
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 14 September 2021
Sec. Cancer Molecular Targets and Therapeutics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.742949
Prion protein has two isoforms including cellular prion protein (PrPC) and scrapie prion protein (PrPSc). PrPSc is the pathological aggregated form of prion protein and it plays an important role in neurodegenerative diseases. PrPC is a glycosylphosphatidylinositol (GPI)-anchored protein that can attach to a membrane. Its expression begins at embryogenesis and reaches the highest level in adulthood. PrPC is expressed in the neurons of the nervous system as well as other peripheral organs. Studies in recent years have disclosed the involvement of PrPC in various aspects of cancer biology. In this review, we provide an overview of the current understanding of the roles of PrPC in proliferation, cell survival, invasion/metastasis, and stem cells of cancer cells, as well as its role as a potential therapeutic target.
Prion protein (PrP) is expressed throughout the whole body. It has two isoforms, cellular prion protein (PrPC) and its pathogenic form-scrapie prion protein (PrPSc) (1, 2). PrPSc is well known for its ability to cause a series of neurodegenerative diseases in human and other mammals (1, 3). It results from post-translational conversion of the glycosylphosphatidylinositol (GPI)-anchored PrPC (4, 5). PrPC, as a scaffold on the cell surface, recruits different partners to execute its functions being involved in signaling pathways (6). The biosynthetic pathway of PrPC is similar to that of other membrane-attached and secreted proteins (5) (Figure 1). It is synthesized in endoplasmic reticulum (ER)-attached ribosomes followed by its import into ER where it is glycosylated and modified by GPI anchor before it is transported into Golgi for further modification. Then PrPC is transported to the cell surface where it can be internalized through endocytic pathway (7). The internalized PrPC can be transported into the lysosome for degradation or be enclosed in exosomes and secreted outside the cells (7). PrPC is mainly attached to lipid rafts on the cell surface via its C-terminal GPI anchor (8, 9). It is also located in the cytosol and the nucleus (10–12). Interestingly, PrPC was found in the exosomes secreted by cancer cells (13).
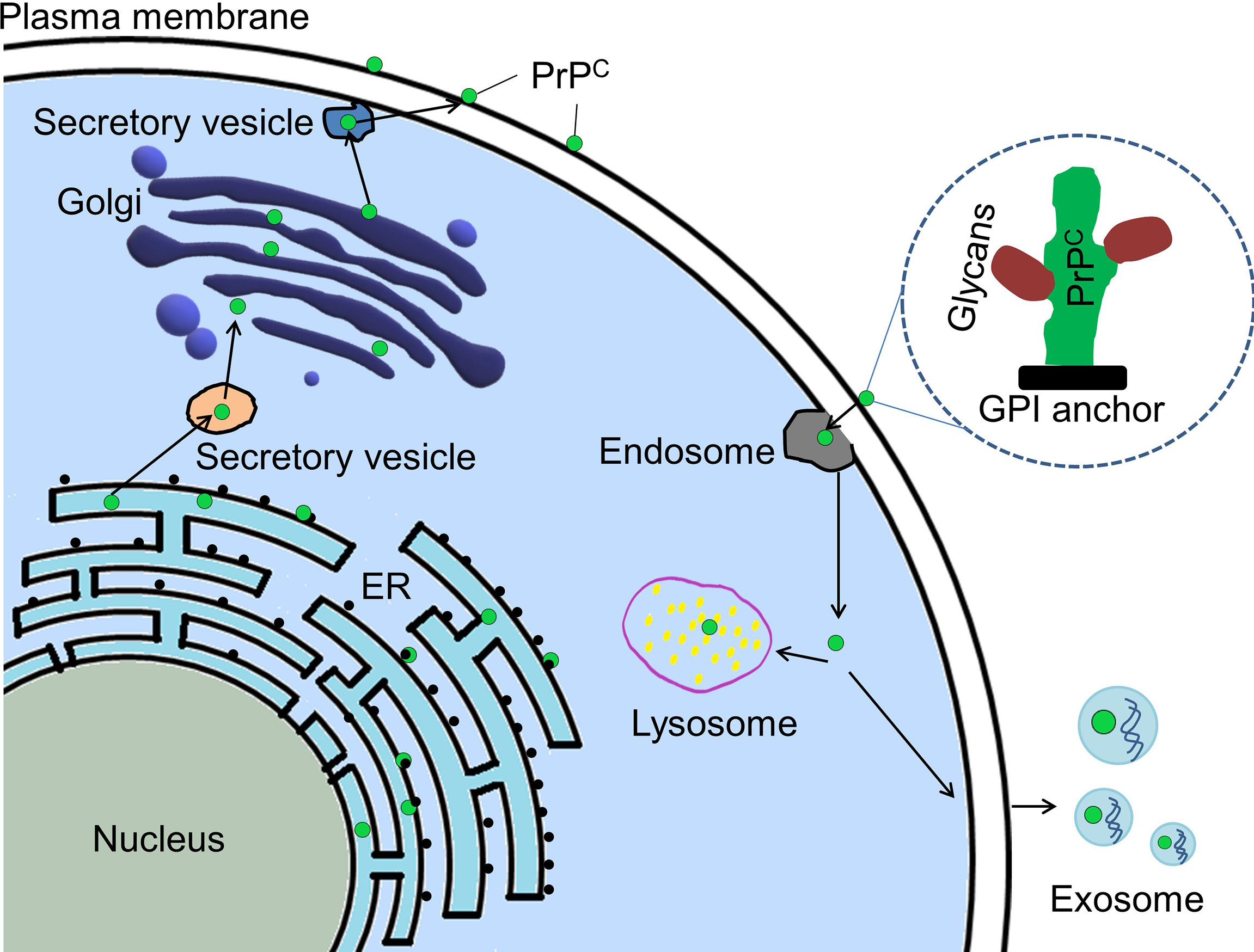
Figure 1 Cellular trafficking pathway of PrPC. PrPC (green dot) is synthesized in ribosome attached to ER (endoplasmic reticulum). PrPC is imported to ER where it will be glycosylated and modified by GPI anchor before it is transported into Golgi apparatus for further modification. Mature PrPC is trafficked to plasma membrane and located there by its GPI anchor. Some mature PrPC could be endocytosed for degradation in the lysosome or for being contained in exosomes and secreted outside the cell. PrPC, Cellular prion protein; GPI, glycosylphosphatidylinositol.
Cancer is the second leading cause of death worldwide. Studies in recent years show that PrPC is involved in various aspects of cancer biology such as cell proliferation, metastasis, cell death, drug resistance and cancer stem cells (14–21). In this review, we summarize the current progress in these aspects.
PrPC can promote proliferation in cancer cells (22). Liang et al. demonstrated that overexpression of PrPC promoted cell proliferation through activation of the phosphatidylinositide 3-kinase (PI3K) pathway and promotion of the G1/S phase transition by upregulating cyclin D1, in gastric cancer cells (22). PrPC is also involved in G1 to S phase transition in renal adenocarcinoma ACHN and colon adenocarcinoma LS 174T cells (23). Knockdown of PrPC inhibited cell proliferation and amplified the inhibitory effect of fucoidan on cell proliferation by suppressing expression of cyclins and cyclin-dependent kinase (CDK), in HT29 colon cancer cells (24). Interaction of PrPC with the co-chaperone Hsp70/90 organizing protein (HOP) promoted proliferation via activating PI3K and extracellular-signal-regulated kinase (ERK1/2) pathways in glioblastomas (GBM) cells (25). Furthermore, HOP-PrPC interaction promoted proliferation of glioblastoma stem-like cells and the decrease expression of PrPC and HOP may work as an effective therapy for GBM in the future (26). Warburg effect refers to the event that cancer cells preferentially use aerobic glycolysis to generate energy and reducing power for their biosynthesis, cell survival and proliferation (27). Overexpression of PrPC mediated Warburg effect by increasing glucose transporter 1 (Glut1) expression which promotes glucose uptake through epigenetic activation of Fyn-HIF-2α-Glut1 pathway in colorectal cancer cells (28). PrPC can also increase cell proliferation by interacting with 37/67 kDa non-integrin laminin receptor (LR/37/67 kDa) and activating downstream ERK1/2 and PI3K/protein kinase B (AKT) signaling pathways in schwannoma cells (29). It promoted proliferation by interacting with Notch1 in pancreatic ductal adenocarcinoma (PDAC) (30). A variant of PrPC with one octapeptide repeat deletion (1-OPRD) is widely present in gastric cancer cell lines and gastric cancer tissues (31). Overexpression of 1-OPRD could promote the proliferation of gastric cancer cells through transcriptional activation of cyclin D3, which facilitated the G1-/S-phase transition in cell cycle (32).
Metastasis leads to more than 90% of cancer-caused death, but its underlying mechanisms still remain poorly understood (33). Christine L et al. divided the process of metastasis into two phases: the first phase is physical translocation of cancer cell from a primary tumor to other distant tissues, and the second phase is colonization of metastatic cancer cells in their new microenvironment (33). EMT refers to epithelial-to-mesenchymal transition (34). Many in vitro models show that EMT act as a key process during cancer metastasis (35, 36). Transcription of Prnp (the gene encoding PrP) considerably increased during EMT (37). Upregulation of PrPC and dedifferentiation of EMT-like cells were observed in invasive colorectal cancer cells (CRC) (18, 38). Overexpression of PrPC by transfecting pCDNA3.0-Prnp in SW480 cells led to EMT whereas, knockdown of Prnp in mesenchymal-like LIM2405 cells caused MET (mesenchymal-to-epithelial transition) (18). The mechanisms underlying EMT enhancement by PrPC are largely unclear.
SATB1 (special AT-rich sequence-binding proteins 1) is a nuclear matrix associated protein. It can induce tumor metastasis by altering chromatin structure and upregulating metastasis-associated genes while downregulating tumour-suppressor genes (39, 40). Knockdown of Prnp resulted in loss of SATB1 expression and reduction of metastatic capacity in CRC with Fyn and specificity protein 1(SP1) being involved in this process, indicating that PrPC may promote tumor metastasis via upregulating the PrPC-Fyn-SP1-SATB1 axis (18). PrPC and γ-Syn are overexpressed in CRC (41, 42). They may be involved in colorectal cancer cell metastasis by inducing an endothelial proliferation to differentiation switch (42, 43).
PrPC is highly expressed in metastatic gastric cancer cells and it may promote invasion and metastasis through activation of the mitogen-activated protein kinases (MEK)/ERK pathway and consequent transactivation of matrix metalloproteinase-11(MMP11) (44). MMP11 can promote matrix degradation, inflammation and tissue remodeling (20, 44). Its N-terminal fragment is essential for transducing invasion-promoting signal of PrPC (20, 44). Tissue Inhibitor of Metalloproteinase (TIMP) is endogenous inhibitor for membrane type1-matrix metalloproteinase (MT1-MMP). The binding of TIMP to the GPI anchor of the prion protein generated a membrane-tethered, high-affinity designer TIMP (named “T1Pr αMT1” hereafter) which is expressed on the cell surface and co-localized with cellular MTI-MMP (45). Therefore, GPI anchor of PrPC might be used as a potential therapy for renal carcinoma (45).
It was reported that PrPC promoted EMT through the activation of the ERK2/mitogen-activated protein kinase (MAPK1) pathway in colorectal cancer stem cells (46). This is consistent with the notion that the appearance of the CSC (cancer stem cell) phenotype and EMT are intimately connected (19). Notch1 is involved in CSCs (47). It is a downstream effector of PrPC both of which colocalizes on the cell membrane and form an interaction network to promote pancreatic cancer cell metastasis (30). Co-treatment with 5-fluorouracil (5-FU) and melatonin could inhibit colon CSC marker octamer-binding transcription factor 4 (Oct4) via downregulation of PrPC-Oct4 pathways (48). Tumor-mediated angiogenesis will be suppressed in this process which suggests that cancer metastasis will be inhibited (48). PrPC-containing exosomes secreted by CRC could also promote tumor metastasis by increasing the permeability of endothelial cells and the secretion of angiogenic factors (49). This study also demonstrated that the combination of anti-PrPC and 5-FU downregulated tumor progression (49).
The immune system is one of the key pathways to control cancer development and metastasis. Regulatory T cells (Tregs), which have immunosuppressive activity (50), are one of the main targets of cancer immunotherapy (51). By constructing a lung metastatic model of melanoma in Prnp0/0 and Tga20 mice, it was demonstrated that the increased expression of PrPC induces the development of Tregs by upregulating transforming growth factor-beta (TGF-β) and programmed death ligand-1(PD-L1), thereby promoting tumor progression (52).
Many studies have demonstrated that PrPC expression promotes cancer cell metastasis (Figure 2). However, one study showed that knockout of Prnp (Prnp0/0) in mesenchymal embryonic mouse cells transformed by Ras/Myc led to more incidence of lung metastasis due to increased expression of αVβ3-integrin (53). This suggest that more studies are required to clarify the roles of PrPC in cancer metastasis.
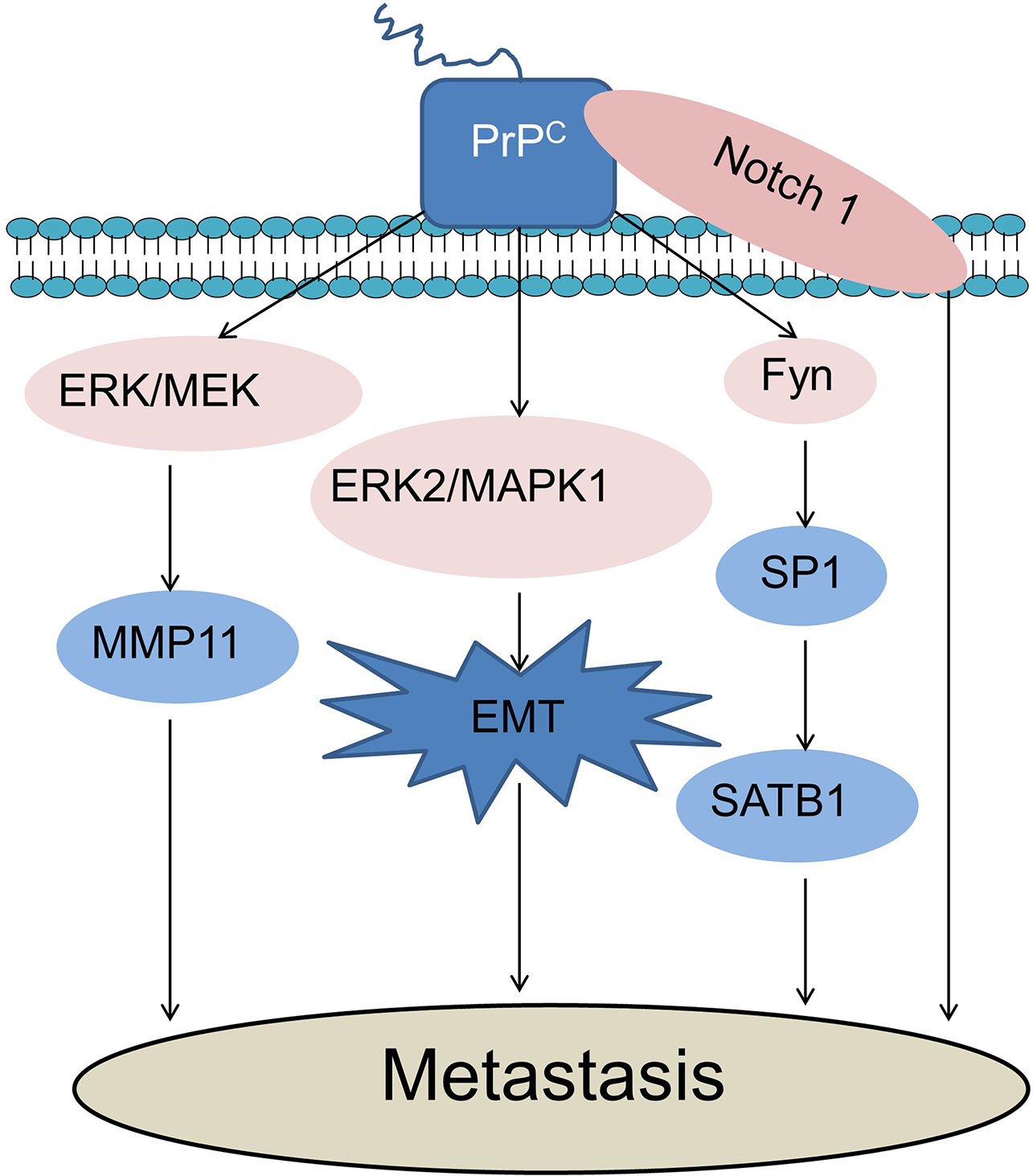
Figure 2 PrPC promotes cancer cell metastasis. PrPC could promote cancer cell metastasis through activation of the MEK/ERK pathway and consequent transactivation of MMP11. PrPC promotes EMT through the activation of the ERK2/MAPK1 pathway during cancer metastasis. PrPC could promote tumor metastasis via up-regulating the PrPC-Fyn-SP1-SATB1 axis. Notch1 and PrPC could form an interaction network to promote cancer cell metastasis. ERK, Extracellular-signal-regulated kinase; MEK, Mitogen-activated protein kinases; MMP11, Matrix metalloproteinase-11; MAPK, Mitogen-activated protein kinase; EMT, Epithelial-mesenchymal transition; SATB1, Special AT-rich sequence-binding proteins 1; SP1, Specificity protein 1.
One major challenge for cancer treatment is drug resistance. Various mechanisms can contribute to cancer drug resistance (54). The most studied mechanisms involving the roles of PrPC in cancer drug resistance include multi-drug resistance (MDR) and inhibition of cell death. Multi-drug resistance (MDR) refers to the ability of cancer cells to survive against a wide range of anti-cancer drugs (55). Cell death can be classified into three main types including apoptosis (Type I programmed cell death), autophagic cell death (Type II programmed cell death) and necrosis (56). Apoptosis is characterized by cell shrinkage, membrane blebbing, chromatin condensation, DNA fragmentation and caspase activation. Autophagic cell death is induced by the over-activation of autophagy that is an intracellular lysosomal degradation process. Necrosis is a non-programmed cell death. It is caused by sudden results to the cells and is characterized by breakage of plasma membrane followed by cytoplasmic leakage.
Upregulation of PrPC can lead to drug resistance in different types of cancers cells (57–59). In colorectal cancer cells, PrPC is involved in 5-FU resistance by increasing cell survival and proliferation via activating PI3K-Akt signaling pathway and the expression of cell cycle-associated proteins (59). PrPC overexpression led to resistance of colorectal cancer LS174T cells to doxorubicin-induced apoptosis by upregulation of the inhibitors of apoptosis proteins (IAPs) (60). Upregulation of PrPC leads to increased superoxide dismutase and catalase activities and decreased endoplasmic reticulum stress and apoptosis, which results in oxaliplatin resistance in colorectal cancer cells (61, 62). In gastric cancer cells, PrPC can promote drug resistance by different mechanisms. PrPC coexists with MGr1-Antigen/37 kDa laminin receptor precursor (MGr1-Ag/37LRP) to promote MDR in gastric cancer cells by inhibiting apoptosis via activation of the PI3K/AKT signaling pathway (63). Octarepeat peptides of PrP may be involved in gastric cancer MDR by increasing the activities of antioxidant enzymes (64). PrPC can promote MDR by upregulating the multidrug resistance protein (P-gp) and suppressing apoptosis in gastric and breast cancer cells (65, 66). Overexpression of PrPC promotes resistance to TNF-α-induced apoptosis by inhibiting Bcl-2-associated X protein (Bax) expression in renal adenocarcinoma ACHN cells (23).
PrPC can be found on the cell surface by attaching to the cell membrane and outside the cells being contained in exosomes which are secreted from the cells (67, 68). The secreted PrPC in tumor microenvironment binds to doxorubicin to prevent it from entering the nucleus and intercalating into DNA to induce cell death; and breast cancer patients with high levels of serum PrPC are at high risk of relapse following doxorubicin treatment (13). PrP synthetic peptide (amino acid residues 105 - 120 of the human prion protein) can protect schwannoma cells from H2O2-mediated cell death (29).
PrPC has been shown to protect cancer cells from apoptosis and autophagic cell death (69). PrPC inhibits apoptosis in neurons and in cancer cells (70). PrPC upregulation inhibits apoptosis induced by Bax expression, serum starvation and anti-cancer drug treatments (57, 70, 71). PrPC can bind to the C-terminus of the anti-apoptotic protein Bcl-2 to form a dimer inhibiting apoptosis (72). When PrPC is upregulated, Bcl-2/Bax ratio increases, resulting in anti-apoptosis in breast carcinoma MCF-7 cells (71). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a ligand for death receptors which can induce cancer cell apoptosis (73). Downregulation of PrPC sensitizes adriamycin-resistant human breast cancer cells to TRAIL-induced apoptosis by increasing Bax/Bcl-2 ratio (58). PrPC inhibited TRAIL-induced apoptosis under hypoxia in human colon carcinoma cells (74). Akt was activated by PrPC to prevent TRAIL-induced apoptosis (75, 76). PrPC also activated PI3K/Akt signaling pathway contributing to its anti-Bax function by preventing the pro-apoptotic conformational changes of Bax at the early step of Bax activation (71). Moreover, PrPC protected lung and pancreatic cancer cells from apoptosis through downregulation of unfolded protein response (UPR) (77).
Autophagy is an evolutionarily conserved catabolic process in eukaryotic cells, in which unnecessary or dysfunctional cytosolic components are degraded and recycled through lysosomes (78). During autophagy (macroautophagy), cytosolic components (cargos) are surrounded by a phagophore which will expands and encloses to form the characteristic double-membraned structure autophagosome. Then, autophagosome will fuse with the lysosome to form autolysosome where cargos are degraded to generate small molecules that can be used for biosynthesis and energy production for cell survival, under stress conditions such as starvation (79). However, when autophagy is over-enhanced, it can induce cell death (autophagic cell death/autophagy-induced cell death) (79). Barbieri et al. demonstrated for the first time that PrPC can modulate autophagic cell death in glial tumor cells (80). They demonstrated that PrPC silencing resulted in inhibition of Mammalian target of rapamycin (mTOR) kinase activity in T98G glioma cells, promoting autophagy leading to autophagic cell death (80). Furthermore, PrPC inhibited autophagy by activating the antioxidant enzyme SOD (81). Since autophagy is mainly a pro-cell survival mechanism, it is expected that PrPC may antagonize drug resistance by inhibiting autophagy in cancer cells.
One study showed that tumor resistance to radiotherapy was also associated with the increased PrPC (82). In neuroblastoma, breast, and colorectal cancer cell lines, ionizing radiation (IR) can increase the expression of PrPC by activating ATM-TAK1-PrPC pathway, thereby leading to the resistance to radiotherapy of tumor cells (82). Taken together, PrPC can modulate various signaling pathways contributing to cancer drug resistance (Figure 3).
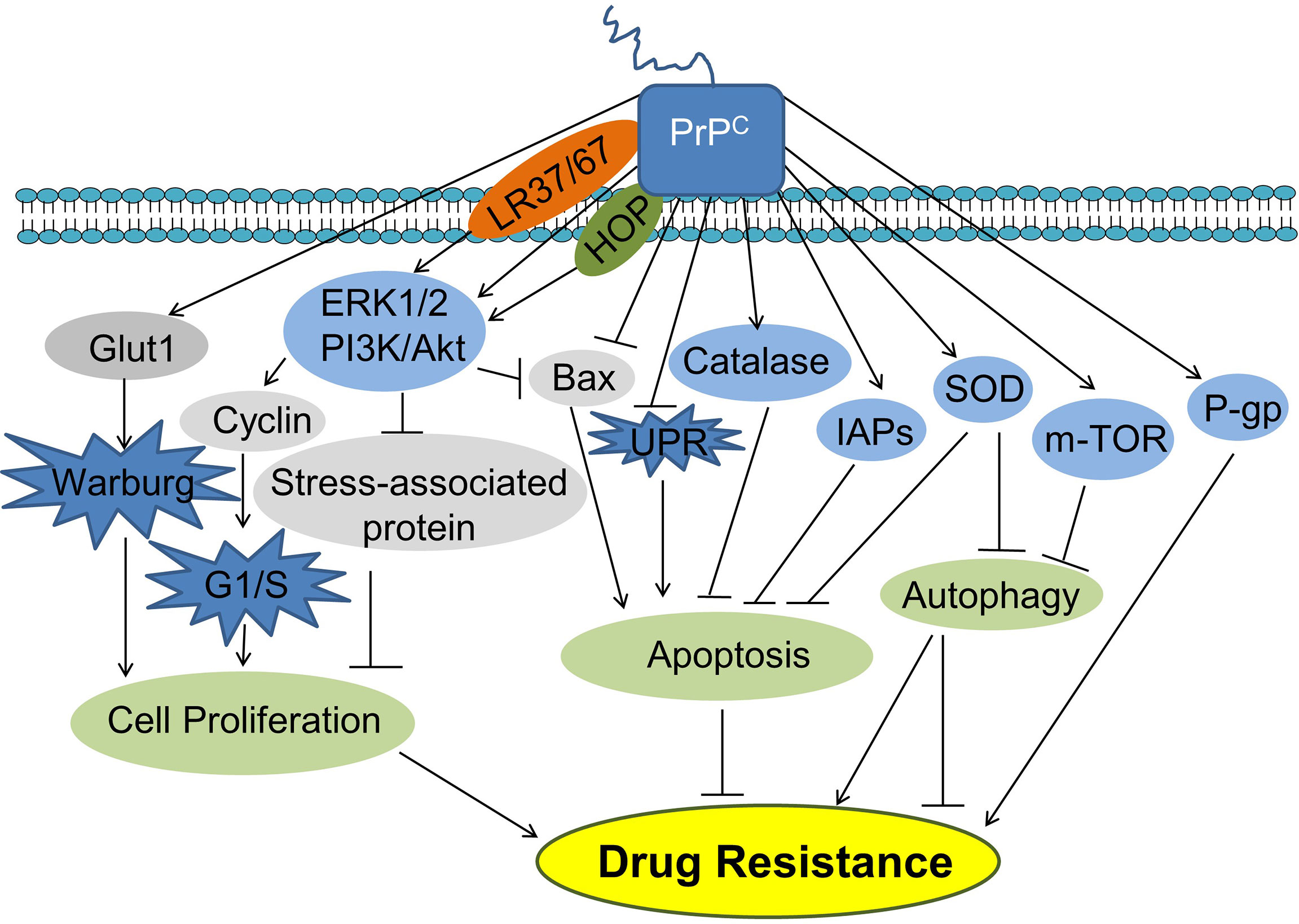
Figure 3 PrPC promotes cancer cell drug resistance. PrPC can promote cancer cell drug resistance by promoting cell proliferation and inhibiting apoptosis. PrPC can also suppress autophagy inhibiting or promoting drug resistance. HOP, Hsp70/90 organizing protein; IAPs, Inhibitors of apoptosis proteins; Glut1, Glucose transporter 1; PI3K, Phosphatidylinositide 3-kinase; AKT, Protein kinase B; Bax, Bcl-2-associated X protein; UPR, Unfolded protein response; SOD, Superoxide dismutase; P-gp, P-glycoprotein.
Although the overexpression of PrPC in cancer cells results in therapy-resistance, researchers have taken advantage of this characteristic to synthesize PrPC-Apt-functionalized doxorubicin-oligomer-AuNPs (PrPC-AptDOa) which could target PrPC-overexpressed CRC (83). PrPC-AptDOa inhibited CRCs proliferation and induced apoptosis more significantly than free Dox at the cellular level (83). However, PrPC is also expressed in normal cells, such as neurons and neuroglia. Therefore, the challenge for cancer treatment is to specifically target PrPC in cancer cells. In addition, further studies of PrPC-AptDOa should be conducted in an animal model and clinical trials to clarify its therapeutic effects and side effects on individuals.
Cancer stem cells (CSCs) are a small subpopulation of cancer cells with the capacities of self-renewal, differentiation and tumorigenicity (84). PrPC is engaged in different types of stem cells, such as hematopoietic stem cells (HSCs), gland stem cells, bone marrow-derived human mesenchymal stem cells (MSCs) and human embryonic stem(ES) cells (85–88). Studies have indicated that PrPC is also involved in CSCs. PrPC protected Oct4, a marker of colon cancer stem cells, from degradation by inducing heat shock protein 1 like (HSPA1L) when in response to co‐treatment with 5‐FU and melatonin (48). One study indicated that PrPC was highly expressed in consensus molecular subgroup (CMS4), a subtype of CRC with higher malignancy, and affected the prognosis of CRC as an upstream molecule in the PrPC-ILK-IDO1 axis (89). PrPC promoted EMT of colorectal cancer stem cells via activation of the ERK2 (MAPK1) pathway to increase cell metastasis (46). CD44 is a CSC marker and critical regulator of cancer stemness (90). PrPC is co-expressed with CD44 in colorectal CSCs (46). PrPC and Hsp70/90 organizing protein (HOP) acted together to regulate self-renewal, proliferation and migration in glioblastoma (GBM) stem-like cells (26). Downregulation of PrPC decreased stem cell-like properties of human GBM CSCs (91). Downregulation of PrPC in models of prion disease through immune, genetic and other mechanisms has achieved some progress. Application of anti-PrP antibodies have been proposed as a promising treatment many decades ago (92, 93). A recent study reported that transgenic mice expressing elk PrP (TgElk) benefited from active PrP vaccination (94). Minikel et al. demonstrated that PrP-lowering antisense oligonucleotides (ASOs) worked via an RNAase-H dependent mechanism and has certain therapeutic effect on prion-infected mice (95). Minikel et al. also proposed that loss-of-function variant of Prnp could be potential targets for prion disease inhibitory drugs (96). The application of these PrPC-lowering approaches may provide novel cancer therapies by targeting CSCs.
Prion protein (PrP) is expressed in nervous system and other organs (97). There are two forms of PrP, including normal PrPC and disease causing PrPSc. PrPC misfolding and aggregation can cause fatal neurodegenerative conditions (98). Studies in recent years show that it also plays a role in cancer. PrPC can stimulate cancer progression by promoting cancer cell proliferation, invasion/metastasis, drug resistance, and cancer stem cell development. Therefore, targeting PrPC is a novel approach for cancer treatment.
MD and YC conceived the topic and designed the outline of this review. MD contributed to the manuscript writing and prepared the figures and tables. YC modified the language. LC, YC and YL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the National Natural Science Foundation of China (82071351).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bax, Bcl-2-associated X protein; Bcl, B cell leukemia oncogene; CRC, Colorectal cancer cell; CSC, Cancer stem cell; EMT, Epithelial-Mesenchymal Transition; ERK, Extracellular-signal-regulated kinase; 5-FU, 5-fluorouracil; GBM, Glioblastomas; GPI, Glycosylphosphatidylinositol; HOP, Hsp70/90 organizing protein; HSCs, Hematopoietic stem cells; MDR, Multi-drug resistance; MET, Mesenchymal-to-epithelial transition; MMP11, Matrix metalloproteinase-11; MSCs, Mesenchymal stem cells; Oct4, Octamer-binding transcription factor 4; 1-OPRD, One octapeptide repeat deletion; PDAC, Pancreatic ductal adenocarcinoma; P-gp, P-glycoprotein; PI3K, Phosphatidylinositol 3 kinase; PrPC, Cellular prion protein; PrPSc, Scrapie prion protein; PrPC-AptDOa, PrPC-Apt-functionalized doxorubicin-oligomer-AuNPs; SATB1, Special AT-rich sequence-binding proteins 1; SOD, Superoxide dismutase; TIMP, Tissue Inhibitor of Metalloproteinase; TNF-α, Tumor Necrosis Factor-α; TRAIL, Tumor necrosis factor-related apoptosis-inducing ligand; UPR, Unfolded protein response.
1. Atkinson CJ, Zhang K, Munn AL, Wiegmans A, Wei MQ. Prion Protein Scrapie and the Normal Cellular Prion Protein. Prion (2016) 10(1):63–82. doi: 10.1080/19336896.2015.1110293
2. Priola SA, Chesebro B, Caughey B. Biomedicine. A View From the Top–Prion Diseases From 10,000 Feet. Sci (N Y NY) (2003) 300(5621):917–9. doi: 10.1126/science.1085920
3. Soto C, Satani N. The Intricate Mechanisms of Neurodegeneration in Prion Diseases. Trends Mol Med (2011) 17(1):14–24. doi: 10.1016/j.molmed.2010.09.001
4. Westergard L, Christensen HM, Harris DA. The Cellular Prion Protein (PrP(C)): Its Physiological Function and Role in Disease. Biochim Biophys Acta (2007) 1772(6):629–44. doi: 10.1016/j.bbadis.2007.02.011
5. Harris DA. Trafficking, Turnover and Membrane Topology of PrP. Br Med Bull (2003) 66:71–85. doi: 10.1093/bmb/66.1.71
6. Linden R, Cordeiro Y, Lima LMTR. Allosteric Function and Dysfunction of the Prion Protein. Cell Mol Life Sci CMLS (2012) 69(7):1105–24. doi: 10.1007/s00018-011-0847-7
7. Alves RN, Iglesia RP, Prado MB, Melo Escobar MI, Boccacino JM, Fernandes CFL, et al. A New Take on Prion Protein Dynamics in Cellular Trafficking. Int J Mol Sci (2020) 21(20):7763. doi: 10.3390/ijms21207763
8. Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie Prion Protein Contains a Phosphatidylinositol Glycolipid. Cell (1987) 51(2):229–40. doi: 10.1016/0092-8674(87)90150-4
9. Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of Detergent-Insoluble Complexes Containing the Cellular Prion Protein and its Scrapie Isoform. J Biol Chem (1997) 272(10):6324–31. doi: 10.1074/jbc.272.10.6324
10. Fioriti L, Dossena S, Stewart LR, Stewart RS, Harris DA, Forloni G, et al. Cytosolic Prion Protein (PrP) Is Not Toxic in N2a Cells and Primary Neurons Expressing Pathogenic PrP Mutations. J Biol Chem (2005) 280(12):11320–8. doi: 10.1074/jbc.M412441200
11. Déry M-A, Jodoin J, Ursini-Siegel J, Aleynikova O, Ferrario C, Hassan S, et al. Endoplasmic Reticulum Stress Induces PRNP Prion Protein Gene Expression in Breast Cancer. Breast Cancer Res BCR (2013) 15(2):R22–R. doi: 10.1186/bcr3398
12. Morel E, Fouquet S, Strup-Perrot C, Pichol Thievend C, Petit C, Loew D, et al. The Cellular Prion Protein PrP(c) Is Involved in the Proliferation of Epithelial Cells and in the Distribution of Junction-Associated Proteins. PloS One (2008) 3(8):e3000–e. doi: 10.1371/journal.pone.0003000
13. Wiegmans AP, Saunus JM, Ham S, Lobb R, Kutasovic JR, Dalley AJ, et al. Secreted Cellular Prion Protein Binds Doxorubicin and Correlates With Anthracycline Resistance in Breast Cancer. JCI Insight (2019) 5(6):e124092. doi: 10.1172/jci.insight.124092
14. Santos TG, Lopes MH, Martins VR. Targeting Prion Protein Interactions in Cancer. Prion (2015) 9(3):165–73. doi: 10.1080/19336896.2015.1027855
15. Mehrpour M, Codogno P. Prion Protein: From Physiology to Cancer Biology. Cancer Lett (2010) 290(1):1–23. doi: 10.1016/j.canlet.2009.07.009
16. Tang Z, Ma J, Zhang W, Gong C, He J, Wang Y, et al. The Role of Prion Protein Expression in Predicting Gastric Cancer Prognosis. J Cancer (2016) 7(8):984–90. doi: 10.7150/jca.14237
17. Kim J-I, Cali I, Surewicz K, Kong Q, Raymond GJ, Atarashi R, et al. Mammalian Prions Generated From Bacterially Expressed Prion Protein in the Absence of Any Mammalian Cofactors. J Biol Chem (2010) 285(19):14083–7. doi: 10.1074/jbc.C110.113464
18. Wang Q, Qian J, Wang F, Ma Z. Cellular Prion Protein Accelerates Colorectal Cancer Metastasis via the Fyn-SP1-SATB1 Axis. Oncol Rep (2012) 28(6):2029–34. doi: 10.3892/or.2012.2025
19. Singh A, Settleman J. EMT, Cancer Stem Cells and Drug Resistance: An Emerging Axis of Evil in the War on Cancer. Oncogene (2010) 29(34):4741–51. doi: 10.1038/onc.2010.215
20. Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: Role in Breast Carcinogenesis, Invasion and Metastasis. Breast Cancer Res BCR (2000) 2(4):252–7. doi: 10.1186/bcr65
21. Medema JP. Cancer Stem Cells: The Challenges Ahead. Nat Cell Biol (2013) 15(4):338–44. doi: 10.1038/ncb2717
22. Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J, et al. Cellular Prion Protein Promotes Proliferation and G1/S Transition of Human Gastric Cancer Cells SGC7901 and AGS. FASEB J Off Publ Fed Am Soc Exp Biol (2007) 21(9):2247–56. doi: 10.1096/fj.06-7799com
23. Yap YH-Y, Say Y-H. Resistance Against Tumour Necrosis Factor α Apoptosis by the Cellular Prion Protein Is Cell-Specific for Oral, Colon and Kidney Cancer Cell Lines. Cell Biol Int (2012) 36(3):273–7. doi: 10.1042/CBI20110088
24. Yun CW, Yun S, Lee JH, Han Y-S, Yoon YM, An D, et al. Silencing Prion Protein in HT29 Human Colorectal Cancer Cells Enhances Anticancer Response to Fucoidan. Anticancer Res (2016) 36(9):4449–58. doi: 10.21873/anticanres.10989
25. Lopes MH, Santos TG, Rodrigues BR, Queiroz-Hazarbassanov N, Cunha IW, Wasilewska-Sampaio AP, et al. Disruption of Prion Protein-HOP Engagement Impairs Glioblastoma Growth and Cognitive Decline and Improves Overall Survival. Oncogene (2015) 34(25):3305–14. doi: 10.1038/onc.2014.261
26. Iglesia RP, Prado MB, Cruz L, Martins VR, Santos TG, Lopes MH. Engagement of Cellular Prion Protein With the Co-Chaperone Hsp70/90 Organizing Protein Regulates the Proliferation of Glioblastoma Stem-Like Cells. Stem Cell Res Ther (2017) 8(1):76–. doi: 10.1186/s13287-017-0518-1
27. Warburg O. The Metabolism of Carcinoma Cells. J Cancer Res (1925) 9:148–63. doi: 10.1158/jcr.1925.148
28. Li Q-Q, Sun Y-P, Ruan C-P, Xu X-Y, Ge J-H, He J, et al. Cellular Prion Protein Promotes Glucose Uptake Through the Fyn-HIF-2α-Glut1 Pathway to Support Colorectal Cancer Cell Survival. Cancer Sci (2011) 102(2):400–6. doi: 10.1111/j.1349-7006.2010.01811.x
29. Provenzano L, Ryan Y, Hilton DA, Lyons-Rimmer J, Dave F, Maze EA, et al. Cellular Prion Protein (PrPc) in the Development of Merlin-Deficient Tumours. Oncogene (2017) 36(44):6132–42. doi: 10.1038/onc.2017.200
30. Wang Y, Yu S, Huang D, Cui M, Hu H, Zhang L, et al. Cellular Prion Protein Mediates Pancreatic Cancer Cell Survival and Invasion Through Association With and Enhanced Signaling of Notch1. Am J Pathol (2016) 186(11):2945–56. doi: 10.1016/j.ajpath.2016.07.010
31. Liang J, Wang JB, Pan YL, Wang J, Liu LL, Guo XY, et al. High Frequency Occurrence of 1-OPRD Variant of PRNP Gene in Gastric Cancer Cell Lines and Chinese Population With Gastric Cancer. Cell Biol Int (2006) 30(11):920–3. doi: 10.1016/j.cellbi.2006.05.015
32. Liang J, Wang J, Luo G, Pan Y, Wang X, Guo C, et al. Function of PrPC (1-OPRD) in Biological Activities of Gastric Cancer Cell Lines. J Cell Mol Med (2009) 13(11-12):4453–64. doi: 10.1111/j.1582-4934.2009.00687.x
33. Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Sci (N Y NY) (2011) 331(6024):1559–64. doi: 10.1126/science.1203543
34. Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-Mesenchymal Transitions in Development and Disease. Cell (2009) 139(5):871–90. doi: 10.1016/j.cell.2009.11.007
35. Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol (2018) 13:395–412. doi: 10.1146/annurev-pathol-020117-043854
36. Nieto MA. The Ins and Outs of the Epithelial to Mesenchymal Transition in Health and Disease. Annu Rev Cell Dev Biol (2011) 27:347–76. doi: 10.1146/annurev-cellbio-092910-154036
37. Mehrabian M, Brethour D, Wang H, Xi Z, Rogaeva E, Schmitt-Ulms G. The Prion Protein Controls Polysialylation of Neural Cell Adhesion Molecule 1 During Cellular Morphogenesis. PloS One (2015) 10(8):e0133741–e. doi: 10.1371/journal.pone.0133741
38. Natalwala A, Spychal R, Tselepis C. Epithelial-Mesenchymal Transition Mediated Tumourigenesis in the Gastrointestinal Tract. World J Gastroenterol (2008) 14(24):3792–7. doi: 10.3748/wjg.14.3792
39. Han H-J, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 Reprogrammes Gene Expression to Promote Breast Tumour Growth and Metastasis. Nature (2008) 452(7184):187–93. doi: 10.1038/nature06781
40. Pavan Kumar P, Purbey PK, Sinha CK, Notani D, Limaye A, Jayani RS, et al. Phosphorylation of SATB1, a Global Gene Regulator, Acts as a Molecular Switch Regulating Its Transcriptional Activity In Vivo. Mol Cell (2006) 22(2):231–43. doi: 10.1016/j.molcel.2006.03.010
41. Antonacopoulou AG, Grivas PD, Skarlas L, Kalofonos M, Scopa CD, Kalofonos HP. POLR2F, ATP6V0A1 and PRNP Expression in Colorectal Cancer: New Molecules With Prognostic Significance? Anticancer Res (2008) 28(2B):1221–7.
42. Ong S-H, Goh K-W, Chieng CK-L, Say Y-H. Cellular Prion Protein and γ-Synuclein Overexpression in LS 174T Colorectal Cancer Cell Drives Endothelial Proliferation-to-Differentiation Switch. PeerJ (2018) 6:e4506–e. doi: 10.7717/peerj.4506
43. Bussolino F, Mantovani A, Persico G. Molecular Mechanisms of Blood Vessel Formation. Trends Biochem Sci (1997) 22(7):251–6. doi: 10.1016/s0968-0004(97)01074-8
44. Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu N, et al. Cellular Prion Protein Promotes Invasion and Metastasis of Gastric Cancer. FASEB J Off Publ Fed Am Soc Exp Biol (2006) 20(11):1886–8. doi: 10.1096/fj.06-6138fje
45. Jiang B, Liu J, Lee MH. Targeting a Designer TIMP-1 to the Cell Surface for Effective MT1-MMP Inhibition: A Potential Role for the Prion Protein in Renal Carcinoma Therapy. Mol (Basel Switzerland) (2019) 24(2):255. doi: 10.3390/molecules24020255
46. Du L, Rao G, Wang H, Li B, Tian W, Cui J, et al. CD44-Positive Cancer Stem Cells Expressing Cellular Prion Protein Contribute to Metastatic Capacity in Colorectal Cancer. Cancer Res (2013) 73(8):2682–94. doi: 10.1158/0008-5472.CAN-12-3759
47. Xiao W, Gao Z, Duan Y, Yuan W, Ke Y. Notch Signaling Plays a Crucial Role in Cancer Stem-Like Cells Maintaining Stemness and Mediating Chemotaxis in Renal Cell Carcinoma. J Exp Clin Cancer Res (2017) 36(1):41–. doi: 10.1186/s13046-017-0507-3
48. Lee JH, Yun CW, Han Y-S, Kim S, Jeong D, Kwon HY, et al. Melatonin and 5-Fluorouracil Co-Suppress Colon Cancer Stem Cells by Regulating Cellular Prion Protein-Oct4 Axis. J Pineal Res (2018) 65(4):e12519–e. doi: 10.1111/jpi.12519
49. Yun CW, Lee JH, Go G, Jeon J, Yoon S, Lee SH. Prion Protein of Extracellular Vesicle Regulates the Progression of Colorectal Cancer. Cancers (Basel) (2021) 13(9):2144. doi: 10.3390/cancers13092144
50. Nishikawa H, Sakaguchi S. Regulatory T Cells in Cancer Immunotherapy. Curr Opin Immunol (2014) 27:1–7. doi: 10.1016/j.coi.2013.12.005
51. Colombo MP, Piconese S. Regulatory-T-Cell Inhibition Versus Depletion: The Right Choice in Cancer Immunotherapy. Nat Rev Cancer (2007) 7(11):880–7. doi: 10.1038/nrc2250
52. Cha S, Sin M-J, Kim M-J, Kim H-J, Kim Y-S, Choi E-K, et al. Involvement of Cellular Prion Protein in Invasion and Metastasis of Lung Cancer by Inducing Treg Cell Development. Biomolecules (2021) 11(2):285. doi: 10.3390/biom11020285
53. Muras AG, Hajj GNM, Ribeiro KB, Nomizo R, Nonogaki S, Chammas R, et al. Prion Protein Ablation Increases Cellular Aggregation and Embolization Contributing to Mechanisms of Metastasis. Int J Cancer (2009) 125(7):1523–31. doi: 10.1002/ijc.24425
54. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Advanced Pharm Bull (2017) 7(3):339–48. doi: 10.15171/apb.2017.041
55. Zahreddine H, Borden KLB. Mechanisms and Insights Into Drug Resistance in Cancer. Front Pharmacol (2013) 4:28. doi: 10.3389/fphar.2013.00028
56. Green DR, Llambi F. Cell Death Signaling. Cold Spring Harbor Perspect Biol (2015) 7(12):a006080. doi: 10.1101/cshperspect.a006080
57. Wang J-H, Du J-P, Zhang Y-H, Zhao X-J, Fan R-Y, Wang Z-H, et al. Dynamic Changes and Surveillance Function of Prion Protein Expression in Gastric Cancer Drug Resistance. World J Gastroenterol (2011) 17(35):3986–93. doi: 10.3748/wjg.v17.i35.3986
58. Meslin F, Hamaï A, Gao P, Jalil A, Cahuzac N, Chouaib S, et al. Silencing of Prion Protein Sensitizes Breast Adriamycin-Resistant Carcinoma Cells to TRAIL-Mediated Cell Death. Cancer Res (2007) 67(22):10910–9. doi: 10.1158/0008-5472.CAN-07-0512
59. Lee JH, Yun CW, Lee SH. Cellular Prion Protein Enhances Drug Resistance of Colorectal Cancer Cells via Regulation of a Survival Signal Pathway. Biomol Ther (2018) 26(3):313–21. doi: 10.4062/biomolther.2017.033
60. Chieng CK-L, Say Y-H. Cellular Prion Protein Contributes to LS 174T Colon Cancer Cell Carcinogenesis by Increasing Invasiveness and Resistance Against Doxorubicin-Induced Apoptosis. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2015) 36(10):8107–20. doi: 10.1007/s13277-015-3530-z
61. Lee JH, Yoon YM, Han Y-S, Yun CW, Lee SH. Melatonin Promotes Apoptosis of Oxaliplatin-Resistant Colorectal Cancer Cells Through Inhibition of Cellular Prion Protein. Anticancer Res (2018) 38(4):1993–2000. doi: 10.21873/anticanres.12437
62. Brown DR, Schulz-Schaeffer WJ, Schmidt B, Kretzschmar HA. Prion Protein-Deficient Cells Show Altered Response to Oxidative Stress Due to Decreased SOD-1 Activity. Exp Neurol (1997) 146(1):104–12. doi: 10.1006/exnr.1997.6505
63. Luo G, Wang W, Wu Q, Lu Y, Su T, Gu N, et al. MGr1-Antigen/37 kDa Laminin Receptor Precursor Promotes Cellular Prion Protein Induced Multi-Drug-Resistance of Gastric Cancer. Oncotarget (2017) 8(42):71630–41. doi: 10.18632/oncotarget.17795
64. Wang JH, Du JP, Li SJ, Zhai LP, Yang XY, Wang ZH, et al. Octarepeat Peptides of Prion are Essential for Multidrug Resistance in Gastric Cancer Cells. J Digestive Dis (2012) 13(3):143–52. doi: 10.1111/j.1751-2980.2011.00563.x
65. Li QQ, Cao XX, Xu JD, Chen Q, Wang WJ, Tang F, et al. The Role of P-Glycoprotein/Cellular Prion Protein Interaction in Multidrug-Resistant Breast Cancer Cells Treated With Paclitaxel. Cell Mol Life Sci CMLS (2009) 66(3):504–15. doi: 10.1007/s00018-008-8548-6
66. Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, et al. Overexpression and Significance of Prion Protein in Gastric Cancer and Multidrug-Resistant Gastric Carcinoma Cell Line SGC7901/ADR. Int J Cancer (2005) 113(2):213–20. doi: 10.1002/ijc.20570
67. Hay B, Prusiner SB, Lingappa VR. Evidence for a Secretory Form of the Cellular Prion Protein. Biochemistry (1987) 26(25):8110–5. doi: 10.1021/bi00399a014
68. Lewis V, Johanssen VA, Crouch PJ, Klug GM, Hooper NM, Collins SJ. Prion Protein "Gamma-Cleavage": Characterizing a Novel Endoproteolytic Processing Event. Cell Mol Life Sci CMLS (2016) 73(3):667–83. doi: 10.1007/s00018-015-2022-z
69. Yang X, Zhang Y, Zhang L, He T, Zhang J, Li C. Prion Protein and Cancers. Acta Biochim Biophys Sin (Shanghai) (2014) 46(6):431–40. doi: 10.1093/abbs/gmu019
70. Kuwahara C, Takeuchi AM, Nishimura T, Haraguchi K, Kubosaki A, Matsumoto Y, et al. Prions Prevent Neuronal Cell-Line Death. Nature (1999) 400(6741):225–6. doi: 10.1038/22241
71. Roucou X, Giannopoulos PN, Zhang Y, Jodoin J, Goodyer CG, LeBlanc A. Cellular Prion Protein Inhibits Proapoptotic Bax Conformational Change in Human Neurons and in Breast Carcinoma MCF-7 Cells. Cell Death Differentiation (2005) 12(7):783–95. doi: 10.1038/sj.cdd.4401629
72. Kurschner C, Morgan JI. Analysis of Interaction Sites in Homo- and Heteromeric Complexes Containing Bcl-2 Family Members and the Cellular Prion Protein. Brain Res Mol Brain Res (1996) 37(1-2):249–58. doi: 10.1016/0169-328x(95)00323-k
73. Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and Characterization of a New Member of the TNF Family That Induces Apoptosis. Immunity (1995) 3(6):673–82. doi: 10.1016/1074-7613(95)90057-8
74. Park J-Y, Jeong J-K, Lee J-H, Moon J-H, Kim S-W, Lee Y-J, et al. Induction of Cellular Prion Protein (PrPc) Under Hypoxia Inhibits Apoptosis Caused by TRAIL Treatment. Oncotarget (2015) 6(7):5342–53. doi: 10.18632/oncotarget.3028
75. Xu J, Zhou J-Y, Wei W-Z, Wu GS. Activation of the Akt Survival Pathway Contributes to TRAIL Resistance in Cancer Cells. PloS One (2010) 5(4):e10226–e. doi: 10.1371/journal.pone.0010226
76. Ramljak S, Herlyn H, Zerr I. Cellular Prion Protein (PrPc) and Hypoxia: True to Each Other in Good Times and in Bad, in Sickness, and in Health. Front Cell Neurosci (2016) 10:292. doi: 10.3389/fncel.2016.00292
77. Gao Z, Peng M, Chen L, Yang X, Li H, Shi R, et al. Prion Protein Protects Cancer Cells Against Endoplasmic Reticulum Stress Induced Apoptosis. Virol Sin (2019) 34(2):222–34. doi: 10.1007/s12250-019-00107-2
78. Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-Term Fasting Induces Profound Neuronal Autophagy. Autophagy (2010) 6(6):702–10. doi: 10.4161/auto.6.6.12376
79. Chen Y, Klionsky DJ. The Regulation of Autophagy - Unanswered Questions. J Cell Sci (2011) 124(Pt 2):161–70. doi: 10.1242/jcs.064576
80. Barbieri G, Palumbo S, Gabrusiewicz K, Azzalin A, Marchesi N, Spedito A, et al. Silencing of Cellular Prion Protein (PrPC) Expression by DNA-Antisense Oligonucleotides Induces Autophagy-Dependent Cell Death in Glioma Cells. Autophagy (2011) 7(8):840–53. doi: 10.4161/auto.7.8.15615
81. Oh J-M, Choi E-K, Carp RI, Kim Y-S. Oxidative Stress Impairs Autophagic Flux in Prion Protein-Deficient Hippocampal Cells. Autophagy (2012) 8(10):1448–61. doi: 10.4161/auto.21164
82. Bernardino-Sgherri J, Siberchicot C, Auvré F, Busso D, Brocas C, El Masri G, et al. Tumor Resistance to Radiotherapy Is Triggered by an ATM/TAK1-Dependent-Increased Expression of the Cellular Prion Protein. Oncogene (2021) 40(19):3460–9. doi: 10.1038/s41388-021-01746-0
83. Go G, Lee SH. The Cellular Prion Protein: A Promising Therapeutic Target for Cancer. Int J Mol Sci (2020) 21(23):9208. doi: 10.3390/ijms21239208
84. Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer Stem Cells. Int J Biochem Cell Biol (2012) 44(12):2144–51. doi: 10.1016/j.biocel.2012.08.022
85. Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion Protein Is Expressed on Long-Term Repopulating Hematopoietic Stem Cells and Is Important for Their Self-Renewal. Proc Natl Acad Sci USA (2006) 103(7):2184–9. doi: 10.1073/pnas.0510577103
86. Liao M-J, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, et al. Enrichment of a Population of Mammary Gland Cells That Form Mammospheres and Have In Vivo Repopulating Activity. Cancer Res (2007) 67(17):8131–8. doi: 10.1158/0008-5472.CAN-06-4493
87. Mohanty ST, Cairney CJ, Chantry AD, Madan S, Fernandes JA, Howe SJ, et al. A Small Molecule Modulator of Prion Protein Increases Human Mesenchymal Stem Cell Lifespan, Ex Vivo Expansion, and Engraftment to Bone Marrow in NOD/SCID Mice. Stem Cells (Dayton Ohio) (2012) 30(6):1134–43. doi: 10.1002/stem.1065
88. Lee YJ, Baskakov IV. The Cellular Form of the Prion Protein Is Involved in Controlling Cell Cycle Dynamics, Self-Renewal, and the Fate of Human Embryonic Stem Cell Differentiation. J Neurochem (2013) 124(3):310–22. doi: 10.1111/j.1471-4159.2012.07913.x
89. Ghazi A, Le Corre D, Pilati C, Taieb J, Aparicio T, Didelot A, et al. Prognostic Value of the PrPc-ILK-IDO1 Axis in the Mesenchymal Colorectal Cancer Subtype. Oncoimmunol (2021) 10(1):1940674. doi: 10.1080/2162402x.2021.1940674
90. Wang L, Zuo X, Xie K, Wei D. The Role of CD44 and Cancer Stem Cells. Methods Mol Biol (Clifton NJ) (2018) 1692:31–42. doi: 10.1007/978-1-4939-7401-6_3
91. Corsaro A, Bajetto A, Thellung S, Begani G, Villa V, Nizzari M, et al. Cellular Prion Protein Controls Stem Cell-Like Properties of Human Glioblastoma Tumor-Initiating Cells. Oncotarget (2016) 7(25):38638–57. doi: 10.18632/oncotarget.9575
92. Heppner FL, Musahl C, Arrighi I, Klein MA, Rülicke T, Oesch B, et al. Prevention of Scrapie Pathogenesis by Transgenic Expression of Anti-Prion Protein Antibodies. Science (2001) 294(5540):178–82. doi: 10.1126/science.1063093
93. White AR, Enever P, Tayebi M, Mushens R, Linehan J, Brandner S, et al. Monoclonal Antibodies Inhibit Prion Replication and Delay the Development of Prion Disease. Nature (2003) 422(6927):80–3. doi: 10.1038/nature01457
94. Abdelaziz DH, Thapa S, Brandon J, Maybee J, Vankuppeveld L, McCorkell R, et al. Recombinant Prion Protein Vaccination of Transgenic Elk PrP Mice and Reindeer Overcomes Self-Tolerance and Protects Mice Against Chronic Wasting Disease. J Biol Chem (2018) 293(51):19812–22. doi: 10.1074/jbc.RA118.004810
95. Minikel EV, Zhao HT, Le J, O’Moore J, Pitstick R, Graffam S, et al. Prion Protein Lowering Is a Disease-Modifying Therapy Across Prion Disease Stages, Strains and Endpoints. Nucleic Acids Res (2020) 48(19):10615–31. doi: 10.1093/nar/gkaa616
96. Minikel EV, Karczewski KJ, Martin HC, Cummings BB, Whiffin N, Rhodes D, et al. Evaluating Drug Targets Through Human Loss-of-Function Genetic Variation. Nature (2020) 581(7809):459–64. doi: 10.1038/s41586-020-2267-z
97. Das AS, Zou W-Q. Prions: Beyond a Single Protein. Clin Microbiol Rev (2016) 29(3):633–58. doi: 10.1128/CMR.00046-15
Keywords: cellular prion protein, cancer, proliferation, metastasis, drug resistance, cancer stem cell
Citation: Ding M, Chen Y, Lang Y and Cui L (2021) The Role of Cellular Prion Protein in Cancer Biology: A Potential Therapeutic Target. Front. Oncol. 11:742949. doi: 10.3389/fonc.2021.742949
Received: 17 July 2021; Accepted: 24 August 2021;
Published: 14 September 2021.
Edited by:
Gabi U. Dachs, University of Otago, Christchurch, New ZealandReviewed by:
Barry Matthew Bradford, University of Edinburgh, United KingdomCopyright © 2021 Ding, Chen, Lang and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Cui, bGN1aUBqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.