- 1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Urology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 3Department of Pathology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Immune checkpoint inhibitors (ICIs) have been proven to be beneficial in multiple advanced malignancies. However, the widespread use of ICIs also occurred with various immune-related adverse events (irAEs). Here, we first report a case of sintilimab-related cystitis/ureteritis. A 53-year-old man with driver gene-negative pulmonary adenocarcinoma (cT1cN3M1c, Stage IVB) was being treated with sintilimab in combination of paclitaxel-albumin and bevacizumab as second-line treatment. He was hospitalized for haematuria, pollakiuria, painful micturition and low back pain after three courses. Urinalysis showed red blood cells (RBCs) and white blood cells (WBCs) were obviously increased, and serum creatinine (sCr) level was also significantly elevated. Urine culture and cytology were both negative, and cystoscopy revealed diffused redness of bladder mucosa. Urinary ultrasonography showed mild hydronephrosis and dilated ureter. The patient was diagnosed as immunotherapy-related cystitis/ureteritis after a multidisciplinary team (MDT) meeting. Once the diagnosis was made, corticosteroid therapy was given, which rapidly resolved the patient’s symptoms and signs. Computer tomography angiography (CTA) and CT urography (CTU) was conducted after sCr level was back to normal and demonstrated ureter dilation and hydroureter. Once symptoms relieved, bladder biopsy was performed and confirmed the bladder inflammation. The patient was subsequently switched to maintenance dose of methylprednisolone and tapered gradually. Since sintilimab has been used in advanced malignancies, we first reported a rare case of sintilimab-induced cystitis/ureteritis and summarized sintilimab-related adverse events to improve the assessment and management of irAEs.
Introduction
Cytotoxic T-lymphocytes-associated protein 4 (CTLA-4) and programmed cell death-1 (PD-1) are receptors that negatively regulate immune responses by inhibiting T-cell activation and proliferation (1). Immune checkpoint inhibitors (ICIs), which improve T-cell cytotoxicity against cancer via CTLA-4, PD-1 or PD-L1 blockade, are known to facilitate immunoactivity against malignancies by disinhibiting the anti-tumour responses of lymphocytes (2). As a member of ICIs, sintilimab (Tyvyt®) is a fully humanized immunoglobulin G4 monoclonal antibody that binding to PD-1, which in turn selectively blocks the interaction between PD-1 and its two known ligands, PD-L1 and PD-L2 (3). Sintilimab has shown efficacy in the treatment of multiple malignancies including lymphoma, lung cancer, hepatocellular carcinoma, and gastric cancer (4). Despite favourable outcomes, it could also lead to a wide spectrum of immune-related adverse events (irAEs) (5, 6). However, sintilimab-associated adverse events affecting the urinary tract have rarely been reported and discussed (5). Here, we first report a case of sintilimab-induced cystitis/ureteritis, and summarize sintilimab-related adverse events to achieve a better management and surveillance of irAEs.
Case Presentation
A 53-year-old man who had no smoking history was admitted to the first Affiliated Hospital of Zhejiang University (Hangzhou, China) with a mass in the posterior segment of the left lower lobe and complaint of upper right abdominal pain while without any respiratory symptoms. On physical examination, superficial right upper quadrant tenderness was noted. The patient was diagnosed with driver gene-negative pulmonary adenocarcinoma (cT1cN3M1c, Stage IVB) according to the eighth edition AJCC staging system. The PD-L1 tumour proportion score (TPS) was 50% using PD-L1 IHC 22C3 pharmDx (Dako North America, Inc., Carpinteria, CA, USA). The patient had a history of laparoscopic cholecystectomy due to gallstone and history of cefalexin allergy, and his family history of genetic diseases was negative. His performance score was 1. Initially, the patient received pemetrexed (Hansoh Pharmaceutical, Co., Ltd, Lianyungang, China) plus carboplatin (Corden Pharma Latina SpA, Sermoneta, Italy) for four cycles, followed by two cycles of pemetrexed and bevacizumab (Qilu Pharmaceutical Co., Ltd, Jinan, China). During first-line treatment, the best effect was assessed as partial response (PR), and the progression free survival (PFS) was about six months. Intravenous ibandronic acid (Institute of Pharmacy, Hebei Medical University, Shijiazhuang, China) was given to reduce metastatic bone pain throughout the first-line regular chemotherapy. Whole brain radiotherapy was conducted for thirteen times (3900 cGy in total) after the first cycle of first-line treatment. However, after six cycles of first-line chemotherapy, chest CT assessment of the patient demonstrated progressive disease according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) (7). The re-assessed staging of the patient was classified as rT1cN3M1c, stage IVB. The patient complained of alleviated upper right abdominal pain without other complaints, and physical examinations demonstrated mild right upper quadrant tenderness with no other alterations. Due to disease progression, he was administered sintilimab (200mg, Q21d, Innovent Biologics, Suzhou, China) in combination of paclitaxel-albumin (Hengrui, Pharmaceutical Co., Ltd, Lianyungang, China) and bevacizumab as second-line therapy. After two cycles of second-line treatment, response to treatment was assessed as PR (Figure 1). Urinalysis results were normal before sintilimab administration. Symptoms with haematuria, pollakiuria and painful micturition developed on the fifth day after the third course of second-line therapy, while no fever was observed. Urinalysis showed red blood cells (RBCs) of 3889.7/µl and white blood cells (WBCs) of 2133.5/µl. Urine culture and serum creatine (sCr) were normal. Urinary bladder ultrasound observed a thickened bladder wall and benign prostatic hyperplasia. Levofloxacin (Daiichi Sankyo (China) Holding Co., Ltd, Beijing, China) was administrated for seven days, which improved symptoms of bloody urine and urinary irritation. These symptoms were originally considered to be the adverse effect of bevacizumab; hence, bevacizumab was immediately discontinued. However, after the fourth cycle of paclitaxel-albumin and sintilimab treatment, similar urinary symptoms and low back pain occurred without fever. Urinalysis showed increased RBCs and WBCs. Urine culture and cytology were both negative. Blood tests showed WBCs of 10100/µl (neutrophils 66.5%, eosinophils 5.5%), C-reactive protein 52.1 mg/L, lactate dehydrogenase 166 U/L, and sCr 299 µmol/L. Urinary ultrasonography showed hydronephrosis and dilated ureter. Cystoscopy revealed diffuse redness of bladder mucosa. A multidisciplinary team (MDT) meeting was organized, and the diagnosis of sintilimab-induced cystitis/ureteritis (Grade 3) was made after exclusion the evidence of urinary infection and metastasis. Methylprednisolone (80mg, 1mg/kg/d, Pfizer Manufacturing Belgium NV, Puurs, Belgium) therapy was performed, and urinary symptoms of the patient was obviously alleviated after two days. The laboratory values were also decreased during corticosteroids treatment (Figure 2A). When sCr level was back to normal, computer tomography angiography (CTA)/CT urography (CTU) was conducted and demonstrated ureter dilation and hydroureter. Once urinary symptoms were alleviated, bladder biopsy was performed and confirmed the bladder lymphocyte-dominant inflammation and interstitial tissue hyperplasia (Figure 3). The patient was then switched to maintenance dose of methylprednisolone and tapered gradually, and the urinary symptoms were completely resolved after seventeen days of corticosteroid treatment. The whole steroid treatment was eight weeks. The timeline of treatment course was summarized in Figure 2B.
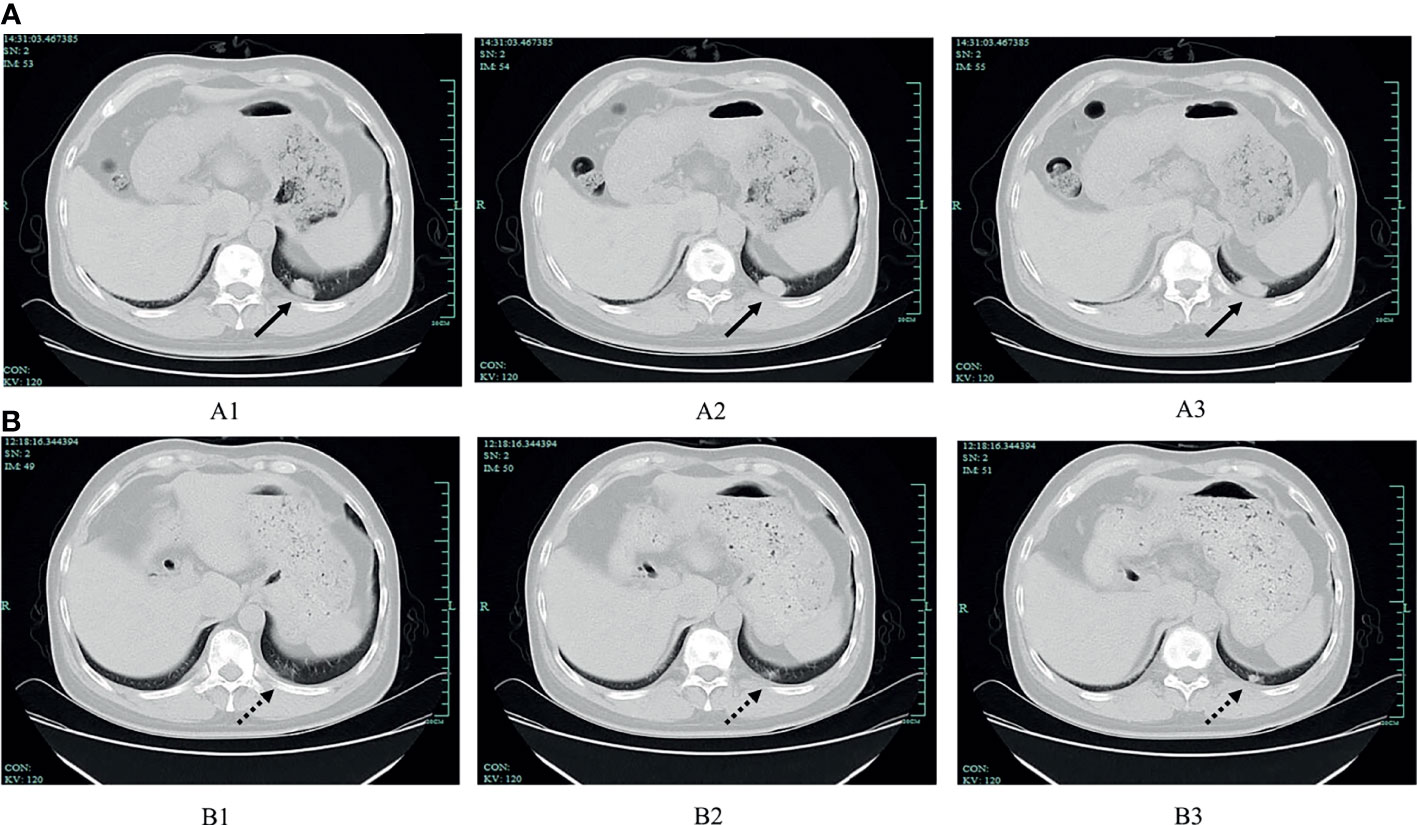
Figure 1 The comparison of chest CT before and after sintilimab treatment. (A) Chest CT scan before sintilimab treatment (July 2020). Images of A1, A2, and A3 consecutively showing the tumour at the posterior segment of the left lower lobe, and the tumour size was 2.1×1.4 cm, 2.4×1.5 cm, 2.7×1.5 cm, respectively (solid lines indicate the tumour). (B) Chest CT scan after sintilimab treatment (September 2020). Images of B1, B2 and B3 showing the tumour at the same location when compared with A1, A2, and A3, separately, and the tumour size was 0.4×0.4 cm, 0.5×0.4 cm, 1.0×0.7 cm, respectively (dotted lines indicate the tumour).
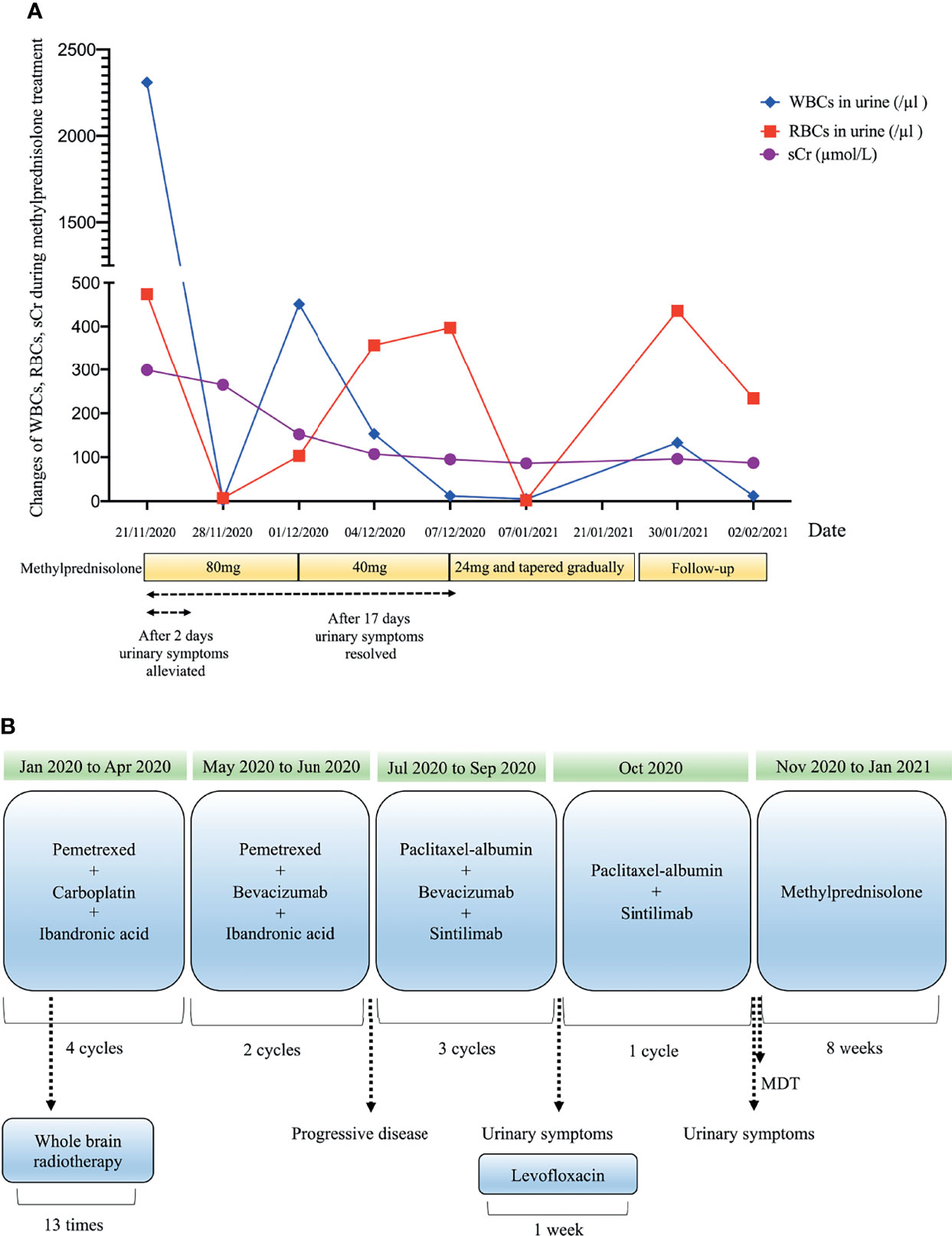
Figure 2 Clinical course over methylprednisolone therapy (A) and timeline of treatment course (B). Drug dosage: bevacizumab, 7.5mg/kg; carboplatin, (area under the concentration-time curve) 4-5 mg/mL/min; ibandronic acid, 4mg; levofloxacin, 500mg; methylprednisolone (initial), 1mg/kg/d; paclitaxel-albumin, 260mg/m2; pemetrexed, 500mg/m2; sintilimab, 200mg.
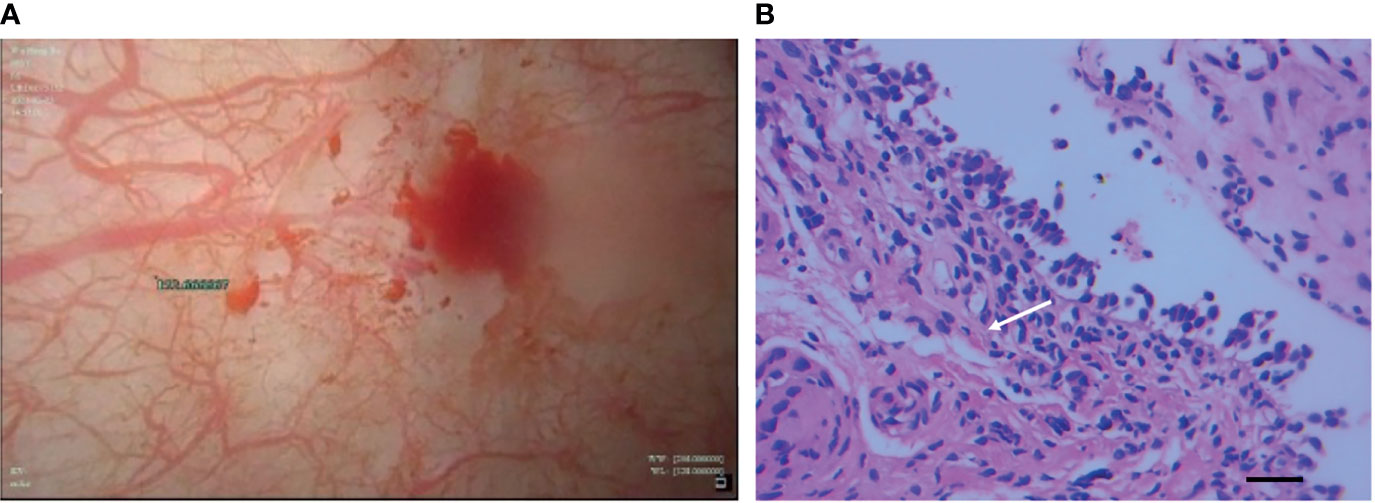
Figure 3 Cystoscopy of bladder (A) revealed diffuse redness of bladder mucosa and histopathological findings (B) of bladder biopsy showed lymphocyte-dominant bladder inflammation and interstitial tissue hyperplasia (white arrow), which was shown in the increased number of fibrocytes (H&E staining, magnification ×400, bar =25μm).
Discussion and Review of the Literature
The wide use of ICIs shows significant benefits for patients with advanced malignancies, however, these agents could induce irAEs with nonspecific clinical symptoms and biomarkers (8). Although irAEs can affect any organ system, irAEs related to the urinary system have been rarely reported and discussed (9–13). Here, we first report an autoimmune cystitis/ureteritis caused by sintilimab.
IrAEs have been suggested to occur at any time, but usually develop within the first few weeks to months after administration initiation (8). In previous case reports of nivolumab-related cystitis, the time from initiation of therapy to development of cystitis ranged from two to twelve months (9–11, 13). In a case report of pembrolizumab-induced cystitis, urinary symptoms occurred after six cycles of treatment (12). In our report, the patient occurred with urinary symptoms after three cycles of sintilimab administration. Hence, it is necessary to aware the possibility of autoimmune cystitis for any newly onset urinary symptoms during ICIs therapy. The patient was diagnosed as autoimmune cystitis/ureteritis based on several reasons. First, the occurrence of urinary symptoms developed during sintilimab treatment, which raised the suspicion of irAEs. Second, there was no evidence of urinary infection and metastasis. Furthermore, the administration of corticosteroid obviously alleviated clinical symptoms and laboratory values of the patient.
PD-1 signalling permits self-tolerance in normal tissues, while tumour cells may hijack this signalling to evade host immune responses and establish a beneficial microenvironment for tumour growth and development (14, 15). Thus, PD-1 blockade could harm protective autoimmunity and lead to different types of irAEs via activated lymphocytes (15). The urinary bladder is immune-privileged because a number of chemicals, toxins and antigens constantly exposed to this site, therefore, it is essential to maintain the integrity and tolerance of the urothelium (16). The pathological features of a nivolumab-induced cystitis have shown epithelial desquamation and interstitial oedema (9). Although the precise mechanisms of ICIs-related cystitis/ureteritis are not fully elucidated, several studies have explored the potential mechanisms of immunotherapy-associated cystis. It has been demonstrated that lymphocytic infiltration, characterized by high CD8 and/or TIA-1 expression, was obviously increased in the urothelium of pembrolizumab-induced cystitis (12). This may indicate that some unknown antigens in the urothelium could be targeted by CD8+ and/or TIA-1+ lymphocytes, which consequently destroy the integrity of urothelial epithelum (12). PD-L1 knockout alone is not sufficient but is required to induce autoimmune cystitis in an animal model, indicating other mechanisms may get involved in the development of immune-cystitis (17). As to the PD-1 blockade will prevent the interaction of PD-1 and its known ligands PD-L1 as well as PD-L2 (3). PD-L2 is constitutively expressed by normal urothelium in healthy individuals and those with urothelial bladder cancer, suggesting a potential role in maintaining tolerance (16), whether PD-1/PD-L2 also participates in the autoimmune course is worthy to be explored.
In published clinical trials, sintilimab-related adverse events have been briefly documented (5). Here, we performed a detailed review of sintilimab-induced adverse events based on available case reports. For the cases reviewed, the demographic features, clinical manifestations and treatment of irAEs were summarized (Table 1). From these case reports, sintilimab has been shown to induce various immune-associated disorders, including autoimmune diabetes (18, 26, 30), pneumonitis (27, 28), pulmonary fibrosis (20), cytokine release syndrome and multiple organ failure (20, 21), myocarditis (24, 29), hypothyroid myopathy (19), myasthenia gravis and myasthenia crisis (22), paraneoplastic neurological syndrome and enteric neuropathy (23), psoriasis exacerbation (25). Some literatures previously indicated that patients with irAEs usually showed a markedly better efficacy than those without irAEs, known as higher overall response rate, longer PFS and better overall survival (31, 32). However, the relationship between new-onset autoimmune cystitis/ureteritis and the therapeutic response of PD-1 inhibitors is currently unknown.
With regard to treatment of irAEs based on Common Terminology Criteria for Adverse Events (CTCAE), irAEs of Grade 2 or higher need to add temporary immunosuppressive agents such as oral glucocorticoids or additional immunomodulators (33). When steroids get involved in ICIs treatment, there is concern that its immunosuppressive effect could jeopardize the therapeutic effect of immunotherapy. However, the effects of steroids on ICIs are still controversial. In a systematic review, Garant et al. (34) demonstrated that the concomitant administration of steroids and ICIs may not result in worse outcomes and Kapoor et al. (35) also showed that the usage of steroid did not make a difference of the overall survival in patients with ICIs treatment. On the other hand, Arbour et al. (36) suggested that the usage of prednisone of more than 10 mg in advance of ICIs could negatively regulate the clinical effect of immunotherapy. The discrepancies between these studies may be ascribed to confounding variables in these studies, and well-designed prospective studies could be helpful to answer this question in the future. Once irAEs are well-controlled, it is reported that ICIs can be administrated again unless disease progression or other uncontrolled drug toxicities. However, sintilimab treatment was discontinued in this patient because of disease progression.
With the widespread use of ICIs in various cancer types, irAEs have raised lots of concerns in clinical practice. Early recognition and intervention of irAEs are crucial and recommended. Due to the complex mechanism and unspecific feature of irAEs, it always goes beyond the field of oncology and demands a collaborative and MDT approach to achieve favourable outcomes (37).
Conclusion
Here, we report a case of nonbacterial cystitis/ureteritis associated with sintilimab and review sintilimab-related adverse events based on published case reports. As sintilimab alone or combined therapies are currently used in various malignancies, it is critical to be aware of its potential irAEs and to achieve adequate assessment and management.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Center for Ethics in the First Affiliated Hospital of Zhejiang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LT designed the case report and drafted the manuscript. YY analyzed the patient data and revised the manuscript. XT made significant contributions to the collection of patient data. ZL provided significant contributions to the interpretation of CTA/CTU imaging. QY contributed to the analysis of pathological data. JZ and ZP provided significant contributions to the analysis of the patient data and designed the case report. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CTA, computer tomography angiography; CTCAE, common terminology criteria for adverse events; CTLA-4, cytotoxic T-lymphocytes-associated protein 4; CTU, computer tomography urography; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events; MDT, multi-disciplinary team; PD-1, programmed cell death-1; PFS, progression free survival; PR, partial response; RBCs, red blood cells; TPS, tumour proportion score; WBCs, white blood cells; sCr, serum creatinine.
References
1. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol Cell Biol (2005) 25(21):9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005
2. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
3. Hoy SM. Sintilimab: First Global Approval. Drugs (2019) 79(3):341–6. doi: 10.1007/s40265-019-1066-z
4. Liu X, Yi Y. Recent Updates on Sintilimab in Solid Tumor Immunotherapy. Biomark Res (2020) 8(1):1–9. doi: 10.1186/s40364-020-00250-z
5. Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, et al. Safety and Activity of Sintilimab in Patients With Relapsed or Refractory Classical Hodgkin Lymphoma (ORIENT-1): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Haematol (2019) 6(1):e12–9. doi: 10.1016/S2352-3026(18)30192-3
6. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: A Randomized, Double-Blind, Phase 3 Study (Oncology Program by InnovENT Anti-PD-1-11). J Thorac Oncol (2020) 15(10):1636–46. doi: 10.1016/j.jtho.2020.07.014
7. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2008) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
8. Postow MA, Sidlow R HM. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
9. Ozaki K, Takahashi H, Murakami Y, Kiyoku H. A Case of Cystitis After Administration of Nivolumab. Int Cancer Conf J (2017) 6(4):164–6. doi: 10.1007/s13691-017-0298-6
10. Shimatani K, Yoshimoto T, Doi Y, Sonoda T, Yamamoto S, Kanematsu A, et al. Two Cases of Nonbacterial Cystitis Associated With Nivolumab, the Anti- Programmed-Death-Receptor-1 Inhibitor. Urol Case Rep (2018) 17:97–9. doi: 10.1016/j.eucr.2017.12.006
11. Yajima S. Improvement of Urinary Symptoms After Bladder Biopsy: A Case of Pathologically Proven Allergy-Related Cystitis During Administration of Nivolumab. IJU Case Rep (2021) 4(4):213–5. doi: 10.1002/iju5.12286
12. Ueki Y, Matsuki M, Kubo T, Morita R, Hirohashi Y, Sato S, et al. Non-Bacterial Cystitis With Increased Expression of Programmed Death-Ligand 1 in the Urothelium: An Unusual Immune-Related Adverse Event During Treatment With Pembrolizumab for Lung Adenocarcinoma. IJU Case Rep (2020) 3(6):266–9. doi: 10.1002/iju5.12211
13. Zhu L, Wang Z, Stebbing J, Wang Z, Peng L. Immunotherapy-Related Cystitis: Case Report and Review of the Literature. Onco Targets Ther (2021) 14:4321–8. doi: 10.2147/OTT.S321965
14. Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue Expression of PD-L1 Mediates Peripheral T Cell Tolerance. J Exp Med (2006) 203(4):883–95. doi: 10.1084/jem.20051776
15. Kennedy LB, Salama AKS. A Review of Cancer Immunotherapy Toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
16. Dowell AC, Munford H, Goel A, Gordon NS, James ND, Cheng KK, et al. PD-L2 Is Constitutively Expressed in Normal and Malignant Urothelium. Front Oncol (2021) 11:1–7. doi: 10.3389/fonc.2021.626748
17. Sugino Y, Nishikawa N, Yoshimura K, Kuno S, Hayashi Y, Yoshimura N, et al. BALB/c-Fcgr2b–/– Pdcd1–/– Mouse Expressing Anti-Urothelial Antibody Is a Novel Model of Autoimmune Cystitis. Sci Rep (2012) 2:1–7. doi: 10.1038/srep00317
18. Wen L, Zou X, Chen Y, Bai X, Liang T. Sintilimab-Induced Autoimmune Diabetes in a Patient With the Anti-Tumor Effect of Partial Regression. Front Immunol (2020) 11:1–4. doi: 10.3389/fimmu.2020.02076
19. Ni J, Zhang L, Zhang X. Marked Elevation of Creatine Phosphokinase Alone Caused by Sintilimab - Beware of Hypothyroid Myopathy. Eur J Cancer (2020) 128:57–9. doi: 10.1016/j.ejca.2019.12.030
20. Hu J, Li Y, Chen X, Luo C, Zuo X. Pulmonary Fibrosis and Cytokine Release Syndrome After Hyperactivation With Sintilimab. J Clin Pharm Ther (2020) 45(6):1474–7. doi: 10.1111/jcpt.13217
21. Gao C, Xu J, Han C, Wang L, Zhou W, Yu Q. An Esophageal Cancer Case of Cytokine Release Syndrome With Multiple-Organ Injury Induced by an Anti-PD-1 Drug: A Case Report. Ann Palliat Med (2020) 9:2393–9. doi: 10.21037/apm-20-1310
22. Xing Q, Zhang ZW, Lin QH, Shen LH, Wang PM, Zhang S, et al. Myositis-Myasthenia Gravis Overlap Syndrome Complicated With Myasthenia Crisis and Myocarditis Associated With Anti-Programmed Cell Death-1 (Sintilimab) Therapy for Lung Adenocarcinoma. Ann Transl Med (2020) 1:6–11. doi: 10.21037/atm.2020.01.79
23. Kang K, Zheng K, Zhang Y. Paraneoplastic Encephalitis and Enteric Neuropathy Associated With Anti-Hu Antibody in a Patient Following. J Immunother (2020) 43(5):165–8. doi: 10.1097/CJI.0000000000000314
24. Liu Y, Jiang L. Tofacitinib for Treatment in Immune-Mediated Myocarditis: The First Reported Cases. J Oncol Pharm Pract (2020) 27(3):739–46. doi: 10.1177/1078155220947141
25. Sui CL, Lin XF, He L, Zhu W. Dermoscopic Features of Acutely Exacerbated Plaque Psoriasis Induced by Anti-Programmed Death-1 for Lung Cancer. Chin (Engl) (2020) 133(17):2123–5. doi: 10.1097/CM9.0000000000000958
26. Huang X, Yang M, Wang L, Li L, Zhong X. Sintilimab Induced Diabetic Ketoacidosis in a Patient With Small Cell Lung Cancer. Med (Baltimore) (2021) 100(19):1–6. doi: 10.1097/MD.0000000000025795
27. Li Y, Zhou Y, Liu YY, Zhang GJ, Xiao L, Li N, et al. Severe Immune-Related Hyperthermia Followed by Immune-Related Pneumonitis With PD-1 Inhibitor (Sintilimab) in Small Cell Lung Cancer: A Case Report. Thorac Cancer (2021) 12(11):1780–3. doi: 10.1111/1759-7714.13967
28. Dai Y, Liu S, Zhang Y, Li X, Zhao Z, Liu P, et al. A False Alarm of COVID−19 Pneumonia in Lung Cancer With Anti−PD−1 Related Pneumonitis: A Case Report and Review of the Literature. J Med Case Rep (2021) 15(1):4–6. doi: 10.1186/s13256-020-02619-y
29. Bi H, Ren D, Wang Q, Ding X, Wang H. Immune Checkpoint Inhibitor-Induced Myocarditis in Lung Cancer Patients: A Case Report of Sintilimab-Induced Myocarditis and a Review of the Literature. Ann Palliat Med (2021) 10(1):793–802. doi: 10.21037/apm-20-2449
30. Fu L, Chen P, Wang S, Liu W, Chen Z, Chen H, et al. Complete Pathological Response With Diabetic Ketoacidosis to the Combination of Sintilimab and Anlotinib in an Unresectable Hepatocellular Carcinoma Patient: A Case Report. Anticancer Drugs (2021) 33(1):e741–6. doi: 10.1097/CAD.0000000000001163
31. Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, et al. Immune-Related Adverse Events Predict the Therapeutic Efficacy of Anti–PD-1 Antibodies in Cancer Patients. Eur J Cancer (2019) 109:21–7. doi: 10.1016/j.ejca.2018.10.014
32. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation Between Immune-Related Adverse Events and E Fficacy in Non- Small Cell Lung Cancer Treated With Nivolumab. Lung Cancer (2018) 115:71–4. doi: 10.1016/j.lungcan.2017.11.019
33. National Institutes of Health and National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Bethesda, Md: U.S Department of Health and Human Services (2017).
34. Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant Use of Corticosteroids and Immune Checkpoint Inhibitors in Patients With Hematologic or Solid Neoplasms: A Systematic Review. Crit Rev Oncol/Hematol (2017) 120:86–92. doi: 10.1016/j.critrevonc.2017.10.009
35. Kapoor A, Noronha V, Patil VM, Joshi A, Menon N, Abraham G, et al. Concomitant Use of Corticosteroids and Immune Checkpoint Inhibitors in Patients With Solid Neoplasms: A Real-World Experience From a Tertiary Cancer Center. Cancer Res Stat Treat (2019) 2(2):204. doi: 10.4103/CRST.CRST_88_19
36. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer. J Clin Oncol (2019) 36(28):2872–8. doi: 10.1200/JCO.2018.79.0006
Keywords: immune checkpoint inhibitor, sintilimab, immune-related adverse events, cystitis, ureteritis
Citation: Tu L, Ye Y, Tang X, Liang Z, You Q, Zhou J and Pan Z (2021) Case Report: A Case of Sintilimab-Induced Cystitis/Ureteritis and Review of Sintilimab-Related Adverse Events. Front. Oncol. 11:757069. doi: 10.3389/fonc.2021.757069
Received: 01 September 2021; Accepted: 06 December 2021;
Published: 23 December 2021.
Edited by:
Maen Abdelrahim, Houston Methodist Research Institute, United StatesReviewed by:
Shamshad Alam, University at Buffalo, United StatesTapas Ranjan Behera, Cleveland Clinic, United States
Copyright © 2021 Tu, Ye, Tang, Liang, You, Zhou and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianying Zhou, emp5aHpAemp1LmVkdS5jbg==; Zhijie Pan, cGFuemoyMDA3MDlAemp1LmVkdS5jbg==
 Lingfang Tu
Lingfang Tu Yuan Ye
Yuan Ye Xiaoping Tang1
Xiaoping Tang1 Jianying Zhou
Jianying Zhou