- 1Department of Neurosurgery, The First Hospital of China Medical University, Shenyang, China
- 2Department of Neurosurgery, Huazhong University of Science and Technology Union Shenzhen Hospital (Nanshan Hospital), Shenzhen, China
- 3Department of Neurosurgery, Shanghai First People’s Hospital of Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Pathology, China Medical University, Shenyang, China
- 5Department of Geriatrics, Shengjing Hospital of China Medical University, Shenyang, China
Purpose: Multifocal and multicentric glioblastomas (mGBMs) are associated with a poorer prognosis compared to unifocal glioblastoma (uGBM). The presence of CD8+ tumor-infiltrating lymphocytes (TILs) is predictive of clinical outcomes in human malignancies. Here, we examined the CD8+ lymphocytic infiltration in mGBMs.
Methods: The clinical data of 57 consecutive IDH wildtype primary mGBM patients with histopathological diagnoses were retrospectively reviewed. CD8+ TILs were quantitatively evaluated by immunohistochemical staining. The survival function of CD8+ TILs was assessed by Kaplan–Meier analysis and Cox proportional hazard models.
Results: No significant difference in the concentration of CD8+ TILs was observed among foci from the same patient (P>0.150). The presence of CD8+ TILs was similar between multifocal and multicentric GBMs (P=0.885). The concentration of CD8+ TILs was significantly lower in mGBMs than in uGBMs (P=0.002). In mGBM patients, the CD8+ TIL level was associated with preoperative KPS (P=0.018). The median overall survival (OS) of the 57 mGBMs was 9 months. A low CD8+ TIL level (multivariate HR 4.404, 95% CI 1.954-9.926, P=0.0004) was an independent predictor of poor OS, while postoperative temozolomide chemotherapy (multivariate HR 6.076, 95% CI 2.330-15.842, P=0.0002) was independently associated with prolonged OS in mGBMs.
Conclusions: Decreased CD8+ TIL levels potentially correlate with unfavorable clinical outcome in mGBMs, suggesting an influence of the local immuno-microenvironment on the progression of mGBMs.
Introduction
Glioblastoma (GBM) is the most common primary malignant tumor in the central nervous system (CNS) and exhibits a poor prognosis (1). GBM is therapeutically intractable and refractory to currently available multimodal treatments including surgery, radiotherapy, and chemotherapy (2). Most newly diagnosed GBMs present as a unifocal GBM (uGBM), whereas GBMs with multiple lesions are relatively rare, as the latter account for only 0.5%–20% of all GBMs based on histopathological or radiological diagnosis (3–9). Multiple GBMs are categorized into multifocal and multicentric GBMs (mGBMs). The foci of multifocal GBMs are close to each other, suggesting a physical connection; in contrast, the foci of multicentric are located in different lobes or hemispheres without any obvious dissemination route (10, 11). Nevertheless, prior publications have shown no significant utility in the distinction between multifocal and multicentric GBMs (5, 7, 10–13). Therefore, per the convention of previous studies (7, 10, 11), we use the term “mGBM” in the present study to represent our cases with multiple GBM lesions within a single patient. According to previous reports, mGBMs are associated with even worse clinical outcome and poorer survival times compared to uGBMs (7, 10, 14, 15). However, the pathogenic mechanisms and clinical characteristics of mGBMs are still largely unclear, and standards of care for mGBMs are not well defined. Therefore, new treatment strategies, including immunotherapies, need to be developed for mGBMs.
The tumor microenvironment (TME) is a critical regulator of pathogenesis and therapeutic responses in GBMs (16). CD8+ tumor-infiltrating lymphocytes (TILs) have been demonstrated to be important components of the TME and predict improved survival in several human malignancies, including breast cancer (17), ovarian cancer (18), and colorectal cancer (19). CD8+ TILs have also been detected in the TME of GBMs; however, the prognostic effect of CD8+ TILs in GBMs is still controversial (20–23). Moreover, the role of CD8+ TILs in mGBMs has not yet been elucidated. Thus, in the present study, we investigated the infiltration of CD8+ lymphocytes in a series of 57 newly diagnosed mGBMs and assessed the effects of CD8+ TILs and other clinical parameters on the prognosis of mGBM patients. Taken together, our findings might shed light on immuno-microenvironmental mechanisms of mGBM biology.
Patients and Methods
Study Patients
This study was approved by the institutional review board of the First Hospital of China Medical University (AF-SOP-07-1.1-01). All methods below were performed in accordance with the relevant guidelines and regulations. From June 2015 to May 2019, 492 patients were newly diagnosed with GBMs at the First Hospital of China Medical University. Among them, 57 consecutive patients (11.6%) had histopathological diagnoses of primary mGBMs and were included in the present study. All the examined foci from the 57 mGBMs were WHO grade IV and IDH wildtype, as determined by next-generation sequencing (Genetron, Shanghai, China). Methylation of the MGMT promoter was detected by a methylation-specific PCR-Fluorescent-Probe Method, as previously described (21). The histopathological diagnoses were reported by the Pathology Department of China Medical University and were further confirmed by two neuropathologists. The clinical data, radiological examinations, and follow-up information were retrospectively reviewed and analyzed. No patients had systemic diseases, other cancers, or other CNS tumors.
Tumor resection was determined by intraoperative observation and postoperative magnetic resonance imaging (MRI) and was defined as gross total resection (GTR, removal of all foci), partial resection (removal of one or more foci) and stereotactic biopsy. Tumor size was calculated as the sum size of all foci, based on preoperative enhanced MRI using the following formula: Σ (anteroposterior diameter × transverse diameter × axial diameter) × π/6. After the histopathological diagnosis was made, adjuvant therapies were determined by multidisciplinary discussion within a brain-tumor team that included neuroradiologists, neuropathologists, radiation oncologists, neurooncologists, and neurosurgeons. The final treatment plan also took the personal decision of each patient into consideration. Ultimately, 16 patients received 3D conformal radiotherapy (60 Gy in 2.0 Gy fractions) as previously described (15), 35 patients received temozolomide (TMZ) chemotherapy according to the Stupp protocol (2), and 15 patients did not receive radiotherapy or chemotherapy.
Immunohistochemistry (IHC)
IHC staining and quantitative evaluation were performed as previously reported (21). Briefly, paraffin-embedded sections underwent deparaffinization with xylene, and rehydration and antigen retrieval was achieved via microwaving in 10 mmol/L of sodium citrate buffer (pH 6.0). After blocking endogenous peroxidase with 3% H2O2 in methanol and blocking non-specific binding with protein-blocking buffer, sections were incubated with primary antibody against CD8 (clone 144B, 1:50; Abcam, Cambridge, UK). Normal mouse serum was used as a negative control. Then, sections were incubated with a horseradish-peroxidase-labeled secondary antibody, colored with diaminobenzidine, and counterstained with hematoxylin. Results were observed and photographed under a light microscope connected to a computer (Olympus, Tokyo, Japan). Each section was assessed using at least five different high-power fields (HPF, 400×) with the most abundant CD8+ TILs. The concentration of CD8+ TILs was independently counted at least three times by two experienced neuropathologists (DZ and JY) blinded to the clinical backgrounds of the patients. To ensure reproducibility, the results were re-examined after a period of time. When a satisfactory intra-observer and inter-observer agreement was obtained, the average of CD8+ TIL counts per field for each patient was utilized for further statistical analysis as previously described (17, 21). Ki-67 IHC staining was routinely performed and reported by the Pathology Department of China Medical University.
Statistical Analysis
The results are presented as the mean ± standard error of the mean (SEM). The t test, analysis of variance, and Mann-Whitney test were used to assess statistical significance. The Kaplan-Meier analysis and log-rank test were used to evaluate survival differences. Multivariate Cox analyses were used to calculate hazard ratios (HRs) of deaths according to different variables. All statistical analyses were performed using SPSS v25.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA). Statistical significance was set at P<0.05 (two-tailed).
Results
Clinical Features
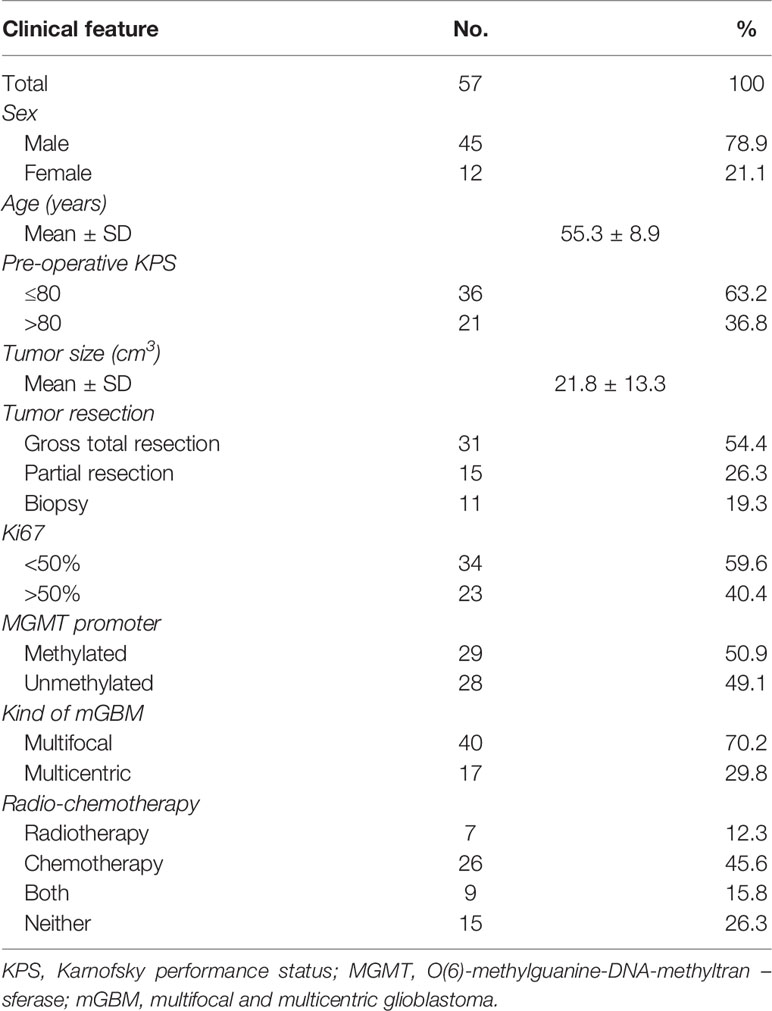
As shown in Table 1, in this series of 57 patients, there were 45 male (78.9%) and 12 female (21.1%) patients, with an average age of 55.3 ± 8.9 years (39–68 years). Forty (70.2%) patients had multifocal GBMs, and seventeen (29.8%) patients had multicentric GBMs (Figure 1A). The mean tumor size was 21.8 ± 13.3 cm3 (0.2–82.3 cm3). Thirty-one (54.4%) patients received GTR, fifteen (26.3%) patients received partial resection, and eleven (19.3%) patients received biopsies. There were 23 (40.4%) cases with Ki-67 >50% and 34 (59.6%) cases with Ki-67 <50%. Furthermore, there were 29 (50.9%) patients with a methylated MGMT promoter. The median follow-up time was 9 months (ranging from 1 to 20 months), during which 35 patients died from GBMs, and no patient was lost to follow-up.
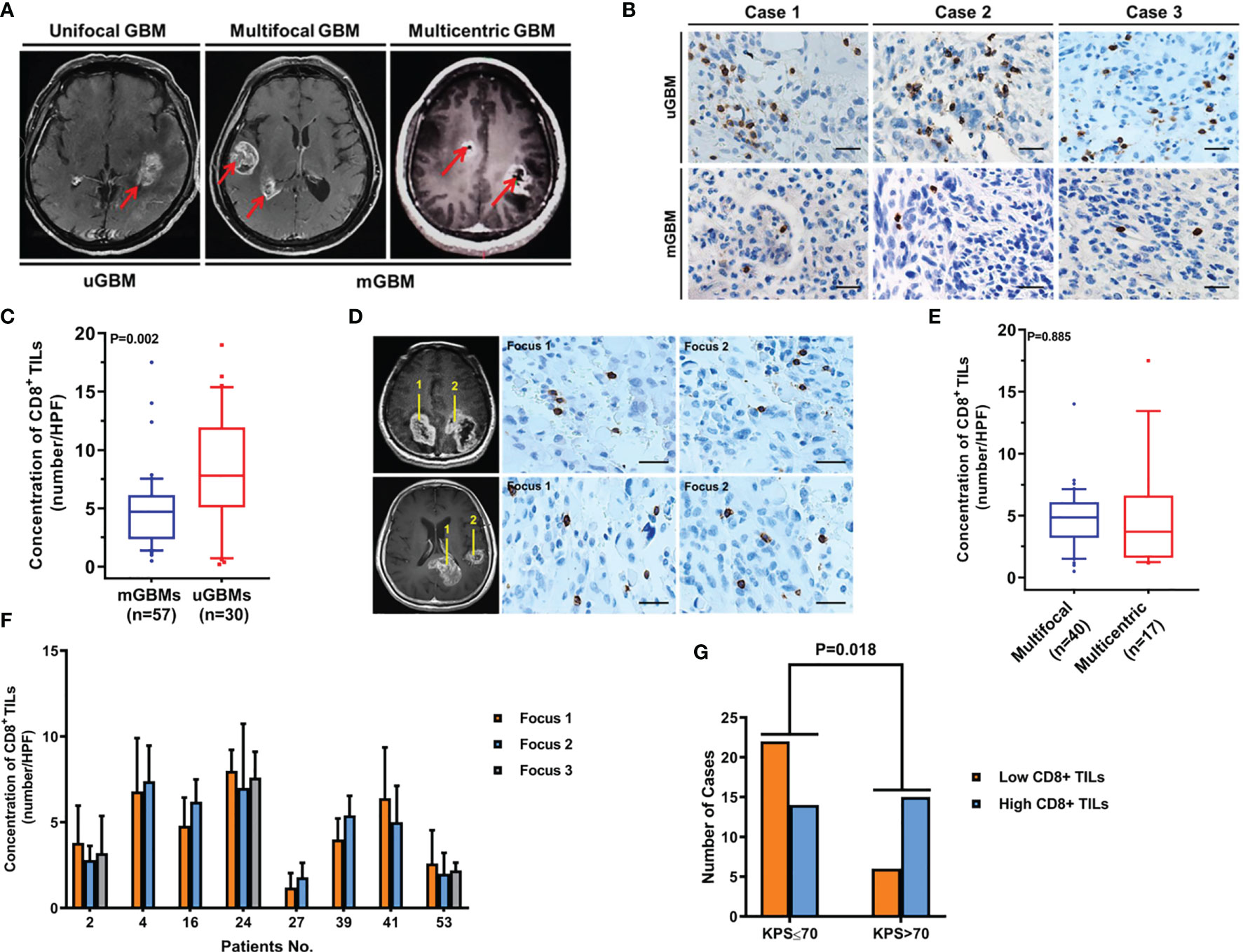
Figure 1 CD8+ TIL levels are decreased in mGBMs. (A) Typical MRIs of a unifocal GBM (uGBM), multifocal GBM, and multicentric GBM (mGBM). (B) Representative CD8+ TIL IHC images of uGBM and mGBM cases. Scale bar, 20 μm. (C) The concentration of CD8+ TILs was significantly lower in mGBMs than in uGBMs. (D) IHC staining of CD8+ TILs in different foci of multicentric and multifocal GBMs. Scale bar, 20 μm. (E) The presence of CD8+ TILs was similar between multifocal and multicentric GBMs. (F) In mGBMs, no significant difference in the concentration of CD8+ TILs was observed among foci from the same patient. (G) CD8+ TIL levels were significantly correlated with preoperative KPS.
CD8+ Lymphocytic Infiltration in mGBMs
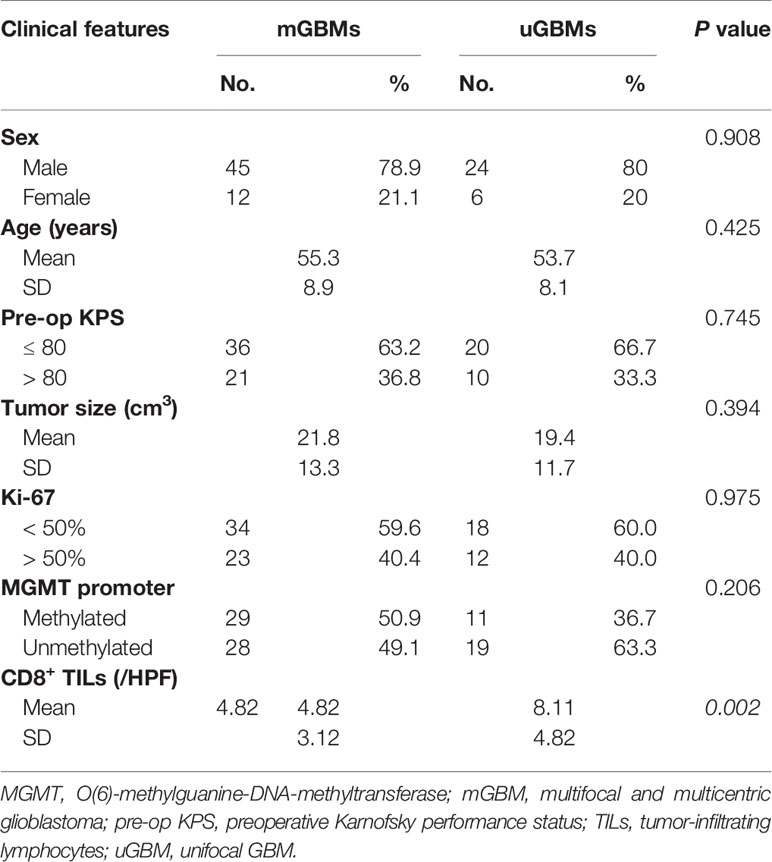
To compare CD8+ lymphocytic infiltration in mGBMs with that in uGBMs, CD8+ TILs were detected in 57 mGBMs and 30 uGBMs. As shown in Table 2, the mGBMs and uGBMs were matched in terms of sex, age, preoperative KPS, tumor size, Ki-67 indexes, and MGMT promoter methylation. The concentration of CD8+ TILs was significantly lower in mGBMs (4.82 ± 3.12/HPF, median 4.70/HPF, interquartile range 2.38–6.12/HPF) than in uGBMs (8.11 ± 4.82/HPF, median 7.80/HPF, interquartile range 5.33–11.70/HPF; P=0.002; Figures 1B, C).
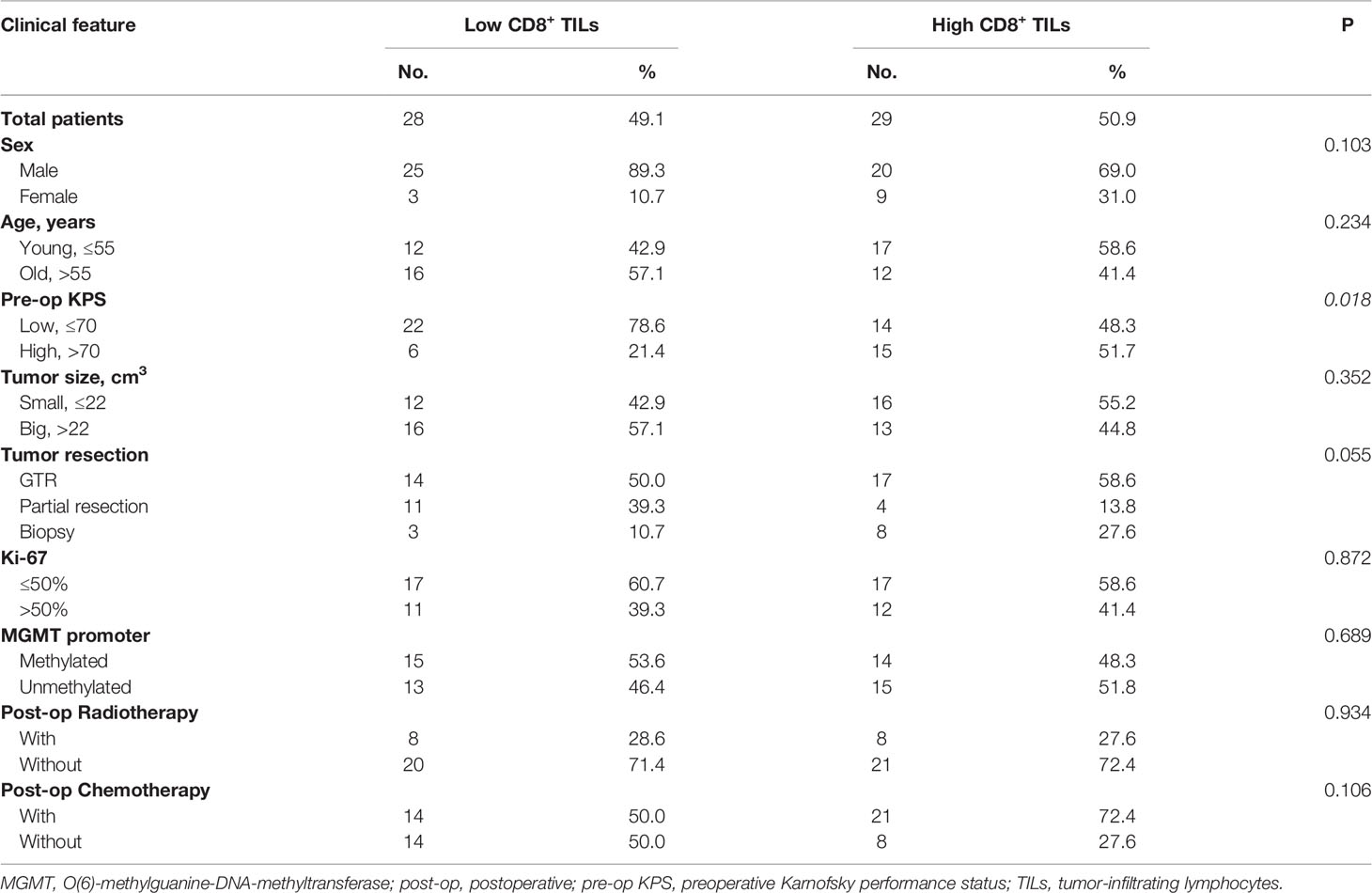
In mGBMs, no significant difference in the concentration of CD8+ TILs was observed among foci from the same patient (P>0.150). The presence of CD8+ TILs was similar between multifocal and multicentric GBMs (4.60 ± 2.63/HPF vs. 4.97 ± 3.07/HPF, P=0.885; Figures 1D–F). The levels of CD8+ TILs did not vary significantly according to sex, age, tumor size, tumor resection, Ki-67, MGMT promoter methylation, or postoperative radiotherapy or chemotherapy. However, CD8+ TIL levels were significantly correlated with preoperative KPS, and low CD8+ lymphocytic infiltration was more frequently found in patients with KPS ≤ 70 (P=0.018; Table 3; Figure 1G).
Prognostic Value of CD8+ TILs in mGBMs
To investigate the prognostic significance of CD8+ TILs in this series of mGBMs, the median concentration of CD8+ TILs (4.70/HPF) was used as the cutoff value to define a low-infiltration group (n=28) and a high-infiltration group (n=29) for survival analysis. In the Kaplan–Meier analysis, low CD8+ TILs were significantly associated with shorter overall survival (OS, median OS 6.3 months vs. 12.5 months, P=0.001; Figure 2A). Meanwhile, postoperative chemotherapy was significantly associated with prolonged OS (median 9.5 months vs. 8.5 months, P<0.001; Figure 2B).
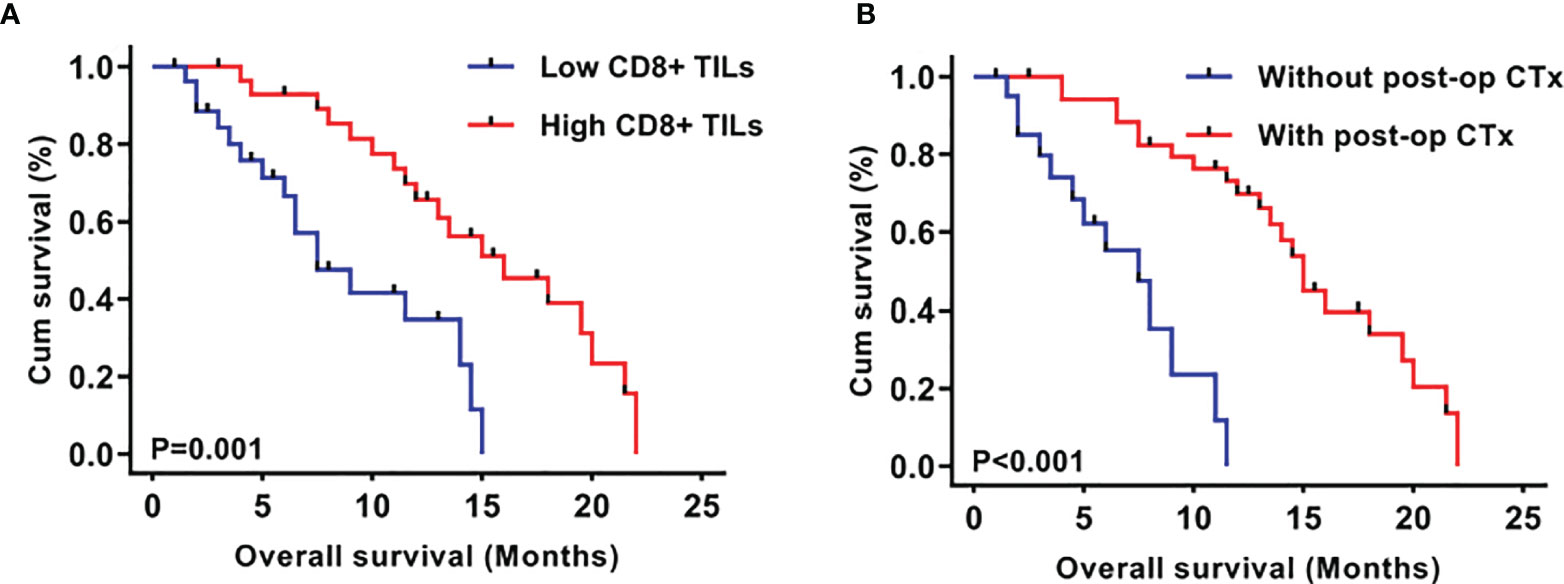
Figure 2 Kaplan–Meier plots of overall survival stratified by CD8+ TIL level (A) and post-operative chemotherapy (B).
As shown in Table 4, in univariate analyses, the P values of postoperative chemotherapy, CD8+ TILs, MGMT promotor methylation, and age were less than 0.2 and were consequently included into multivariate analyses. Multivariate Cox analyses demonstrated that CD8+ TILs (P=0.0004, HR 4.404, 95% CI 1.954-9.926) and postoperative chemotherapy (P=0.0002, HR 6.076, 95% CI 2.330-15.842) were independent prognostic factors in mGBMs. Consistent with previous reports (7, 10), no significant difference was observed in the OS between patients with multifocal and multicentric GBMs (P=0.462).
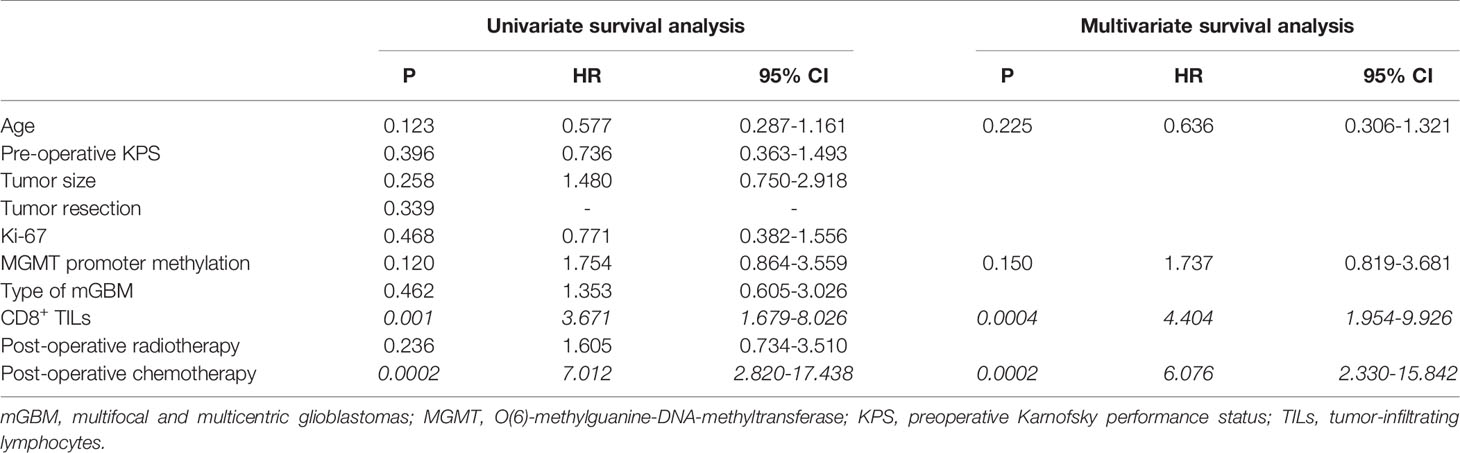
Table 4 Univariate and multivariate analyses of different prognostic parameters for overall survival of 57 mGBM patients.
Discussion
Although GBMs have been extensively investigated, few studies have focused on multifocal and multicentric GBMs (mGBMs) possibly due to their rare occurrences. Since the majority of newly diagnosed GBMs are unifocal, mGBMs may have distinct molecular mechanisms, microenvironmental characteristics, and clinical courses (10, 11), which have not been clearly elucidated. Moreover, current standard radio-chemotherapy shows inferior therapeutic effects in mGBMs compared with those in uGBMs (15). Therefore, the development of novel mGBM-targeted treatment strategies is clinically relevant.
Previous studies have demonstrated that the immunosuppressive TME plays an important role in the development and progression of GBMs (24, 25). A hallmark of GBM-induced immunosuppression is the inhibition of CD8+ effector T-cell mediated anti-tumor responses (26, 27). A number of GBM-derived factors trigger reprogramming of immune cells and inhibit the accumulation and activation of cytotoxic CD8+ TILs (24, 28). Although some studies have failed to show a relation between CD8+ TILs and clinical outcomes (20, 21), several studies have observed a significant correlation between decreased CD8+ TILs and poorer patient survival in GBMs (22, 23, 29). In addition, previous studies did not investigate mGBMs and uGBMs separately. Therefore, in the present study, we specifically examined the CD8+ TILs in mGBMs. We found that mGBMs were associated with decreased CD8+ lymphocytic infiltration compared with uGBMs. Moreover, lower CD8+ TIL levels predicted shorter survival times in mGBM patients. Decreased CD8+ TIL levels indicate a more serious local immunosuppressive TME, which may facilitate the development of multifocality and promote the immune evasion of tumor cells. Therefore, inhibition of CD8+ lymphocytic infiltration might provide an immuno-microenvironmental basis for the formation and progression of multiple lesions of mGBMs. Moreover, our present results showed that CD8+ TIL levels were associated with preoperative KPS in mGBM patients, which suggests an interaction of systemic status and the local immune TME. Nevertheless, further studies are required to better understand the intrinsic mechanisms of this immunosuppression. Our present results also indicate that the immunomodulatory mechanism employed by mGBMs poses a considerable challenge to immunotherapy, since effective immunotherapy often requires the participation of functionally active CD8+ T cells (28, 30). Thus, counteracting the impairment of CD8+ TILs and promoting a sustained tumor-cell-directed cytotoxic T-cell response are necessary to overcome such immunosuppression and to establish efficacious immunotherapeutic treatments in mGBM patients.
Currently, specific clinical guidelines for the standard treatment of mGBMs are still unavailable (1). Unfortunately, mGBMs are treated in the same way as uGBMs, although they are biologically and clinically different from each other (5, 10, 11). Therefore, the efficacies of surgical treatment and radio-chemotherapy should be re-evaluated in mGBM patients. For example, the role of surgery for uGBMs has been well demonstrated by previous studies, and GTR significantly improves OS (31, 32). However, the optimal surgical management of mGBMs remains controversial (5). Some authors support stereotactic biopsy as the first choice, showing that radical resection increases postoperative morbidity without survival benefits (33–35); while other authors propose aggressive resection, reporting that cytoreductive surgery strongly influences OS in mGBM patients (6, 36, 37). In addition, the prognostic value of radiation therapy has not been clarified in mGBM patients, and the efficacies of whole-brain radiotherapy and 3D conformal radiotherapy are still under debate (13, 38, 39). In the present study, we found that TMZ chemotherapy was associated with improved OS of patients with mGBMs, which is consistent with findings from previous reports (15, 38). Therefore, TMZ systemic therapy is effective and beneficial and should be a major component of forthcoming therapeutic strategies for mGBMs.
Limitations
The present study had some limitations: This was a retrospective study, and the number of included cases was relatively small. Whenever possible, prospective studies with large sample sizes should be performed to draw stronger conclusions. Given that mGBMs rarely occur, accumulation of such cases is clinically relevant and important. Moreover, the quantification of CD8+ TILs is difficult and our approach may still introduce various kinds of bias, including sampling bias, to the study.
Conclusions
In this study, we demonstrated decreased CD8+ lymphocytic infiltration in mGBMs and potential prognostic significance of CD8+ TIL levels in mGBM patients. Our results suggest that the local immunosuppressive TME might affect the development and progression of mGBMs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the institutional review board of the First Hospital of China Medical University (AF-SOP-07-1.1-01).
Authors Contributions
RW, YS, TH, XW, and LK cooperated to complete the experiment. RW, TH, YJ, DZ, JY, and SH contributed to the collection and analysis of data. RW, YS, TH, XW, and YJ participated in drafting the text and figures. SH and LK designed the study and gave indispensable guidance in drafting the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Liaoning Revitalization Talents Program (no. XLYC1807253) and the National Natural Science Foundation of China (nos. 81772653 and 81402045).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Qingchang Li at the Department of Pathology, China Medical University, for technological support with immunohistochemical analysis. And we thank Junqi Wu, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, for assistance with statistical and survival analyses.
References
1. Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European Association for Neuro-Oncology (EANO) Guideline on the Diagnosis and Treatment of Adult Astrocytic and Oligodendroglial Gliomas. Lancet Oncol (2017) 18:e315–29. doi: 10.1016/S1470-2045(17)30194-8
2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352:987–96. doi: 10.1056/NEJMoa043330
3. Thomas RP, Xu LW, Lober RM, Li G, Nagpal S. The Incidence and Significance of Multiple Lesions in Glioblastoma. J Neurooncol (2013) 112:91–7. doi: 10.1007/s11060-012-1030-1
4. Lasocki A, Gaillard F, Tacey MA, Drummond KJ, Stuckey SL. The Incidence and Significance of Multicentric Noncontrast-Enhancing Lesions Distant From a Histologically-Proven Glioblastoma. J Neurooncol (2016) 129:471–8. doi: 10.1007/s11060-016-2193-y
5. Di Carlo DT, Cagnazzo F, Benedetto N, Morganti R, Perrini P. Multiple High-Grade Gliomas: Epidemiology, Management, and Outcome. A Systematic Review and Meta-Analysis. Neurosurg Rev (2019) 42:263–75. doi: 10.1007/s10143-017-0928-7
6. Hassaneen W, Levine NB, Suki D, Salaskar AL, de Moura Lima A, McCutcheon IE, et al. Multiple Craniotomies in the Management of Multifocal and Multicentric Glioblastoma. Clin Article J Neurosurg (2011) 114:576–84. doi: 10.3171/2010.6.JNS091326
7. Patil CG, Yi A, Elramsisy A, Hu J, Mukherjee D, Irvin DK, et al. Prognosis of Patients With Multifocal Glioblastoma: A Case-Control Study. J Neurosurg (2012) 117:705–11. doi: 10.3171/2012.7.JNS12147
8. Djalilian HR, Shah MV, Hall WA. Radiographic Incidence of Multicentric Malignant Gliomas. Surg Neurol (1999) 51:554–8. doi: 10.1016/s0090-3019(98)00054-8
9. Stark AM, Nabavi A, Mehdorn HM, Blomer U. Glioblastoma Multiforme-Report of 267 Cases Treated at a Single Institution. Surg Neurol (2005) 63:162–9. doi: 10.1016/j.surneu.2004.01.028
10. Liu Q, Liu Y, Li W, Wang X, Sawaya R, Lang FF, et al. Genetic, Epigenetic, and Molecular Landscapes of Multifocal and Multicentric Glioblastoma. Acta Neuropathol (2015) 130:587–97. doi: 10.1007/s00401-015-1470-8
11. Abou-El-Ardat K, Seifert M, Becker K, Eisenreich S, Lehmann M, Hackmann K, et al. Comprehensive Molecular Characterization of Multifocal Glioblastoma Proves Its Monoclonal Origin and Reveals Novel Insights Into Clonal Evolution and Heterogeneity of Glioblastomas. Neuro Oncol (2017) 19:546–57. doi: 10.1093/neuonc/now231
12. Kyritsis AP, Bondy ML, Xiao M, Berman EL, Cunningham JE, Lee PS, et al. Germline P53 Gene Mutations in Subsets of Glioma Patients. J Natl Cancer Inst (1994) 86:344–9. doi: 10.1093/jnci/86.5.344
13. Showalter TN, Andrel J, Andrews DW, Curran WJ Jr, Daskalakis C, Werner-Wasik M. Multifocal Glioblastoma Multiforme: Prognostic Factors and Patterns of Progression. Int J Radiat Oncol Biol Phys (2007) 69:820–4. doi: 10.1016/j.ijrobp.2007.03.045
14. Ahmadipour Y, Jabbarli R, Gembruch O, Pierscianek D, Darkwah Oppong M, Dammann P, et al. Impact of Multifocality and Molecular Markers on Survival of Glioblastoma. World Neurosurg (2019) 122:e461–6. doi: 10.1016/j.wneu.2018.10.075
15. Syed M, Liermann J, Verma V, Bernhardt D, Bougatf N, Paul A, et al. Survival and Recurrence Patterns of Multifocal Glioblastoma After Radiation Therapy. Cancer Manag Res (2018) 10:4229–35. doi: 10.2147/CMAR.S165956
16. Quail DF, Joyce JA. The Microenvironmental Landscape of Brain Tumors. Cancer Cell (2017) 31:326–41. doi: 10.1016/j.ccell.2017.02.009
17. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J Clin Oncol (2011) 29:1949–55. doi: 10.1200/JCO.2010.30.5037
18. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/regulatory T Cell Ratio Are Associated With Favorable Prognosis in Ovarian Cancer. Proc Natl Acad Sci USA (2005) 102:18538–43. doi: 10.1073/pnas.0509182102
19. Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients With Early-Stage Colorectal Cancer. J Clin Oncol (2009) 27:5944–51. doi: 10.1200/JCO.2008.19.6147
20. Kim YH, Jung TY, Jung S, Jang WY, Moon KS, Kim IY, et al. Tumour-Infiltrating T-Cell Subpopulations in Glioblastomas. Br J Neurosurg (2012) 26:21–7. doi: 10.3109/02688697.2011.584986
21. Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, et al. Tumour-Infiltrating CD4(+) and CD8(+) Lymphocytes as Predictors of Clinical Outcome in Glioma. Br J Cancer (2014) 110:2560–8. doi: 10.1038/bjc.2014.162
22. Zhang W, Wu S, Guo K, Hu Z, Peng J, Li J. Correlation and Clinical Significance of LC3, CD68+ Microglia, CD4+ T Lymphocytes, and CD8+ T Lymphocytes in Gliomas. Clin Neurol Neurosurg (2018) 168:167–74. doi: 10.1016/j.clineuro.2018.02.044
23. Pereira MB, Barros LRC, Bracco PA, Vigo A, Boroni M, Bonamino MH, et al. Transcriptional Characterization of Immunological Infiltrates and Their Relation With Glioblastoma Patients Overall Survival. Oncoimmunology (2018) 7:e1431083. doi: 10.1080/2162402X.2018.1431083
24. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune Microenvironment of Gliomas. Lab Invest (2017) 97:498–518. doi: 10.1038/labinvest.2017.19
25. Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A, et al. Programmed Death Ligand 1 Expression and Tumor-Infiltrating Lymphocytes in Glioblastoma. Neuro Oncol (2015) 17:1064–75. doi: 10.1093/neuonc/nou307
26. Mohme M, Schliffke S, Maire CL, Rünger A, Glau L, Mende KC, et al. Immunophenotyping of Newly Diagnosed and Recurrent Glioblastoma Defines Distinct Immune Exhaustion Profiles in Peripheral and Tumor-Infiltrating Lymphocytes. Clin Cancer Res (2018) 24:4187–200. doi: 10.1158/1078-0432.CCR-17-2617
27. Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-Cell Exhaustion Signatures Vary With Tumor Type and Are Severe in Glioblastoma. Clin Cancer Res (2018) 24:4175–86. doi: 10.1158/1078-0432.CCR-17-1846
28. Mohme M, Maire CL, Geumann U, Schliffke S, Dührsen L, Fita KD, et al. Local Intracerebral Immunomodulation Using Interleukin-Expressing Mesenchymal Stem Cells in Glioblastoma. Clin Cancer Res (2020) 26:2626–39. doi: 10.1158/1078-0432.CCR-19-0803
29. Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and Prognostic Impact of FoxP3+ Regulatory T Cells in Human Gliomas. Clin Cancer Res (2008) 14:5166–72. doi: 10.1158/1078-0432.CCR-08-0320
30. Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively Personalized Vaccination Trial for Newly Diagnosed Glioblastoma. Nature (2019) 565:240–5. doi: 10.1038/s41586-018-0810-y
31. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A Multivariate Analysis of 416 Patients With Glioblastoma Multiforme: Prognosis, Extent of Resection, and Survival. J Neurosurg (2001) 95:190–8. doi: 10.3171/jns.2001.95.2.0190
32. Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the Extent of Tumor Volume Resection and Patient Survival in Surgery of Glioblastoma Multiforme With High-Field Intraoperative MRI Guidance. Neuro Oncol (2011) 13:1339–48. doi: 10.1093/neuonc/nor133
33. Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, et al. Extent of Resection in Patients With Glioblastoma: Limiting Factors, Perception of Resectability, and Effect on Survival. J Neurosurg (2012) 117:851–9. doi: 10.3171/2012.8.JNS12234
34. Nerland US, Jakola AS, Giannadakis C, Solheim O, Weber C, Nygaard ØP, et al. The Risk of Getting Worse: Predictors of Deterioration After Decompressive Surgery for Lumbar Spinal Stenosis: A Multicenter Observational Study. World Neurosurg (2015) 84:1095–102. doi: 10.1016/j.wneu.2015.05.055
35. Paulsson AK, Holmes JA, Peiffer AM, Miller LD, Liu W, Xu J, et al. Comparison of Clinical Outcomes and Genomic Characteristics of Single Focus and Multifocal Glioblastoma. J Neurooncol (2014) 119:429–35. doi: 10.1007/s11060-014-1515-1
36. di Russo P, Perrini P, Pasqualetti F, Meola A, Vannozzi R. Management and Outcome of High-Grade Multicentric Gliomas: A Contemporary Single-Institution Series and Review of the Literature. Acta Neurochir (Wien) (2013) 155:2245–51. doi: 10.1007/s00701-013-1892-9
37. Salvati M, Caroli E, Orlando ER, Frati A, Artizzu S, Ferrante L. Multicentric Glioma: Our Experience in 25 Patients and Critical Review of the Literature. Neurosurg Rev (2003) 26:275–9. doi: 10.1007/s10143-003-0276-7
38. Lahmi L, Idbaih A, Rivin Del Campo E, Hoang-Xuan K, Mokhtari K, Sanson M, et al. Whole Brain Radiotherapy With Concurrent Temozolomide in Multifocal and/or Multicentric Newly Diagnosed Glioblastoma. J Clin Neurosci (2019) 68:39–44. doi: 10.1016/j.jocn.2019.07.065
Keywords: Multifocal glioma, CD8, tumor-infiltrating lymphocytes (TILs), chemotherapy, prognosis
Citation: Wang R, Song Y, Hu T, Wang X, Jiang Y, Zhang D, Yu J, Han S and Kan L (2021) Decreased CD8+ Lymphocytic Infiltration in Multifocal and Multicentric Glioblastomas. Front. Oncol. 11:748277. doi: 10.3389/fonc.2021.748277
Received: 27 July 2021; Accepted: 10 September 2021;
Published: 27 September 2021.
Edited by:
Carsten Friedrich, Klinikum Oldenburg AöR, GermanyReviewed by:
Jing Xu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaFlorian Andreas Gessler, University Hospital Rostock, Germany
Copyright © 2021 Wang, Song, Hu, Wang, Jiang, Zhang, Yu, Han and Kan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Han, aGFuc2hlbmcyMDAxX3hAYWxpeXVuLmNvbQ==; Liang Kan, a2FubGlhbmczMUBzaW5hLmNvbQ==
 Run Wang
Run Wang Yifu Song1
Yifu Song1 Yang Jiang
Yang Jiang Sheng Han
Sheng Han