
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 02 September 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.728854
 Ning Li1†
Ning Li1† Wenying Deng1†
Wenying Deng1† Guifang Zhang2†
Guifang Zhang2† Yali Du1
Yali Du1 Yanwei Guo3
Yanwei Guo3 Yijie Ma1
Yijie Ma1 Chen Wei1
Chen Wei1 Liangyu Bie1
Liangyu Bie1 Chi Zhang1
Chi Zhang1 Tao Song4
Tao Song4 Suxia Luo1*
Suxia Luo1* Baijun Fang5*
Baijun Fang5*Purpose: Apatinib is an approved third-line treatment for metastatic gastric cancer in China and demonstrates good safety, tolerability, and efficacy in other advanced solid tumors. The aim of this prospective, single-arm, multicenter, phase 2 study was to assess the efficacy and safety of low-dose apatinib combined with S-1 in the treatment of refractory mCRC.
Patients and Methods: Patients with refractory mCRC were enrolled and administered apatinib combined with S-1 until disease progression, patient decision to withdraw, or unacceptable toxic effects. The primary endpoint was investigator-evaluated progression-free survival (PFS) and the secondary endpoints were overall survival (OS), objective response rate (ORR), disease control rate (DCR).
Results: From December 2017 to December 2018, 30 patients were enrolled and 29 patients were eligible for the evaluation of efficacy and safety. The median PFS (mPFS) and OS (mOS) were 7.9 and 12.9 months, respectively. Exploratory analysis revealed that patients administered S-1 ≥ 70 days achieved longer mPFS and mOS. Four patients achieved a partial response, 22 achieved stable disease, and three had progressive disease, attributing to an ORR of 13.79% and a DCR of 89.66%. Ten grade 3 adverse events were reported and the frequency of each grade 3 adverse event was less than 5%. No grade 4 side events were observed.
Conclusions: These results indicated that apatinib combined with S-1 showed promising efficacy and manageable toxicity in patients with progressive mCRC after at least 2 prior lines of therapy, making it a promising therapeutic option for mCRC treatment.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT03397199, identifier NCT03397199.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer deaths worldwide (1). According to the 2015 Chinese Cancer Statistics, the incidence and mortality of CRC increased (2). Surgical resection and preoperative chemoradiotherapy are radical measures for patients in the early or locally advanced stages (3). However, approximately one-quarter of patients present with metastatic disease at diagnosis, and another quarter eventually develop metastases during the course of their treatment. Systemic chemotherapy regimens (fluorouracil with leucovorin in addition to either irinotecan or oxaliplatin) remain the standard treatment for most patients with metastatic CRC (mCRC) (4). Chemotherapy in combination with agents targeting the vascular endothelial growth factor (VEGF) pathway or the epidermal growth factor receptor (EGFR) also improve outcomes for mCRC (5). In addition to third-line treatment options for Chinese patients with mCRC including trimethoprim fluorouracil (TAS-102) and regorafenib, treatment options are still limited for patients with mCRC who are refractory to standard first- and second-line therapy. Therefore, developing third-line therapeutic strategies for mCRC is necessary, especially in China.
Angiogenesis, mainly regulated by the VEGF pathway, plays important roles in the development of colorectal cancer, and anti-angiogenesis has been an important treatment strategy for patients with mCRC (6). For instance, bevacizumab, anti-VEGF antibody drug, has been used to treat mCRC in both first- and second-line settings in combination with chemotherapy (7, 8). Regorafenib and fruquintinib, as small-molecule multi-receptor tyrosine kinase inhibitors, are recommended as third-line therapies, however, their benefits are only modestly improved and adverse effects might be difficult to manage (9–11).
Apatinib, a novel small-molecule tyrosine kinase inhibitor (TKI), which selectively binds to and strongly inhibits vascular epidermal growth factor receptor 2 (VEGFR2), has shown efficacy in various types of cancers (12). A Phase III study showed that apatinib improves overall survival (OS) and progression-free survival (PFS) of patients with heavily pretreated gastric cancer (13) and it was approved as a subsequent-line therapy for gastric cancer by the China State Food and Drug Administration. Furthermore, several phase II/III trials have proven its satisfactory efficacy and tolerable adverse events in many other cancer types, such as metastatic triple-negative breast cancer (14), non-small-cell lung cancer (15), and ovarian cancer (16). Recently, two prospective studies reported that apatinib monotherapy in a third-line setting displays promising activities in patients with refractory colorectal cancer (17, 18). Further, several retrospective studies evaluated the safety and efficacy of apatinib in patients with heavily treated mCRC and suggested that apatinib is effective as a third-line treatment (19–21).
The oral fluoropyrimidine S-1, as a prodrug of 5-fluorouracil (5-FU), has exhibited comparable efficacy results compared to 5-FU and capecitabine in mCRC (22–24), with a lower incidence of the hand-foot syndrome and reduced gastrointestinal toxicities (23, 25). Previous studies showed S-1 combined with irinotecan to have an equal effect as FOLFIRI as second-line chemotherapy for mCRC (24).
Based on these studies, here, we aimed to report the efficacy and safety of apatinib combined with S1 in patients with mCRC after the failure of at least two lines of previous treatments.
This investigator-initiated, multicenter, prospective phase 2 study was open to patients with refractory metastatic colorectal cancer. Between December 2017 and December 2018, a total of 30 patients from three oncology centers in China were enrolled in this trial.
The criteria for patient inclusion were as follows: (1) Aged 18-75 years, diagnosed with mCRC, and received more than two lines of standard chemotherapy (irinotecan and oxaliplatin). 20 patients previously treated with targeted therapy. The targeted therapy drugs were bevacizumab in 19 patients (65%), cetuximab in 2 patients (7%); (2) an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1; (3) at least one measurable lesion evaluated according to the Response Evaluation Criteria in Solid Tumors (RESIST) criteria (1.1); (4) absolute neutrophil count ≥ 1.5× 109 cells per L, and platelet count ≥ 80 × 109 cells per L; Key exclusion criteria were; previous exposure to apatinib, allergies to any of the excipients, uncontrolled blood pressure, any history of surgery or gastrointestinal perforation, and bleeding or obstruction within 28 days before day 1 of the enrollment. Patients with a history of any one or more of the following conditions within the past 6 months before day 1 of this study were also excluded; central nervous system metastasis, myocardial infarction, unstable angina, and congestive heart failure.
Study procedures were approved by the Research Ethics Board of Henan Cancer Hospital and were following the Declaration of Helsinki. All patients provided written informed consent before enrollment in the study.
The study was a single-arm, multicenter, phase II clinical trial. This study aimed to assess the safety and efficacy of apatinib combined with S-1 in the treatment of mCRC patients. All patients received oral apatinib (HengRui Medicine Co., Ltd., China) 250 mg daily, combined with S1 (40 mg for BSA < 1.2, 50 mg for 1.2 < BSA < 1.5, or 60 mg for BSA >1.5; between days 1-14, for 3 weeks) until disease progression or intolerable toxic effects. If intolerable toxic effects were observed (hypertension, hand and foot symptom, albuminuria), apatinib was temporarily discontinued until the patient had recovered from toxicity. If side effects were caused by S-1, the dose was reduced. If toxicity abated with the dose reduction, the dose of S-1 would not return to the initial dose. Regardless of permanent discontinuation of Apatinib or S-1, patients continued monotherapy with another drug. Patients who completed the first 28 days of treatment were evaluated for efficacy and re-evaluate every 56 days. The chest/abdominal/pelvic CT scan and traditional biomarkers [carcinoembryonic antigen (CEA), cancer antigen 72-4 (CA724), and CA199] were rechecked for disease assessment. The CT images were assessed by professional radiologists who have been involved in the evaluation of several clinical studies at our center, and who were blinded to the therapeutic outcomes and clinicopathological features.
The primary analysis endpoint was PFS and secondary analysis endpoints were disease control rate (DCR), objective response rate (ORR), and OS. PFS was calculated from the date of oral treatment to the date of disease progression, death, or the last follow-up. OS was determined as the period between the date of oral treatment and the date of death or the last follow-up. Tumor response was evaluated by radiologists and oncologists according to RECIST version 1.1 as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). DCR was defined as the percentage of patients with CR, PR, and SD. Adverse events were reviewed and classified from patients’ medical history and telephone follow-up according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 5.0 (CTCAE 5.0).
PASS 15 software and a single-sample log-rank test were used to calculate the sample size. Given that the expected median PFS (mPFS) of 5.5 months with 80% power, α=0.05 (two-sided) and β=0.20, an enrollment period of 24 months, and a follow-up period of 6 months, we estimated that at least 29 subjects needed to be enrolled. PFS was defined as the time from the start of low-dose apatinib combined with S-1 therapy to the first disease progression or death from any cause. OS was evaluated as the time from the start of low-dose apatinib combined with S-1 therapy to death from any cause.
All data were statistically analyzed using the Statistical Package for the Social Sciences (SPSS) 22.0 software (SPSS Inc. Chicago IL, USA). The Kaplan-Meier method and the log rank test were used to analyze the OS and PFS. Multivariate analyses of cox regression model were applied to evaluate the effects of multiple covariates on survival.
Of the 32 patients screened, 30 were enrolled in the study and one was excluded because of failure to follow-up. The main clinical features of the 29 patients are shown in Table 1. The median age of the patients was 53 (range 25-73) years, while 17 males and 12 females were included in this study. The proportion of patients with ECOG PS scores of 0 and 1 were 3.4% (1/29) and 96.6% (28/29), respectively. (5) All patients were tested for KRAS, BRAF V600E, and MSI status. We identified 8 patients (28%) with KRAS mutations. All patients with BRAF V600E wild type and MSS status were detected. The anti-VEGF agent used was bevacizumab in 19 patients (65%) prior to the use of apatinib. 16 patients received apatinib as the third-line treatment, 13 patients received apatinib as the fourth- or higher-line therapy. Eleven patients were diagnosed with primary colon adenocarcinoma and 18 patients with rectal cancer. Most patients (23/29, 79.3%) were tested for KRAS mutation.
The date cut-off date for the analyses was January 30, 2021. At the time of analysis 29 events were observed. Total of 29 patients was evaluated for therapeutic efficacy and adverse events after taking apatinib combined with S1. The median duration of treatment was 4.6 months. Among all 29 patients, no patient achieved CR, four patients achieved PR, and 22 patients were SD. The waterfall plots of evaluable patients showing the best change compared with the baseline are shown in Figure 1 and the treatment response is shown in Figure 2. An ORR of 13.79% and a DCR of 89.66% were achieved, and two patients developed a cavity in metastatic lung disease. Median PFS was 7.9 months (95% confidence interval [CI]: 4.92-10.88) and median OS (mOS) was 12.9 months (95% CI: 9.576-16.224). Kaplan-Meier curves for PFS and OS are shown in Figure 3.
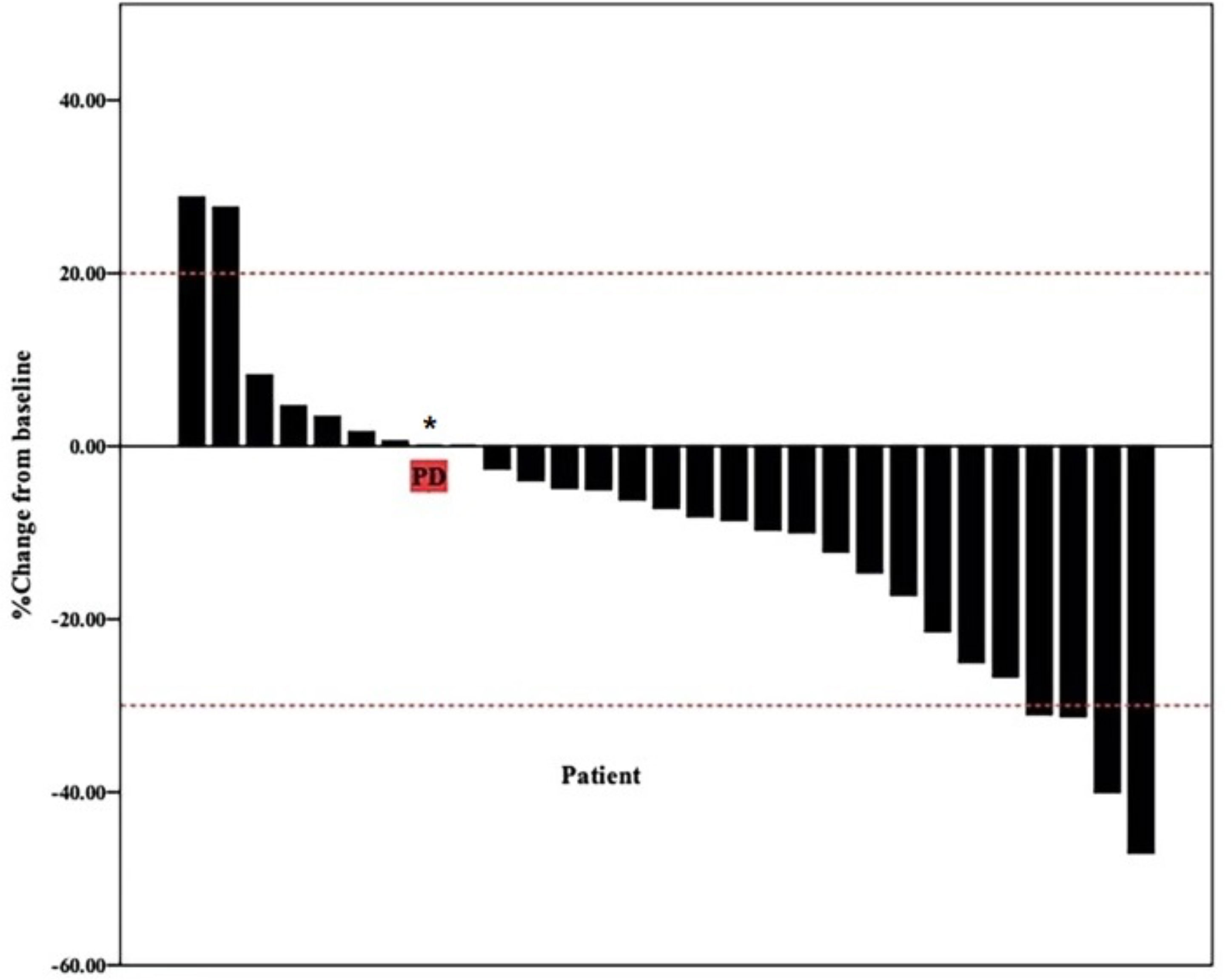
Figure 1 Waterfall plot of best reduction in tumor size compared with baseline. Bar graph depicting the best percentage change in tumor size compared to baseline in 29 patients. * patient evaluation of tumor is SD, but pleural effusion increased significantly.
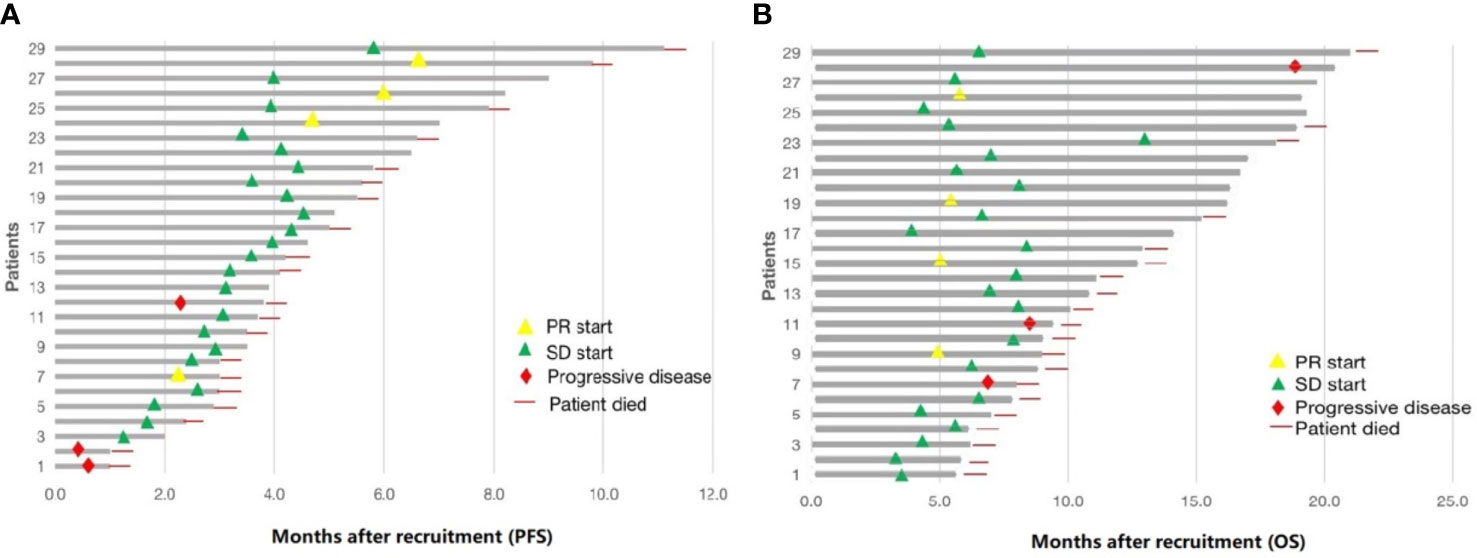
Figure 2 Summary of 29 patients treated with low-dose apatinib and S1. (A) progression-free survival (PFS). (B) overall survival (OS).
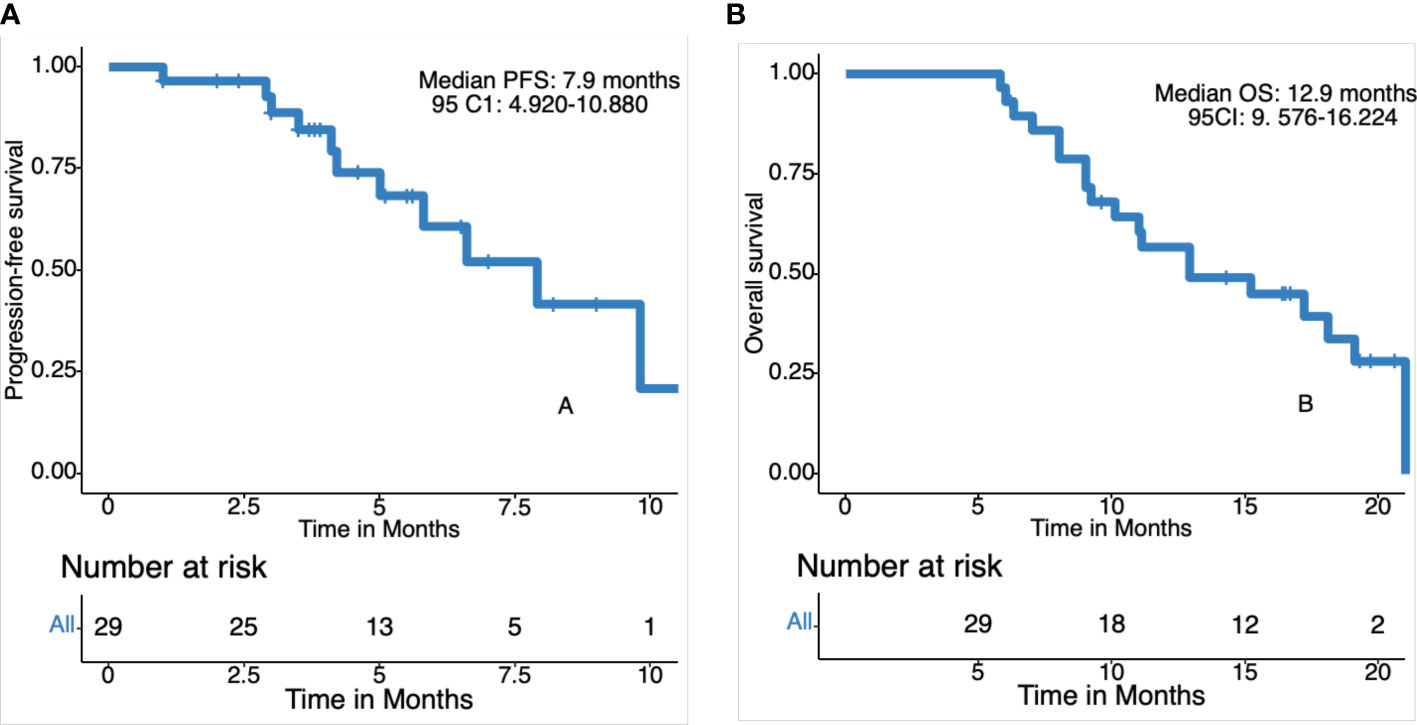
Figure 3 Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS). (A) Progression free survival in 29 patients. (B) Overall survival in 29 patients.
Subgroups analysis showed that a longer mPFS and mOS were observed in patients who received S-1 treatment time≥ 70 days, compared to those who received S-1 treatment time< 70 days (9.8 vs 4.2 months, p=0.001; 19.1 vs 9.2 months, p=0.008). Patients with a lower neutrophil-to-lymphocyte ratio also exhibited better mPFS and mOS than those with higher neutrophil-to-lymphocyte ratio (5.6 vs 3.5 months, p=0.006; 19.1 vs 8 months, p=0.005). Low D-dimer was associated with a better OS than a high one (15.2 vs 8 months, p=0.033). Pre-treatment with or without targeted therapy did not affect survival. Exploratory subgroup analysis is shown in Table 2. The Kaplan-Meier curves for PFS and OS in different subgroups are shown in Supplementary Figures 4, 5. Univariate analysis showed that the D-dimer, S-1 treatment, and neutrophil-to-lymphocyte ratio were significantly associated with OS (hazard ratio [HR], 3.23, 0.25, and 4.22, respectively; Supplementary Table 1). We then performed Cox multivariate regression analysis. Only the neutrophil-to-lymphocyte ratio remained significantly associated with OS (HR, 2.71; Supplementary Table 1).
Total of 29 patients was eligible for the safety analysis. And 28 (96.6%) patients experienced adverse events during treatment and the incidence of different adverse events is summarized in Table 3. The most common adverse events at all grades were thrombocytopenia (37.93%), followed by leukopenia and elevated transaminase with same incidence rates of 27.59%. A total of ten grade 3 adverse events were reported and the frequency of each grade 3 adverse event was less than 5%. Grade 4 toxicities did not occur in any of the patients. No serious hepatic or renal function impairment was reported and no toxicity-related deaths occurred during the study.
The most common apatinib-related adverse events were hypertension (20.69%), proteinuria (6.90%), and fatigue (13.79%). Overall, 5 (17.24%) of the 29 patients had drug-related grade 3 adverse events, and 2 (6.90%) experienced treatment modifications because of apatinib-related proteinuria.
Anemia and thrombocytopenia developed in 10.34 and 37.93% of the patients, respectively. One patient had grade 3 thrombocytopenia, and one patient, who experienced grade 3 anemia, received a red blood cell transfusion.
The most common nonhematological toxicities were fatigue, nausea, and elevated transaminase. Among them, the incidence of grade 1-2 fatigue, nausea, and elevated transaminase were 13.79, 10.34, and 24.14%, respectively. Only one patient had grade 3 elevated transaminase.
To the best of our knowledge, this is the first prospective study to evaluate the efficacy and safety of combination therapy with apatinib, an oral agent with anti-angiogenic properties, and S1 in patients with chemo refractory mCRC. Promising results were observed using this treatment regimen. More than half of the patients had been treated with chemotherapy and targeted therapy and their prognosis was very poor because of the absence of effective regimens. However, the patients co-treated with apatinib and S1 achieved an unexpected objective response and the DCR. More importantly, mPFS and mOS were also promising. Furthermore, the combination of apatinib and S-1 had manageable toxicity. Consequently, patients with mCRC, who had progressed disease after being exposed to at least 2 prior lines of therapy, could benefit from the combination therapy with apatinib and S1.
S-1 showed superiority in terms of cost-effectiveness and patient preference. A phase II trial reported an ORR of 14.3% and a PFS of 3.03 months for patients with mCRC who were treated with oral S-1 monotherapy as a third-line therapy (26). In addition, apatinib as a third-line monotherapy in patients with mCRC achieved an ORR of 8.3-13.9%, an mPFS of 3.7-4.8 months, and an mOS of 7.3-9.1 months (17–21). Although cross-trial comparisons were tough, our study presented that the mPFS and mOS of apatinib combined with S-1 were superior and the ORR was similar to those observed in previous studies.
Numerous patients with mCRC having disease progression after receiving all standard therapies still exhibit an adequate ECOG PS to tolerate further treatment. Recent drugs regorafenib, fruquintinib, and TAS-102 have superior efficacy compared with the placebo, therefore they would be suitable for third-line therapy in mCRC (11, 27). Unfortunately, the survival effect of the third-line CRC treatment in phase 3 trials is modest. In the CORRECT trial, regorafenib monotherapy yielded an mOS of 6.4 months and an mPFS of 1.9 months, which was also validated in an Asian population in the CONCUR trial (PFS: 3.2 months; OS: 8.8 months) (9, 10). The phase III FRESCO trial of fruquintinib monotherapy reported an ORR of 4.7%, an mOS of 9.3 months, and an mPFS of 3.7 months (11). In the RECOURSE trial, TAS-102 monotherapy achieved an ORR of 1.6%, a DCR of 44%, an mOS of 7.1 months, and an mPFS of 2.0 months (28). In the trial that tested TAS-102 with or without bevacizumab in patients with chemo refractory mCRC, an ORR of 2.1%, an mOS of 9.6 months, and an mPFS of 4.6 months were reported (29). In our study, we observed an ORR of 13.79%, an mPFS of 7.9 months, and an mOS of 12.9 months. Compared to the patients included in our clinical trials, approximately 50% of patients in the RECOURSE and CORRECT trials were treated in 4 or more lines. Third-line or post-third-line treatment may increase OS expectancy in patients of mCRC.
The safety profile of available third-line/further-line mCRC therapies may help treatment selection for patients when the quality of life is threatened. In this study, there were no grade 4 adverse events or treatment-related deaths and no grade 3/4 neutropenia occurred in any patients. In contrast, grade 3 adverse events were observed in almost 50% of the patients administered regorafenib (mostly hand-foot skin reaction, fatigue, diarrhea, hypertension, and rash) (9). Cetuximab and panitumumab cause almost 30% of grade 3-4 toxicities (mainly rash and dermatitis acneiform) in patients (30). In the study conducted by Li et al., the most frequently observed drug-related adverse events were hypertension (69.5%), proteinuria (47.8%), and hand-foot syndrome (45.6%) (31). In another study, the incidences of the three most common grade 3/4 adverse events were higher than those in our study (hypertension, 7%; hand-foot syndrome, 6%; proteinuria, 4%) (32). In the present study, most adverse events were of grades 1-2 and were well tolerated and controlled, maybe because we used low-dose apatinib.
It must be pointed out that exploratory analysis revealed that patients that received S-1 treatment ≥ 70 days achieved longer mPFS and mOS, suggesting that patients obtained extended survival benefits. Additionally, patients with hypertension showed a tendency of prolonged PFS and OS which was consistent with the finding that anti-angiogenic adverse events could be predictive biomarkers for treatment efficacy (33).
We acknowledge that this study had some limitations. This was a single-arm study without a control group for comparison, and thus selection bias could not be excluded due to the non-randomized design. Considering that fewer adverse events depend on administering low-doses of apatinib, further studies are needed to validate our results. The phase III trial of low-dose apatinib with S1 is ongoing and good results are expected.
In conclusion, this study is the first to report a combination therapy with apatinib and S1 in patients with refractory mCRC. We expect apatinib plus S-1 to demonstrate promising efficacy and manageable toxicity as a third-line therapy for mCRC, which warrants further investigation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The Research Ethics Board of Henan Cancer Hospital approved the protocol and the study was in accordance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conception and design: NL, SL, and BF. Development of methodology: WD. Acquisition of data (provided acquired and managed patients, provided facilities, etc.): GZ, YD, YG, and YM. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): CW. Writing, review, and/or revision of the manuscript: LB. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): CZ. Study supervision: TS. All authors contributed to the article and approved the submitted version.
Key Scientific and Technological Projects in Henan Province (202102310111); Key projects of Henan Provincial Department of Education (13A320440). Joint Funds of the National Natural Science Foundation of China (U2004132)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully thank the patients, their families, and the institutions for their participation. We also thank Cunnan Dong and Guoliang Chen for the editing support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.728854/full#supplementary-material
Supplementary Table 1 | Univariate and Multivariate Cox analyses of the S1 time, D dimer and NLR.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA: Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer. Ann Oncol (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
4. Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian Adapted ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer: A JSMO-ESMO Initiative Endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol (2018) 29:44–70. doi: 10.1093/annonc/mdx738
5. Xie YH, Chen YX, Fang JY. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct Target Ther (2020) 5:22. doi: 10.1038/s41392-020-0116-z
6. Tampellini M, Sonetto C, Scagliotti GV. Novel Anti-Angiogenic Therapeutic Strategies in Colorectal Cancer. Expert Opin Investig Drugs (2016) 25:507–20. doi: 10.1517/13543784.2016.1161754
7. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab Plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N Engl J Med (2004) 350:2335–42. doi: 10.1056/NEJMoa032691
8. Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in Combination With Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol (2007) 25:1539–44. doi: 10.1200/JCO.2006.09.6305
9. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet (2013) 381:303–12. doi: 10.1016/S0140-6736(12)61900-X
10. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib Plus Best Supportive Care Versus Placebo Plus Best Supportive Care in Asian Patients With Previously Treated Metastatic Colorectal Cancer (CONCUR): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2015) 16:619–29. doi: 10.1016/S1470-2045(15)70156-7
11. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA (2018) 319:2486–96. doi: 10.1001/jama.2018.7855
12. Li J, Tang Z, Wang H, Wu W, Zhou F, Ke H, et al. CXCL6 Promotes non-Small Cell Lung Cancer Cell Survival and Metastasis via Down-Regulation of Mir-515-5p. Biomed Pharmacotherapy = Biomed Pharmacotherapie (2018) 97:1182–8. doi: 10.1016/j.biopha.2017.11.004
13. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol (2016) 34:1448–54. doi: 10.1200/JCO.2015.63.5995
14. Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, et al. Multicenter Phase II Study of Apatinib, a Novel VEGFR Inhibitor in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. Int J Cancer (2014) 135:1961–9. doi: 10.1002/ijc.28829
15. Liu Z, Ou W, Li N, Wang SY. Apatinib Monotherapy for Advanced non-Small Cell Lung Cancer After the Failure of Chemotherapy or Other Targeted Therapy. Thorac Cancer (2018) 9:1285–90. doi: 10.1111/1759-7714.12836
16. Lan CY, Wang Y, Xiong Y, Li JD, Shen JX, Li YF, et al. Apatinib Combined With Oral Etoposide in Patients With Platinum-Resistant or Platinum-Refractory Ovarian Cancer (AEROC): A Phase 2, Single-Arm, Prospective Study. Lancet Oncol (2018) 19:1239–46. doi: 10.1016/S1470-2045(18)30349-8
17. Wang F, Yuan X, Jia J, Bi X, Zhou Z, Zhou Q, et al. Apatinib Monotherapy for Chemotherapy-Refractory Metastatic Colorectal Cancer: A Multi-Centre, Single-Arm, Prospective Study. Sci Rep (2020) 10:6058. doi: 10.1038/s41598-020-62961-5
18. Chen X, Qiu T, Zhu Y, Sun J, Li P, Wang B, et al. Phase II Study of Apatinib in Refractory Metastatic Colorectal Cancer. Oncologist (2019) 24:883–e407. doi: 10.1634/theoncologist.2019-0164
19. Li A, Wang K, Xu A, Wang G, Miao Y, Sun Z, et al. Apatinib as an Optional Treatment in Metastatic Colorectal Cancer. Med (Baltimore) (2019) 98:e16919. doi: 10.1097/MD.0000000000016919
20. Liang L, Wang L, Zhu P, Xia Y, Qiao Y, Wu J, et al. A Pilot Study of Apatinib as Third-Line Treatment in Patients With Heavily Treated Metastatic Colorectal Cancer. Clin Colorectal Cancer (2018) 17:e443–9. doi: 10.1016/j.clcc.2018.02.011
21. Gou M, Si H, Zhang Y, Qian N, Wang Z, Shi W, et al. Efficacy and Safety of Apatinib in Patients With Previously Treated Metastatic Colorectal Cancer: A Real-World Retrospective Study. Sci Rep (2018) 8:4602. doi: 10.1038/s41598-018-22302-z
22. Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, et al. Leucovorin, Fluorouracil, and Oxaliplatin Plus Bevacizumab Versus s-1 and Oxaliplatin Plus Bevacizumab in Patients With Metastatic Colorectal Cancer (SOFT): An Open-Label, Non-Inferiority, Randomised Phase 3 Trial. Lancet Oncol (2013) 14:1278–86. doi: 10.1016/S1470-2045(13)70490-X
23. Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim KP, et al. S-1 Plus Oxaliplatin Versus Capecitabine Plus Oxaliplatin for First-Line Treatment of Patients With Metastatic Colorectal Cancer: A Randomised, non-Inferiority Phase 3 Trial. Lancet Oncol (2012) 13:1125–32. doi: 10.1016/S1470-2045(12)70363-7
24. Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, et al. Irinotecan Plus s-1 (IRIS) Versus Fluorouracil and Folinic Acid Plus Irinotecan (FOLFIRI) as Second-Line Chemotherapy for Metastatic Colorectal Cancer: A Randomised Phase 2/3 non-Inferiority Study (FIRIS Study). Lancet Oncol (2010) 11:853–60. doi: 10.1016/S1470-2045(10)70181-9
25. Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a Novel Form of an Oral 5-Fluorouracil Derivative (s-1) Directed to the Potentiation of the Tumor Selective Cytotoxicity of 5-Fluorouracil by Two Biochemical Modulators. Anticancer Drugs (1996) 7:548–57. doi: 10.1097/00001813-199607000-00010
26. Jeung HC, Rha SY, Cho BC, Yoo NC, Roh JK, Roh WJ, et al. A Phase II Trial of s-1 Monotherapy in Metastatic Colorectal Cancer After Failure of Irinotecan- and Oxaliplatin-Containing Regimens. Br J Cancer (2006) 95:1637–41. doi: 10.1038/sj.bjc.6603468
27. Walter T, Hawkins NS, Pollock RF, Colaone F, Shergill S, Ross PJ. Systematic Review and Network Meta-Analyses of Third-Line Treatments for Metastatic Colorectal Cancer. J Cancer Res Clin Oncol (2020) 146(10):2575—87. doi: 10.1016/j.jval.2020.04.1546
28. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N Engl J Med (2015) 372:1909–19. doi: 10.1056/NEJMoa1414325
29. Pfeiffer P, Yilmaz M, Moller S, Zitnjak D, Krogh M, Petersen LN, et al. TAS-102 With or Without Bevacizumab in Patients With Chemorefractory Metastatic Colorectal Cancer: An Investigator-Initiated, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol (2020) 21:412–20. doi: 10.1016/S1470-2045(19)30827-7
30. Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab Versus Cetuximab in Patients With Chemotherapy-Refractory Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer (ASPECCT): A Randomised, Multicentre, Open-Label, non-Inferiority Phase 3 Study. Lancet Oncol (2014) 15:569–79. doi: 10.1016/S1470-2045(14)70118-4
31. Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, et al. Safety and Pharmacokinetics of Novel Selective Vascular Endothelial Growth Factor Receptor-2 Inhibitor YN968D1 in Patients With Advanced Malignancies. BMC Cancer (2010) 10:529. doi: 10.1186/1471-2407-10-529
32. Sun D, Hou H, Zhang C, Zhang X. The Efficacy and Safety of Apatinib for Refractory Malignancies: A Review and Meta-Analysis. Onco Targets Ther (2018) 11:6539–54. doi: 10.2147/OTT.S176429
Keywords: apatinib, S-1, colorectal cancer, metastatic, efficacy
Citation: Li N, Deng W, Zhang G, Du Y, Guo Y, Ma Y, Wei C, Bie L, Zhang C, Song T, Luo S and Fang B (2021) Low-Dose Apatinib Combined With S-1 in Refractory Metastatic Colorectal Cancer: A Phase 2, Multicenter, Single-Arm, Prospective Study. Front. Oncol. 11:728854. doi: 10.3389/fonc.2021.728854
Received: 22 June 2021; Accepted: 17 August 2021;
Published: 02 September 2021.
Edited by:
Bhawna Sirohi, Apollo Proton Cancer Centre, IndiaCopyright © 2021 Li, Deng, Zhang, Du, Guo, Ma, Wei, Bie, Zhang, Song, Luo and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suxia Luo, emx5eWx1b3N1eGlhMDM2MUB6enUuZWR1LmNu; Baijun Fang, ZmFuZ2JhaWp1bkAxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.