- 1Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 2Division of Hematology and Oncology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 3Department of Urology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 4Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Recent developments in prostate-specific membrane antigen (PSMA) targeted diagnostic imaging and therapeutics (theranostics) promise to advance the management of primary, biochemically recurrent, and metastatic prostate cancer. In order to maximize the clinical impact of PSMA-targeted theranostics, a coordinated approach between the clinical stakeholders involved in prostate cancer management is required. Here, we present a vision for multidisciplinary use of PSMA theranostics from the viewpoints of nuclear radiology, medical oncology, urology, and radiation oncology. We review the currently available and forthcoming PSMA-based imaging and therapeutics and examine current and potential impacts on prostate cancer management from early localized disease to advanced treatment-refractory disease. Finally, we highlight the clinical and research opportunities related to PSMA-targeted theranostics and describe the importance of multidisciplinary collaboration in this space.
Introduction
Prostate cancer is the second most common malignancy in men and the fifth leading cause of cancer-related death worldwide (1). Localized indolent disease has a good prognosis; however, advanced localized, recurrent and metastatic disease often portend poor outcomes (1, 2). Prostate-specific membrane antigen (PSMA) is increasingly appreciated as a promising imaging and therapeutic target for prostate cancer (3). As these agents become FDA-approved and clinically available, opportunities and challenges will arise to incorporate them appropriately into the management armamentarium for prostate cancer. Success in this endeavor will require coordination and collaboration among the clinical stakeholders in prostate cancer management, including imaging physicians, medical oncologists, urologists and radiation oncologists. In this review, we provide a multidisciplinary viewpoint of how PSMA-targeting agents will advance clinical management of prostate cancer. We outline the PSMA-targeted agents for imaging and therapy and their roles in the management of both localized and metastatic disease. Finally, we identify opportunities for cross-specialty collaboration to advance the utility of PSMA-targeted agents for prostate cancer management.
PSMA: A Promising Imaging/Therapeutic Target
PSMA is expressed at 100–1000-fold higher levels in prostate cancer compared to healthy prostate tissue, and importantly, shows highest expression in high-grade and castration-resistant prostate cancer (3, 4). Multiple studies have demonstrated correlation between PSMA expression and prostate cancer aggressiveness (5), Gleason score (6), metastatic potential (7) and castration resistance (8, 9), suggesting that PSMA is a promising imaging/therapeutic target.
PSMA-Targeted Imaging Agents
The first FDA-approved molecular imaging agent developed to target PSMA was the radiolabeled monoclonal antibody indium-111 (111In)-capromab pendetide (ProstaScint) for single-photon emission computed tomography (SPECT) imaging detection of sites of biochemical recurrence (10). Clinical adoption of ProstaScint has remained low due to the relatively poor resolution of SPECT imaging as well as limited sensitivity due to an unfavorable biodistribution and the antibody targeting an intracellular epitope of PSMA (11–13).
Several SPECT-imaging agents targeting PSMA were developed after ProstaScint, including agents labeled with 99mTc (14–16) and 123I (17). However, more recent attention has been focused on positron emission tomography (PET) agents, which offer higher sensitivity and spatial resolution compared to SPECT (4, 18).
The pharmacokinetics of small molecules with their fast clearance and good tumor penetration results in a high tumor to background ratio (19). These properties make them ideal as imaging agents. Table 1 lists the most common PSMA-targeting small molecule agents that are actively used in trials and clinically worldwide. At the time of writing, two of these agents, 68Ga-PSMA-11 and 18F-DCFPyL, have been FDA-approved (but awaiting CMS approval). Beyond favorable imaging profiles (20), these agents have been demonstrated in multiple retrospective and prospective studies to be superior compared to standard cross-sectional imaging, (CT/MRI) (21, 22) nuclear medicine assays (bone scintigraphy) (23, 24), 18F-fluciclovine (25–29), 11C-choline PET/CT (30, 31), and other modalities (32, 33) for characterizing disease burden across the spectrum of the disease (Figure 1). Applications include the localized disease setting for both intraprostatic localization and staging (34–36), detection of lesions during biochemical recurrence (22, 37), and for stratification and treatment monitoring in metastatic disease (38). Furthermore, PSMA-targeted imaging has shown synergy with other modalities such as multiparametric prostate MRI (39, 40) and FDG-PET for improved characterization of disease burden (41) and image guidance for bone biopsies (42).

Table 1 Clinically relevant PSMA-targeted imaging and radio-therapeutic agents (Active at the time of review).
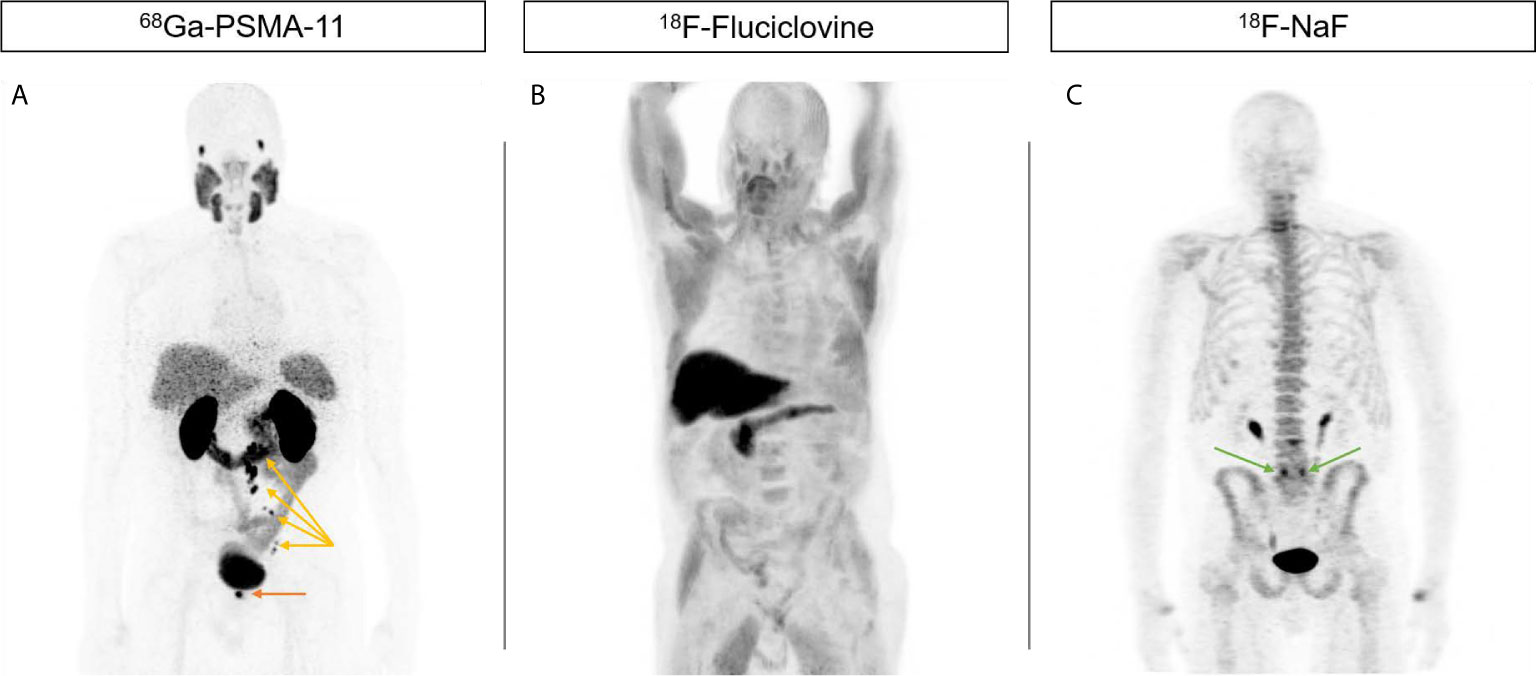
Figure 1 Increased sensitivity of PSMA-targeted imaging compared to current alternatives. 71-year-old male presented initially with T3 N0 M0 Gleason 4 + 5 = 9 PSA 9.42 prostate adenocarcinoma who declined local therapy and was managed with ADT alone, subsequently with castration-resistant progression. (A) 68Ga-PSMA-11 PET showing focal uptake in the right prostate bed (orange arrow) as well as left pelvic and retroperitoneal nodes (yellow arrows). (B) 18F-Fluciclovine PET do not show any abnormal uptake. (C) 18F-NaF PET do not show any abnormal uptake suspicious for metastases. Uptake at L5/S1 facets is due to degenerative change (green arrows).
Role of PSMA Imaging in Localized Disease
Accurate staging is critical for risk stratification and treatment decisions. Surgery and radiation therapy are curative treatments for localized disease, offer potential cure for biochemically recurrent disease (i.e., salvage radiotherapy or salvage prostatectomy), and can offer durable control in the oligometastatic disease setting. To the extent PSMA imaging can identify micrometastatic disease and reclassify clinical stage, patient selection for local therapies can be expected to improve. Further, the success of radiation largely centers on accurate identification and encompassing of disease within a radiation field in the setting of localized or salvage radiation, or to precisely target disease with stereotactic ablative radiation therapy (SABR) for patients with oligometastatic prostate cancer. Conventional imaging has low sensitivity and low specificity for detection of prostate cancer spread. Thus, PSMA imaging is being explored to determine its role in early stage disease, including for accurate assessment of intraprostatic tumor burden, with higher PSMA uptake previously shown to be associated with histological identification of focal lesions (39, 43, 44). This can guide focal SABR escalation at these sites (45, 46).
High-Risk Disease
Early data exploring the role of PSMA PET/CT in high-risk disease suggest that it can lead to changes in treatment decisions. The proPSMA trial recruited men with high-risk localized prostate cancer randomized to either conventional imaging or PSMA PET/CT as first-line imaging, followed by second-line cross-over imaging for patients with fewer than three distant metastases (21). PSMA PET/CT as first-line imaging led to change in management in 28% of patients (compared to 15% following conventional imaging), half of which comprised a change in surgical or radiotherapy technique. In patients who underwent second-line imaging, PSMA PET/CT similarly led to a change in management in 25% of patients, compared to only 5% following conventional imaging. A separate retrospective study of 138 prostate cancer patients who underwent 68Ga-PSMA-PET/CT imaging at initial diagnosis evaluated the number and anatomical location of PSMA-positive lymph nodes (47). Overall, 441 PSMA-positive lymph node metastases were identified (most frequently of which were internal iliac lymph nodes [25%]). The PSMA-positive lymph nodes were mapped onto a CT planning scan and the standard pelvic radiotherapy fields were overlaid on top for comparison. Extending the cranial border of the pelvic field from L5/S1 to L4/L5 increased accuracy of covering potentially involved nodes. Another recent study used data from two prospective trials with PSMA PET/CT imaging in high-risk individuals with cN0M0 disease per conventional imaging to develop a nomogram to help identify high-risk patients who might benefit from the addition of a PSMA PET/CT (48).
Localized Salvage Therapy
Biochemical recurrence after radical prostatectomy or primary radiation can potentially be cured with localized salvage therapy such as pelvic-targeted radiation or salvage prostatectomy (49). Biochemical recurrence can now be detected at earlier PSA values, with the definition of failure at 0.2 ng/mL (50). At these low PSA levels, conventional imaging has poor sensitivity for detecting sites of recurrence. PSMA imaging has been shown to be more sensitive in this setting in multiple prospective studies (27, 37, 51–53). The enhanced detection of local and distant lesions with PSMA-targeted imaging has ramifications for treatment planning, including choice of localized vs. systemic therapy. For example, a study in 79 radio-recurrent patients using 18F-DCFPyL PET/CT not only showed superior disease detection compared to conventional imaging (87% vs. 67% overall, 30% vs. 15% for identifying distant metastases), but changed the proposed management in 43% of patients (54). However, it currently remains unknown whether these changes in management are appropriate or will improve overall disease outcomes.
Oligometastatic Disease
The role of surgery and radiation therapy is evolving in the management of low-burden metastatic disease, also known as oligometastatic disease. Early data suggest that aggressive radiation targeted at metastatic lesions may improve outcomes (55–57). PSMA imaging may contribute to increased detection of metastatic disease and thus increased number of patients classified as oligometastatic prostate cancer. A phase II trial evaluating use of metastasis-directed therapy (MDT) to PSMA-defined oligorecurrent prostate cancer demonstrated that, of 37 patients undergoing MDT (stereotactic ablative body radiotherapy [SABR] or surgery), 22% were rendered biochemically disease-free (58). The ORIOLE trial, a randomized phase 2 trial, evaluated men with 1-3 lesions defined on conventional imaging, randomizing to standard-of-care treatment versus SABR to all detectable lesions. Patients who underwent SABR also had PSMA PET/CT at baseline and day 180. Overall, 16/36 SABR patients had 1 or more PSMA-positive lesions that were not included as part of the SABR-directed therapy. Of those who had no untreated lesions, the proportion with progression at 6 months was 1/19 (5%) compared to 6/16 (38%) with any untreated lesion. Men who had all PSMA-positive lesions treated were less likely to have new lesions at 6 months (3 of 19, 15.8% versus 10 of 16, 62.5%, p=0.006) (59). Taken together, these data support that aggressive metastasis-directed treatment to all PSMA PET-avid lesions may be curative in a subset of patients with low-burden metastatic disease.
Surgical Guidance With PSMA-Imaging
Molecular imaging approaches are increasingly being adopted for surgical guidance (60). Pelvic lymph node dissection (PLND) is the standard approach for nodal staging or management of local lymphatic metastases (61). PSMA-targeted radiolabeled and fluorescent probes are being tested for identifying lymph node metastases intraoperatively during PLND, for confirming appropriate surgical margins, and for correlation with pathological assessment (62–65). These approaches may improve surgical outcomes by increasing the likelihood that all clinically significant disease is resected at the time of surgery.
Therapeutic Role of PSMA in Metastatic Disease
PSMA-Targeted Radioligand Therapy
Systemically delivered radiotherapies already play a key role in metastatic prostate cancer management, especially with the use of 223Radium for management of osseous lesions (66). PSMA-targeted radiotherapies are poised to offer an even more impactful alternative, being effective for both PSMA-expressing bone and soft tissue metastases (67). To date, the most tested PSMA-targeted agent is 177Lu-PSMA-617, among other agents outlined in Table 1, with several clinical studies showing significant treatment response, both by imaging and PSA monitoring (68–70). The largest randomized phase III trial comparing 177Lu PSMA-617 to standard of care alone in 831 patients with advanced metastatic castration-resistant prostate cancer (mCRPC) (VISION) demonstrated that 177Lu PSMA-617 significantly improves overall survival (OS, median, 15.3 vs. 11.3 months) and progression-free survival (rPFS, median, 8.7 vs. 3.4 months) in patients with PSMA-positive mCRPC (71, 72). Based on the promising results of this trial, regulatory approval for this agent is expected to be imminent. Another randomized phase II trial (TheraP) demonstrated that 177Lu PSMA-617 compared with cabazitaxel in men with mCRPC led to a higher PSA response and fewer grade 3 or 4 adverse effects (73). Several studies have also extended the use of these agents for management of micrometastases in the setting of localized disease (74) and oligometastases (75). Correlation with PSMA imaging is key for patient stratification since patients with high PSMA expression level and low tumor heterogeneity show better outcomes (76, 77).
PSMA radioligand therapy is an area of active investigation, with most notable areas focused on testing various choices of radionuclides and ligands to improve outcomes and reduce toxicity (78). For instance, several radiopharmaceuticals have been engineered to enable radiolabeling of the same ligand using both imaging and therapeutic radionuclides, allowing an accurate pharmacokinetic readout using imaging prior to radioligand therapy (75) (4). Other areas of investigation focus on the development of therapeutic agents with more favorable pharmacokinetics for therapeutic payload delivery such as antibody constructs with longer biological half-lives and different organ toxicity profiles (79, 80). Additionally, the optimal choice of radionuclides is also being assessed. Commonly used radionuclides including 177Lu and 90Y for PSMA-therapy predominantly exert their cytotoxic actions via beta particle emission, with spatial range of action on the order of mm. Alpha particles, such a 225Ac or 209Pb, can confer higher linear energy transfer (up to 20x) compared to beta particles, but act on a shorter spatial range (81). 225Ac-PSMA-617 alone or in tandem with 177Lu-PSMA-617 have been studied clinically, with promising results (82). Auger emitters, which impart high energy at a shorter range than alpha particles, may also be useful in the setting of micro-metastases (83). Further preclinical and clinical studies are needed to understand and optimize the interplay between these design parameters, and their effects on efficacy.
PSMA-Targeted Bispecific Agents
Multiple PSMA-targeted bispecific molecules have advanced to early phase clinical evaluation in patients with mCRPC. These antibody-derived bispecific molecules bind to PSMA and a T-cell-specific antigen such as CD3 or CD28, resulting in activation of T-cell response to PSMA-expressing prostate cancer cells. PSMA-targeted bispecific agents are being developed as monotherapies and in combination with immune checkpoint inhibitors.
Pasotuxizumab (AMG 212) is a bispecific T-cell engager (BiTE) engineered to engage PSMA and CD3 and demonstrated reasonable tolerability, immunogenicity, and clinical activity in a phase 1 dose-escalation study in mCRPC patients (84). PSA declines ≥50% (PSA50) occurred in 29% and 19% of patients treated with subcutaneous and intravenous dosing, respectively, including 2 long-term responders (11-17 months to tumor progression). Pasotuxizumab was limited by a short half-life, and a half-life extended anti-PSMA x CD3 BiTE acapatamab (AMG 160) was developed for further clinical evaluation. Preliminary results from the phase 1 study of acapatamab in heavily pretreated mCRPC patients showed promising activity and manageable toxicity (85). PSA50 responses were seen in 34% of evaluable patients, including a patient who previously progressed on lutetium-PSMA therapy. Cytokine release syndrome (CRS) was observed in 91% of patients, but most cases were grade 1-2 and decreased in severity after cycle 1. Combination therapy with anti-PD-1 immune checkpoint inhibitors, abiraterone, or enzalutamide is planned (85, 86).
HPN424 is a PSMA-targeting T-cell engager with three binding domains: anti-PSMA, anti-CD3, and anti-albumin for half-life extension (87). Preliminary results from the phase 1/2 study of HPN424 in mCRPC patients demonstrated PSA50 responses in 3 (5%) patients. In the highest fixed dose cohort evaluated to date, 3 of 7 patients had PSA declines and 1 patient had a confirmed partial response by RECIST. CRS events occurred in 63% of patients, with 4% of patients experiencing grade 3 CRS. The study continues in dose escalation.
Additional PSMA-targeted bispecific agents are entering the clinical setting. REGN5678 is a first-in-class human IgG4-based bispecific engineered to target PSMA and the T-cell costimulatory receptor CD28, and will be evaluated in a phase 1/2 first-in-human study as monotherapy and in combination with the anti-PD-1 antibody cemiplimab (88). TNB-585 and CCW702 are anti-PSMA x CD3 bispecific agents entering phase 1 evaluation (89, 90).
PSMA-Targeted CAR-T
Chimeric antigen receptor (CAR) T cell therapies are a powerful class of genetically-engineered T cells with synthetic receptors that redirect their specificity, function, and metabolism, and represent a major advancement in the treatment of certain refractory hematologic malignancies (91). Prostate cancer serves as an attractive target for evaluation of CAR-T therapy in solid tumors due to the relative specificity of PSMA as target antigen. An early generation PSMA-targeted CAR-T was evaluated in a small phase 1 study that reported clinical partial responses in 2 of 5 mCRPC patients, with PSA declines of 50% and 70% (92). A second generation PSMA-targeted CAR-T demonstrated evidence of cytokine activation and prolonged stable disease for >6 months in 2 of 7 patients dosed (93).
More modern CAR-T therapies are now entering clinical evaluation for mCRPC patients, with some reporting very preliminary results to date. CART-PSMA-TGFβRDN cells involving autologous T cells engineered to express a dominant negative form of TGFβRII and a CAR with specificity to PSMA reported PSA50 decreases in 2 of 3 patients with one-month follow-up, including a patient with >95% PSA decline (94). However, one patient developed grade 2 CRS that progressed to fatal encephalopathy and multi-organ failure despite aggressive immunosuppressive therapy. A second CART-PSMA-TGFβRdn study has reported early results with PSA50 decline in 1 of 10 patients (98% decline) and PSA30 decline in 3 additional patients (95). Grade 2+ CRS was seen in 5 of 7 patients treated at higher dose. However, the therapy was associated with lethal neurotoxicity and sepsis. P-PSMA-101 is an autologous CAR-T product being evaluated in the U.S. (NCT04249947), while several PSMA-targeted CAR-T products are in clinical trials in China (NCT04053062, NCT04768608, NCT04429451). PSMA-imaging has also been harnessed as a means to track CAR-T trafficking (96).
PSMA-Targeted Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) comprise a monoclonal antibody binding to a target antigen that is highly specific to tumor cells, a synthetic linker domain, and a potent cytotoxic chemotherapy payload (97). ADCs can deliver chemotherapeutics in a more targeted manner to tumor cells, while sparing normal cells. PSMA represents a rationale target for the development of ADCs.
MLN2704, PSMA ADC, and MEDI3726 are three PSMA-targeted ADCs that have undergone clinical investigation to date. MLN2704 is comprised of a de-immunized anti-PSMA monoclonal antibody (J591) with high affinity to the external domain of PSMA complexed via a thiopentanoate linker to maytansinoid-1, a potent anti-microtubule chemotherapeutic (98). PSMA ADC is a fully human immunoglobulin G1 anti-PSMA monoclonal antibody complexed to the anti-mitotic agent monomethyl auristatin E via a valine-citrulline linker, which is more stable than thiol linkers in plasma (99). MEDI3726 is comprised of J591 conjugated to the DNA cross-linking agent pyrrolobenzodiazepine (100). These PSMA-targeted ADCs have been evaluated in separate early phase clinical trials in mCRPC patients, with MLN2704 and PSMA ADC treatments associated with PSA50 response in 8% and 14% of patients, respectively, while MEDI3726 reported a modest 12% composite response rate involving radiographic, PSA50, and circulating tumor cell (CTC) responses. However, these PSMA-targeted ADCs have been limited by neuropathy, skin toxicities, and cytopenias. Nonetheless, the clinical studies further validate PSMA as a therapeutic target in mCRPC, and future development of ADCs may focus on improving synthetic linkers that limit deconjugation of the chemotherapeutic payload outside of the tumor microenvironment.
Multidisciplinary Opportunities and Challenges in the Era of PSMA-Targeted Prostate Cancer Management
PSMA-targeted imaging and therapy are poised to play key roles in the management of prostate cancer. Evaluation of their clinical utility will require high-level evidence from prospective clinical studies (101). Precision medicine principles guided by theranostics should be incorporated in the design of these trials, and will require collaboration across radiology/nuclear medicine, urology, medical and radiation oncology. Standardized acquisition methods, and interpretation criteria of PSMA-based imaging exams, such as with recently proposed criteria like the PROMISE staging system (102) or PSMA-RADS (103) will be paramount in this regard. In addition, collaborative efforts at both pre-clinical and clinical levels to examine combination treatments involving the different PSMA-targeting modalities are vital to understanding their optimal role in the treatment armamentarium for prostate cancer.
In summary, exciting opportunities abound with the multiple PSMA-targeted imaging and therapy agents in the clinical pipeline. Collaboration across the different clinical disciplines in the prostate cancer management team will be crucial to maximize the potential of these agents.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
This work is supported in part by a Thrall Innovation Grant from the Department of Radiology, Massachusetts General Hospital (TN) and 1K08CA249047-01(PH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rawla P. Epidemiology of Prostate Cancer. World J Oncol (2019) 10(2):63–89. doi: 10.14740/wjon1191
2. La Manna F, Karkampouna S, Zoni E, De Menna M, Hensel J, Thalmann GN, et al. Metastases in Prostate Cancer. Cold Spring Harb Perspect Med (2018) 9(3):a033688. doi: 10.1101/cshperspect.a033688
3. Chang SS. Overview of Prostate-Specific Membrane Antigen. Rev Urol (2004) 6 Suppl 10(Suppl 10):S13–S8.
4. Lawhn-Heath C, Salavati A, Behr SC, Rowe SP, Calais J, Fendler WP, et al. Prostate-Specific Membrane Antigen PET in Prostate Cancer. Radiology (2021) 299(2):248–60. doi: 10.1148/radiol.2021202771
5. Gao J, Zhang C, Zhang Q, Fu Y, Zhao X, Chen M, et al. Diagnostic Performance of 68Ga-PSMA PET/CT for Identification of Aggressive Cribriform Morphology in Prostate Cancer With Whole-Mount Sections. Eur J Nucl Med Mol Imaging (2019) 46(7):1531–41. doi: 10.1007/s00259-019-04320-9
6. Ross JS, Sheehan CE, Fisher HA, Kaufman RP Jr., Kaur P, Gray K, et al. Correlation of Primary Tumor Prostate-Specific Membrane Antigen Expression With Disease Recurrence in Prostate Cancer. Clin Cancer Res (2003) 9(17):6357–62.
7. Koerber SA, Boesch J, Kratochwil C, Schlampp I, Ristau J, Winter E, et al. Predicting the Risk of Metastases by PSMA-PET/CT-Evaluation of 335 Men With Treatment-Naïve Prostate Carcinoma. Cancers (Basel) (2021) 13(7):1508. doi: 10.3390/cancers13071508
8. Thang SP, Violet J, Sandhu S, Iravani A, Akhurst T, Kong G, et al. Poor Outcomes for Patients With Metastatic Castration-Resistant Prostate Cancer With Low Prostate-Specific Membrane Antigen (PSMA) Expression Deemed Ineligible for (177)Lu-Labelled PSMA Radioligand Therapy. Eur Urol Oncol (2019) 2(6):670–6. doi: 10.1016/j.euo.2018.11.007
9. Fourquet A, Aveline C, Cussenot O, Créhange G, Montravers F, Talbot JN, et al. (68)Ga-PSMA-11 PET/CT in Restaging Castration-Resistant Nonmetastatic Prostate Cancer: Detection Rate, Impact on Patients’ Disease Management and Adequacy of Impact. Sci Rep (2020) 10(1):2104. doi: 10.1038/s41598-020-58975-8
10. Taneja SS. ProstaScint(R) Scan: Contemporary Use in Clinical Practice. Rev Urol (2004) 6 Suppl 10(Suppl 10):S19–28.
11. Schwarzenboeck SM, Rauscher I, Bluemel C, Fendler WP, Rowe SP, Pomper MG, et al. PSMA Ligands for PET Imaging of Prostate Cancer. J Nucl Med (2017) 58(10):1545–52. doi: 10.2967/jnumed.117.191031
12. Rauscher I, Maurer T, Souvatzoglou M, Beer AJ, Vag T, Wirtz M, et al. Intrapatient Comparison of 111In-PSMA I&T SPECT/CT and Hybrid 68ga-HBED-CC PSMA PET in Patients With Early Recurrent Prostate Cancer. Clin Nucl Med (2016) 41(9):e397–402. doi: 10.1097/rlu.0000000000001273
13. Wallitt KL, Khan SR, Dubash S, Tam HH, Khan S, Barwick TD. Clinical PET Imaging in Prostate Cancer. Radiographics (2017) 37(5):1512–36. doi: 10.1148/rg.2017170035
14. Urbán S, Meyer C, Dahlbom M, Farkas I, Sipka G, Besenyi Z, et al. Radiation Dosimetry of Tc99m-PSMA I&S: A Single-Center Prospective Study. J Nucl Med (2020) jnumed.120.253476. doi: 10.2967/jnumed.120.253476
15. Vats K, Agrawal K, Sharma R, Sarma HD, Satpati D, Dash A. Preparation and Clinical Translation of 99mtc-PSMA-11 for SPECT Imaging of Prostate Cancer. MedChemComm (2019) 10(12):2111–7. doi: 10.1039/C9MD00401G
16. Osborne J, Akhtar NH, Vallabhajosula S, Nikolopoulou A, Maresca KP, Hillier SM, et al. Tc-99m Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen (PSMA): New Molecular Imaging Probes to Detect Metastatic Prostate Adenocarcinoma (PC). J Clin Oncol (2012) 30(5_suppl):173–. doi: 10.1200/jco.2012.30.5_suppl.173
17. Hillier S, Kern A, Maresca K, Marquis J, Eckelman W, Joyal J, et al. I-123-MIP-1072, a Small-Molecule Inhibitor of Prostate-Specific Membrane Antigen, Is Effective at Monitoring Tumor Response to Taxane Therapy. J Nucl Med: Off Publication Soc Nucl Med (2011) 52:1087–93. doi: 10.2967/jnumed.110.086751
18. Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA Theranostics: Current Status and Future Directions. Mol Imaging (2018) 17:1536012118776068. doi: 10.1177/1536012118776068
19. Wittrup KD, Thurber GM, Schmidt MM, Rhoden JJ. Chapter Ten - Practical Theoretic Guidance for the Design of Tumor-Targeting Agents. In: Wittrup KD, Verdine GL, eds. Methods Enzymol, vol. 503. San Diego: Academic Press (2012). p. 255–68.
20. Rauscher I, Krönke M, König M, Gafita A, Maurer T, Horn T, et al. Matched-Pair Comparison of (68)Ga-PSMA-11 PET/CT and (18)F-PSMA-1007 PET/CT: Frequency of Pitfalls and Detection Efficacy in Biochemical Recurrence After Radical Prostatectomy. J Nucl Med (2020) 61(1):51–7. doi: 10.2967/jnumed.119.229187
21. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-Specific Membrane Antigen PET-CT in Patients With High-Risk Prostate Cancer Before Curative-Intent Surgery or Radiotherapy (proPSMA): A Prospective, Randomised, Multicentre Study. Lancet (2020) 395(10231):1208–16. doi: 10.1016/s0140-6736(20)30314-7
22. Sawicki LM, Kirchner J, Buddensieck C, Antke C, Ullrich T, Schimmöller L, et al. Prospective Comparison of Whole-Body MRI and (68)Ga-PSMA PET/CT for the Detection of Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. Eur J Nucl Med Mol Imaging (2019) 46(7):1542–50. doi: 10.1007/s00259-019-04308-5
23. Acar E, Bekiş R, Polack B. Comparison of Bone Uptake in Bone Scan and Ga-68 PSMA PET/CT Images in Patients With Prostate Cancer. Curr Med Imaging Rev (2019) 15(6):589–94. doi: 10.2174/1573405615666190225155254
24. Simsek DH, Sanli Y, Civan C, Engin MN, Isik EG, Ozkan ZG, et al. Does Bone Scintigraphy Still Have a Role in the Era of 68 Ga-PSMA PET/CT in Prostate Cancer? Ann Nucl Med (2020) 34(7):476–85. doi: 10.1007/s12149-020-01474-7
25. Tan N, Oyoyo U, Bavadian N, Ferguson N, Mukkamala A, Calais J, et al. PSMA-Targeted Radiotracers Versus 18F Fluciclovine for the Detection of Prostate Cancer Biochemical Recurrence After Definitive Therapy: A Systematic Review and Meta-Analysis. Radiology (2020) 296(1):44–55. doi: 10.1148/radiol.2020191689
26. Turkbey B, Choyke PL. 18F-Fluciclovine PET or PSMA PET for Prostate Cancer Imaging? Nat Rev Urol (2020) 17(1):9–10. doi: 10.1038/s41585-019-0255-6
27. Pernthaler B, Kulnik R, Gstettner C, Salamon S, Aigner RM. Kvaternik H. A Prospective Head-To-Head Comparison of 18F-Fluciclovine With 68ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT. Clin Nucl Med (2019) 44(10):e566–e73. doi: 10.1097/rlu.0000000000002703
28. Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a Case Series of 10 Patients With Prostate Cancer Recurrence. J Nucl Med (2018) 59(5):789–94. doi: 10.2967/jnumed.117.203257
29. Savir-Baruch B, Choyke PL, Rowe SP, Schuster DM, Subramaniam RM, Jadvar H. Role of 18F-Fluciclovine and Prostate-Specific Membrane Antigen PET/CT in Guiding Management of Oligometastatic Prostate Cancer: AJR Expert Panel Narrative Review. Am J Roentgenol (2021) 216(4):851–9. doi: 10.2214/AJR.20.24711
30. Treglia G, Pereira Mestre R, Ferrari M, Bosetti DG, Pascale M, Oikonomou E, et al. Radiolabelled Choline Versus PSMA PET/CT in Prostate Cancer Restaging: A Meta-Analysis. Am J Nucl Med Mol Imaging (2019) 9(2):127–39.
31. Moghul M, Somani B, Lane T, Vasdev N, Chaplin B, Peedell C, et al. Detection Rates of Recurrent Prostate Cancer: (68)Gallium (Ga)-Labelled Prostate-Specific Membrane Antigen Versus Choline PET/CT Scans. A Systematic Review. Ther Adv Urol (2019) 11:1756287218815793. doi: 10.1177/1756287218815793
32. Regula N, Kostaras V, Johansson S, Trampal C, Lindström E, Lubberink M, et al. Comparison of 68Ga-PSMA-11 PET/CT With 11C-Acetate PET/CT in Re-Staging of Prostate Cancer Relapse. Sci Rep (2020) 10(1):4993. doi: 10.1038/s41598-020-61910-6
33. Piccardo A, Paparo F, Puntoni M, Righi S, Bottoni G, Bacigalupo L, et al. 64cucl2 PET/CT in Prostate Cancer Relapse. J Nucl Med (2018) 59(3):444–51. doi: 10.2967/jnumed.117.195628
34. Hicks RM, Simko JP, Westphalen AC, Nguyen HG, Greene KL, Zhang L, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET/MRI Compared With Multiparametric MRI in the Detection of Prostate Cancer. Radiology (2018) 289(3):730–7. doi: 10.1148/radiol.2018180788
35. Muehlematter UJ, Burger IA, Becker AS, Schawkat K, Hötker AM, Reiner CS, et al. Diagnostic Accuracy of Multiparametric MRI Versus 68Ga-PSMA-11 PET/MRI for Extracapsular Extension and Seminal Vesicle Invasion in Patients With Prostate Cancer. Radiology (2019) 293(2):350–8. doi: 10.1148/radiol.2019190687
36. Zhao J, Mangarova DB, Brangsch J, Kader A, Hamm B, Brenner W, et al. Correlation Between Intraprostatic PSMA Uptake and MRI PI-RADS of [(68)Ga]Ga-PSMA-11 PET/MRI in Patients With Prostate Cancer: Comparison of PI-RADS Version 2.0 and PI-RADS Version 2.1. Cancers (Basel) (2020) 12(12):3523. doi: 10.3390/cancers12123523
37. Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol (2019) 5(6):856–63. doi: 10.1001/jamaoncol.2019.0096
38. Kuten J, Sarid D, Yossepowitch O, Mabjeesh NJ, Even-Sapir E. [(68)Ga]Ga-PSMA-11 PET/CT for Monitoring Response to Treatment in Metastatic Prostate Cancer: Is There Any Added Value Over Standard Follow-Up? EJNMMI Res (2019) 9(1):84. doi: 10.1186/s13550-019-0554-1
39. Spohn S, Jaegle C, Fassbender TF, Sprave T, Gkika E, Nicolay NH, et al. Intraindividual Comparison Between 68Ga-PSMA-PET/CT and mpMRI for Intraprostatic Tumor Delineation in Patients With Primary Prostate Cancer: A Retrospective Analysis in 101 Patients. Eur J Nucl Med Mol Imaging (2020) 47(12):2796–803. doi: 10.1007/s00259-020-04827-6
40. Murthy V, Sonni I, Jariwala N, Juarez R, Reiter RE, Raman SS, et al. The Role of PSMA PET/CT and PET/MRI in the Initial Staging of Prostate Cancer. Eur Urol Focus (2021) 7(2):258–66. doi: 10.1016/j.euf.2021.01.016
41. Zukotynski KA, Jadvar H, Cho SY, Kim CK, Cline K, Emmenegger U, et al. FDG and PSMA PET in Metastatic Castration-Resistant Prostate Cancer (mCRPC). J Clin Oncol (2020) 38(6_suppl):23–. doi: 10.1200/JCO.2020.38.6_suppl.23
42. de Jong AC, Smits M, van Riet J, Fütterer JJ, Brabander T, Hamberg P, et al. (68)Ga-PSMA-Guided Bone Biopsies for Molecular Diagnostics in Patients With Metastatic Prostate Cancer. J Nucl Med (2020) 61(11):1607–14. doi: 10.2967/jnumed.119.241109
43. Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of Intraprostatic Tumor Extent With ⁶⁸Ga-PSMA Distribution in Patients With Prostate Cancer. J Nucl Med (2016) 57(4):563–7. doi: 10.2967/jnumed.115.169243
44. Kostyszyn D, Fechter T, Bartl N, Grosu AL, Gratzke C, Sigle A, et al. Intraprostatic Tumour Segmentation on PSMA-PET Images in Patients With Primary Prostate Cancer With a Convolutional Neural Network. J Nucl Med (2020) 62(6):823–8. doi: 10.2967/jnumed.120.254623
45. Zamboglou C, Thomann B, Koubar K, Bronsert P, Krauss T, Rischke HC, et al. Focal Dose Escalation for Prostate Cancer Using (68)Ga-HBED-CC PSMA PET/CT and MRI: A Planning Study Based on Histology Reference. Radiat Oncol (2018) 13(1):81. doi: 10.1186/s13014-018-1036-8
46. Zschaeck S, Wust P, Beck M, Wlodarczyk W, Kaul D, Rogasch J, et al. Intermediate-Term Outcome After PSMA-PET Guided High-Dose Radiotherapy of Recurrent High-Risk Prostate Cancer Patients. Radiat Oncol (2017) 12(1):140. doi: 10.1186/s13014-017-0877-x
47. Onal C, Ozyigit G, Guler OC, Hurmuz P, Torun N, Tuncel M, et al. Role of 68-Ga-PSMA-PET/CT in Pelvic Radiotherapy Field Definitions for Lymph Node Coverage in Prostate Cancer Patients. Radiother Oncol: J Eur Soc Ther Radiol Oncol (2020) 151:222–7. doi: 10.1016/j.radonc.2020.08.021
48. Ma TM, Gafita A, Shabsovich D, Juarez J, Grogan TR, Thin P, et al. Identifying the Best Candidates for Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography as the Primary Staging Approach Among Men With High-Risk Prostate Cancer and Negative Conventional Imaging. Eur Urol Oncol (2021) S2588-9311(21)00030-4. doi: 10.1016/j.euo.2021.01.006
49. Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or Early Salvage Radiotherapy for the Treatment of Localised and Locally Advanced Prostate Cancer: A Prospectively Planned Systematic Review and Meta-Analysis of Aggregate Data. Lancet (2020) 396(10260):1422–31. doi: 10.1016/s0140-6736(20)31952-8
50. Lowrance W, Breau RH, Chou R, Chapin BF, Crispino T, dreicer R, et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J Urol (2021) 205(14):14–21. doi: 10.1097/JU.0000000000001375
51. Song H, Harrison C, Duan H, Guja K, Hatami N, Franc BL, et al. Prospective Evaluation of (18)F-DCFPyL PET/CT in Biochemically Recurrent Prostate Cancer in an Academic Center: A Focus on Disease Localization and Changes in Management. J Nucl Med (2020) 61(4):546–51. doi: 10.2967/jnumed.119.231654
52. Emmett L, Tang R, Nandurkar R, Hruby G, Roach P, Watts JA, et al. 3-Year Freedom From Progression After (68)Ga-PSMA PET/CT-Triaged Management in Men With Biochemical Recurrence After Radical Prostatectomy: Results of a Prospective Multicenter Trial. J Nucl Med (2020) 61(6):866–72. doi: 10.2967/jnumed.119.235028
53. Koschel S, Taubman K, Sutherland T, Yap K, Chao M, Guerrieri M, et al. Patterns of Disease Detection Using [(18)F]DCFPyL PET/CT Imaging in Patients With Detectable PSA Post Prostatectomy Being Considered for Salvage Radiotherapy: A Prospective Trial. Eur J Nucl Med Mol Imaging (2021). doi: 10.1007/s00259-021-05354-8
54. Liu W, Zukotynski K, Emmett L, Chung HT, Chung P, Wolfson R, et al. A Prospective Study of 18F-DCFPyL PSMA PET/CT Restaging in Recurrent Prostate Cancer Following Primary External Beam Radiotherapy or Brachytherapy. Int J Radiat Oncol Biol Phys (2020) 106(3):546–55. doi: 10.1016/j.ijrobp.2019.11.001
55. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic Ablative Radiotherapy Versus Standard of Care Palliative Treatment in Patients With Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet (2019) 393(10185):2051–8. doi: 10.1016/s0140-6736(18)32487-5
56. Kamran SC, Zietman AL. Curing Metastatic Disease With Ablative Radiation Therapy: Separating Truth From Wish. Int J Radiat Oncol Biol Phys (2020) 107(3):433–6. doi: 10.1016/j.ijrobp.2020.02.468
57. Kamran SC, Efstathiou JA. Current State of Personalized Genitourinary Cancer Radiotherapy in the Era of Precision Medicine. Front Oncol (2021) 11:675311. doi: 10.3389/fonc.2021.675311
58. Glicksman RM, Metser U, Vines D, Valliant J, Liu Z, Chung PW, et al. Curative-Intent Metastasis-Directed Therapies for Molecularly-Defined Oligorecurrent Prostate Cancer: A Prospective Phase II Trial Testing the Oligometastasis Hypothesis. Eur Urol (2021) S0302-2838(21)00151-2. doi: 10.1016/j.eururo.2021.02.031
59. Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol (2020) 6(5):650–9. doi: 10.1001/jamaoncol.2020.0147
60. Solomon SB, Cornelis F. Interventional Molecular Imaging. J Nucl Med (2016) 57(4):493–6. doi: 10.2967/jnumed.115.161190
61. Bianchi L, Gandaglia G, Fossati N, Suardi N, Moschini M, Cucchiara V, et al. Pelvic Lymph Node Dissection in Prostate Cancer: Indications, Extent and Tailored Approaches. Urologia (2017) 84(1):9–19. doi: 10.5301/uro.5000139
62. Maurer T, Graefen M, van der Poel H, Hamdy F, Briganti A, Eiber M, et al. Prostate-Specific Membrane Antigen-Guided Surgery. J Nucl Med (2020) 61(1):6–12. doi: 10.2967/jnumed.119.232330
63. Jilg CA, Reichel K, Stoykow C, Rischke HC, Bartholomä M, Drendel V, et al. Results From Extended Lymphadenectomies With [111In]PSMA-617 for Intraoperative Detection of PSMA-PET/CT-Positive Nodal Metastatic Prostate Cancer. EJNMMI Res (2020) 10(1):17. doi: 10.1186/s13550-020-0598-2
64. Derks YHW, Löwik DWPM, Sedelaar JPM, Gotthardt M, Boerman OC, Rijpkema M, et al. PSMA-Targeting Agents for Radio- and Fluorescence-Guided Prostate Cancer Surgery. Theranostics (2019) 9(23):6824–39. doi: 10.7150/thno.36739
65. van Leeuwen FWB, van Oosterom MN, Meershoek P, van Leeuwen PJ, Berliner C, van der Poel HG, et al. Minimal-Invasive Robot-Assisted Image-Guided Resection of Prostate-Specific Membrane Antigen–Positive Lymph Nodes in Recurrent Prostate Cancer. Clin Nucl Med (2019) 44(7):580–1. doi: 10.1097/RLU.0000000000002600
66. Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. New Engl J Med (2013) 369(3):213–23. doi: 10.1056/NEJMoa1213755
67. Herrmann K, Schwaiger M, Lewis JS, Solomon SB, McNeil BJ, Baumann M, et al. Radiotheranostics: A Roadmap for Future Development. Lancet Oncol (2020) 21(3):e146–e56. doi: 10.1016/S1470-2045(19)30821-6
68. Sun M, Niaz MO, Nelson A, Skafida M, Niaz MJ. Review of 177Lu-PSMA-617 in Patients With Metastatic Castration-Resistant Prostate Cancer. Cureus (2020) 12(6):e8921. doi: 10.7759/cureus.8921
69. Privé BM, Peters SMB, Muselaers CHJ, van Oort IM, Janssen MJR, Sedelaar JPM, et al. Lutetium-177-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer: A Prospective Pilot Study. Clin Cancer Res (2021) 27(13):3595–601. doi: 10.1158/1078-0432.Ccr-20-4298
70. Rasul S, Hacker M, Kretschmer-Chott E, Leisser A, Grubmüller B, Kramer G, et al. Clinical Outcome of Standardized 177Lu-PSMA-617 Therapy in Metastatic Prostate Cancer Patients Receiving 7400 MBq Every 4 Weeks. Eur J Nucl Med Mol Imaging (2020) 47(3):713–20. doi: 10.1007/s00259-019-04584-1
71. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. New Engl J Med (2021). doi: 10.1056/NEJMoa2107322
72. Sartor AO, Morris MJ, Messman R, Krause BJ. VISION: An International, Prospective, Open-Label, Multicenter, Randomized Phase III Study of 177Lu-PSMA-617 in the Treatment of Patients With Progressive PSMA-Positive Metastatic Castration-Resistant Prostate Cancer (mCRPC). J Clin Oncol (2020) 38(6_suppl):TPS259–TPS. doi: 10.1200/JCO.2020.38.6_suppl.TPS259
73. Hofman MS, Emmett L, Violet J, YZ A, Lawrence NJ, Stockler M, et al. TheraP: A Randomized Phase 2 Trial of (177) Lu-PSMA-617 Theranostic Treatment vs Cabazitaxel in Progressive Metastatic Castration-Resistant Prostate Cancer (Clinical Trial Protocol ANZUP 1603). BJU Int (2019) 124 Suppl 1:5–13. doi: 10.1111/bju.14876
74. Dhiantravan N, Violet J, Eapen R, Alghazo O, Scalzo M, Jackson P, et al. Clinical Trial Protocol for LuTectomy: A Single-Arm Study of the Dosimetry, Safety, and Potential Benefit of ≪Sup<177≪/Sup<Lu-PSMA-617 Prior to Prostatectomy. Eur Urol Focus (2021) 7(2):234–7. doi: 10.1016/j.euf.2020.09.021
75. Privé BM, Janssen MJR, van Oort IM, Muselaers CHJ, Jonker MA, de Groot M, et al. Lutetium-177-PSMA-I&T as Metastases Directed Therapy in Oligometastatic Hormone Sensitive Prostate Cancer, a Randomized Controlled Trial. BMC Cancer (2020) 20(1):884. doi: 10.1186/s12885-020-07386-z
76. Prasad V, Huang K, Prasad S, Makowski MR, Czech N, Brenner W. In Comparison to PSA, Interim Ga-68-PSMA PET/CT Response Evaluation Based on Modified RECIST 1.1 After 2nd Cycle Is Better Predictor of Overall Survival of Prostate Cancer Patients Treated With 177lu-PSMA. Front Oncol (2021) 11:578093. doi: 10.3389/fonc.2021.578093
77. Seifert R, Kessel K, Schlack K, Weber M, Herrmann K, Spanke M, et al. PSMA PET Total Tumor Volume Predicts Outcome of Patients With Advanced Prostate Cancer Receiving [(177)Lu]Lu-PSMA-617 Radioligand Therapy in a Bicentric Analysis. Eur J Nucl Med Mol Imaging (2021) 48(4):1200–10. doi: 10.1007/s00259-020-05040-1
78. Czerwińska M, Bilewicz A, Kruszewski M, Wegierek-Ciuk A, Lankoff A. Targeted Radionuclide Therapy of Prostate Cancer-From Basic Research to Clinical Perspectives. Molecules (2020) 25(7):1743. doi: 10.3390/molecules25071743
79. Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I Trial of 177Lutetium-Labeled J591, a Monoclonal Antibody to Prostate-Specific Membrane Antigen, in Patients With Androgen-Independent Prostate Cancer. J Clin Oncol (2005) 23(21):4591–601. doi: 10.1200/jco.2005.05.160
80. Nauseef JT, Bander NH, Tagawa ST. Emerging Prostate-Specific Membrane Antigen-Based Therapeutics: Small Molecules, Antibodies, and Beyond. Eur Urol Focus (2021) 7(2):254–7. doi: 10.1016/j.euf.2021.02.006
81. Kratochwil C, Haberkorn U, Giesel FL. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin Nucl Med (2020) 50(2):133–40. doi: 10.1053/j.semnuclmed.2020.02.004
82. Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. (225)Ac-PSMA-617/(177)Lu-PSMA-617 Tandem Therapy of Metastatic Castration-Resistant Prostate Cancer: Pilot Experience. Eur J Nucl Med Mol Imaging (2020) 47(3):721–8. doi: 10.1007/s00259-019-04612-0
83. Shen CJ, Minn I, Hobbs RF, Chen Y, Josefsson A, Brummet M, et al. Auger Radiopharmaceutical Therapy Targeting Prostate-Specific Membrane Antigen in a Micrometastatic Model of Prostate Cancer. Theranostics (2020) 10(7):2888–96. doi: 10.7150/thno.38882
84. Hummel HD, Kufer P, Grüllich C, Seggewiss-Bernhardt R, Deschler-Baier B, Chatterjee M, et al. Pasotuxizumab, a BiTE(®) Immune Therapy for Castration-Resistant Prostate Cancer: Phase I, Dose-Escalation Study Findings. Immunotherapy (2021) 13(2):125–41. doi: 10.2217/imt-2020-0256
85. Tran B, Horvath L, Dorff T, Rettig M, Lolkema MP, Machiels J, et al. Results From a Phase I Study of AMG 160, a Half-Life Extended (HLE), PSMA-Targeted, Bispecific T-Cell Engager (BiTE®) Immune Therapy for Metastatic Castration-Resistant Prostate Cancer (mCRPC). Ann Oncol (2020) 31(suppl_4):S507–49. doi: 10.1016/annonc/annonc275
86. Subudhi SK, Siddiqui BA, Maly JJ, Nandagopal L, Lam ET, Whang YE, et al. Safety and Efficacy of AMG 160, a Half-Life Extended BiTE Immune Therapy Targeting Prostate-Specific Membrane Antigen (PSMA), and Other Therapies for Metastatic Castration-Resistant Prostate Cancer (mCRPC). J Clin Oncol (2021) 39(suppl 15; abstr TPS5088). doi: 10.1200/JCO.2021.39.15_suppl.TPS5088
87. de Bono JS, Fong L, Beer TM, Gao X, Geynisman DM, Burris HA, et al. Results of an Ongoing Phase 1/2a Dose Escalation Study of HPN424, a Tri-Specific Half-Life Extended PSMA-Targeting T-Cell Engager, in Patients With Metastatic Castration-Resistant Prostate Cancer (mCRPC). J Clin Oncol (2021) 39(suppl 15; abstr 5013). doi: 10.1200/JCO.2021.39.15_suppl.5013
88. Zhang J, Stein MN, Kelly WK, Tsao CK, Falchook GS, Xu Y, et al. A Phase I/II Study of REGN5678 (Anti-PSMAxCD28, a Costimulatory Bispecific Antibody) With Cemiplimab (Anti–PD-1) in Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol (2021) 39(suppl 6; abstr TPS174). doi: 10.1200/JCO.2021.39.6_suppl.TPS174
89. Buelow B, Dalvi P, Dang K, Patel A, Johal K, Pham D, et al. TNB585.001: A Multicenter, Phase 1, Open-Label, Dose-Escalation and Expansion Study of Tnb-585, a Bispecific T-Cell Engager Targeting PSMA in Subjects With Metastatic Castrate Resistant Prostate Cancer. J Clin Oncol (2021) 39(suppl 15; abstr TPS5092). doi: 10.1200/JCO.2021.39.15_suppl.TPS5092
90. Markowski MC, Kilari D, Eisenberger MA, McKay RR, Dreicer R, Trikha M, et al. Phase I Study of CCW702, a Bispecific Small Molecule-Antibody Conjugate Targeting PSMA and CD3 in Patients With Metastatic Castration-Resistant Prostate Cancer (mCRPC). J Clin Oncol (2021) 39(suppl 15; abstr TPS5094). doi: 10.1200/JCO.2021.39.15_suppl.TPS5094
91. June CH, Sadelain M. Chimeric Antigen Receptor Therapy. New Engl J Med (2018) 379(1):64–73. doi: 10.1056/NEJMra1706169
92. Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo AS, et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate (2016) 76(14):1257–70. doi: 10.1002/pros.23214
93. Slovin SF, Wang X, Hullings M, Arauz G, Bartido S, Lewis JS, et al. Chimeric Antigen Receptor (CAR+) Modified T Cells Targeting Prostate Specific Membrane Antigen (PSMA) in Patients (Pts) With Castrate Metastatic Prostate Cancer (CMPC). J Clin Oncol (2013) 31(suppl; abstr TPS3115). doi: 10.1200/jco.2013.31.15_suppl.tps3115
94. Carabasi MH, McKean M, Stein MN, Schweizer MT, Luke JJ, Narayan V, et al. PSMA Targeted Armored Chimeric Antigen Receptor (CAR) T-Cells in Patients With Advanced mCRPC: A Phase I Experience. J Clin Oncol (2021) 39(suppl 15; abstr 2534). doi: 10.1200/JCO.2021.39.15_suppl.2534
95. Narayan V, Barber-Rotenberg J, Fraietta J, Hwang WT, Lacey SF, Plesa G, et al. A Phase I Clinical Trial of PSMA-Directed/Tgfβ-Insensitive CAR-T Cells in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol (2021) 39(suppl 6; abstr 125). doi: 10.1200/JCO.2021.39.6_suppl.125
96. Minn I, Huss DJ, Ahn H-H, Chinn TM, Park A, Jones J, et al. Imaging CAR T Cell Therapy With PSMA-Targeted Positron Emission Tomography. Sci Adv (2019) 5(7):eaaw5096–eaaw. doi: 10.1126/sciadv.aaw5096
97. Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and Challenges for the Next Generation of Antibody–Drug Conjugates. Nat Rev Drug Discovery (2017) 16(5):315–37. doi: 10.1038/nrd.2016.268
98. Milowsky MI, Galsky MD, Morris MJ, Crona DJ, George DJ, Dreicer R, et al. Phase 1/2 Multiple Ascending Dose Trial of the Prostate-Specific Membrane Antigen-Targeted Antibody Drug Conjugate MLN2704 in Metastatic Castration-Resistant Prostate Cancer. Urol Oncol (2016) 34(12):530 e15– e21. doi: 10.1016/j.urolonc.2016.07.005
99. Petrylak DP, Vogelzang NJ, Chatta K, Fleming MT, Smith DC, Appleman LJ, et al. PSMA ADC Monotherapy in Patients With Progressive Metastatic Castration-Resistant Prostate Cancer Following Abiraterone and/or Enzalutamide: Efficacy and Safety in Open-Label Single-Arm Phase 2 Study. Prostate (2020) 80(1):99–108. doi: 10.1002/pros.23922
100. de Bono JS, Fleming MT, Wang JS, Cathomas R, Miralles MS, Bothos J, et al. Phase I Study of MEDI3726: A Prostate-Specific Membrane Antigen-Targeted Antibody–Drug Conjugate, in Patients With mCRPC After Failure of Abiraterone or Enzalutamide. Clin Cancer Res (2021) 27(13):3602–9. doi: 10.1158/1078-0432.ccr-20-4528
101. Shaygan B, Zukotynski K, Bénard F, Ménard C, Sistani G, Bauman G, et al. Canadian Urological Association Best Practice Report: Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography (PSMA PET/CT) and PET/magnetic Resonance (MR) in Prostate Cancer. Can Urol Assoc J (2021) 15(6):162–72. doi: 10.5489/cuaj.7268
102. Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J Nucl Med (2018) 59(3):469–78. doi: 10.2967/jnumed.117.198119
Keywords: PSMA, PET, prostate cancer, radiation, theranostics, therapy, molecular imaging
Citation: Ng TSC, Gao X, Salari K, Zlatev DV, Heidari P and Kamran SC (2021) Incorporating PSMA-Targeting Theranostics Into Personalized Prostate Cancer Treatment: a Multidisciplinary Perspective. Front. Oncol. 11:722277. doi: 10.3389/fonc.2021.722277
Received: 08 June 2021; Accepted: 12 July 2021;
Published: 28 July 2021.
Edited by:
Trevor Royce, University of North Carolina at Chapel Hill, United StatesReviewed by:
Luca Faustino Valle, University of California, Los Angeles, United StatesSimon Spohn, University of Freiburg Medical Center, Germany
Copyright © 2021 Ng, Gao, Salari, Zlatev, Heidari and Kamran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophia C. Kamran, c2thbXJhbkBtZ2guaGFydmFyZC5lZHU=
 Thomas S. C. Ng
Thomas S. C. Ng Xin Gao2
Xin Gao2 Sophia C. Kamran
Sophia C. Kamran