- 1Department of Diagnostic Radiology, Humanitas Clinical and Research Center Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS), Milan, Italy
- 2Otorhinolaryngology Unit, Humanitas Clinical and Research Centre Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS), Milan, Italy
- 3Department of Biomedical Sciences, Humanitas University, Milan, Italy
- 4Department of Supply Chain, Humanitas Clinical and Research Center Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS), Rozzano, Milan, Italy
- 5Department of Computer Science (DI), Università degli Studi di Milano, Milan, Italy
- 6Department of Pathology, Humanitas Clinical and Research Center Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS), Milan, Italy
- 7Department of Radiology, Azienda Socio Sanitaria Territoriale di Bergamo Ovest, Treviglio, Italy
Background: A surgical margin is the apparently healthy tissue around a tumor which has been removed. In oral cavity carcinoma, a negative margin is considered ≥ 5 mm, a close margin between 1 and 5 mm, and a positive margin ≤ 1 mm. Currently, the intraoperative surgical margin status is based on the visual inspection and tissue palpation by the surgeon and intraoperative histopathological assessment of the resection margins by frozen section analysis (FSA). FSA technique is limited and susceptible to sampling errors. Definitive information on the deep resection margins requires postoperative histopathological analysis.
Methods: We described a novel approach for the assessment of intraoperative surgical margins by examining a surgical specimen oriented through a 3D-printed specific patient tongue with real-time Magnetic Resonance Imaging (MRI). We reported the preliminary results of a case series of 10 patients, prospectively enrolled, with oral tongue carcinoma who underwent surgery between February 2020 and April 2021. Two radiologists with 5 and 10 years of experience, respectively, in Head and Neck radiology in consensus evaluated specimen MRI and measured the distance between the tumor and the specimen surface. We performed intraoperative bedside FSA. To compare the performance of bedside FSA and MRI in predicting definitive margin status we computed the weighted sensitivity (SE), specificity (SP), accuracy (ACC), area under the ROC curve (AUC), F1-score, Positive Predictive Value (PPV), and Negative Predictive Value (NPV). To express the concordance between FSA and ex-vivo MRI we reported the jaccard index.
Results: Intraoperative bedside FSA showed SE of 90%, SP of 100%, F1 of 95%, ACC of 0.9%, PPV of 100%, NPV (not a number), and jaccard of 90%, and ex-vivo MRI showed SE of 100%, SP of 100%, F1 of 100%, ACC of 100%, PPV of 100%, NPV of 100%, and jaccard of 100%. These results needed to be validated in a larger sample size of 21- 44 patients.
Conclusion: The presented method allows a more accurate evaluation of surgical margin status, and the first clinical experiences underline the high potential of integrating FSA with ex-vivo MRI of the fresh surgical specimen.
Introduction
A surgical margin is the apparently healthy tissue around a tumor that has been surgically removed. Most commonly, in oral cavity carcinoma, a margin larger than or equal to 5 mm is considered as “negative”, a margin between 1 and 5 mm as “close”, and a margin less than 1 mm as “positive” (1, 2). Radicality and negative margin status represent the most successful outcome in oral cancer surgery. Close or positive margins require re-resection or adjuvant (chemo)radiotherapy contributing to costs, morbidity, and reduced quality of life of the patients who have to undergo these treatments. Mitchell et al. (3) showed that in oral carcinoma five-year survival was 81%, 75%, and 54% for clear, close, and involved margins, respectively, which highlights the importance of clear margins.
Currently, the intraoperative surgical margin status is based on the visual inspection and tissue palpation by the surgeon during surgery and intraoperative histopathological assessment of the resection margins by frozen section analysis (FSA). FSA technique is limited and susceptible to sampling errors (4, 5). Definitive information on the deep resection margins requires postoperative histopathological analysis.
The margin revision of initially positive margins to ‘‘clear’’ based on FSA guidance does not equate to an initially negative margin and does not significantly improve local control. Prospective studies should determine what system of resected specimen analysis best predicts completeness of resection (6).
Real-time Magnetic Resonance Imaging (MRI) on surgical specimen has been used in a few previous studies (7, 8). In particular, Heidkamp et al. (8) showed a positive predictive value (PPV) and negative predictive value (NPV) for Oral Squamous Cell Carcinoma (OSCC) localization of 87-96% and 75-79%, respectively, and a PPV and NPV for identification of margins <5 mm of 5-38% and 87-91%.
When studying correlations between imaging and histological data, the different spatial resolution of the two methods can increase bias, and the evaluation of the piece of the organ as opposed to the whole organ without orientation references could be challenging for the pathologist. The introduction of a 3D-printed anatomic model of the tongue of the patient, obtained from staging MRI for surgical specimen orientation, reproducing the anatomic context from which the specimen has been excised could improve surgeon, pathologist, and radiologist communication in the assessment of margins.
The employment of MRI to examine the surgical specimen oriented through the 3D model could allow for a better macroscopic radial margin evaluation and measurement of the distance to all margins, avoiding sampling errors of FSA.
The purpose of this paper was to report the preliminary results of the diagnostic accuracy of FSA and MRI in evaluating intraoperative surgical margins in oral tongue carcinoma, by examining the surgical specimen oriented through the 3D-printed specific patient tongue model.
Materials and Equipment
We described the steps of the process in a sequential workflow, diagrammed in a flow chart (Figure 1), to clarify how these steps have been executed.
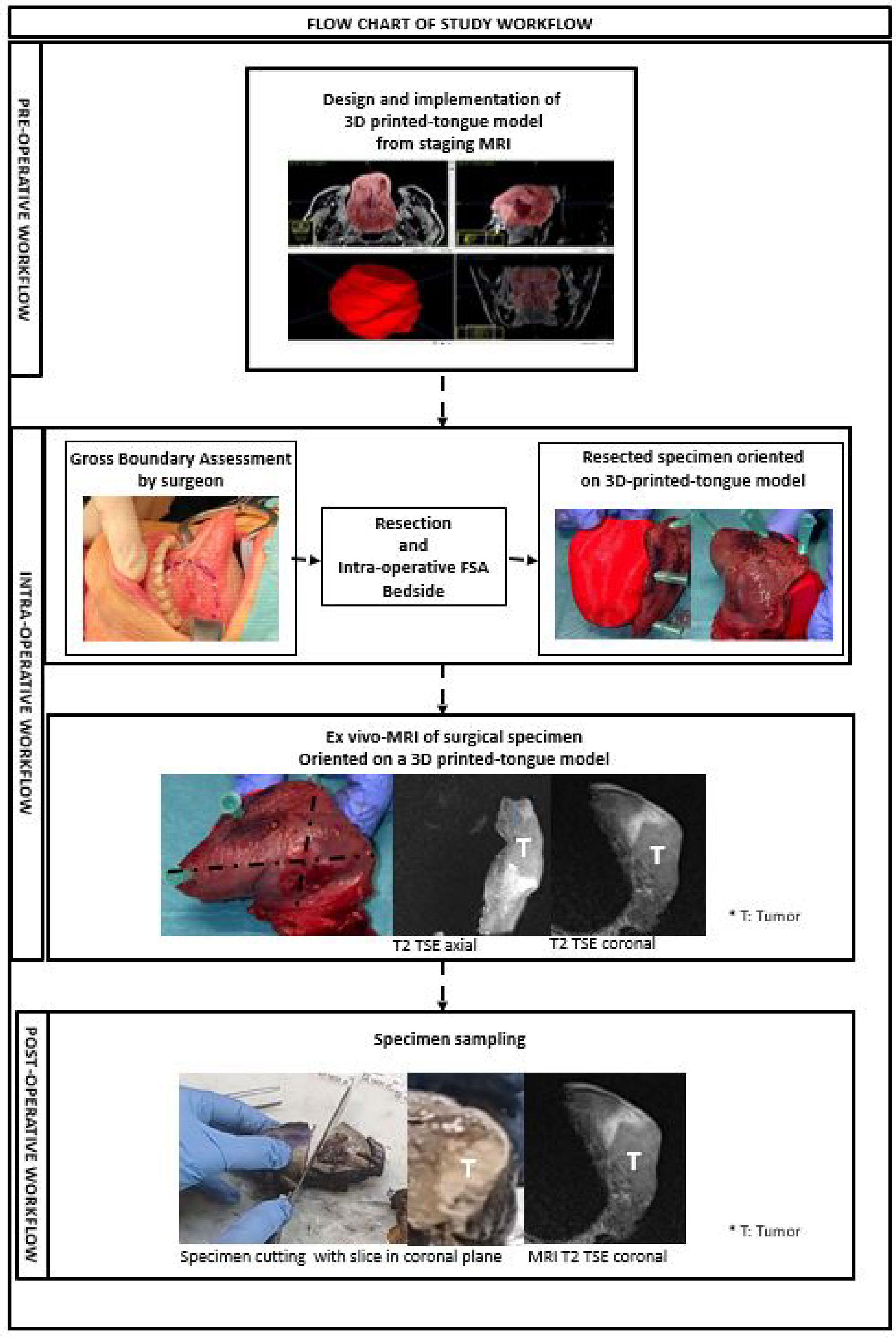
Figure 1 This figure shows pre-operative, intra-operative and post-operative steps of the study workflow.
Pre-Operative Workflow: Design and Implementation of 3D Printed-Tongue Model
We obtained a 3D-printed model of the patient’s tongue on which the area which should have been resected has been reproduced. We implemented the model by the 3D post-contrast fat suppressed gradient-echo T1weighted sequence (VIBE) of staging MRI examinations on a 1.5T MRI System (Magnetom Aera, Siemens Healthineers, Erlangen, Germany) using a phased array surface coil. 3D post-contrast anonymized images demonstrating a good contrast between the tumor and the tongue parenchyma were selected and transferred into ITK-SNAP, a software application used to segment structures in 3D medical images (http://www.itksnap.org/pmwiki/pmwiki.php). The tongue tumor and tongue parenchyma were segmented as separate anatomical regions of interest (ROIs). For all ROIs, both threshold and manual editing were performed to ensure that only the anatomy of interest would be selected. Each ROI was converted to a separate 3D object and combined into a 3D virtual model. The segmentation data, in DICOM format, were converted to STL format so that the 3D printer could recognize them. Surgeons and radiologists segmented tongue tumor, and a 3D virtual model (Figure 2) of the tongue with the pathological area was printed by a 3D printer (VERVE, Kentstrapper, Florence Italy).

Figure 2 Lateral view of the 3D-printed model shows the protuted tongue (inside the dotted line), the floor of the mouth (outside the dotted line), and the tumor in red.
Intraoperative Workflow: Intraoperative FSA and Ex Vivo-MRI of Surgical Specimen Oriented on a 3D Printed-Tongue Model
The surgeon determined the intended boundaries of resection and, following resection, sampled the tumor bed to establish intraoperative margin status. The positive margins at FSA have been radicalized. While maintained at the operating room, fresh specimens were fixed on the 3D-printed model for the correct orientation of the resected surgical specimen (Figures 3, 4). The specimen was immersed in perfluoropolyether (Galden, Solvay Solexis, Thorofare, New Jersey) to eliminate magnetic susceptibility artifacts arising from the air tissue transition (7).The specimen oriented on the 3D model was placed on an MRI phantom with a reference placed on the tumor. A four channel phased array surface carotid coil (Magnetom Aera, Siemens, Erlangen, Germany) was mounted underneath and on top of the 3D model which was positioned in a 1.5 T clinical MRI system (Magnetom Aera, Siemens, Erlangen, Germany). Axial, Coronal, Sagittal T2‐weighted (T2W) turbo spin echo (TSE) sequences (using Field of View (FoV) read 130 mm, FoV phase 100.0%, slice thickness 3.0 mm, TR 3430.0 ms, TE 92.0 ms, Averages 3) and Diffusion weighted spin‐echo echo planar images (using FoV read 140 mm, FoV phase 100.0%, slice thickness 3.0 mm, TR 3500 ms,TE 55.0 ms) were acquired. Apparent diffusion coefficient (ADC) maps were calculated based on acquired b values of 50, 500, and1000 s/mm2 using the standard post processing available on the MRI system.

Figure 3 Superior (A) and Lateral (B) views of surgical specimen of a left hemiglossectomy oriented on a 3D-printed model of the protuted tongue and the floor of the mouth obtained from the staging MRI of the patient.
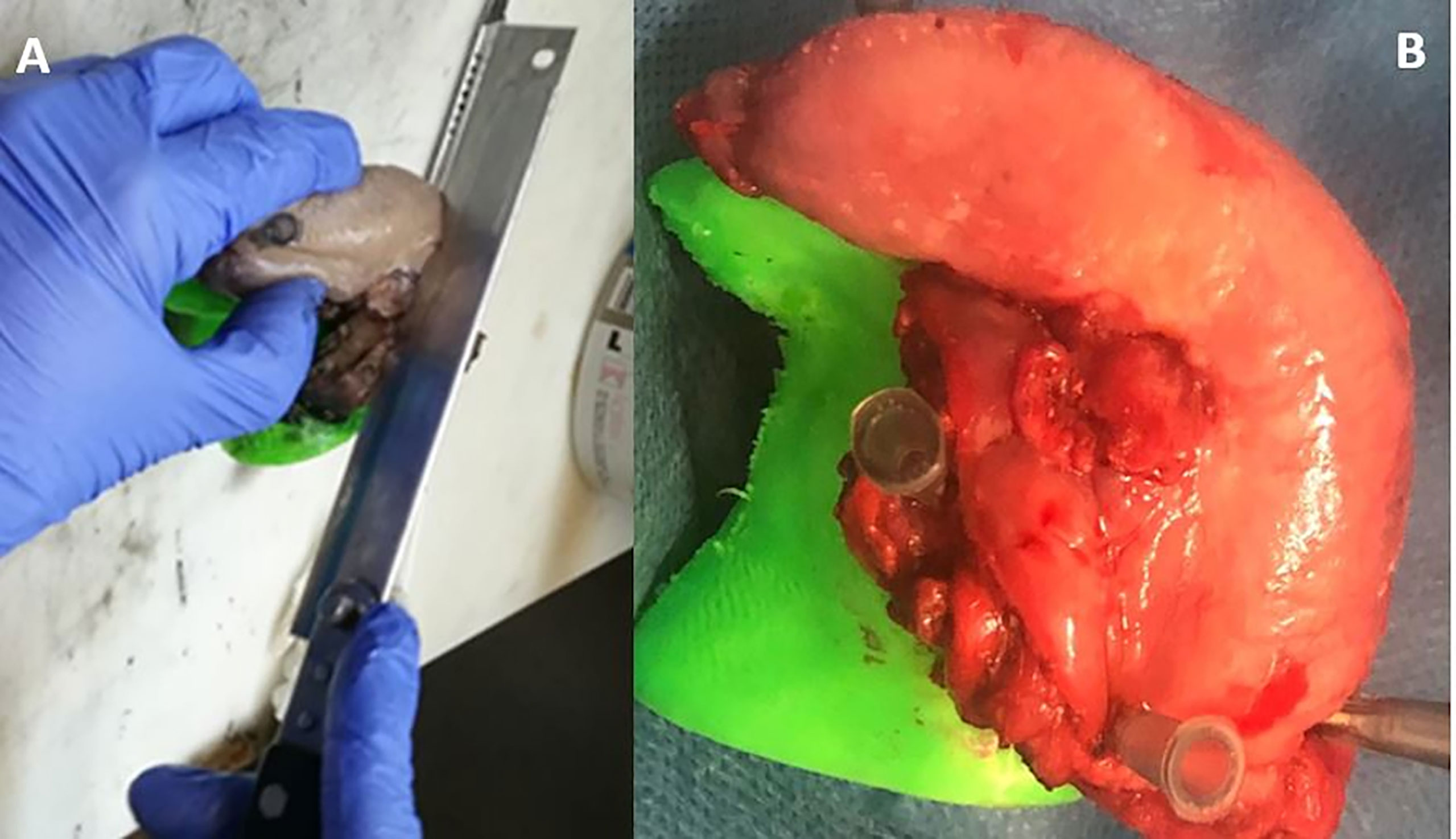
Figure 4 Dissection of the specimen into 3–5 mm slices (A, B) parallel to MRI planes to evaluate the distance from the tumour to the surface of the specimen.
Post-Operative Workflow: Specimen Sampling
Following MRI acquisition, surgical specimen oriented on the 3D-printed tongue model was transported to the pathology laboratory for formalin fixation. Next, the specimen oriented on the 3D model was completely cut up into 4 mm thick slices parallel to the coronal plane of MRI evaluation (Figure 4) and whole-mount paraffin was embedded to evaluate the macroscopic depth of invasion of the tumor and the radial distance of the tumor to all of the margins. In addition to this, the radial margin at the periphery has been submitted. The serial slices of the specimen were sequentially laid out, numbered, and photographed.
Methods
Study Design and Patients
This was a pilot observational prospective mono-institutional cohort study including all consecutive adult patients with a histological diagnosis of OSCC who underwent a primary surgical treatment of lingual resection (hemiglossectomy or partial glossectomy) between February 2020 and April 2021. We prospectively included all the cases with an intraoperative margin status evaluation by FSA and ex-vivo MRI. Institutional review board approval and informed consent for all the patients enrolled were obtained.
Qualitative and Quantitative Analysis of Ex-Vivo MRI
The mean time duration of Ex-vivo MRI examination was 22.7 minutes (range 16-40 minutes).
For the qualitative analysis, two radiologists (CG and LB, dedicated head and neck radiologists with respectively 5 and more than 20 years experience), in consensus, evaluated the image quality of the acquired MRI series, enabling visualization of even small structures.
They, blinded to histological results, radially measured the distance (in millimeters) from all the margins to the tumor (Figure 5).

Figure 5 Coronal, sagittal, and axial T2 TSE MRI sequences of the surgical specimen oriented on a 3D-printed model. Multiplanar MRI sequences show the tumor (T) and its macroscopic radial distance (dotted line) from the surface of the specimen in all the planes.
To differentiate tumor from edema they calculated ADC values within ROI drawn on the tumor and surrounding tissue.
They considered a margin clear if > 5mm, close if =1–5 mm, and involved if <1 mm.
The patients were stratified into three groups depending on the analysis of their margins. The three groups were negative, close, and involved.
Reference Standard: Radio-Pathological Correlation
A dedicated head and neck pathologist with more than of ten years of experience (BF) without knowledge of the MRI results annotated tumor location and measured the distance from the tumor to the margins, which was considered the gold standard.
The serial coronal slices were correlated with the T2 coronal sequences of ex-vivo MRI of specimen oriented on the 3D-printed tongue model (Figures 1, 4).
The patients were stratified into three groups depending on the final analysis of their margins on the specimen. The three groups were negative, close, and involved.
Statistical Analysis
Diagnostic Accuracy
To compare the performance of the initial bedside FSA and ex-vivo MRI in predicting definitive negative, close, and involved margins we computed the weighted sensitivity(SE), specificity(SP), accuracy(ACC), area under the ROC curve (AUC), F1-score, Positive Predictive Value (PPV), and Negative Predictive Value (NPV), where the weights are proportional to the cardinality of each class.
In addition to this, to express the concordance between FSA and ex-vivo MRI, we reported the jaccard index, computed as the number of equal classifications, divided by the number of total samples. Final pathological diagnosis was the gold standard.
Intended Sample Size for Conclusive Results
In a study published in the literature (6), 640 consecutive patients over an 11-year period with at least five years’ follow up were studied. A total of 213 patients (33%) had resection margins that were clear (5 mm or more), 314 (49%) were close (1 - 4.9 mm), and 113 (18%) were involved (0 - 0.9 mm). The required sample size was determined in order to detect a significant difference with an accuracy 0.2-0.3 times greater than 0.50 (which practically corresponds to a random classifier) with a power of 0.90. Assuming equal variance, a total of 21-44 patients should be enrolled to report conclusive results.
Results
Participants
We enrolled 10 patients (6 females and 4 males with a median age 53.1 years, range 30-89) with a histological diagnosis of squamous cell carcinoma of the oral cavity (OSCC) who underwent surgery (5 hemiglossectomies and 5 partial glossectomies) for oral tongue squamous carcinoma between February and April 2021. The definitive margin status was negative in nine cases and positive in one case. In ex-vivo MRI, margin status was negative in nine cases and positive in one case (revision was not performed in this case because FSA was negative). The final pathological T status was T1 in one case, T2 in seven cases, and T3 in two cases. In the 10 cases, the mean maximum diameter of the tumor at the final histological diagnosis was 22.3 (range 10-37 mm). The average time taken for each MRI examination was 24.7 minutes (range 16-38 minutes). The T2 series of MRI was therefore the series that was matched to histology and was subsequently annotated.
Preliminary Results
MRI succeeded in margin prediction in all the cases, and FSA failed in one case.
In this group of patients initial bedside FSA showed SE of 90%, SP of 100%, F1 of 95%, ACC of 90%, PPV of 100%, NPV (not a number), and jaccard of 90%, and ex-vivo MRI showed SE of 100%, SP of 100%, F1 of 100%, ACC of 100%, PPV of 100%, NPV of 100%, and jaccard of 100%.
These results needed to be validated in a larger sample size of 21- 44 patients.
Discussion
Intraoperative assessment of the resection margins can provide valuable information, enabling additive resection to obtain negative margin status. Despite this, the clinical value and the method of assessing the intraoperative margin are not well defined (5, 9, 10). Many factors influence the evaluation of surgical margins, making it more or less adequate; these include the sampling of the margins (from the block sample compared to the surgical defect alone), the ability and methods used to determine the distance to margins, the communication between the surgeon and the pathologist involving the specimen, orientation and areas of concern, and the subsite in the head and neck (11).
Several techniques aiming for intraoperative assessment of surgical margins in oral cavity/tongue squamous cell carcinoma have been investigated. These include elastic scattering spectroscopy (12) fluorescence (13–15), hyperspectral imaging (16), optical coherence tomography (17), spectroscopy (18), ultrasound (19, 20) and intraoperative slicing of the whole specimen by the pathologist (21). Only MRI, ultrasound, and intraoperative slicing of the whole specimen by the pathologist can allow sampling of the entire specimen and/or probing depth of the lesion.
With our study, we have found it helpful to report and demonstrate to our surgical team the gross distance to all margins by using the intraoperative ex- vivo MRI, and to improve surgeon and pathologist communication by introducing a 3D-printed tongue model to allow pathologists to understand specimen orientation and to learn what margins will be revised based on gross impression.
To our knowledge this is the first study in which a surgeon provided orientation of the specimen on the 3D-printed model of the tongue with the reproduced bed of resection. Generally, the surgeon provides orientation by designating one or two points on the specimen.
The specimen orientation on the 3D-printed model from patient MRI facilitates review and correlation.
By maintaining the orientation of the specimen, the pathologist is facilitated in noting the distance of the tumor from each margin and in communicating the site of positive and close margins to the submitting surgeon.
While most surgeons sample margins only from the surgical bed without margin assessment from the resection specimen, as demonstrated by a survey of American Head and Neck Society members (22), we introduced ex-vivo MRI to outcome the limit of the lack of a true measurement of the distance of the invasive tumor from the resection margin. According to FSA, a margin can only be determined as positive or negative and the margin presented separately can be thin <5 mm in thickness. Additionally, without a defined margin orientation it is not clear how the separately submitted margins reflect the true areas of the en block specimen that are noted to be close or positive. In our preliminary experience, ex vivo MRI was more accurate than intraoperative FSA in predicting margin status.
The results of a previous study (7) showed high specificity and low sensitivity of MRI in identifying margins less than 5 mm. According to these results, ex-vivo MRI assessment of the pathologic en bloc specimen could direct intra-operative defect-derived FSA margin assessment and margin revision, determining the closest margins on gross assessment and requiring separately submitted tissues of correct size to have negative margins. The orientation of the specimen on the 3D model could allow a better match between the separately submitted margins and the true areas of the en bloc specimen that are noted to be close or positive. This could be because the revised margin is not taken from the correct location (23).
Some limitations of our study should be discussed. First, the sample size was small and heterogeneous. Our cohort contained a broad range of cases with various T classifications. Furthermore, a relatively small proportion of the margins were less than 5 mm. As established in our statistical analysis on intended sample size, our preliminary results should be validated in a larger cohort of patients.
The limitation of ex- vivo MRI could be the lack of identification of microscopic tissue changes. Conventional MRI could not eneable thin differentiations between tumor and surrounding tissue in the presence of edema. Diffusion weighted imaging (DWI) can be used to evaluate the rate of microscopic water diffusion within tissues. DWI may be measured by means of apparent diffusion coefficient (ADC). Areas of decreased ADC values within tumors could be used as a powerful imaging biomarker of cancer (24, 25).
Tumor shrinkage after formalin fixation could lead to an underestimation of tumor margins (26). This could be demonstrated by performing ex-vivo MRI of the specimen after formalin fixation. We performed MRI after formalin fixation in one case but the changes of the signal intensity of the specimen did not allow us to evaluate the effect of shrinkage.
Other limitations were the inexperience of the MRI readers, who had experience with in vivo applications of MRI but not in ex-vivo MRI of tongue resection specimens, and the fact that only one pathologist evaluated histopathology.
Ex-vivo MRI is a costly and time consuming process. It requires the use of MRI equipment, subtracting time for performing diagnostic tests. It also requires excellent communication and coordination between surgeons, operating room staff, pathologists, and radiologists to avoid additional operating time. Considering that the operating times for these types of complex resections and reconstructions, with the maximum operating time of 160 minutes in which flap reconstruction was not required and 700 minutes of microvascular reconstruction, MRI does not unreasonably lengthen the time in the operating room (21). This intervention has the potential to both reduce rates of close pathological margins and the need for postoperative radiotherapy, as shown in our preliminary experience with the detection of positive margins not detected by intraoperative FSA.
In conclusion, considering the staffing and expensive nature of integrating FSA with ex-vivo MRI of the fresh specimen for evaluation on macroscopic proximity of the tumor to the margins of resection, this intervention can be performed with some planning and coordination between the radiologists, pathologists, and surgeons in a selected subset of patients most likely to benefit by avoiding adjuvant radiotherapy. Finally, we would recommend consideration of intraoperative MRI tumor margin assessment for selected cases potentially be cured with surgery alone, i.e. T1/T2N0 tumor.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors by contacting the corresponding author.
Ethics Statement
The study was reviewed and approved by the Humanitas Research Hospital, Rozzano. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CG, LB, GS, and GM conceived of the presented idea. CG developed the theory and performed the computations. CG, GM, LB, and FF verified the analytical methods. GS encouraged CG to investigate margin status and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript. CG, GM, LD, FC, MC, FG, RB, and BF carried out the experiment. CG wrote the manuscript with support from GM. RB and PO fabricated the 3D model sample. AD, FR, AE, FF, and LT helped supervise the project. CG and EC developed the theoretical formalism, performed the analytic calculations and performed the numerical simulations and contributed to the interpretation of the results. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge our radiology technicians for the acquisition of MRI examinations (Giuseppe Morrone, Pasquala Ragucci, Giacomo Marconi, Riccardo Ramberti, Giulio Belice, Riccardo Fabbri) coordinated by Simona Superbi and Antonio Spinillo; all the ENT surgeons of Humanitas Clinical and Research Center IRCCS; Clarissa Nigro of the nursing staff for the coordination of the workflow between the operating room and the MRI room; and Giovanna Giannitto for English language editing and Massimiliano Battaglia and Federica Fici for editing.
References
1. Helliwell T, Woolgar J. Standards and Datasets for Reporting Cancers [Internet]. In: Dataset for Histopathology Reportingofmucosal Malignancies of the Oral Cavity. Carlton House Terrace, London: The Royal College of Pathologists 2. (2013). Available at: https://www.rcpath.org/uploads/assets/c4a9faf7-393a-4ba8-9532f719d8cdff3b/Dataset-for-histopathology-reporting-of-mucosal-malignancies-of-the-oral-cavity.pdf.
2. Hinni ML, Ferlito A, Brandwein-Gensler MS, Takes RP, Silver CE, Westra WH, et al. Surgical Margins in Head and Neck Cancer: A Contemporary Review. Head Neck (2013) 35(9):1362–70. doi: 10.1002/hed.23110
3. Mitchell DA, Kanatas A, Murphy C, Chengot P, Smith AB, Ong TK. Margins and Survival in Oral Cancer. Br J Oral Maxillofac Surg (2018) 56(9):820–9. doi: 10.1016/j.bjoms.2018.06.021
4. DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, Utility,and Cost of Frozen Section Margins in Head and Neck Cancersurgery. Laryngoscope (2000) 110(10):1773–6. doi: 10.1097/00005537-200010000-00039
5. Gerber S, Gengler C, Grätz KW, Kruse AL. The Impact of Frozensections on Final Surgical Margins in Squamous Cell Carcinoma of the Oral Cavity and Lips: A Retrospective Analysis Over an 11 Years Period. Head Neck Oncol (2011) 3(1):56. doi: 10.1186/1758-3284-3-56
6. Azzopardi S, Lowe D, Rogers S. Audit of the Rates of Re-Excision for Close or Involved Margins in the Management of Oral Cancer. Br J Oral Maxillofacial Surg (2019) 57(7):678–81. doi: 10.1016/j.bjoms.2019.05.006
7. Steens SCA, Bekers EM, Weijs WLJ, Litjens GJS, Veltien A, Maat A, et al. Evaluation of Tongue Squamous Cell Carcinoma Resection Margins Using Ex-Vivo MR. Int J Comput Assist Radiol Surg (2017) 12(5):821–8. doi: 10.1007/s11548-017-1524-6
8. Heidkamp J, Weijs WLJ, van Engen-van Grunsven ACH, de Laak-de Vries I, Maas MC, Rovers MM, et al. Assessment of Surgical Tumor-Free Resection Margins in Fresh Squamous-Cell Carcinoma Resection Specimens of the Tongue Using a Clinical MRI System. Head Neck (2020) 42(8):2039–49. doi: 10.1002/hed.26125
9. Gokavarapu S, Chandrasekhara Rao LM, Patnaik SC, Parvataneni N, Raju KVVN, Chander R. Prognostic Value of Frozen Section in T1, T2 Carcinoma of Oral Cavity. Indian J Otolaryngol Head Neck Surg (2015) 67(Suppl 1):86–90. doi: 10.1007/s12070-014-0783-6
10. Chaturvedi P, Datta S, Nair S, Nair D, Pawar P, Vaishampayan S, et al. Gross Examination by the Surgeon as an Alternative to Frozen Section for Assessment of Adequacy of Surgical Margin in Head and Neck Squamous Cell Carcinoma: Gross Examination Versus Frozen Section for Assessing Surgical Margin of HNSCC. Head Neck (2014) 36(4):557–63. doi: 10.1002/hed.23313
11. MD. Determining Adequate Margins in Head and Neck Cancers: Practice and Continued Challenges. Curr Oncol Rep (2016) 18(9):54. doi: 10.1007/s11912-016-0540-y
12. Grillone GA, Wang Z, Krisciunas GP. The Color of Cancer: Margin Guidance for Oral Cancer Resection Using Elastic Scatteringspectroscopy. Laryngoscope (2017) 127(suppl 4):S1–9. doi: 10.1002/lary.26763
13. JM, de Boer E, van Dam GM, Moore LS, Bevans SL, Walsh EM, et al. Fluorescence Imaging to Localize Head and Neck Squamous Cell Carcinoma for Enhanced Pathological Assessment: Fluorescence-Guided Pathology. J Pathol Clin Res (2016) 2(2):104–12. doi: 10.1002/cjp2.40
14. van Keulen S, Nishio N, Birkeland A, Fakurnejad S, Martin B, Forouzanfar T, et al. The Sentinel Margin: Intraoperative Ex Vivo Specimen Mapping Using Relative Fluorescence Intensity. Clin Cancer Res (2019) 25(15):4656–62. doi: 10.1158/1078-0432.CCR-19-0319
15. RW, Teraphongphom NT, Berg NS. Determinationof Tumor Margins With Surgical Specimen Mapping Using Near-Infrared Fluorescence. Cancer Res (2018) 78(17):5154. doi: 10.1158/0008-5472.CAN-18-0878
16. Fei B, Lu G, Wang X, Zhang H, Little JV, Patel MR, et al. Label-Free Reflectance Hyperspectral Imaging for Tumor Margin Assessment: A Pilot Study on Surgical Specimens of Cancer Patients. J BioMed Opt (2017) 22(8):1–7. doi: 10.1117/1.JBO.22.8.086009
17. Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C. Opticalcoherence Tomography in the Assessment of Oral Squamous Cell Carcinoma Resection Margins. Photodiagnosis Photodyn Ther (2016) 13:211–7. doi: 10.1016/j.pdpdt.2015.07.170
18. Barroso EM, Smits RWH, van Lanschot CGF, Caspers PJ, Ten Hove I, Mast H, et al. Water Concentration Analysis by Raman Spectroscopy to Determine the Location of the Tumor Border in Oral Cancer Surgery. Cancer Res (2016) 76(20):5945–53. doi: 10.1158/0008-5472.CAN-16-1227
19. O, Kanumuri V, Juliano AF, Faquin WC, Cunnane ME, Varvares MA. Intraoperative Ultrasound in Oral Tongue Cancer Resection: Feasibility Study and Early Outcomes. Otolaryngol Head Neck Surg (2018) 158(4):645–8. doi: 10.1177/0194599817742856
20. de Koning KJ, Koppes SA, de Bree R, Dankbaar JW, Willems SM, van Es RJJ, et al. Feasibility Study of Ultrasound-Guided Resection of Tongue Cancer With Immediate Specimen Examination to Improve Margin Control - Comparison With Conventional Treatment. Oral Oncol (2021) 116(105249):105249. doi: 10.1016/j.oraloncology.2021.105249
21. Smithers FAE, Haymerle G, Palme CE, Low T-HH, Froggatt C, Gupta R, et al. A Prospective Study of Intraoperative Assessment of Mucosal Squamous Cell Carcinoma Margins in the Head and Neck. Head Neck (2021) 43(2):590–600. doi: 10.1002/hed.26517
22. Meier JD, Oliver DA, Varvares MA. Surgical Margin Determination in Head and Neck Oncology: Current Clinical Practice. The Results of an International American Head and Neck Society Member Survey. Head Neck (2005) 27(11):952–8. doi: 10.1002/hed.20269
23. Scholl P, Byers RM, Batsakis JG. Microscopic Cut-Through of Cancer in the Surgical Treatment of Squamous Carcinoma of Thetongue. Prognostic Ther Implications Am J Surg (1986) 152(4):354–60. doi: 10.1016/0002-9610(86)90304-1
24. Hermans R, de keyzer F, Vandecaveye V, Carp L. Imaging Techniques. In: Head and Neck Cancer Imaging. Berlin/Heidelberg, Germany: Springer (2012). p. 33–54.
25. Bonello L, Preda L, Conte G, et al. Squamous Cell Carcinoma of the Oral Cavity and Oropharynx: What Does the Apparent Diffusion Coefficient Tell Us About Its Histology? Acta Radiol (2016) 57(11):1344–51. doi: 10.1177/0284185115587734
Keywords: head and neck, virtual surgical planning, 3D printing, tumor, resection, surgical margins, ex-vivo
Citation: Giannitto C, Mercante G, Disconzi L, Boroni R, Casiraghi E, Canzano F, Cerasuolo M, Gaino F, De Virgilio A, Fiamengo B, Ferreli F, Esposito AA, Oliva P, Ronzoni F, Terracciano L, Spriano G and Balzarini L (2021) Frozen Section Analysis and Real-Time Magnetic Resonance Imaging of Surgical Specimen Oriented on 3D Printed Tongue Model to Assess Surgical Margins in Oral Tongue Carcinoma: Preliminary Results. Front. Oncol. 11:735002. doi: 10.3389/fonc.2021.735002
Received: 01 July 2021; Accepted: 10 November 2021;
Published: 09 December 2021.
Edited by:
Richard Yuxiong Su, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Marco Artico, Sapienza University of Rome, ItalyAkshat Malik, Max Institute of Cancer Care, India
Copyright © 2021 Giannitto, Mercante, Disconzi, Boroni, Casiraghi, Canzano, Cerasuolo, Gaino, De Virgilio, Fiamengo, Ferreli, Esposito, Oliva, Ronzoni, Terracciano, Spriano and Balzarini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caterina Giannitto, Y2F0ZXJpbmEuZ2lhbm5pdHRvQGh1bWFuaXRhcy5pdA==
†These authors share first authorship
‡These authors share senior authorship
 Caterina Giannitto
Caterina Giannitto Giuseppe Mercante
Giuseppe Mercante Luca Disconzi1,3
Luca Disconzi1,3 Elena Casiraghi
Elena Casiraghi Fabio Ferreli
Fabio Ferreli Flavio Ronzoni
Flavio Ronzoni