
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 02 September 2021
Sec. Genitourinary Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.713645
This article is part of the Research TopicNephrectomy in Localized Renal Cell CarcinomaView all 6 articles
Background: Partial nephrectomy (PN) is the recommended treatment for T1 renal cell carcinoma (RCC). Compared with suture PN, sutureless PN reduces the difficulty and time of operation, but the safety and feasibility have been controversial. This meta-analysis was conducted to compare the function and perioperative outcomes of suture and sutureless PN for T1 RCC.
Methods: Systematic literature review was performed up to April 2021 using multiple databases to identify eligible comparative studies. According to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria, identification and selection of the studies were conducted. Meta-analysis was performed for studies comparing suture to sutureless PN for both T1a and T1b RCC. In addition, subgroup analysis was performed on operation time, warm ischemia time, estimated blood loss, and postoperative complications. Sensitivity analysis was used in analysis with high heterogeneity (operation time and estimated blood loss).
Results: Eight retrospective studies were included with a total of 1,156 patients; of the 1,156 patients, 499 received sutureless PN and 707 received suture PN. The results showed that sutureless PN had shorter operative time (I2 = 0%, P < 0.001), warm ischemia time (I2 = 97.5%, P < 0.001), and lower clamping rate (I2 = 85.8%, P = 0.003), but estimated blood loss (I2 = 76.6%, P = 0.064) had no difference. In the comparison of perioperative outcomes, there was no significant difference in postoperative complications (I2 = 0%, P = 0.999), positive surgical margins (I2 = 0%, P = 0.356), postoperative estimated glomerular filtration rat (eGFR) (I2 = 0%, P = 0.656), and tumor recurrence (I2 = 0%, P = 0.531).
Conclusions: In T1a RCC with low RENAL score, sutureless PN is a feasible choice, whereas it should not be overestimated in T1b RCC.
Partial nephrectomy (PN), in the surgical treatment of T1 renal cell carcinoma (RCC), is recommended according to the guidelines of the American Urology Association (AUA) and European Association of Urology (EAU) (1, 2). PN can provide better protection of renal function (3, 4), reduce the risk of severe cardiovascular events, and improve overall survival (5). However, the learning curve of PN is steep (6). In order to avoid the warm ischemic injury, the ischemic time usually limit to 25 min (7). Complete tumor resection, hemostasis, and suture in a limited time is a great challenge.
In recent years, some urologists have been focused on sutureless PN and tried to prove its safety and feasibility (2, 8–17). Without suture, the difficulty of operation is reduced, and the operation time is saved. However, the lack of reliable renal parenchyma suture may increase the potential risk of postoperative complications, which is controversial.
The superiority of PN in the surgical treatment of T1 RCC has been fully confirmed; by contrast, there is no meta-analysis comparing suture and sutureless PN. To fill this gap, we designed the present meta-analysis to compare the function and perioperative outcomes of the two techniques in PN for T1 RCC.
A literature search was performed in multiple databases (PubMed, Embase, and Web of Science) up to April 2021 to identify studies comparing suture to sutureless PN for T1 RCC. The diagnosis (kidney neoplasms, kidney cancer, renal carcinoma, renal tumor) and intervention (partial nephrectomy, nephron preservation and suture, surureless, and suture free) were used, respectively. We carried on the reference list search and citation literature retrieval for the full-text literature that met the research selection criteria.
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria were used for article selection (Figure 1), which was performed by two investigators (WZ and BC). The following study types were included: original studies comparing suture and sutureless PN were included regardless of the technique. All titles were screened for manuscripts written in the English language and only on adult patients. All studies were determined to include perioperative or functional outcomes. The titles of the articles were first reviewed to determine whether they might potentially fit the inclusion criteria. After assessing the abstract, a more comprehensive assessment was conducted by viewing the full text to determine whether the study should be included. Studies without primary data (i.e., case report reviews, commentaries, letters, and conference abstract) were excluded, but the reference lists were examined to identify that additional studies of interest had been included. References from the included studies were manually reviewed to identify additional studies of interest. Disagreement on whether or not an article should be included was resolved using a third reviewer (KT).
The quality of each study was determined using the Newcastle–Ottawa Scale (NOS) for nonrandomized controlled trials. The maximum score of the scale is 9. A total score of 5 or lower is considered low quality, 6–7 is considered intermediate quality, and 8–9 is considered high quality. Two researchers evaluated each study independently.
Data were extracted using a predefined data extraction form. Baseline demographics (age, T stage, RENAL Score, and baseline renal function), perioperative data (operative time, warm ischemia time, estimated blood loss, postoperative complications, surgical margins, and clamping rate), functional parameters [postoperative estimated glomerular filtration rat (eGFR)] and oncological outcome parameters (tumor recurrence) were extracted from the studies whenever available. For continuous outcomes, weighted mean difference (WMD) was used to measure differences; the risk ratio (RR) with 95% confidence interval (CI) was calculated for binary variables. For studies reporting medians and ranges [or interquartile ranges (IQR)], validated mathematical models were used to convert the median (range or IQR) to the mean [standard deviation (SD)] (18, 19). Between-study statistical heterogeneity was assessed using I2 and the Cochrane Q test. If there is no significant heterogeneity between the studies (P > 0.10, I2 < 50%), pooled estimates were calculated using a fixed-effects model; otherwise, using random effects model (P < 0.10, I2 > 50%). The significance level was set to α = 0.05, and 95%CI was taken. Begg’s and Egger’s tests were used to detect publication bias. Sensitivity analysis was used in high heterogeneity analysis to test the stability of the conclusion. This research used statistical software Stata12.0 to merge data.
This analysis included eight retrospective case–control studies with a total of 1,156 patients. All the included studies were compared the sutureless and suture techniques in laparoscopic PN for T1 tumors. Two of the studies used propensity score to match the preoperative baseline characteristics (Table 1). Subgroup analysis was performed between uncomplex (studies only included T1a RCC and RENAL score < 6) and complex subgroups (studies included T1b RCC and RENAL score ≥ 6), including operation time, warm ischemia time, estimated blood loss, and postoperative complications. Among the selected article, postoperative follow-up observation was carried out in six articles; only two articles reported recurrence. In addition, four articles compared postoperative eGFR. Due to different renal function evaluation criteria, we only selected two articles with the same criteria for meta-analysis. We analyzed the baseline characteristics according to tumor size, age, and preoperative renal function and evaluated selection bias to obtain more convincing conclusions (Table 2).
In perioperative outcomes, the operation time was different (I2 = 0; WMD, −24.836; 95%CI, −28.727, −20.945; P < 0.001), and subgroup analysis showed that sutureless PN has shorter operation time than suture PN in uncomplex subgroup (I2 = 0; WMD, −20.053; 95%CI, −35.599 to −4.507; P = 0.011) and complex subgroup (I2 = 0; WMD, −25.155; 95%CI, −29.174 to −21.137; P < 0.001). Difference also existed in the warm ischemia time (I2 = 97.5; WMD, −8.335; 95%CI, −12.254 to −4.416; P < 0.001). In subgroup analysis, the ischemia time of sutureless PN was shorter than that of suture PN in the uncomplex subgroup (I2 = 97.9%; WMD, −6.575; 95%CI, −7.852 to −5.298; P < 0.001) and complex subgroup (I2 = 0; WMD, −9.306; 95%CI, −14.433 to −4.179; P < 0.001) (Figure 2). In addition, the rate of warm ischemia in the sutureless PN was lower than that in the suture PN (I2 = 85.8%; RR, 0.447; 95%CI, 0.264, 0.756; P = 0.003). There was no difference in estimated blood loss (I2 = 76.6%; WMD, −27.529; 95%CI, −56.645 to 1.588; P = 0.064); further subgroup analysis found sutureless PN had less blood loss in the complex subgroup (I2 = 0%; WMD, −105.175; 95%CI, −156.824 to −53.527; P < 0.001), while blood loss in the uncomplex subgroup had no difference (I2 = 77.8%; WMD, −10.198; 95%CI, −56.645 to 1.588; P = 0.064) (Figure 3). There was no significant difference in postoperative complications (I2 = 0; RR, 0.915; 95%CI, 0.578–1.449; P = 0.999), positive surgical margin (I2 = 0; RR, 0.604; 95%CI, 0.207–1.761; P = 0.356), postoperative renal function (I2 = 0; WMD, −1.491; 95%CI, −8.049 to 5.066; P = 0.656), and tumor recurrence (I2 = 0; RR, 0.55; 95%CI, −0.085 to 3.573; P = 0.531) (Figures 4 and 5).
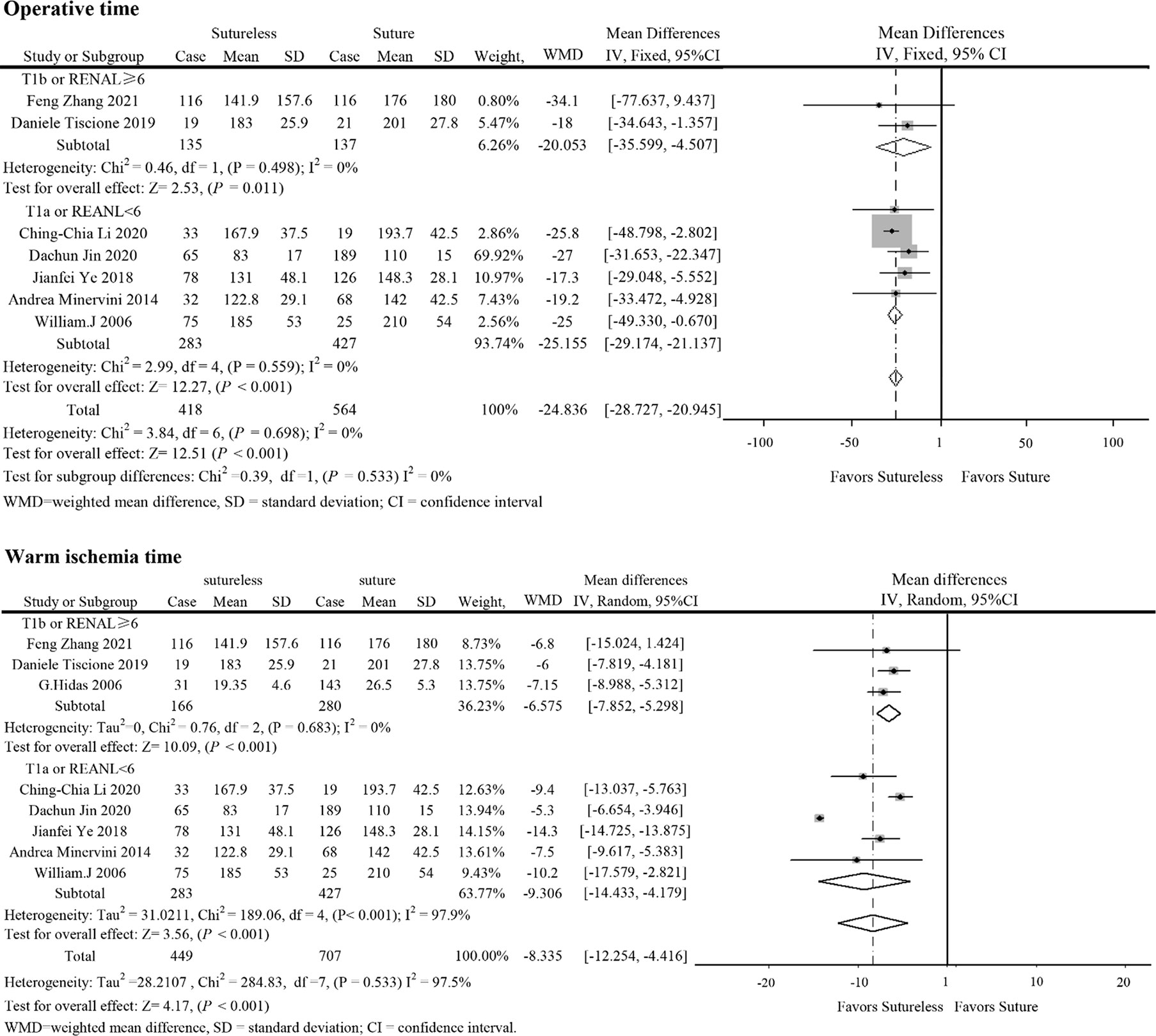
Figure 2 Forest plots of operative time and warm ischemia time for sutureless versus suture partial nephrectomy.
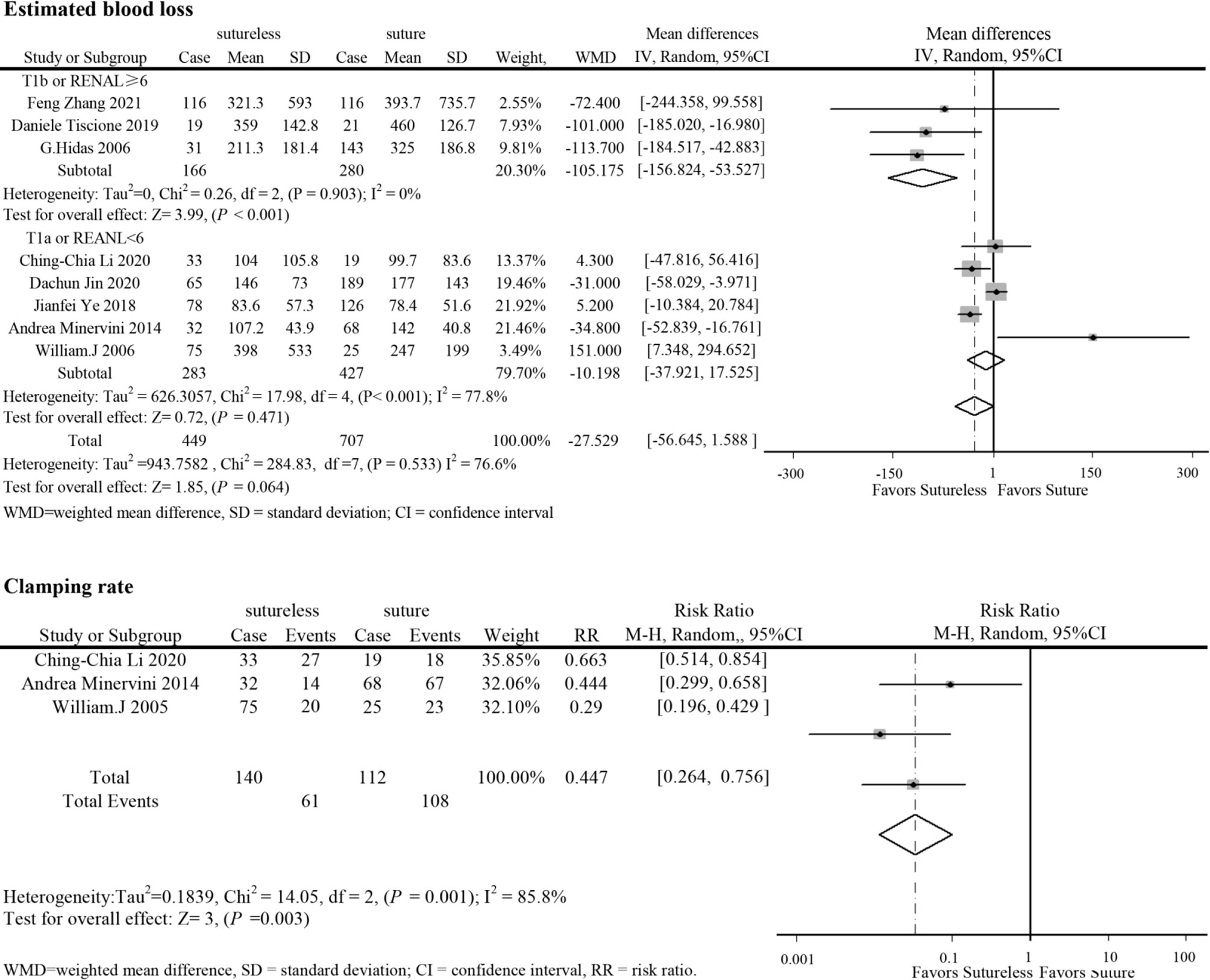
Figure 3 Forest plots of estimated blood loss and clamping rate for sutureless versus suture partial nephrectomy.
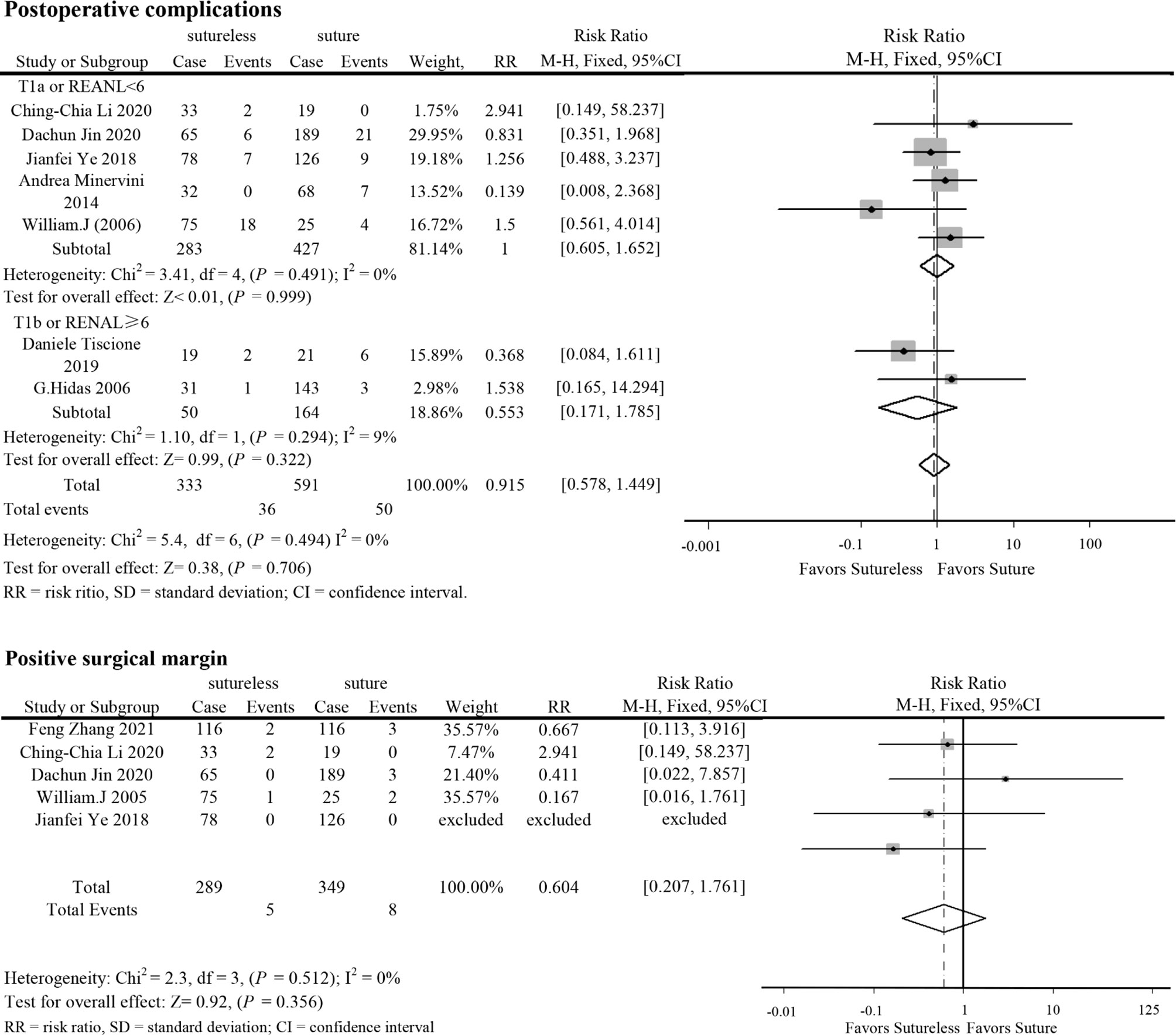
Figure 4 Forest plots of postoperative complications and positive surgical margin for sutureless versus suture partial nephrectomy.
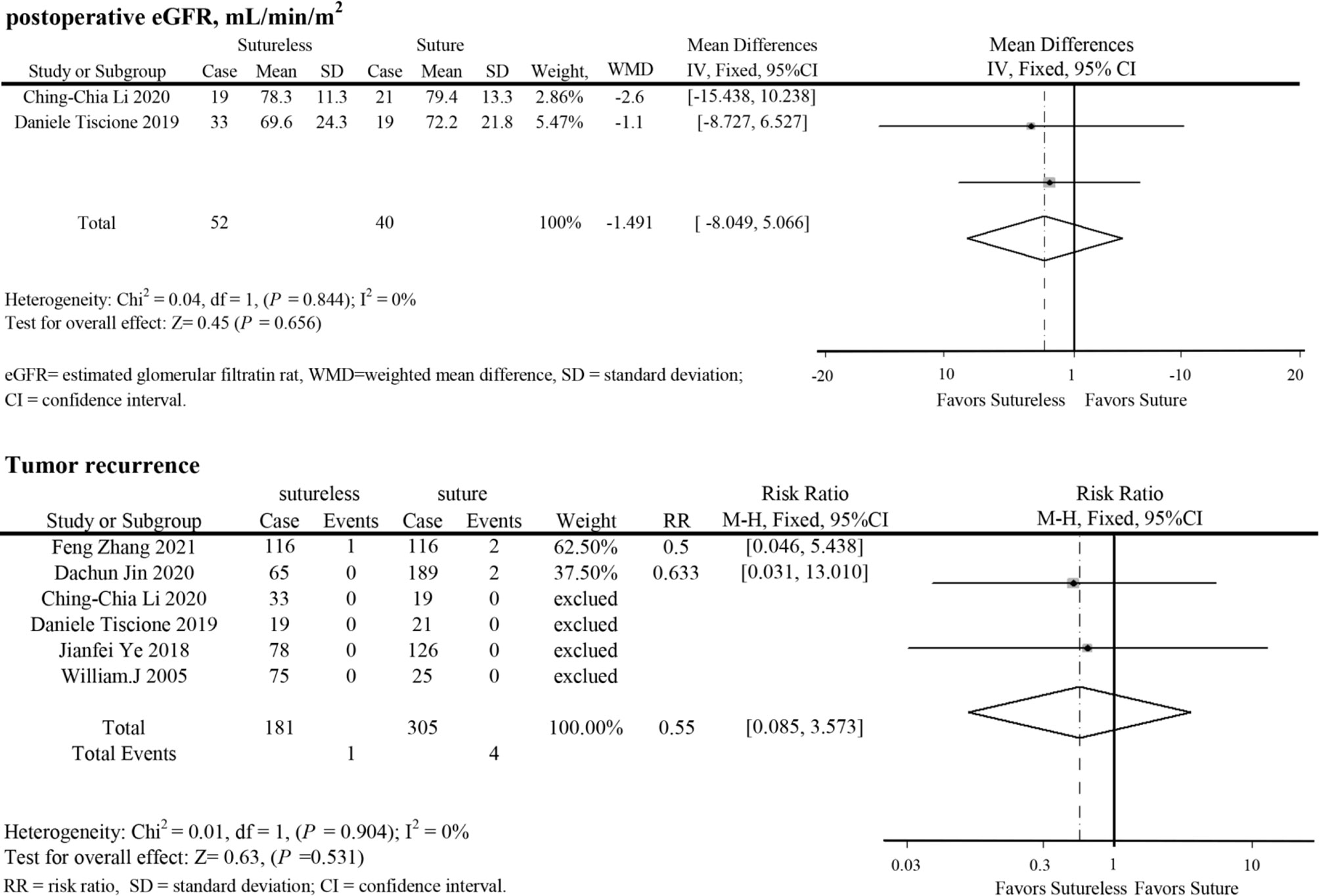
Figure 5 Forest plots of postoperative eGFR and tumor recurrence for sutureless versus suture partial nephrectomy.
Sensitivity analysis was used in analysis with high heterogeneity (operation time and estimated blood loss). After excluding the study of Jianfei Ye et al. (13), the heterogeneity of warm ischemia time decreased (I2 from 97.5% to 25.3%), but the results were consistent with previous studies. In addition, in comparing operation time, after excluding the study of Dachun Jin et al. (10), the results were also consistent with the previous studies.
Begg’s and Egger’s tests found no significant publication bias (Table 3).
At present, PN is the recommended method for surgical treatment of T1 tumors (1). A successful PN operation included surgical margin, slight decrease in renal function, and no serious postoperative complications (20). Maximum preservation of renal function is the original intention of PN (21). Saving the time of ischemia could reduce renal damage (22). In this study, sutureless PN had advantages in shortening the ischemia time and reducing operative blood loss. In addition, the rate of complete renal artery clamping was lower in the sutureless PN, which might have a potential advantage in the protection of postoperative renal function (23, 24).
Without reconstruction, sutureless PN could reduce the operation difficulty, which could reduce operation time. Although the lack of reconstruction might bring potential risks, in comparison of operative blood loss and postoperative complications, the application of biomaterials and electrocoagulation hemostasis in sutureless PN seems to have similar operative outcomes as suture PN. However, there was no difference in surgical blood loss between the two techniques in uncomplex subgroup, which reflected that reduction in clamping rate might lead to more blood loss. Due to the bias of retrospective study and the technical differences between each center that might directly affect the operative blood loss, operation time, and ischemia time, it cannot fully prove that sutureless PN has more advantages. In addition, with the continuous optimization and maturity of suture PN, the difference in operative blood loss and ischemia time might be reduced (25).
Segmental renal artery ligation caused by the suture might lead to the loss of functional renal parenchyma (26–28). Sutureless PN seems to reduce the potential impact on postoperative renal function, but no difference in postoperative eGFR was found in our study. In the study of Zhang et al. and Jin et al., it was shown that the decline in renal function after sutureless PN was lower than that after suture PN (10, 11). Due to the different diagnostic criteria in the included studies, we could not evaluate the difference in renal function decline to prove that sutureless technique is better than suture technique in renal function protection. In this study, the low positive surgical margin and tumor recurrence reported in the included studies might benefit from the resection near the pseudocapsule of renal tumor, which had been proven to be safe for oncology outcomes (29, 30). Due to the incomplete follow-up data in the included studies, more detailed data are needed to assess the long-term prognosis of sutureless and suture PN.
In this study, outcomes of sutureless and suture PN in perioperative were similar, and sutureless PN might have potential advantages in protecting renal function. However, in larger and more complex T1b RCC, which requires more extensive parenchyma resection and reconstruction, the potential risk may be greater. Overall, under strict preoperative and intraoperative evaluation, sutureless PN could be a feasible choice for smaller T1a RCC, whereas should not be overestimated in T1b RCC.
Our research has some limitations. First, the meta-analysis of retrospective studies, which have an inherent bias, could not fully compare the pros and cons between the two techniques. Second, the technical differences and diagnostic criteria between different time periods and different centers and heterogeneity between studies were inevitable. Analysis of the sources of heterogeneity requires more detailed subgroup data. Third, due to the lack of oncology outcome-related data, we cannot better evaluate the surgical prognosis. Lastly, only eight retrospective studies are included in this analysis, and more high-quality studies are needed in the future.
Our meta-analysis suggested that the two surgical techniques have similar perioperative outcomes in T1a RCC with low RENAL score. In addition, sutureless PN might have potential advantages in the protection of renal function. In some cases, sutureless PN is a feasible choice under strict preoperative and intraoperative evaluation. However, application in larger and more complex T1b needs to be cautiously made. More well-designed prospective randomized clinical trials are needed for further research in the future.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Study concept and design: KT and WZ. Data acquisition: WZ, BC, SX, YM, and JH. Data analysis: WZ, BC, and SX. Drafting of manuscript: WZ. Critical revision of the manuscript: KT. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Fund Project of Guizhou Health Commission (gzwkj2021-211).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol (2019) 75:799–810. doi: 10.1016/j.eururo.2019.02.011
2. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol (2017) 198:520–9. doi: 10.1016/j.juro.2017.04.100
3. Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic Kidney Disease After Nephrectomy in Patients With Renal Cortical Tumours: A Retrospective Cohort Study. Lancet Oncol (2006) 7:735–40. doi: 10.1016/S1470-2045(06)70803-8
4. Zabell JR, Wu J, Suk-Ouichai C, Campbell SC. Renal Ischemia and Functional Outcomes Following Partial Nephrectomy. Urol Clin North Am (2017) 44:243–55. doi: 10.1016/j.ucl.2016.12.010
5. Patel HD, Pierorazio PM, Johnson MH, Sharma R, Iyoha E, Allaf ME, et al. Renal Functional Outcomes After Surgery, Ablation, and Active Surveillance of Localized Renal Tumors: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol (2017) 12:1057–69. doi: 10.2215/CJN.11941116
6. Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A. Autorino R. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-Analysis of Comparative Studies. Eur Urol (2017) 71:606–17. doi: 10.1016/j.eururo.2016.08.060
7. Volpe A, Blute ML, Ficarra V, Gill IS, Kutikov A, Porpiglia F, et al. Renal Ischemia and Function After Partial Nephrectomy: A Collaborative Review of the Literature. Eur Urol (2015) 68:61–74. doi: 10.1016/j.eururo.2015.01.025
8. Simone G, Papalia R, Guaglianone S, Gallucci M. ’Zero Ischaemia’, Sutureless Laparoscopic Partial Nephrectomy for Renal Tumours With a Low Nephrometry Score. BJU Int (2012) 110:124–30. doi: 10.1111/j.1464-410X.2011.10782.x
9. Dell’Atti L, Scarcella S, Manno S, Polito M, Galosi AB. Approach for Renal Tumors With Low Nephrometry Score Through Unclamped Sutureless Laparoscopic Enucleation Technique: Functional and Oncologic Outcomes. Clin Genitourin Cancer (2018) 16:e1251–6. doi: 10.1016/j.clgc.2018.07.020
10. Jin D, Ren D, Zhang J, Xu G, Ge C, Jiang Q, et al. A Propensity Score-Matched Comparison Between Sutureless and Suture Techniques in Laparoscopic Nephron-Sparing Surgery: A Retrospective Non-Randomized Observational Study. J Laparoendosc Adv Surg Tech A (2020) 30:1314–9. doi: 10.1089/lap.2020.0187
11. Zhang F, Gao S, Zhao Y, Wu B, Chen X. Comparison of Sutureless and Conventional Laparoscopic Partial Nephrectomy: A Propensity Score-Matching Analysis. Front Oncol (2021) 11:649356. doi: 10.3389/fonc.2021.649356
12. Johnston WR, Montgomery JS, Seifman BD, Hollenbeck BK, Wolf JJ. Fibrin Glue V Sutured Bolster: Lessons Learned During 100 Laparoscopic Partial Nephrectomies. J Urol (2005) 174:47–52. doi: 10.1097/01.ju.0000162041.64143.08
13. Ye J, Zhang S, Tian X, Wang G, Zhao L, Ma L. Knotless Retroperitoneoscopic Nephron-Sparing Surgery for Small Renal Masses: Comparison of Bipolar Sutureless Technique and Barbed Suture Technique. J Int Med Res (2018) 46:1649–56. doi: 10.1177/0300060518760737
14. Li CC, Chien TM, Huang SP, Yeh HC, Lee HY, Ke HL, et al. Single-Site Sutureless Partial Nephrectomy for Small Exophytic Renal Tumors. J Clin Med (2020) 9:3658. doi: 10.3390/jcm9113658
15. Minervini A, Siena G, Tuccio A, Lapini A, Serni S, Carini M. Sutureless Hemostatic Control During Laparoscopic NSS for the Treatment of Small Renal Masses. Surg Innov (2014) 21:32–8. doi: 10.1177/1553350613484823
16. Tiscione D, Cai T, Luciani LG, Puglisi M, Mattevi D, Nesi G, et al. Sutureless Laparoscopic Partial Nephrectomy Using Fibrin Gel Reduces Ischemia Time While Preserving Renal Function. Arch Ital Urol Androl (2019) 91:30–4. doi: 10.4081/aiua.2019.1.30
17. Hidas G, Kastin A, Mullerad M, Shental J, Moskovitz B, Nativ O. Sutureless Nephron-Sparing Surgery: Use of Albumin Glutaraldehyde Tissue Adhesive (BioGlue). Urology (2006) 67:697–700; discussion 700. doi: 10.1016/j.urology.2005.10.064
18. Wan X, Wang W, Liu J, Tong T. Estimating the Sample Mean and Standard Deviation From the Sample Size, Median, Range and/or Interquartile Range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
19. Hozo SP, Djulbegovic B, Hozo I. Estimating the Mean and Variance From the Median, Range, and the Size of a Sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
20. Hung AJ, Cai J, Simmons MN, Gill IS. "Trifecta" in Partial Nephrectomy. J Urol (2013) 189:36–42. doi: 10.1016/j.juro.2012.09.042
21. Mir MC, Ercole C, Takagi T, Zhang Z, Velet L, Remer EM, et al. Decline in Renal Function After Partial Nephrectomy: Etiology and Prevention. J Urol (2015) 193:1889–98. doi: 10.1016/j.juro.2015.01.093
22. Porpiglia F, Fiori C, Bertolo R, Angusti T, Piccoli GB, Podio V, et al. The Effects of Warm Ischaemia Time on Renal Function After Laparoscopic Partial Nephrectomy in Patients With Normal Contralateral Kidney. World J Urol (2012) 30:257–63. doi: 10.1007/s00345-011-0729-5
23. Simone G, Gill IS, Mottrie A, Kutikov A, Patard JJ, Alcaraz A, et al. Indications, Techniques, Outcomes, and Limitations for Minimally Ischemic and Off-Clamp Partial Nephrectomy: A Systematic Review of the Literature. Eur Urol (2015) 68:632–40. doi: 10.1016/j.eururo.2015.04.020
24. Klatte T, Ficarra V, Gratzke C, Kaouk J, Kutikov A, Macchi V, et al. Gill IS. A Literature Review of Renal Surgical Anatomy and Surgical Strategies for Partial Nephrectomy. Eur Urol (2015) 68:980–92. doi: 10.1016/j.eururo.2015.04.010
25. Bertolo R, Campi R, Klatte T, Kriegmair MC, Mir MC, Ouzaid I, et al. Suture Techniques During Laparoscopic and Robot-Assisted Partial Nephrectomy: A Systematic Review and Quantitative Synthesis of Peri-Operative Outcomes. Bju Int (2019) 123:923–46. doi: 10.1111/bju.14537
26. Bahler CD, Sundaram CP. Effect of Renal Reconstruction on Renal Function After Partial Nephrectomy. J Endourol (2016) 30(Suppl 1):S37–41. doi: 10.1089/end.2016.0055
27. Dong W, Wu J, Suk-Ouichai C, Caraballo AE, Remer E, Li J, et al. Devascularized Parenchymal Mass Associated With Partial Nephrectomy: Predictive Factors and Impact on Functional Recovery. J Urol (2017) 198:787–94. doi: 10.1016/j.juro.2017.04.020
28. Porpiglia F, Bertolo R, Amparore D, Fiori C. Nephron-Sparing Suture of Renal Parenchyma After Partial Nephrectomy: Which Technique to Go For? Some Best Practices. Eur Urol Focus (2019) 5:600–3. doi: 10.1016/j.euf.2017.08.006
29. Azhar RA, de Castro AA, Broxham E, Sherrod A, Ma Y, Cai J, et al. Histological Analysis of the Kidney Tumor-Parenchyma Interface. J Urol (2015) 193:415–22. doi: 10.1016/j.juro.2014.08.010
Keywords: partial nephrectomy, renal cell carcinoma, meta-analysis, suture, sutureless
Citation: Zhang W, Che B, Xu S, Mu Y, He J and Tang K (2021) Comparison of Sutureless Versus Suture Partial Nephrectomy for Clinical T1 Renal Cell Carcinoma: A Meta-Analysis of Retrospective Studies. Front. Oncol. 11:713645. doi: 10.3389/fonc.2021.713645
Received: 23 May 2021; Accepted: 13 August 2021;
Published: 02 September 2021.
Edited by:
Antonio Augusto Ornellas, National Cancer Institute (INCA), BrazilReviewed by:
Ari Adamy, Santa Casa Hospital, BrazilCopyright © 2021 Zhang, Che, Xu, Mu, He and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaifa Tang, dGFuZ2thaWZhQGdtYy5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.