
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 December 2021
Sec. Surgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.726569
 Yan-Jun Xiang1,2†
Yan-Jun Xiang1,2† Kang Wang2†
Kang Wang2† Yi-Tao Zheng1
Yi-Tao Zheng1 Hong-Ming Yu2
Hong-Ming Yu2 Yu-Qiang Cheng2
Yu-Qiang Cheng2 Wei-Jun Wang2
Wei-Jun Wang2 Yun-Feng Shan1*
Yun-Feng Shan1* Shu-Qun Cheng1,2*
Shu-Qun Cheng1,2*Background: Microvascular invasion (MVI) is a significant risk factor affecting survival outcomes of patients after R0 liver resection (LR) for hepatocellular carcinoma (HCC). However, whether the existing staging systems of hepatocellular carcinoma can distinguish the prognosis of patients with MVI and the prognostic value of MVI in different subtypes of hepatocellular carcinoma remains to be clarified.
Methods: A dual-center retrospective data set of 1,198 HCC patients who underwent R0 LR was included in the study between 2014 and 2016. Baseline characteristics and staging information were collected. Homogeneity and modified Akaike information criterion (AICc) were compared between each system. And the prognostic significance of MVI for overall survival (OS) was studied in each subgroup.
Results: In the entire cohort, there were no significant survival differences between Cancer of the Liver Italian Program (CLIP) score 2 and 3 (p = 0.441), and between Taipei Integrated Scoring System (TIS) score 3 and 4 (p = 0.135). In the MVI cohort, there were no significant survival differences between Barcelona Clinic Liver Cancer stages B and C (p=0.161), CLIP scores 2 and 3 (p = 0.083), TIS scores 0 and 1 (p = 0.227), TIS scores 2 and 3 (p =0.794), Tokyo scores 3 and 4 (p=0.353), and American Joint Committee on Cancer Tumor-Node-Metastasis 7th stage I and II (p=0.151). Among the eight commonly used HCC staging systems, the Hong Kong Liver Cancer (HKLC) staging system showed the highest homogeneity and the lowest AICc value in both the entire cohort and MVI cohort. In each subgroup of the staging systems, MVI generally exhibited poor survival outcomes.
Conclusions: The HKLC staging system was the most accurate model for discriminating the prognosis of MVI patients, among the eight staging systems. Meanwhile, our findings suggest that MVI may be needed to be incorporated into the current HCC staging systems as one of the grading criteria.
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related death and responsible for more than 700,000 deaths annually (1). Liver resection (LR) or liver transplantation remains the first-line treatment method for patients with early or intermediate stage of HCC (2–4). Unfortunately, the 5-year recurrence rate is as high as 70–80% after curative liver resection, which severely limits the long-term survival of patients with HCC (5, 6).
Microvascular invasion (MVI), defined as “a cancer cell nest with >50 cells in the endothelial vascular lumen under microscopy” (7), is considered an early means of cancer cell spread through the vasculature and a key factor affecting the recurrence and long-term survival of patients with HCC (8–12). However, some authors have recently suggested that MVI is not a prognostic factor for all HCC patients. The long-term survival of small HCC (≤2 cm) is excellent and not influenced by MVI (13), and the clinical value of MVI in patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B is limited (12, 14). Thus, the prognostic significance of MVI in various HCC staging systems still needs further investigation.
To date, several staging systems have been proposed to stratify HCC patients into different subgroups for better treatment decision-making and prognostic prediction (15). Among these, the BCLC staging system is recommended by the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) (2, 3). Compared with the BCLC system, the recently proposed Hong Kong Liver Cancer (HKLC) staging system provides better prognostic ability and a more aggressive treatment algorithm (16). In addition to the BCLC and HKLC systems, multiple staging systems have been proposed, including Cancer of the Liver Italian Program (CLIP), Taipei Integrated Scoring System (TIS), Tumor-Node-Metastasis (TNM) by Liver Cancer Study Group of Japan (LCSGJ), Tokyo Score, American Joint Cancer Committee (AJCC) TNM 7th edition, and Okuda staging system (17–21). Pursuing an optimal staging system for HCC has generated a gradually upward interest over the past two decades, and this lack of consensus may stem from the heterogeneity of the underlying liver diseases and different preferences for treatment modalities worldwide (22, 23). A recent study showed that the CLIP staging system is the most stable and optimal model (24). However, none of the above staging systems include MVI status in their staging criteria.
This study aimed to investigate which staging system was the relative optimal one for HCC patients with MVI and to evaluate whether MVI was an independent risk factor in various subgroups of the eight existing staging systems, and attempt to find the basis for integrating MVI into the above staging systems.
A retrospective study was conducted on consecutive HCC patients who underwent LR with curative intent at the First Affiliated Hospital of Wenzhou Medical University from March 2014 to March 2016 and the Eastern Hepatobiliary Surgery Hospital from February 2014 to January 2015. This study was approved by the Institutional Ethics Committees of the First Affiliated Hospital of Wenzhou Medical University and the Eastern Hepatobiliary Surgery Hospital. As patients’ identities were anonymized, the requirement for informed consent was waived by the Ethics Committees.
The inclusion criteria were patients with (I) HCC confirmed by postoperative histopathology and cytology, (II) well preserved liver function with Child-Pugh class A or B7, (III) LR with R0 status (no gross residual tumor under visual observation, and negative resection margins under microscopy), and (IV) preoperative imaging data of contrast-enhanced magnetic resonance imaging (MRI) of abdomen. The exclusion criteria were patients with (I) extrahepatic metastasis, (II) preoperative radiofrequency ablation, (III) recurrent HCC, (IV) a previous history of other malignancies, and (V) incomplete clinical data.
Eight staging systems include BCLC, HKLC, CLIP score, TIS score, LCSGJ, Tokyo score, AJCC TNM 7th edition, and Okuda staging systems (16–21, 25). Table 1 summarizes the key characteristics of each staging system. The detailed staging criteria are presented in the supplementary material.
MVI was defined as “a cancer cell nest with >50 cells in the endothelial vascular lumen under microscopy” (7). In this study, the method to detect MVI was the 7-point sampling protocol (7). Macrovascular invasion, including portal vein tumor thrombus (PVTT) and hepatic vein tumor thrombus (HVTT), was defined as radiological evidence of tumor invasion into the major vasculatures or their main branches. Bile duct tumor thrombus (BDTT) was defined as radiological evidence of tumor invasion into the bile duct.
Routine preoperative investigations included blood tests, coagulation profile, liver and kidney functions, hepatitis serology, serum alpha-fetoprotein (AFP), abdominal ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT) scanning. Preoperative diagnosis of HCC was based on the criteria proposed by the AASLD (2). Hepatectomy was performed as previously described (26–28). In both surgical centers in this study, anatomical resection is the first choice for a single tumor, or multiple tumors located in a single liver segment or adjacent segments. For multiple tumors involving the right and left hemilivers, anatomical resection is used for the main tumor, while non-anatomical resection with an adequate resection margin for satellite nodules (29). For patients with an insufficient residual liver volume, non-anatomical resection is used to achieve a negative resection margin. A negative margin was defined as the lack of tumor cells on microscopic examination of the resected margins of the specimen. For patients with combined macrovascular invasion or BDTT, the tumor thrombus would be removed intraoperatively either by thrombectomy or by concomitant extrahepatic bile duct resection (30–32).
All patients were regularly followed up in the outpatient clinic once every 1–3 months after discharge from hospital. At each follow-up visit, there were routine medical history taking, physical examination, laboratory blood tests, and abdominal ultrasonography or contrast enhanced CT/MRI. The primary end point of this study was overall survival (OS), which was defined as the time from initial hepatectomy to the date of death or the date of last follow-up. Disease-free survival (DFS) was defined as the time from hepatic resection to the diagnosis of tumor recurrence.
All clinical data were analyzed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) or R 4.0 software (http://www.r-project.org/). Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Univariate Cox regression analysis was used to evaluate the potential significance of each variable in the entire cohort. All variables that were significantly related to OS (p<0.05) were incorporated into the multivariate Cox regression analysis (backward stepwise selection process, p<0.05). Corrected Akaike information criterion (AICc) was obtained to reveal how staging systems were correlated with the patients’ survival. Homogeneity was measured by Wald χ2 test to assess the differences in survival of patients in the same stage within each system (33).
Of 1,198 patients at the First Affiliated Hospital of Wenzhou Medical University and the Eastern Hepatobiliary Surgery Hospital with complete clinicopathological and follow-up data, there were 510 (42.6%) patients with MVI and 688 (58.4%) patients without MVI. Table 2 summarizes the clinicopathological features of these patients. The median age was 51 years, with the majority of male (83%). Nine hundred ninety-four (83%) patients were HBV positive, 18 (2%) patients had BDTT, and 110 (9%) patients had macrovascular invasion. There were 114 (10%) patients who received neoadjuvant transcatheter arterial chemoembolization (TACE) and 507 (42%) patients who underwent adjuvant TACE. There were some differences at baseline between patients with MVI and those without MVI (Supplemental Table 1). The median follow-up for the entire cohort was 34 months.
Univariate regression analysis revealed that sex, antiviral therapy, current smoking, ascites, albumin, alanine aminotransferase (ALT), prealbumin, AFP, varicose veins of gastric fundus, BDTT, macrovascular invasion, MVI, tumor number, and maximal tumor diameter were potential risk factors of survival in HCC patients (Table 3). Multivariate regression analysis of these factors showed that current smoking, albumin, prealbumin, AFP, varicose veins of gastric fundus, BDTT, macrovascular invasion, MVI, tumor number, and maximal tumor diameter as independent risk factors of survival of patients with HCC.
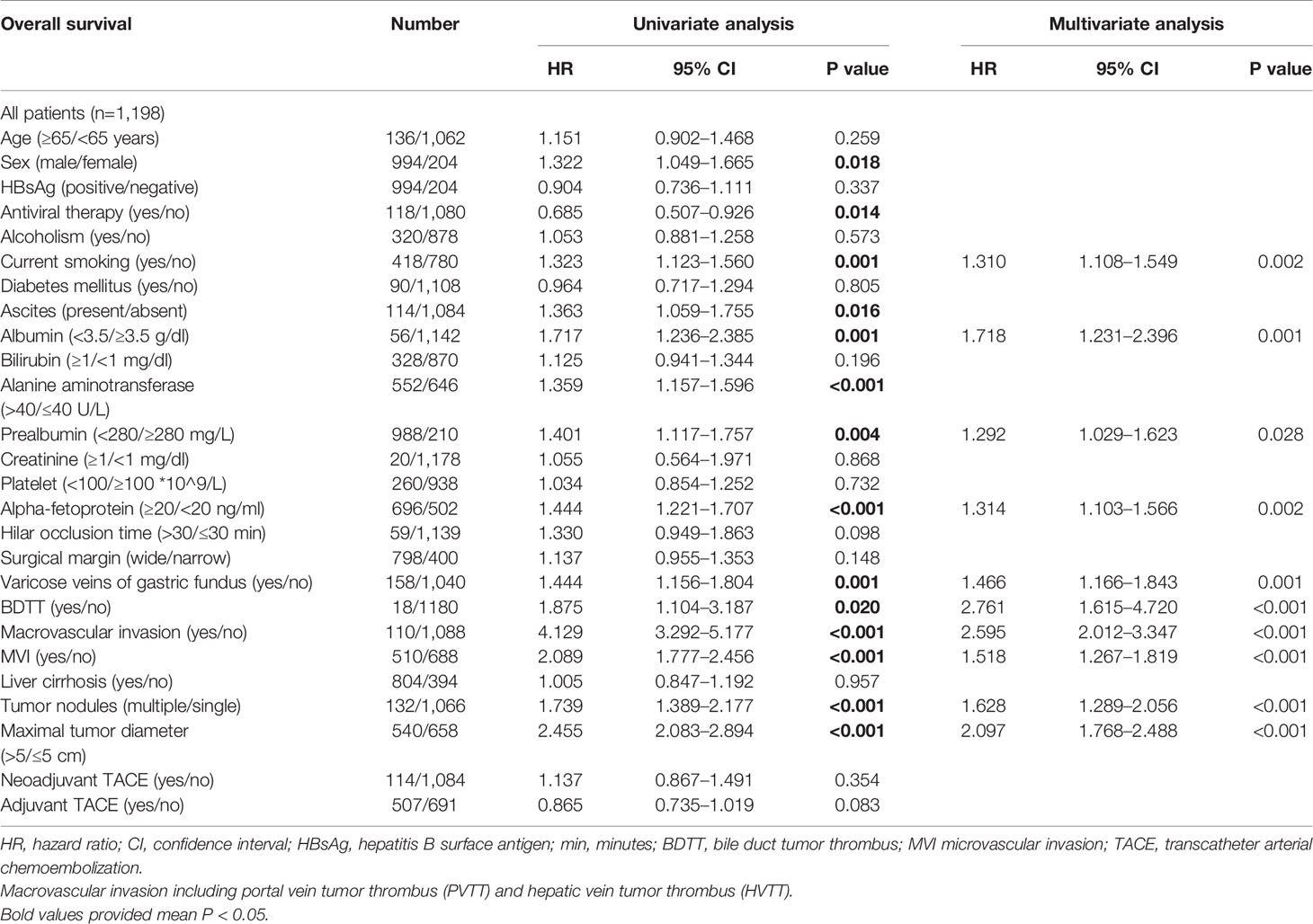
Table 3 Univariate and multivariate survival analysis in the entire hepatocellular carcinoma cohort.
The eight common HCC staging systems were evaluated respectively with Kaplan-Meier survival analysis. In the entire cohort, significant differences in survival distribution were observed for all stages of BCLC, HKLC, CLIP score, TIS score, LCSGJ, Tokyo score, TNM, and Okuda staging system (p<0.05). There were no significant survival differences between CLIP scores 2 and 3 (p = 0.441), and between TIS scores 3 and 4 (p = 0.135) (Figure 1). The role of CLIP score, TIS score, and Tokyo score in discriminating DFS was limited (Supplementary Figure 1). In the MVI cohort, significant differences in survival distribution were also found for all stages of BCLC, HKLC, CLIP score, TIS score, LCSGJ, Tokyo score, TNM, and Okuda staging system (p<0.05). There were no significant survival differences between BCLC stages B and C (p=0.161), CLIP scores 2 and 3 (p = 0.083), TIS scores 0 and 1 (p = 0.227), TIS scores 2 and 3 (p = 0.794), Tokyo scores 3 and 4 (p=0.353), and TNM stages I and II (p=0.151) (Figure 2). The discrimination ability for DFS of the eight staging systems in patients with MVI is detailed in Supplementary Figure 2.
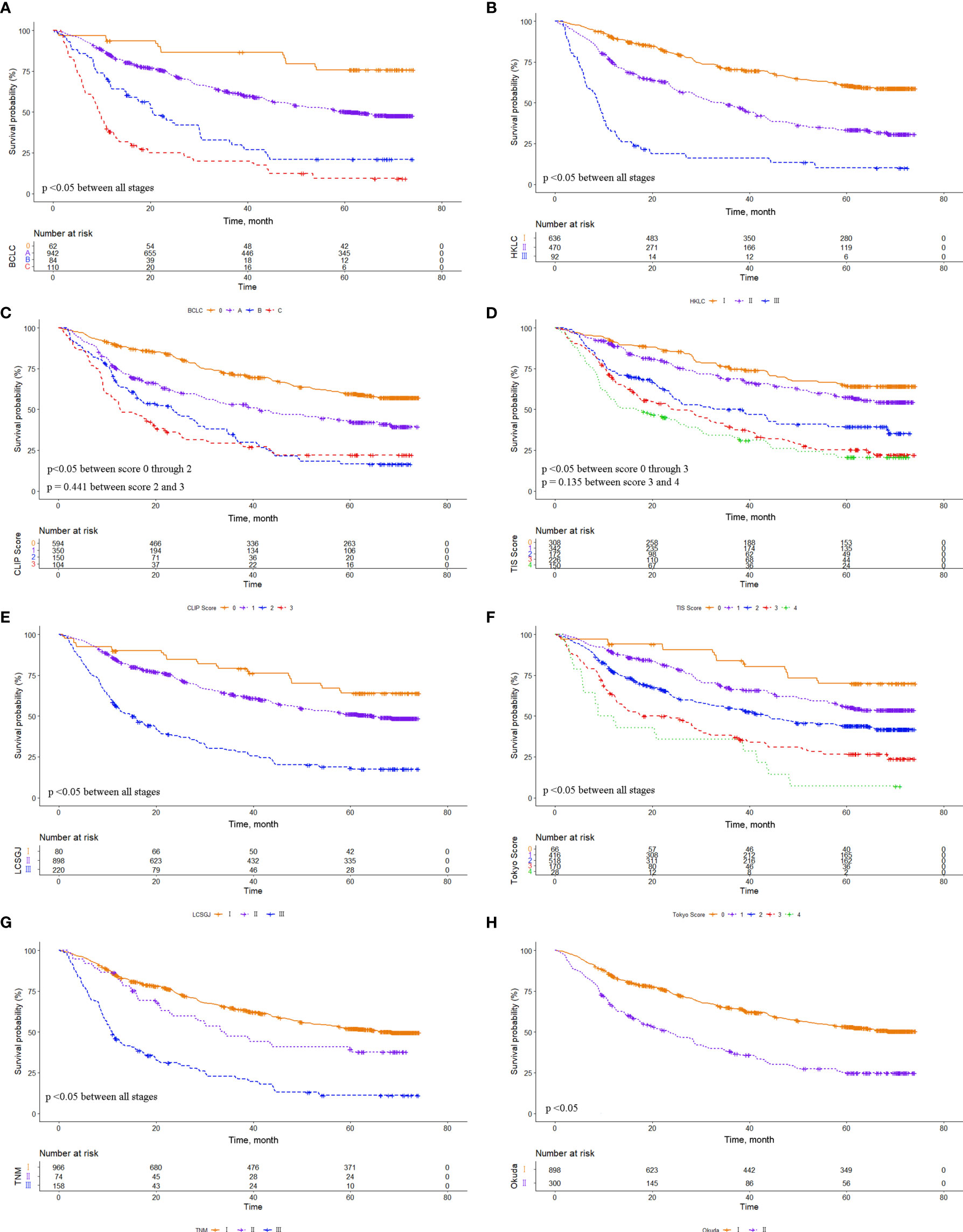
Figure 1 Comparison of overall survival distributions by (A) Barcelona Clinic Liver Cancer, (B) Hong Kong Liver Cancer, (C) Cancer of the Liver Italian Program, (D) Taipei Integrated Scoring, (E) Tumor-Node-Metastasis by Liver Cancer Study Group of Japan, (F) Tokyo, (G) Tumor-Node-Metastasis by American Joint Cancer Committee 7th edition, and (H) Okuda staging systems in the entire cohort.
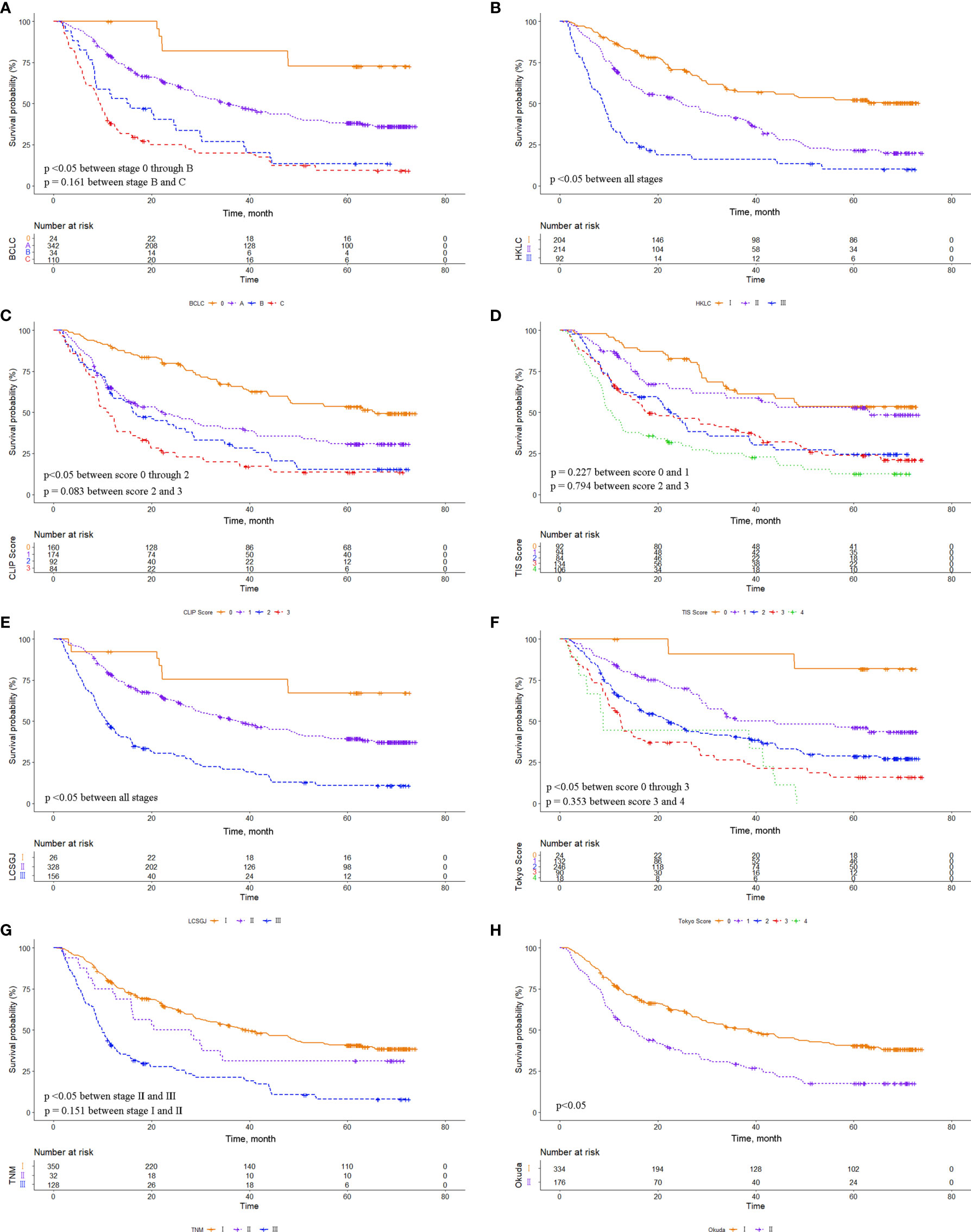
Figure 2 Comparison of overall survival distributions by (A) Barcelona Clinic Liver Cancer, (B) Hong Kong Liver Cancer, (C) Cancer of the Liver Italian Program, (D) Taipei Integrated Scoring, (E) Tumor-Node-Metastasis by Liver Cancer Study Group of Japan, (F) Tokyo, (G) Tumor-Node-Metastasis by American Joint Cancer Committee 7th edition, and (H) Okuda staging systems in the microvascular invasion (MVI) cohort.
The prognostic performance of the eight staging systems is shown in Table 4. In all patient cohorts, the HKLC system provided the lowest AICc value and the highest homogeneity, followed by the TIS and BCLC system. In the MVI cohorts, the HKLC was consistently associated with the lowest AICc value and the highest homogeneity.
In BCLC staging system, there was no significant difference in survival between patients with and without MVI in stages 0 (p=0.75) and B (p=0.1), and patients with MVI had worse survival in stage A (p<0.001). Notably, we detected MVI in all patients with macrovascular invasion. In HKLC staging system, patients with MVI had worse survival in stages I and II (p<0.001). In CLIP scoring system, there was no significant difference in score 2 (p=0.17), and patients with MVI had worse survival in scores 0, 1, and 3 (p<0.05) (Supplementary Figure 3). In TIS scoring system, there was no significant difference in scores 1 (p=0.062) and 3 (p=0.28), and patients with MVI had worse survival in scores 0, 2, and 4 (p<0.05). In LCSGJ staging system, there was no significant difference in stage I (p=0.78), and patients with MVI had worse survival in stages II and III (p<0.001) (Supplementary Figure 4). In Tokyo scoring system, there was no significant difference in scores 0 (p=0.12) and 4 (p=0.56), and patients with MVI had worse survival in scores 1, 2, and 3 (p<0.001). In TNM staging system, there was no significant difference in stage II (p=0.087), and patients with MVI had worse survival in stages I and III (p<0.05) (Supplementary Figure 5). And in Okuda staging system, patients with MVI had worse survival for stages I and II (p<0.001) (Supplementary Figure 6).
We adjusted the effect of MVI on overall survival in all subgroups. The adjusted HR was calculated by multivariable COX regression model with covariates listed in Supplementary Table 1. There was no significant difference in CLIP score 0 (adjusted HR 1.273, 95% CI 0.942–1.720, p=0.116), while patients with MVI had worse survival in CLIP scores 2 (adjusted HR 1.868, 95% CI 1.111–3.141, p=0.018) and TIS score 1 (adjusted HR 1.675, 95% CI 1.105–2.539, p=0.015).
MVI is one of the most important prognostic factors in patients with HCC (8–12). At present, there are many models to predict the occurrence of MVI before operation and to assess the prognosis of MVI patients (26, 34, 35). The predictors in these models include tumor diameter, tumor number, and other factors that have also existed in the current HCC staging systems. Therefore, whether the current HCC staging systems are able to distinguish the prognosis of MVI patients and whether the prognostic significance of MVI in different subgroups of HCC staging systems are worth exploring. In this study, we collected data of HCC patients who underwent radical surgery from two high-volume clinical centers. Possible prognostic factors were examined, and eight staging systems were evaluated. We confirmed that the key prognostic factors of HCC include current smoking, albumin, prealbumin, AFP, varicose veins of gastric fundus, BDTT, macrovascular invasion, MVI, tumor number, and maximal tumor diameter. We also demonstrated that among the eight staging systems currently used, HKLC is the best prognostic model and provides a better prognostic prediction ability. The results were consistent in the MVI cohort.
In this study, important prognostic factors for HCC were identified. There is no doubt that patients’ bad living habits, such as smoking, will reduce their survival (36, 37). Albumin and prealbumin levels are closely related to the severity of liver cirrhosis, and it is not surprising that they can predict adverse outcomes in patients with HCC (24). Alpha-fetoprotein level, macrovascular invasion, MVI, and tumor load have been considered as important prognostic indexes (38, 39). Consistent with our previous studies, our results showed that gastric varices and BDTT reflect poor survival outcomes in HCC patients (26, 40).
Using Kaplan-Meier survival analysis, we showed that all eight HCC staging systems were associated with a trend of gradually decreasing survival from early to advanced stages. The survival difference was obvious for each system in the whole cohort. However, the survival difference was insignificant between CLIP score 2/3, TIS score 3/4, which may be related to the fact that our included patients all underwent LR, and most patients were subject to HBV infection. In the MVI cohort, there were no significant survival differences among BCLC stage B/C, CLIP score 2/3, TIS score 0/1, TIS score 2/3, Tokyo score 2/3, and TNM 7th stage I/II, which may be attributed to the fact that MVI decreased overall patient survival, and the prognostic value of MVI differed among the various subgroups.
In subgroup analysis, we found that patients in BLCL stages 0 and B, CLIP score 0, TIS scores 3, LCSGJ stage I, Tokyo scores 0 and 4, and TNM 7th stage II, with or without MVI, had no significant impact on patients’ survival. To the best of our knowledge, this phenomenon of different prognostic significance of MVI among various subgroups has been previously documented. Huang et al. reported that the clinical value of microvascular invasion in HCC patients at BCLC stage 0 or B was limited (12). Different surgical approaches and various resection ranges of patients may markedly affect the MVI status in the residual liver, and there are some other clinical factors that will affect the prognosis of HCC patients at different stages. Chan et al. refined the seventh edition of AJCC TNM staging and incorporated MVI status into the T staging, and the updated T staging can better stratify HCC patients into subsets with distinct long-term prognosis (41). In addition, the TNM staging of the eighth edition AJCC describes vascular invasion as “For pathological classification, vascular invasion includes gross as well as microscopic involvement of vessels.” These data indicate that MVI can influence the accuracy of the existing HCC staging systems, and imply that the modification of the existing HCC staging system is imperative.
Several limitations of this study must be acknowledged. First, this is a retrospective study with its inherent defects. Second, because MVI can only be diagnosed postoperatively, it is not allowed to discuss this for patients who are not candidates for surgery. Third, the used method to diagnose MVI was the 7-point sampling protocol, which can lead to under-diagnosis of MVI. Fourth, this study was conducted in China, where HBV infection rate is high, and HBV is associated with a high incidence of MVI (42–45). The results of this study may not be applicable to HCC patients with other etiologic factors.
In summary, our results suggested that the HKLC staging system is the most accurate prognostic model among the eight commonly used HCC staging systems. In each subgroup of the staging systems, although MVI showed different prognostic value, it generally exhibited poor survival outcomes. At the same time, our results showed that MVI may be needed to be incorporated into the current HCC staging systems as one of the grading criteria.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Ethics Committees of the First Affiliated Hospital of Wenzhou Medical University and the Eastern Hepatobiliary Surgery Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conception and Design: S-QC, Y-FS, Y-JX, and KW. Financial Support: S-QC Y-FS, and KW. Provision of Study Materials or Patients: Y-JX, KW, Y-TZ, and H-MY. Collection and Assembly of Data: Y-JX, KW, Y-QC, and W-JW. Data Analysis and Interpretation: Y-JX, KW, and Y-TZ. Manuscript Writing: All authors. Final Approval of Manuscript: All authors. All authors contributed to the article and approved the submitted version.
This work was supported by the Clinical Research Plan of SHDC (No. SHDC2020CR1004A), the State Key Program of National Natural Science Foundation of China (No: 81730097), the National Natural Science Foundation of China (No: 82072618 and 81770630), and the Science and Technology Commission Foundation of Shanghai Municipality (No: 19411967300).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.726569/full#supplementary-material
Supplementary Figure 1 | Comparison of disease-free survival distributions by (A) Barcelona Clinic Liver Cancer, (B) Hong Kong Liver Cancer, (C) Cancer of the Liver Italian Program, (D) Taipei Integrated Scoring, (E) Tumor-Node-Metastasis by Liver Cancer Study Group of Japan, (F) Tokyo, (G) Tumor-Node-Metastasis by American Joint Cancer Committee 7th edition, and (H) Okuda staging systems in the entire cohort.
Supplementary Figure 2 | Comparison of disease-free survival distributions by (A) Barcelona Clinic Liver Cancer, (B) Hong Kong Liver Cancer, (C) Cancer of the Liver Italian Program, (D) Taipei Integrated Scoring, (E) Tumor-Node-Metastasis by Liver Cancer Study Group of Japan, (F) Tokyo, (G) Tumor-Node-Metastasis by American Joint Cancer Committee 7th edition, and (H) Okuda staging systems in the microvascular invasion (MVI) cohort.
Supplementary Figure 3 | Cumulative overall survival (OS) curves of patients with or without microvascular invasion (MVI). (A) BCLC stage 0, (B) BCLC stage A, (C) BCLC stage B, (D) HKLC stage I, (E) HKLC stage II, (F) CLIP score 0, (G) CLIP score 1, (H) CLIP score 2, (I) CLIP score 3. BCLC, Barcelona Clinic Liver Cancer; HKLC, Hong Kong Liver Cancer; CLIP, Cancer of the Liver Italian Program.
Supplementary Figure 4 | Cumulative overall survival (OS) curves of patients with or without microvascular invasion (MVI). (A) TIS score 0, (B) TIS score 1, (C) TIS score 2, (D) TIS score 3, (E) TIS score 4, (F) LCSGJ stage I, (G) LCSGJ stage II, (H) LCSGJ stage III. TIS, Taipei Integrated Scoring System; LCSGJ, Liver Cancer Study Group of Japan.
Supplementary Figure 5 | Cumulative overall survival (OS) curves of patients with or without microvascular invasion (MVI). (A) Tokyo score 0, (B) Tokyo score 1, (C) Tokyo score 2, (D) Tokyo score 3, (E) Tokyo score 4, (F) AJCC TNM 7th stage I, (G) AJCC TNM 7th stage II, (H) AJCC TNM 7th stage III. AJCC, American Joint Cancer Committee; TNM, Tumor-Node-Metastasis.
Supplementary Figure 6 | Cumulative overall survival (OS) curves of patients with or without microvascular invasion (MVI). (A) Okuda stage I, (B) Okuda stage II.
HCC, hepatocellular carcinoma; LR, liver resection; MVI, microvascular invasion; BCLC, Barcelona Clinic Liver Cancer; EASL, European Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; HKLC, Hong Kong Liver Cancer; CLIP, Cancer of the Liver Italian Program; TIS, Taipei Integrated Scoring System; TNM, Tumor-Node-Metastasis; LCSGJ, Liver Cancer Study Group of Japan; AJCC, American Joint Cancer Committee; PVTT, portal vein tumor thrombus; HVTT, hepatic vein tumor thrombus; BDTT, bile duct tumor thrombus; AFP, alpha-fetoprotein; MRI, magnetic resonance imaging; CT, computed tomography; OS, overall survival; AICc, Corrected Akaike information criterion; ALT, alanine aminotransferase.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
3. European Association for the Study of the Liver, Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
4. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese Clinical Guidelines for the Management of Hepatocellular Carcinoma: Updates and Insights. Hepatobiliary Surg Nutr (2020) 9(4):452–63. doi: 10.21037/hbsn-20-480
5. Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, et al. Improved Results of Liver Resection for Hepatocellular Carcinoma on Cirrhosis Give the Procedure Added Value. Ann Surg (2001) 234(1):71–8. doi: 10.1097/00000658-200107000-00011
6. Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic Review of Outcomes of Liver Resection for Early Hepatocellular Carcinoma Within the Milan Criteria. Br J Surg (2012) 99(12):1622–9. doi: 10.1002/bjs.8915
7. Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, et al. Practice Guidelines for the Pathological Diagnosis of Primary Liver Cancer: 2015 Update. World J Gastroenterol (2016) 22(42):9279–87. doi: 10.3748/wjg.v22.i42.9279
8. Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A Systematic Review of Microvascular Invasion in Hepatocellular Carcinoma: Diagnostic and Prognostic Variability. Ann Surg Oncol (2013) 20(1):325–39. doi: 10.1245/s10434-012-2513-1
9. Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular Invasion is a Better Predictor of Tumor Recurrence and Overall Survival Following Surgical Resection for Hepatocellular Carcinoma Compared to the Milan Criteria. Ann Surg (2011) 254(1):108–13. doi: 10.1097/SLA.0b013e31821ad884
10. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting Survival After Liver Transplantation in Patients With Hepatocellular Carcinoma Beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol (2009) 10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5
11. Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular Invasion in Patients With Hepatocellular Carcinoma and its Predictable Clinicopathological Factors. Ann Surg Oncol (2008) 15(5):1375–82. doi: 10.1245/s10434-008-9846-9
12. Huang C, Zhu XD, Ji Y, Ding GY, Shi GM, Shen YH, et al. Microvascular Invasion has Limited Clinical Values in Hepatocellular Carcinoma Patients at Barcelona Clinic Liver Cancer (BCLC) Stages 0 or B. BMC Cancer (2017) 17(1):58. doi: 10.1186/s12885-017-3050-x
13. Shindoh J, Andreou A, Aloia TA, Zimmitti G, Lauwers GY, Laurent A, et al. Microvascular Invasion Does Not Predict Long-Term Survival in Hepatocellular Carcinoma Up to 2 Cm: Reappraisal of the Staging System for Solitary Tumors. Ann Surg Oncol (2013) 20(4):1223–9. doi: 10.1245/s10434-012-2739-y
14. Shen J, Wen J, Li C, Wen T, Yan L, Li B, et al. The Prognostic Value of Microvascular Invasion in Early-Intermediate Stage Hepatocelluar Carcinoma: A Propensity Score Matching Analysis. BMC Cancer (2018) 18(1):278. doi: 10.1186/s12885-018-4196-x
15. Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, et al. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg (2016) 263(6):1112–25. doi: 10.1097/SLA.0000000000001556
16. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer Staging System With Treatment Stratification for Patients With Hepatocellular Carcinoma. Gastroenterology (2014) 146(7):1691–700 e3. doi: 10.1053/j.gastro.2014.02.032
17. Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, et al. Proposal of a New Prognostic Model for Hepatocellular Carcinoma: An Analysis of 403 Patients. Gut (2005) 54(3):419–25. doi: 10.1136/gut.2003.035055
18. Kudo M, Chung H, Osaki Y. Prognostic Staging System for Hepatocellular Carcinoma (CLIP Score): Its Value and Limitations, and a Proposal for a New Staging System, the Japan Integrated Staging Score (JIS Score). J Gastroenterol (2003) 38(3):207–15. doi: 10.1007/s005350300038
19. Chevret S, Trinchet J-C, Mathieu D, Rached AA, Beaugrand M, Chastang C. A New Prognostic Classification for Predicting Survival in Patients With Hepatocellular Carcinoma. J Hepatol (1999) 31(1):133–41. doi: 10.1016/s0168-8278(99)80173-1
20. Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, et al. A New Prognostic Model for Hepatocellular Carcinoma Based on Total Tumor Volume: The Taipei Integrated Scoring System. J Hepatol (2010) 53(1):108–17. doi: 10.1016/j.jhep.2010.01.038
21. Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of Primary Hepatocellular Carcinoma. Hepatology (1984) 4(1 Suppl):3S–6S. doi: 10.1002/hep.1840040703
22. Chapiro J, Geschwind JF. Hepatocellular Carcinoma: Have We Finally Found the Ultimate Staging System for HCC? Nat Rev Gastroenterol Hepatol (2014) 11(6):334–6. doi: 10.1038/nrgastro.2014.67
23. Subramaniam S, Kelley RK, Venook AP. A Review of Hepatocellular Carcinoma (HCC) Staging Systems. Chin Clin Oncol (2013) 2(4):33. doi: 10.3978/j.issn.2304-3865.2013.07.05
24. Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, et al. Prognosis of Hepatocellular Carcinoma: Assessment of Eleven Staging Systems. J Hepatol (2016) 64(3):601–8. doi: 10.1016/j.jhep.2015.10.029
25. Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of Hepatocellular Carcinoma. Hepatology (2005) 42(5):1208–36. doi: 10.1002/hep.20933
26. Zhang XP, Wang K, Wei XB, Li LQ, Sun HC, Wen TF, et al. An Eastern Hepatobiliary Surgery Hospital Microvascular Invasion Scoring System in Predicting Prognosis of Patients With Hepatocellular Carcinoma and Microvascular Invasion After R0 Liver Resection: A Large-Scale, Multicenter Study. Oncologist (2019) 24(12):e1476–e88. doi: 10.1634/theoncologist.2018-0868
27. Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who Have Hepatocellular Carcinoma With Microvascular Invasion. Ann Surg Oncol (2016) 23(4):1344–51. doi: 10.1245/s10434-015-5008-z
28. Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial Hepatectomy With Wide Versus Narrow Resection Margin for Solitary Hepatocellular Carcinoma: A Prospective Randomized Trial. Ann Surg (2007) 245(1):36–43. doi: 10.1097/01.sla.0000231758.07868.71
29. Zhang YF, Zhou J, Wei W, Zou RH, Chen MS, Lau WY, et al. Intermediate-Stage Hepatocellular Carcinoma Treated With Hepatic Resection: The NSP Score as an Aid to Decision-Making. Br J Cancer (2016) 115(9):1039–47. doi: 10.1038/bjc.2016.301
30. Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, et al. Effects of Location and Extension of Portal Vein Tumor Thrombus on Long-Term Outcomes of Surgical Treatment for Hepatocellular Carcinoma. Ann Surg Oncol (2006) 13(7):940–6. doi: 10.1245/ASO.2006.08.007
31. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Liver Resection for Hepatocellular Carcinoma Associated With Hepatic Vein Invasion: A Japanese Nationwide Survey. Hepatology (2017) 66(2):510–7. doi: 10.1002/hep.29225
32. Kasai Y, Hatano E, Seo S, Taura K, Yasuchika K, Uemoto S. Hepatocellular Carcinoma With Bile Duct Tumor Thrombus: Surgical Outcomes and the Prognostic Impact of Concomitant Major Vascular Invasion. World J Surg (2015) 39(6):1485–93. doi: 10.1007/s00268-015-2985-9
33. Pourhoseingholi MA, Hajizadeh E, Moghimi Dehkordi B, Safaee A, Abadi A, Zali MR. Comparing Cox Regression and Parametric Models for Survival of Patients With Gastric Carcinoma. Asian Pac J Cancer Prev (2007) 8(3):412–6.
34. Li X, Huang H, Yu X, Chen P, Ouyang J, Huang B. A Novel Prognostic Nomogram Based on Microvascular Invasion and Hematological Biomarkers to Predict Survival Outcome for Hepatocellular Carcinoma Patients. Surg Oncol (2020) 33:51–7. doi: 10.1016/j.suronc.2020.01.006
35. Wang ZX, Peng W, Zhang XY, Wen TF, Li C. Prognostic Significance of Postoperative Change of PALBI Grade for Patients With Hepatocellular Carcinoma After Hepatectomy. Medicine (Baltimore) (2021) 100(11):e24476. doi: 10.1097/MD.0000000000024476
36. Soerjomataram I, Bray F. Planning for Tomorrow: Global Cancer Incidence and the Role of Prevention 2020-2070. Nat Rev Clin Oncol (2021) 18(10):663–72. doi: 10.1038/s41571-021-00514-z
37. Rutledge SM, Asgharpour A. Smoking and Liver Disease. Gastroenterol Hepatol (N Y) (2020) 16(12):617–25.
38. Toyoda H, Kumada T, Tada T, Yama T, Mizuno K, Sone Y, et al. Differences in the Impact of Prognostic Factors for Hepatocellular Carcinoma Over Time. Cancer Sci (2017) 108(12):2438–44. doi: 10.1111/cas.13406
39. Zhang LX, Luo PQ, Chen L, Song DD, Xu AM, Xu P, et al. Model to Predict Overall Survival in Patients With Hepatocellular Carcinoma After Curative Hepatectomy. Front Oncol (2020) 10:537526. doi: 10.3389/fonc.2020.537526
40. Sun J, Wu J, Liu C, Shi J, Wei Y, Zhou J, et al. Typing of Biliary Tumor Thrombus Influences the Prognoses of Patients With Hepatocellular Carcinoma. Cancer Biol Med (2021) 18(3):808–15. doi: 10.20892/j.issn.2095-3941.2020.0202
41. Chan AC, Fan ST, Poon RT, Cheung TT, Chok KS, Chan SC, et al. Evaluation of the Seventh Edition of the American Joint Committee on Cancer Tumour-Node-Metastasis (TNM) Staging System for Patients Undergoing Curative Resection of Hepatocellular Carcinoma: Implications for the Development of a Refined Staging System. HPB (Oxford) (2013) 15(6):439–48. doi: 10.1111/j.1477-2574.2012.00617.x
42. Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg (2016) 151(4):356–63. doi: 10.1001/jamasurg.2015.4257
43. Wei X, Li N, Li S, Shi J, Guo W, Zheng Y, et al. Hepatitis B Virus Infection and Active Replication Promote the Formation of Vascular Invasion in Hepatocellular Carcinoma. BMC Cancer (2017) 17(1):304. doi: 10.1186/s12885-017-3293-6
44. Xu J, Liu H, Chen L, Wang S, Zhou L, Yun X, et al. Hepatitis B Virus X Protein Confers Resistance of Hepatoma Cells to Anoikis by Up-Regulating and Activating P21-Activated Kinase 1. Gastroenterology (2012) 143(1):199–212 e4. doi: 10.1053/j.gastro.2012.03.053
Keywords: microvascular invasion, hepatocellular carcinoma, staging system, prognosis, bi-centeric
Citation: Xiang Y-J, Wang K, Zheng Y-T, Yu H-M, Cheng Y-Q, Wang W-J, Shan Y-F and Cheng S-Q (2021) Prognostic Value of Microvascular Invasion in Eight Existing Staging Systems for Hepatocellular Carcinoma: A Bi-Centeric Retrospective Cohort Study. Front. Oncol. 11:726569. doi: 10.3389/fonc.2021.726569
Received: 17 June 2021; Accepted: 29 November 2021;
Published: 16 December 2021.
Edited by:
Gianluca Rompianesi, University of Naples Federico II, ItalyCopyright © 2021 Xiang, Wang, Zheng, Yu, Cheng, Wang, Shan and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Feng Shan, c2hhbnl1bmZlbmdAd211LmVkdS5jbg==; Shu-Qun Cheng, Y2hlbmdzaHVxdW5Ac21tdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.