- 1Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of VIP Medical Services, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objective: The optimal treatment for resectable esophageal squamous cell carcinoma (ESCC) remains controversial. Surgery is the primary treatment but with poor results. Attempts to improve patient survival have been made by introducing chemotherapy, radiotherapy, or both. However, randomized comparisons for all these strategies are not always available. This network meta-analysis compared the overall survival of neoadjuvant and adjuvant therapy with surgery alone to identify the most effective approach.
Methods: We systematically searched electronic databases (PubMed, Embase, and Cochrane Library) for relevant studies published before April 2021. Only phase II and III randomized controlled trials comparing the following treatments were included: surgery alone, neoadjuvant chemotherapy (NCT), radiotherapy (NRT) or chemoradiotherapy (NCRT), adjuvant chemotherapy (ACT), radiotherapy (ART), or chemoradiotherapy (ACRT). The hazard ratios (HR) and 95% confidence intervals (CIs) of overall survival (OS) was identified as the measurement of effectiveness. A network meta-analysis was conducted to synthesize the evidence under the Bayesian framework, and the relative effects of all possible comparisons were made. The ranking analysis was performed to support the decision in clinical practice.
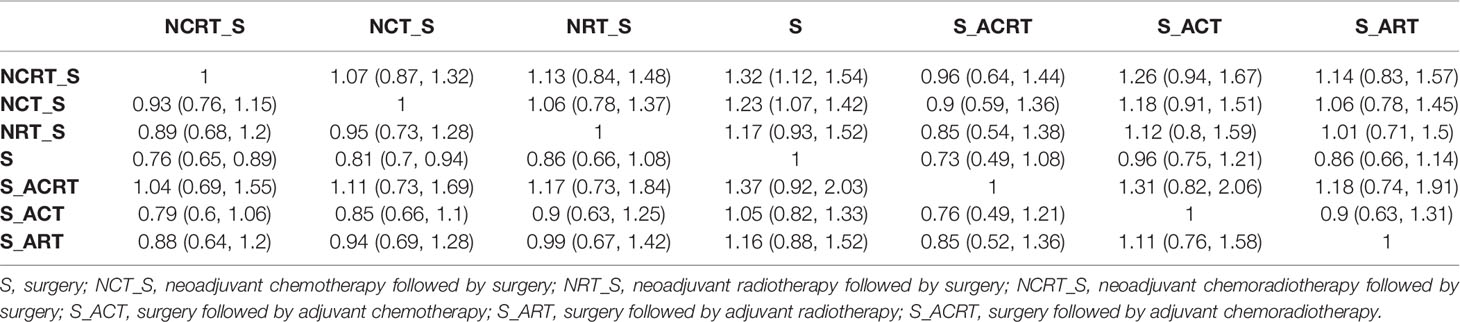
Results: A total of 19 relevant trials with 3,749 patients were identified. Compared with surgery alone, NCRT (HR 0.76, 95% CI 0.65–0.89) and NCT (HR 0.81, 95% CI 0.70–0.94) significantly improved OS, while other treatments, including NRT (HR 0.86, 95% CI 0.66–1.08), ACRT (HR 0.73, 95% CI 0.49–1.08), ACT (HR 0.96, 95% CI 0.75–1.21), and ART (HR 0.86, 95% CI 0.66–1.14), provided no significant survival advantage. None of the neoadjuvant and adjuvant treatments showed a statistically significant difference in OS to each other when compared in pairs.
Conclusion: For resectable esophageal squamous cell carcinoma, this network meta-analysis showed that NCRT may be the optimal strategy, NCT may be the second choice, while other multimodality treatments could not improve OS compared with surgery alone. It remains unclear whether ESCC will benefit from adding radiotherapy into the neoadjuvant treatment.
Systematic Review Registration: We registered this meta-analysis protocol at the prospective register of systematic reviews, PROSPERO https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=172745 (Identification code: CRD42020172745).
Introduction
Esophageal cancer is the sixth most common cause of cancer-related death worldwide, and esophageal squamous cell carcinoma (ESCC) is the most common histology subtype (1). Surgery is the mainstream treatment for resectable ESCC; however, the overall survival remains unsatisfactory if treated with surgery alone (2). Multimodality treatments based on surgery have been investigated to improve the overall survival (OS), including neoadjuvant or adjuvant chemotherapy, radiotherapy, or chemoradiotherapy. However, randomized comparisons for all these strategies are not always available. The optimal treatment for resectable ESCC remains controversial.
Network meta-analysis could simultaneously compare the overall survival of different treatments through direct and indirect comparisons to identify the most effective approach. Several network meta-analyses were conducted but with inconsistent results and some major drawbacks (3–5), resulting in insufficient evidence. Besides, several essential trials have been published in recent years (6–9), and therefore, it is necessary to perform a rigorous and updated review. This network meta-analysis compared overall survival after multimodality treatments or surgery alone to identify the most effective approach.
Methods
Systematic Review
Searching Literature
We carried out a systematic search of available literature and reported results in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (10) and AMSTAR (Assessing the methodological quality of systematic reviews) (11) Guidelines. We registered this meta-analysis protocol at the prospective register of systematic reviews, PROSPERO (Identification code: CRD42020172745). According to the pre-specified searching strategy and inclusion criteria, we systematically searched electronic databases (PubMed, Embase, and Cochrane Library) for relevant studies published before April 2021. The searching strategy was provided in Supplement 1.
Inclusion Criteria
1) Phase II/III RCTs with a sample size larger than 50 patients;
2) Trials that enrolled patients with resectable thoracic ESCC;
3) Trials that compared different treatments [surgery alone, neoadjuvant chemotherapy (NCT), radiotherapy (NRT) or chemoradiotherapy (NCRT), adjuvant chemotherapy (ACT), radiotherapy (ART) or chemoradiotherapy (ACRT)];
4) Total radiation dose should be more than 30 Gy;
5) Trials that designed OS as the primary endpoint;
6) Only full-text articles were included; if multiple publications of the same trial were retrieved, the most recent and informative paper was included.
Quality Assessment
Two reviewers independently assessed the quality of all trials using a revised Cochrane risk of bias tool for randomized trials (12), while another reviewer served as a referee in case of controversies. The full scale is constituted by the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall bias. According to the detailed guidance of RoB 2, each domain could be judged as any of the three levels: low risk, high risk, or unclear risk of bias.
The funnel plot was used to evaluate potential publication bias (13).
Data Extraction
Two reviewers extracted the data independently. Any discrepancies were discussed with a third reviewer and reached a consensus ultimately. The data obtained from each article are listed as follows: (1) first author’s name, (2) publication year, (3) sample size, (4) tumor location, (5) treatment regimens, and (6) survival data.
Statistical Analysis
Overall survival (OS) was identified as the measurement of effectiveness and expressed as hazard ratios (HR), which considered the timing and censoring of survival status. To obtain the HR and its standard error, three approaches were applied: (1) For studies that reported the summary statistics, HR was directly collected, and standard error was calculated from 95% confidence interval (CI). (2) In the absence of summary statistics, some studies published the Kaplan–Meier curve with at-risk table. Using Tierney’s method, survival rate and number at risk were extracted from the plot based on the time intervals divided schematically. The number of survival patients, deaths, and censors were estimated for every time interval. HR and standard error were calculated by combining all time intervals. (3) For studies that published the Kaplan–Meier curve with the follow-up information instead of at-risk table, similar steps were applied, whereas the estimate of censor is approximated based on a linear pattern.
Bayesian network meta-analysis was carried out to synthesize all therapeutic options within a mixed treatment comparison framework using R 3.6.0. The random-effects model was prioritized to address the study-specific effects, which were components of the overarching distribution. Uninformative prior distribution was given to all parameters. The node-split method was used to assess the inconsistency. The estimates of relative effects and 95% CI were reported. Besides, the surface under the cumulative ranking scores were calculated as well.
Results
Description of Studies Included and Quality Assessment
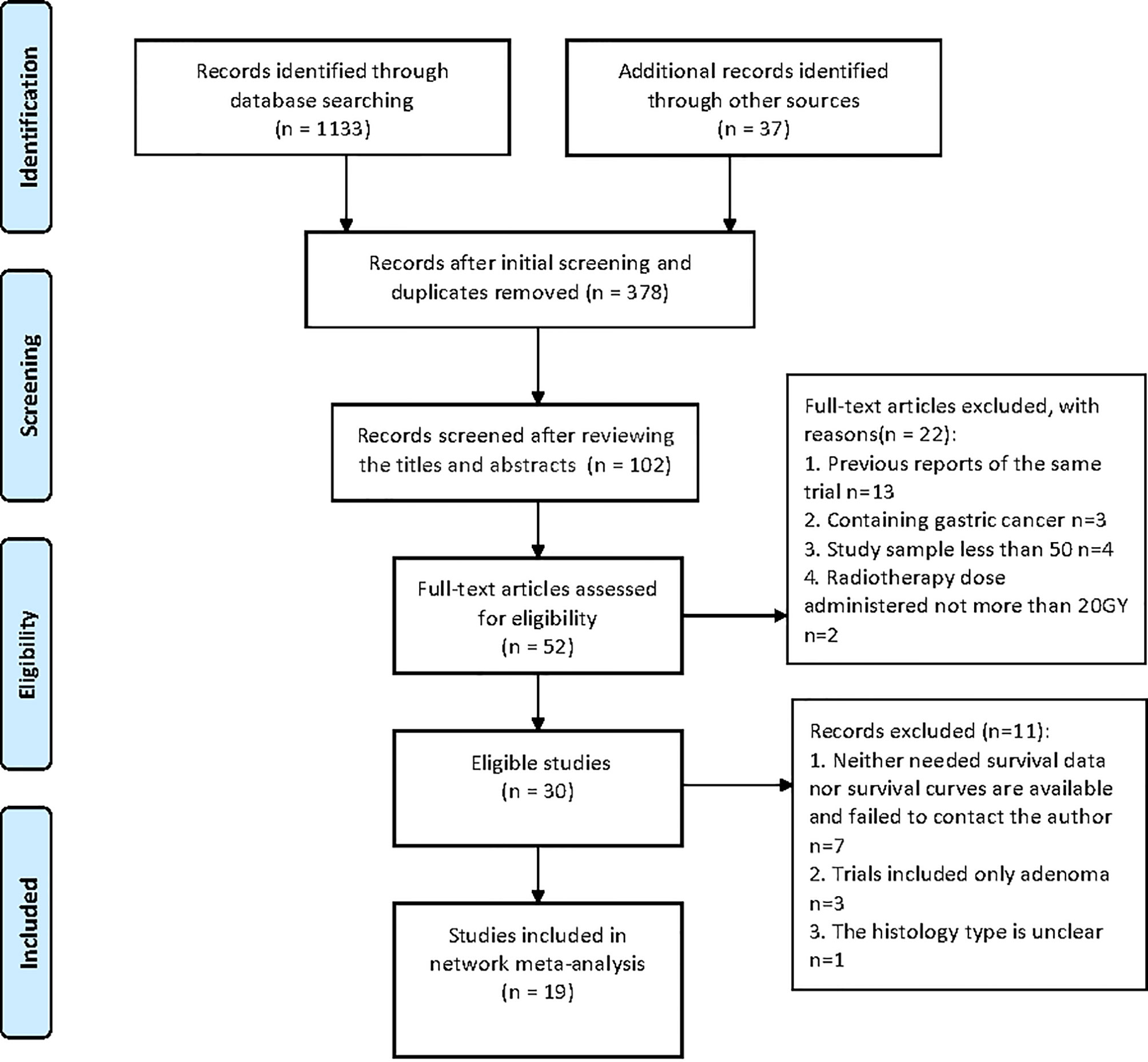
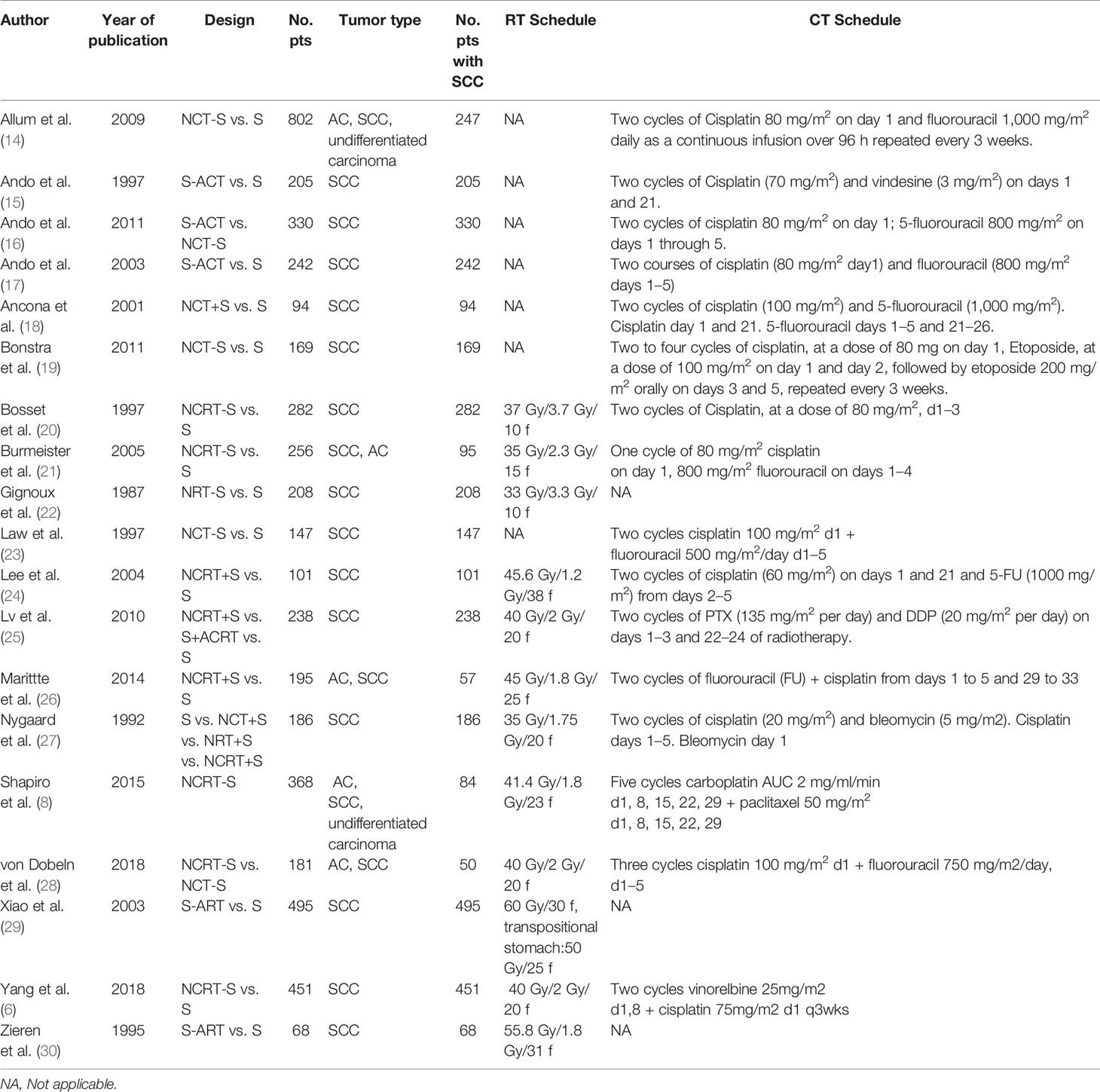
The search and selection process was displayed in a PRISMA diagram (Figure 1). Nineteen eligible trials with 3,749 patients were included, and 21 comparisons were generated (Network plot, Figure 2). The main features of the studies are shown in Table 1.
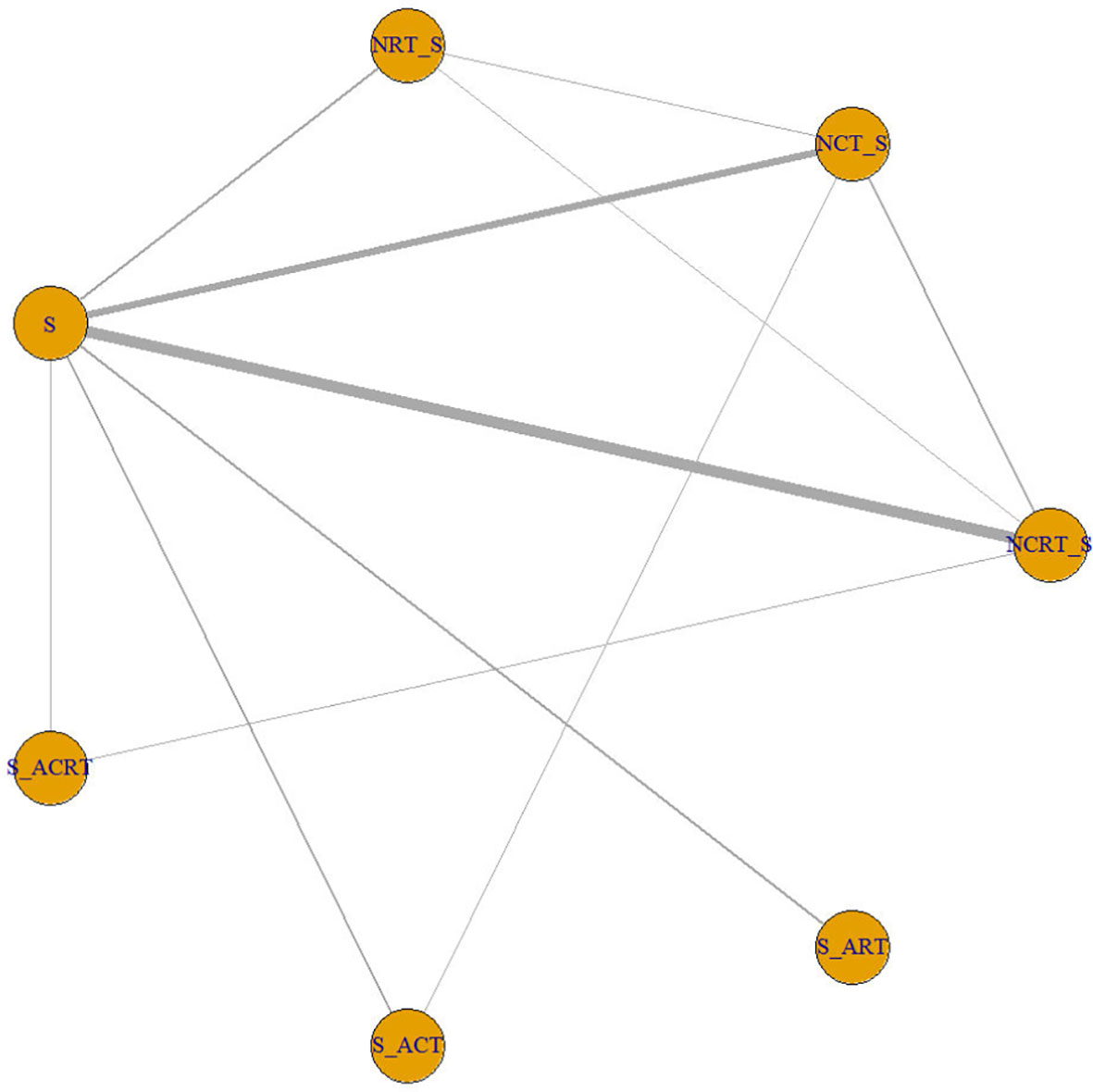
Figure 2 Network plot showing the following different treatment modalities for patients with esophageal squamous carcinoma. The node size is proportional to the total number of patients receiving specific treatment. Each line represents a type of head-to-head comparison. The width of lines is proportional to the number of trials comparing the connected treatments. S, surgery; NCT_S, neoadjuvant chemotherapy followed by surgery; NRT_S, neoadjuvant radiotherapy followed by surgery; NCRT_S, neoadjuvant chemoradiotherapy followed by surgery; S_ACT, surgery followed by adjuvant chemotherapy; S_ART, surgery followed by adjuvant radiotherapy; S_ACRT, surgery followed by adjuvant chemoradiotherapy.
We did not find an obvious risk of bias (Supplement 2). All included trials were generally comparable in terms of clinical features. We did not find a violation of the transitivity assumption. Inconsistency between direct and indirect evidence was investigated with node-split models. The network design could identify seven closed loops, and a different model was built for each of them. We did not detect a significant difference between direct and indirect treatment, except for the comparison between NRT and NCRT (p > 0.05 for all models, Supplement 3). We made a funnel plot and found no publication bias (Supplement 4).
Network Meta-Analysis of the Overall Results
The results are shown in Table 2. Compared with surgery alone, NCRT (HR 0.76, 95% credible interval 0.65–0.89) and NCT (HR 0.81, 0.70–0.94) showed favorable OS with significant difference, while NRT (HR 0.86, 0.66–1.08), ACRT (HR 0.73, 0.49–1.08), ACT (HR 0.96, 0.75–1.21), and ART (HR 0.86, 0.66–1.14) provided no significant survival advantage. Besides, multimodality treatment did not show a statistically significant difference from each other.
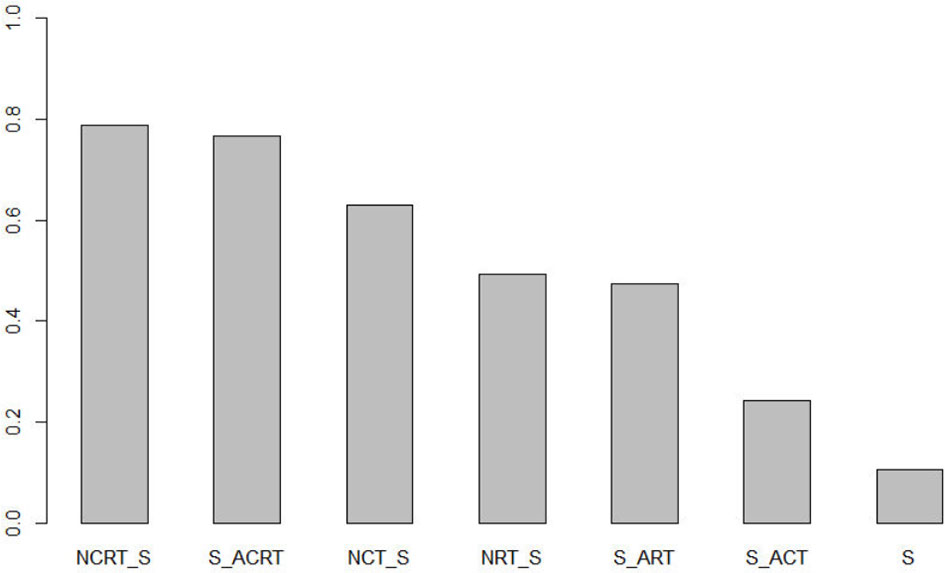
Ranking analysis (Figure 3) was used to provide a hierarchy of treatments. The surface under the cumulative ranking curve value in NCRT was 0.79, which was the highest among all seven treatments, followed by ACRT (0.77), NCT (0.63), NRT (0.50), ART (0.48), ACT (0.25), and surgery alone (0.11). NCRT is most likely to be the optimal treatment modality.
Discussion
Several previous network meta-analyses (3–5) were conducted but with inconsistent results and some major drawbacks: mixed with adenocarcinoma; including trials with low radiotherapy dose, which may overlook the benefit of radiotherapy adding to surgery; including trials with a small sample size that may not be well randomized; dating back to the 1980s, the time interval between trials was significant, which would enhance heterogeneity; outdated since several essential trials have been published in recent years (6–9). Therefore, we performed a rigorous and updated network meta-analysis including 19 RCTs.
We found that NCRT and NCT showed significantly better survival than surgery alone, while NRT and adjuvant treatments failed to show a survival benefit. Ranking analysis showed that NCRT may be the optimal treatment. This conclusion further strengthens the role of NCRT as the preferred treatment modality in resectable ESCC.
As for neoadjuvant treatments, we found that NCRT and NCT presented a survival benefit compared with surgery alone. Neoadjuvant treatments may have several advantages: tumor downstaging makes the surgical procedure more accessible and improves the radical resection rate; patients could complete the treatment plan before surgery more easily; chemotherapeutic drugs can reach the target before the local blood supply is destroyed. NRT failed to show a survival benefit compared with surgery alone. As a local treatment, radiotherapy plus surgery may exacerbate local toxicity and could not control distant metastasis.
Although NCRT ranked first in the ranking analysis, NCRT did not show a significant survival advantage compared with NCT. Several previous meta-analyses and clinical trials showed the efficacy of NCRT but not NCT. The NeoRes trial (9) revealed slightly improved survival with NCRT among patients with SCC. A meta-analysis (31) showed that NCRT had a significant survival advantage (HR 0.72; 95% CI 0.52–0.99) over NCT in SCC. Several previous meta-analyses and our study showed OS benefit for NCRT is slightly higher than NCT, but the significance for NCRT was not confirmed. Network meta-analyses are generally associated with broader confidence intervals than pairwise comparisons, partly explaining the lack of significance. Nevertheless, these results can only serve as a hypothesis-generating source. It is still not clear whether ESCC will benefit from adding radiotherapy into the neoadjuvant treatment.
Combining chemotherapy and radiotherapy in neoadjuvant therapy may have several advantages: chemotherapy can control distant metastatic tumor cells, and radiotherapy can control regional tumors; therefore, spatial cooperation may enhance antitumor efficacy. Nonetheless, NCRT was also associated with a higher risk of postoperative mortality than neoadjuvant CT or surgery alone (32). The National Comprehensive Cancer Network (NCCN) guidelines recommend NCRT for patients with localized esophageal cancer. In contrast, Guidelines in Japan recommended neoadjuvant chemotherapy for resectable stage II or III thoracic esophageal carcinoma, and for local residual tumor or local recurrence, postoperative irradiation was suggested (33). Therefore, we should balance the benefit and toxicity risks to make individualized treatment choices. Further study is needed to compare survival and toxicity differences between NCRT and NCT to identify whether radiotherapy is indispensable in neoadjuvant treatment.
Adjuvant therapy failed to show a survival benefit compared with surgery alone, though ACRT showed a marginally significant survival advantage and ranked second in the ranking analysis. Adjuvant therapy may eliminate the residual tumor, lymph node metastasis, and possible micrometastasis. However, no visible target could be used to evaluate the effect of postoperative adjuvant therapy, and compliance is poor. Thus, ACRT could be reserved for patients who did not receive neoadjuvant therapy.
There are some limits to our study. First, some of the selected studies contained AC; survival data were extracted from the subgroup analysis for these studies. Despite the random allocation of the studies, the SCC subgroup’s randomization may not be well guaranteed. Second, our report contained trials in a considerable time interval. With the development of surgery techniques, radiotherapy technology, and chemotherapy regimens, heterogeneity between studies cannot be avoided despite careful consideration. Third, our analysis contained only one NRT study. The limited number of patients included should be considered, so the result of NRT compared with other treatment modalities should be interpreted with caution. Last, this study used aggregated data rather than individual patient data; thus, unknown confounding factors may exist.
Conclusions
For resectable esophageal squamous cell carcinoma, NCRT may be the optimal strategy, and NCT may be the second choice, while other multimodality treatments could not improve OS compared with surgery alone. It remains unclear whether ESCC will benefit from adding radiotherapy into the neoadjuvant treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
ZM, MY, YB, and ZH contributed to the conception and design of the study. ZM, MY, and YB organized the database. YW performed the statistical analysis. ZM wrote the first draft of the manuscript. ZM and YW wrote sections of the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
This study is supported by the National Key Research and Development Program (2017YFC1311000 and 1311002) and CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-1-011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.728185/full#supplementary-material
References
1. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology (2020) 159(1):335–49.e15. doi: 10.1053/j.gastro. 2020.02.068
2. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal Carcinoma. Lancet (2013) 381(9864):400–12. doi: 10.1016/S0140-6736(12)60643-6
3. Huang Y, Wang H, Luo G, Zhang Y, Wang L, Li K. A Systematic Review and Network Meta-Analysis of Neoadjuvant Therapy Combined With Surgery for Patients With Resectable Esophageal Squamous Cell Carcinoma. Int J Surg (2017) 38:41–7. doi: 10.1016/j.ijsu.2016.12.035
4. Zhao Y, Wang Y, Shan L, Peng C, Zhang W, Zhao X. A Network Meta-Analysis for Neoadjuvant and Adjuvant Treatments for Resectable Squamous Cell Carcinoma of Esophagus. Sci Rep (2021) 11(1):6800. doi: 10.1038/s41598-021-86102-8
5. Montagnani F, Fornaro L, Frumento P, Vivaldi C, Falcone A, Fioretto L. Multimodality Treatment of Locally Advanced Squamous Cell Carcinoma of the Oesophagus: A Comprehensive Review and Network Meta-Analysis. Crit Rev Oncol Hematol (2017) 114:24–32. doi: 10.1016/j.critrevonc.2017.03.024
6. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/JCO.2018.79.1483
7. Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, et al. Preoperative Chemotherapy Versus Chemoradiotherapy in Locally Advanced Adenocarcinomas of the Oesophagogastric Junction (POET): Long-Term Results of a Controlled Randomised Trial. Eur J Cancer (Oxford Engl 1990) (2017) 81:183–90. doi: 10.1016/j.ejca.2017.04.027
8. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant Chemoradiotherapy Plus Surgery Versus Surgery Alone for Oesophageal or Junctional Cancer (CROSS): Long-Term Results of a Randomised Controlled Trial. Lancet Oncol (2015) 16(9):1090–8. doi: 10.1016/S1470-2045(15)00040-6
9. Klevebro F, Alexandersson von Döbeln G, Wang N, Johnsen G, Jacobsen AB, Friesland S, et al. A Randomized Clinical Trial of Neoadjuvant Chemotherapy Versus Neoadjuvant Chemoradiotherapy for Cancer of the Oesophagus or Gastro-Oesophageal Junction. Ann Oncol (2016) 27(4):660–7. doi: 10.1093/annonc/mdw010
10. Liberati A, Altman DGH, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
11. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ (2017) 358:j4008. doi: 10.1136/bmj.j4008
12. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
13. Stuck AE, Rubenstein LZ, Wieland D. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Asymmetry Detected in Funnel Plot was Probably Due to True Heterogeneity. BMJ Br Med J (1998) 316(7129):469. doi: 10.1136/bmj.315.7109.629
14. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J Clin Oncol (2009) 27(30):5062–7. doi: 10.1200/JCO.2009.22.2083
15. Ando N, Iizuka T, Kakegawa T, Isono K, Watanabe H, Ide H, et al. A Randomized Trial of Surgery With and Without Chemotherapy for Localized Squamous Carcinoma of the Thoracic Esophagus: The Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg (1997) 114(2):205–9. doi: 10.1016/S0022-5223(97)70146-6
16. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A Randomized Trial Comparing Postoperative Adjuvant Chemotherapy With Cisplatin and 5-Fluorouracil Versus Preoperative Chemotherapy for Localized Advanced Squamous Cell Carcinoma of the Thoracic Esophagus (JCOG9907). Ann Surg Oncol (2012) 19(1):68–74. doi: 10.1245/s10434-011-2049-9
17. Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, et al. Surgery Plus Chemotherapy Compared With Surgery Alone for Localized Squamous Cell Carcinoma of the Thoracic Esophagus: A Japan Clinical Oncology Group Study–Jcog9204. J Clin Oncol (2003) 21(24):4592–6. doi: 10.1200/JCO.2003.12.095
18. Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, et al. Only Pathologic Complete Response to Neoadjuvant Chemotherapy Improves Significantly the Long Term Survival of Patients With Resectable Esophageal Squamous Cell Carcinoma: Final Report of a Randomized, Controlled Trial of Preoperative Chemotherapy Versus Surgery Alone. Cancer (2001) 91(11):2165–74.
19. Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, van Berge Henegouwen MI, Ten Kate FJ, et al. Chemotherapy Followed by Surgery Versus Surgery Alone in Patients With Resectable Oesophageal Squamous Cell Carcinoma: Long-Term Results of a Randomized Controlled Trial. BMC Cancer (2011) 11:181–1. doi: 10.1186/1471-2407-11-181
20. Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al. Chemoradiotherapy Followed by Surgery Compared With Surgery Alone in Squamous-Cell Cancer of the Esophagus. N Engl J Med (1997) 337(3):161–7. doi: 10.1056/NEJM199707173370304
21. Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Resectable Cancer of the Oesophagus: A Randomised Controlled Phase III Trial. Lancet Oncol (2005) 6(9):659–68. doi: 10.1016/S1470-2045(05)70288-6
22. Gignoux M, Roussel A, Paillot B, Gillet M, Schlag P, Favre JP, et al. The Value of Preoperative Radiotherapy in Esophageal Cancer: Results of a Study of the E.O.R.T.C. World J Surg (1987) 11(4):426–32. doi: 10.1007/BF01655805
23. Law S, Fok M, Chow S, Chu KM, Wong J. Preoperative Chemotherapy Versus Surgical Therapy Alone for Squamous Cell Carcinoma of the Esophagus: A Prospective Randomized Trial. J Thorac Cardiovasc Surg (1997) 114(2):210–7. doi: 10.1016/S0022-5223(97)70147-8
24. Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, et al. A Single Institutional Phase III Trial of Preoperative Chemotherapy With Hyperfractionation Radiotherapy Plus Surgery Versus Surgery Alone for Resectable Esophageal Squamous Cell Carcinoma. Ann Oncol (2004) 15(6):947–54. doi: 10.1093/annonc/mdh219
25. Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-Term Efficacy of Perioperative Chemoradiotherapy on Esophageal Squamous Cell Carcinoma. World J Gastroenterol (2010) 16(13):1649–54. doi: 10.3748/wjg.v16.i13.1649
26. Mariette C, Dahan L, Mornex F, Maillard E, Thomas P-A, Meunier B, et al. Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Stage I and II Esophageal Cancer: Final Analysis of Randomized Controlled Phase III Trial FFCD 9901. J Clin Oncol (2014) 32(23):2416–22. doi: 10.1200/JCO.2013.53.6532
27. Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, et al. Pre-Operative Radiotherapy Prolongs Survival in Operable Esophageal Carcinoma: A Randomized, Multicenter Study of Pre-Operative Radiotherapy and Chemotherapy. The Second Scandinavian Trial in Esophageal Cancer. World J Surg (1992) 16(6):1104–9; discussion 1110. doi: 10.1016/S0022-5223(97)70147-8
28. von Döbeln GA, Klevebro F, Jacobsen AB, Johannessen HO, Nielsen NH, Johnsen G, et al. Neoadjuvant Chemotherapy Versus Neoadjuvant Chemoradiotherapy for Cancer of the Esophagus or Gastroesophageal Junction: Long-Term Results of a Randomized Clinical Trial. Dis Esophagus (2019) 32(2):1–11. doi: 10.1093/dote/doy078
29. Xiao ZF, Yang ZY, Liang J, Miao YJ, Wang M, Yin WB, et al. Value of Radiotherapy After Radical Surgery for Esophageal Carcinoma: A Report of 495 Patients. Ann Thorac Surg (2003) 75(2):331–6. doi: 10.1016/S0003-4975(02)04401-6
30. Zieren HU, Müller JM, Jacobi CA, Pichlmaier H, Müller RP, Staar S, et al. Adjuvant Postoperative Radiation Therapy After Curative Resection of Squamous Cell Carcinoma of the Thoracic Esophagus: A Prospective Randomized Study. World J Surg (1995) 19(3):444–9. doi: 10.1007/BF00299187
31. Li F, Ding N, Zhao Y, Yuan L, Mao Y. The Current Optimal Multimodality Treatments for Oesophageal Squamous-Cell Carcinoma: A Systematic Review and Meta-Analysis. Int J Surg (2018) 60:88–100. doi: 10.1016/j.ijsu.2018.10.037
32. Jin H-L, Zhu H, Ling T-S, Zhang H-J, Shi R-H. Neoadjuvant Chemoradiotherapy for Resectable Esophageal Carcinoma: A Meta-Analysis. World J Gastroenterol (2009) 15(47):5983. doi: 10.1093/dote/doy078
Keywords: esophageal squamous cell carcinoma, treatment, systemic review, network meta-analysis, survival
Citation: Ma Z, Yuan M, Bao Y, Wang Y, Men Y and Hui Z (2021) Survival of Neoadjuvant and Adjuvant Therapy Compared With Surgery Alone for Resectable Esophageal Squamous Cell Carcinoma: A Systemic Review and Network Meta-Analysis. Front. Oncol. 11:728185. doi: 10.3389/fonc.2021.728185
Received: 22 June 2021; Accepted: 23 September 2021;
Published: 20 October 2021.
Edited by:
Cornelis F.M. Sier, Leiden University, NetherlandsReviewed by:
Alessandro Boscarelli, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyHui Zhu, Shandong University, China
Jianzhong Cao, Shanxi Provincial Cancer Hospital, China
Copyright © 2021 Ma, Yuan, Bao, Wang, Men and Hui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhouguang Hui, ZHJodWl6Z0AxNjMuY29t
†These authors have contributed equally to this work
 Zeliang Ma
Zeliang Ma Meng Yuan
Meng Yuan Yongxing Bao
Yongxing Bao Yang Wang1
Yang Wang1 Yu Men
Yu Men Zhouguang Hui
Zhouguang Hui