatin-Induced Acute Thrombocytopenia. J Cancer Res Ther (2016) 12(2):509–14. doi: 10.4103/0973-1482.154056
12. Kang L, Tian Y, Xu S, Chen H. Oxaliplatin−induced Peripheral Neuropathy: Clinical Features, Mechanisms, Prevention and Treatment. J Neurol (2021) 268(9):3269–82. doi: 10.1007/s00415-020-09942-w
13. Aroldi F, Prochilo T, Bertocchi P, Zaniboni A. Oxaliplatin-Induced Hypersensitivity Reaction: Underlying Mechanisms and Management. J Chemother (2015) 27(2):63–6. doi: 10.1179/1973947814Y.0000000204
14. Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved Overall Survival With Oxaliplatin, Fuorouracil, and Leucovorin as Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J Clin Oncol (2009) 27(19):3109–16. doi: 10.1200/JCO.2008.20.6771
15. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
16. Hoff PM, Saad ED, Costa F, Coutinho AK, Caponero R, Prolla G, et al. Literature Review and Practical Aspects on the Management of Oxaliplatin-Associated Toxicity. Clin Colorectal Cancer (2012) 11(2):93–100. doi: 10.1016/j.clcc.2011.10.004
17. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
18. Messersmith WA. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J Natl Compr Cancer Netw (2019) 17(5.5):599–601. doi: 10.6004/jnccn.2019.5014
19. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2016) 14(10):1286–312. doi: 10.6004/jnccn.2016.0137
20. Cassidy J, Misset JL. Oxaliplatin-Related Side Effects:Characteristics and Management. Semin Oncol (2002) 29(5 Suppl 15):11–20. doi: 10.1053/sonc.2002.35524
21. Oun R, Moussa YE, Wheate NJ. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans (2018) 47(19):6645–53. doi: 10.1039/c8dt00838h
22. Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw (2017) 15(7):883–93. doi: 10.6004/jnccn.2017.0117
23. Xu N, Fang WJ, Zhang XC, Yu LF, Bao HY, Shi GM, et al. A Phase II Trial of Oxaliplatin, Folinic Acid, and 5-Fluorouracil (FOLFOX4) as First-Line Chemotherapy in Advanced Colorectal Cancer: A China Single-Center Experience. Cancer Investig (2007) 25(7):599–605. doi: 10.1080/07357900701470739
24. Kamimura K, Matsumoto Y, Zhou Q, Moriyama M, Saijo Y. Myelosuppression by Chemotherapy in Obese Patients With Gynecological Cancers. Cancer Chemother Pharmacol (2016) 78(3):633–41. doi: 10.1007/s00280-016-3119-2
25. Jardim DL, Rodrigues CA, Novis YA, Rocha VG, Hoff PM. Oxaliplatin-Related Thrombocytopenia. Ann Oncol (2012) 23(8):1937–42. doi: 10.1093/annonc/mds074
26. Phull P, Quillen K, Hartshorn KL. Acute Oxaliplatin-Induced Hemolytic Anemia, Thrombocytopenia, and Renal Failure: Case Report and a Literature Review. Clin Colorectal Canc (2016) S1533-0028(16):30259-6. doi: 10.1016/j.clcc.2016.11.005
27. Polyzos A, Tsavaris N, Gogas H, Souglakos J, Vambakas L, Vardakas N, et al. Clinical Features of Hypersensitivity Reactions to Oxaliplatin: A 10-Year Experience. Oncology (2009) 76(1):36–41. doi: 10.1159/000178163
28. Bautista MA, Stevens WT, Chen CS, Curtis BR, Aster RH, Hsueh CT. Hypersensitivity Reaction and Acute Immunemediated Thrombocytopenia From Oxaliplatin:Two Case Reports and a Review of the Literature. J Hematol Oncol (2010) 3:12. doi: 10.1186/1756-8722-3-12
29. Gebremedhn EG, Shortland PJ, Mahns DA. The Incidence of Acute Oxaliplatin-Induced Neuropathy and its Impact on Treatment in the First Cycle: A Systematic Review. BMC Cancer (2018) 18(1):410. doi: 10.1186/s12885-018-4185-0
30. Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, et al. Sinusoidal Obstruction Syndrome and Nodular Regenerative Hyperplasia are Frequent Oxaliplatin-Associated Liver Lesions and Partially Prevented by Bevacizumab in Patients With Hepatic Colorectal Metastasis. Histopathology (2010) 56(4):430–9. doi: 10.1111/j.1365-2559.2010.03511.x
31. Lu QY, Zhao AL, Deng W, Li ZW, Shen L. Hepatic Histopathology and Postoperative Outcome After Preoperative Chemotherapy for Chinese Patients With Colorectal Liver Metastases. World J Gastrointest Surg (2013) 5(3):30–6. doi: 10.4240/wjgs.v5.i3.30
32. Liu F, Cao X, Ye J, Pan XL, Kan XF, Song YH. Oxaliplatin-Induced Hepatic Sinusoidal Obstruction Syndrome in a Patient With Gastric Cancer: A Case Report. Mol Clin Oncol (2018) 8(3):453–6. doi: 10.3892/mco.2017.1540
33. van Mierlo KM, Zhao J, Kleijnen J, Rensen SS, Schaap FG, Dejong CH, et al. The Influence of Chemotherapy-Associated Sinusoidal Dilatation on Short-Term Outcome After Partial Hepatectomy for Colorectal Liver Metastases: A Systematic Review With Meta-Analysis. Surg Oncol (2016) 25(3):298–307. doi: 10.1016/j.suronc.2016.05.030
34. Zhao J, van Mierlo KMC, Gómez-Ramírez J, Kim H, Pilgrim CHC, Pessaux P, et al. Systematic Review of the Influence of Chemotherapy-Associated Liver Injury on Outcome After Partial Hepatectomy for Colorectal Liver Metastases. Br J Surg (2017) 104(8):990–1002. doi: 10.1002/bjs.10572
35. Hisaka T, Ishikawa H, Sakai H, Kawahara R, Goto Y, Nomura Y, et al. Sinusoidal Obstruction Syndrome and Postoperative Complications Resulting From Preoperative Chemotherapy for Colorectal Cancer Liver Metastasis. Anticancer Res (2019) 39(8):4549–54. doi: 10.21873/anticanres.13632
36. Bano N, Najam R, Qazi F, Mateen A. Clinical Features of Oxaliplatin Induced Hypersensitivity Reactions and Therapeutic Approaches. Asian Pac J Cancer Prev (2016) 17(4):1637–41. doi: 10.7314/apjcp.2016.17.4.1637
37. Brandi G, Pantaleo MA, Galli C, Falcone A, Antonuzzo A, Mordenti P, et al. Hypersensitivity Reactions Related to Oxaliplatin (OHP). Br J Cancer (2003) 89(3):477–81. doi: 10.1038/sj.bjc.6601155
38. Shibata Y, Ariyama H, Baba E, Takii Y, Esaki T, Mitsugi K, et al. Oxaliplatin-Induced Allergic Reaction in Patients With Colorectal Cancer in Japan. Int J Clin Oncol (2009) 14(5):397–401. doi: 10.1007/s10147-009-0883-6
39. Parel M, Ranchon F, Nosbaum A, You B, Vantard N, Schwiertz V, et al. Hypersensitivity to Oxaliplatin: Clinical Features and Risk Factors. BMC Pharmacol Toxicol (2014) 15:1. doi: 10.1186/2050-6511-15-1
40. Okayama T, Ishikawa T, Sugatani K, Yoshida N, Kokura S, Matsuda K, et al. Hypersensitivity Reactions to Oxaliplatin: Identifying the Risk Factors and Judging the Efficacy of a Desensitization Protocol. Clin Ther (2015) 37(6):1259–69. doi: 10.1016/j.clinthera.2015.03.012
41. Yamauchi H, Goto T, Takayoshi K, Sagara K, Uoi M, Kawanabe C, et al. A Retrospective Analysis of the Risk Factors for Allergic Reactions Induced by the Administration of Oxaliplatin. Eur J Cancer Care (2015) 24(1):111–6. doi: 10.1111/ecc.12156
42. Shao YY, Hu FC, Liang JT, Chiu WT, Cheng AL, Yang CH. Characteristics and Risk Factors of Oxaliplatin-Related Hypersensitivity Reactions. J Formos Med Assoc (2010) 109(5):362–8. doi: 10.1016/S0929-6646(10)60064-2
43. Kim BH, Bradley T, Tai J, Budman DR. Hypersensitivity to Oxaliplatin: An Investigation of Incidence and Risk Factors, and Literature Review. Oncology (2009) 76(4):231–8. doi: 10.1159/000205263
44. Li M, Jiang C, Yang JY, Yu ZQ, Li W, Zhao L, et al. Clinical Features of Oxaliplatin-Related Hypersensitivity Reactions in Chinese Patients: A Retrospective Multicenter Analysis. Curr Med Sci (2021) 41(4):827–31. doi: 10.1007/s11596-021-2387-1
45. Wang JH, King TM, Chang MC, Hsu CW. Oxaliplatin-Induced Severe Anaphylactic Reactions in Metastatic Colorectal Cancer:Case Series Analysis. World J Gastroenterol (2012) 18(38):5427–33. doi: 10.3748/wjg.v18.i38.5427
46. Siu SW, Chan RT, Au GK. Hypersensitivity Reactions to Oxaliplatin: Experience in a Single Institute. Ann Oncol (2006) 17(2):259–61. doi: 10.1093/annonc/mdj042
Keywords: oxaliplatin, cancer, post-marketing surveillance, safety, Chinese
Citation: Yu Z, Huang R, Zhao L, Wang X, Shangguan X, Li W, Li M, Yin X, Zhang C and Liu D (2021) Safety Profile of Oxaliplatin in 3,687 Patients With Cancer in China: A Post-Marketing Surveillance Study. Front. Oncol. 11:757196. doi: 10.3389/fonc.2021.757196
Received: 11 August 2021; Accepted: 06 October 2021;
Published: 21 October 2021.
Edited by:
Miao Yan, Central South University, ChinaReviewed by:
Xianglin Du, University of Texas Health Science Center at Houston, United StatesBiswajit Dubashi, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), India
Copyright © 2021 Yu, Huang, Zhao, Wang, Shangguan, Li, Li, Yin, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengliang Zhang, Y2x6aGFuZ0B0amgudGptdS5lZHUuY24=; Dong Liu, bGQyMDY5QG91dGxvb2suY29t
†These authors have contributed equally to this work and share first authorship
 Zaoqin Yu1†
Zaoqin Yu1† Chengliang Zhang
Chengliang Zhang Dong Liu
Dong Liu