- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Neurosurgery, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
- 3China National Clinical Research Center for Neurological Diseases, Beijing, China
- 4Beijing Key Laboratory of Brain Tumor, Beijing, China
Objective: Primary squamous cell carcinomas (PSCCs) arising in intracranial epidermoid cysts (IECs) are very rare, and their management and prognostic factors remain unclear. This study aimed to enunciate the clinical features and suggest a treatment protocol based on cases from the literature and the cases from our institution.
Methods: The clinicoradiological data were obtained from nine patients with PSCCs arising in IECs, who underwent surgical treatment at Beijing Tiantan Hospital between July 2012 and June 2018. We also searched the PubMed database using the keywords “epidermoid cyst(s)” or “epidermoid tumor(s)” combined with “malignant” or “malignancy” or “intracranial” or “brain” or “squamous cell carcinoma” between 1960 and 2020. Risk factors for overall survival (OS) were evaluated in the pooled cohort.
Results: The mean age of our cohort was 51.2 ± 8.3 years (range: 39–61 years), which included eight males and one female. Gross total resection (GTR) was achieved in three patients, while non-GTR was achieved in six patients. Radiotherapy was administered to five patients. After a median follow-up of 16.7 ± 21.6 months (range: 3–72 months), eight patients died with a mean OS time of 9.75 ± 6.6 months (range: 3–23 months). In the literature between 1965 and 2020, 45 cases of PSCCs arising in IECs were identified in 23 males and 22 females with a mean age of 55.2 ± 12.4 years. GTR, non-GTR, and biopsy were achieved in six (13.3%), 36 (80%), and three (6.7%) cases, respectively. After a mean follow-up of 12.7 ± 13.4 months (range: 0.33–60 months), 54.1% (20/37) patients died, and recurrence occurred in 53.6% (15/28) patients. A multivariate analysis demonstrated that postoperative radiotherapy (p = 0.002) was the only factor that favored OS. The Kaplan–Meier analysis showed that, compared with no radiotherapy (median survival time: 4 months), radiotherapy (median survival time: 24 months) had significantly prolonged OS (p = 0.0011), and GTR could not improve OS (p = 0.5826), compared with non-GTR. The 1-year OS of patients with or without radiotherapy was 72.5% or 18.2%, respectively.
Conclusion: Malignant transformation of IEC into PSCC was prevalent in elderly patients, with slight male predominance. GTR of previous benign IECs is recommended. For remnant benign IECs, close follow-up should be performed. Postoperative radiotherapy for PSCCs could bring survival benefit. GTR of these malignant intracranial tumors is difficult when they involve important brain structures. Future studies with larger cohorts are necessary to verify our findings.
Introduction
Intracranial epidermoid cysts (IECs) are benign tumors that typically develop in the cerebellopontine angle (CPA) region (1). The IEC represents about 0.2%–1.8% of all brain tumors, and its malignant transformation into SCC is a rare occurrence (2, 3). Most of these tumors are regarded as congenital lesions arising from heterotopic epithelial cells misplaced in the neural tube when the latter separates from the ectoderm between the third and fifth weeks of embryonal life (4–6). Ernst reported the first case of such malignant transformation in 1912 (2), and a few cases that met the Garcia criteria have been subsequently reported. Garcia et al. (7) defined the criteria for the malignant transformation of IECs as follows: the tumor had to be restricted to the intracranial or intradural compartment without invasion or extension beyond the dura or cranial bones. Also, there must be no extension or invasion through the intracranial orifices; no communication or connection with the middle ear, air sinuses, or sella turcica; and no evidence of nasopharyngeal tumor. According to Hamlat (8), primary squamous cell carcinoma (PSCC) was classified into five groups: 1) initial malignant transformation of a benign cyst, 2) malignant transformation from a remnant cyst, 3) malignant transformation of a dermoid and epithelial cyst, 4) malignant transformation with leptomeningeal carcinomatosis, and 5) other malignancies arising from benign cysts. Herein, we described nine cases of PSCCs arising in IECs at our center and also reviewed relevant literature from the PubMed database.
Methods
Nine patients who underwent surgical treatment were pathologically confirmed to have PSCCs at Beijing Tiantan Hospital between July 2012 and June 2018. According to Hamlat (8), PSCC could arise in an untreated benign IEC or a remnant benign IEC. Among our nine cases, four had a history of previous resection of benign ECs. The other five patients did not have a history of surgery and were considered as having malignant transformation of IECs due to coexistence of benign ECs and squamous cell carcinomas in the lesion without the evidence of metastatic diseases. The following information was collected: age, sex, symptoms, symptom duration, imaging characteristics, extent of tumor excision, pathological results, treatment, and prognosis. Pre- and postoperative MRI scans were performed to determine the extent of tumor resection, which was defined as gross total resection (GTR) and non-GTR. The follow-up was performed by telephone interview every six months. This study was approved by the Beijing Tiantan Hospital Research Ethics Committee.
Then we searched the PubMed database using the keywords “epidermoid cyst(s)” or “epidermoid tumor(s)” combined with “malignant” or “malignancy” or “intracranial” or “brain” or “squamous cell carcinoma” between 1960 and 2020. A total of 45 articles in English language that met the Garcia criteria were included.
The risk factors for overall survival (OS) were evaluated with univariate and multivariate Cox regression analyses of a total of 46 cases (including 37 cases from the literature and nine cases from our center). The survival curves were performed by the Kaplan–Meier method. Analyses were performed using SPSS Statistical Package software (version 22.0, IBM Corp.), with the significance set at p < 0.05.
Results
Cases From Our Institution
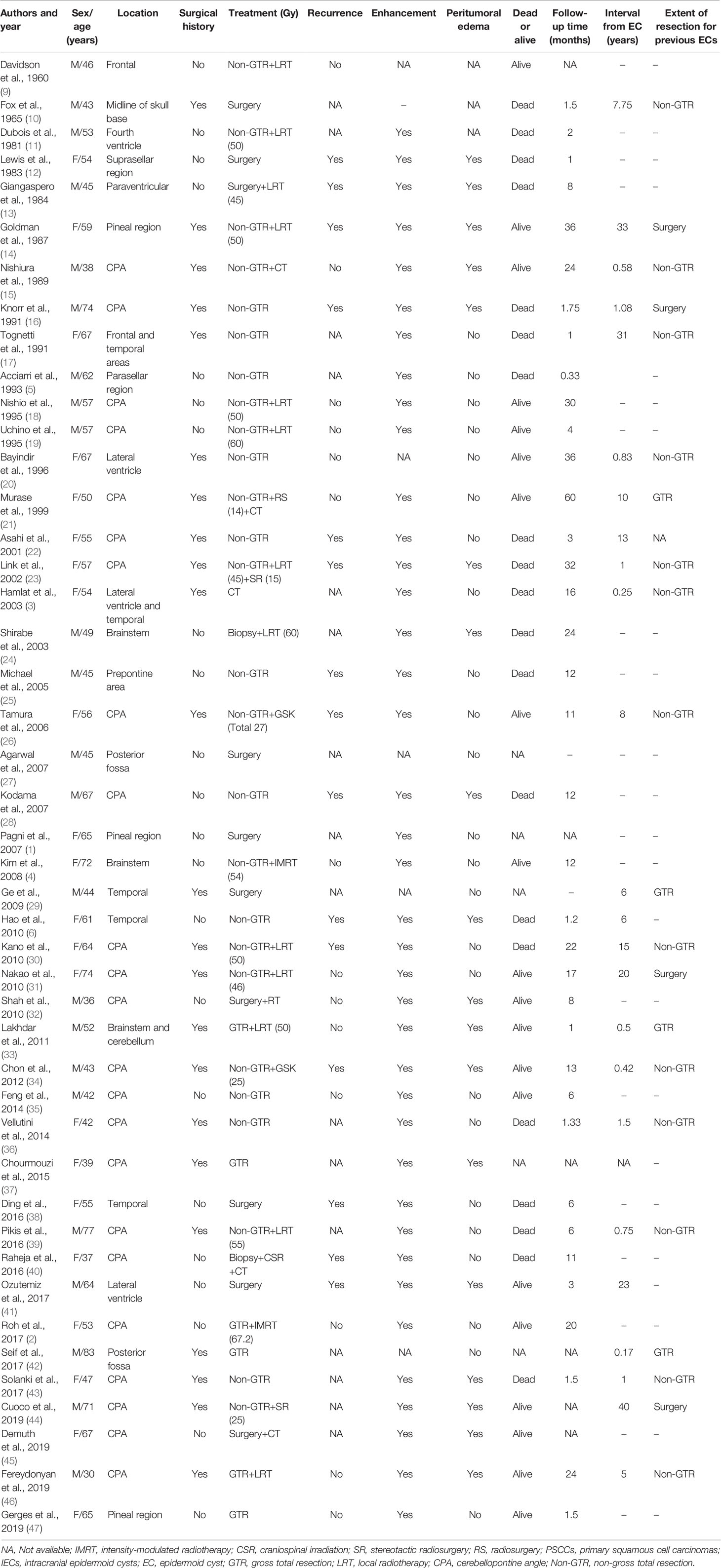
The cases from our institute included eight males and one female with a mean age 51.2 ± 8.3 years (range: 39–61 years). The duration of symptoms ranged from 2 to 12 months with a mean length of 9.33 ± 3.74 months. The preoperative symptoms included headache (n = 3), facial numbness (n = 3), trouble coughing (n = 3), diplopia (n = 2), limb weakness (n = 2), facial paralysis (n = 2), tinnitus (n = 1), ataxia (n = 1), ptosis (n = 1), hearing loss (n = 1), and vomiting (n = 1). Five patients experienced natural malignant transformation of benign intracranial ECs, and four patients suffered malignant transformation of remnant benign IECs. The interval from EC was 3–28 years (Table 1). MRI showed that the tumors were most commonly located in the CPA region (n = 8), and only one case was located in the suprasellar region. On contrast-enhanced MRI, enhancement was observed in all nine cases, and peritumoral edema was observed in six (66.7%) cases. All patients accepted surgical treatment. GTR was achieved in three patients, and non-GTR was achieved in six patients. Radiotherapy was administered to five patients, of whom one patient accepted gamma knife therapy, one patient accepted proton beam therapy, and three patients accepted external beam radiation. After a mean follow-up of 16.7 ± 21.6 months, eight patients died, and the mean OS was of 9.75 ± 6.6 months. Among them, seven patients died of tumor recurrence, and one patient died of intracranial infection. Postoperative pathological examination revealed squamous cell carcinoma. Immunohistochemical staining showed that the Ki67 proliferative indexes ranged from 10% to 90% (Table 1).
Cases From the Literature
A total of 45 patients (23 were males and 22 were females) diagnosed with intracranial PSCCs were identified between June 1960 and July 2020 (Table 2). The mean age of patients was 55.2 ± 12.4 years (range 30–83 years). On contrast-enhanced MRI or CT, enhancement was observed in all 39 available cases, and the tumors were typically located in the CPA region (n = 23). Peritumoral edema was observed in 18 (42.9%) cases. Surgical resection was performed as GTR in six patients and non-GTR in 36 patients. Twenty-three patients had a surgical history, and the pathological results were benign ECs. The interval from EC was 0.17–40 years with a mean time of 9.4 ± 11.8 years. Postoperative radiotherapy was administered to 21 patients (46.8%), of whom three patients accepted gamma knife therapy. Chemotherapy was administered to five patients (8.5%). After a mean follow-up of 13.0 ± 13.3 months (range: 0.33–60 months), 52.6% (20/38) patients died, while 53.6% (15/28) patients suffered from recurrence (Table 3).
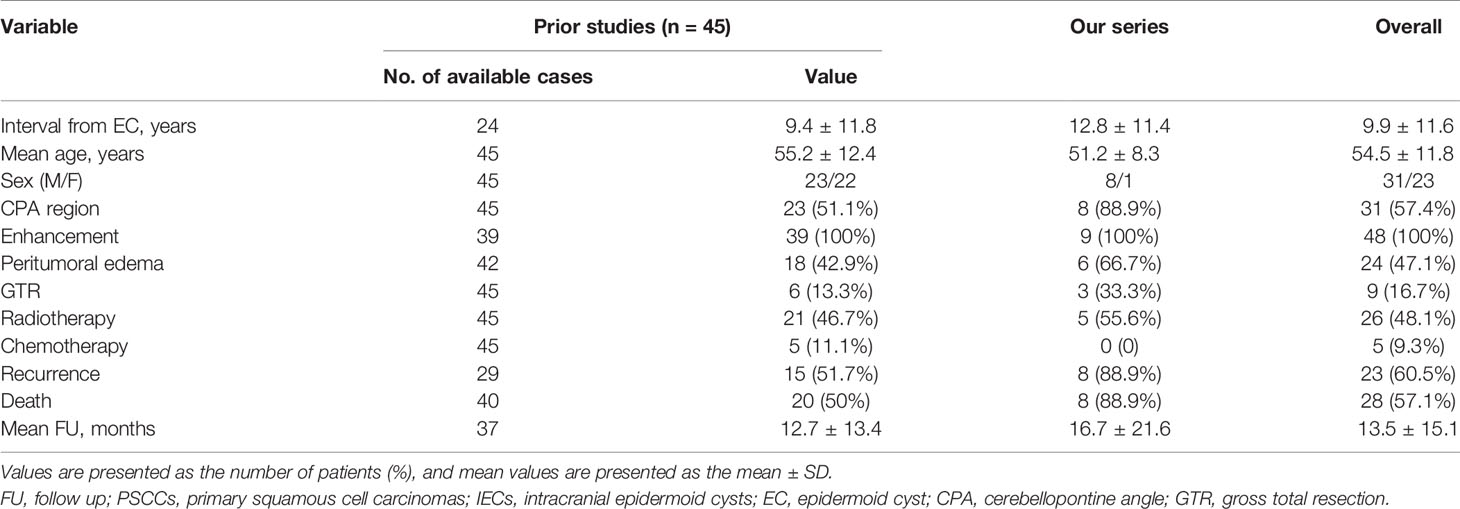
Table 3 Summary of clinical characteristics of PSCCs arising in IECs from literature and our institute.
Statistical Analysis of Prognostic Factors for Overall Survival
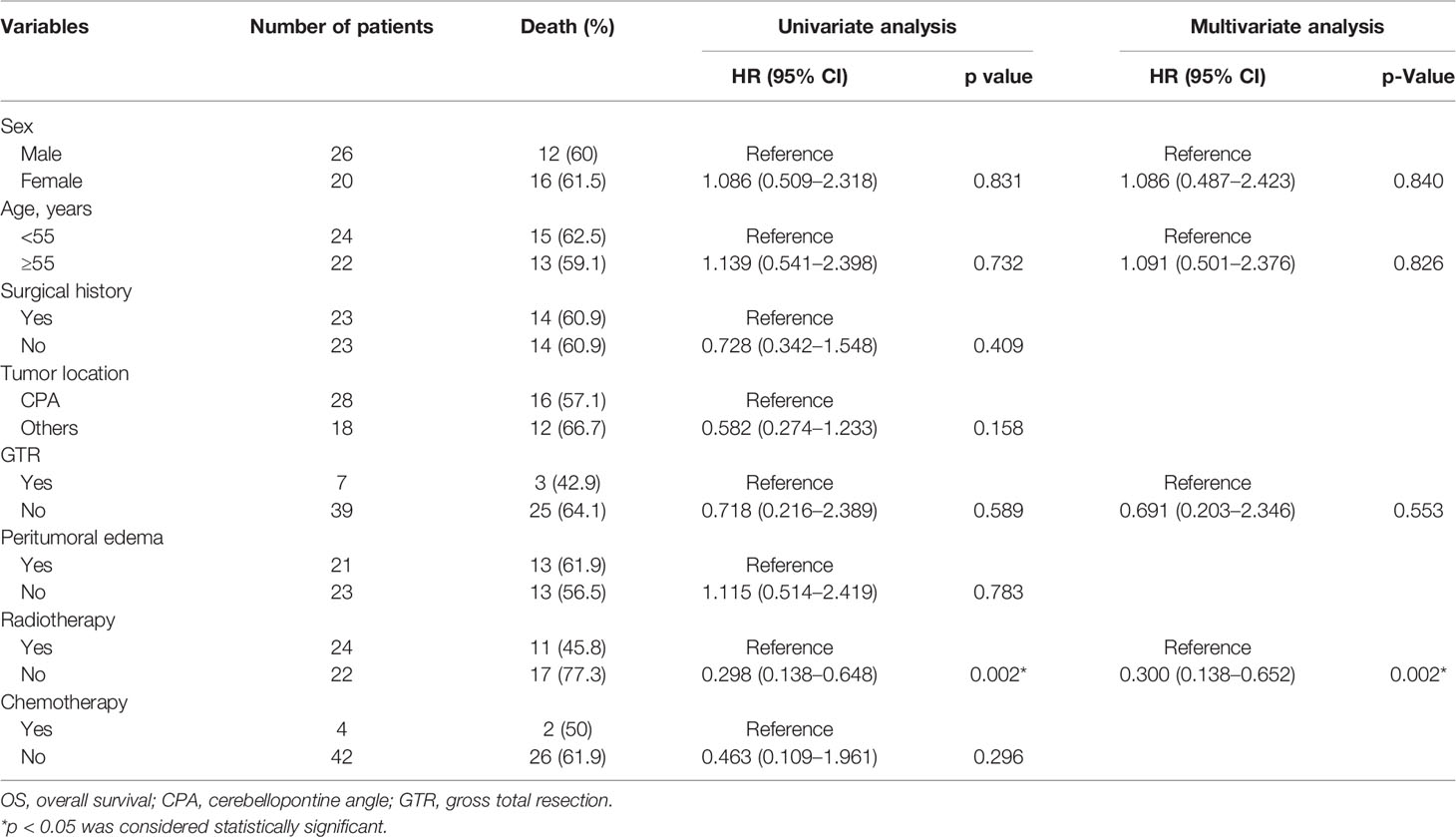
The mean follow-up in the pooled cohort (46 cases) was 13.5 ± 15.1 months. The results of univariate Cox regression analysis (including sex, age, surgical history, tumor location, peritumoral edema, extent of tumor resection, radiotherapy, and chemotherapy) showed that compared with no postoperative radiotherapy, postoperative radiotherapy had significantly prolonged OS (HR 0.298, 95% CI 0.138–0.648, p = 0.002). Other factors, including sex (HR 1.086, 95% CI 0.509–2.318, p = 0.831), age (HR 1.139, 95% CI 0.541–2.398, p = 0.732), surgical history (HR 0.728, 95% CI 0.342–1.548, p = 0.409), tumor location (HR 0.582, 95% CI 0.274–1.233, p = 0.158), GTR (HR 0.718, 95% CI 0.216–2.389, p = 0.589), peritumoral edema (HR 1.115, 95% CI 0.514–2.419, p = 0.783), and chemotherapy (HR 0.463, 95% CI 0.109–1.961, p = 0.296) were not significant. Then we added sex, age, GTR, and radiotherapy to the multivariate Cox regression analysis. Radiotherapy (HR 0.300, 95% CI 0.138–0.652, p = 0.002) was the only protective factor (Table 4). The Kaplan–Meier analysis showed that compared with no radiotherapy (median survival time: 4 months), radiotherapy (median survival time: 24 months) significantly prolonged OS of patients (p = 0.0011) (Figure 1A) , and GTR could not improve OS (p = 0.5826) (Figure 1B). The 1-year OS in patients with or without radiotherapy was 72.5% or 18.2%, respectively.
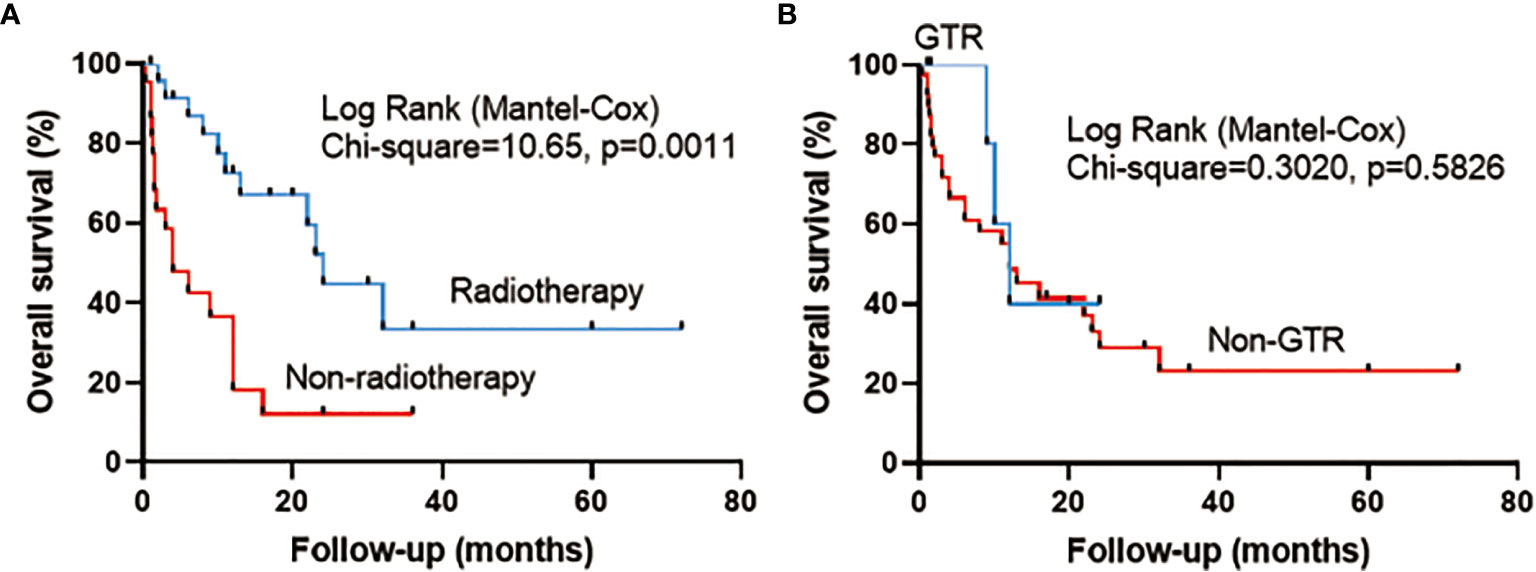
Figure 1 Survival curve analysis (log-rank test) illustrating the different overall survival (OS) rates in pooled cases between (A) radiotherapy and no radiotherapy and between (B) gross total resection (GTR) and non-gross total resection (Non-GTR).
Illustrative Case
Case 8
A 61-year-old male presented with dizziness and hearing loss for over 1 year and developed left hemiparesis for 2 weeks. Neurological examination revealed dysfunction of right cranial nerve VIII and weakness of left limb. A T2-weighted MRI showed a heterogeneous signal of the lesion located at the right CPA region. Diffusion-weighted imaging (DWI) revealed a high-signal-intensity lesion located in the right CPA cistern, prepontine cistern, and right cisterna ambiens and low signal intensity in the right pons. A T1-weighted gadolinium-enhanced MRI showed an intra-axial ring-like enhanced mass in the right brainstem, while the high-signal-intensity lesion on DWI showed no enhancement. Peritumoral edema was also observed (Figures 2A–C). A right sub-temporal craniotomy was performed. During surgery, the tumor (outside the brainstem) was found to be visually white and pearly-like with less blood supply. The tumor outside the brainstem was easily removed, and the tumor inside the brainstem was also totally resected (Figures 2I, J). His postoperative course was uneventful. Postoperative MRI showed no tumor residue (Figures 2D–F). The result of pathological examination showed EC (outside the brainstem) and SCC (inside the brainstem) (Figures 2G, H). Immunohistochemistry revealed that the Ki67 proliferation index was approximately 90%, which may indicate aggressive biological behavior of the tumor. The patient also underwent a whole-body positron emission tomography, and the results did not show any evidence of metastatic disease. A diagnosis of PSCC arising in IEC was made. We advised the patient to consult radiotherapists for further treatment. The patient refused to undergo radiotherapy and died of tumor recurrence 12 months after surgery.
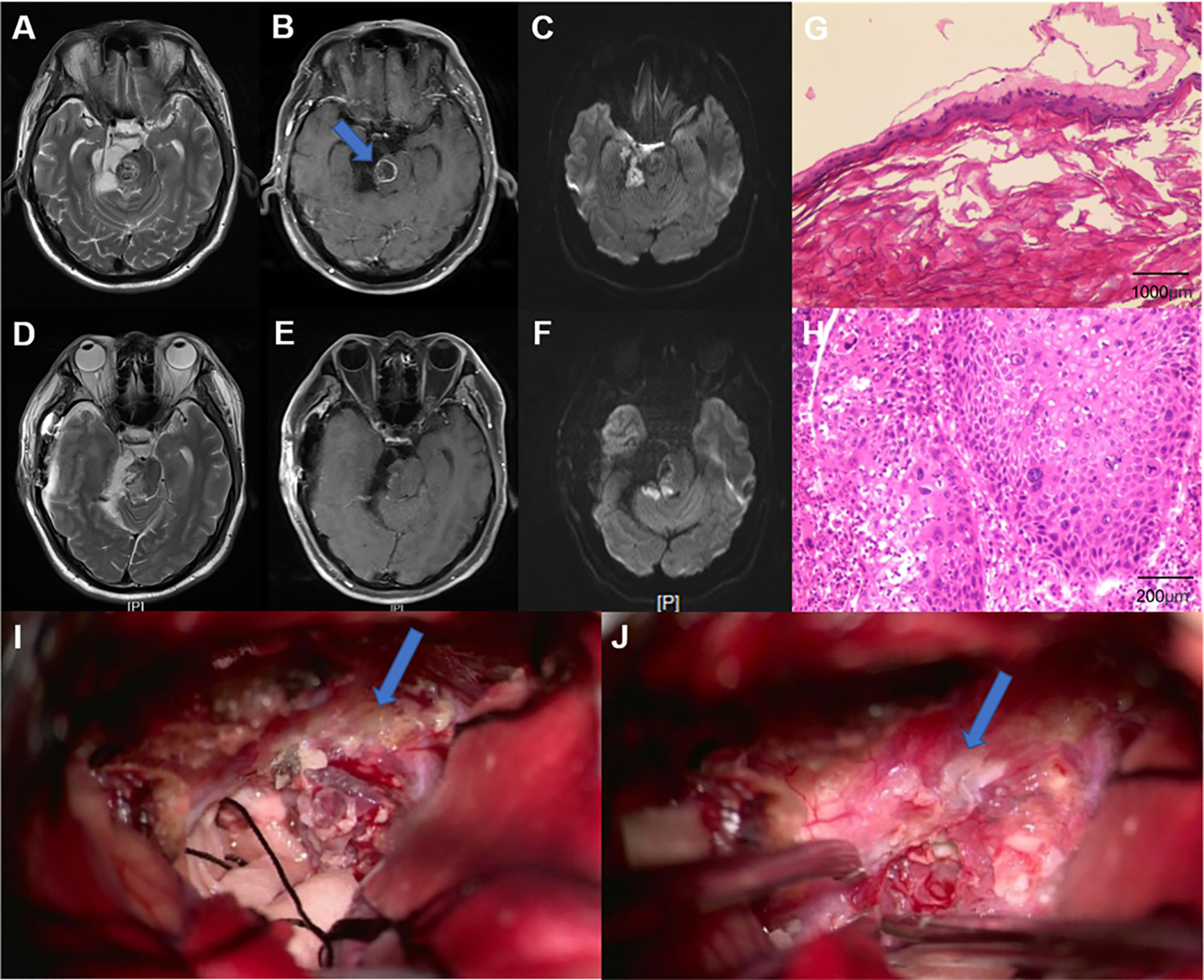
Figure 2 (A–C) Preoperative MRI. (A) Axial, T2-weighted MRI showed a heterogeneous signal of the lesion located at the right cerebellopontine angle (CPA) region. (B) Axial, contrast-enhanced MRI revealed relatively regular, ring-like enhanced lesion in the right brainstem. (C) Axial, diffusion-weighted imaging (DWI) showed a heterogeneous signal of the lesion. (D–F) Postoperative MRI. The solid mass was totally resected. Pathological results indicated a benign EC (G) (specimen from outside the brainstem) (H&E, ×40) and squamous cell carcinoma (H) (specimen from inside the brainstem) (H&E, ×200). (I, J) Images during the surgery. (I) The resection of the benign epidermoid cyst (as pointed by an arrow). (J) A part of the capsule that adhered tightly to the brainstem (as pointed by an arrow).
Discussion
The IECs, sometimes termed as intracranial pearly tumors or cholesteatomas, are rare lesions that represent approximately 7% of the CPA tumors, and the malignancy of IECs is an extreme occurrence (2, 28). At our institute, 1,095 cases of IECs were pathologically confirmed between July 2012 and June 2018. Among them, nine cases showed PSCCs arising in IECs, and the malignancy of IECs was approximately 0.82% at our center. In this study, we discussed the clinical features of intracranial PSCCs and proposed a treatment protocol based on 54 cases (including 45 cases from the literature and nine cases from our institution).
It was reported that these tumors often affected elderly patients with female predominance (33); however, all nine cases from our hospital and the pooled 45 cases showed a slight male predominance (57.4%). Similar to previous studies (26, 33), the interval to malignant transformation was relatively long, ranging from 0.17 to 40 years with a mean time 9.9 ± 11.6 years. Malignant transformation usually occurred within the primary location of the lesions, which were primarily located in the CPA area but also found in the parapontine, intraventricular, and parasellar regions (26, 43). The growth rate of IECs was linear rather than exponential, and rapid progression of symptoms and signs may indicate the malignant transformation of the lesion (31). PSCC could arise in a natural malignant transformation of IEC or a remnant benign IEC. Of the pooled analysis, 27 cases experienced natural malignant transformation of IEC, and another 27 cases had a surgical history, and there was no statistical difference in OS between the two groups (p = 0.409). Although the first case of malignancy of IEC was reported over a century ago, the mechanisms underlying this transformation remain unclear. Some potential mechanisms include a chronic inflammatory response due to repeated cystic rupture or subtotal resection of the cystic wall (4). In this study, 21 patients (84%) with non-GTR and four (16%) patients with GTR suffered malignant transformation of previous benign IECs. Hence, we hypothesized that non-GTR of previous benign IECs might be a risk factor for the malignant transformation. The mechanism of malignancy may be the recurrent inflammatory stimulation of the residual tumor. Close follow-up should be performed for those who did not achieve GTR of benign IECs. Repeated cystic rupture may be another explanation for the malignant transformation of ECs. In 2010, Hao et al. reported a natural malignant transformation of an IEC without a history of surgery and suggested that spontaneous rupture of the EC may contribute to the malignant transformation (6). We found that some benign ECs had thickened capsules. Postoperative pathological examination showed epithelial dysplasia in these capsules. We speculated that the epithelial dysplasia may lead to PSCC.
Radiologically, typical benign IECs appear as low-density lesions in the subarachnoid space without contrast enhancement on CT or MRI and hyperintense on DW-MRI. The malignant transformation of intracranial epithelial cysts appears as apparent enhancement by contrast medium on CT or T1-weighted MRI and hypointense on DWI (30, 31). In this study, significant enhancement on MRI or CT was observed in all cases (48 cases were available). Although some enhanced lesions can exist adjacent to or within benign epidermoid cysts without malignant transformation (28), appearance of a significant enhancement in epidermoid cyst should alert the neurosurgeon of a malignant transformation. Peritumoral edema was also found in these tumors (47.1%). Interestingly, univariate analysis showed that absence of peritumoral edema (p = 0.783) could not predict a better OS. Histopathologically, the epidermoid cyst wall comprises benign squamous epithelium, and the cysts have keratin debris and squamous epithelium but lack malignant cells. The primary intracranial squamous cell carcinomas have poorly differentiated epithelial cells with pleomorphic nuclei, and they display stromal invasion. Among eight patients from our institute, the Ki67 index was relatively high, ranging from 10% to 90%, which indicated a relatively aggressive disease course. Our study failed to confirm the positive efficacy of GTR for survival benefit. Limited cases with GTR (n = 7) may cause potential bias of pooled analysis. In addition, GTR of intracranial PSCCs was controversial. Given the adherent nature of the cyst wall, it may adhere tightly to some important structures, such as brainstem or the adjacent cranial nerves, which would make GTR impossible. The modalities of adequate chemotherapy have been examined in various studies, but the effectiveness remains uncertain (8). Radiotherapy after the surgery of intracranial PSCCs seems effective (31). Nagasawa et al. reviewed 36 cases of epidermoid tumors with malignant transformation and compared survival outcomes between the surgery alone group and the surgery plus radiotherapy group. OS of patients treated with postoperative radiotherapy was 6.1 months longer than that of patients treated with surgery alone (48). The survival rate was increased in those receiving radiotherapy than in those who achieved no further treatment after the surgery (4). The stereotactic radiosurgery has also been used in some cases with satisfactory survival benefits (23). Our study also confirmed the positive effect of postoperative radiotherapy with larger cohorts (p = 0.002), and the mean OS of patients with radiotherapy was 20 months longer than that of patients with no radiotherapy. It is noted that some patients did not accept radiotherapy because of severe neurological dysfunction after surgery. We recommend that when the tumor involves the brainstem and/or cranial nerves, the operation should not be aggressive. The proton beam therapy might be effective. Chen et al. reported a 43-year-old male who accepted proton beam therapy after surgery with no evidence of tumor recurrence for 2 years (49). Coincidentally, the patient accepted surgery at our hospital in September 2015, and we continued to follow up this patient. Through telephone interview, he was uneventful until February 2021, but 1 month later, he presented with dizziness, headache, and dysfunction of cranial nerves IX, X and XI; and the MRI scan revealed tumor recurrence. He has been alive for 72 months now. To the best of our knowledge, this case has the longest OS in the literature.
In addition, it was reported that IECs could transform into melanoma. Kaif et al. (50) reported a 26-year-old female with malignant melanoma arising in a cerebellopontine EC; and Meng et al. (51) described a case of fulminant leptomeningeal carcinomatosis from a malignant melanoma arising in a cerebellopontine epidermoid cyst. Contrast enhancement on MRI and rapid progression of clinical symptoms may indicate malignant transformation of IECs.
Conclusion
Malignant transformation of IEC into PSCC is very rare and has a poor clinical outcome. We described nine cases of malignant IECs at our institute over a period of 6 years and reviewed the relevant literature. These lesions often affect elderly patients. Contrast enhancement on MRI and rapid progression of clinical symptoms may indicate malignant transformation of IECs. Non-GTR of previously benign IECs may have potential risk for malignant transformation. For remnant benign IECs, close follow-up should be performed. Postoperative radiotherapy for PSCCs could bring survival benefit. GTR of these malignant intracranial tumors is difficult when they involve critical brain structures. Future studies with larger cohorts are necessary to verify these findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
PCZ: writing—original draft and conceptualization. TS: formal analysis and investigation. YW: literature review. YG: follow-up. PZ: literature review. ZW: methodology and resources. JZ: methodology and resources. LZ: writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Multicenter clinical big data study and multi-path tumorigenesis mechanisms and precision treatment research on brainstem glioma (JINGYIYAN2018-7) and Special Fund of the Pediatric Medical Coordinated Development Center of Beijing Hospitals Authority (XTYB201822).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pagni F, Brenna A, Leone BE, Vergani F, Isimbaldi G. Malignant Epidermoid Cyst of the Pineal Region With Lumbar Metastasis. Neuropathology: Off J Japanese Soc Neuropathol (2007) 27(6):566–9. doi: 10.1111/j.1440-1789.2007.00820.x
2. Roh TH, Park YS, Park YG, Kim SH, Chang JH. Intracranial Squamous Cell Carcinoma Arising in a Cerebellopontine Angle Epidermoid Cyst: A Case Report and Literature Review. Med (Baltimore) (2017) 96(51):e9423. doi: 10.1097/md.0000000000009423
3. Hamlat A, Hua ZF, Saikali S, Egreteau J, Guegan Y. Malignant Transformation of Intracranial Epidermoid Cyst With Leptomeningeal Carcinomatosis: Case Report. Acta Neurologica Belgica (2003) 103(4):221–4.
4. Kim MS, Kim OL. Primary Intracranial Squamous Cell Carcinoma in the Brain Stem With a Cerebellopontine Angle Epidermoid Cyst. J Korean Neurosurg Soc (2008) 44(6):401–4. doi: 10.3340/jkns.2008.44.6.401
5. Acciarri N, Padovani R, Foschini MP, Giulioni M, Finizio FS. Intracranial Squamous Cell Carcinoma Arising in an Epidermoid Cyst. Br J Neurosurg (1993) 7(5):565–9. doi: 10.3109/02688699308995081
6. Hao S, Tang J, Wu Z, Zhang L, Zhang J, Wang Z. Natural Malignant Transformation of an Intracranial Epidermoid Cyst. J Formosan Med Assoc (2010) 109(5):390–6. doi: 10.1016/s0929-6646(10)60068-x
7. Garcia CA, McGarry PA, Rodriguez F. Primary Intracranial Squamous Cell Carcinoma of the Right Cerebellopontine Angle. J Neurosurg (1981) 54(6):824–8. doi: 10.3171/jns.1981.54.6.0824
8. Hamlat A, Hua ZF, Saikali S, Laurent JF, Gedouin D, Ben-Hassel M, et al. Malignant Transformation of Intra-Cranial Epithelial Cysts: Systematic Article Review. J Neurooncol (2005) 74(2):187–94. doi: 10.1007/s11060-004-5175-4
9. Davidson SI, Small JM. Malignant Change in an Intracranial Epidermoid. J Neurol Neurosurg Psychiatry (1960) 23(2):176–8. doi: 10.1136/jnnp.23.2.176
10. Fox H, South EA. Squamous Cell Carcinoma Developing In An Intracranial Epidermoid Cyst (Cholesteatoma). J Neurol Neurosurg Psychiatry (1965) 28(3):276–81. doi: 10.1136/jnnp.28.3.276
11. Dubois PJ, Sage M, Luther JS, Burger PC, Heinz ER, Drayer BP. Case Report. Malignant Change in an Intracranial Epidermoid Cyst. J Comput Assisted Tomography (1981) 5(3):433–5. doi: 10.1097/00004728-198106000-00025
12. Lewis AJ, Cooper PW, Kassel EE, Schwartz ML. Squamous Cell Carcinoma Arising in a Suprasellar Epidermoid Cyst. Case Report. J Neurosurg (1983) 59(3):538–41. doi: 10.3171/jns.1983.59.3.0538
13. Giangaspero F, Manetto V, Ferracini R, Piazza G. Squamous Cell Carcinoma of the Brain With Sarcoma-Like Stroma. Virchows Archiv A Pathological Anat Histopathol (1984) 402(4):459–64. doi: 10.1007/bf00734642
14. Goldman SA, Gandy SE. Squamous Cell Carcinoma as a Late Complication of Intracerebroventricular Epidermoid Cyst. Case Report. J Neurosurg (1987) 66(4):618–20. doi: 10.3171/jns.1987.66.4.0618
15. Nishiura I, Koyama T, Handa J, Amano S. Primary Intracranial Epidermoid Carcinoma–Case Report. Neurologia Medico-Chirurgica (1989) 29(7):600–5. doi: 10.2176/nmc.29.600
16. Knorr JR, Ragland RL, Smith TW, Davidson RI, Keller JD. Squamous Carcinoma Arising in a Cerebellopontine Angle Epidermoid: CT and MR Findings. AJNR Am J Neuroradiol (1991) 12(6):1182–4.
17. Tognetti F, Lanzino G, Manetto V, Calbucci F. Intracranial Squamous Cell Carcinoma Arising in Remnant of Extirpated Epidermoid Cyst. Br J Neurosurg (1991) 5(3):303–5. doi: 10.3109/02688699109005191
18. Nishio S, Takeshita I, Morioka T, Fukui M. Primary Intracranial Squamous Cell Carcinomas: Report of Two Cases. Neurosurgery (1995) 37(2):329–32. doi: 10.1227/00006123-199508000-00021
19. Uchino A, Hasuo K, Matsumoto S, Uda K, Moriguchi M, Nishio T, et al. Intracranial Epidermoid Carcinoma: CT and MRI. Neuroradiology (1995) 37(2):155–8. doi: 10.1007/bf00588635
20. Bayindir C, Balak N, Karasu A. Micro-Invasive Squamous Cell Carcinoma Arising in a Pre-Existing Intraventricular Epidermoid Cyst. Case Report and Literature Review. Acta Neurochir (Wien) (1996) 138(8):1008–12. doi: 10.1007/bf01411292
21. Murase S, Yamakawa H, Ohkuma A, Sumi Y, Kajiwara M, Takami T, et al. Primary Intracranial Squamous Cell Carcinoma–Case Report. Neurologia Medico-Chirurgica (1999) 39(1):49–54. doi: 10.2176/nmc.39.49
22. Asahi T, Kurimoto M, Endo S, Monma F, Ohi M, Takami M. Malignant Transformation of Cerebello-Pontine Angle Epidermoid. J Clin Neurosci (2001) 8(6):572–4. doi: 10.1054/jocn.2000.0856
23. Link MJ, Cohen PL, Breneman JC, Tew JM Jr. Malignant Squamous Degeneration of a Cerebellopontine Angle Epidermoid Tumor. Case Report. J Neurosurg (2002) 97(5):1237–43. doi: 10.3171/jns.2002.97.5.1237
24. Shirabe T, Fukuoka K, Watanabe A, Imamura K, Ishii R. Primary Squamous Cell Carcinoma of the Brain. A Rare Autopsy Case. Neuropathology: Off J Japanese Soc Neuropathology (2003) 23(3):225–9. doi: 10.1046/j.1440-1789.2003.00499.x
25. Michael LM 2nd, Moss T, Madhu T, Coakham HB. Malignant Transformation of Posterior Fossa Epidermoid Cyst. Br J Neurosurg (2005) 19(6):505–10. doi: 10.1080/02688690500495356
26. Tamura K, Aoyagi M, Wakimoto H, Tamaki M, Yamamoto K, Yamamoto M, et al. Malignant Transformation Eight Years After Removal of a Benign Epidermoid Cyst: A Case Report. J Neurooncol (2006) 79(1):67–72. doi: 10.1007/s11060-005-9117-6
27. Agarwal S, Rishi A, Suri V, Sharma MC, Satyarthi GD, Garg A, et al. Primary Intracranial Squamous Cell Carcinoma Arising in an Epidermoid Cyst–a Case Report and Review of Literature. Clin Neurol Neurosurg (2007) 109(10):888–91. doi: 10.1016/j.clineuro.2007.07.026
28. Kodama H, Maeda M, Hirokawa Y, Suzuki H, Hori K, Taki W, et al. MRI Findings of Malignant Transformation of Epidermoid Cyst: Case Report. J Neurooncol (2007) 82(2):171–4. doi: 10.1007/s11060-006-9255-5
29. Ge P, Luo Y, Fu S, Ling F. Recurrent Epidermoid Cyst With Malignant Transformation Into Squamous Cell Carcinoma. Neurologia Medico-Chirurgica (2009) 49(9):442–4. doi: 10.2176/nmc.49.442
30. Kano T, Ikota H, Kobayashi S, Iwasa S, Kurosaki S, Wada H. Malignant Transformation of an Intracranial Large Epidermoid Cyst With Leptomeningeal Carcinomatosis: Case Report. Neurologia Medico-Chirurgica (2010) 50(4):349–53. doi: 10.2176/nmc.50.349
31. Nakao Y, Nonaka S, Yamamoto T, Oyama K, Esaki T, Tange Y, et al. Malignant Transformation 20 Years After Partial Removal of Intracranial Epidermoid Cyst–Case Report. Neurologia Medico-Chirurgica (2010) 50(3):236–9. doi: 10.2176/nmc.50.236
32. Shah A, Goel A, Goel N. A Case of Cerebellopontine Angle Epidermoid Tumor and Brainstem Squamous Cell Carcinoma Presenting as Collision Tumor. Acta Neurochir (Wien) (2010) 152(6):1087–8. doi: 10.1007/s00701-010-0606-9
33. Lakhdar F, Hakkou el M, Gana R, Maaqili RM, Bellakhdar F. Malignant Transformation Six Months After Removal of Intracranial Epidermoid Cyst: A Case Report. Case Rep Neurological Med (2011) 2011:525289. doi: 10.1155/2011/525289
34. Chon KH, Lee JM, Koh EJ, Choi HY. Malignant Transformation of an Epidermoid Cyst in the Cerebellopontine Angle. J Korean Neurosurg Soc (2012) 52(2):148–51. doi: 10.3340/jkns.2012.52.2.148
35. Feng R, Gu X, Hu J, Lang L, Bi H, Guo J, et al. Surgical Treatment and Radiotherapy of Epidermoid Cyst With Malignant Transformation in Cerebellopontine Angle. Int J Clin Exp Med (2014) 7(1):312–5.
36. Vellutini EA, de Oliveira MF, Ribeiro AP, Rotta JM. Malignant Transformation of Intracranial Epidermoid Cyst. Br J Neurosurg (2014) 28(4):507–9. doi: 10.3109/02688697.2013.869552
37. Chourmouzi D, Papadopoulou E, Karkavelas G, Drevelegas A. Imaging Findings of an Epidermoid Cyst Undergoing Malignant Transformation. J Belgian Soc Radiol (2015) 1599(1):42–5. doi: 10.5334/jbr-btr.829
38. Ding S, Jin Y, Jiang J. Malignant Transformation of an Epidermoid Cyst in the Temporal and Prepontine Region: Report of a Case and Differential Diagnosis. Oncol Lett (2016) 11(5):3097–100. doi: 10.3892/ol.2016.4368
39. Pikis S, Margolin E. Malignant Transformation of a Residual Cerebellopontine Angle Epidermoid Cyst. J Clin Neurosci (2016) 33:59–62. doi: 10.1016/j.jocn.2016.04.008
40. Raheja A, Eli IM, Bowers CA, Palmer CA, Couldwell WT. Primary Intracranial Epidermoid Carcinoma With Diffuse Leptomeningeal Carcinomatosis: Report of Two Cases. World Neurosurg (2016) 88:692.e9–692.e16. doi: 10.1016/j.wneu.2015.11.039
41. Ozutemiz C, Ada E, Ersen A, Ozer E. Imaging Findings of an Epidermoid Cyst With Malignant Transformation to Squamous Cell Carcinoma. Turkish Neurosurg (2017) 27(2):312–5. doi: 10.5137/1019-5149.Jtn.12722-14.0
42. Seif B, Pourkhalili R, Shekarchizadeh A, Mahzouni P. Malignant Transformation of an Intracranial Extradural Epidermoid Cyst Into Squamous Cell Carcinoma Presented With Cerebrospinal Fluid Leakage. Advanced Biomed Res (2017) 6:16. doi: 10.4103/2277-9175.200791
43. Solanki SP, Maccormac O, Dow GR, Smith S. Malignant Transformation of Residual Posterior Fossa Epidermoid Cyst to Squamous Cell Carcinoma. Br J Neurosurg (2017) 31(4):497–8. doi: 10.3109/02688697.2015.1125445
44. Cuoco JA, Rogers CM, Busch CM, Apfel LS, Entwistle JJ, Marvin EA. Intracranial Squamous Cell Carcinoma Arising From a Cerebellopontine Angle Epidermoid Cyst Remnant Four Decades After Partial Resection. Front Oncol (2019) 9:694. doi: 10.3389/fonc.2019.00694
45. Demuth S, Lasry DE, Obaid S, Letourneau-Guillon L, Chasse M, Belanger K, et al. Pseudo-Chemical Meningitis and the Malignant Transformation of an Epidermoid Cyst. Can J Neurological Sci Le J Canadien Des Sci Neurologiques (2019) 46(5):642–4. doi: 10.1017/cjn.2019.235
46. Fereydonyan N, Taheri M, Kazemi F. Cerebellar Squamous Cell Carcinoma Due to Malignant Transformation of Cerebellopontine Angle Epidermoid Cyst, Report an Interesting Case and Review the Literature. Prague Med Rep (2019) 120(2-3):95–102. doi: 10.14712/23362936.2019.14
47. Gerges MM, Godil SS, Rumalla K, Liechty B, Pisapia DJ, Magge RS, et al. Genomic Profile of a Primary Squamous Cell Carcinoma Arising From Malignant Transformation of a Pineal Epidermoid Cyst. Acta Neurochir (Wien) (2019) 161(9):1829–34. doi: 10.1007/s00701-019-03983-5
48. Nagasawa D, Yew A, Spasic M, Choy W, Gopen Q, Yang I. Survival Outcomes for Radiotherapy Treatment of Epidermoid Tumors With Malignant Transformation. J Clin Neurosci (2012) 19(1):21–6. doi: 10.1016/j.jocn.2011.06.002
49. Chen Z, Araya M, Onishi H. Proton Beam Therapy for Malignant Transformation of Intracranial Epidermoid Cyst. BMJ Case Rep (2019) 17:12(7). doi: 10.1136/bcr-2019-229388
50. Kaif M, Neyaz A, Shukla S, Husain N. Fulminant Leptomeningeal Carcinomatosis From a Malignant Melanoma Arising in a Cerebellopontine Epidermoid Cyst: A Rare Case With Diagnostic Pointers. Neuropathology: Off J Japanese Soc Neuropathol (2018) 38(5):503–9. doi: 10.1111/neup.12480
Keywords: intracranial epidermoid cysts, malignant transformation, gross total resection, radiotherapy, the cerebellopontine angle
Citation: Zuo P, Sun T, Wang Y, Geng Y, Zhang P, Wu Z, Zhang J and Zhang L (2021) Primary Squamous Cell Carcinomas Arising in Intracranial Epidermoid Cysts: A Series of Nine Cases and Systematic Review. Front. Oncol. 11:750899. doi: 10.3389/fonc.2021.750899
Received: 31 July 2021; Accepted: 30 September 2021;
Published: 26 October 2021.
Edited by:
Brad E. Zacharia, Penn State Milton S. Hershey Medical Center, United StatesReviewed by:
Subhas K. Konar, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaErin Bonner, Children’s National Health System, United States
Copyright © 2021 Zuo, Sun, Wang, Geng, Zhang, Wu, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liwei Zhang, emhhbmdsaXdlaXR0eXlAMTYzLmNvbQ==
 Pengcheng Zuo1
Pengcheng Zuo1 Zhen Wu
Zhen Wu Junting Zhang
Junting Zhang Liwei Zhang
Liwei Zhang