- Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
Introduction: HR+/HER2− breast cancer (BC) has a much lower pathological complete response (pCR) rate to neoadjuvant chemotherapy (NAC). Therefore, to better stratify the relapse risk for HR+/HER2− non-pCR populations, it is essential to accurate identification new prognostic markers.
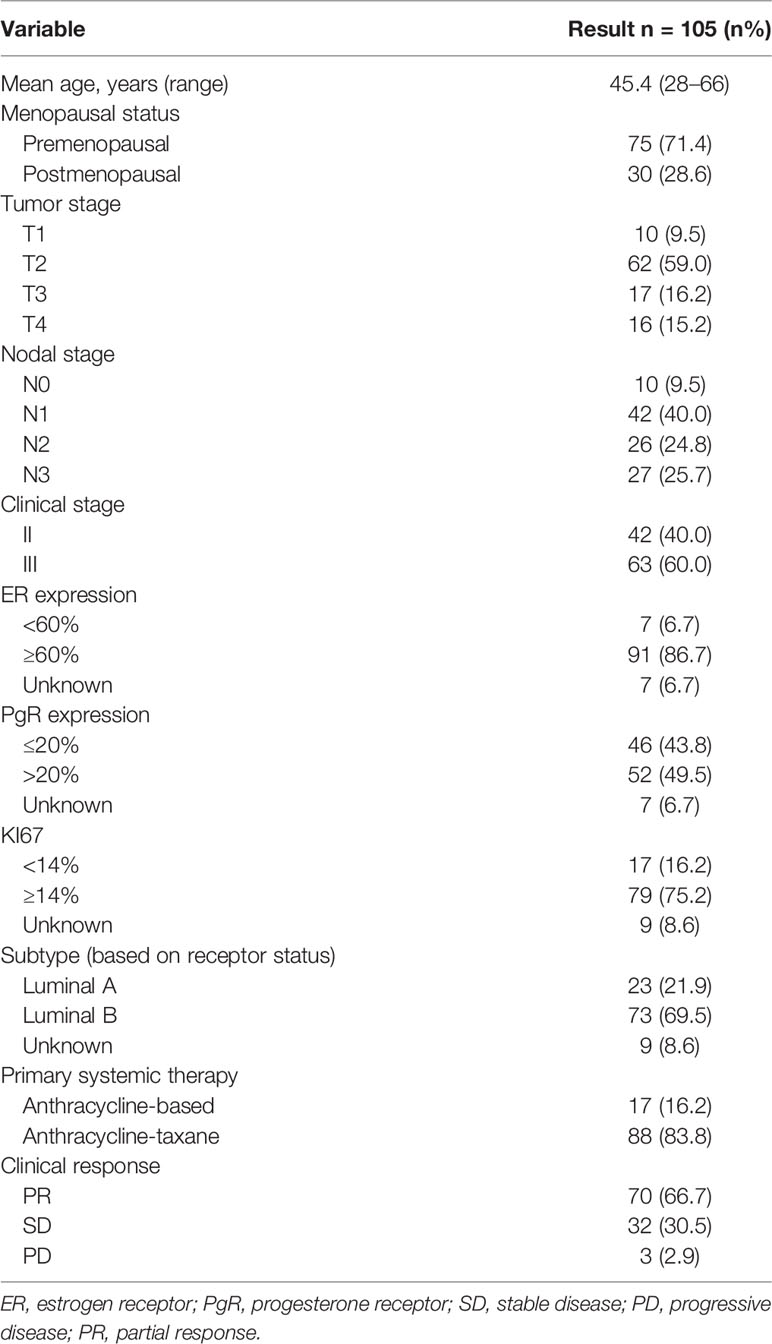
Materials and Methods: The study retrospectively analyzed 105 stage II–III patients who were diagnosed with HR+/HER2− BC and received NAC followed by breast and axilla surgery between 2013 and 2019 in Sun Yat-Sen University Cancer Center. The Miller–Payne (MP) grading system was used to evaluate pathological responses to NAC. The 70-gene signature was used to classify the prognosis signatures.
Results: Among the 105 patients, the study demonstrated that larger tumor size and lower progesterone receptor level at baseline and larger tumor size postoperative were statistically significantly associated with worse disease-free survival (DFS) (p = 0.004, p = 0.021, and p = 0.001, respectively). Among 54 patients who underwent the 70-gene assays, 26 (48.1%) had a low-risk signature; 28 (51.9%) patients had a high-risk signature. Patients with poor response (MP grades 1–2) were more likely to with a high-risk 70-gene signature than those with good response (MP grades 4–5). The final analysis showed that DFS was longer in the low-risk group than in the high-risk group [52.4 vs. 36.1 months of the median DFS, hazard ratio (HR) for recurrence, 0.29; 95% confidence interval (CI), 0.10–0.80; p = 0.018]. DFS was longer in the good response (MP grades 3–4) group than in the poor response (MP grades 1–2) group (94.7% vs. 60% of the patients free from recurrence; HR, 0.16; 95% CI, 0.05–0.47; p = 0.037). When stratified by MP grades combined with the 70-gene signature, subgroup analyses showed the good-response low-risk group with the best DFS, whereas the poor-response high-risk group showed the worst DFS (p = 0.048). Due to the short median follow-up time of 34.5 months (5.9–75.1 months), MP grades and the 70-gene signature did not show significant prognostic value for overall survival.
Conclusion: The study showed that analysis of MP grades combined with the 70-gene signature with residual NAC-resistant breast samples has a significant correlation with DFS.
Introduction
Hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) accounts for about 50–60% of all invasive breast carcinomas (1). HR+/HER2− patients with locally advanced stages may receive neoadjuvant chemotherapy (NAC) to reduce tumor burden and potentially facilitate breast or axillary conservation (2). Previous studies have demonstrated higher rates of pathological complete response (pCR) to NAC in triple-negative and HER2+ BC, but with a much lower rate in HR+/HER2− patients (3–5). However, pCR is not a surrogate endpoint for prediction of long-term clinical benefit in HR+/HER2− patients, such as disease-free survival (DFS) and overall survival (OS) (6). In particular, for HR+/HER2− BC, various multigene expression assays have been developed and validated to have prognostic value (7, 8). Patients who have residual invasive carcinoma after the receipt of NAC for HR+/HER2− BC have poor prognoses. Therefore, to better stratify the relapse risk for HR+/HER2− non-pCR populations, it is essential to accurate identification new prognostic markers.
The 70-gene signature (MammaPrint) is a prognostic tool that classifies tumors into groups that are associated with a good prognosis or a poor prognosis based on the risk of distant metastases at 5 and at 10 years (7). MammaPrint has been approved by the Food and Drug Administration (FDA), which can distinguish patients who are at significant risk for distant metastases and death from those at low risk (9). The prognostic value of 70-gene signature has been validated in a range of studies (9, 10). Furthermore, the results showed that the 70-gene signature increased independent prognostic information to that provided by commonly used clinicopathological factors. The Miller–Payne (MP) grading system is a widely accepted and frequently used method, and it is an independent predictor of DFS or OS (11). Prognostic value of the 70-gene signature in HR+/HER2− early-stage BC after NCT is unclear. Therefore, the 70-gene assay combined with MP grading system to assess the prognosis value of HR+/HER2− early-stage BC has great clinical significance.
Integrating tumor size and nodal status with tumor grade and genomic signatures could provide accurate prognostic estimates for HR+/HER2− BC (12, 13). The risk factors for early recurrence and for late recurrence are largely the same (14, 15). There is a need for more accurate predictive markers to guide intensive adjuvant therapy in HR+/HER2− BC after NAC. However, integrating the 70-gene signature with MP grading system for HR+/HER2− early-stage BC after NAC prognostic estimates is uncertain. Here, we designed this study to examine prognosis value of the 70-gene signature combined with MP grading system in patients who underwent NAC for HR+/HER2− early-stage BC.
Materials and Methods
Patient Material
The flowchart of the study is shown in Figure 1. The clinical study has been approved by the ethical committee of Sun Yat-Sen University Cancer Center (ethics approval number of clinical study project: B2020-329). Formalin-fixed paraffin-embedded tumor specimens and clinical data were retrospectively collected from a consecutive of 105 HR+/HER2− BC who received NAC between 2013 and 2019. The criteria for inclusion were as follows: (1) histologically confirmed invasive carcinoma at core needle biopsy; (2) clinical stages II–III; (3) histochemical examination of ER ≥ 10%, HER2 negative; (4) both NAC and surgical procedures performed in our hospital; (5) MP grades available after surgery; and (6) never received chemotherapy, radiation, and endocrine therapy before NAC. All patients were accepted at least two cycles of NAC. Patients with no or insufficient tumor samples as determined by a pathologist and insufficient RNA for testing were excluded, resulting in a total of 54 patients enrolled suitable for the 70-gene assay.
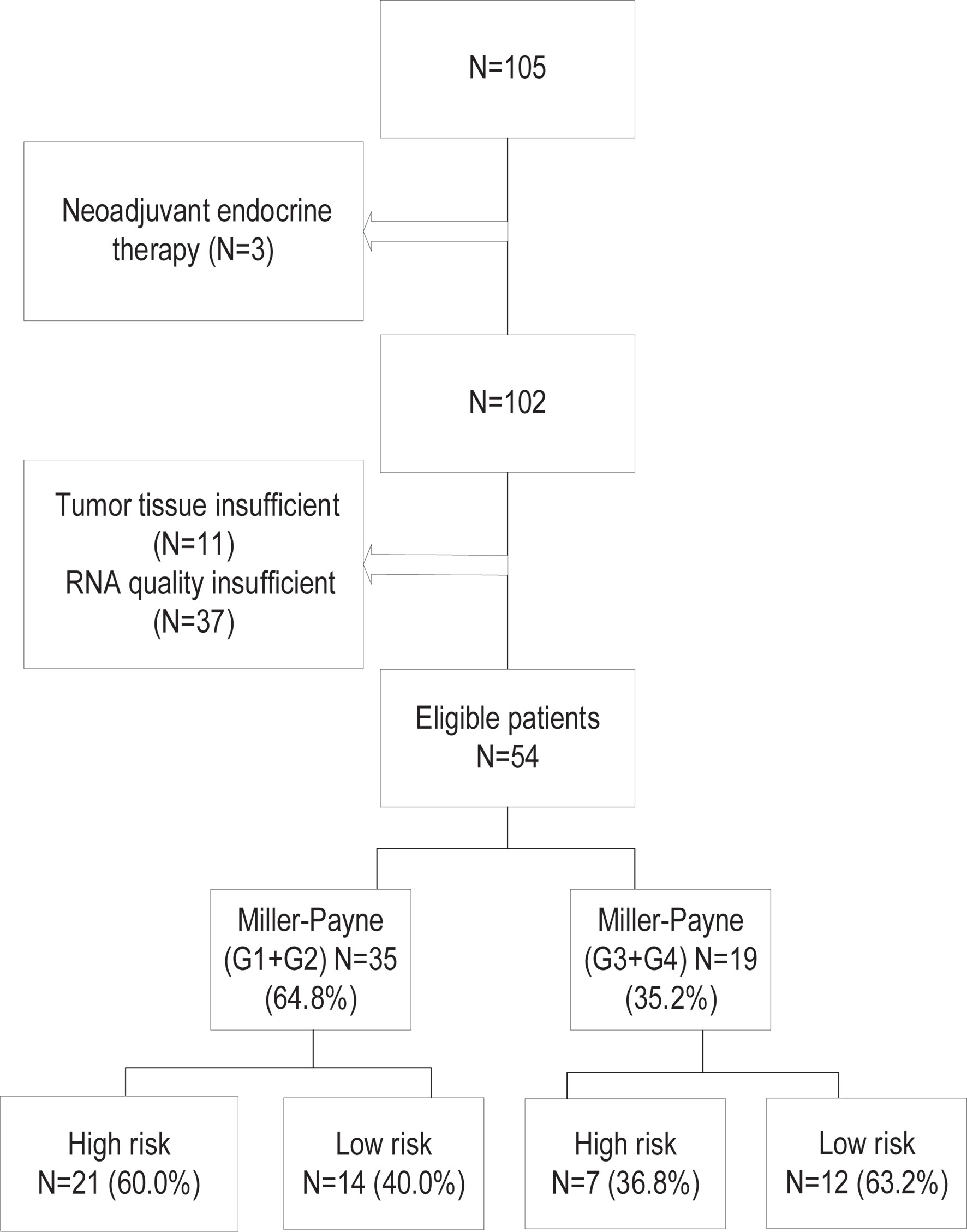
Figure 1 The patient selection and the classification of the 70-gene signature and Miller–Payne pathological grades.
Molecular and Clinical Characteristics
The 70-gene expression profile was assessed from the formalin-fixed paraffin-embedded resection breast samples blinded to clinical and pathological data. The paraffin blocks were cut into tissue sections with a thickness of 3–5 µm to make white slices without cover glass and then dewaxed to obtain enough tumor RNA for genetic testing (16, 17). RNA extraction and amplification were performed as previously described (18). Only specimens with at least 50% tumor cells were further analyzed. To assess the mRNA expression level of the 70 genes, RNA was hybridized with a standard reference to the custom-designed diagnostic chip, each containing oligonucleotide probes for the profiles in triplicate or more. Tumors were classified as high or low genomic risk as described previously (7).
The pathological response to NAC was assessed by the MP grading system (11), compared with the pathology changes in the resected tumor specimens with the tissues before chemotherapy:
Grade 1: No change or some alteration to individual malignant cells but no reduction in overall cellularity;
Grade 2: A minor loss of tumor cells but overall cellularity still high; up to 30% loss;
Grade 3: Between an estimated 30% and 90% reduction in tumor cells;
Grade 4: A marked disappearance of tumor cells such that only small clusters or widely dispersed individual cells remain; more than 90% loss of tumor cells;
Grade 5: No malignant cells identifiable in sections from the site of the tumor, only vascular fibroelastotic stroma remains often containing macrophages. However, ductal carcinoma in situ (DCIS) may be present.
pCR was defined as no histological evidence of malignancies or only in situ residuals in breast tissue after surgery and complete disappearance of lymph node metastasis.
Grades 1 and 2 are categorized as poor response, and Grades 3–5 are categorized as good response.
Following Up
The follow-up data including the dates of recurrence and death were collected through the outpatient service, telephone, and the way to the hospital review, until death or the date of the last follow-up (on May 1, 2021). The median follow-up time was 37.5 months (2.4–92.3 months). DFS was calculated from the date of surgery to the date of disease relapse (local or distant relapse or death from any cause). OS was calculated from the date of surgery to the date of death or the latest follow-up.
Statistical Analysis
Analyses were performed using SPSS version 26. The differences in patients and tumor characteristics between the 70-gene high- and low-risk signature were tested using Fisher’s exact test. Logistic regression was applied to identify variables predictive of MP grades. The results of the model were expressed as odds ratio (OR) and relative 95% confidence interval (CI). Univariate and multivariate analyses were performed with the Cox proportional model to determine the effects of independent prognostic factors on DFS and OS. Calculating the exponential of the regression coefficients from the Cox model provided an estimate of the HR and the 95% CI. DFS and OS curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Two-tailed p < 0.05 were considered statistically significant.
Results
Patient Characteristics and Responses
We collected the information of 105 BC patients who received NAC before surgery (Tables 1, 2). The median age was 45.4 years (range, 28–66 years). All patients were diagnosed with stage II or III disease, and 71.4% of them were premenopausal at diagnosis. The clinical response to NAC was assessed for each patient and summarized by the RECIST 1.1 criteria. The overall clinical PR rate was 66.7% (70/105), and the clinical SD rate was 30.5% (32/105), and the remainder of the patients had PD. The pathological responses were assessed by MP grades. Six patients (5.7%) had an MP grade 5 response, 9 patients (8.6%) had a grade 4 response, 40 patients (38.1%) had a grade 3 response, 36 patients (34.3%) had a grade 2 response, and 14 patients (13.3%) had a grade 1 response. The 105 patients were classified into two subtypes: Luminal-A (21.9%, n = 23), 1 patient (4.3%) was grade 4, 8 patients (34.8%) were grade 3, 8 patients (34.8%) were grade 2, and 6 patients (26.1%) were MP grade 1; Luminal-B (69.5%, n = 73), 5 patients (6.8%) were grade 5, 7 patients (9.6%) were grade 4, 30 patients (41.1%) were grade 3, 25 patients (34.2%) were grade 2, and 6 patients (8.2%) were MP grade 1. The overall pCR was 3.8% (4/105), considering both MP grades and residual nodes. Thirty-eight patients (36.2%) received adjuvant chemotherapy; 90 patients (85.7%) received adjuvant radiotherapy. Type of endocrine therapy used was tamoxifen in 33 (31.4%), aromatase inhibitor (AI) + ovarian function suppression in 47 (44.8%), and AI alone in 31 (29.5%). A total of 54 patients had available postoperative tissue for the 70-gene signature (Supplementary Table S1).
Logistic Analysis of Pretreatment Factors and MP Grades
Univariate logistic regression analysis revealed that good response (Grades 3–5) to NAC in early-stage HR+/HER2− BC was significantly associated with N-stage 2–3, Anthracycline-taxane or clinical PR (N-stage: p = 0.042, OR 2.25, 95% CI 1.03–4.92; primary systemic therapy: p = 0.014, OR 4.48, 95% CI 1.35–14.84; clinical response: p = 0.010, OR 0.33, 95% CI 0.14–0.77), while it was unrelated to age, clinical tumor stage, clinical stage, PR, Ki-67, and molecular subtypes (Table 3).
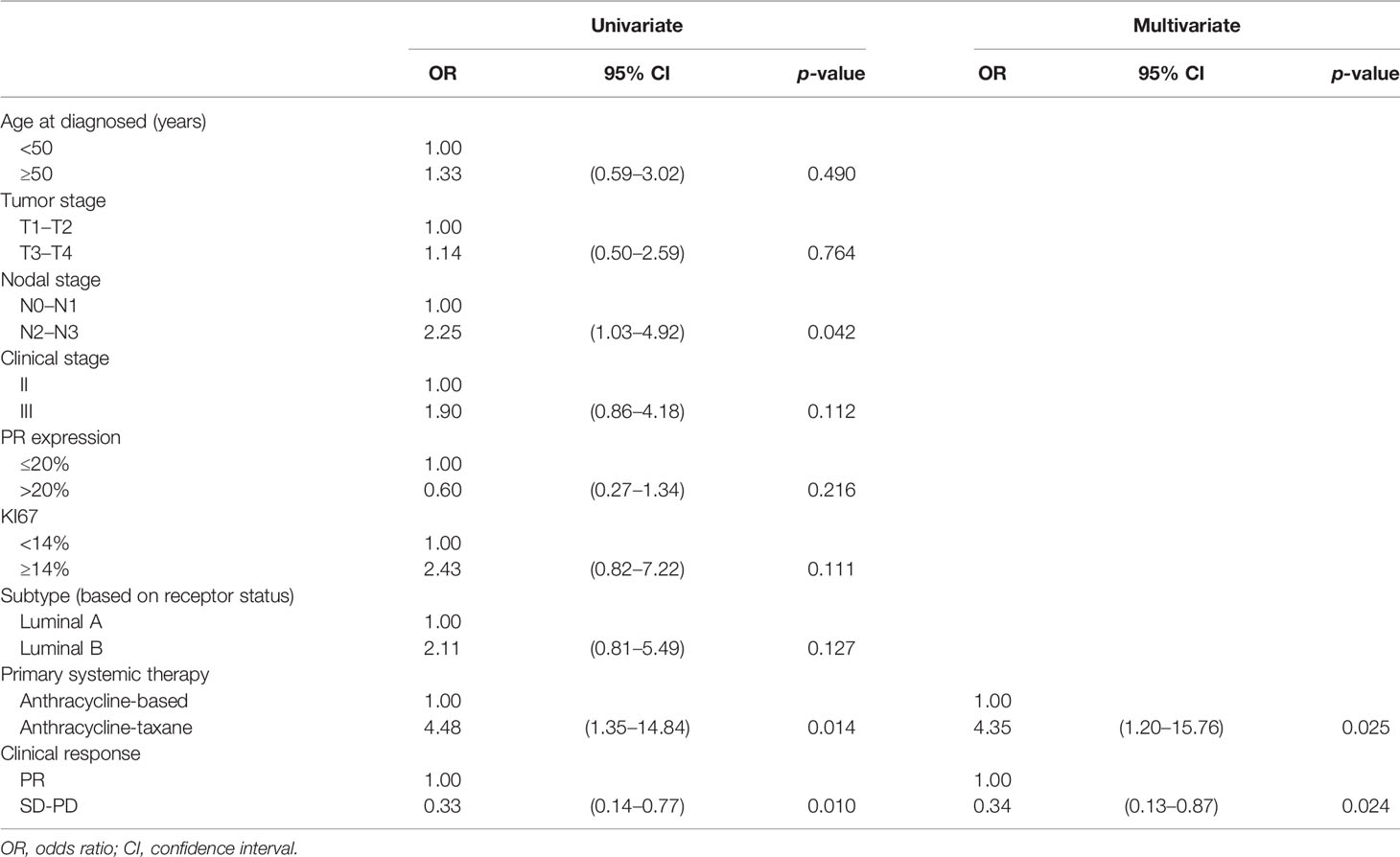
Table 3 Logistic univariate and multivariate analyses for the relationship between clinicopathological characteristics pretreatment and Miller–Payne grades in 105 patients.
Multivariate analysis was performed using baseline characteristics with p < 0.15 in the univariate analysis. The results showed that good response to NAC in early-stage HR+/HER2− BC was significantly associated with anthracycline-taxane chemotherapy or clinical PR (p < 0.05). The other factors were not related to good response. ER could not be analyzed for MP grades because most patients had tumors with high ER expression.
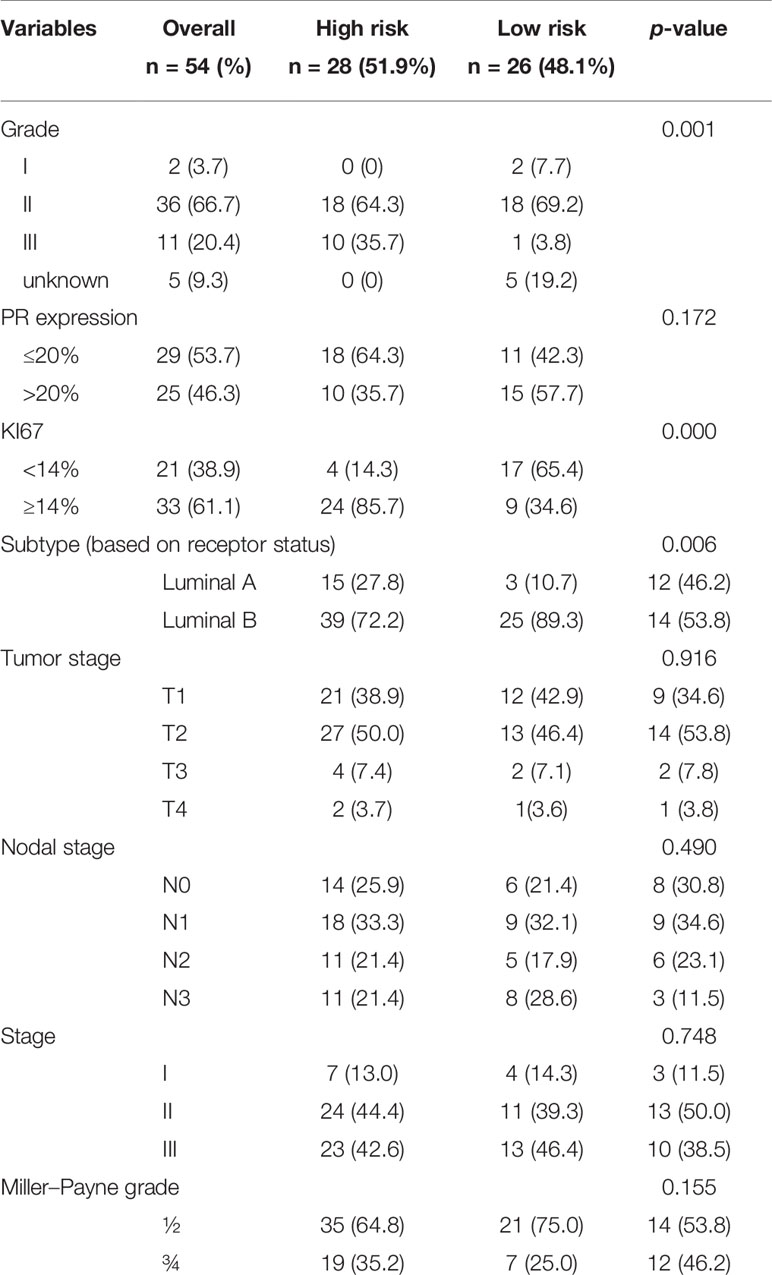
Characteristics of the Postoperative Tumor in the MammaPrint Cohort
The 70-gene signature was analyzed in 54 patients; the tumor characteristics are presented in Supplementary Table S1 and Table 4. The 54 patients were classified into two subtypes: Luminal-A (18.5%, n = 10), 6 patients (60.0%) had low- and 4 patients (40.0%) had high-risk signature; Luminal-B (74.1%, n = 40), 18 patients (45.0%) had low- and 22 patients (55.0%) had high-risk signature. Among the 54 early HR+/HER2− patients, 26 (48.1%) had a low-risk signature, whereas 28 (51.9%) patients had a high-risk signature. Tumors with a high-risk signature were of higher grade, higher Ki67 level, and were more often classified as Luminal B tumors. Figure 2 shows the relation between the classification of the 70-gene profile as a continuous variable and MP grades (good response: grades 3–4; poor response: grades 1–2). Patients with a good response have a higher probability to with a high MammaPrint Index.
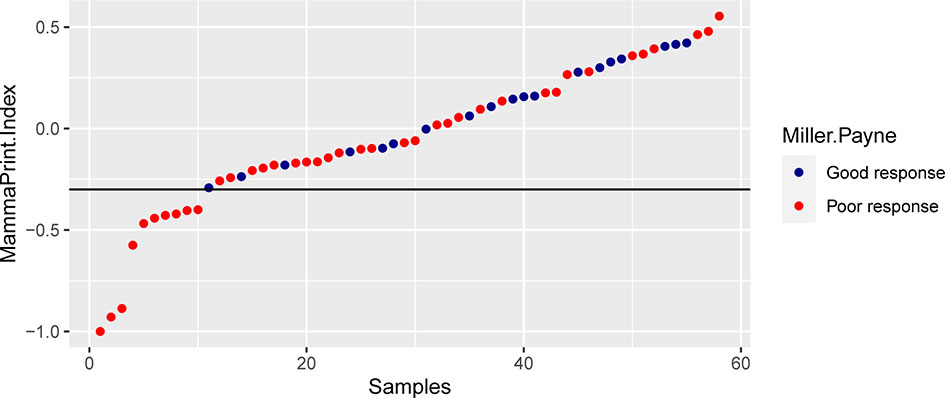
Figure 2 The association between the classification of the 70-gene recurrence risk and the pathological response.
Survival Analysis
Of the 105 early-stage HR+/HER2− cases, the magnitude of impact on different prognosis including DFS and OS differed depending on factors (Supplementary Table S2). Multivariate analysis showed that larger tumor size and lower PR level at baseline and larger tumor size postoperative were independent predictor of DFS.
Among 54 patients who underwent the 70-gene assays, there were four incidences of death. The median follow-up time was 34.5 months (5.9–75.1 months). Postoperative clinicopathological factors were analyzed for survival (Table 5). Univariate analysis showed that DFS was significantly associated with KI67, pathological tumor stage, and MammaPrint signature. OS was related to the pathological tumor stage (p < 0.05); however, it was unrelated to histological grade, PR, KI67, molecular subtypes, nodal stage, pathological stage, MP grades, and MammaPrint signature (Figure 3). Multivariate analysis was performed using postoperative characteristics with p < 0.15 in the univariate analysis. The results showed that pathological tumor stage was independent predictor of DFS and OS in early-stage HR+/HER2− BC after NAC.
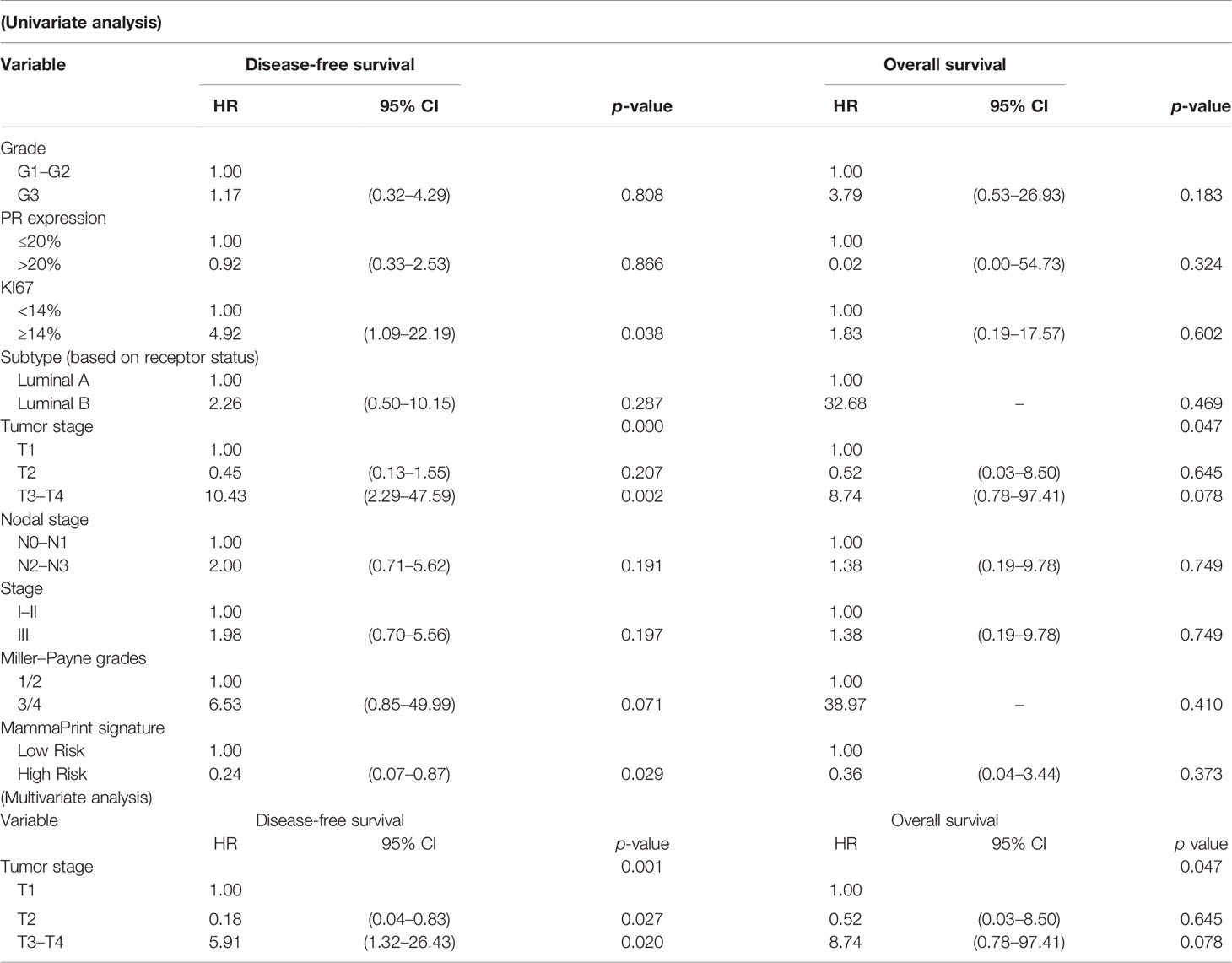
Table 5 Univariate and multivariate analysis of postoperative factors predictive of disease-free survival and overall survival in MammaPrint group.

Figure 3 Disease-free survival (DFS) and overall survival (OS) according to Miller–Payne grades (A) and MammaPrint evaluation (B). Kaplan-Meier DFS and OS estimates for combinations of Miller–Payne grades and MammaPrint evaluation (C).
The study showed that DFS was longer in the good response (MP grades 3–4) group than in the poor response (MP grades 1–2) group (94.7% vs. 60% of the patients free from recurrence; HR, 0.16; 95% CI, 0.05–0.47; p = 0.037) (Figure 3A). DFS was longer in the low-risk group than in the high-risk group (52.4 vs. 36.1 months of the median DFS; HR for recurrence, 0.29; 95% CI, 0.10–0.80; p = 0.018) (Figure 3B). When stratified by MP grades and the 70-gene signature, subgroup analyses showed the -response low-risk group with the best DFS, whereas the poor-response high-risk group showed the worst DFS (p = 0.048, Figure 3C).
Discussion
The pCR rate is associated with tumor subtypes; the HER2+ and triple-negative subtypes had higher pCR rates than the HR+/HER2− subtype (3, 19). In this study, a series of 105 patients with stage II–III primary invasive HR+/HER2− BC who received NAC were described. The overall pCR was 3.8% (4/105), which was consistent with previous studies. Therefore, pCR cannot be used to evaluate pathological responses to HR+/HER2− BC after NAC, and more accurate prediction alternative markers are needed. The overall loss of cellularity after NAC is not always reflected by a decrease in tumor size, as the fibrous stroma still exists. The MP grading system is based on the degree of tumor cell loss, which occurs within the tumor during NAC. Therefore, MP grades can accurately evaluate the main manifestation of pathological response to NAC and identify patients with a better prognosis (11, 20). Our study showed that only six patients (5.7%) had a MP grade 5 response, many patients exhibiting residual tumor cells were present, and the reaction to NAC may correlated with survival benefit. Multivariate analysis showed that anthracycline-taxane chemotherapy or clinical response was significantly associated with good response (MP grades 3–5). Therefore, anthracycline-taxane regimen was a better choice for HR+/HER2− BC who received NAC.
This study demonstrated that larger tumor size and lower progesterone receptor level at baseline and larger tumor size of postoperative were statistically significantly associated with worse DFS (p = 0.004, p = 0.021 and p = 0.001, respectively). However, a previous study showed that lower estrogen receptor level after 2 weeks of endocrine therapy is associated with poorer recurrence-free survival (21). In our study, ER could not be analyzed as most patients had tumors with high ER expression. A possible explanation for this phenomenon may be that significant changes in tumor hormone receptors status occur following NAC (22).
Patients with residual BC after NAC are at a high risk of recurrence of metastases, which make these patients ideal applicants for prognostic analysis. Residual cancer burden (RCB) score can be as a prognostic marker for long-term survival after NAC independent of the molecular subtype (23). The prognostic value of posttreatment Oncotype DX Recurrence Score (RS) results in tumor specimens from patients receiving neoadjuvant endocrine therapy (NET) was evaluated in a study by Ueno et al. (24), which demonstrated that posttreatment RSs was significantly associated with DFS. Next-generation sequencing-based multigene assay have been validated to have prognostic in early-stage HR+/HER2− BC (8). A study showed that neoadjuvant therapy can significantly altered MammaPrint index and molecular subtype, indicating that the genetic characteristics of the residual tumor are different from pretreatment genomic profile (25). To better delineate the relapse risk in patients after NAC with residual disease, it is necessary to develop a multigene assay to predict prognosis.
The residual chemotherapy-resistant cancer cells in the breast and nodes after NAC may harbor different gene expression patterns and DNA mutational signatures, which would result in differential survival. In this study, 54 resection breast samples met the 70-gene profile quality requirements and performed genomic assays. The proportion of tumors with a low-risk signature was 48.1%; that of high-risk signature was 51.9%. We observed that tumors with poor response (MP Grades 1–2) were more likely to have a high-risk 70-gene signature than those with good response (MP Grades 4–5) (Figure 1). Our study showed that MP grades and the 70-gene signature were statistically significantly associated with DFS (p = 0.037, p = 0.018, respectively). Stratifying by MP grades and the 70-gene signature also showed marked prognostic value (p = 0.048). The poor-response high-risk group showed the worst DFS, whereas the good-response low-risk group showed the best DFS. All patients received tamoxifen or aromatase inhibitors combined with ovarian function suppression adjuvant endocrine therapy. The study showed that active endocrine intervention for the poor-response high-risk group was not enough; the addition of chemotherapy or intensive endocrine therapy (e.g., cyclin-dependent kinase 4/6 inhibitors, CDK4/6i) in the adjuvant setting may prolong DFS and improve prognosis for these patients. Due to the short follow-up time, MP grades and the 70-gene signature did not show significant prognostic value for OS. A previous study used tumor samples prior to NAC for the 70-gene, which signature showed good prognostic value and predictive chemotherapeutic sensitivity (26). However, this study used residual chemotherapy-resistant breast samples, which showed similar prognostic value. This finding has important clinical implication. Based on the prognostic value of the 70-gene signature in HR+/HER2− early-stage BC who have residual invasive carcinoma after NCA; further clinical studies of intensive adjuvant therapy in high-risk patients should be conducted.
There were several limitations that should be considered while interpreting the study findings. One critical limitation was the small sample size that restricted our analysis for the subgroup based on MP grades and the 70-gene signature. Another limitation was that a longer follow-up will be required to confirm that MP grades and the 70-gene signature were associated with long-term survival. Different NAC regimens and cycles were another issue. In the present study, most patients received different cycles of anthracycline-based or anthracycline-taxane NAC, which may affect a reduction in tumor load of the primary tumor. Despite these limitations, our study has several strengths. To our knowledge, this is the first study that provides an analysis of MP grades combined with the 70-gene signature associated with prognostic in HR+/HER2− non-pCR populations. Integration of MP grades and the 70-gene signature to determine the appropriate options of adjuvant treatment may prolong DFS and improve prognosis. Further studies are warranted to confirm the clinical utility of MP grades combined with the 70-gene signature in early-stage HR+/HER2− breast cancer.
In conclusion, this study showed that analysis of MP grades combined with the 70-gene signature with residual neoadjuvant chemotherapy-resistant breast samples has a significant correlation with DFS. In particular, subgroup analyses based on MP grades and the 70-gene signature also showed markedly prognostic value.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
Conception and design of the study: FX and SW. Acquisition of clinical data: LW, QL, KJ, RH, KL, PZ, and DZ. Miller–Payne grades pathological evaluation: RL. Analysis and interpretation of the data: LW and QL. Manuscript drafting and revision: LW, QL, FX, and SW. All authors contributed to the article and approved the submitted version.
Funding
The work was funded by the Sun Yat-sen University Clinical Research 5010 Program (2017010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the staff members of the Department of Pathology, Sun Yat-sen University Cancer Center for their assistance with the preparation and preservation of paraffin-embedded breast cancer samples.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.735670/full#supplementary-material
References
1. Li T, Mello-Thoms C, Brennan PC. Descriptive Epidemiology of Breast Cancer in China: Incidence, Mortality, Survival and Prevalence. Breast Cancer Res Treat (2016) 159(3):395–406. doi: 10.1007/s10549-016-3947-0
2. Burstein HJ. Systemic Therapy for Estrogen Receptor-Positive, HER2-Negative Breast Cancer. N Engl J Med (2020) 383(26):2557–70. doi: 10.1056/NEJMra1307118
3. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J Clin Oncol (2012) 30(15):1796–804. doi: 10.1200/JCO.2011.38.8595
4. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic Complete Response After Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-Analysis. Clin Cancer Res (2020) 26(12):2838–48. doi: 10.1158/1078-0432.CCR-19-3492
5. Schettini F, Pascual T, Conte B, Chic N, Braso-Maristany F, Galvan P, et al. HER2-Enriched Subtype and Pathological Complete Response in HER2-Positive Breast Cancer: A Systematic Review and Meta-Analysis. Cancer Treat Rev (2020) 84:101965. doi: 10.1016/j.ctrv.2020.101965
6. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet (London England) (2014) 384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8
7. Cardoso F, van't Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med (2016) 375(8):717–29. doi: 10.1056/NEJMoa1602253
8. Lee HB, Lee SB, Kim M, Kwon S, Jo J, Kim J, et al. Development and Validation of a Next-Generation Sequencing-Based Multigene Assay to Predict the Prognosis of Estrogen Receptor-Positive, HER2-Negative Breast Cancer. Clin Cancer Res (2020) 26(24):6513–22. doi: 10.1158/1078-0432.CCR-20-2107
9. Buyse M, Loi S, van't Veer L, Viale G, Delorenzi M, Glas AM, et al. Validation and Clinical Utility of a 70-Gene Prognostic Signature for Women With Node-Negative Breast Cancer. J Natl Cancer Institute (2006) 98(17):1183–92. doi: 10.1093/jnci/djj329
10. Mook S, Schmidt MK, Viale G, Pruneri G, Eekhout I, Floore A, et al. The 70-Gene Prognosis-Signature Predicts Disease Outcome in Breast Cancer Patients With 1-3 Positive Lymph Nodes in an Independent Validation Study. Breast Cancer Res Treat (2009) 116(2):295–302. doi: 10.1007/s10549-008-0130-2
11. Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A New Histological Grading System to Assess Response of Breast Cancers to Primary Chemotherapy: Prognostic Significance and Survival. Breast (2003) 12(5):320–7. doi: 10.1016/s0960-9776(03)00106-1
12. Sestak I, Buus R, Cuzick J, Dubsky P, Kronenwett R, Denkert C, et al. Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol (2018) 4(4):545–53. doi: 10.1001/jamaoncol.2017.5524
13. Dowsett M, Turner N. Estimating Risk of Recurrence for Early Breast Cancer: Integrating Clinical and Genomic Risk. J Clin Oncol (2019) 37(9):689–92. doi: 10.1200/JCO.18.01412
14. Mauriac L, Keshaviah A, Debled M, Mouridsen H, Forbes JF, Thurlimann B, et al. Predictors of Early Relapse in Postmenopausal Women With Hormone Receptor-Positive Breast Cancer in the BIG 1-98 Trial. Ann Oncol (2007) 18(5):859–67. doi: 10.1093/annonc/mdm001
15. Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, Cowens JW, et al. Factors Predicting Late Recurrence for Estrogen Receptor-Positive Breast Cancer. J Natl Cancer Institute (2013) 105(19):1504–11. doi: 10.1093/jnci/djt244
16. Choi Y, Kim A, Kim J, Lee J, Lee SY, Kim C. Optimization of RNA Extraction From Formalin-Fixed Paraffin-Embedded Blocks for Targeted Next-Generation Sequencing. J Breast Cancer (2017) 20(4):393–9. doi: 10.4048/jbc.2017.20.4.393
17. William A. <510(K) Substantial Equivalence Determination Decision Memorandum.Pdf. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K201902.
18. Delahaye LJ, Wehkamp D, Floore AN, Bernards R, Van't Veer LJ, Glas AM. Performance Characteristics of the MammaPrint((R)) Breast Cancer Diagnostic Gene Signature. Per Med (2013) 10(8):801–11. doi: 10.2217/pme.13.88
19. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-Analysis of the Association of Breast Cancer Subtype and Pathologic Complete Response to Neoadjuvant Chemotherapy. Eur J Cancer (Oxford Engl 1990) (2012) 48(18):3342–54. doi: 10.1016/j.ejca.2012.05.023
20. Chen S, Liu Y, Ouyang QW, Huang L, Luo RC, Shao ZM. Clinical and Pathological Response to Neoadjuvant Chemotherapy Based on Primary Tumor Reduction is Correlated to Survival in Hormone Receptor-Positive But Not Hormone Receptor-Negative Locally Advanced Breast Cancer. Ann Surg Oncol (2015) 22(1):32–9. doi: 10.1245/s10434-014-3894-0
21. Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A'Hern R, et al. Prognostic Value of Ki67 Expression After Short-Term Presurgical Endocrine Therapy for Primary Breast Cancer. J Natl Cancer Institute (2007) 99(2):167–70. doi: 10.1093/jnci/djk020
22. Gahlaut R, Bennett A, Fatayer H, Dall BJ, Sharma N, Velikova G, et al. Effect of Neoadjuvant Chemotherapy on Breast Cancer Phenotype, ER/PR and HER2 Expression - Implications for the Practising Oncologist. Eur J Cancer (Oxford Engl 1990) (2016) 60:40–8. doi: 10.1016/j.ejca.2016.03.006
23. Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol (2017) 35(10):1049–60. doi: 10.1200/JCO.2015.63.1010
24. Ueno T, Saji S, Masuda N, Iwata H, Kuroi K, Sato N, et al. Changes in Recurrence Score by Neoadjuvant Endocrine Therapy of Breast Cancer and Their Prognostic Implication. ESMO Open (2019) 4(1):e000476. doi: 10.1136/esmoopen-2018-000476
25. Beitsch P, Whitworth P, Baron P, Pellicane J, Treece T, Yoder E, et al. Genomic Impact of Neoadjuvant Therapy on Breast Cancer: Incomplete Response Is Associated With Altered Diagnostic Gene Signatures. Ann Surg Oncol (2016) 23(10):3317–23. doi: 10.1245/s10434-016-5329-6
Keywords: breast cancer, neoadjuvant chemotherapy, Miller–Payne system, gene expression signature, prognostic
Citation: Wang L, Luo R, Lu Q, Jiang K, Hong R, Lee K, Zhang P, Zhou D, Wang S and Xu F (2021) Miller–Payne Grading and 70-Gene Signature Are Associated With Prognosis of Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Early-Stage Breast Cancer After Neoadjuvant Chemotherapy. Front. Oncol. 11:735670. doi: 10.3389/fonc.2021.735670
Received: 03 July 2021; Accepted: 03 September 2021;
Published: 24 September 2021.
Edited by:
Mariana Segovia, National Autonomous University of Mexico, MexicoReviewed by:
Sahar Hamed, Mansoura University, EgyptYeon Hee Park, Sungkyunkwan University, South Korea
Copyright © 2021 Wang, Luo, Lu, Jiang, Hong, Lee, Zhang, Zhou, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Xu, eHVmZWlAc3lzdWNjLm9yZy5jbg==; Shusen Wang, d2FuZ3Noc0BzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Liye Wang
Liye Wang Rongzhen Luo†
Rongzhen Luo† Kuikui Jiang
Kuikui Jiang Kaping Lee
Kaping Lee Danyang Zhou
Danyang Zhou Shusen Wang
Shusen Wang Fei Xu
Fei Xu