- 1Human Cancer Genomic Research, Research Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 2Department of Pediatric Hematology-Oncology, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 3Department of Surgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 4Department of Pathology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Background: Papillary Thyroid Cancer (PTC) is the most common endocrine malignancy, with recurrence rate as high as 30%. A great deal of controversy surrounds the significance of microscopic extrathyroidal extension (m-ETE) as a prognostic factor. The most recent edition (8th) of American Joint Committee on Cancer (AJCC) staging system has removed m-ETE from the definition of pT3, which suggests that m-ETE may lack prognostic impact in PTC patients. Moreover, data about m-ETE prevalence and clinical impact on Middle Eastern PTC remains unknown. We therefore investigate the prevalence of m-ETE and its clinico-pathological correlation and prognostic impact in Middle Eastern PTC. We also compared the AJCC 7th and 8th staging systems and their prognostic performance.
Methods: PTCs from 1430 consecutive adult (> 18 years) patients from single tertiary care hospital were included in this study. A retrospective analysis of PTC patients’ survival and recurrence were compared between AJCC 8th and AJCC 7th staging systems using Proportion of Variation Explained (PVE) and Harrell’s C-index.
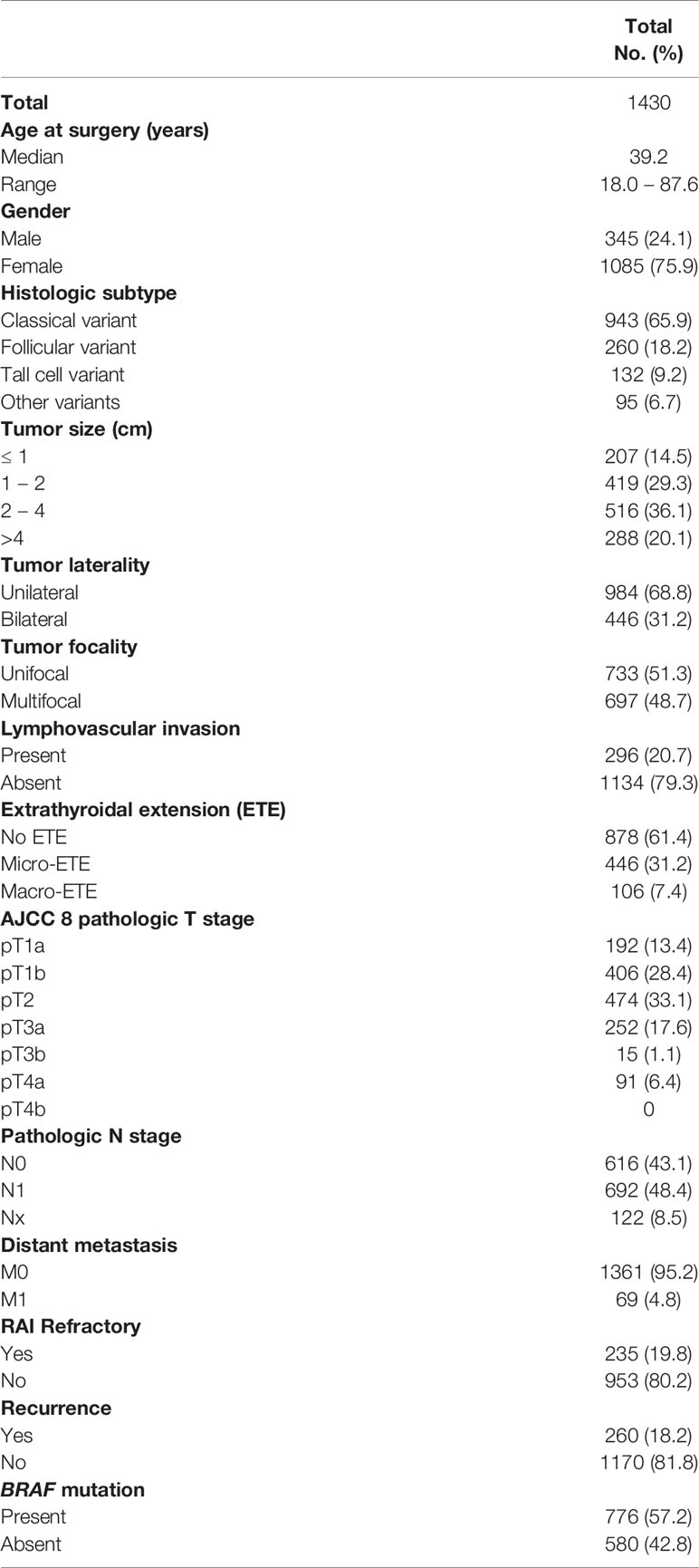
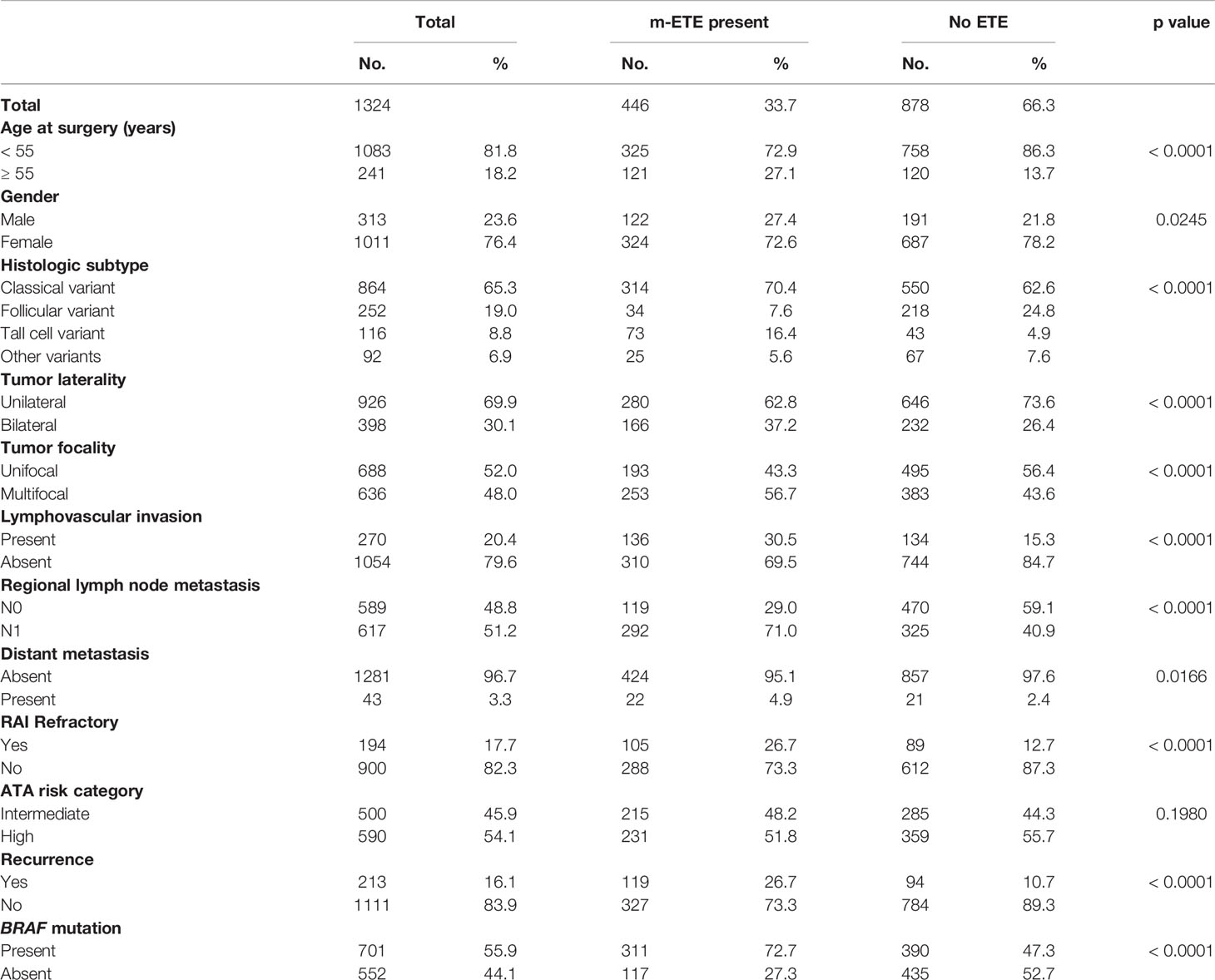
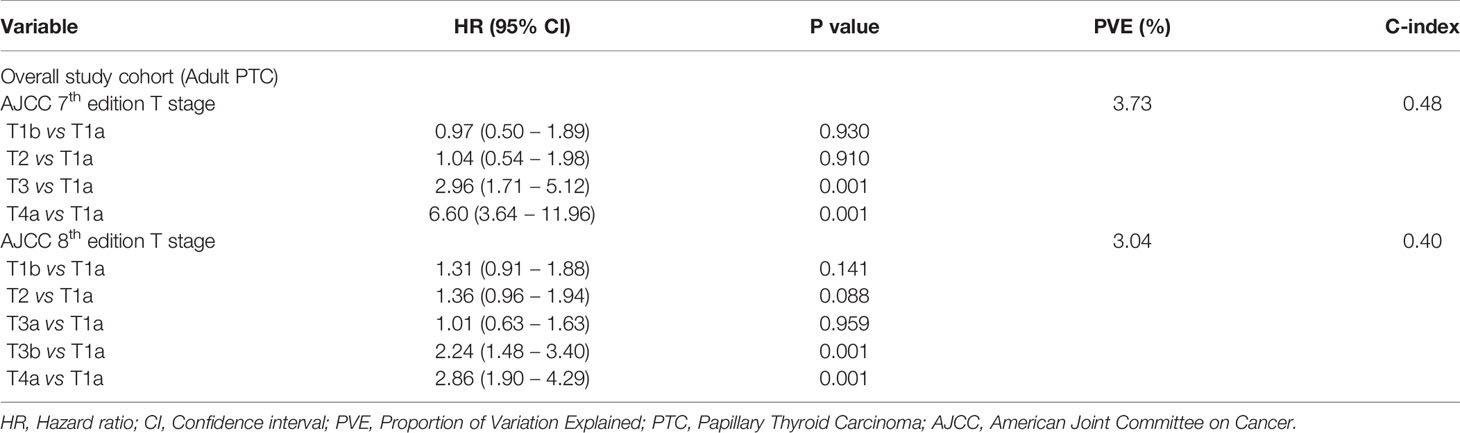
Results: Median follow up of the study cohort was 9.3 years. 31.2% (446/1430) of patients had m-ETE. In the overall cohort, m-ETE was associated with multiple adverse features such as older age (p < 0.0001), male sex (p = 0.0245), tall cell variant (p < 0.0001), bilateral tumors (p < 0.0001), multifocality (p < 0.0001), lymphovascular invasion (p < 0.0001), lymph node metastasis (p < 0.0001), distant metastasis (p = 0.0166), tumor recurrence (p < 0.0001), radioactive iodine refractoriness (p < 0.0001), BRAF mutation (p < 0.0001) and reduced recurrence-free survival (RFS; HR = 1.75; 95% CI = 1.30 – 2.35; p < 0.0001) irrespective of tumor size. Of the 611 patients with T3 disease based on AJCC 7th edition, 359 (58.8%) were down-staged in AJCC 8th edition classification. Overall, the prognostic performance of AJCC 8th edition was inferior to AJCC 7th on the basis of lower PVE (3.04% vs. 3.73%) and lower C-index (0.40 vs. 0.48).
Conclusions: In Middle Eastern PTC, m-ETE is significantly associated with compromised survival and acts as an independent predictor of RFS. Given these findings, m-ETE should be included in the thyroid cancer treatment guidelines.
Introduction
The incidence of thyroid cancer has rapidly increased over the past two decades (1, 2). Papillary thyroid cancer (PTC) is the most common thyroid malignancy and generally carries favorable prognosis (3–5), whereby low risk PTC patients have excellent outcome with conservative treatment, such as adequate surgery and TSH suppressive therapy (6, 7). However, a subset of PTC patients present with aggressive disease and experience recurrence, leading to poor prognosis (8–10). Therefore, identifying tumors with potentially aggressive behavior and increased likelihood of recurrence is crucial for therapeutic decision-making and appropriate patient management. Interestingly, PTC is one of the most common cancers in Saudi Arabia and is the second commonest cancer affecting Saudi females (11). Middle Eastern PTCs show a relatively higher rate of recurrence than in Western countries (12–15). Therefore, identifying patients at risk of recurrence is a critical step in the management of high risk PTCs, so that appropriate treatment can be initiated.
A number of prognostic factors, including age, sex, histology, tumor size, vascular invasion, lymph node metastasis and extra-thyroidal extension (ETE) have been identified as predictors of recurrence and patient outcome (16–20). However, there has been considerable controversy regarding microscopic ETE (m-ETE) (as determined histologically using microscopic evaluation) and its prognostic significance, as well as its association with recurrence (21–23). The American Thyroid Association (ATA) guidelines for predicting recurrence considers patients with m-ETE to be at an intermediate risk for recurrence (6), suggesting that these patients should be treated with more aggressive treatment and radioiodine (RAI) ablation. In agreement with this, the European Thyroid Association is also in favor of RAI ablation for PTC patients presenting with m-ETE (24).
Although ATA guidelines are widely used, the American Joint Committee on Cancer (AJCC) TNM staging remains the most commonly used system for PTC staging. The most recent edition (8th) of AJCC staging system has removed m-ETE from the definition of pT3 disease, and minimized the clinical impact of m-ETE, down staging it from T3 to T1/2 classification compared to the 7th edition (25). These changes reflect doubts on the ability to accurately identify m-ETE by histopathologist and its clinical prognostic impact in PTC. Whether the presence of m-ETE directly impacts clinical outcome and patient management is a matter of strong debate. While some studies have reported that m-ETE does not affect the disease free survival or risk of recurrence (22, 26–28), others report a negative impact on the clinical outcome of PTCs with m-ETE (21, 29, 30).
However, none of the above studies were conducted on PTCs from Middle Eastern ethnicity and the clinico-pathological associations as well as prognostic impact of m-ETE still remains unknown in this ethnicity. Therefore, we carried out this study to investigate the incidence and clinical impact of m-ETE as predictor of patient’s prognosis in Middle Eastern PTCs treated at our institute whilst also comparing the prognostic performance of AJCC 7th (pT-7) and AJCC 8th (pT-8) edition T staging systems.
Materials and Methods
Patient Selection
One thousand four-hundred and thirty consecutive unselected adult PTC patients (> 18 years) diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were included in the study. Cases were identified based on clinical history followed by fine needle aspiration cytology for confirmation. The Institutional Review Board of the hospital approved this study and the Research Advisory Council (RAC) provided waiver of consent under project RAC # 2110 031 and 2211 168.
Clinico-Pathological Data
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Extra-thyroidal extension was further classified, based on previous publications (31–33), as follows: microscopic ETE was defined as tumor extending beyond the thyroid capsule into the surrounding peri-thyroidal soft tissues of fat and/or skeletal muscle, without visual evidence of this invasion and macroscopic ETE defined as visual evidence of tumor invasion into strap muscles, subcutaneous soft tissue, larynx, trachea, esophagus, recurrent laryngeal nerve or prevertebral fascia. Staging of PTC was performed using the AJCC seventh and eighth edition staging systems. Only structural recurrence (local, regional or distant) was considered for analysis. Recurrence was defined as any newly detected tumor or metastatic lymph node based on ultrasound and/or imaging studies in patients who had been previously free of disease following initial treatment. RAI refractory disease and ATA risk categories were defined based on 2015 ATA guidelines (6).
BRAF Mutation Analysis
BRAF mutation data for the entire PTC cohort was available from our previous study (34).
Follow-Up and Study Endpoint
Patients were regularly followed by both physical examinations and imaging studies to identify tumor recurrence. The median follow-up was 9.3 years (range 1.0 – 30.1 years). The primary study endpoint for our analysis was recurrence-free survival (RFS). RFS was defined as the time (in months) from date of initial surgery to the occurrence of any tumor recurrence (local, regional or distant). In case of no recurrence, date of last follow-up was the study endpoint.
Statistical Analysis
The associations between clinico-pathological variables and extrathyroidal extension was performed using contingency table analysis and Chi square tests. Mantel-Cox log-rank test was used to evaluate recurrence-free survival. Survival curves were generated using the Kaplan-Meier method. Cox proportional hazards model was used for multivariate analysis. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP11.0 (SAS Institute, Inc., Cary, NC) software package.
The relative prognostic performance of each T staging system was evaluated using the Proportion of Variation Explained (PVE) and Harrell’s Concordance Index (C-index). The PVE in Cox-proportional hazard model was calculated to compare the relative validity of models with AJCC 7th and 8th T stages. The PVE ranges from 0% to 100%, with a higher number indicating better predictability (35). Additionally, we evaluated the predictive capacity of the two models using the Harrell’s C-index. It is commonly used to evaluate risk models in survival analysis (36, 37). A model with perfect predictive capacity (sensitivity and specificity of 100%) would have a Harrell’s C-index of 1·00; a category that exhibited a higher Harrell’s c-index was considered to exhibit a more accurate predictive capacity. C-index and PVE were calculated using R version 4.0.1.
Results
Patient and Tumor Characteristics
Median age of the study population was 39.2 years (range: 18 – 88 years), with a male to female ratio of 1:3. The majority of tumors were classical variant of PTC (65.9%; 943/1430). 31.2% (446/1430) of tumors were bilateral and 48.7% (697/1430) were multifocal. m-ETE was noted in 31.2% (446/1430), whereas 7.4% (106/1430) of tumors showed macroscopic ETE. Tumor recurrence was seen in 18.2% (260/1430) (Table 1).
Clinico-Pathological Associations of Microscopic Extrathyroidal Extension
We examined the clinico-pathological associations of m-ETE in our cohort. For this purpose, we excluded patients with macroscopic ETE (n = 106). Of the remaining 1324 PTCs, 33.7% (446/1324) showed m-ETE, whereas 66.3% (878/1324) had no ETE. m-ETE was significantly associated with adverse clinico-pathological characteristics such as age ≥ 55 years (p < 0.0001), male sex (p = 0.0245), tall cell variant (p < 0.0001), bilateral tumors (p < 0.0001), multifocality (p < 0.0001), lymphovascular invasion (p < 0.0001), regional lymph node metastasis (p < 0.0001), distant metastasis (p = 0.0166), poor RAI response (p < 0.0001) and tumor recurrence (p < 0.0001). We also found a significant association between m-ETE and BRAF mutation (p < 0.0001) (Table 2). Furthermore, patients exhibiting m-ETE showed a significantly reduced RFS (p < 0.0001) (Figure 1A). Since the AJCC 8th classification preferred tumor size over m-ETE for classifying patients as T3a, we sought to determine whether tumor size or m-ETE was an independent predictor of RFS. On multivariate analysis, m-ETE was an independent predictor of RFS (HR = 1.75; 95% CI = 1.30 – 2.35; p < 0.0001), irrespective of tumor size (Table 3). We also analyzed the overall survival (OS) and found that m-ETE was associated with poor OS only on univariate analysis (p < 0.0001) (Figure 1B) but not on multivariate analysis (HR = 1.73; 95% CI = 0.82 – 3.77; p = 0.1503).
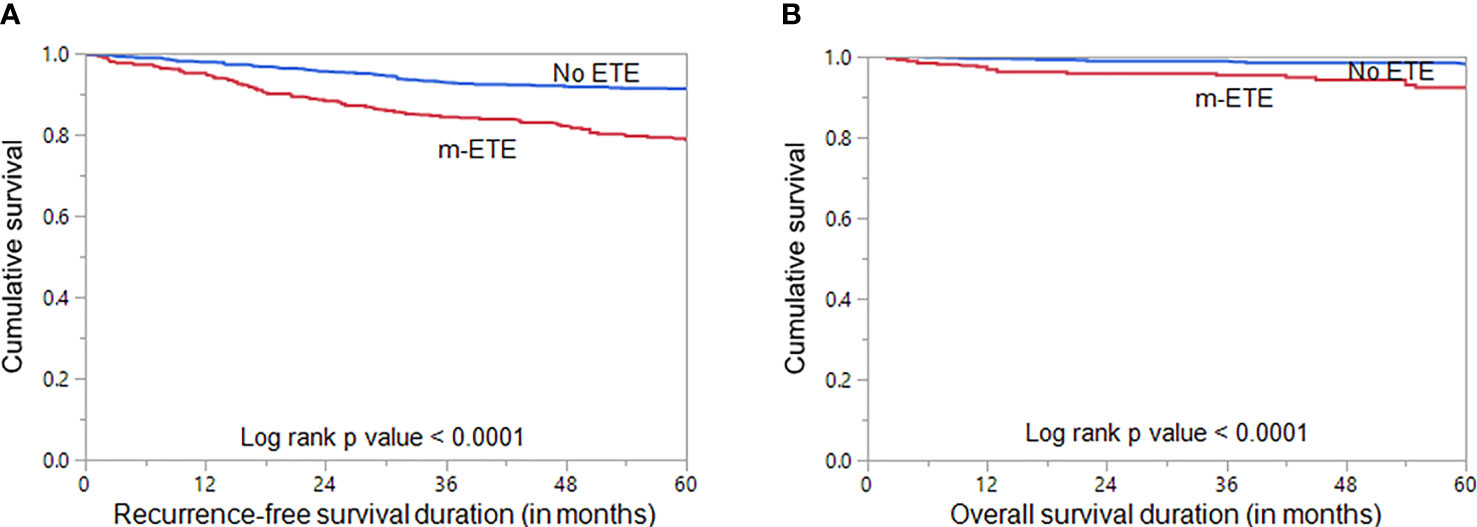
Figure 1 Survival Analysis of microscopic extrathyroidal extension (m-ETE). Kaplan Meier survival plot showing statistically significant poor (A) recurrence-free survival (p < 0.0001) and (B) overall survival (p < 0.0001) in PTC patients with m-ETE compared to those with no ETE.
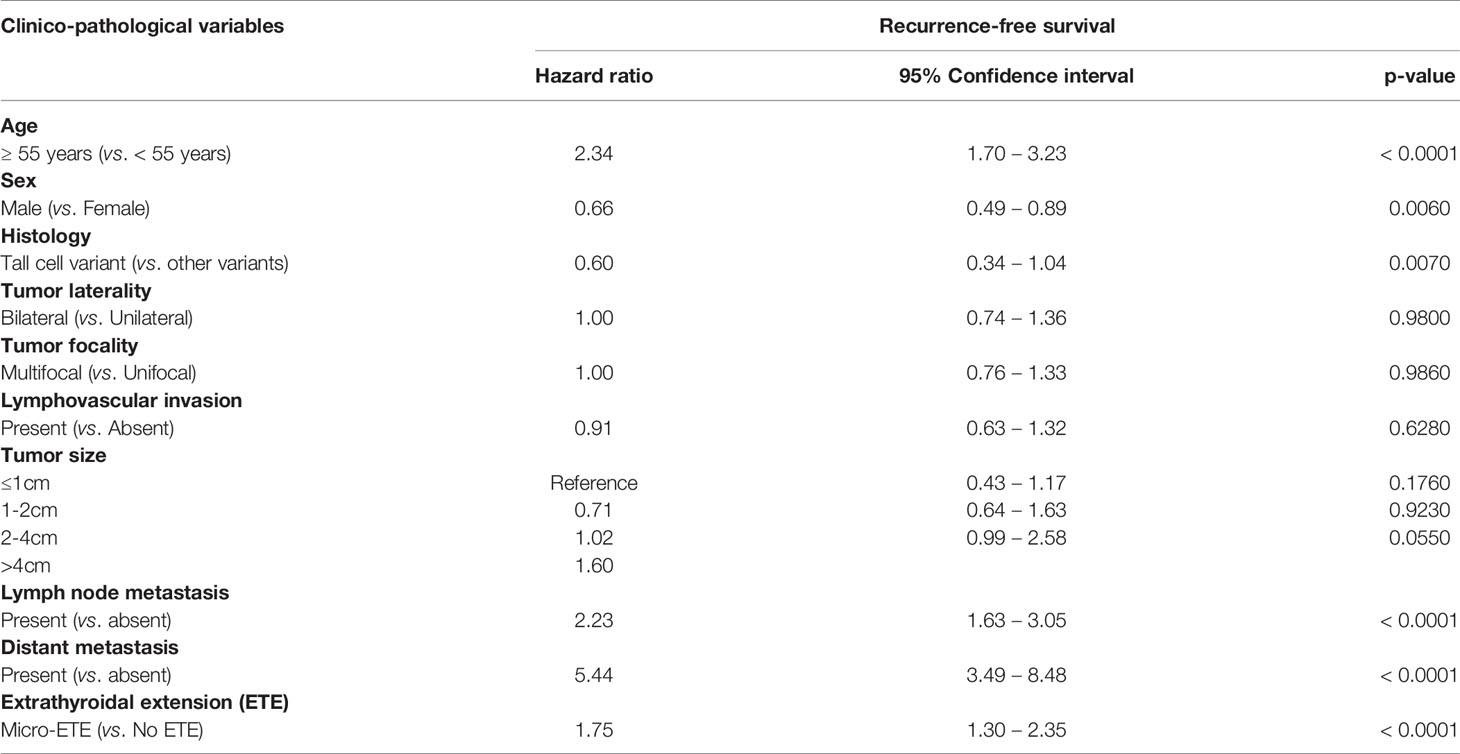
Table 3 Multivariate analysis of microscopic extrathyroidal extension using Cox Proportional Hazard Model for Recurrence-free survival.
Prognostic Performance of AJCC 7 and 8 Classifications
Of 611 patients with T3 disease based on AJCC 7th edition classification, 359 (58.8%) were down-staged in the AJCC 8th edition classification. Among the 359 T3 patients who were down-staged in AJCC8 classification, 166 patients were downstaged to T1 and 193 patients were downstaged to T2. Twenty-two percent (79/359) of the downstaged patients developed tumor recurrence. Recurrence was noted in 18.7% (31/166) of patients who were downstaged from T3 to T1 and in 24.2% (48/193) of patients who were downstaged from T3 to T2 (Table 4). Since the proportion of recurrence in downstaged patients was higher than the overall cohort (22.0% vs. 18.2%), we next analyzed the prognostic performance of AJCC pT-7 and AJCC pT-8 classifications. Overall, the prognostic performance of pT-8 was inferior to pT-7 on the basis of lower PVE (3.04% vs 3.73%) and lower C-index (0.40 vs 0.48) (Table 5).
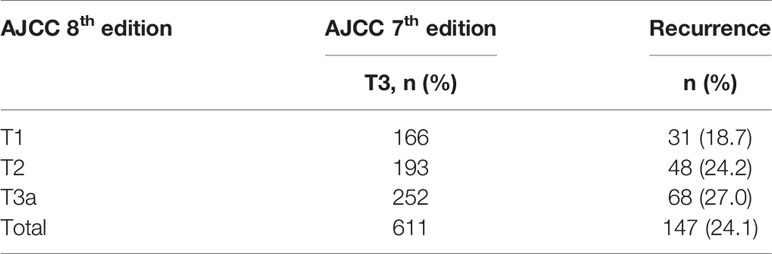
Table 4 Pathological tumor stage migration of AJCC 7th edition T3 tumors and incidence of recurrence.
Discussion
In the era of personalized medicine and risk-tailored management, risk stratification is necessary to provide appropriate therapy and predict response to the initial treatment. Recent research has indicated that m-ETE only exerts minor effect on patient prognosis and therapy decisions (28, 38, 39). The updated 8th edition of the AJCC staging system removed the sub classification of m-ETE, resulting in down staging of T3 tumors. Therefore, we conducted this study to evaluate, for the first time, the clinico-pathological characteristics and clinical impact of m-ETE on patient outcome in a large cohort of Middle Eastern PTC.
In our study, the overall incidence of m-ETE was 31.2% in PTCs. Interestingly, m-ETE was associated with several adverse clinico-pathological factors such as older age group, tall cell variant, multifocality, lymphovascular invasion, regional lymph node metastasis, and radioiodine therapy refractiveness. Moreover, strong correlation between the presence of m-ETE and BRAF mutation was noted, with 72.7% of m-ETE PTC having concurrent BRAF mutations.
There is considerable controversy regarding the prognostic role of m-ETE. We found m-ETE to be associated with poor OS in univariate analysis only. Previous studies have found contrasting results, with some showing an unfavorable impact of m-ETE on OS (33, 40), whereas others found no association (28). Interestingly, in the present research, m-ETE was associated with higher risk of recurrence compared to PTCs without m-ETE and multivariate analysis revealed that m-ETE was an independent prognostic marker for poor RFS. This raises the likelihood that patients presenting with m-ETE might have more aggressive disease and are therefore less likely to respond well to initial treatment. With a median follow-up of 9.3 years, the present study revealed more recurrent disease among patients with m-ETE. Indeed, we showed that the presence of m-ETE conferred a 2.5-fold increased risk of recurrence for all ages (p < 0.0001; Table 2). Several previous attempts to identify the prognostic impact of m-ETE on patient outcome have shown conflicting results. Park et al. (41) analyzed a cohort of 734 PTC patients and found that m-ETE was an important prognostic factor associated with RFS. Similar studies by Radowsky et al. (21) and Tran et al. (42), on large cohorts of PTC, also showed that m-ETE was associated with recurrence and RFS. In contrast, other studies failed to find any prognostic impact for m-ETE. Nixon et al. (22) studied 984 patients (115 with m-ETE and 869 without m-ETE) and found no significant difference in 10-year disease-specific survival or RFS between the two groups. Hay et al. (38), Arora et al. (43) and Shin et al. (44), in large cohorts of PTC, also found no difference in RFS between patients with m-ETE and no ETE. Our results are the first to show that m-ETE is an independent predictor of poor RFS in this study population, irrespective of tumor size, and supports the inclusion of m-ETE in future AJCC T staging.
Although the AJCC staging system is primarily used as a predictor of mortality, recent studies have shown the utility of this staging system as a novel tool for predicting PTC recurrence (42, 45, 46). In addition, we were intrigued by the exclusion of m-ETE from the AJCC 8th edition staging system and hence sought to determine which of the two staging systems (AJCC 7 or AJCC 8 T stage) was a better predictor of recurrence. Therefore, we further compared the prognostic performance of the AJCC 7th and 8th edition staging system in the whole cohort and found the ability of pT-7 to predict RFS was superior to pT-8 based on different models’ performance regardless of age or the presence of distant metastasis. The superiority of the prognostic impact of pT-7 and pT-8 was highlighted in a previous study (42), where however, it was seen only in PTC patients ≥ 55 years old without distant metastasis. Another important highlight of this study is the significant association between m-ETE and poor response to RAI, which could reflect the potential influence of m-ETE on treatment decision. However, this needs to be confirmed by further studies, since our data only showed this correlation on univariate analysis. Collectively, our results support the inclusion of m-ETE in risk stratification, as in previous AJCC TNM editions and the ATA risk of recurrence guidelines.
Despite the obvious strengths of our study, including the use of more than 1400 PTCs from a unique ethnicity, the presence of comprehensive clinical and follow-up data and the finding of m-ETE to be a robust independent prognostic factor for RFS, irrespective of tumor size, our study should be viewed in light of a few limitations. Our study was a retrospective and single center study, which could carry selection bias, and hence more prospective multicenter studies in Middle Eastern population are needed. Additionally, we do acknowledge that inter-observer variability for the interpretation of m-ETE is a source of debate (47). However, the histopathologic sections have been reviewed by at least two pathologists to minimize the inter-observer and intra-observer variability. Despite our efforts, we cannot deny the effect of inter-observer and intra-observer variability in our study. Therefore, our conclusions should be interpreted with caution.
In conclusion, our study shows that m-ETE plays an important role in PTC patients from Middle Eastern ethnicity. We found that m-ETE alone is associated with aggressive PTC markers and is an independent marker for poor RFS. Thus omitting minimal ETE from the definition of T3 disease could compromise patient care and management, resulting in these patients being less likely to undergo RAI therapy. Therefore, our results support the inclusion of m-ETE in risk stratification models, such as the AJCC 7th edition and the ATA risk of recurrence guidelines, for Middle Eastern PTC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The Institutional Review Board of King Faisal Specialist Hospital and Research Centre approved this study and the Research Advisory Council (RAC) provided waiver of consent under project RAC # 2110 031 and 2211 168. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SP and AS analyzed the clinical data, designed and wrote the manuscript. ZQ and KS performed statistical analysis. FD performed clinical data abstraction. SA-S and FA-D contributed samples and analyzed clinical data. KA-K designed, implemented the study, wrote and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid Cancer Incidence Trends in the United States: Association With Changes in Professional Guideline Recommendations. Thyroid (2020) 30(8):1132–40. doi: 10.1089/thy.2019.0415
2. Kitahara CM, Sosa JA. The Changing Incidence of Thyroid Cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
3. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Minimal Extrathyroid Extension Does Not Affect the Relapse-Free Survival of Patients With Papillary Thyroid Carcinoma Measuring 4 Cm or Less Over the Age of 45 Years. Surg Today (2006) 36(1):12–8. doi: 10.1007/s00595-005-3090-8
4. Ito Y, Miyauchi A, Kihara M, Fukushima M, Higashiyama T, Miya A. Overall Survival of Papillary Thyroid Carcinoma Patients: A Single-Institution Long-Term Follow-Up of 5897 Patients. World J Surg (2018) 42(3):615–22. doi: 10.1007/s00268-018-4479-z
5. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
7. Iñiguez-Ariza NM, Brito JP. Management of Low-Risk Papillary Thyroid Cancer. Endocrinol Metab (2018) 33(2):185. doi: 10.3803/EnM.2018.33.2.185
8. Coca-Pelaz A, Shah JP, Hernandez-Prera JC, Ghossein RA, Rodrigo JP, Hartl DM, et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv Ther (2020) 37:3112–28. doi: 10.1007/s12325-020-01391-1
9. Ritter A, Mizrachi A, Bachar G, Vainer I, Shimon I, Hirsch D, et al. Detecting Recurrence Following Lobectomy for Thyroid Cancer: Role of Thyroglobulin and Thyroglobulin Antibodies. J Clin Endocrinol Metab (2020) 105(6):e2145–e51. doi: 10.1210/clinem/dgaa152
10. Póvoa AA, Teixeira E, Bella-Cueto MR, Melo M, Oliveira MJ, Sobrinho-Simões M, et al. Clinicopathological Features as Prognostic Predictors of Poor Outcome in Papillary Thyroid Carcinoma. Cancers (2020) 12(11):3186. doi: 10.3390/cancers12113186
11. Alrawaji, Alshahrani, Alzahrani, Alomran, Almadouj, Alshehri, et al. Cancer Incidence Report Saudi Arabia 2015. Council SH, editor. Riyadh: Saudi Cancer Registry (2018).
12. Siraj AK, Parvathareddy SK, Qadri Z, Siddiqui K, Al-Sobhi SS, Al-Dayel F, et al. Annual Hazard Rate of Recurrence in Middle Eastern Papillary Thyroid Cancer Over a Long-Term Follow-Up. Cancers (2020) 12(12):3624. doi: 10.3390/cancers12123624
13. Alzahrani AS, Alomar H, Alzahrani N. Thyroid Cancer in Saudi Arabia: A Histopathological and Outcome Study. Int J Endocrinol (2017) 8423147. doi: 10.1155/2017/8423147
14. Nixon IJ, Ganly I, Patel SG, Palmer FL, Di Lorenzo MM, Grewal RK, et al. The Results of Selective Use of Radioactive Iodine on Survival and on Recurrence in the Management of Papillary Thyroid Cancer, Based on Memorial Sloan-Kettering Cancer Center Risk Group Stratification. Thyroid (2013) 23(6):683–94. doi: 10.1089/thy.2012.0307
15. Zahedi A, Bondaz L, Rajaraman M, Leslie WD, Jefford C, Young JE, et al. Risk for Thyroid Cancer Recurrence Is Higher in Men Than in Women Independent of Disease Stage at Presentation. Thyroid (2020) 30(6):871–7. doi: 10.1089/thy.2018.0775
16. Barbosa MP, Momesso D, Bulzico DA, Farias T, Dias F, Lima RA, et al. Metastatic Lymph Node Characteristics as Predictors of Recurrence/Persistence in the Neck and Distant Metastases in Differentiated Thyroid Cancer. Arch Endocrinol Metab (2017) 61(6):584–9. doi: 10.1590/2359-3997000000307
17. Chen D, Huang L, Chen S, Huang Y, Hu D, Zeng W, et al. Innovative Analysis of Distant Metastasis in Differentiated Thyroid Cancer. Oncol Lett (2020) 19(3):1985–92. doi: 10.3892/ol.2020.11304
18. Lang BH-H, Wong KP, Cheung CY, Wan KY, Lo C-Y. Evaluating the Prognostic Factors Associated With Cancer-Specific Survival of Differentiated Thyroid Carcinoma Presenting With Distant Metastasis. Ann Surg Oncol (2013) 20(4):1329–35. doi: 10.1245/s10434-012-2711-x
19. Kim Y, Roh JL, Song D, Cho KJ, Choi SH, Nam SY, et al. Predictors of Recurrence After Total Thyroidectomy Plus Neck Dissection and Radioactive Iodine Ablation for High-Risk Papillary Thyroid Carcinoma. J Surg Oncol (2020) 122(5):906–13. doi: 10.1002/jso.26090
20. Ryu YJ, Cho JS, Park MH, Yoon JH. Identifying Risk Factors of Recurrence for Clinically Node Negative Papillary Thyroid Carcinoma With Pathologic N1a. BMC Surg (2019) 19(1):1–9. doi: 10.1186/s12893-019-0541-5
21. Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of Degree of Extrathyroidal Extension of Disease on Papillary Thyroid Cancer Outcome. Thyroid (2014) 24(2):241–4. doi: 10.1089/thy.2012.0567
22. Nixon IJ, Ganly I, Patel S, Palmer FL, Whitcher MM, Tuttle RM, et al. The Impact of Microscopic Extrathyroid Extension on Outcome in Patients With Clinical T1 and T2 Well-Differentiated Thyroid Cancer. Surgery (2011) 150(6):1242–9. doi: 10.1016/j.surg.2011.09.007
23. Rivera M, Ricarte-Filho J, Tuttle RM, Ganly I, Shaha A, Knauf J, et al. Molecular, Morphologic, and Outcome Analysis of Thyroid Carcinomas According to Degree of Extrathyroid Extension. Thyroid (2010) 20(10):1085–93. doi: 10.1089/thy.2010.0174
24. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European Consensus for the Management of Patients With Differentiated Thyroid Carcinoma of the Follicular Epithelium. Eur J Endocrinol (2006) 154(6):787–803. doi: 10.1530/eje.1.02158
25. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer: What Changed and Why? Thyroid (2017) 27(6):751–6. doi: 10.1089/thy.2017.0102
26. Kluijfhout WP, Pasternak JD, Kwon JS, Lim J, Shen WT, Gosnell JE, et al. Microscopic Positive Tumor Margin Does Not Increase the Risk of Recurrence in Patients With T1–T2 Well-Differentiated Thyroid Cancer. Ann Surg Oncol (2016) 23(5):1446–51. doi: 10.1245/s10434-015-4998-x
27. Woo CG, Sung CO, Choi YM, Kim WG, Kim TY, Shong YK, et al. Clinicopathological Significance of Minimal Extrathyroid Extension in Solitary Papillary Thyroid Carcinomas. Ann Surg Oncol (2015) 22(3):728–33. doi: 10.1245/s10434-015-4659-0
28. Al-Qurayshi Z, Shama MA, Randolph GW, Kandil E. Minimal Extrathyroidal Extension Does Not Affect Survival of Well-Differentiated Thyroid Cancer. Endocr Relat Cancer (2017) 24(5):221–6. doi: 10.1530/ERC-16-0509
29. Santos MJ, Bugalho MJ. Papillary Thyroid Carcinoma: Different Clinical Behavior Among Pt3 Tumors. Endocrine (2016) 53(3):754–60. doi: 10.1007/s12020-016-0927-4
30. Youngwirth LM, Adam MA, Scheri RP, Roman SA, Sosa JA. Extrathyroidal Extension Is Associated With Compromised Survival in Patients With Thyroid Cancer. Thyroid (2017) 27(5):626–31. doi: 10.1089/thy.2016.0132
31. McLeod DS. Papillary Thyroid Cancer With Microscopic Extra-Thyroidal Extension. Thyroid Cancer: Springer (2021) p:41–53. doi: 10.1007/978-3-030-61919-0_6
32. Park J-W, Lee O-J, Son S-M, Woo C-G. Impact of Minimal Extrathyroidal Extension on Recurrence in Papillary Thyroid Carcinoma Measuring 4 Cm or Less Without Clinical Lymph Node Metastasis. J Endocr Surg (2019) 19(1):11–7. doi: 10.16956/jes.2019.19.1.11
33. Bortz MD, Kuchta K, Winchester DJ, Prinz RA, Moo-Young TA. Extrathyroidal Extension Predicts Negative Clinical Outcomes in Papillary Thyroid Cancer. Surgery (2021) 169(1):2–6. doi: 10.1016/j.surg.2020.04.003
34. Siraj AK, Parvathareddy SK, Pratheeshkumar P, Divya SP, Al-Sobhi SS, Al-Dayel F, et al. PD-L1 Is an Independent Prognostic Marker in Middle Eastern PTC and Its Expression Is Upregulated by BRAFV600E Mutation. Cancers (2021) 13(3):555. doi: 10.3390/cancers13030555
35. Schemper M, Stare J. Explained Variation in Survival Analysis. Stat Med (1996) 15(19):1999–2012. doi: 10.1002/(SICI)1097-0258(19961015)15:19<1999::AID-SIM353>3.0.CO;2-D
36. Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the Yield of Medical Tests. Jama (1982) 247(18):2543–6. doi: 10.1001/jama.247.18.2543
37. Harrell FE Jr, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med (1996) 15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
38. Hay ID, Johnson TR, Thompson GB, Sebo TJ, Reinalda MS. Minimal Extrathyroid Extension in Papillary Thyroid Carcinoma Does Not Result in Increased Rates of Either Cause-Specific Mortality or Postoperative Tumor Recurrence. Surgery (2016) 159(1):11–21. doi: 10.1016/j.surg.2015.05.046
39. Doescher J, Veit JA, Hoffmann TK. The 8th Edition of the AJCC Cancer Staging Manual: Updates in Otorhinolaryngology, Head and Neck Surgery. Hno (2017) 65(12):956–61. doi: 10.1007/s00106-017-0391-3
40. Liu Z, Huang Y, Chen S, Hu D, Wang M, Zhou L, et al. Minimal Extrathyroidal Extension Affects the Prognosis of Differentiated Thyroid Cancer: Is There a Need for Change in the AJCC Classification System? PloS One (2019) 14(6):e0218171. doi: 10.1371/journal.pone.0218171
41. Park YM, Lee DY, Oh KH, Cho J-G, Baek S-K, Kwon S-Y, et al. Clinical Implications of Pathologic Factors After Thyroid Lobectomy in Patients With Papillary Thyroid Carcinoma. Oral Oncol (2017) 75:1–5. doi: 10.1016/j.oraloncology.2017.10.012
42. Tran B, Roshan D, Abraham E, Wang L, Garibotto N, Wykes J, et al. An Analysis of the American Joint Committee on Cancer 8th Edition T Staging System for Papillary Thyroid Carcinoma. J Clin Endocrinol Metab (2018) 103(6):2199–206. doi: 10.1210/jc.2017-02551
43. Arora N, Turbendian HK, Scognamiglio T, Wagner PL, Goldsmith SJ, Zarnegar R, et al. Extrathyroidal Extension Is Not All Equal: Implications of Macroscopic Versus Microscopic Extent in Papillary Thyroid Carcinoma. Surgery (2008) 144(6):942–8. doi: 10.1016/j.surg.2008.07.023
44. Shin JH, Ha TK, Park HK, Ahn MS, Kim KH, Bae KB, et al. Implication of Minimal Extrathyroidal Extension as a Prognostic Factor in Papillary Thyroid Carcinoma. Int J Surg (2013) 11(9):944–7. doi: 10.1016/j.ijsu.2013.06.015
45. Chereau N, Oyekunle T, Zambeli-Ljepović A, Kazaure H, Roman S, Menegaux F, et al. Predicting Recurrence of Papillary Thyroid Cancer Using the Eighth Edition of the AJCC/UICC Staging System. J Br Surg (2019) 106(7):889–97. doi: 10.1002/bjs.11145
46. Nam SH, Bae MR, Roh J-L, Gong G, Cho K-J, Choi S-H, et al. A Comparison of the 7th and 8th Editions of the AJCC Staging System in Terms of Predicting Recurrence and Survival in Patients With Papillary Thyroid Carcinoma. Oral Oncol (2018) 87:158–64. doi: 10.1016/j.oraloncology.2018.11.003
Keywords: microscopic extrathyroidal extension, papillary thyroid carcinoma, recurrence, AJCC staging, recurrence-free survival
Citation: Parvathareddy SK, Siraj AK, Qadri Z, DeVera F, Siddiqui K, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2021) Microscopic Extrathyroidal Extension Results in Increased Rate of Tumor Recurrence and Is an Independent Predictor of Patient’s Outcome in Middle Eastern Papillary Thyroid Carcinoma. Front. Oncol. 11:724432. doi: 10.3389/fonc.2021.724432
Received: 13 June 2021; Accepted: 15 November 2021;
Published: 01 December 2021.
Edited by:
Alvaro Sanabria, University of Antioquia, ColombiaReviewed by:
Hugo Fontan Köhler, A. C. Camargo Cancer Center, BrazilGiacomo Accardo, University of Campania Luigi Vanvitelli, Italy
Copyright © 2021 Parvathareddy, Siraj, Qadri, DeVera, Siddiqui, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, a2t1cmF5YUBrZnNocmMuZWR1LnNh
†These authors have contributed equally to this work
 Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Khawla S. Al-Kuraya
Khawla S. Al-Kuraya