- 1Department of Clinical Laboratory, Shantou Hospital Traditional Chinese Medicine, Shantou, China
- 2Department of Thoracic Surgery, Cancer Hospital of Shantou University Medical College, Shantou, China
Background: The goal of this study was to investigate the impact of mean corpuscular volume (MCV) in patients with esophageal squamous cell carcinoma (ESCC) who underwent surgical resection.
Methods: A total of 615 patients with ESCC who underwent esophagectomy were analyzed. Patients were divided into two groups according to the standard MCV: the high MCV group (>100 fl) and the low MCV group (≤100 fl). Survival analyses were performed to calculate overall survival (OS) and cancer-specific survival (CSS) and investigate the independent prognostic factors.
Results: Fifty-one patients (8.3%) were in the high MCV group, and the other 564 patients (91.7%) were defined as the low MCV group. MCV was significantly correlated with sex, habitual alcohol or tobacco use, tumor length, body mass index, and multiple primary malignancies (P < 0.05). Elevated MCV was significantly correlated with poor survival in univariate and multivariate analyses. However, in subgroup analyses, MCV was found to be correlated with survival only in patients with alcohol or tobacco consumption and not in patients without alcohol or tobacco consumption.
Conclusions: Pretreatment MCV was correlated with survival in ESCC patients after esophagectomy. However, its prognostic value might only exist in patients with alcohol or tobacco consumption.
Introduction
Esophageal carcinoma is a common digestive system malignancy with high mortality (1). Esophagectomy remains the most important tool for treatment in resectable cases, while neoadjuvant chemoradiotherapy is recommended for locally advanced diseases (2). The identification of factors associated with high risk prior to treatment is important for planning the individual therapeutic strategy for patients with malignancies. Currently, the TNM staging system is widely used for predicting the outcomes of esophageal cancer and other malignancies. Although a separate clinical stage (cTNM) was provided in the 8th edition for ESCC to be used as a prognostic indicator before treatment, its predictive value is still limited. Therefore, we think that it is necessary to develop other easily accessible and effective indicators to predict the outcome of esophageal cancer patients before treatment, which may help to improve individualize their treatment.
Mean corpuscular volume (MCV) is a measure of the average volume of erythrocytes. Elevated MCV is always associated with deficiency of folate and vitamin B12, which may be seen in patients with a history of gastrectomy, malnutrition due to alcohol abuse, and so on (3). Recently, MCV was found to be correlated with the survival of patients with several malignancies, such as liver cancer, gastroesophageal adenocarcinomas, colorectal cancer, and head and neck cancer (3–8).
Alcohol and tobacco abuse have been recognized as risk factors for various cancers. Previous studies also showed that elevated MCV was more often found in patients with high alcohol or tobacco consumption (9, 10). To the best of our knowledge, only two studies have evaluated the predictive significance of MCV in patients with esophageal cancer who have undergone surgical resection (11, 12). However, neither of these studies evaluated the prognostic value of MCV according to the status of alcohol and tobacco consumption.
In this study, we investigated the prognostic value of MCV in patients with esophageal squamous cell carcinoma (ESCC) who underwent surgical resection and tried to elucidate the prognostic significance of MCV in these patients according to the history of alcohol and tobacco consumption.
Patients and Methods
Patients
Between September 2014 and December 2017, 817 patients with esophageal cancer underwent esophagectomy at Shantou University Medical College Cancer Hospital. Only patients with ESCC who underwent surgery as their initial treatment were included in this study. Patients with a past history of gastrectomy were also excluded from this study. This study was approved by the Ethics Committee of our hospital. Written informed consent was signed for all patients.
Data Collection
All clinicopathological data and laboratory data were obtained from the patients’ medical records. The stage of the tumor was classified based on the 8th edition American Joint Committee on Cancer TNM staging system for ESCC. Habitual alcohol use was defined as drinking ≥1 time per week, and habitual tobacco use was defined as smoking at least 1 cigarette per day for at least one year. Weight, height, and laboratory data were collected within 1 week before surgery.
The cutoff values of MCV and serum albumin were determined according to the standard value in our hospital. Patients were divided into a high MCV group (>100 fl) and a low MCV group (≤100 fl) as well as a high serum albumin group (≥40 g/L) and a low serum albumin group (<40 g/L). Anemia was defined as Hb < 12 g/dL for women and Hb <13 g/dL for men according to the World Health Organization guidelines (13).
Surgery
Most of the patients underwent esophagectomy through a right thoracotomy, while other patients underwent a left thoracotomy. For lymphadenectomy, the regional lymph nodes in the middle mediastinal, lower mediastinal, and upper abdominal regions were routinely dissected in all patients. For patients who underwent esophagectomy through a right thoracotomy, the lymph nodes around the left and right recurrent laryngeal nerves were also dissected. Postoperative morbidity was defined as a state where the Clavien–Dindo classification was II or higher (14).
Statistical Analyses
The smoking index was calculated by daily cigarette intake × number of years smoking. The alcohol index was calculated as daily ethanol intake (g) × number of years drinking. Continuous variables were compared using Student’s t-test. Categorical variables were compared by the χ2 test or Fisher’s exact test. Overall survival (OS) time was calculated from the date of operation to the date of death or most recent follow-up. Cancer-specific survival (CSS) was defined as survival from the date of operation until death due to esophageal carcinoma in the absence of other causes. Survival was calculated using the Kaplan-Meier method, and the differences in survival between groups were compared by the log-rank test. Multivariate Cox regression analyses were applied to identify independent prognostic factors. P< 0.05 was set as significant. All statistical analyses were conducted in SPSS 20.0 software (IBM, Armonk, New York, USA).
Results
Patient Characteristics
Of the 817 patients with esophageal carcinoma who underwent esophagectomy between September 2014 and December 2017, 761 patients were diagnosed with ESCC. One hundred sixteen patients who received neoadjuvant therapy were excluded from this study (including 94 patients with neoadjuvant chemoradiotherapy, 13 patients with neoadjuvant radiotherapy, and 9 patients with neoadjuvant chemotherapy). Five patients with a past history of gastrectomy and 25 patients lacking any follow-up data were also excluded. Thus, 615 patients were enrolled for analysis in this study. There were 472 men and 143 women, and the median age was 61 years (range, 38 to 84 years). The mean number of lymph nodes dissected was 26.9 ± 11.0, and the median number was 26 (range, 6-74). Based on the 8th edition of the TNM staging system, 283 patients (46.0%) had pN0 disease, 206 patients (33.5%) had pN1 disease, 99 patients (16.1%) had pN2 disease, and 27 patients (4.4%) had pN3 disease. Radical resection was achieved in 590 patients (95.9%), while palliative resection was performed in 25 patients (4.1%). The postoperative morbidity rate was 8.3% (51/615). The hospital mortality rate was 0.5% (3/615).
One hundred and sixty-eight patients received postoperative adjuvant therapy, including 64 cases of postoperative chemotherapy, 79 cases of postoperative radiotherapy, and 25 cases of postoperative chemoradiotherapy. The most common regimen for chemotherapy was Paclitaxel + Cisplatin or 5-Fu + Cisplatin. A total dose of 40–66 Gy (median 50Gy) irradiation (2 Gy/day, 5 days per week) was administered for postoperative therapy.
Two hundred twenty-five patients habitually drank alcohol, while the other 390 patients did not. The mean alcohol index for these 225 patients with habitual alcohol consumption was 3344.3 ± 117.6. Four hundred thirty-three patients had habitual tobacco use, while the other 182 patients did not. The mean smoking index for these 433 patients with habitual tobacco consumption was 932.5 ± 22.2. Twenty-two patients had multiple primary malignancies (including 5 patients with synchronous malignancy and 17 patients with metachronous malignancy). One hundred twenty-five patients were in the low serum albumin group, while the other 490 patients were defined as the high serum albumin group. Fifty-one patients (8.3%) were in the high MCV group, and the other 564 patients (91.7%) were in the low MCV group.
Correlation Between MCV and Clinicopathological Factors
Table 1 shows patient clinicopathological factors stratified by MCV. MCV was significantly correlated with sex, habitual alcohol or tobacco use, tumor length, body mass index (BMI), and multiple primary malignancies (P < 0.05). None of the female patients (0/143) had high MCV in this study, compared with 10.8% of the male patients (51/472) with high MCV (P<0.001). A total of 11.3% of the patients with habitual tobacco use had high MCV, which was significantly higher than the 1.1% of patients without habitual tobacco use (P<0.001). The incidence of elevated MCV was higher in patients with habitual alcohol use than in patients without habitual alcohol use (18.2% vs 2.6%, P<0.001). Higher MCV was also more often found in patients with longer tumor length, lower BMI, and multiple primary malignancies.
Survival and Prognostic Factors
The last follow-up was conducted in December 2020, after a mean follow-up time of 34.7 months (range, 1-69 months). Two hundred thirteen patients died, and 10 patients were lost to follow-up (1.6%).
The 1-, 3- and 5-year OS rates for all patients were 88.6%, 66.0% and 61.3%, respectively. The 1-, 3- and 5-year CSS rates were 88.8%, 66.4% and 62.2%, respectively. The correlations between the clinicopathological factors and survival are shown in Table 2. In univariate analysis, the variables correlated with OS and CSS were tumor length, thoracotomy, resection margin, pT category, pN category, pTNM stage, multiple primary malignancies, and MCV. The 5-year OS and CSS for patients in the low MCV group were 63.2% and 64.1%, respectively, which were significantly higher than the 41.3% and 41.3% for patients in the high MCV group (P<0.05, Figure 1). Other factors correlated with poor survival were longer tumor length, lower serum albumin, left thoracotomy, palliative resection, advanced pT category, advanced pN category, advanced pTNM stage, and multiple primary malignancies.
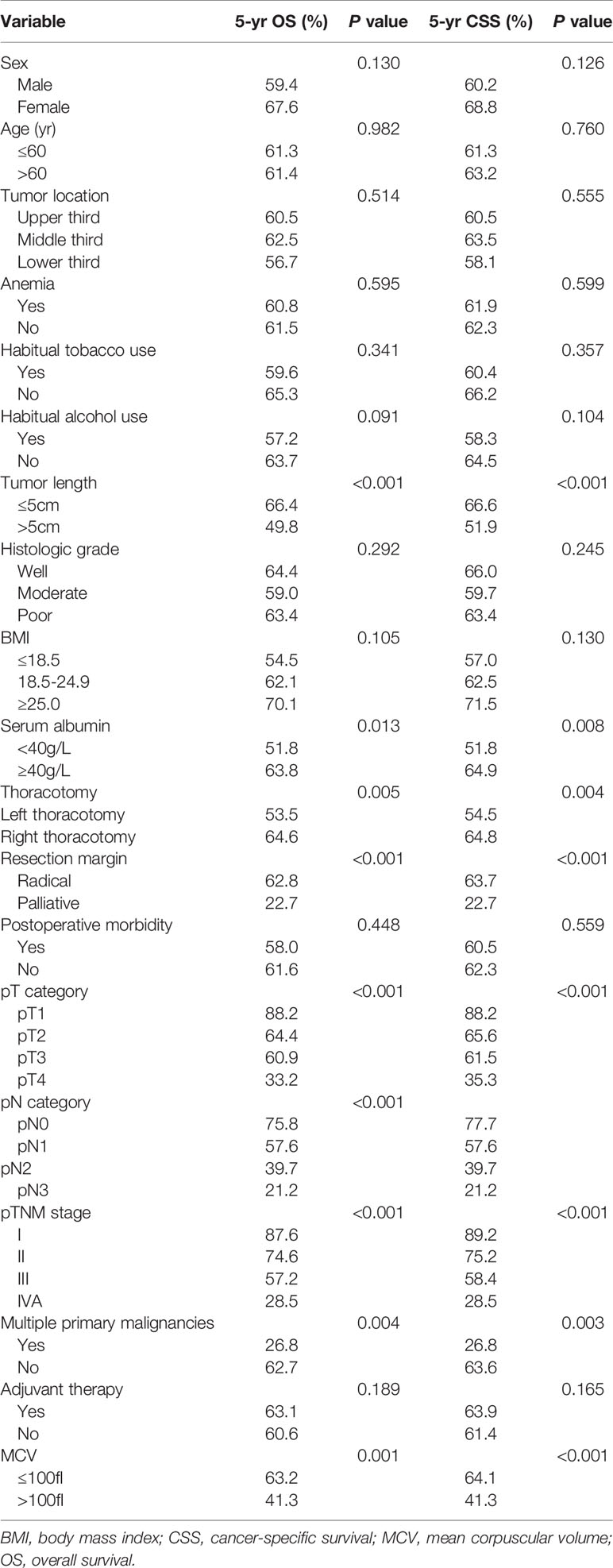
Table 2 Univariate analysis in regard to overall survival and cancer-specific survival according to clinicopathological factors.
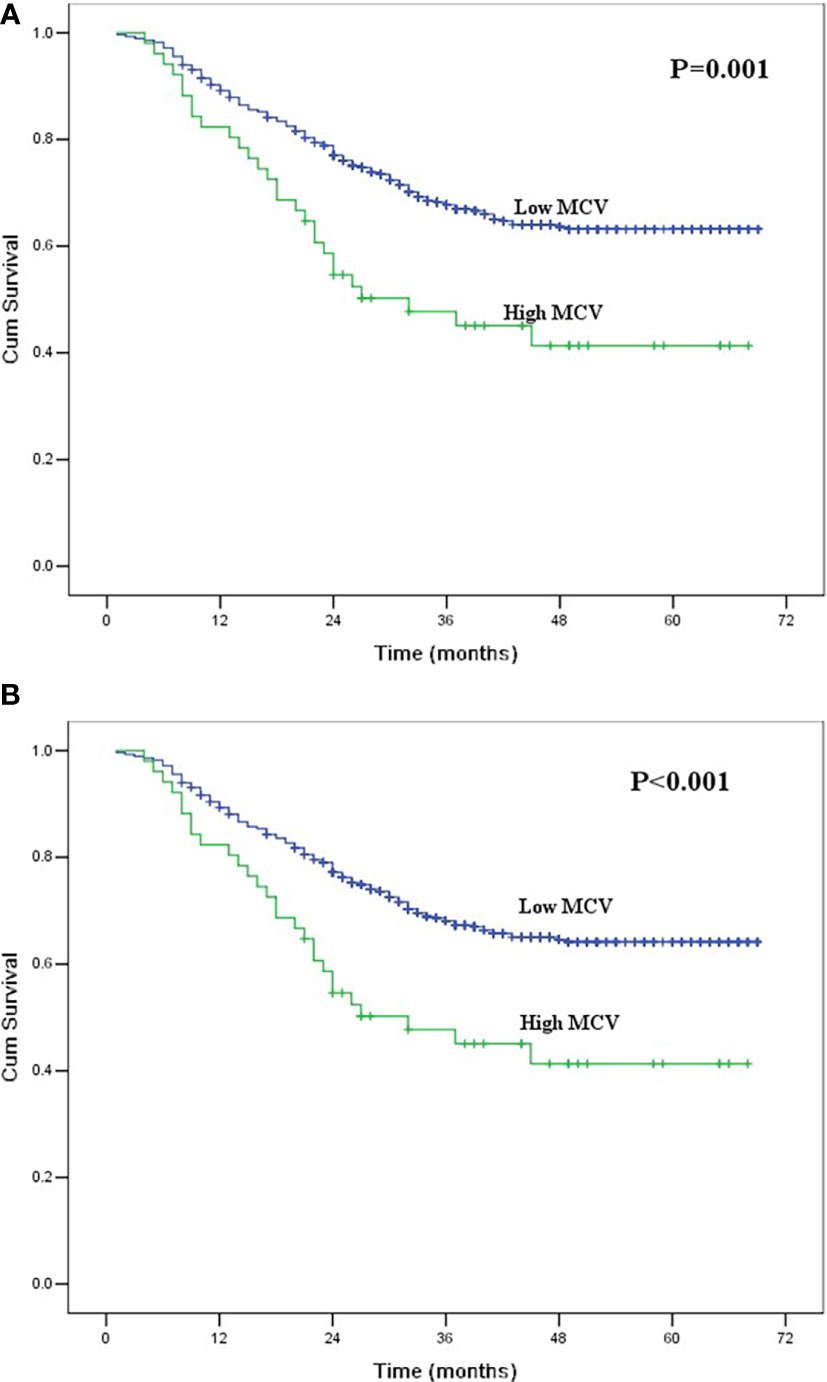
Figure 1 (A) Kaplan-Meier curves for overall survival of the entire group according to mean corpuscular volume. The survival difference was significant (P = 0.001). (B) Kaplan-Meier curves for cancer-specific survival of the entire group according to mean corpuscular volume. The survival difference was significant (P < 0.001).
The multivariate analysis incorporated factors that were significant in the univariate analyses (Table 3). Thoracotomy, resection margin, pT category, pN category, and MCV were independent prognostic factors for OS and CSS in this study. However, tumor length, serum albumin, pTNM stage, and multiple primary malignancies were not independent risk factors.
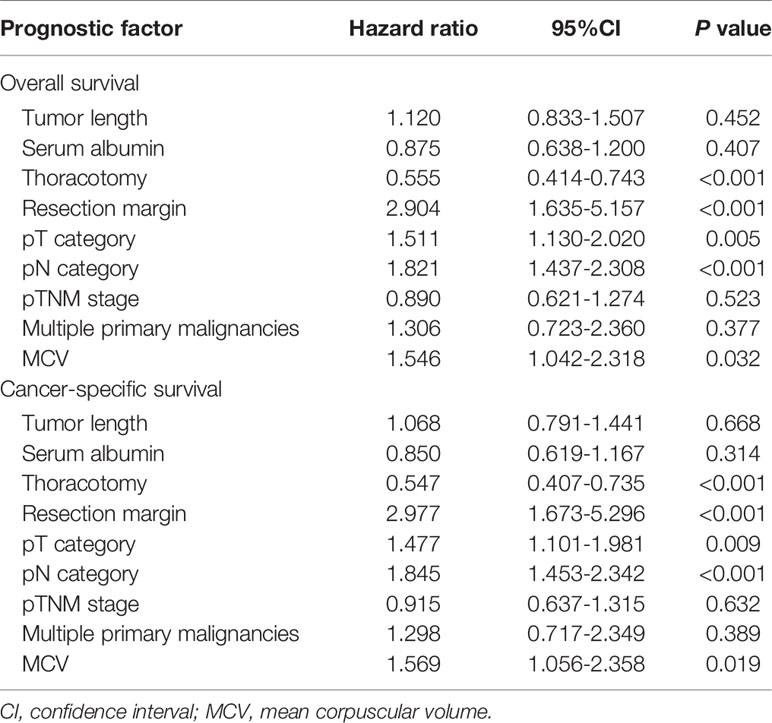
Table 3 Multivariate analysis in regard to overall survival and cancer-specific survival of the 615 patients with esophageal squamous cell carcinoma.
Prognostic Value of MCV According to Alcohol and Tobacco Consumption
We further conducted subgroup analyses to identify the value of MCV according to the history of alcohol and tobacco consumption. Of 225 patients with habitual alcohol use, 41 patients had high MCV, and 184 patients had low MCV. The mean alcohol index for patients with high MCV was 4213.4 ± 426.1, which was higher than the 3150.7 ± 180.4 for patients with low MCV (P = 0.016). High MCV was found to be significantly correlated with poor OS and CSS in ESCC patients with habitual alcohol use (P<0.05, Figure 2). However, MCV was not correlated with survival in the 390 patients without habitual alcohol use (P>0.05, Figure 2).
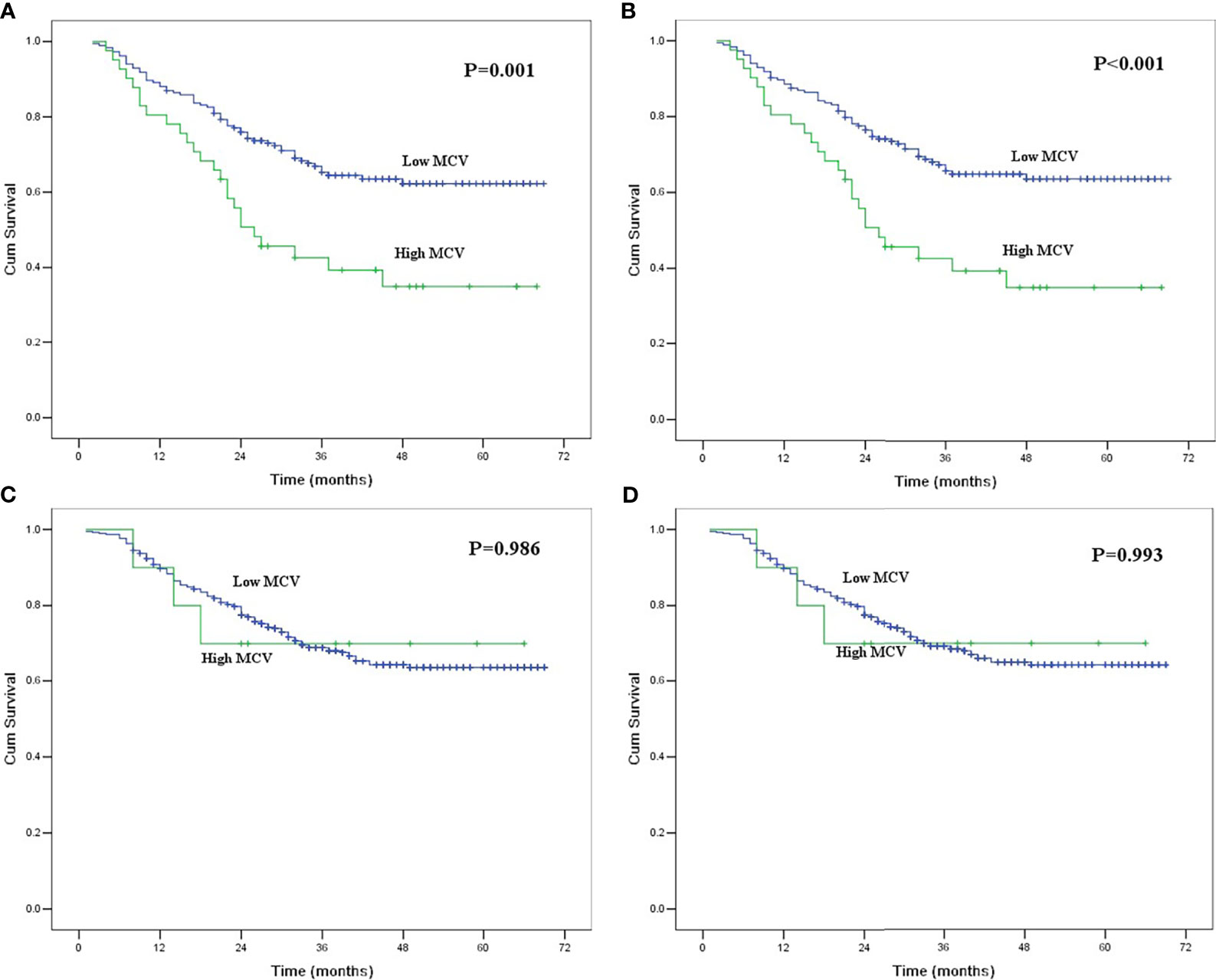
Figure 2 (A) Kaplan-Meier curves for overall survival of the patients with habitual alcohol use according to mean corpuscular volume. The survival difference was significant (P = 0.001). (B) Kaplan-Meier curves for cancer-specific survival of the patients with habitual alcohol use according to mean corpuscular volume. The survival difference was significant (P < 0.001). (C) Kaplan-Meier curves for overall survival of the patients without habitual alcohol use according to mean corpuscular volume. The survival difference was not significant (P = 0.986). (D) Kaplan-Meier curves for cancer-specific survival of the patients without habitual alcohol use according to mean corpuscular volume. The survival difference was not significant (P = 0.993).
Of the 433 patients with habitual tobacco use, 49 patients had high MCV, and 384 patients had low MCV. The mean smoking index for patients with high MCV was 1079.6 ± 71.4, which was higher than the 913.7 ± 23.2 for patients with low MCV (P = 0.018). In survival analysis, MCV was also found to be correlated with survival in ESCC patients with habitual tobacco use but not in patients without habitual tobacco use (Figure 3).
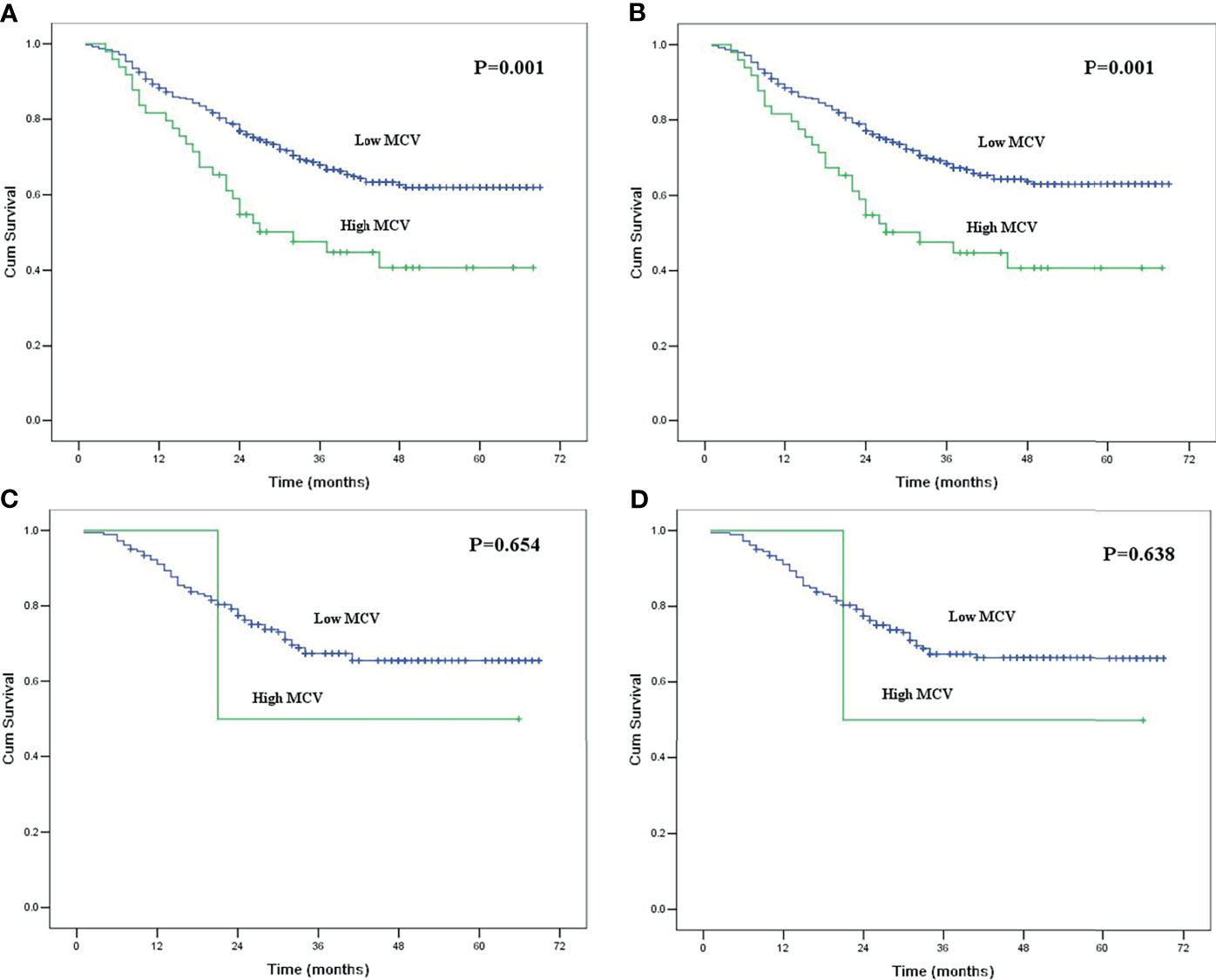
Figure 3 (A) Kaplan-Meier curves for overall survival of the patients with habitual tobacco use according to mean corpuscular volume. The survival difference was significant (P = 0.001). (B) Kaplan-Meier curves for cancer-specific survival of the patients with habitual tobacco use according to mean corpuscular volume. The survival difference was significant (P = 0.001). (C) Kaplan-Meier curves for overall survival of the patients without habitual tobacco use according to mean corpuscular volume. The survival difference was not significant (P = 0.654). (D) Kaplan-Meier curves for cancer-specific survival of the patients without habitual tobacco use according to mean corpuscular volume. The survival difference was not significant (P = 0.638).
Discussion
Preoperative malnutrition has been reported to be a predictor of outcomes in cancer patients and can be used as a prognostic indicator (15–17). Recently, MCV has been found to be another nutrition parameter correlated with the survival of various solid malignancies (3–8). Previous studies showed that elevated MCV was always observed in patients with malnutrition due to alcohol abuse (3). Our current study also showed that MCV was significantly correlated with habitual alcohol or tobacco use in patients with ESCC. Moreover, elevated MCV was more often seen in malnutrition patients with low BMI. Surprisingly, none of the 143 female patients in the current study had high MCV, compared with 10.8% among male patients. The explanation for this result may be that few females had habitual alcohol and tobacco abuse in this study. Only 1 female patient with habitual alcohol use and 2 female patients with habitual tobacco use were enrolled in our current study.
To date, only two studies have evaluated the value of MCV in patients with esophageal carcinoma who underwent surgical resection (11, 12), and both of these studies found that elevated MCV was an independent negative prognostic factor. Our current study reported a same result. The 5-year OS and CSS for patients with high MCV were significantly lower than those for patients with low MCV, and MCV was found to be an independent prognostic factor for patients with ESCC after esophagectomy.
Alcohol and tobacco abuse are leading risk factors for death globally and have been found to be correlated with multiple carcinomas, including ESCC (18–21). Elevated MCV has been recognized as a biomarker for habitual alcohol and tobacco abuse (22, 23). Our study also found that elevated MCV was more often seen in patients with alcohol and tobacco consumption, especially in patients with a higher alcohol or smoking index.
Although MCV has been reported to be correlated with the survival of patients with esophageal carcinoma, no studies have investigated the prognostic value of MCV in ESCC patients who had habitual alcohol or tobacco abuse. To the best of our knowledge, our study is the first to demonstrate that MCV was correlated with survival only in ESCC patients with habitual alcohol or tobacco use and not in patients without. Combined with the fact that MCV is more often found in patients with heavy alcohol or tobacco consumption, we can assume that MCV is not only a biomarker for habitual alcohol and tobacco abuse, but also a prognostic factor for ESCC patients with alcohol or tobacco consumption. When we use MCV as a prognostic factor for patients with ESCC, alcohol and tobacco consumption should be taken into account. We think that more studies should be conducted to confirm our findings.
The mechanism by which elevated MCV leads to a poor prognosis of ESCC patients with alcohol or tobacco consumption is still not clear, but there are several possible explanations. First, elevated MCV is always correlated with malnutrition due to a rough lifestyle. In our current study, we found that elevated MCV was more often found in patients with lower BMI, which was an indicator of malnutrition. Previous studies found that malnutrition might be correlated with poor survival in patients with ESCC (24–26). Second, elevated MCV is more often seen in patients with higher alcohol or smoking index. Recent study showed that heavy drinking (>60g per day) represented the largest burden of alcohol-attributable cancer death globally, while esophageal cancer was the most common alcohol-attributable cancer (21). Third, patients with elevated MCV might have a higher incidence of multiple primary malignancies. In our study, six of the 51 patients (11.8%) in the high MCV group developed multiple primary malignancies, while only 2.8% of the patients in the low MCV group developed multiple primary malignancies. A previous study found that the occurrence of multiple primary cancers was associated with poor prognosis in patients with esophageal cancer (27). More studies should be conducted to elucidate the mechanism by which MCV correlates with poor outcomes in ESCC patients.
There are several limitations of this study. First, it was a retrospective study from a single center, which undermined its power. Second, the patient number in some subgroups was too small, which limited the statistical power. For example, there were only two patients with high MCV in the no-habitual-tobacco use group. Third, we did not consider the synergistic effect between alcohol and tobacco in this study, as many patients may consumed both alcohol and tobacco. We think that further studies with larger cohorts are needed to confirm our findings and investigate the mechanism by which MCV relates to the prognosis of ESCC patients.
In conclusion, our study demonstrated that pretreatment MCV was correlated with survival in ESCC patients after esophagectomy. However, its prognostic value might only exist in patients with alcohol or tobacco consumption and not in patients without alcohol or tobacco consumption. Due to the small number of patients with high MCV in the no-habitual-tobacco use group and no-habitual-alcohol use group, we think that a multicenter study with larger cohorts are needed to confirm our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shantou University Medical College Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
S-jH and P-fZ contributed equally to this article. S-bC designed the research, analyzed the data and wrote part of the paper. S-jH and P-fZ analyzed the data and wrote part of the paper. All authors contributed to the article and approved the submitted version.
Funding
The Medical Scientific Research Foundation of Guangdong Province of China (B2019070).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank American Journal Experts (www.aje.cn) for English language editing.
References
1. Rustgi AK, El-Serag HB. Esophageal Carcinoma. N Engl J Med (2014) 371(26):2499–509. doi: 10.1056/NEJMra1314530
2. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
3. Yoon HJ, Kim K, Nam YS, Yun JM, Park M. Mean Corpuscular Volume Levels and All-Cause and Liver Cancer Mortality. Clin Chem Lab Med (2016) 54(7):1247–57. doi: 10.1515/cclm-2015-0786
4. Jomrich G, Hollenstein M, John M, Ristl R, Paireder M, Kristo I, et al. High Mean Corpuscular Volume Predicts Poor Outcome for Patients With Gastroesophageal Adenocarcinoma. Ann Surg Oncol (2019) 26(4):976–85. doi: 10.1245/s10434-019-07186-1
5. Mizuno H, Yuasa N, Takeuchi E, Miyake H, Nagai H, Yoshioka Y, et al. Blood Cell Markers That can Predict the Long-Term Outcomes of Patients With Colorectal Cancer. PLoS One (2019) 14(8):e0220579. doi: 10.1371/journal.pone.0220579
6. Liu Q, Yang Y, Li X, Zhang S. Implications of Habitual Alcohol Intake With the Prognostic Significance of Mean Corpuscular Volume in Stage II-III Colorectal Cancer. Front Oncol (2021) 11:681406. doi: 10.3389/fonc.2021.681406
7. Borsetto D, Polesel J, Tirelli G, Menegaldo A, Baggio V, Gava A, et al. Pretreatment High MCV as Adverse Prognostic Marker in Nonanemic Patients With Head and Neck Cancer. Laryngoscope (2021) 131(3):E836–45. doi: 10.1002/lary.28882
8. Nagai H, Yuasa N, Takeuchi E, Miyake H, Yoshioka Y, Miyata K. The Mean Corpuscular Volume as a Prognostic Factor for Colorectal Cancer. Surg Today (2018) 48(2):186–94. doi: 10.1007/s00595-017-1575-x
9. Zhang J, Zhang S, Song Y, Ma G, Meng Y, Ye Z, et al. Facial Flushing After Alcohol Consumption and the Risk of Cancer: A Meta-Analysis. Med (Baltimore) (2017) 96(13):e6506. doi: 10.1097/MD.0000000000006506
10. Malenica M, Prnjavorac B, Bego T, Dujic T, Semiz S, Skrbo S, et al. Effect of Cigarette Smoking on Haematological Parameters in Healthy Population. Med Arch (2017) 71(2):132–6. doi: 10.5455/medarh.2017.71.132-136
11. Zheng YZ, Dai SQ, Li W, Cao X, Li Y, Zhang LJ, et al. Prognostic Value of Preoperative Mean Corpuscular Volume in Esophageal Squamous Cell Carcinoma. World J Gastroenterol (2013) 19(18):2811–7. doi: 10.3748/wjg.v19.i18.2811
12. Yoshida N, Kosumi K, Tokunaga R, Baba Y, Nagai Y, Miyamoto Y, et al. Clinical Importance of Mean Corpuscular Volume as a Prognostic Marker After Esophagectomy for Esophageal Cancer: A Retrospective Study. Ann Surg (2020) 271(3):494–501. doi: 10.1097/SLA.0000000000002971
13. World Health Organization (WHO). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: World Health Organization (2011).
14. Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
15. Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, et al. Impact of Body Mass Index on Perioperative Outcomes in Patients Undergoing Major Intra-Abdominal Cancer Surgery. Ann Surg Oncol (2008) 15(8):2164–72. doi: 10.1245/s10434-008-9990-2
16. Masoomi H, Nguyen B, Smith BR, Stamos MJ, Nguyen NT. Predictive Factors of Acute Respiratory Failure in Esophagectomy for Esophageal Malignancy. Am Surg (2012) 78(10):1024–8. doi: 10.1177/000313481207801002
17. Pausch T, Hartwig W, Hinz U, Swolana T, Bundy BD, Hackert T, et al. Cachexia But Not Obesity Worsens the Postoperative Outcome After Pancreatoduodenectomy in Pancreatic Cancer. Surgery (2012) 152(3 Suppl 1):S81–88. doi: 10.1016/j.surg.2012.05.028
18. GBD 2016 Alcohol Collaborators. Alcohol Use and Burden for 195 Countries and Territories, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet (2018) 392(10152):1015–35. doi: 10.1016/S0140-6736(18)31310-2
19. Rehm J, Shield KD, Weiderpass E. Alcohol Consumption. A Leading Risk Factor for Cancer. Chem Biol Interact (2020) 331:109280. doi: 10.1016/j.cbi.2020.109280
20. Zheng W, McLerran DF, Rolland BA, Fu Z, Boffetta P, He J, et al. Burden of Total and Cause-Specific Mortality Related to Tobacco Smoking Among Adults Aged 45 Years in Asia: A Pooled Analysis of 21 Cohorts. PLoS Med (2014) 11(4):e1001631. doi: 10.1371/journal.pmed.1001631
21. Rumgay H, Shield K, Charvat H, Ferrari P, Sornpaisarn B, Obot I, et al. Global Burden of Cancer in 2020 Attributable to Alcohol Consumption: A Population-Based Study. Lancet Oncol (2021) 22(8):1070–81. doi: 10.1016/S1470-2045(21)00279-5
22. Niemelä O. Biomarkers in Alcoholism. Clin Chim Acta (2007) 377(1-2):39–49. doi: 10.1016/j.cca.2006.08.035
23. Yokoyama M, Yokoyama A, Yokoyama T, Hamana G, Funazu K, Kondo S, et al. Mean Corpuscular Volume and the Aldehyde Dehydrogenase-2 Genotype in Male Japanese Workers. Alcohol Clin Exp Res (2003) 27(9):1395–401. doi: 10.1097/01.ALC.0000085589.47243.8D
24. Wang P, Li Y, Sun H, Liu S, Zhang R, Liu X, et al. Predictive Value of Body Mass Index for Short-Term Outcomes of Patients With Esophageal Cancer After Esophagectomy: A Meta-Analysis. Ann Surg Oncol (2019) 26(7):2090–103. doi: 10.1245/s10434-019-07331-w
25. Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, et al. Preoperative Prognostic Nutritional Index Predicts Long-Term Surgical Outcomes in Patients With Esophageal Squamous Cell Carcinoma. World J Surg (2018) 42(7):2199–208. doi: 10.1007/s00268-017-4437-1
26. Migita K, Matsumoto S, Wakatsuki K, Ito M, Kunishige T, Nakade H, et al. The Prognostic Significance of the Geriatric Nutritional Risk Index in Patients With Esophageal Squamous Cell Carcinoma. Nutr Cancer (2018) 70(8):1237–45. doi: 10.1080/01635581.2018.1512640
Keywords: esophageal neoplasm, mean corpuscular volume, prognosis, squamous cell carcinoma, surgery
Citation: Huang S-j, Zhan P-f and Chen S-b (2021) Mean Corpuscular Volume as a Prognostic Factor for Patients With Habitual Alcohol or Tobacco Use After Esophagectomy. Front. Oncol. 11:752229. doi: 10.3389/fonc.2021.752229
Received: 02 August 2021; Accepted: 29 October 2021;
Published: 16 November 2021.
Edited by:
Mingran Xie, Anhui Medical University, ChinaReviewed by:
Peng Lin, Sun Yat-sen University Cancer Center, ChinaXie Xuan, Sun Yat-sen Memorial Hospital, China
Copyright © 2021 Huang, Zhan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-bin Chen, Y2hlbnNiNTM1MTc2QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Shu-jie Huang
Shu-jie Huang Peng-fei Zhan
Peng-fei Zhan Shao-bin Chen
Shao-bin Chen