- 1Department of Pharmacy, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
- 2Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, United States
- 3Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
- 4University of Pittsburgh Medical Center (UPMC) Hillman Cancer Center, Pittsburgh, PA, United States
Background: Anti-PD-1 immune checkpoint inhibitor (ICI) therapy has revolutionized the treatment of melanoma by producing durable long-term responses in a subset of patients. ICI-treated patients develop unique toxicities - immune related adverse events (irAEs) – that arise from unrestrained immune activation. The link between irAE development and clinical outcome in melanoma and other cancers is inconsistent; and little data exists on the occurrence of multiple irAEs. We sought to characterize development of single and multiple irAEs, and association of irAE(s) development with clinical variables and impact upon outcomes in advanced melanoma patients treated with anti-PD-1 ICIs.
Methods: We conducted a retrospective study of 190 patients with metastatic melanoma treated with single-agent anti-PD-1 ICI therapy between June 2014 and August 2020 at a large integrated network cancer center identified through retrospective review of pharmacy records. irAEs were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Results: 190 patients were evaluated of whom 114 patients (60.0%) experienced ≥1 irAE, including 30 (15.8%) with grade 3/4 irAEs. The occurrence of any irAE was strongly associated with the development of investigator-assessed response to anti-PD-1 therapy (p < 0.0001); whether evaluated by current (p=0.0082) or best (p=0.0001) response. In patients with ≥2 irAEs, distinct patterns were observed. Median progression-free survival (PFS) and overall survival (OS) were greater in those with any irAE compared to those without (PFS, 28 months vs. 5 months, p < 0.0001; OS, not reached vs. 9 months, p < 0.0001). Development of ≥2 irAEs had a trend towards improved PFS and OS compared to those who developed a single irAE, although this did not reach statistical significance (p=0.2555, PFS; p=0.0583, OS). Obesity but not age or gender was distinctly associated with irAE development.
Conclusions: In this study, we demonstrated that irAE occurrence was significantly associated with response to anti-PD-1 therapy and improved PFS/OS. Those who developed multiple irAEs had a trend towards improved PFS and OS compared to those who developed only a single irAE. Increased BMI but neither age nor gender were associated with irAE development. Distinct patterns of irAEs observed suggest shared etiopathogenetic mechanisms.
Introduction
Programmed cell death protein 1 (PD-1) is a receptor expressed on activated T cells which binds to PD ligand 1 (PD-L1, B7-H1) and PD ligand 2 (PD-L2, B7-DC) (1–4). The interaction between PD-1 and PD-L1 negatively regulates T cell function (1–4). Within tumors, tumor cells and tumor-infiltrating immune cells including macrophages and dendritic cells (DCs) express PD-L1 (5); and the PD-1/PD-L1 interaction down-regulates host immune responses in peripheral tissues, representing a means by which tumors subvert anti-tumor immune responses. PD-1/PD-L1 blockade increases the number and function of tumor antigen (TA)-specific CD8+ T cells preclinically and in ex vivo melanoma (6, 7). Therapeutic blockade of the PD-1/PD-L1 interaction by immune checkpoint inhibitors (ICI) has transformed the management of advanced melanoma; with objective responses in 35-40% of patients (8, 9), with 35% progression-free at 1-year (10, 11), and 34% alive at 5-years (12).
Immune-related adverse events (irAE) represent a unique spectrum of toxicities observed in patients treated with ICIs targeting cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) and PD-1 receptors. irAEs can affect any organ system with unique patterns and incidence depending on whether patients are treated with single agent or dual ICI. irAEs most commonly involve skin, endocrine glands, gastrointestinal tract and liver (13, 14). irAE occurrence has been associated with improved outcomes for patients treated with anti-PD-(L)1 or anti-CTLA-4 ICI primarily in the context of melanoma (15–19), non-small cell lung cancer (NSCLC) (13, 20, 21), and urothelial cancer (19). Separately, while certain demographic variables including body mass index (BMI) (22, 23), and gender (24), have been associated with ICI outcomes; the association of these with irAE occurrence have not been studied.
In this study, we report upon the association with irAE occurrence and outcome to PD-1 blockade in patients with advanced melanoma treated with nivolumab or pembrolizumab. We evaluate the temporal pattern of irAE occurrence, relationship of irAE occurrence in relation to outcomes including objective response, progression-free and overall survival, and relationship of irAE occurrence. We perform separate analyses by gender and BMI and investigate the influence of systemic steroid use upon the stated outcomes.
Materials And Methods
Patient Selection
Approval was obtained from the University of Pittsburgh Cancer Institute (UPCI) Institutional Review Board (IRB) for a retrospective analysis of cancer patients who had received treatment with anti-PD-1 and/or anti-CTLA-4 ICI (IRB number PRO18080253) identified based on retrospective review of pharmacy records. For the purposes of this analysis, only patients with advanced metastatic melanoma treated with single-agent anti-PD-1 ICI were considered. Inclusion criteria were diagnosis of unresectable (stage III) or metastatic (stage IV) cutaneous, mucosal, or unknown primary melanoma, treatment with at least one dose of anti-PD-1 therapy administered singly, and at least one restaging scan available to assess disease response. Patients with uveal melanoma were excluded. A prespecified sample size was not determined. 190 melanoma patients treated between June 2014 and August 2020 with either nivolumab or pembrolizumab in the advanced setting were included, although patients who received adjuvant nivolumab or pembrolizumab were not included. Patients were treated with intravenous pembrolizumab (2mg/kg every 21 days; 200mg every 21 days) or nivolumab (3mg/kg every 14 days; 240mg every 14 days; 480mg every 28 days) under a range of doses and schedules as indicated. Treatment duration was not specified a priori but was typically administered for up to 24 months, disease progression, or unacceptable toxicity.
Patient demographics and baseline characteristics, along with data regarding prior systemic therapy (if any) and performance status (PS) on the Eastern Cooperative Oncology Group (ECOG) scale were collected. Mutational status as determined on primary or metastatic tumor tissue was recorded. Disease stage at start of anti-PD-1 therapy was assigned using the American Joint Committee on Cancer (AJCC) 8th edition staging classification (25).
Radiographic response was assessed using computed tomography (CT) scans or positron emission tomography (PET) scans with CT attenuation performed at baseline and every 3 months as assessed by the treating investigator (D.D., Y.N., J.J.L., or J.M.K.). Clinical response to treatment was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to the Response Evaluation Criteria in Solid Tumors (RECIST) v 1.1 criteria (26). Patients who were not radiologically evaluable were not included in this analysis. Best overall response was defined as the best achieved response observed during treatment period; while current objective response rate (ORR) was defined as the proportion of patients with CR or PR at the most recent radiographic assessment. Progression-free survival (PFS) was defined as time from date on which immunotherapy was started to date of first radiographic or clinical progression. Patients who had not progressed were censored at the date of last follow up. Overall survival (OS) was calculated from date on which immunotherapy was started to date of death; and patients who were alive were censored at the date of last follow up.
Immune-Related Adverse Event (irAE) Assessment
irAE terms were defined as any AE that previously had been reported to be associated with the mechanism of action of pembrolizumab or nivolumab ICI therapy. The following groups of irAEs were considered based on a prespecified list and were grouped as follows (1): endocrine irAE (hypothyroidism, hyperthyroidism, hypophysitis, type 1 diabetes, or adrenal insufficiency) (2); cutaneous irAE (macular rash, papular rash, bullous disease, vitiligo excluding pruritus alone) (3); rheumatologic irAE (myalgia, arthritis, myositis, temporal arteritis, xerostomia) (4); gastrointestinal irAE (colitis, hepatitis, pancreatitis) (5); other irAEs (pneumonitis, immune thrombocytopenia, nephritis, aseptic meningitis). We specifically only evaluated irAEs that occurred after receipt of anti-PD-1 therapy and any event temporally linked to this; but specifically excluded any irAE that occurred following receipt of any subsequent therapy (immune, targeted therapy or other). We excluded AEs – such as fatigue or pruritus – that are not classically considered immune mediated but could conceivably result from an immune mediated process. irAEs that occurred between the start and last dose of treatment were tabulated. irAEs severity was graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0. Management of irAEs was obtained based on chart review. Treatment-related irAEs were typically managed following published American Society of Clinical Oncology (ASCO) clinical practice guidelines and use of steroid and steroid-sparing agents was documented (27, 28).
To describe patterns of multiple irAEs, we evaluated all patients with >1 irAE (N=44) that developed during treatment and/or follow-up period. irAE categories were grouped and between-category relationships (rheumatologic-endocrine, cutaneous-rheumatologic, etc.) were considered regardless of number, and/or temporality and subsequently enumerated (xCy, where x is the number of irAE categories, and y is the number of unique rearrangements). This generated 3 relationships per individual from those with 3 irAEs (N=10), and 6 relationships per individual from those with 4 irAEs (N=4). The total number of unique two-dimensional relationships so derived (78) were plotted on a chord gram using Flourish studio (https://app.flourish.studio/@daan/chord-diagram).
Statistical Analysis
Data were summarized using descriptive statistics. Normality testing (D’Agostino and Pearson test) was performed to determine whether data were sampled from a Gaussian distribution. The Mann–Whitney U test was used to compare continuous non-normally distributed variables. Incidence of events among the groups was analyzed for statistical significance using the Fisher exact test. Logistic regression models were used to study the effect of explanatory variables on binary outcomes, and odds ratios (OR) and 95% confidence intervals (CI) were calculated based on these models. Survival outcomes were evaluated with the Kaplan–Meier method. The relationship between survival outcomes with risk factors was studied with Cox models. Hazard ratio (HR) and 95% CI were calculated for each risk factor. Univariate and multivariate analysis were performed when appropriate, using Cox proportional hazard model. All p values were two-sided; p values < 0.05 were statistically significant. Statistical analysis was performed with SAS version 9.4 (SAS institute Inc, Cary, NC), GraphPad Prism version 6.0 for Mac (GraphPad Software, San Diego CA), and IBM-Microsoft SPSS (SPSS Statistics. International Business Machines Corporation IBM. 2013. Armonk, USA) version 20.0 for Mac.
Results
Patients and Clinical Characteristics
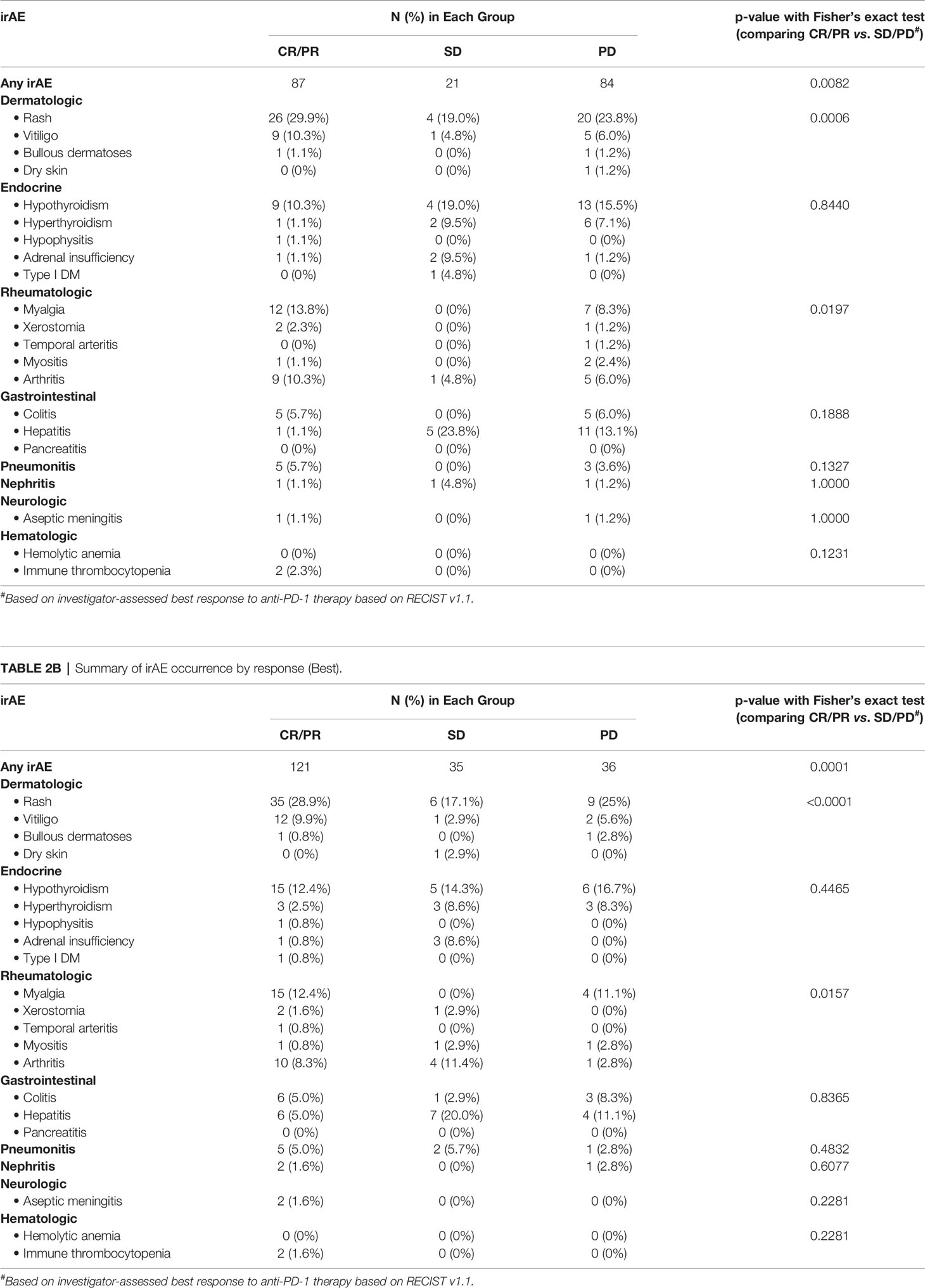
190 patients with advanced/unresectable cutaneous, mucosal, or unknown primary melanoma who met the inclusion criteria were included in the present analysis (119 males, 71 females). The median age was 68 (range 20.0 to 91.0 years), and the median duration of follow-up for all patients was 18.0 months. 162 (85.3%) of patients had stage IV disease while 28 (14.7%) had unresectable stage III disease. 157 (82.6%) of patients were treated with pembrolizumab; while 33 (17.4%) received nivolumab. Anti-PD-1 therapy was typically administered in the front-line setting (107 patients, 56.3%), and less commonly in the 2nd (47 patients, 24.7%) or ≥3rd line (36 patients, 19.0%) settings. 68 (35.8%) of patients were exposed to ipilimumab previously in either the adjuvant or metastatic settings. The overall rate of progression was 59.5%, and patients who progressed were treated with either targeted therapy, or investigational immunotherapy. Baseline characteristics are tabulated in Table 1.
IrAE Incidence, Temporal Relationship and Resolution
Treatment-related irAEs of any grade occurred in 114 (60.0%) of patients, and 44 (23.2%) of patients experienced more than one AE (Figure 1). The vast majority of all irAEs were Grade 1-2 and comprised: dermatologic irAEs [61/68 (89.7%)], endocrinologic irAEs [39/41, (95.1%)], rheumatologic irAEs [34/41, (82.9%)]. Grade 3-4 irAEs occurred in 30 (15.8%) patients; while no incidence of grade 5 irAEs were noted. Anti-PD-1 therapy resulted in treatment discontinuation in 30 (15.8%) of patients, with the most common etiology being pneumonitis in 6 patients (20.0%), colitis in 5 patients (16.7%), and hepatitis in 5 patients (16.7%).
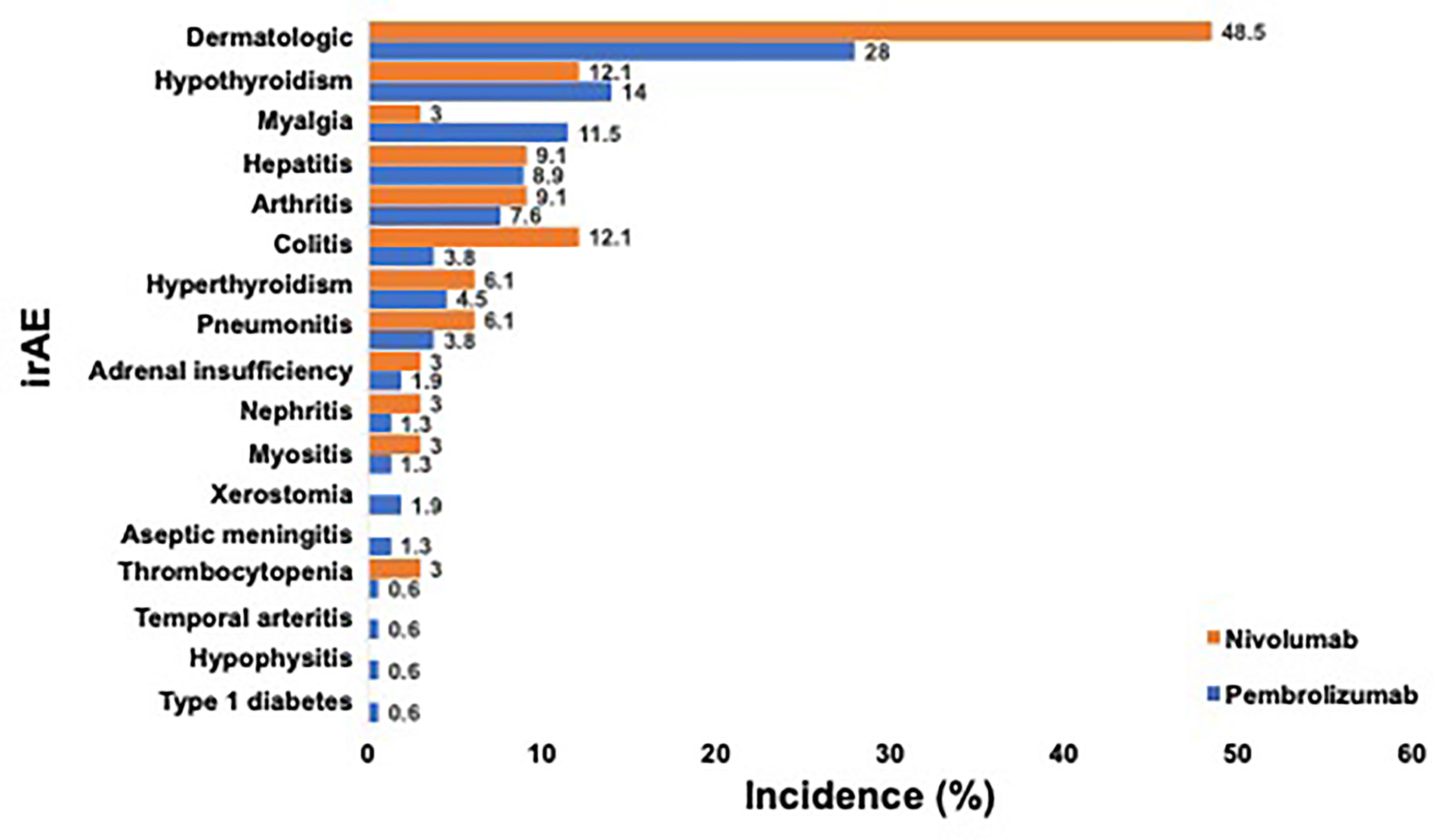
Figure 1 Incidence of Any-grade irAE Occurring in at least One PD-1 Treated Advanced Melanoma Patient. a. Legend: Incidence of any-grade irAE that occurred in ≥1 patient after anti-PD-1 therapy for advanced melanoma is shown. Patients who received pembrolizumab (blue) and nivolumab (orange) are depicted separately. Incidence of each irAE is listed on the right.
When we evaluated the various irAEs in relation to start of therapy, we observed differing times to occurrence among the various categories of irAEs (Figure 2). Dermatologic irAEs tended to occur relatively soon after initiation of therapy (median 12.0 weeks) while neurologic and pulmonary irAEs tended to occur relatively late (median 40.6 weeks and 62.4 weeks respectively) (Figure 2). The median time (in weeks) to onset of the most common irAEs (incidence ≥5%) were as follows: hyperthyroidism (6.3 weeks), dermatologic (12.0 weeks), myalgia (12.0 weeks), arthritis (18.4 weeks), hypothyroidism (20.4 weeks), colitis (21.6 weeks), hepatitis (24.0 weeks), and pneumonitis (62.4 weeks) (Figure 2); qualitatively similar to prior reports (14). At the time of this analysis, all irAEs had completely resolved except for vitiligo, myalgias and endocrine irAEs requiring permanent hormone replacement therapy.
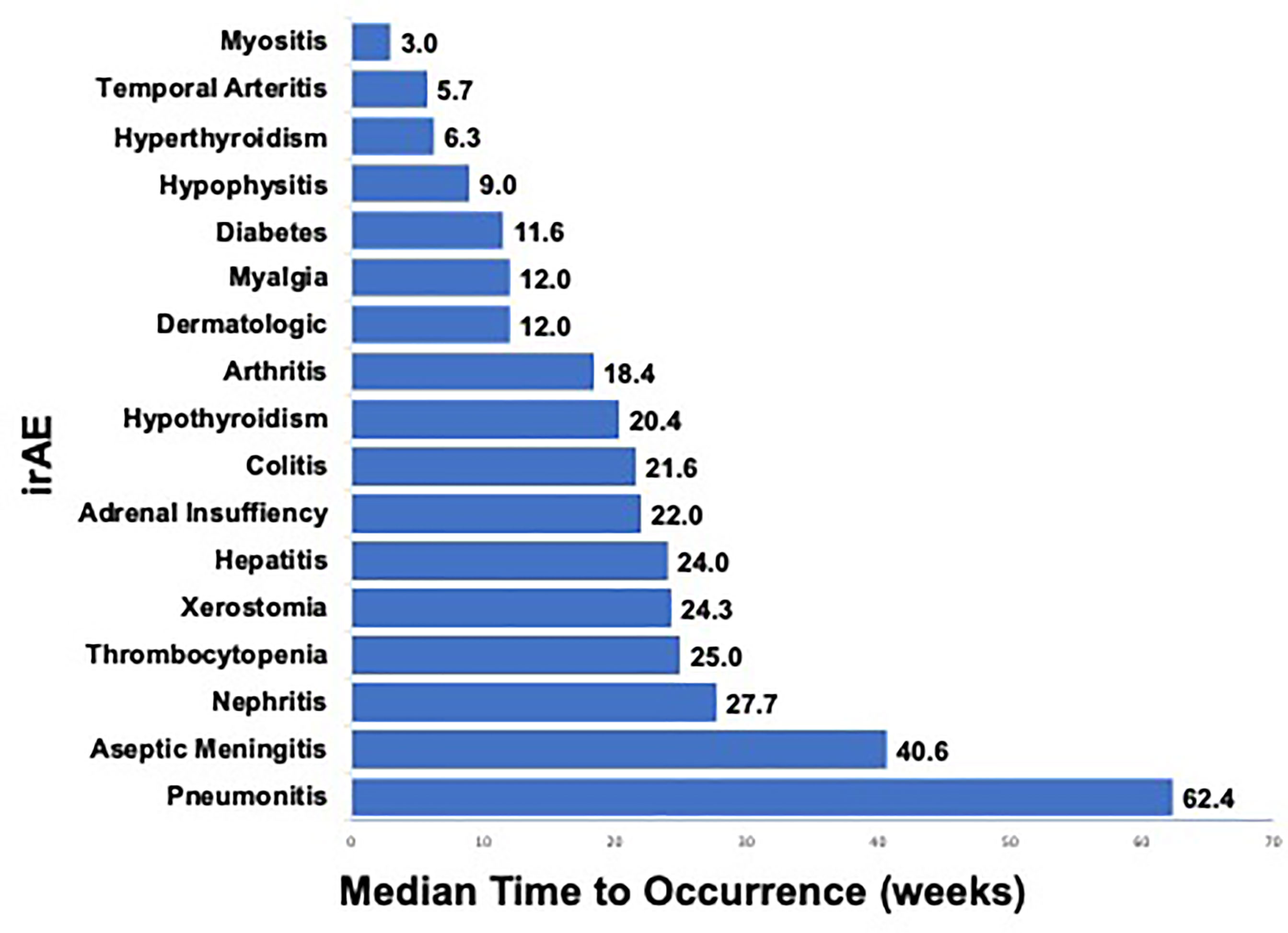
Figure 2 Median Time (weeks) to irAE Occurrence in PD-1 Treated Advanced Melanoma Patients. a. Legend: Median time (in weeks) from start of therapy to development of any-grade irAE that occurred in ≥1 patient after anti-PD-1 therapy for advanced melanoma is shown. Range (in weeks) from start of therapy to development of irAE is shown on right.
IrAE Occurrence and Response/Survival to Anti-PD-1 Therapy
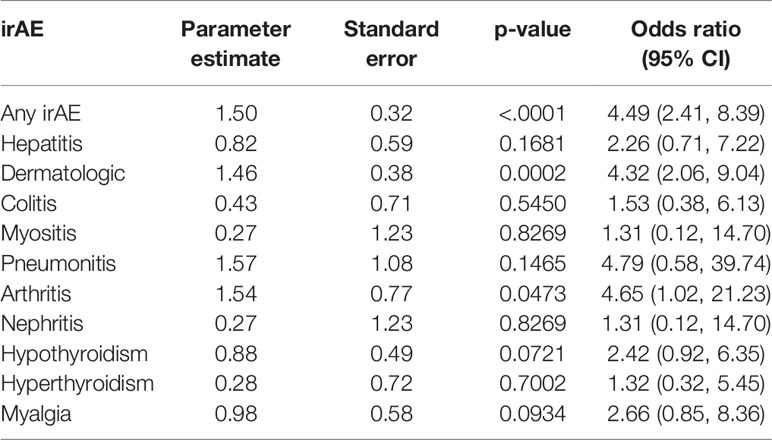
The occurrence of any irAE was strongly associated with the development of investigator-assessed response to anti-PD-1 therapy, defined as CR or PR assessed by RECIST v1.1 (p<0.0001); whether evaluated by current (p=0.0082) or best (p=0.0001) response, summarized in Table 2 and Supplementary Table 1. Of the various irAEs, only dermatologic and rheumatologic irAEs were significantly associated with current response (dermatologic, p=0.0006; rheumatologic, p=0.0197), and best response (dermatologic, p<0.0001; rheumatologic, p=0.0157). The odds of response in patients who developed any irAE, a dermatologic irAE, or arthritis irAE were 4.49 (95% CI 2.41, 8.39), 4.32 (95% CI 2.06, 9.04), and 4.65 (95% CI 1.02, 21.23), respectively, summarized in Table 3 and Figure 3. These results underscore similar observations by other groups in the setting of melanoma patients treated with anti-PD-1 ICIs both in the adjuvant and advanced settings (16, 19, 29–32).
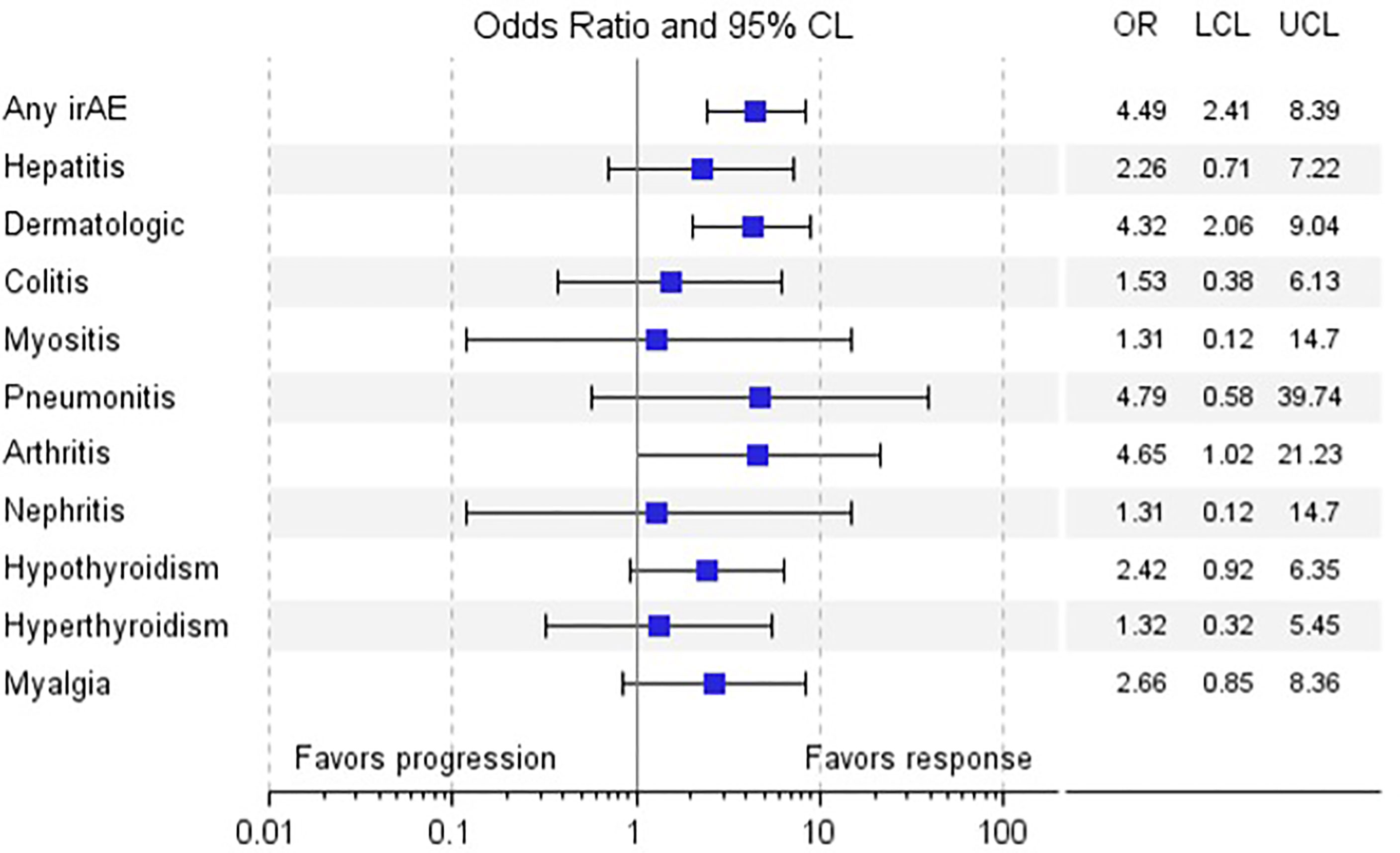
Figure 3 Odds Ratio of Response vs. Non-response by irAE. a. Legend: Forest plot of odds ratio of response vs. non-response by various irAE.
Concordant with the positive association of irAE and objective response to anti-PD-1 therapy, irAE occurrence was associated with improved PFS and OS. Median PFS (mPFS) and OS (mOS) were significantly greater in those who developed irAE than in those who did not (mPFS 28 months vs. 5 months, p < 0.0001; mOS not reached vs. 9 months, p < 0.0001) (Figures 4A, B).
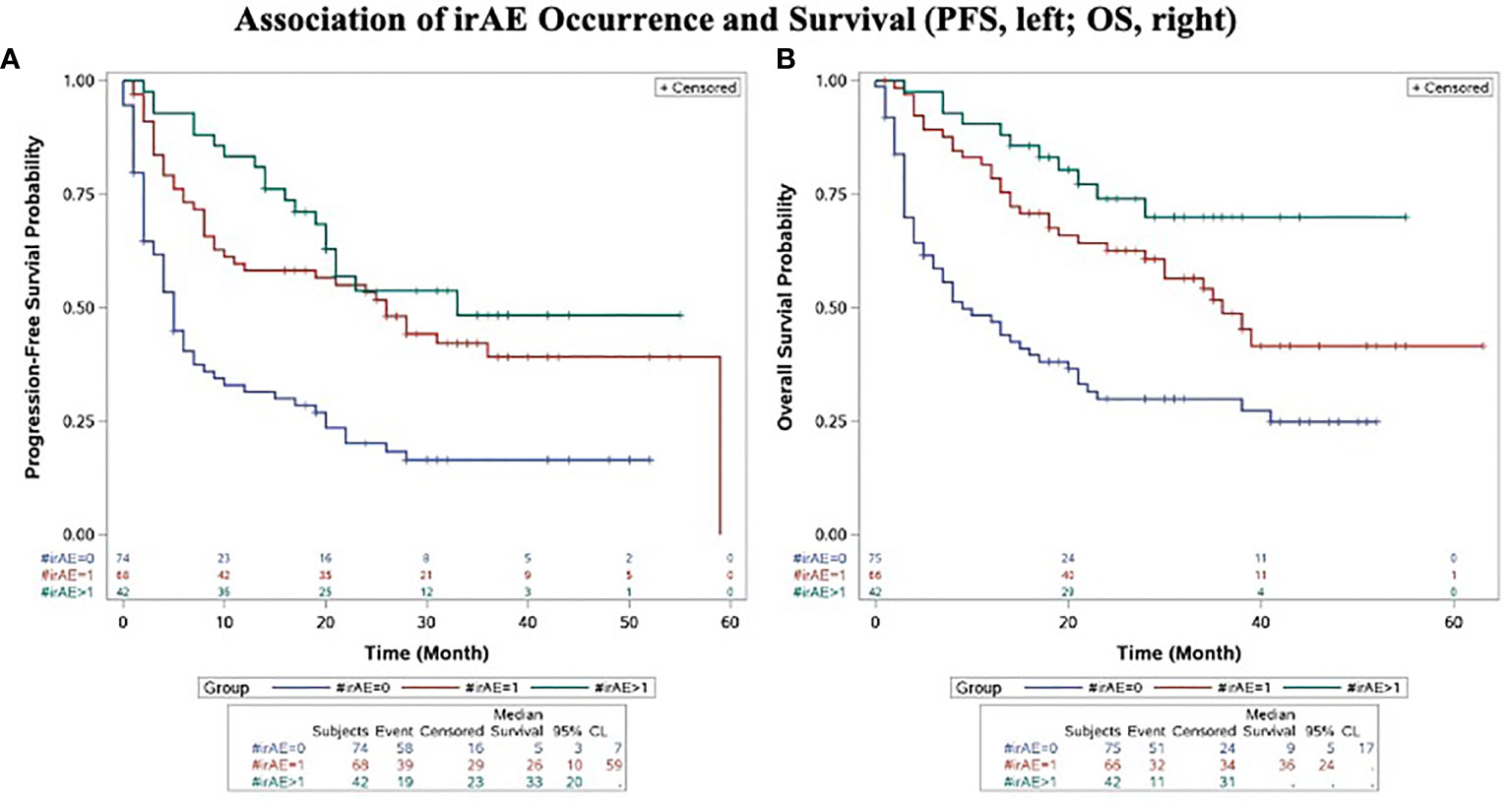
Figure 4 Association of irAE Occurrence and Progression-Free Survival (A) and Overall Survival (B). Kaplan-Meier plots of progression-free survival (A) and overall survival (B) in advanced melanoma patients treated with anti-PD-1 therapy compared by development and extent of irAE (0 vs. single vs. multiple). All p-values significant and unadjusted for multiple comparisons.
The proportion of patients who were BRAF mutant was 27.4%, while 22.6% of patients were NRAS mutant. Oncogenic BRAF mutations were associated with lower odds of ORR (odds ratio 0.37, p=0.0049) and irAE development (odds ratio 0.45, p=0.0180). Conversely, we observed greater ORR (odds ratio 2.53, p=0.0177) and irAE development (odds ratio 1.49, p=0.2844) in NRAS mutant patients; concordant with prior observations by other groups (33). Given that this was a retrospective study wherein mutation status was not prospectively collected with consequent imbalances, the associations of oncogenic drivers with irAE occurrence must be interpreted cautiously.
The longitudinal nature of the analysis permitted us to characterize patients who developed multiple irAEs. Overall, 42 (22.1%) unique patients developed 100 unique irAEs, grouped by organ system involvement in Supplementary Table 2. Three patients experienced four distinct irAEs; while 10 distinct patients developed three distinct irAEs (Supplementary Table 2). The commonest irAE doublets were dermatitis-endocrinopathy (15, 19.5%) and dermatitis-rheumatologic (15, 19.5%), followed by endocrinopathy-hepatitis (7, 9.1%) and endocrinopathy-rheumatologic (6, 7.8%) – a pattern reflected partially by the overall incidence of organ-specific irAEs. These relationships are depicted using a chord diagram in Supplementary Figure 1.
Patients who developed multiple irAEs had improved PFS and OS compared to those who did not develop an irAE (p<0.0001, PFS; p<0.0001, OS). Compared to those who developed a single irAE, patients who developed multiple irAEs had a trend towards improved PFS and OS, although this did not reach statistical significance for the OS and the PFS comparisons (p=0.2555, PFS; p=0.0583, OS) (Figures 4A, B).
Relationship of Steroid Use Upon irAE Resolution and Relationship of Gender, Age and BMI on irAE Development
50 (26.3%) patients required steroids for treatment of irAEs, of whom 7 (14.0%) patients failed steroid management and required biologics. Measures of response to therapy including objective response rate (ORR), median progression-free survival (PFS) and median overall survival (OS) were similar among patients who received steroids for irAE management compared to those who did not: ORR 47.1% vs. 52.9% (p = 0.2825); mPFS 31 months vs. 26 months (p = 0.5186); and mOS not reached vs. 38 months (p = 0.2593) (Supplementary Table 3).
We investigated the influence of gender, age at treatment onset and pre-treatment BMI upon irAE development. While irAE occurrence was not affected by sex (p=0.9026) or age (p=0.2130), we observed that increasing BMI (OR 1.06, p = 0.0206) increased the likelihood of irAE occurrence (Supplementary Table 4). Herein, we observed that the odds of irAE development were greater in BMI >25.0 (OR 1.97, 95% CI 1.04, 3.74) compared to patients with BMI ≤25.0. These data are in line with other reports including a meta-analysis linking increased BMI with a greater risk of irAE development in patients treated with anti-PD-1 and/or anti-CTLA-4 ICI (34–38).
Discussion
Several single-center and pooled analyses have evaluated the incidence of irAE and outcomes to anti-PD-1 therapy in melanoma in multiple settings including adjuvant therapy and advanced disease (15–19, 39). However, this real-world single-center analysis is the first study to our knowledge that evaluates the longitudinal development of irAE in anti-PD-1 treated melanoma patients and their relationship to response and survival outcomes along with other demographic determinants of response and autoimmunity including age, gender and BMI. Given the inherent difficulty in classifying and attributing irAEs to antecedent anti-PD-1 therapy, it is not surprising that the data on the incidence and severity of immune-related toxicities is disparate.
Our data show that most irAEs from anti-PD-1 monotherapy were low-grade. The incidence, time to occurrence and profile of irAEs and grade 3/4 irAEs were similar to what had been previously reported for pembrolizumab and nivolumab in the setting of advanced melanoma (8, 9, 39). The majority of irAEs we observed resolved without sequelae, except for endocrine irAEs such as adrenal insufficiency and hypothyroidism, which often required long-term endocrine therapy. The longitudinal follow up at a single center afforded us the ability to examine the temporal trends of irAEs along with the co-occurrence of multiple irAEs. While dermatologic and endocrine irAEs were common events that occurred early, we did note moderately frequent co-occurrence of dermatologic-rheumatologic and dermatologic-endocrine manifestations - suggestive of the presence of a shared factor (or factors) at the level of the host shared among these patients such as: HLA polymorphisms (40), tumor antigenic mimicry (20), and/or intestinal microbiota (41–43). Prospective studies integrating phenotypic information with other biomarkers are needed to further characterize these patterns.
The association between irAE occurrence and improved benefit to anti-PD-1 ICI in terms of response and PFS has previously been reported in ICI-treated melanoma patients (14, 17); although a recent pooled analysis of KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 studies that corrected for immortal time bias suggested similar ORR, PFS and OS statistics among patients who did or did not experience irAEs (39). While we observed that irAE occurrence was associated with response to anti-PD-1 ICI, this association was driven overwhelmingly by the positive association between radiographic response and irAEs belonging to either dermatologic or rheumatologic categories. The positive association between cutaneous adverse events (particularly vitiligo) and favorable therapeutic outcomes in melanoma has been shown with various agents including biochemotherapy (44, 45), interferon (46), ipilimumab (47), and anti-PD-1 ICI (48, 49). Given the known associations between cutaneous and autoimmune diseases with joint/muscle involvement, these observations suggest a shared etiopathogenetic mechanism between cutaneous and rheumatologic irAEs wherein exposure to anti-PD-1 ICI unmasks a latent reactivity to shared antigen(s) (50).
Female gender, increasing age and greater BMI are all associated with increased risk of autoimmunity and autoimmune diseases (51–53). In the context of gender, this is likely related to the effects of endogenous estrogen and/or prolactin upon self-reactive B and T cells (54–56), and/or skewed X-chromosome inactivation (XCI) that results in loss of immunological tolerance to self-antigens (57). While several reports have suggested improved immunotherapy efficacy in men (24), other meta-analyses have derived opposite conclusions (58, 59). We did not observe any differences in irAE incidence by sex.
Both ageing and obesity are tightly associated with “inflammaging” – a chronic low-grade inflammatory state characterized by inflammatory cytokine production and a dynamic reorganization of the adaptive immune compartment associated with co-emergence of exhausted T cells expressing inhibitory receptors including PD-1 and CD4+CD25highFoxP3+ regulatory T cells (60, 61). Consequently, both increased age (62–64), and elevated BMI (65, 66), have independently been linked with improved outcomes to ICI. Consistent with a prior report, we did not observe an increase in the risk of irAE with increased age (62). We observed a significant association between irAEs and higher BMI – consistent with results from retrospective studies evaluating anti-PD-1 ICI-treated cancer patients (34, 38), along with a recent meta-analysis and systemic review (36).
This study has several limitations. Similar to other retrospective analyses, this study is susceptible to inherent biases including reporter bias, although detailed chart review by a trained oncology pharmacist was utilized to minimize this. As we sought to characterize irAEs in patients treated with anti-PD-1 ICI singly, we restricted our analyses to melanoma patients which limits the generalizability of these results to other histologies and patients treated with combinations including ICI/chemotherapy and ICI combinations. Although our focus was development of irAEs following anti-PD-1 monotherapy, we did consider the impact of prior anti-CTLA-4 therapy on clinical outcomes. However, upon review of the data, we identified significant heterogeneity in CTLA-4 treated patients (i.e. ipilimumab dose, number of cycles given, timing of ipilimumab in respect to subsequent anti-PD-1 therapy, and line of therapy). The retrospective nature of this analyses implied a lack of balance in the number of patients in each CTLA-4 subgroup, precluding statistical analyses. However, given the significant role of CTLA-4 blockade in the melanoma armamentarium hitherto limited by irAEs, and the observations that the irAE incidence of CTLA-4 may be ameliorated by low-dose combinations, future efforts should be directed at evaluating the detailed incidence of irAEs in CTLA-4 treated patients by dose (67). To increase statistical power, we grouped individual irAEs into linked irAE categories. While this approach has been adopted by others, there is the chance that in doing so, irAEs with diametric effects upon response were grouped, negating the overall effect of the irAE category upon response, a particular consideration with endocrinopathies.
In conclusion, the detailed longitudinal analyses in this single-center study permitted deep phenotypic characterization and uncovered previously unreported relationships of multiple temporally separated irAEs; and their relationship to response and survival outcomes in anti-PD-1 treated melanoma patients. These observations suggest that the incidence of certain irAEs may be underreported and that irAEs linked to shared etiopathogenetic mechanisms may co-occur in the same individual weeks to months apart that should be the focus of future analyses.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Pittsburgh Cancer Institute (UPCI) Institutional Review Board (IRB) (IRB number PRO18080253). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MB, DF, ZR, and HW acquired, analyzed and interpreted data, and helped to draft the manuscript. DD conceived of the study; participated in study design and coordination; acquired, analyzed and interpreted data; and helped to draft the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Melanoma Research Foundation Breakthrough Consortium (MRFBC) (DD); Award Number P50 CA254865-01A1 from the National Cancer Institute (JK, HZ, DD, and JL); Award Number R01 CA257265-01 from the National Cancer Institute (DD, HZ).
Conflict of Interest
YN: Research Funding (Merck, Pfizer, and Bristol-Myers Squibb). JL: Stock and Other Ownership Interests (RefleXion); Consulting/Advisory Role (7 Hills, Abbvie, Alnylam, Actym, Alphamab Oncology, Arch Oncology, Array, Bayer, Bristol-Myers Squibb, Checkmate Pharmaceuticals, Cstone, Eisai, EMD Serono, Flame,Fstar, Gilead, Kadmon, KSQ, Knaph, Janssen, Immunocore, Inzen, Macrogenics,Mavu, Merck, Mersana, Nektar, Novartis, Onc.AI, Pfizer, Pyxis, Regeneron, Ribon, Rubius, Silicon, Synlogic, TRex, Tempest, Werewolf, Xilio, Xencor); Research Funding (AbbVie, Agios, Array, Astellas, Bristol-Myers Squibb, Corvus, EMD Serono, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, MAcrogenics, Merck, Moderna, Nektar, Numab, Replimmune, Rubius, Spring Bank, Synlogic, Takeda, Trishula, Tizona, Xencor). JK: Consulting/Advisory Role (Amgen, Bristol-Myers Squibb, Checkmate Pharmaceuticals, and Novartis); Research Funding (Amgen, Bristol-Myers Squibb, Castle Biosciences, Checkmate Pharmaceuticals, Immunocore LLC, Iovance, and Novartis). HZ: Consulting/Advisory Role (Bristol Myers Squibb, Checkmate Pharmaceuticals, GlaxoSmithKline/Tesaro and Vedanta Biosciences); Research Funding (Bristol Myers Squibb, Checkmate Pharmaceuticals, and GlaxoSmithKline/Tesaro). DD: Consulting/Advisory Role (Ascendis Pharma, Bristol Myers Squibb, Checkmate Pharmaceuticals, Shionogi, Vedanta Biosciences); Research Funding (Arcus, Checkmate Pharmaceuticals, Cellsight Technologies, GlaxoSmithKline/Tesaro, Merck Inc.).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.749064/full#supplementary-material
Abbreviations
AJCC, American Joint Committee on Cancer; CR, complete response; CI, confidence intervals; CTLA-4, cytotoxic T-lymphocyte antigen 4; ICI, immune checkpoint inhibitor; IRB, Institutional Review Board; LDH, lactate dehydrogenase; OS, overall survival; PD-1, programmed death 1; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; ULN, upper limit normal; HCC, Hillman Cancer Center.
References
1. Dong H, Zhu G, Tamada K, Chen L. B7-H1, A Third Member of the B7 Family, Co-Stimulates T-Cell Proliferation and Interleukin-10 Secretion. Nat Med (1999) 5(12):1365–9. doi: 10.1038/70932
2. Freeman GJ, Borriello F, Hodes RJ, Reiser H, Hathcock KS, Laszlo G, et al. Uncovering of Functional Alternative CTLA-4 Counter-Receptor in B7-Deficient Mice. Science (1993) 262(5135):907–9. doi: 10.1126/science.7694362
3. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nat Immunol (2001) 2(3):261–8. doi: 10.1038/85330
4. Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a New Dendritic Cell Molecule With Potent Costimulatory Properties for T Cells. J Exp Med (2001) 193(7):839–46. doi: 10.1084/jem.193.7.839
5. Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential Expression of Immune-Regulatory Genes Associated With PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res (2015) 21(17):3969–76. doi: 10.1158/1078-0432.CCR-15-0244
6. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor Antigen-Specific CD8 T Cells Infiltrating the Tumor Express High Levels of PD-1 and Are Functionally Impaired. Blood Aug 20 PMCID PMC2927090 (2009) 114(8 SRC - GoogleScholar):1537–44. doi: 10.1182/blood-2008-12-195792
7. Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, et al. PD-1 Is a Regulator of NY-ESO-1-Specific CD8+ T Cell Expansion in Melanoma Patients. J Immunol (2009) 182(9):5240–9. doi: 10.4049/jimmunol.0803245
8. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma Without BRAF Mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
9. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma. N Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
10. Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, et al. Efficacy and Safety of Nivolumab in Patients With BRAF V600 Mutant and BRAF Wild-Type Advanced Melanoma: A Pooled Analysis of 4 Clinical Trials. JAMA Oncol (2015) 1(4):433–40. doi: 10.1001/jamaoncol.2015.1184
11. Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA (2016) 315(15):1600–9. doi: 10.1001/jama.2016.4059
12. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol (2019) 5(10):1411–20. doi: 10.1001/jamaoncol.2019.2187
13. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Multiorgan Immune-Related Adverse Events During Treatment With Atezolizumab. J Natl Compr Canc Netw (2020) 18(9):1191–9. doi: 10.6004/jnccn.2020.7567
14. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol (2017) 35(7):785–92. doi: 10.1200/JCO.2015.66.1389
15. Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity Correlates With Tumor Regression in Patients With Metastatic Melanoma Treated With Anti-Cytotoxic T-Lymphocyte Antigen-4. J Clin Oncol (2005) 23(25):6043–53. doi: 10.1200/JCO.2005.06.205
16. Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol (2020) 6(4):519–27. doi: 10.1001/jamaoncol.2019.5570
17. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association With Outcomes. Clin Cancer Res (2016) 22(4):886–94. doi: 10.1158/1078-0432.CCR-15-1136
18. Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, De Braud F, et al. Immune-Related Adverse Events Correlate With Improved Survival in Patients Undergoing Anti-PD1 Immunotherapy for Metastatic Melanoma. J Cancer Res Clin Oncol (2019) 145(2):511–21. doi: 10.1007/s00432-018-2819-x
19. Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J Clin Oncol (2019) 37(30):2730–7. doi: 10.1200/JCO.19.00318
20. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol (2018) 4(3):374–8. doi: 10.1001/jamaoncol.2017.2925
21. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of Immune-Related Adverse Events on Survival in Patients With Advanced Non-Small Cell Lung Cancer Treated With Nivolumab: Long-Term Outcomes From a Multi-Institutional Analysis. J Cancer Res Clin Oncol (2019) 145(2):479–85. doi: 10.1007/s00432-018-2805-3
22. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A Multicenter Study of Body Mass Index in Cancer Patients Treated With Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors: When Overweight Becomes Favorable. J Immunother Cancer (2019) 7(1):57. doi: 10.1186/s40425-019-0527-y
23. McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of Body-Mass Index and Outcomes in Patients With Metastatic Melanoma Treated With Targeted Therapy, Immunotherapy, or Chemotherapy: A Retrospective, Multicohort Analysis. Lancet Oncol (2018) 19(3):310–22. doi: 10.1016/S1470-2045(18)30078-0
24. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer Immunotherapy Efficacy and Patients' Sex: A Systematic Review and Meta-Analysis. Lancet Oncol (2018) 19(6):737–46. doi: 10.1016/S1470-2045(18)30261-4
25. Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann Surg Oncol (2018) 25(8):2105–10. doi: 10.1245/s10434-018-6513-7
26. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
27. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
28. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1. 2020 J Natl Compr Canc Netw (2020) 18(3):230–41. doi: 10.6004/jnccn.2020.0012
29. Cortellini A, Buti S, Agostinelli V, Bersanelli M. A Systematic Review on the Emerging Association Between the Occurrence of Immune-Related Adverse Events and Clinical Outcomes With Checkpoint Inhibitors in Advanced Cancer Patients. Semin Oncol (2019) 46(4-5):362–71. doi: 10.1053/j.seminoncol.2019.10.003
30. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations Between the Immune-Related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients. Clin Lung Cancer (2019) 20(4):237–47.e1. doi: 10.1016/j.cllc.2019.02.006
31. Petrelli F, Grizzi G, Ghidini M, Ghidini A, Ratti M, Panni S, et al. Immune-Related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J Immunother (2020) 43(1):1–7. doi: 10.1097/CJI.0000000000000300
32. Verzoni E, Carteni G, Cortesi E, Giannarelli D, De Giglio A, Sabbatini R, et al. Real-World Efficacy and Safety of Nivolumab in Previously-Treated Metastatic Renal Cell Carcinoma, and Association Between Immune-Related Adverse Events and Survival: The Italian Expanded Access Program. J Immunother Cancer (2019) 7(1):99. doi: 10.1186/s40425-019-0579-z
33. Johnson DB, Lovly CM, Flavin M, Panageas KS, Ayers GD, Zhao Z, et al. Impact of NRAS Mutations for Patients With Advanced Melanoma Treated With Immune Therapies. Cancer Immunol Res (2015) 3(3):288–95. doi: 10.1158/2326-6066.CIR-14-0207
34. Cortellini A, Bersanelli M, Santini D, Buti S, Tiseo M, Cannita K, et al. Another Side of the Association Between Body Mass Index (BMI) and Clinical Outcomes of Cancer Patients Receiving Programmed Cell Death Protein-1 (PD-1)/ Programmed Cell Death-Ligand 1 (PD-L1) Checkpoint Inhibitors: A Multicentre Analysis of Immune-Related Adverse Events. Eur J Cancer (2020) 128:17–26. doi: 10.1016/j.ejca.2019.12.031
35. Guzman-Prado Y, Ben Shimol J, Samson O. Body Mass Index and Immune-Related Adverse Events in Patients on Immune Checkpoint Inhibitor Therapies: A Systematic Review and Meta-Analysis. Cancer Immunol Immunother (2021) 70(1):89–100. doi: 10.1007/s00262-020-02663-z
36. Rogado J, Romero-Laorden N, Sanchez-Torres JM, Ramos-Levi AM, Pacheco-Barcia V, Ballesteros AI, et al. Effect of Excess Weight and Immune-Related Adverse Events on the Efficacy of Cancer Immunotherapy With Anti-PD-1 Antibodies. Oncoimmunology (2020) 9(1):1751548. doi: 10.1080/2162402X.2020.1751548
37. Daly LE, Power DG, O'Reilly A, Donnellan P, Cushen SJ, O'Sullivan K, et al. The Impact of Body Composition Parameters on Ipilimumab Toxicity and Survival in Patients With Metastatic Melanoma. Br J Cancer (2017) 116(3):310–7. doi: 10.1038/bjc.2016.431
38. Eun Y, Kim IY, Sun JM, Lee J, Cha HS, Koh EM, et al. Risk Factors for Immune-Related Adverse Events Associated With Anti-PD-1 Pembrolizumab. Sci Rep (2019) 9(1):14039. doi: 10.1038/s41598-019-50574-6
39. Robert C, Hwu WJ, Hamid O, Ribas A, Weber JS, Daud AI, et al. Long-Term Safety of Pembrolizumab Monotherapy and Relationship With Clinical Outcome: A Landmark Analysis in Patients With Advanced Melanoma. Eur J Cancer (2020) 144:182–91. doi: 10.1016/j.ejca.2020.11.010
40. Hasan Ali O, Berner F, Bomze D, Fassler M, Diem S, Cozzio A, et al. Human Leukocyte Antigen Variation Is Associated With Adverse Events of Checkpoint Inhibitors. Eur J Cancer (2019) 107:8–14. doi: 10.1016/j.ejca.2018.11.009
41. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat Commun (2016) 7:10391. doi: 10.1038/ncomms10391
42. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated With Ipilimumab. Ann Oncol (2017) 28(6):1368–79. doi: 10.1093/annonc/mdx108
43. Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut Microbiota Signatures Are Associated With Toxicity to Combined CTLA-4 and PD-1 Blockade. Nat Med (2021) 8:1432–41. doi: 10.1038/s41591-021-01406-6.
44. Boasberg PD, Hoon DS, Piro LD, Martin MA, Fujimoto A, Kristedja TS, et al. Enhanced Survival Associated With Vitiligo Expression During Maintenance Biotherapy for Metastatic Melanoma. J Invest Dermatol (2006) 126(12):2658–63. doi: 10.1038/sj.jid.5700545
45. Richards JM, Mehta N, Ramming K, Skosey P. Sequential Chemoimmunotherapy in the Treatment of Metastatic Melanoma. J Clin Oncol (1992) 10(8):1338–43. doi: 10.1200/JCO.1992.10.8.1338
46. Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, et al. Prognostic Significance of Autoimmunity During Treatment of Melanoma With Interferon. N Engl J Med (2006) 354(7):709–18. doi: 10.1056/NEJMoa053007
47. Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer Regression and Autoimmunity Induced by Cytotoxic T Lymphocyte-Associated Antigen 4 Blockade in Patients With Metastatic Melanoma. Proc Natl Acad Sci USA (2003) 100(14):8372–7. doi: 10.1073/pnas.1533209100
48. Bottlaender L, Amini-Adle M, Maucort-Boulch D, Robinson P, Thomas L, Dalle S. Cutaneous Adverse Events: A Predictor of Tumour Response Under Anti-PD-1 Therapy for Metastatic Melanoma, a Cohort Analysis of 189 Patients. J Eur Acad Dermatol Venereol (2020) 34(9):2096–105. doi: 10.1111/jdv.16311
49. Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol (2016) 152(1):45–51. doi: 10.1001/jamadermatol.2015.2707
50. Arad-Dann H, Isenberg DA, Shoenfeld Y, Offen D, Sperling J, Sperling R. Autoantibodies Against a Specific Nuclear RNP Protein in Sera of Patients With Autoimmune Rheumatic Diseases Associated With Myositis. J Immunol (1987) 138(8):2463–8.
51. Vadasz Z, Haj T, Kessel A, Toubi E. Age-Related Autoimmunity. BMC Med (2013) 11:94. doi: 10.1186/1741-7015-11-94
52. Voskuhl R. Sex Differences in Autoimmune Diseases. Biol Sex Differ (2011) 2(1):1. doi: 10.1186/2042-6410-2-1
53. Zhou Y, Sun M. A Meta-Analysis of the Relationship Between Body Mass Index and Risk of Rheumatoid Arthritis. EXCLI J (2018) 17:1079–89. doi: 10.17179/excli2018-1763
54. Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen Alters Thresholds for B Cell Apoptosis and Activation. J Clin Invest (2002) 109(12):1625–33. doi: 10.1172/JCI0214873
55. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-Specific Disease Provoked by Systemic Autoimmunity. Cell (1996) 87(5):811–22. doi: 10.1016/S0092-8674(00)81989-3
56. Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of Castration and Sex Hormone Treatment on Survival, Anti-Nucleic Acid Antibodies, and Glomerulonephritis in NZB/NZW F1 Mice. J Exp Med (1978) 147(6):1568–83. doi: 10.1084/jem.147.6.1568
57. Chitnis S, Monteiro J, Glass D, Apatoff B, Salmon J, Concannon P, et al. The Role of X-Chromosome Inactivation in Female Predisposition to Autoimmunity. Arthritis Res (2000) 2(5):399–406. doi: 10.1186/ar118
58. Wallis CJD, Butaney M, Satkunasivam R, Freedland SJ, Patel SP, Hamid O, et al. Association of Patient Sex With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5(4):529–36. doi: 10.1001/jamaoncol.2018.5904
59. Grassadonia A, Sperduti I, Vici P, Iezzi L, Brocco D, Gamucci T, et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J Clin Med (2018) 7(12):542. doi: 10.3390/jcm7120542
60. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: A New Immune-Metabolic Viewpoint for Age-Related Diseases. Nat Rev Endocrinol (2018) 14(10):576–90. doi: 10.1038/s41574-018-0059-4
61. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic Inflammation in the Etiology of Disease Across the Life Span. Nat Med (2019) 25(12):1822–32. doi: 10.1038/s41591-019-0675-0
62. Ben-Betzalel G, Steinberg-Silman Y, Stoff R, Asher N, Shapira-Frommer R, Schachter J, et al. Immunotherapy Comes of Age in Octagenarian and Nonagenarian Metastatic Melanoma Patients. Eur J Cancer (2019) 108:61–8. doi: 10.1016/j.ejca.2018.12.012
63. Betof AS, Nipp RD, Giobbie-Hurder A, Johnpulle RAN, Rubin K, Rubinstein SM, et al. Impact of Age on Outcomes With Immunotherapy for Patients With Melanoma. Oncologist (2017) 22(8):963–71. doi: 10.1634/theoncologist.2016-0450
64. Kugel CH 3rd, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age Correlates With Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin Cancer Res (2018) 24(21):5347–56. doi: 10.1158/1078-0432.CCR-18-1116
65. An Y, Wu Z, Wang N, Yang Z, Li Y, Xu B, et al. Association Between Body Mass Index and Survival Outcomes for Cancer Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J Transl Med (2020) 18(1):235. doi: 10.1186/s12967-020-02404-x
66. Chen H, Wang D, Zhong Q, Tao Y, Zhou Y, Shi Y. Pretreatment Body Mass Index and Clinical Outcomes in Cancer Patients Following Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancer Immunol Immunother (2020) 69(12):2413–24. doi: 10.1007/s00262-020-02680-y
67. Kleef R, Nagy R, Baierl A, Bacher V, Bojar H, McKee DL, et al. Low-Dose Ipilimumab Plus Nivolumab Combined With IL-2 and Hyperthermia in Cancer Patients With Advanced Disease: Exploratory Findings of a Case Series of 131 Stage IV Cancers - A Retrospective Study of a Single Institution. Cancer Immunol Immunother (2021) 70(5):1393–403. doi: 10.1007/s00262-020-02751-0
Keywords: melanoma, metastatic, immunotherapy, CTLA-4, PD-1, immune related adverse events, irAE, autoimmune
Citation: Bastacky ML, Wang H, Fortman D, Rahman Z, Mascara GP, Brenner T, Najjar YG, Luke JJ, Kirkwood JM, Zarour HM and Davar D (2021) Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis. Front. Oncol. 11:749064. doi: 10.3389/fonc.2021.749064
Received: 28 July 2021; Accepted: 03 September 2021;
Published: 26 November 2021.
Edited by:
William L. Redmond, Earle A. Chiles Research Institute, United StatesReviewed by:
Brendan Curti, Earle A. Chiles Research Institute, United StatesTibor Bakacs, Alfred Renyi Institute of Mathematics, Hungary
Copyright © 2021 Bastacky, Wang, Fortman, Rahman, Mascara, Brenner, Najjar, Luke, Kirkwood, Zarour and Davar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diwakar Davar, ZGF2YXJkQHVwbWMuZWR1
 Melissa L. Bastacky1
Melissa L. Bastacky1 Hong Wang
Hong Wang Gerard P. Mascara
Gerard P. Mascara John M. Kirkwood
John M. Kirkwood Hassane M. Zarour
Hassane M. Zarour Diwakar Davar
Diwakar Davar