
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 26 August 2021
Sec. Skin Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.733917
This article is part of the Research Topic The Evolving Role of Immunotherapy in Non-Melanoma Skin Cancers View all 12 articles
 Andrea Boutros1,2*
Andrea Boutros1,2* Federica Cecchi1
Federica Cecchi1 Enrica Teresa Tanda1,3
Enrica Teresa Tanda1,3 Elena Croce1,2
Elena Croce1,2 Riccardo Gili1,2
Riccardo Gili1,2 Luca Arecco2,4
Luca Arecco2,4 Francesco Spagnolo1†
Francesco Spagnolo1† Paola Queirolo5†
Paola Queirolo5†Cutaneous squamous cell carcinoma (CSCC) accounts for approximately 20% of all keratinocytic tumors. In most cases, the diagnosis and treatments are made on small, low-risk lesions. However, in about 5% of cases, CSCC may present as either locally advanced or metastatic (i.e. with locoregional lymph nodes metastases or distant localizations). Prior to the introduction of immunotherapy in clinical practice, the standard treatment of advanced CSCC was not clearly defined, and up to 60% of patients received no systemic therapy. Thanks to a strong pre-clinical rationale, clinical trials led to the FDA (Food and Drug Administration) and EMA (European Medicines Agency) registration of cemiplimab, a PD-1 inhibitor that achieved encouraging results in terms of objective response, overall survival, and quality of life. Subsequently, the anti-PD-1 pembrolizumab received the approval for the treatment of advanced CSCC by the FDA only. In this review, we will focus on the definition of advanced CSCC and on the current and future therapeutic options, with a particular regard for immunotherapy.
Cutaneous squamous cell carcinoma (CSCC) is a non-melanoma skin cancer of keratinocytic origin, and accounts for approximately 20% of all keratinocytic cancers, standing as the second most common neoplasm after basal cell carcinoma (BCC) (1). The main risk factors are chronic exposure to ultraviolet (UV) radiation, followed by age, fair phototype and immunosuppression [specifically related to solid organ transplantation (2), chronic lymphocytic leukemia (3), and HIV infection (4)] (5). Other risk factors like the exposure to arsenic and polyaromatic hydrocarbons can be considered occupational (6).
CSCC is characterized by a high tumor mutational burden (TMB) (7) with a large amount of UV radiation-related mutations, most notably C>T and CC>TT dinucleotide mutations (8). However, genetic mutations that could lead to a targeted treatment are infrequent, and may include PIK3CA, fibroblast growth factor receptor 3 (FGFR3), BRAF and EGFR (9).
Some hereditary syndromes may increase the risk of developing CSCC such as xeroderma pigmentosum, epidermolysis bullosa, oculocutaneous albinism, Lynch syndrome, and Fanconi syndrome (1).
Due to the heterogeneity of clinical and histologic presentations, therapeutic options, and low mortality rates, accurate data on the incidence and prevalence of CSCC are not available to-date. In Australia, where the highest incidence of skin cancer is generally recorded, there are an estimated 387 cases per 100,000 (10). In the United States, more than 700.000 new cases of CSCC are diagnosed annually, and about 3900-8800 people die each year due to this disease (11). In Europe, the incidence of CSCC ranges across different latitudes from 9 to 96 per 100.000 for male individuals and 5 to 68 per 100.000 for females (12–15).
In more than 90% of cases, the prognosis is good and treatment consists of minimally invasive surgical procedures or, in selected cases, other local therapy modalities (16). In case of primary CSCC for which curative surgery is not indicated, definitive radiotherapy (RT) may be considered as a primary treatment. Despite the lack of randomized trials comparing the outcomes of RT versus surgery and other local therapy modalities, in a systematic review and pooled analysis of 7 observational studies for a total of 761 primary CSCCs, the local relapse with RT was as low as 6.4% (17). However, especially in the immunocompromised patient population, in case of social difficulties, lack of caregiver support, and/or in presence of multiple comorbidities, CSCC can manifest in locally advanced or metastatic forms representing an emerging clinical problem (5). In these cases, local treatments are no longer indicated to achieve an appropriate disease control. Until few years ago, the only available therapeutic options were chemotherapy and targeted therapy (i.e., EGFR inhibitors), with poor response rates and duration of response, and frequently at the cost of unacceptable toxicities for such a frail population. With the approval by the Food And Drug Administration (FDA) and European Medicines Agency (EMA) of the anti-PD-1 cemiplimab in 2018, and of the anti-PD-1 pembrolizumab by the FDA only in 2020, immunotherapy has become the standard of care for patients with CSCC who are not eligible for curative surgery or radiotherapy (18).
In this review, we will discuss the main criteria for the identification of CSCC patients who are at high risk of relapse, and the multidisciplinary definition of locally advanced CSCC, according to the most recent guidelines. In addition to that, the results of main systemic treatment regimens will be discussed, with a focus on immunotherapy, especially regarding the key findings on the new therapeutic options and future therapeutic landscapes.
In most cases, CSCCs are detected as small or early-stage lesions that have a low risk of recurrence after an appropriate surgical treatment (16). Specifically, the overall recurrence rate has been shown in several studies to be between 2.1% and 4.6% (19). Although only few CSCC have a high risk of local or distant recurrence, it is essential to identify the high-risk patient group for a proper diagnostic and therapeutic workup, and an individualized follow-up. Risk factors can be either tumor-related (clinical or pathological) or patient-related, as indicated by the European Dermatology Forum (EDF), European Association of Dermato-Oncology (EADO), and European Organization for Research and Treatment of Cancer (EORTC) guidelines (20, 21). However, the impact of each individual risk factor is not entirely clear. In a recent meta-analysis, published data on risk factors for recurrence, metastasis, and disease-specific death of CSCC were systematically analyzed. The main results of this work were summarized in Table 1 (22). Briefly, tumor depth was associated with the highest risk ratio of local recurrence and metastasis, while a tumor diameter > 20 mm was associated with the highest risk ratio of disease-specific death (22).
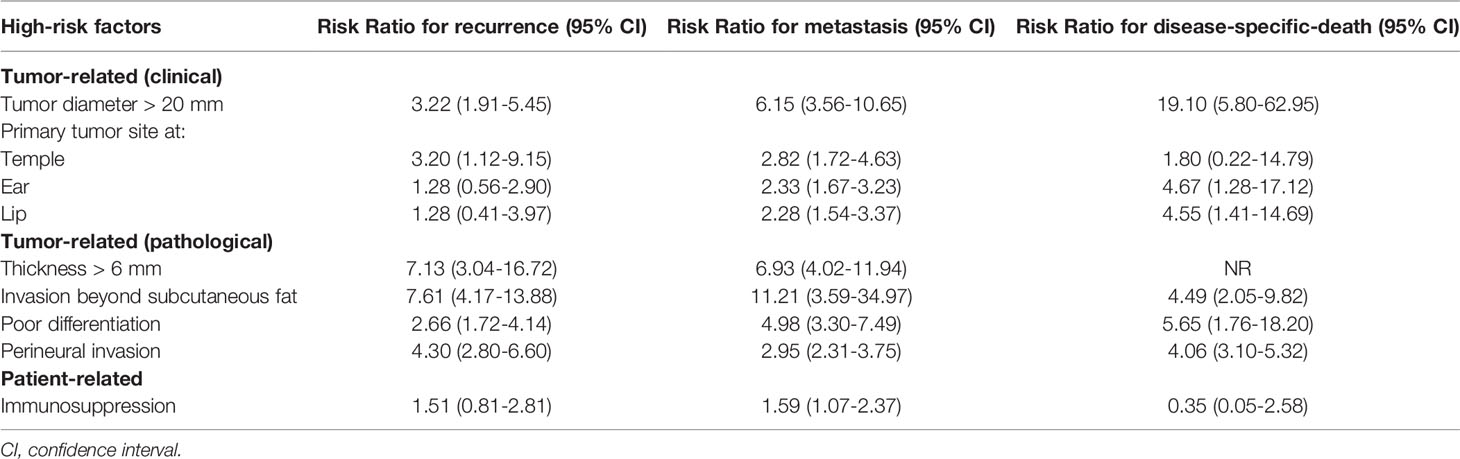
Table 1 Risk ratios for recurrence, metastasis, and disease-specific death for some of the most relevant high-risk factors (22).
There are several available staging systems for CSCC but each of them presents some important pitfalls and may not be able to fully provide an adequate risk stratification for all cases. The American Joint Committee on Cancer (AJCC) 8th edition classification does not perform well especially regarding T stage, as few tumors fit the criteria for T4, but most T2 tumors actually turn out to be associated with poor outcomes (23). Brigham and Women’s Hospital (BWH) and the Breuninger systems are more accurate in stratifying the risk of T stage but are limited to the classification of primary tumors only (24). Finally, neither the AJCC nor the BWH staging systems consider immunosuppression, which is included as a major high-risk factor in the EADO and NCCN guidelines (20, 21, 25). Indeed, immunosuppression associated with conditions such as solid organ transplantation (26), HIV infection, and chronic lymphocytic leukemia (CLL), is not only a risk factor for increased incidence of CSCC, but also a risk factor for a more unfavorable outcome (20). Therefore, further efforts are needed to develop a dedicated classification for CSCC that could be more useful in daily clinical practice for risk stratification and early identification of high-risk CSCC (20).
Advanced CSCC is defined as a tumor for which neither surgery nor radiation therapy with curative intent is indicated (21). This broad definition is driven by the fact that there is no precise consensus on when CSCC can be considered advanced (27). In addition, contraindication to surgery or radiation therapy with curative intent may be due to several reasons which include not only the anatomic extent of the tumor, but also the patient’s clinical condition, comorbidities, the risk of mutilation or severe functional loss due to the surgery, previous treatments performed, and patient preference (27).
The advanced form can be divided into locally advanced and metastatic (loco-regional and distant). Advanced forms are considered rare; it is estimated that only about 5% of total CSCC cases may become advanced, with the limitations of missing epidemiologic data (20). Unfortunately, while the definition of metastatic CSCC (mCSCC) implies the dissemination of tumor cells through locoregional lymph nodes or both distant lymph node and other visceral sites, there are no precise parameters for defining the locally advanced forms, and a multidisciplinary discussion is essential for defining the best diagnostic and therapeutic strategies. In general, a locally advanced CSCC (laCSCC) is a tumor which is no longer eligible for either surgery or curative radiation therapy due to multiple recurrences, large extension, bone erosion and/or deep infiltration beyond the subcutaneous tissue into muscles/nerve. Moreover, the definition of laCSCC could fit tumor masses where curative resection may lead to unacceptable complications, morbidity or deformity (27). Finally, multiple CSCCs related to genetic syndromes as xeroderma pigmentosum and those related to chronic conditions such as chronic lymphocytic leukemia (CLL) may be included in these criteria (27). Patient-related features, such as age, comorbidities and patient preferences, may also play a role in the choice of either surgery or immunotherapy.
Before immunotherapy, in addition to palliative radiotherapy, chemotherapy and targeted therapy with EGFR (Epidermal Growth Factor Receptor) inhibitors were the only available therapeutic options for advanced CSCC (21). In particular, chemotherapy can be considered in different treatment settings depending on the therapeutic purpose: (1) curative intent concurrent with radiation therapy, based on squamous cell carcinoma of the head and neck (HNSCC) clinical trials data. In fact, Pignon and colleagues published a meta-analysis conducted on 17,346 patients with HNSCC, demonstrating a survival benefit of concurrent chemoradiation (28). Notably, this benefit was not significant in the population over 70 years of age (28); (2) postoperative concomitant chemoradiation. A chemoradiation approach versus radiotherapy alone in a postoperative setting has been evaluated in a study including a population with at least one of the following high-risk features: intraparotid nodal disease, cervical nodal disease, primary tumor > 5 cm, primary tumor invading surrounding cartilage, skeletal muscle, or bone, and in-transit metastases. The study showed no differences between the two study arms in terms of either locoregional relapse or OS (29); (3) palliative intent, with questionable benefit in terms of quality of life (QoL) and overall survival (OS). Retrospective data showed that platinum derivatives appear to be the most active drugs in terms of progression-free survival (PFS) of 9.8 months and OS of 15.2 months (30). Other therapeutic options may be fluoropyrimidines (capecitabine), taxanes, bleomycin, adriamycin, and methotrexate, with PFS of approximately 5.5 months and OS of 10.9 months (30).
Regarding EGFR inhibitors, there are limited data in the curative and postoperative setting. Specifically, postoperative cetuximab concurrent with radiotherapy (n=29) versus radiation therapy alone (n=39) in patients with high-risk head and neck CSCC (high grade of differentiation, perineural or lymphovascular invasion, positive surgical margins, lymph node involvement, tumor recurrence, immunosuppression, localization to ear, cheek, lip), showed an advantage in terms of both freedom from local recurrence and freedom from distant recurrence (31). In the advanced setting, a phase II study including 36 patients with CSCC showed a response rate of 28% with a median duration of response of 6.8 months (32). Similar results were also observed with dacomitinib, with grade 3-4 adverse events being observed in 36% of patients, and 16% of patients discontinuing treatment because of drug-related toxicity (33). Finally, in a large retrospective case series, both chemotherapy and targeted therapy for the treatment of advanced CSCC showed response rates of less than 20%, with overall survivals of less than 20 months (34).
In summary, these treatment approaches were unsatisfactory, both in their impact on survival and quality of life (21), and a standard regimen for the treatment of advanced CSCC was not clearly defined, with up to 60% of patients with locally advanced CSCC not receiving any systemic therapy at all (35).
The therapeutic paradigm of CSCC has been radically changed in recent years with the introduction of immunotherapy (21). For this reason, it is crucial to discuss each advanced case in a multidisciplinary setting to properly balance the risks and benefits of this treatment in a population commonly affected by severe comorbidities and to assess the most appropriate therapeutic strategy.
Immunotherapy is considered the breakthrough in the treatment of advanced CSCC. The available clinical evidence is supported by a strong preclinical rationale. UV radiation is the most relevant risk factor for CSCC, which in fact is among the tumors with the highest rate of somatic mutations (36). The high tumor mutational burden (TMB) sets the background for a large number of neoantigens that can be recognized by the immune system. The high number of somatic mutations found in CSCC provided the strong biological rationale for the development of immunological therapies. Indeed, several studies observed that CSCC is the tumor with the highest TMB (7), with a linear relationship between tumor mutational burden and immunotherapy efficacy (37). Moreover, CSCC is a typical tumor of the elderly, with a mean age of onset of 70 years, while it is extremely rare in subjects younger than 45 years of age. Some evidence suggests that the chance of obtaining benefit from immunotherapy may increase with age. In a study involving more than 500 melanoma patients treated with PD-1 inhibitors, the risk of disease progression decreased by 13% for each decade of age (38). Finally, CSCC is characterized by high expression of Programmed Death-Ligand 1 (PD-L1) (39). The interaction of this ligand with Programmed Death-1 (PD-1) results in the inhibition of the anti-tumor immune T cell response (40). This immune checkpoint is exploited by cancer cells to escape the immune response and is one of the mechanisms underlying the rationale for the use of PD-1 inhibitors in the treatment of CSCC.
One of the first clinical evidence supporting the use of the anti-PD-1 immunotherapy for the treatment of advanced CSCC was provided by the CARSKIN trial, where first-line therapy with pembrolizumab in patients with unresectable CSCC demonstrated an objective response rate at week 15 of treatment (ORRW15) of 55% in PD-L1+ patients versus 17% in PD-L1- patients (41). In the subsequent phase II KEYNOTE-629 trial, 105 patients with locally advanced, metastatic, or relapsed CSCC received pembrolizumab as a first-line treatment (13%) or subsequent to another systemic therapy (87%) achieving an ORR of 34% and disease control rate (DCR) of 52%. The safety profile was also acceptable and consistent with that observed in previous trials with pembrolizumab (42). As already mentioned, the results of this phase 2 trial led to the approval by the FDA of pembrolizumab for the treatment of advanced CSCC.
Before that, cemiplimab was approved by the FDA in 2018, and then by EMA, for the treatment of both mCSCC (nodal or distant metastases) and laCSCC (locally advanced) which are not eligible for curative surgery or radiation therapy, following the results of a phase 1 study that showed durable responses in 50% of 26 treated patients (18). These results were confirmed in the phase 2, open-label, non-randomized EMPOWER-CSCC 1 trial, where 193 patients with advanced, non-eligible for curative surgery or radiotherapy CSCC were enrolled. In the locally advanced CSCC group, patients were considered non-eligible for surgery if the anatomical location of the tumor would cause serious functional and aesthetic consequences (38% of cases). Other causes of inoperability were previous recurrences of the same lesion (32%) and the impossibility to obtain a complete surgical resection due to severe local invasiveness (26%). The most frequent cause of contraindication to radiotherapy was an unfavorable risk/benefit ratio (49% of cases) (18). At the last update presented at ASCO Annual Meeting 2020, the pooled ORR was 46.1% with a DCR of 72.5% (43). Clinical activity was observed regardless of PD-L1 expression (43). In addition, approximately half of patients achieved an anti-tumor response within the first 2 months, and nearly 80% within the first 4 months (43). The study showed that patients with laCSCC receiving cemiplimab after more than one recurrence after surgical excision had less than half the probability of achieving a response if compared to patients receiving upfront immunotherapy (43). This makes it essential, in the case of lesions that are potentially resectable but for which a curative outcome cannot be reasonably expected with surgery (i.e., in the presence of major risk factors), a careful multidisciplinary evaluation considering cemiplimab as a first-line treatment.
Cemiplimab has shown benefits not only in terms of clinical activity and efficacy, but also in terms of safety and quality of life. In fact, the toxicity profile of cemiplimab is comparable to that observed with other PD-1 inhibitors. Only 5% of patients had to discontinue therapy due to an adverse event of grade 3 or higher (18). According to health-related quality of life data, cemiplimab led to a clinically relevant improvement in terms of both QLQ-C30 pain scale and QLQ-C30 global health status (44).
Regarding special populations such as organ transplant patients, limited data are available. A recent systematic review showed that among 57 transplanted patients who received an immune checkpoint inhibitor for advanced malignancies, 37% experienced organ rejection, and 14% died due to rejection (45). Most of the observed rejections were among kidney (40%), liver (35%), and heart (20%) transplant patients (45). The overall response rate was 30-40% for PD-1 inhibitors (45). In case of advanced CSCC, a careful multidisciplinary approach is required to assess the risk of organ rejection and the benefit of PD-1 inhibitor treatment. In addition, patients should be fully informed of the possible risks and benefits before starting treatment with immune checkpoint inhibitors. In addition, retrospective data of 12 patients with HIV infection and advanced malignancies treated with PD-1/PD-L1 inhibitor therapy showed objective responses without unexpected adverse events nor significant impact on HIV viremia (38). In another study, pembrolizumab showed to be safe in HIV-infected patients, in particular in maintaining CD4+ T-cell count and viral suppression (46).
The ongoing clinical studies recruiting patients with advanced or high-risk CSCC are summarized in Table 2. In most trials a treatment regimen including a PD-1 inhibitor is being investigated, and especially in earlier settings, such as high-risk CSCC. Most significantly, in the R2810-ONC-1788 study (NCT03969004), patients with high-risk CSCC are randomized to receive cemiplimab for 1 year versus placebo after surgery and adjuvant radiation therapy. The primary endpoint is disease-free survival (DFS). Cemiplimab is also being investigated in the neoadjuvant setting. Specifically, Gross and colleagues presented at the European Society of Medical Oncology (ESMO) meeting 2019 data from a phase 2 study (NCT03565783) where 20 patients with stage III/IV (M0) (AJCC 8th edition) CSCC of the head and neck received 2 doses of preoperative cemiplimab achieving a 55% of pathological complete response (pCR) and a major pathology response (MPR) in 15% (47). There were no grade ≥ 3 adverse events (47).
Immunotherapy has led to pivotal changes in advanced CSCC both in terms of objective responses, survival, and improved quality of life. However, patients with advanced CSCC receiving immunotherapy after more than one recurrence after surgical excision had less than half the probability of achieving an objective response (43). This could be related to primary or secondary resistance to immunotherapy (48). For this reason, clinical trials are ongoing with the aim of overcoming resistance to immunotherapy. In fact, the combination of PD-1 or PD-L1 inhibitors with other agents (such as radiotherapy, oncolytic viruses, or EGFR inhibitors) is being investigated to overcome primary or secondary resistance to immunotherapy, such as in the I-Tackle trial (NCT03666325) with the addition of cetuximab to pembrolizumab at primary or acquired resistance; or in the UNSCARRed study (NCT03737721) with the addition of radiotherapy to avelumab.
Cutaneous squamous cell carcinoma is a common condition, although it remains rare in its advanced stages; high-risk cases require multidisciplinary care due to the complexity associated with both the disease and the often frail population (27). Before the introduction of immunotherapy in clinical practice, a standard of care for advanced CSCC was not clearly defined, and up to 60% of patients with advanced CSCC did not receive any systemic therapy at all, due to the low clinical activity and high risk of severe toxicities (21). Based on a strong preclinical rationale, clinical trials were conducted leading to the registration by the regulatory authorities of anti-PD-1 immunotherapy in patients with advanced CSCC (21). Cemiplimab was the first PD-1 inhibitor receiving an indication in CSCC after showing in a clinical trial rapid and durable responses in more than 40% of patients (in Figures 1, 2 we reported two clinical cases of rapid clinical response), with a favorable safety profile. In addition to that, cemiplimab led to an improvement in health-related quality of life with a reduction in cancer-related pain after a few cycles of therapy (18, 43, 44).
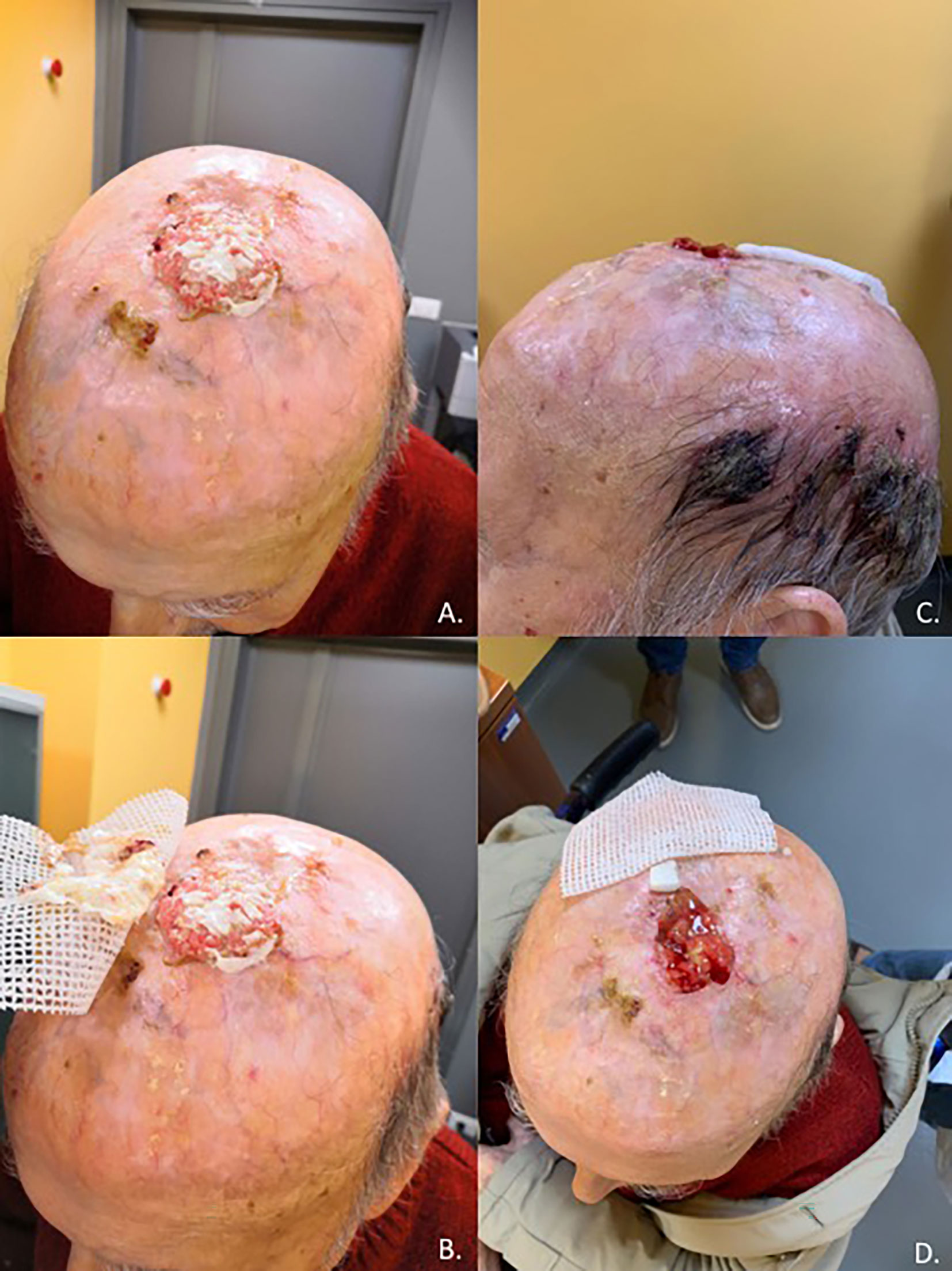
Figure 1 Case report of a 92-year-old man with unresectable, non-eligible to curative radiotherapy, locally advanced CSCC invading the skullcap and leptomeningeal membrane (A, B) who achieved a rapid clinical response after one cycle of Cemiplimab (C, D).
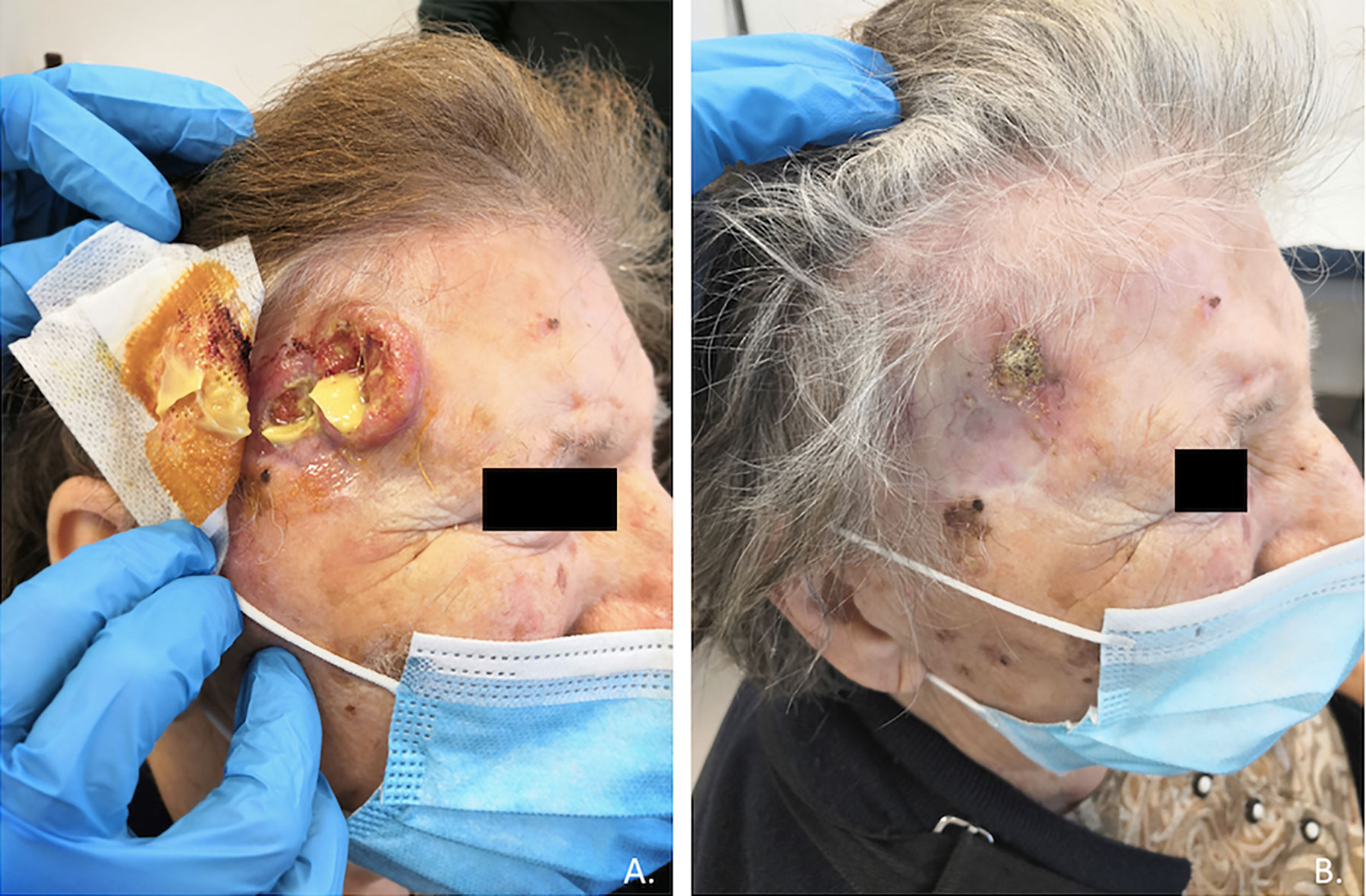
Figure 2 Case report of a rapid clinical response, after only one course of therapy with cemiplimab, in an 83-year-old patient with locally advanced recurrence of cutaneous squamous carcinoma of the right temporal region (A,B).
Anti-PD-1 drugs are the backbone of current clinical investigation in patients with CSCC. Specifically, several clinical trials with PD-1 inhibitors are currently underway investigating the activity, efficacy, and safety of adjuvant approaches in individuals with high-risk CSCC, and neoadjuvant approaches in patients with advanced CSCC. Based on the results of these studies, anti-PD-1 drugs may soon become standard of care in the adjuvant and neoadjuvant settings.
FS and PQ jointly supervised this work. All authors contributed to the article and approved the submitted version.
FS received honoraria for presentations or lectures from Sanofi, Roche, BMS, Novartis, Merk, SunPharma, MSD, Pierre Fabre, and surved on advisory boards of Novartis, Philogen, SunPharma and MSD; PQ reports consulting or advisory role for Bristol Myers Squibb, Merck & Co., Novartis, Pierre Favre, Roche/Genentech, and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nagarajan P, Asgari MM, Green AC, Guhan SM, Arron ST, Proby CM, et al. Keratinocyte Carcinomas: Current Concepts and Future Research Priorities. Clin Cancer Res (2019) 25(8):2379–91. doi: 10.1158/1078-0432.CCR-18-1122
2. Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, et al. Incidence of and Risk Factors for Skin Cancer in Organ Transplant Recipients in the United States. JAMA Dermatol (2017) 153(3):296–303. doi: 10.1001/jamadermatol.2016.4920
3. Brewer JD, Shanafelt TD, Khezri F, Sosa Seda IM, Zubair AS, Baum CL, et al. Increased Incidence and Recurrence Rates of Nonmelanoma Skin Cancer in Patients With Non-Hodgkin Lymphoma: A Rochester Epidemiology Project Population-Based Study in Minnesota. J Am Acad Dermatol (2015) 72(2):302–9. doi: 10.1016/j.jaad.2014.10.028
4. Omland SH, Ahlström MG, Gerstoft J, Pedersen G, Mohey R, Pedersen C, et al. Risk of Skin Cancer in Patients With HIV: A Danish Nationwide Cohort Study. J Am Acad Dermatol (2018) 79(4):689–95. doi: 10.1016/j.jaad.2018.03.024
5. Que SKT, Zwald FO, Schmults CD. Cutaneous Squamous Cell Carcinoma. J Am Acad Dermatol (2018) 78(2):237–47. doi: 10.1016/j.jaad.2017.08.059
6. Green AC, Olsen CM. Cutaneous Squamous Cell Carcinoma: An Epidemiological Review. Br J Dermatol (2017) 177(2):373–81. doi: 10.1111/bjd.15324
7. Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, et al. Mutational Landscape of Aggressive Cutaneous Squamous Cell Carcinoma. Clin Cancer Res (2014) 20(24):6582–92. doi: 10.1158/1078-0432.CCR-14-1768
8. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of Mutational Processes in Human Cancer. Nature (2013) 500(7463):415–21. doi: 10.1038/nature12477
9. Al-Rohil RN, Tarasen AJ, Carlson JA, Wang K, Johnson A, Yelensky R, et al. Evaluation of 122 Advanced-Stage Cutaneous Squamous Cell Carcinomas by Comprehensive Genomic Profiling Opens the Door for New Routes to Targeted Therapies. Cancer (2016) 122(2):249–57. doi: 10.1002/cncr.29738
10. Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-Melanoma Skin Cancer in Australia: The 2002 National Survey and Trends Since 1985. Med J Aust (2006) 184(1):6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x
11. Karia PS, Han J, Schmults CD. Cutaneous Squamous Cell Carcinoma: Estimated Incidence of Disease, Nodal Metastasis, and Deaths From Disease in the United States, 2012. J Am Acad Dermatol (2013) 68(6):957–66. doi: 10.1016/j.jaad.2012.11.037
12. Katalinic A, Kunze U, Schäfer T. Epidemiology of Cutaneous Melanoma and Non-Melanoma Skin Cancer in Schleswig-Holstein, Germany: Incidence, Clinical Subtypes, Tumour Stages and Localization (Epidemiology of Skin Cancer). Br J Dermatol (2003) 149(6):1200–6. doi: 10.1111/j.1365-2133.2003.05554.x
13. Brewster DH, Bhatti LA, Inglis JHC, Nairn ER, Doherty VR. Recent Trends in Incidence of Nonmelanoma Skin Cancers in the East of Scotland, 1992–2003. Br J Dermatol (2007) 156(6):1295–300. doi: 10.1111/j.1365-2133.2007.07892.x
14. Andersson EM, Paoli J, Wastensson G. Incidence of Cutaneous Squamous Cell Carcinoma in Coastal and Inland Areas of Western Sweden. Cancer Epidemiol (2011) 35(6):e69–74. doi: 10.1016/j.canep.2011.05.006
15. de Vries E, Trakatelli M, Kalabalikis D, Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, et al. Known and Potential New Risk Factors for Skin Cancer in European Populations: A Multicentre Case–Control Study. Br J Dermatol (2012) 167(s2):1–13. doi: 10.1111/j.1365-2133.2012.11081.x
16. Brougham NDLS, Dennett ER, Cameron R, Tan ST. The Incidence of Metastasis From Cutaneous Squamous Cell Carcinoma and the Impact of Its Risk Factors. J Surg Oncol (2012) 106(7):811–5. doi: 10.1002/jso.23155
17. Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for Non-Metastatic Squamous Cell Carcinoma of the Skin: Systematic Review and Pooled Analysis of Observational Studies. BMJ (2013) 347:f6153. doi: 10.1136/bmj.f6153
18. Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in Locally Advanced Cutaneous Squamous Cell Carcinoma: Results From an Open-Label, Phase 2, Single-Arm Trial. Lancet Oncol (2020) 21(2):294–305. doi: 10.1016/S1470-2045(19)30728-4
19. Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors Predictive of Recurrence and Death From Cutaneous Squamous Cell Carcinoma: A 10-Year, Single-Institution Cohort Study. JAMA Dermatol (2013) 149(5):541. doi: 10.1001/jamadermatol.2013.2139
20. Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 1. Epidemiology, Diagnostics and Prevention. Eur J Cancer (2020) 128:60–82. doi: 10.1016/j.ejca.2020.01.007
21. Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 2. Treatment. Eur J Cancer (2020) 128:83–102. doi: 10.1016/j.ejca.2020.01.008
22. Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-Analysis. JAMA Dermatol (2016) 152(4):419. doi: 10.1001/jamadermatol.2015.4994
23. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging: The Eighth Edition AJCC Cancer Staging Manual. CA: A Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, Hwang W-T, Gelfand JM, Whalen FM, et al. Evaluation of AJCC Tumor Staging for Cutaneous Squamous Cell Carcinoma and a Proposed Alternative Tumor Staging System. JAMA Dermatol (2013) 149(4):402. doi: 10.1001/jamadermatol.2013.2456
25. National Comprehensive Cancer Network. Squamous Cell Skin Cancer. In: NCCN Clinical Practice Guidelines in Oncology, vol. version 2 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). (2018). Available at: www.NCCN.org.
26. Euvrard S, Kanitakis J, Claudy A. Skin Cancers After Organ Transplantation. N Engl J Med (2003) 348(17):1681–91. doi: 10.1056/NEJMra022137
27. Soura E, Gagari E, Stratigos A. Advanced Cutaneous Squamous Cell Carcinoma: How Is It Defined and What New Therapeutic Approaches Are Available? Curr Opin Oncol (2019) 31(5):461–8. doi: 10.1097/CCO.0000000000000566
28. Pignon J-P, Maître A, Maillard E, Bourhis J. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 93 Randomised Trials and 17,346 Patients. Radiother Oncol (2009) 92(1):4–14. doi: 10.1016/j.radonc.2009.04.014
29. Porceddu SV, Bressel M, Poulsen MG, Stoneley A, Veness MJ, Kenny LM, et al. Postoperative Concurrent Chemoradiotherapy Versus Postoperative Radiotherapy in High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck: The Randomized Phase III TROG 05.01 Trial. JCO (2018) 36(13):1275–83. doi: 10.1200/JCO.2017.77.0941
30. Jarkowski A 3rd, Hare R, Loud P, Skitzki JJ, Kane JM 3rd, May KS, et al. Systemic Therapy in Advanced Cutaneous Squamous Cell Carcinoma (CSCC): The Roswell Park Experience and a Review of the Literature. Am J Clin Oncol (2016) 39(6):545–8. doi: 10.1097/COC.0000000000000088
31. Palmer JD, Schneider CJ, Hockstein N, Hanlon AL, Silberg J, Strasser J, et al. Combination of Post-Operative Radiotherapy and Cetuximab for High-Risk Cutaneous Squamous Cell Cancer of the Head and Neck: A Propensity Score Analysis. Oral Oncol (2018) 78:102–7. doi: 10.1016/j.oraloncology.2018.01.015
32. Maubec E, Petrow P, Scheer-Senyarich I, Duvillard P, Lacroix L, Gelly J, et al. Phase II Study of Cetuximab As First-Line Single-Drug Therapy in Patients With Unresectable Squamous Cell Carcinoma of the Skin. JCO (2011) 29(25):3419–26. doi: 10.1200/JCO.2010.34.1735
33. Cavalieri S, Perrone F, Miceli R, Ascierto PA, Locati LD, Bergamini C, et al. Efficacy and Safety of Single-Agent Pan-Human Epidermal Growth Factor Receptor (HER) Inhibitor Dacomitinib in Locally Advanced Unresectable or Metastatic Skin Squamous Cell Cancer. Eur J Cancer (2018) 97:7–15. doi: 10.1016/j.ejca.2018.04.004
34. Cowey CL, Robert NJ, Espirito JL, Davies K, Frytak J, Lowy I, et al. Clinical Outcomes Among Unresectable, Locally Advanced, and Metastatic Cutaneous Squamous Cell Carcinoma Patients Treated With Systemic Therapy. Cancer Med (2020) 9(20):7381–7. doi: 10.1002/cam4.3146
35. Hillen U, Leiter U, Haase S, Kaufmann R, Becker J, Gutzmer R, et al. Advanced Cutaneous Squamous Cell Carcinoma: A Retrospective Analysis of Patient Profiles and Treatment Patterns—Results of a Non-Interventional Study of the DeCOG. Eur J Cancer (2018) 96:34–43. doi: 10.1016/j.ejca.2018.01.075
36. Marks R, Rennie G, Selwood TS. MALIGNANT TRANSFORMATION OF SOLAR KERATOSES TO SQUAMOUS CELL CARCINOMA. Lancet (1988) 331(8589):795–7. doi: 10.1016/S0140-6736(88)91658-3
37. Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med (2017) 377(25):2500–1. doi: 10.1056/NEJMc1713444
38. Kugel CH, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age Correlates With Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin Cancer Res (2018) 24(21):5347–56. doi: 10.1158/1078-0432.CCR-18-1116
39. Patel R, Chang ALS. Immune Checkpoint Inhibitors for Treating Advanced Cutaneous Squamous Cell Carcinoma. Am J Clin Dermatol (2019) 20(4):477–82. doi: 10.1007/s40257-019-00426-w
40. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
41. Maubec E, Boubaya M, Petrow P, Beylot-Barry M, Basset-Seguin N, Deschamps L, et al. Phase II Study of Pembrolizumab As First-Line, Single-Drug Therapy for Patients With Unresectable Cutaneous Squamous Cell Carcinomas. JCO (2020) 38(26):3051–61. doi: 10.1200/JCO.19.03357
42. Grob J-J, Gonzalez R, Basset-Seguin N, Vornicova O, Schachter J, Joshi A, et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). JCO (2020) 38(25):2916–25. doi: 10.1200/JCO.19.03054
43. Rischin D, Khushalani NI, Schmults CD, Guminski AD, Chang ALS, Lewis KD, et al. Phase II Study of Cemiplimab in Patients (Pts) With Advanced Cutaneous Squamous Cell Carcinoma (CSCC): Longer Follow-Up. JCO (2020) 38(15_suppl):10018–8. doi: 10.1200/JCO.2020.38.15_suppl.10018
44. Migden MR, Rischin D, Sasane M, Mastey V, Pavlick A, Schmults CD, et al. Health-Related Quality of Life (HRQL) in Patients With Advanced Cutaneous Squamous Cell Carcinoma (CSCC) Treated With Cemiplimab: Post Hoc Exploratory Analyses of a Phase II Clinical Trial. JCO (2020) 38(15_suppl):10033–3. doi: 10.1200/JCO.2020.38.15_suppl.10033
45. Fisher J, Zeitouni N, Fan W, Samie FH. Immune Checkpoint Inhibitor Therapy in Solid Organ Transplant Recipients: A Patient-Centered Systematic Review. J Am Acad Dermatol (2020) 82(6):1490–500. doi: 10.1016/j.jaad.2019.07.005
46. Anti-PD-1 Therapy OK for Most With HIV. Cancer Discovery (2018) 8(2):130–1. doi: 10.1158/2159-8290.CD-NB2017-174
47. Gross N, Ferrarotto R, Nagarajan P, Bell D, El-Naggar A, Johnson JM, et al. Phase II Study of Neoadjuvant Cemiplimab Prior to Surgery in Patients With Stage III/IV (M0) Cutaneous Squamous Cell Carcinoma of the Head and Neck (CSCC-Hn). Ann Oncol (2019) 30:v910. doi: 10.1093/annonc/mdz394.071
Keywords: immunotherapy, skin cancer, CSCC, cutaneous squamous cell carcinoma, cemiplimab, non-melanoma skin cancer, anti-PD-1 (programmed cell death-1 protein) monoclonal antibody, keratinocyte carcinomas
Citation: Boutros A, Cecchi F, Tanda ET, Croce E, Gili R, Arecco L, Spagnolo F and Queirolo P (2021) Immunotherapy for the Treatment of Cutaneous Squamous Cell Carcinoma. Front. Oncol. 11:733917. doi: 10.3389/fonc.2021.733917
Received: 30 June 2021; Accepted: 09 August 2021;
Published: 26 August 2021.
Edited by:
Samisubbu Naidu, Indiana University, United StatesReviewed by:
Brian C. Capell, University of Pennsylvania, United StatesCopyright © 2021 Boutros, Cecchi, Tanda, Croce, Gili, Arecco, Spagnolo and Queirolo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Boutros, Ym91dHJvcy5hbmRyZWFAZ21haWwuY29t
†These authors jointly supervised this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.