
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 November 2021
Sec. Cancer Metabolism
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.749881
This article is part of the Research Topic Double-Edged Swords: Important Factors Connecting Metabolic Disorders and Cancer Development - From Basic Research to Translational Applications View all 11 articles
 Ying Shan1,2†
Ying Shan1,2† Meng Qin1,2†
Meng Qin1,2† Jie Yin1,2
Jie Yin1,2 Yan Cai1,2
Yan Cai1,2 Yan Li1,2
Yan Li1,2 Yu Gu1,2
Yu Gu1,2 Wei Wang1,2
Wei Wang1,2 Yong-xue Wang1,2
Yong-xue Wang1,2 Jia-yu Chen1,2
Jia-yu Chen1,2 Ying Jin1,2*
Ying Jin1,2* Ling-ya Pan1,2*
Ling-ya Pan1,2*Objective: To investigate the oncologic and reproductive outcomes of fertility-sparing treatments (FSTs) in atypical endometrial hyperplasia (AEH) and endometrial cancer (EC) patients with excess weight (EW).
Methods: This retrospective study comprised patients with AEH or EC who achieved a complete response (CR) after FST from 2010 to 2018. The clinical characteristics, oncological and reproductive outcomes were compared between the excess weight (EW) group (body mass index (BMI)≥25 kg/m2) and normal weight (NW) group (BMI<25 kg/m2). The risk factors associated with recurrence and unsuccessful pregnancy in patients with EW were analyzed.
Results: Overall, 227 patients were enrolled, including 139 (61.2%) in EW group and 88 (38.8%) in NW group. In patients with EW, the pregnancy rate, the live birth rate and the relapse rate were 29.8%, 23.4%, and 30.9%, respectively. In patients with NW, these rates were 61.1%, 47.2%, and 31.8%, respectively. No significant differences were observed in the time to remission (P=0.865) and disease-free survival (DFS) (P=0.750). Patients in NW group achieved a better pregnancy rate than patients in the EW group (P=0.034). The patients with EW using ovulation induction to increase fertility tended to have a shorter time to pregnancy (P=0.042). However, no significant risk factors associated with unsuccessful pregnancy were identified after the multivariate analysis. In terms of DFS, the combination of gonadotropin-releasing hormone agonist (GnRH-a) and LNG-IUD was better for patients with EW than GnRH-a or oral progestin therapy alone (P=0.044, adjusted hazard ratio (HR)=0.432, 95% confidence interval (CI): 0.152-1.229), especially for patients with EW diagnosed with EC (P=0.032).
Conclusion: FSTs for overweight and obese patients should be more individualized. GnRH-a and/or LNG-IUD may be options prior to FSTs in patients with EW. Further prospective studies are needed.
Endometrial cancer (EC) is one of the most common malignant tumors in females (1). EC usually arises in postmenopausal women, but approximately 10% of EC patients are younger than 40 years old (2). Atypical endometrial hyperplasia (AEH) prior to EC represents a continuously changing disease process as AEH is a precancerous lesion of EC. The risk of AEH progressing to EC within fifteen years has been reported to be as high as 29.0% (3, 4). Thus, therapy for both AEH and EC should warrant attention.
In recent years, the incidence of young patients with AEH and EC has increased worldwide, and fertility-sparing treatments (FSTs) to preserve reproductive function are urgently needed (5). Approximately 80% of young EC patients have well-differentiated type I disease at a very early stage and high-estrogen exposure backgrounds, presenting the possibility of progestin-based therapy (6). The National Comprehensive Cancer Network (NCCN) has provided FST options for the management of AEH and EC patients who meet five specific criteria (7). The common recommended conservative treatments for AEH and EC include high-dose oral progestin and levonorgestrel intrauterine devices (LNG-IUDs). After FST, most AEH and EC patients can achieve a higher complete response (CR) rate and lower relapse rate and then successfully undergo delivery (8). Nevertheless, approximately one-quarter of females still suffer from recurrence problems. Gallos et al. showed that the relapse rates of patients with EC and AEH after FST were 40.6% and 26%, respectively, in a meta-analysis of 34 observational studies (9). The curative effect and prognostic outcomes after recurrence were not satisfactory. Therefore, the risk factors for recurrence must be identified to decrease the risk of recurrence.
The relationship between obesity and endometrial cancer (EC) has been established and accepted for decades. Overweight and obesity are evaluated by body mass index (BMI), which is a measurement of a person’s weight with respect to his or her height. The World Health Organization (WHO) defines an adult who has a BMI between 25 kg/m2 and 29.9 kg/m2 as overweight and an adult who has a BMI of 30 kg/m2 or higher as obese (10). On the one hand, the risk of EC increases with increasing weight according to several previous reports (11). Compared with normal-weight women, the relative risk (RR) and odds ratio for developing EC were 1.34 and 1.43 in overweight women and 2.54 and 3.33 in obese women, respectively (12). The previous study from our single team reported that age ≥35 years, obesity, prolonged time to CR, and consistent infertility after conservative treatment were associated with an increased risk of recurrence (9). In addition, although the mortality rate of EC is low, the RR of death is significantly higher for obese EC patients than for those with a normal BMI (RR 2.53 for BMI 30–34 kg/m2, RR 6.25 for BMI > 40 kg/m2) (13). Thus, we can solve most of treatment and recurrence problems related to FSTs if we can solve the treatment problems encountered in obese patients with AEH and EC. On the other hand, FSTs for obese patients are challenging. Obese patients with EC often have multiple complications, such as polycystic ovarian syndrome (PCOS), diabetes mellitus (DM), and hypertension. Moreover, obesity not only is a risk factor for developing EC but may also significantly impact pregnancy (14). High-dose oral progestin, as the most common FST, has side effects, including weight gain, abnormal lipid metabolism, and compromised liver function. These side effects limit its application in overweight patients and may lead to an increased risk of recurrence (15). Recently, the use of gonadotropin-releasing hormone agonist (GnRH-a) has become widely popularized for obese patients in clinical practice, but NCCN guidelines do not provide specific treatment recommendations (16).
Therefore, we performed this retrospective study to explore the oncologic and reproductive results of FSTs in patients with excess weight (EW) with AEH and EC, as well as risk factors for unsuccessful and recurrent pregnancy. Developing more suitable management strategies is important for such populations.
This retrospective study included all patients with AEH and EC who received FSTs between January 2010 and December 2018 at Peking Union Medical College Hospital (PUMCH). Patients with EW were defined as having a BMI equal to or greater than 25 kg/m2. In our institution, patients were considered candidates for FST when they met the following criteria, which were almost consistent with NCCN guidelines (7): 1) age younger than 40 years old and a strong desire for fertility preservation; 2) a diagnosis of EC of a well-differentiated type (G1) or AEH through dilation and curettage (D&C) with or without hysteroscopy, with confirmation of the pathological diagnosis by at least two experienced gynecological pathologists; 3) a tumor confined to the endometrium with no evidence of myometrial invasion as evaluated by transvaginal ultrasonography and pelvic magnetic resonance imaging (MRI); 4) Estrogen receptor (ER) and progesterone receptor (PR) positivity; 5) a normal serum CA125 level; 6) no contraindications for progestin therapy or other medical therapy; 7) fertility function assessment prior to FST; and 8) an understanding through counseling that fertility-sparing option is not a standard of care for the treatment of EC and provision of written informed consent. This study was conducted with the approval of the Ethics Committee of PUMCH.
All included patients were provided counseling regarding their FST options, including the side effects of the drugs and potential risks of recurrence or progression. Patients who met the study inclusion criteria were divided into two groups: the excess weight (EW) group, BMI of which was equal to or more than 25 kg/m2; the normal weight (NW) group, BMI of which was less than 25 kg/m2. In this study, the treatment methods were divided into four groups: 1) MPA; 2) MA; 3) GnRH-a; and 4) GnRH-a+LNG-IUD. Oral progestin therapy is one of the most common primary FSTs and includes medroxyprogesterone acetate (MPA) at doses of 250-500 mg/day or megestrol acetate (MA) at doses of 160-480 mg/day. If a patient with EC or with extended lesion, GnRH-a was administered for three to six cycles (3.6 mg/3.75 mg) by subcutaneous injection as the first treatment according to experience. In addition, an LNG-IUD could be placed in combination for treatment. The patients using common therapy in combination with letrozole or only LNG-IUD were in a small number, which were excluded from this study to avoid results bias. The response to treatment was assessed every 3-6 months using pathological specimens, which were obtained via D&C and hysteroscopy. CR was defined as the absence of endometrial hyperplasia or carcinoma. Patients were recommended to receive regular maintenance treatment after CR while waiting for fertility or not having fertility willing, which included LNG-IUD or low-dose oral progestin. The ultrasound should be administered every 3 to 6 months during maintenance therapy.
All willing patients with immediate fertility after a CR were transferred to the specialized reproductive center to undergo counseling regarding reproductive treatment options. In a general way, the patients with a preferable ovarian reserve and successful ovulation, as well as smooth fallopian tubes, were encouraged to conceive spontaneously. Patients with anovulation were recommended track their sex life and induce ovulation with letrozole, which was administered at a dose of 2.5 mg/day for 5 days. For patients with a reduced ovarian reserve, anovulation, or PCOS, assisted reproductive technology (ART) was encouraged as early as possible. These methods included intrauterine insemination (IUI) or in vitro fertilization and embryo transfer (IVF-ET). The time to pregnancy was defined as the time interval between the date that CR was achieved and the date that a pregnancy was confirmed by ultrasound examination or the final follow-up.
Recurrence was defined as initial lesions (AEH or EC) reappearing in the specimen after complete remission or a disease lesion reappearing in the endometrium and/or myometrium on imaging examination. Patients with EW were divided into two groups: the recurrence group and the control group. Disease-free survival (DFS) was defined as the time interval between the date that CR was achieved and the date of recurrence or the final follow-up. For this study, patients with any of the following characteristics were excluded: 1) received other treatments except MA, MPA and GnRHa ± LNG-IUD; 2) did not achieve CR after FST; 3) were not regularly evaluated every three months via D&C or hysteroscopy during the treatment period; and 4) were not followed up regularly after CR at PUMCH or a local hospital.
All statistical analyses were performed using SPSS software (version 23.0; SPSS Inc., Chicago, IL, USA), and graphs were generated using GraphPad Prism software for Macbook (version 7.0; GraphPad software Inc., San Diego, USA). Student’s t-tests and Mann-Whitney U tests were used to compare continuous variables. Pearson’s chi-squared tests and Fisher’s exact tests were used to compare categorical variables (17). Survival analysis was performed using Kaplan-Meier curves and the log-rank test. Each factor related to survival outcomes was individually evaluated using a Cox regression model in a univariate analysis. Then, all variables with P values <0.200 and meaningful variables based on the univariate analysis were included in the Cox proportional hazards regression model in a multivariate analysis. The associations of these variables with follow-up outcomes was evaluated by hazard ratios (HRs) and 95% confidence intervals (CIs). Statistical significance was set at P<0.050.
Table 1 shows the clinical and pathological characteristics of AEH and EC patients after FST between EW and the NW group. Overall, 227 patients who met the inclusion criteria were included in this retrospective analysis. The NW group contained 88 (38.8%) patients, while the EW group contained 139 (61.2%) patients, including 74 (53.2%) patients with a 25≤BMI<30 kg/m2 and 65 (46.8%) patients with a BMI≥30 kg/m2. The mean BMI of all patients was 26.8( ± 5.2) kg/m2 (ranging from 18.0 to 46.5 kg/m2), and the mean age was 31.5 ( ± 4.7) years. A total of 29.1% of patients had complications, including PCOS (20.7%), DM (5.3%), and others (3.1%). A total of 63.0% of patients were diagnosed with AEH, and 37.0% were diagnosed with EC. The median follow-up time was 41.7 ( ± 23.0) months. No significant differences were observed in complications (P=0.120), menstruation cycle (P=0.190), previous pregnancy (P=0.050), previous delivery (P=0.063), and histology (P=0.902). Therefore, most of variables were equally comparable for the survival analysis.
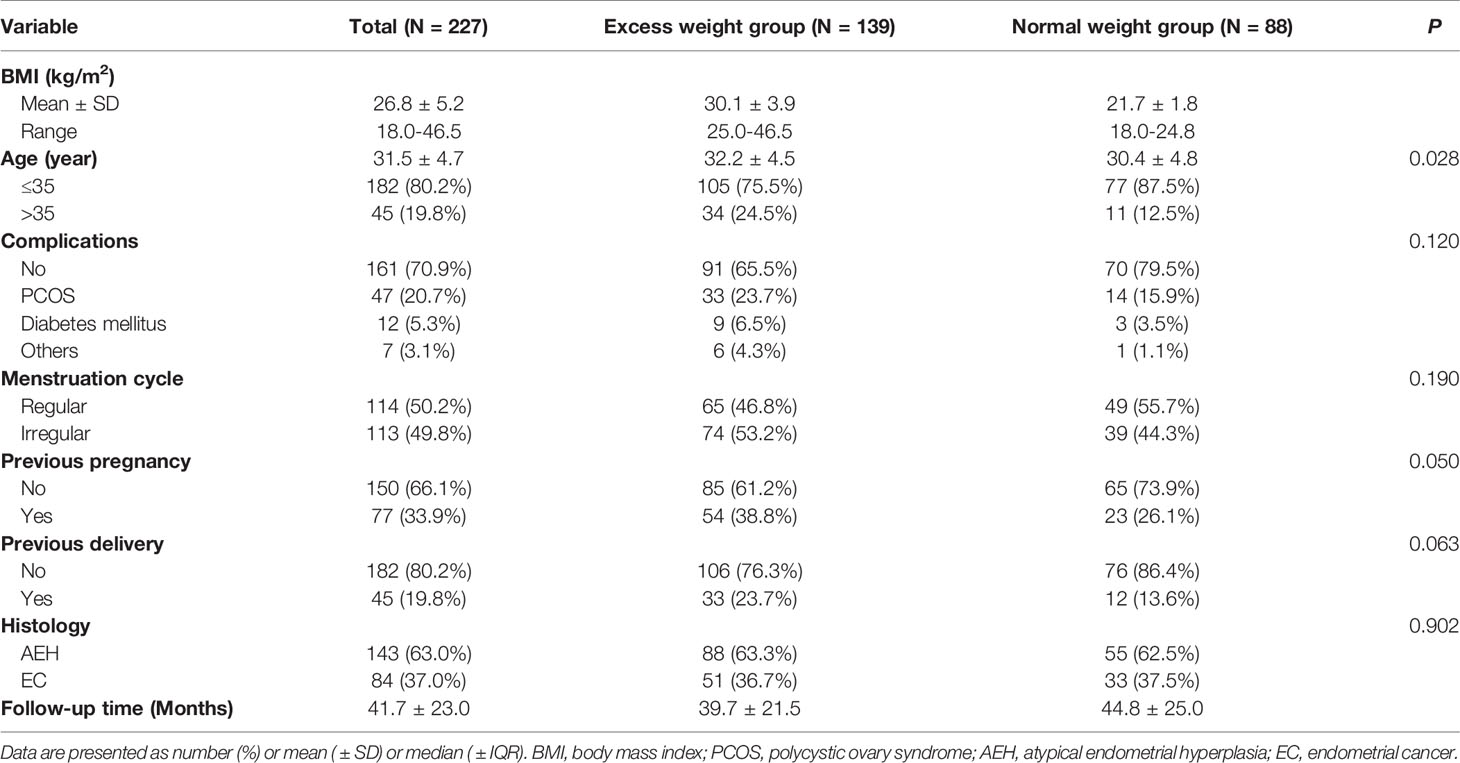
Table 1 The clinical characteristics of AEH and EC patients after FST between the excess weight group and normal weight group.
The treatment, follow-up and reproductive outcomes of the included patients are shown in Table 2. The treatment methods were divided into four groups: MPA (40.1%), MA (17.2%), GnRH-a (17.6%), and GnRH-a+LNG-IUD (25.1%). A total of 51.1% of patients received regular maintenance treatment, including LNG-IUDs (73.3%) and Duphaston (26.7%). The mean time to a CR was 8.7 ( ± 5.6) months, and 22% patients need more than 12 months. The time to remission among the four treatment types did not significantly differ (P=0.597) in patients with EW, as shown in Figure 2A. A total of 33.8% of patients with EW had an immediate pregnancy intention after remission and attempted to become pregnant by different methods. Among these patients with EW, 27.7% of patients spontaneously became pregnant, 23.4% used ovulation induction, and the others used IVF-ET to improve their chances of conceiving. In patients with EW, the pregnancy rate, the live birth rate and the relapse rate were respectively 29.8%, 23.4%, and 30.9%. While in patients with NW, the pregnancy rate, the live birth rate and the relapse rate were respectively 61.1%, 47.2%, and 31.8%. There were no significant differences of the time to remission between EW group and NW group in Figure 1A (P=0.865). The patients in NW group showed similar DFS with patients in EW group in Figure 1B (P=0.750, HR=0.926, 95% CI: 0.577-1.485). However, the patients in NW group had better pregnancy rate than in EW group (P=0.034, HR=2.023, 95% CI: 1.047-3.909), as shown in Figure 1C.
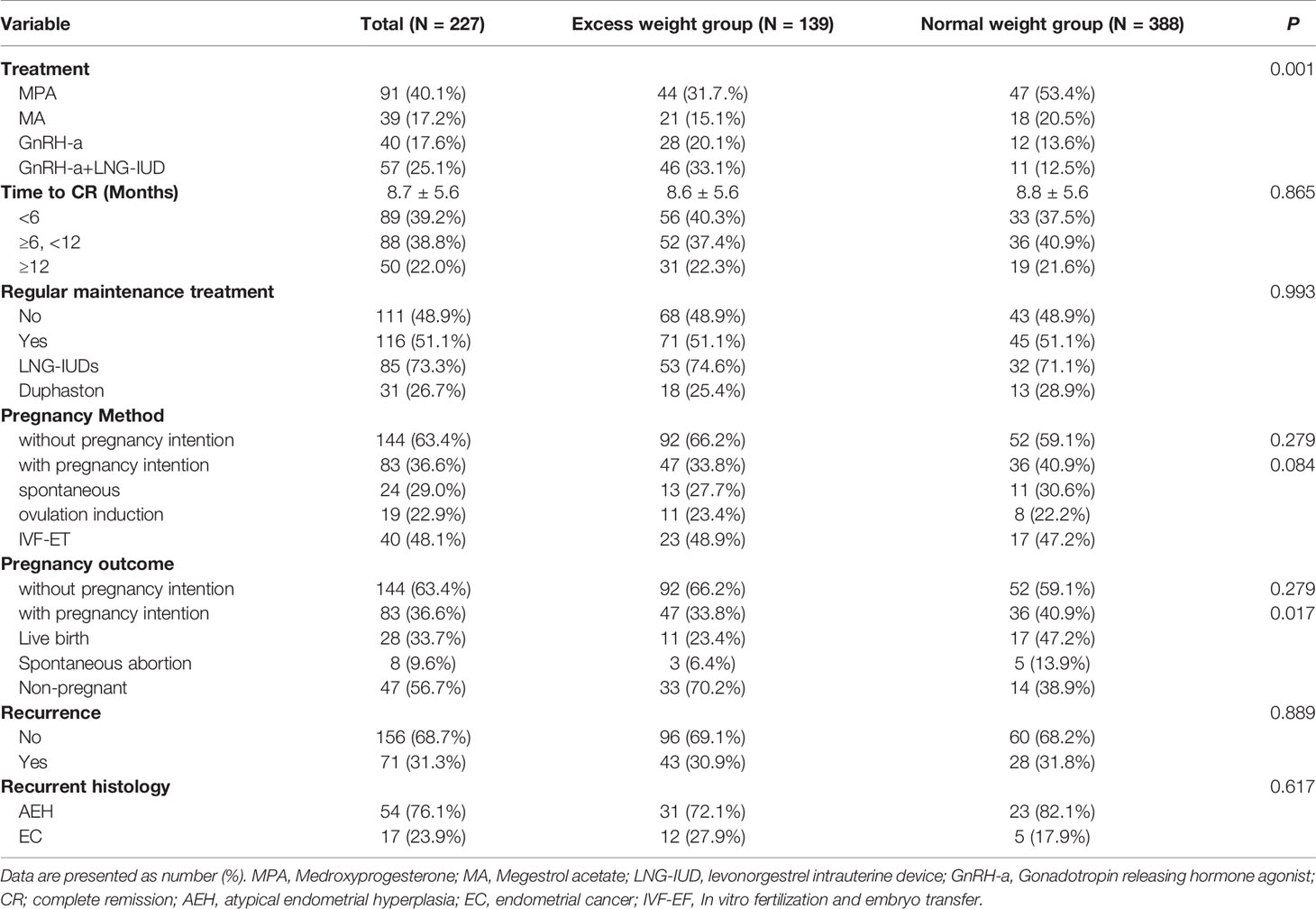
Table 2 The treatment, follow-up and reproductive outcomes of AEH and EC patients after FST between the excess weight group and normal weight group.
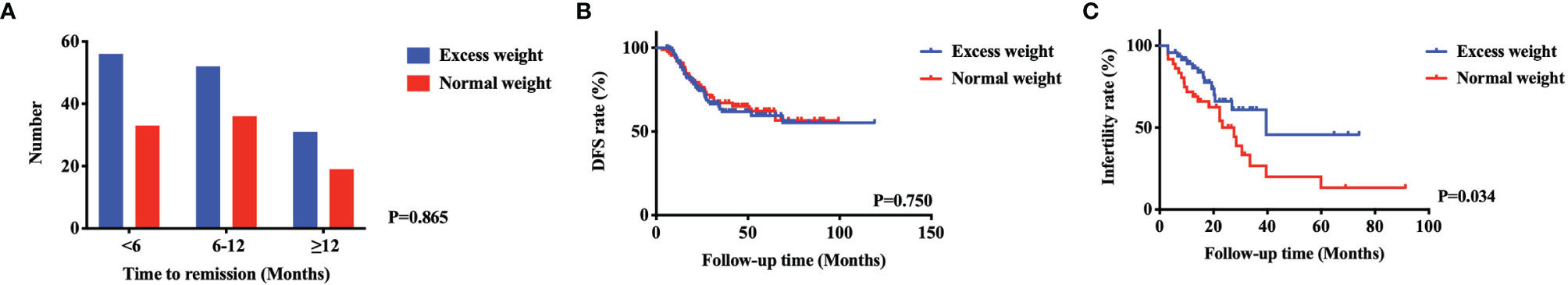
Figure 1 The comparison of AEH and EC patients after FST between excess weight group and normal weight group. There were no significant differences of the time to remission (A) and DFS (B) between two groups. The patients in NW group had better pregnancy rate than in EW group (C).
The risk factors associated with unsuccessful pregnancy among AEH and EC patients with EW after FSTs with pregnancy intention were further analyzed. In the univariate analysis shown in Table 3, the P values of the following two factors were less than 0.050: histology (P=0.009), and pregnancy method (P=0.042). In Figure 2B, the patients with EW who used letrozole for ovulation induction to promote conception had the shortest time to pregnancy, followed by those who used IVF-ET and those who achieved spontaneous pregnancy. However, there were no significant risk factors associated with unsuccessful pregnancy after the multivariate analysis.
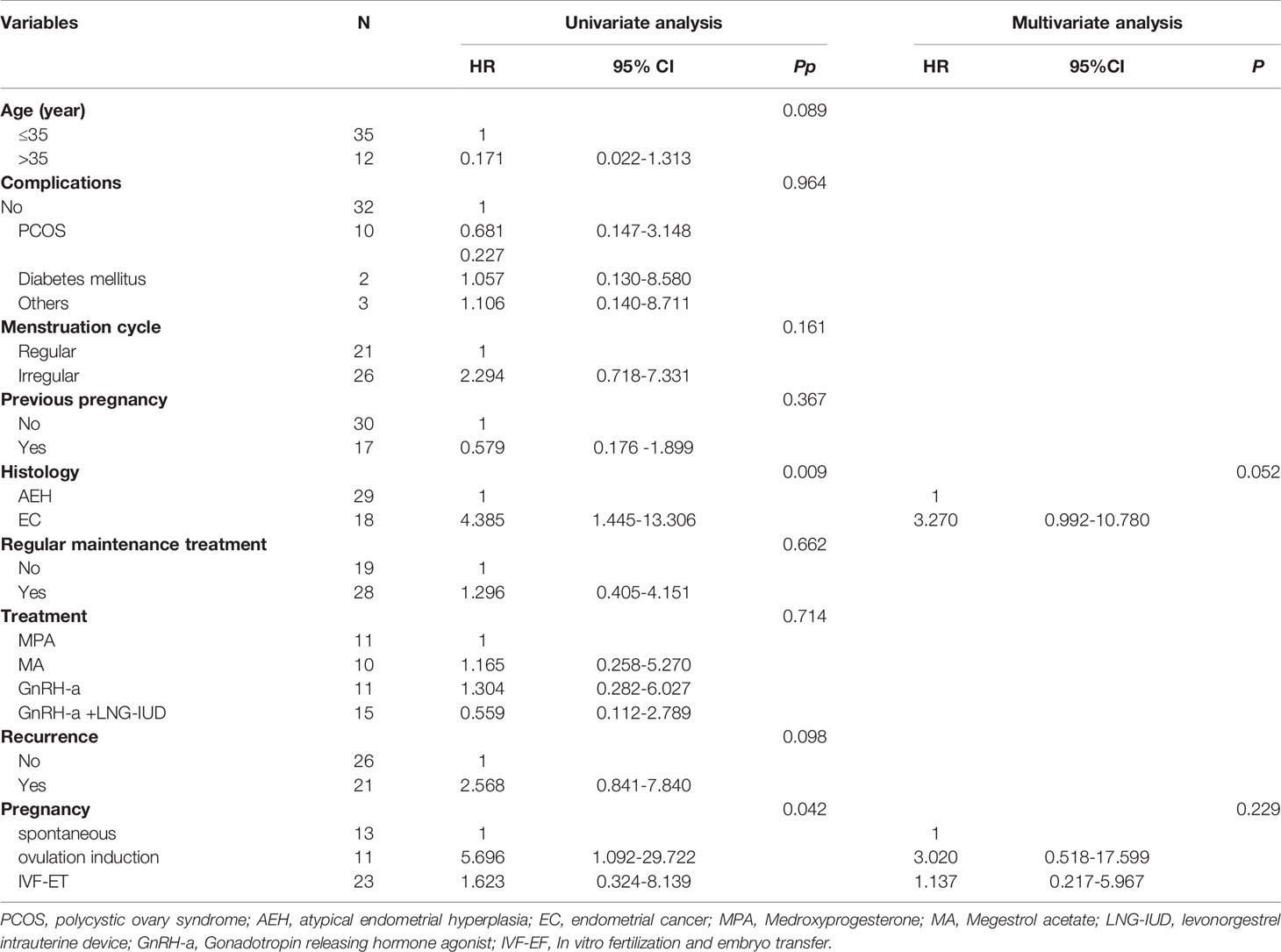
Table 3 The univariate analysis and multivariate analysis of risk factors associated with infertility for AEH and EC patients of excess weight after FST with pregnancy intention.
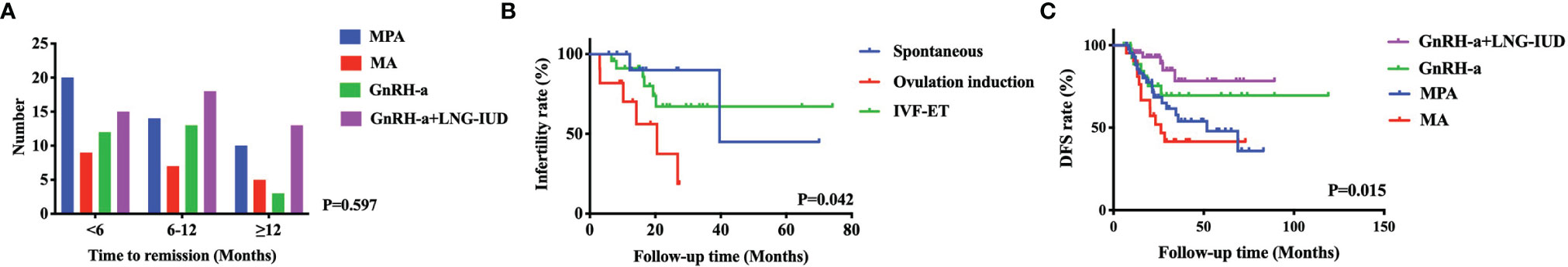
Figure 2 The risk factors associated with infertility for AEH and EC patients of excess weight with pregnancy intention. The time to remission among the four treatment types did not significantly differ in patients with EW (A). In the univariate analysis, the patients with EW who used ovulation induction to promote conception had the shortest time to pregnancy, followed by those who used IVF-ET and those who achieved spontaneous pregnancy (B). The patients with EW treated with GnRH-a+LNG-IUD had the best DFS, followed by those treated with GnRH-a and MPA, and the worst DFS was observed in patients treated with MA (C).
Similarly, in the subgroup analysis of initial pathology, the oncologic outcomes of the included patients with EW characterized by the pregnancy method were further analyzed. There were no significant differences among three pregnancy methods in AEH (P=0.456) and EC (P=0.111) patients with EW, which were respectively shown in Figures S1A, B.
We analyzed the risk factors associated with recurrence for AEH and EC patients with EW after FSTs. In the univariate analysis, as shown in Table 4, the P values of the following two factors were less than 0.050: the absence of regular maintenance treatment (P=0.017) and treatment type (P=0.015). In Figure 2C, the patients with EW treated with GnRH-a+LNG-IUD had the best DFS (HR=0.309, 95%CI: 0.122-0.778), followed by those treated with GnRH-a and MPA, and the worst DFS was observed in patients treated with MA. After multivariate analysis, the treatment type was the risk factors associated with recurrence for AEH and EC patients with EW (P=0.044, adjusted HR=0.432, 95% CI: 0.152-1.229).
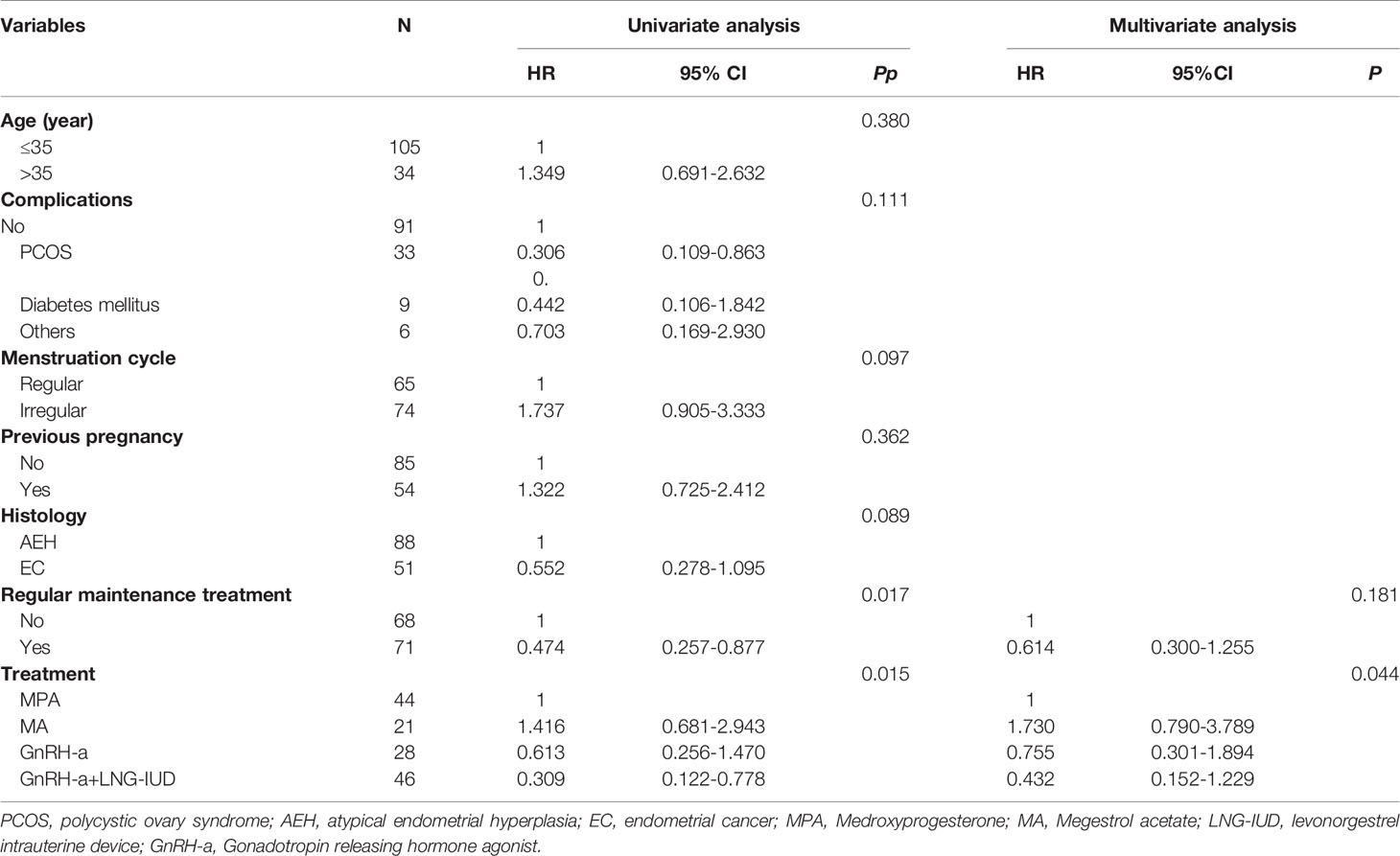
Table 4 The univariate analysis and multivariate analysis of risk factors associated with recurrence for AEH and EC patients of excess weight after FST.
In the subgroup analysis of initial pathology, we further analyzed the oncologic outcomes of the included patients with EW characterized by treatment method. Similar tendencies for oncologic outcomes among EC patients with EW (P=0.032) are shown in Figure S1D. However, there was no significant difference among four treatment methods in AEH patients with EW (P=0.289), as shown in Figure S1C.
BMI plays an important role in the occurrence and development of AEH and EC. Approximately two-thirds of EC patients are obese, and the risk of EC is 2- to 5-times higher among obese women, which can be explained by the fact that increased sex hormone production from adipose tissue causes unopposed estrogen stimulation in the endometrial lining, similar to what occurs in breast cancer (6). Our previous study reported that obesity is not a risk factor for recurrence in AEH and EC patients after FST, although EW led to a higher incidence rate of EC and AEH (9). Gonthier et al. also showed that obese patients with AEH and EC also had similar CR rates and relapse rates compared with nonobese patients (18). However, a study performed by von Greunigen and the Gynecologic Oncology Group (GOG) showed that obesity increases the risk of mortality among women with a diagnosis of EC (19). In addition, obese patients often also have insulin resistance or cardiovascular diseases, which may lead to a longer therapeutic duration and poor prognosis for AEH and EC patients after FST (20). Therefore, overweight and obesity are nonnegligible risk factors during therapy for AEH and EC.
For AEH or EC patients without any contraindications receiving FSTs, the common treatment is high-dose progestin (21). However, the most common side effect of high-dose oral progestin is an increase in BMI, which creates a very large challenge for overweight patients trying to control their body weight (22). In addition, high-dose oral progestin may lead to elevated liver enzymes, which is also a very unfavorable condition for obese patients, especially those with DM or hypertension (23). Cholakian et al. showed that in patients with a BMI ≥ 35 kg/m2, MA was associated with more weight gain than LNG-IUDs (+2.2 vs -5.40 kg, P=0.05) (24). Regarding conservative treatment for EC, weight change is one of the evaluation indices for treatment effect and prognostic outcomes (25). Park et al. reported that a BMI≥25 kg/m2 before and after treatment is an important predictor for poor treatment response and high recurrence rate (25). The treatments for obesity include diet control, exercise, drugs, and bariatric surgery (26). The NCCN guidelines have indicated the importance of effective weight control and healthy lifestyle management for overweight patients. In our institution, overweight patients are also required to seek advice for weight loss in the nutrition department while undergoing FSTs. It is essential for patients to maintain a normal BMI during progestin treatment. Therefore, oral progestin may not be an optimal option for overweight AEH and EC patients, as the most common side effect is weight gain (22).
GnRH-a and LNG-IUDs as effective and acceptable forms of treatment have been used for multiple purposes by thousands of women worldwide. In our study, the combined use of GnRH-a and LNG-IUDs yielded the best DFS trend among treatments, especially for overweight EC patients. On the one hand, this result can be explained by a lower probability of weight gain with an GnRH-a and LNG-IUD. Cholakian et al. reported that the median weight change during therapy was greater with MA than with LNG-IUDs (+2.95 vs. +0.05 kg, P=0.03) (24). On the other hand, LNG-IUDs played an important role in maintaining endometrial thinning. Additionally, GnRH-a can inhibit the hypothalamic pituitary-gonadal axis in the central nervous system. Thus, the combination of LNG-IUDs and GnRH-a was a comparably effective method to suppress the production of estrogen from both the ovaries and peripheral tissue. Other studies have reported results similar to those of our study. A systematic review of 19 articles showed that LNG-IUDs had an advantage over oral progestin (27). Women with AEH were more likely to show regression with an LNG-IUD than with oral progestin. Furthermore, GnRH is an effective fertility-sparing strategy for women with AEH and EC due to the low recurrence rate of these diseases and the absence of progressive disease; this method achieved a good long-term uterine preservation rate and a high pregnancy rate (16, 28). Therefore, the abovementioned studies and our study provide evidence that LNG-IUDs and GnRH-a are preferred in the treatment of overweight and obese patients with AEH and EC (16).
On the one hand, letrozole belongs to a class of medications known as aromatase inhibitors and acts by blocking estrogen production and causing the pituitary gland to increase its stimulation of ovarian follicles (29). NCCN guidelines have recommended that letrozole can be used to enhance ovulation as the first-line treatment (7). Additionally, letrozole has been reported to be an effective ovulation induction agent in higher-BMI women (30). Obese patients usually have PCOS or ovulation disorders, which are important risk factors for infertility. Insulin resistance caused by obesity may exacerbate hyperandrogenism, and hyperandrogenism can increase the resistance to insulin, thus forming a vicious cycle (31). Letrozole can inhibit the growth of nondominant follicles and promote the development of single follicles (32). Letrozole has a short drug half-life and is thought to cause fewer antiestrogenic side effects on estrogen target organs than clomiphene citrate because ERs are not directly affected (33). In summary, letrozole can increase the sensitivity of patients to gonadotropin and improve impaired ovarian function due to obesity (34, 35). In our study, overweight patients using letrozole for ovulation induction to promote conception may have improved pregnancy outcomes to some extent, while IVF-ET was not an optimal choice. This can be explained by the fact that most patients using IVF-ET may indeed have infertility syndromes, and the successful pregnancy rate was originally lower. According to guidelines recommendations, obese patients should not undergo IVF until their BMI drops to below 30 kg/m2. On the other hand, letrozole can be used for FST in patients with AEH and EC from European Society of Gynecological Oncology (ESGO) guideline (36). This kind of treatment has been reported in our institution, as well as in some literatures (37–40). A previous study reported that the combination of GnRH-a and aromatase inhibitors showed a beneficial long-term outcome in young obese EC patients who wished to receive FST (40). However, the use of letrozole for FST was in a small number, and not more high evidence-based studies supported. Thus, in our institution, letrozole was mainly applied to induce ovulation in most overweight patients.
The results of our study showed that for patients with EW, maintenance treatment tend to reduce the recurrence rate of AEH and EC and increase the likelihood of maintaining regular menstruation, regardless of pregnancy intention after CR (21). The main maintenance treatments in this study were LNG-IUDs or low-dose oral progestin. Our previous studies have confirmed that regular oral progestin also significantly prolonged the DFS (RR=4.726; 95% CI: 2.672– 8.359) of young patients with AEH and EC (41). Wang et al. also showed that maintenance therapy was an independent protective factor for recurrence (P=0.001), while DM was an independent risk factor for recurrence (P=0.003) (42). Park et al. reported that if patients want to maintain fertility after childbirth, they can choose to use periodic oral contraceptives or LNG-IUDs to prevent recurrence (43). Therefore, it is considerable to use regular maintenance treatment after remission, regardless of whether the patients are considered normal weight or excess weight.
Nonetheless, there are several limitations in this study. First, unknown potential confounders and selection biases may be present in this retrospective institutional study due to the long period of data collection. However, we attempted to define the patient inclusion criteria carefully to ensure that all data were collected in a similar way and to always ensure uniformity between the two groups stratified by BMI. Moreover, we balanced confounding factors in the EW group with a Cox multivariate regression analysis when heterogeneities were present in the baseline factors. We also divided the dataset into homogenous subgroups and performed a stratification analysis. Second, the conservative treatments for AEH and EC patients are relatively unique. There has been no uniform standard for FST until now. This study retrospectively summarized the characteristics of existing cases and proposed guidelines. In clinical practice, gynecologic oncologists should pay more attention to the weight loss of overweight patients and adopt more personalized treatment options for such patients (11). For obese patients who have no histological response to the primary therapy for over 6-12 months, an alternative therapy strategy should be actively applied. Overweight patients should be informed that they may have an elevated risk of failed conservative treatment (44). In the future, we will continue to analyze more AEH and EC patients after FSTs and collect more information from new patients.
In conclusion, most patients with AEH and EC who undergo FSTs are overweight and obese. The combination of GnRH-a and LNG-IUD produced better outcomes in patients with EW than GnRH-a or oral progestin therapy alone, especially for patients with EW diagnosed with EC. GnRH-a and/or LNG-IUD may be options prior to FSTs in patients with EW due to the low relapse rates of AEC and EC. Furthermore, patients with EW using ovulation induction to boost fertility tend to have a shorter time to pregnancy. The use of regular maintenance treatment after remission is recommended. Fertility-sparing management should not necessarily be contraindicated in overweight and obese patients, but the therapy and reproductive strategy should be more individualized. Further prospective studies are needed to investigate the underlying factors associated with oncologic and pregnancy outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was conducted with the approval of the Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Study concepts: L-yP, and YJ. Study design: YS, MQ, L-yP, and YJ. Data acquisition: YS, MQ, YG, and WW. Quality control of data and algorithms: JY, Y-yC, and YL. Data analysis and interpretation: YS, MQ, and Y-xW. Statistical analysis: YS, MQ, and YC. Manuscript preparation: YS and MQ. Manuscript editing: All authors. Manuscript review: L-yP, YJ, and JY. All authors contributed to the article and approved the submitted version.
This project was supported by The Fund of The National Key R&D Program of China 2016YFC1303700 (Affiliated project 2016YFC1303701, 2016.9-2020.12). Furthermore, this project was also supported by CAMS Innovation Fund for Medical Sciences (CIFMS-2017-I2M-1-002, 2017.01-2020.12). Besides, MQ was supported by China Scholarship Council (201906210463).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.749881/full#supplementary-material
1. Zhu G, Falahat R, Wang K, Mailloux A, Artzi N, Mule JJ. Tumor-Associated Tertiary Lymphoid Structures: Gene-Expression Profiling and Their Bioengineering. Front Immunol (2017) 8:767. doi: 10.3389/fimmu.2017.00767
2. Tangjitgamol S, Anderson BO, See HT, Lertbutsayanukul C, Sirisabya N, Manchana T, et al. Management of Endometrial Cancer in Asia: Consensus Statement From the Asian Oncology Summit 2009. Lancet Oncol (2009) 10(11):1119–27. doi: 10.1016/S1470-2045(09)70290-6
3. Committee on Gynecologic Practice Society of Gynecologic Oncology. The American College of Obstetricians and Gynecologists Committee Opinion No. 631. Endometrial Intraepithelial Neoplasia. Obstetrics Gynecol (2015) 125(5):1272–8. doi: 10.1097/01.aog.0000465189.50026.20
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J For Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
5. Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and Management of Endometrial Cancer. Am Family Physician (2016) 93(6):468–74.
6. Lee WL, Lee FK, Su WH, Tsui KH, Kuo CD, Hsieh SL, et al. Hormone Therapy for Younger Patients With Endometrial Cancer. Taiwanese J Obstetrics Gynecol (2012) 51(4):495–505. doi: 10.1016/j.tjog.2012.09.003
7. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Network JNCCN (2018) 16(2):170–99. doi: 10.6004/jnccn.2018.0006
8. Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, Relapse, and Live Birth Rates With Fertility-Sparing Therapy for Endometrial Cancer and Atypical Complex Endometrial Hyperplasia: A Systematic Review and Metaanalysis. Am J Obstetrics Gynecol (2012) 207(4):266. doi: 10.1016/j.ajog.2012.08.011
9. Yim GW, Kim SW, Nam EJ, Kim S, Kim YT. Learning Curve Analysis of Robot-Assisted Radical Hysterectomy for Cervical Cancer: Initial Experience at a Single Institution. J Gynecol Oncol (2013) 24(4):303–12. doi: 10.3802/jgo.2013.24.4.303
10. Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of Body Mass Index in Diagnosing Obesity in the Adult General Population. Int J Obes (Lond) (2008) 32(6):959–66. doi: 10.1038/ijo.2008.11
11. Papatla K, Huang M, Slomovitz B. The Obese Endometrial Cancer Patient: How do We Effectively Improve Morbidity and Mortality in This Patient Population? Ann Oncol (2016) 27(11):1988–94. doi: 10.1093/annonc/mdw310
12. Jenabi E, Poorolajal J. The Effect of Body Mass Index on Endometrial Cancer: A Meta-Analysis. Public Health (2015) 129(7):872–80. doi: 10.1016/j.puhe.2015.04.017
13. Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol (2019) 5(1):37–44. doi: 10.1001/jamaoncol.2018.4280
15. Bouwman F, Smits A, Lopes A, Das N, Pollard A, Massuger L, et al. The Impact of BMI on Surgical Complications and Outcomes in Endometrial Cancer Surgery–An Institutional Study and Systematic Review of the Literature. Gynecologic Oncol (2015) 139(2):369–76. doi: 10.1016/j.ygyno.2015.09.020
16. Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-Releasing Hormone Agonist Combined With a Levonorgestrel-Releasing Intrauterine System or Letrozole for Fertility-Preserving Treatment of Endometrial Carcinoma and Complex Atypical Hyperplasia in Young Women. Int J Gynecological Cancer (2017) 27(6):1178–82. doi: 10.1097/igc.0000000000001008
17. du Prel J-B, Röhrig B, Hommel G, Blettner M. Choosing Statistical Tests: Part 12 of a Series on Evaluation of Scientific Publications. Dtsch Arztebl Int (2010) 107(19):343–8. doi: 10.3238/arztebl.2010.0343
18. Gonthier C, Walker F, Luton D, Yazbeck C, Madelenat P, Koskas M. Impact of Obesity on the Results of Fertility-Sparing Management for Atypical Hyperplasia and Grade 1 Endometrial Cancer. Gynecologic Oncol (2014) 133(1):33–7. doi: 10.1016/j.ygyno.2013.11.007
19. von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment Effects, Disease Recurrence, and Survival in Obese Women With Early Endometrial Carcinoma: A Gynecologic Oncology Group Study. Cancer (2006) 107(12):2786–91. doi: 10.1002/cncr.22351
20. Yang B, Xie L, Zhang H, Zhu Q, Du Y, Luo X, et al. Insulin Resistance and Overweight Prolonged Fertility-Sparing Treatment Duration in Endometrial Atypical Hyperplasia Patients. J Gynecologic Oncol (2018) 29(3):e35. doi: 10.3802/jgo.2018.29.e35
21. Gressel GM, Parkash V, Pal L. Management Options and Fertility-Preserving Therapy for Premenopausal Endometrial Hyperplasia and Early-Stage Endometrial Cancer. Int J Gynaecol Obstetrics (2015) 131(3):234–9. doi: 10.1016/j.ijgo.2015.06.031
22. Li M, Guo T, Cui R, Feng Y, Bai H, Zhang Z. Weight Control Is Vital for Patients With Early-Stage Endometrial Cancer or Complex Atypical Hyperplasia Who Have Received Progestin Therapy to Spare Fertility: A Systematic Review and Meta-Analysis. Cancer Manage Res (2019) 11:4005–21. doi: 10.2147/cmar.s194607
23. Raffone A, Travaglino A, Saccone G, Di Maio A, Mollo A, Mascolo M, et al. Diabetes Mellitus and Responsiveness of Endometrial Hyperplasia and Early Endometrial Cancer to Conservative Treatment. Gynecological Endocrinol (2019) 35(11):932–7. doi: 10.1080/09513590.2019.1624716
24. Cholakian D, Hacker K, Fader AN, Gehrig PA, Tanner EJ 3rd. Effect of Oral Versus Intrauterine Progestins on Weight in Women Undergoing Fertility Preserving Therapy for Complex Atypical Hyperplasia or Endometrial Cancer. Gynecologic Oncol (2016) 140(2):234–8. doi: 10.1016/j.ygyno.2015.12.010
25. Park JY, Seong SJ, Kim TJ, Kim JW, Bae DS, Nam JH. Significance of Body Weight Change During Fertility-Sparing Progestin Therapy in Young Women With Early Endometrial Cancer. Gynecologic Oncol (2017) 146(1):39–43. doi: 10.1016/j.ygyno.2017.05.002
26. Ward KK, Roncancio AM, Shah NR, Davis MA, Saenz CC, McHale MT, et al. Bariatric Surgery Decreases the Risk of Uterine Malignancy. Gynecologic Oncol (2014) 133(1):63–6. doi: 10.1016/j.ygyno.2013.11.012
27. Nwanodi O. Progestin Intrauterine Devices and Metformin: Endometrial Hyperplasia and Early Stage Endometrial Cancer Medical Management. Healthcare (Basel Switzerland) (2017) 5(3):30. doi: 10.3390/healthcare5030030
28. Tock S, Jadoul P, Squifflet JL, Marbaix E, Baurain JF, Luyckx M. Fertility Sparing Treatment in Patients With Early Stage Endometrial Cancer, Using a Combination of Surgery and GnRH Agonist: A Monocentric Retrospective Study and Review of the Literature. Front Med (2018) 5:240. doi: 10.3389/fmed.2018.00240
29. Bedaiwy MA, Mousa NA, Esfandiari N, Forman R, Casper RF. Follicular Phase Dynamics With Combined Aromatase Inhibitor and Follicle Stimulating Hormone Treatment. J Clin Endocrinol Metab (2007) 92(3):825–33. doi: 10.1210/jc.2006-1673
30. McKnight KK, Nodler JL, Cooper JJ Jr, Chapman VR, Cliver SP, Bates GW Jr. Body Mass Index-Associated Differences in Response to Ovulation Induction With Letrozole. Fertil steril (2011) 96(5):1206–8. doi: 10.1016/j.fertnstert.2011.08.002
31. Okamura Y, Saito F, Takaishi K, Motohara T, Honda R, Ohba T, et al. Polycystic Ovary Syndrome: Early Diagnosis and Intervention Are Necessary for Fertility Preservation in Young Women With Endometrial Cancer Under 35 Years of Age. Reprod Med Biol (2017) 16(1):67–71. doi: 10.1002/rmb2.12012
32. Tanbo T, Mellembakken J, Bjercke S, Ring E, Åbyholm T, Fedorcsak P. Ovulation Induction in Polycystic Ovary Syndrome. Acta Obstetricia Gynecol Scand (2018) 97(10):1162–7. doi: 10.1111/aogs.13395
33. Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The Management of Anovulatory Infertility in Women With Polycystic Ovary Syndrome: An Analysis of the Evidence to Support the Development of Global WHO Guidance. Hum Reprod Update (2016) 22(6):687–708. doi: 10.1093/humupd/dmw025
34. Costello MF, Garad RM, Hart R, Homer H, Johnson L, Jordan C, et al. A Review of Second- and Third-Line Infertility Treatments and Supporting Evidence in Women With Polycystic Ovary Syndrome. Med Sci (Basel Switzerland) (2019) 7(7):75. doi: 10.3390/medsci7070075
35. Casper RF, Mitwally MF. A Historical Perspective of Aromatase Inhibitors for Ovulation Induction. Fertil Steril (2012) 98(6):1352–5. doi: 10.1016/j.fertnstert.2012.10.008
36. Rodolakis A, Biliatis I, Morice P, Reed N, Mangler M, Kesic V, et al. European Society of Gynecological Oncology Task Force for Fertility Preservation: Clinical Recommendations for Fertility-Sparing Management in Young Endometrial Cancer Patients. Int J Gynecological Cancer (2015) 25(7):1258–65. doi: 10.1097/igc.0000000000000493
37. Barker LC, Brand IR, Crawford SM. Sustained Effect of the Aromatase Inhibitors Anastrozole and Letrozole on Endometrial Thickness in Patients With Endometrial Hyperplasia and Endometrial Carcinoma. Curr Med Res Opin (2009) 25(5):1105–9. doi: 10.1185/03007990902860549
38. Tabatabaie A, Karimi Zarchi M, Dehghani-Tafti M, Miratashi-Yazdi A, Teimoori S, Dehghani A. Comparing Letrozole With Medroxyprogesterone Acetate (MPA) as Hormonal Therapy for Simple Endometrial Hyperplasia Without Atypia in Adult and Middle-Aged Women. Eur J Gynaecol Oncol (2013) 34(6):552–5.
39. Azim A, Oktay K. Letrozole for Ovulation Induction and Fertility Preservation by Embryo Cryopreservation in Young Women With Endometrial Carcinoma. Fertil Steril (2007) 88(3):657–64. doi: 10.1016/j.fertnstert.2006.12.068
40. Zhang Z, Huang H, Feng F, Wang J, Cheng N. A Pilot Study of Gonadotropin-Releasing Hormone Agonist Combined With Aromatase Inhibitor as Fertility-Sparing Treatment in Obese Patients With Endometrial Cancer. J Gynecologic Oncol (2019) 30(4):e61. doi: 10.3802/jgo.2019.30.e61
41. Yin J, Ma S, Shan Y, Wang Y, Li Y, Jin Y, et al. Risk Factors for Recurrence in Patients With Atypical Endometrial Hyperplasia and Endometrioid Adenocarcinoma After Fertility-Sparing Treatments. Cancer Prev Res (Philadelphia Pa) (2020) 13(4):403–10. doi: 10.1158/1940-6207.capr-19-0399
42. Wang Y, Zhou R, Wang H, Liu H, Wang J. Impact of Treatment Duration in Fertility-Preserving Management of Endometrial Cancer or Atypical Endometrial Hyperplasia. Int J Gynecol Cancer (2019) 29(4):699–704. doi: 10.1136/ijgc-2018-000081
43. Park H, Seok JM, Yoon BS, Seong SJ, Kim JY, Shim JY, et al. Effectiveness of High-Dose Progestin and Long-Term Outcomes in Young Women With Early-Stage, Well-Differentiated Endometrioid Adenocarcinoma of Uterine Endometrium. Arch Gynecol Obstetrics (2012) 285(2):473–8. doi: 10.1007/s00404-011-1959-x
Keywords: fertility-sparing treatments, atypical endometrial hyperplasia (AEH), endometrial cancer (EC), excess weight, levonorgestrel intrauterine devices, gonadotropin-releasing hormone agonist (GnRH- a)
Citation: Shan Y, Qin M, Yin J, Cai Y, Li Y, Gu Y, Wang W, Wang Y-x, Chen J-y, Jin Y and Pan L-y (2021) Effect and Management of Excess Weight in the Context of Fertility-Sparing Treatments in Patients With Atypical Endometrial Hyperplasia and Endometrial Cancer: Eight-Year Experience of 227 Cases. Front. Oncol. 11:749881. doi: 10.3389/fonc.2021.749881
Received: 30 July 2021; Accepted: 22 October 2021;
Published: 05 November 2021.
Edited by:
Che-Pei Kung, Washington University School of Medicine in St. Louis, United StatesReviewed by:
Gulzhanat Aimagambetova, Nazarbayev University, KazakhstanCopyright © 2021 Shan, Qin, Yin, Cai, Li, Gu, Wang, Wang, Chen, Jin and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-ya Pan, cGFubHlAcHVtY2guY24=; Ying Jin, amlueWluZ0BwdW1jaC5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.