- 1OMFS IMPATH Research Group, Department of Imaging & Pathology, Faculty of Medicine, KU Leuven, Leuven, Belgium
- 2Department of Oral and Maxillofacial Surgery, University Hospitals Leuven, Leuven, Belgium
- 3Department of Plastic, Reconstructive, and Aesthetic Surgery, University Hospitals Leuven, Leuven, Belgium
- 4Department of Dental Medicine, Karolinska Institutet, Stockholm, Sweden
Objective: To investigate the adherence to initially planned maxillofacial reconstructions using computer-assisted surgery (CAS) and to identify the influential factors affecting its compliance for maxillofacial reconstruction.
Patients and Methods: A retrospective analysis of 136 computer-assisted maxillofacial reconstructive surgeries was conducted from January 2014 to June 2020. The categorical parameters involved age, gender, disease etiology, disease site, defect size, bone flap segments, and flap type. Apart from descriptive data reporting, categorical data were related by applying the Fisher-exact test, and a p-value below 5% was considered statistically significant (P < 0.05).
Results: The main reasons for partial or non-adherence included unfitness, patient health condition, and other subjective reasons. Out of the total patient population, 118 patients who underwent mandibular reconstruction showed higher CAS compliance (83.9%) compared to the 18 midface reconstruction (72.2%) without any statistically significant difference (p = 0.361). Based on the size of the defect, a significantly higher CAS compliance (p = 0.031) was observed with a minor defect (80.6%) compared to the large-sized ones (74.1%). The bone flaps with two or more segments were significantly (p = 0.003) prone to observe a partial (15.4%) or complete (12.8%) discard of the planned CAS compared to the bone flaps with less than two segments. The malignant tumors showed the lowest CAS compliance when compared to other disorders without any significant difference (p = 0.1).
Conclusion: The maxillofacial reconstructive surgical procedures offered optimal compliance to the initially planned CAS. However, large-sized defects and multiple bone flap segments demonstrated a higher risk of partial or complete abandonment of the CAS.
Introduction
Reconstructive maxillofacial surgery following tumor resection, trauma, osteonecrosis, and other infectious diseases is vital for restoring facial aesthetics, function, oral rehabilitation and improving the patient’s quality of life (QOL) (1). Depending on the complexity of the defect, the reconstruction might range from a local flap with secondary bone grafting to microvascular free flap surgery. The maxillofacial region mandates special care from a surgeon as it occupies a central position concerning the aesthetics and functionality, as an inadequate reconstruction might negatively influence the final outcome (2).
Previously, maxillofacial reconstruction with the traditional freehand technique offered a challenge for optimally repositioning the grafted segments and maintaining facial symmetry. However, with the advent of computer-assisted surgery (CAS) and three-dimensional (3D) printing, the reconstructive surgical accuracy and patient- and surgery-related outcomes have significantly improved (3, 4). Additionally, CAS has also played a vital role in improving the oral rehabilitation by increasing the predictability of replacing missing teeth with both first- and second-stage dental implant placement in the grafted region (5). Thereby, making CAS an indispensable tool for reconstructive surgery.
Over the past few years, the significant technological advancements and availability of surgeon-friendly software programs have led to the domination of CAS for maxillofacial reconstruction compared to its conventional counterpart by offering multiple advantages, which commonly include, improved resection accuracy, reduction in the operation, ischemia and hospitalization time, improved functional and aesthetic outcomes and minimization of the intersegmental gap size (6–8). At the same instance, the disadvantages such as preparation and planning time, and cost aspects cannot be ignored (9–11). Although, multiple centers now offer in-house CAS services for decreasing the time to therapy initiation (TTI) (12). However, an issue still exists where certain centers with low-volume of reconstruction cases rely on out-of-house services, which might cause a delay in the delivery and treatment time, in turn leading to further growth of the tumor (13). All these limiting CAS factors should be taken into consideration, as TTI has been known to be an influential factor for pathologic tumor upstaging, where an untimely intervention might lead to further tumor progression and increased mortality (14, 15).
Various studies have focussed on the accuracy and reproducibility of the CAS for maxillofacial reconstruction. However, a lack of evidence exists pertaining to the CAS compliance during the reconstructive procedures. It is questionable whether a surgeon completely adheres to the planned CAS (16). Previous studies reporting on the CAS compliance have only briefly reported whether the planning was executed entirely, partially, or abandoned and also failed to assess the factors which might influence its adherence.
Therefore, the present study was conducted to investigate the CAS compliance for initially planned maxillofacial reconstruction and to identify potential influential factors that might affect its adherence to the initially planned CAS.
Material and Methods
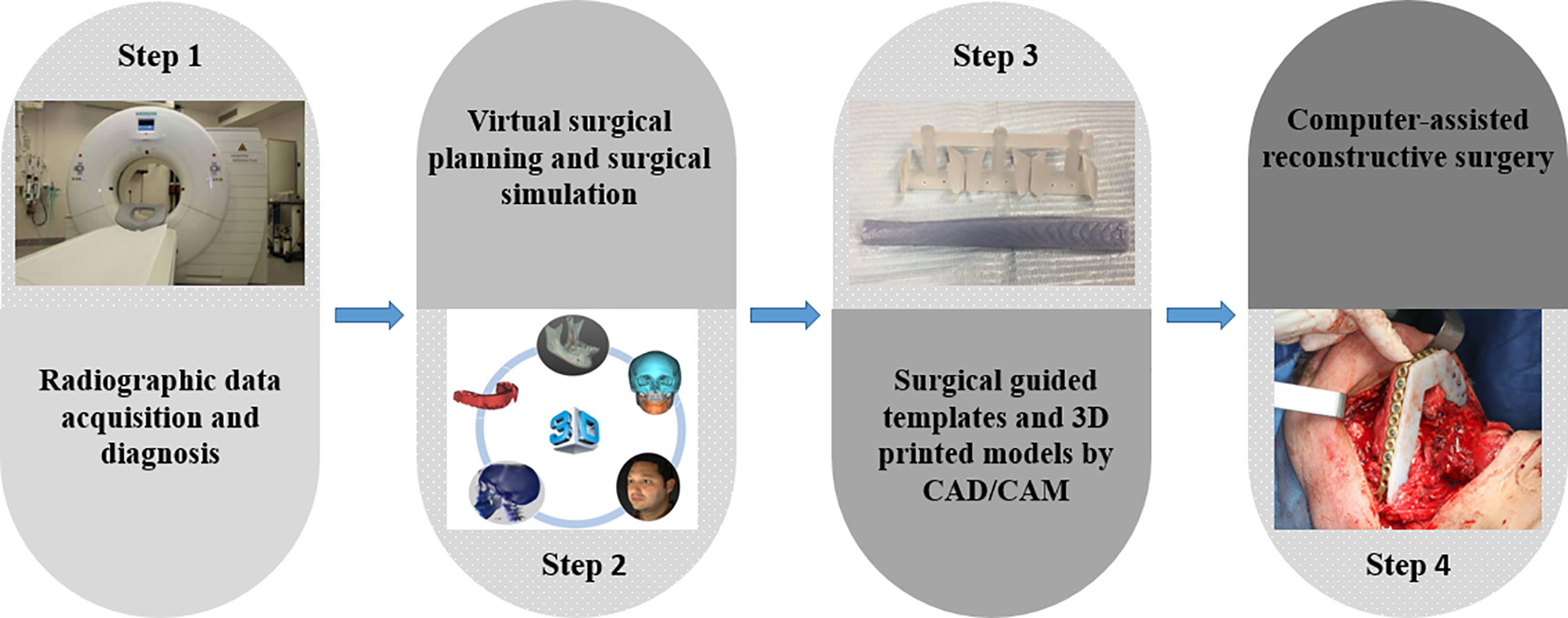
The Local Ethics Committee approved the study (reference no.: S63615) and was conducted in compliance with the World Medical Association Declaration of Helsinki on medical research (clinicaltrials.gov, NCT04895319). A total of 210 patients who underwent CAS-based maxillofacial reconstruction were screened from January 2014 to June 2020. The inclusion criteria involved patients undergoing maxillofacial reconstruction with CAS, which included virtual surgical planning, CAD-CAM surgical guides/templates, and pre-bent plates on 3D printed models. The workflow in our single-center was illustrated in Figure 1. Reasons for reconstruction were oncologic, osteoradionecrosis, trauma, and osteoporosis. Patients undergoing computer-assisted implant surgery and orthognathic surgery were excluded.
All computer-assisted surgeries were planned by an experienced clinical engineer in discussion with the oral and maxillofacial surgical team. The virtual planning was performed to determine the resection, cut margins, and localize the optimal angles for performing osteotomies. After that, surgical cutting guides were designed utilizing a 3D designing software (3-Matic, Version 9.0-13.0, Materialise, Leuven, Belgium). The generated virtual templates and the planned 3D skeletal model were exported in a Standard Tessellation Language (STL) format and printed with a professional 3D printer (Connex 350 3D printer, Stratasys, Eden Prairie, MN, USA). The reconstructive plates were pre-bent on the 3D-printed model. A fixation tray was applied for the guided placement of the reconstructive plates. The screw holes’ locations were drilled and marked onto the surgical template by the surgeon (Figure 2).
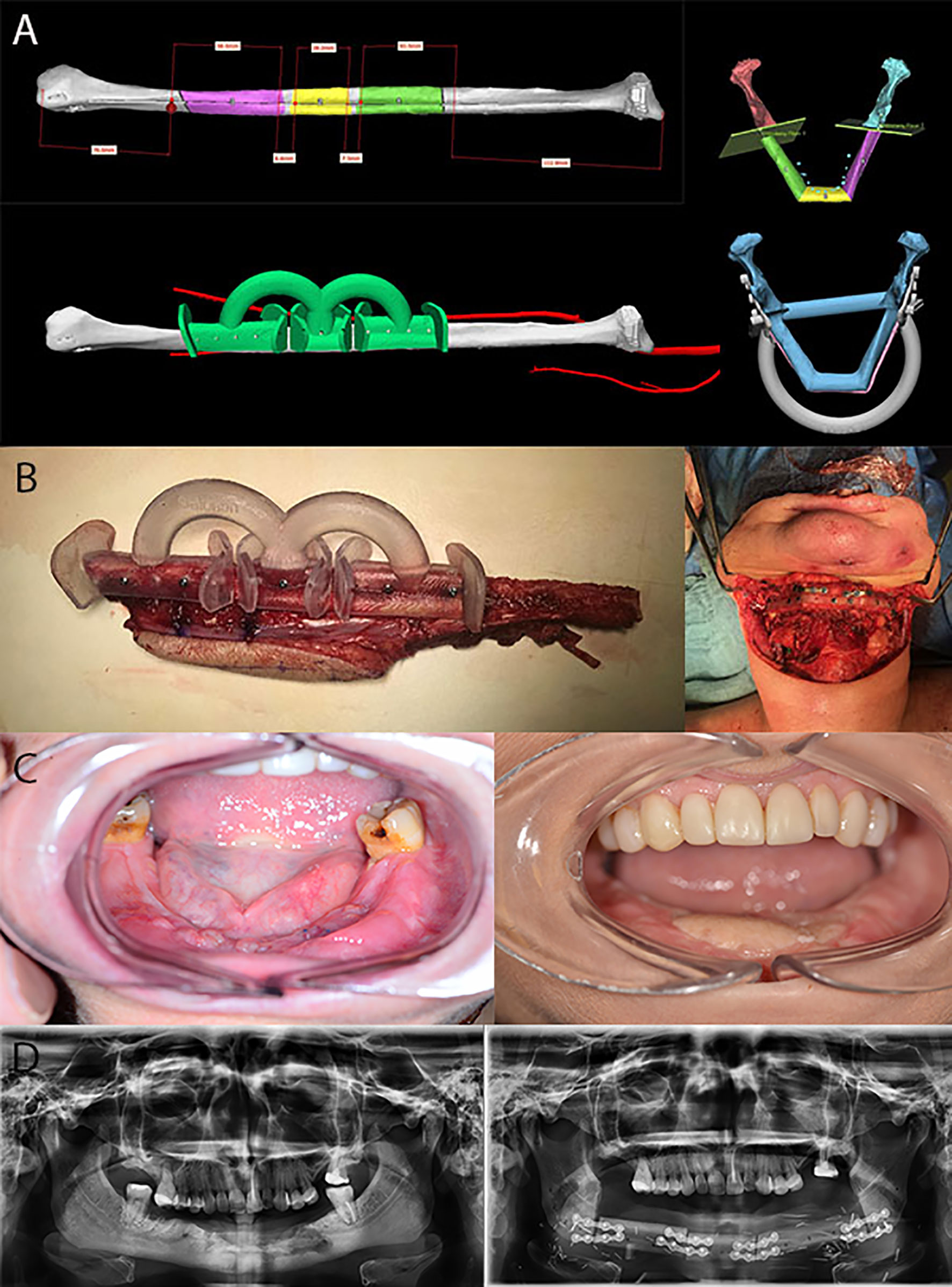
Figure 2 Computer-assisted surgical planning and execution for a squamous cell carcinoma reconstruction. (A) Preoperative virtual analysis and planning. (B) Fibular graft fabrication assisted by guided templates. (C) Preoperative and postoperative intraoral photos of squamous cell carcinoma resection with mandibular reconstruction. (D) Preoperative and postoperative panoramic radiographs.
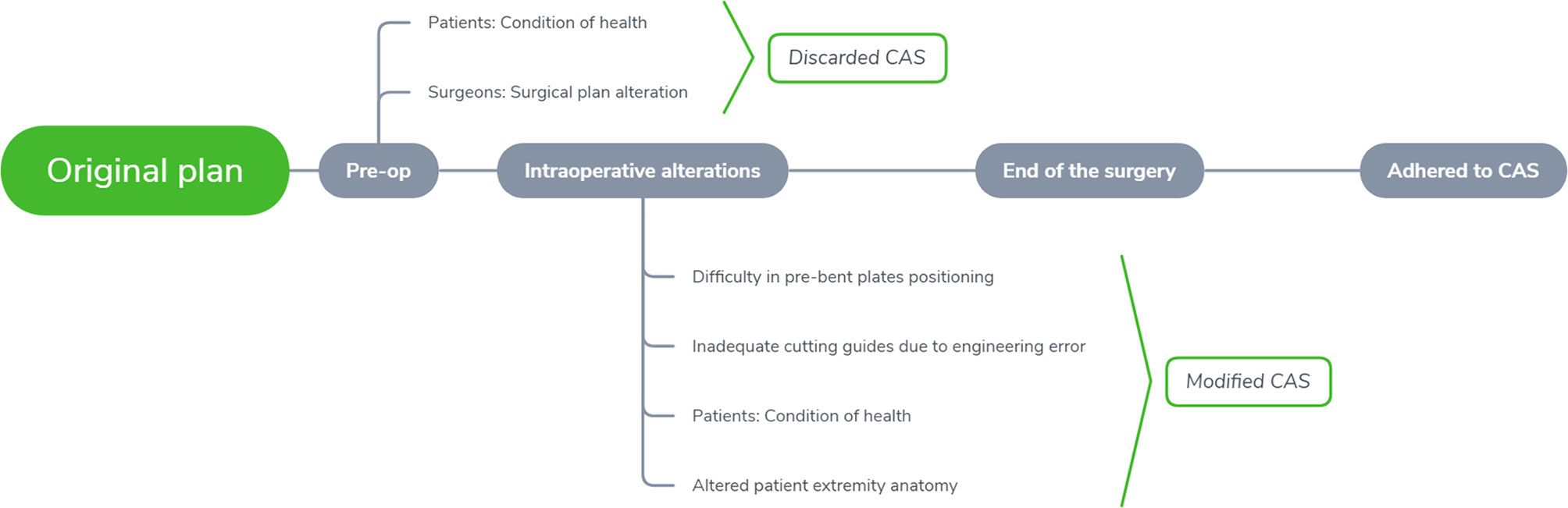
The patients were divided into three groups depending on the CAS compliance either during the pre-operative or intra-operatively, which included; complete adherence, partial adherence, and no adherence (Figure 3). The recorded categorical parameters involved disease etiology classified by either malignant or non-malignant tumor, disease site (mandible or midface), bone flap segments (< 2 or ≥ 2 segments), and flap type (bone flap or others). (The defects were classified based on Brown classification, where class I, II of mandibular defect and class I, II, V, VI of maxillary and midface defect were defined as a small defect; Class III, IV of the mandibular defect and class III, IV of maxillary and midface defect were defined as a large defect (17, 18).
Statistical Analysis
Data were analyzed using IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY: IBM Corp, USA). Mean values and standard deviation were recorded for all parameters. The categorical data were compared by applying the Fisher-exact test. A p-value below 5% was considered statistically significant (p < 0.05).
Results
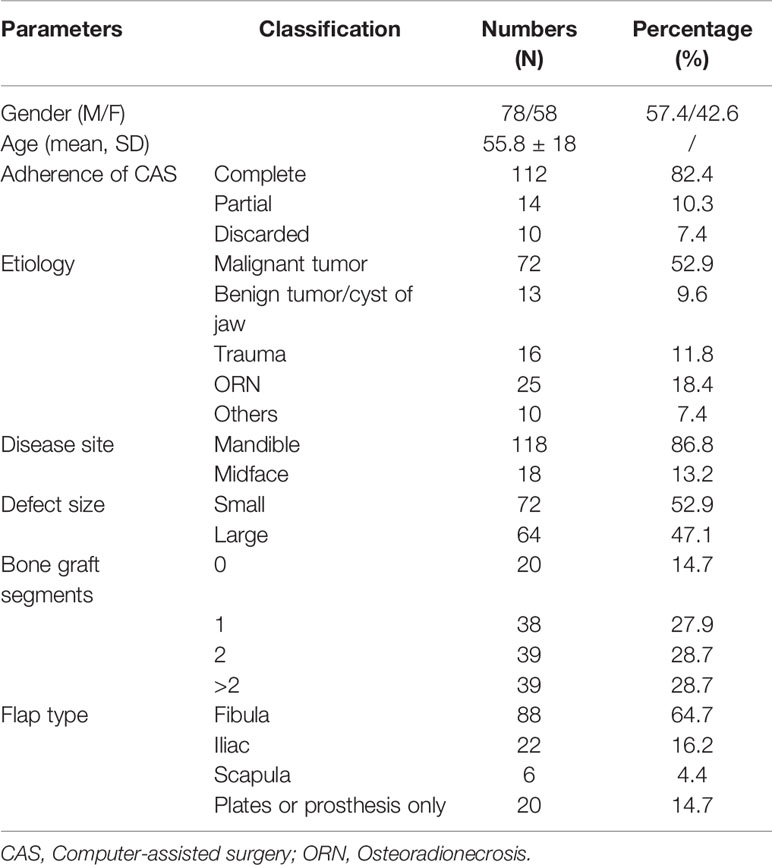
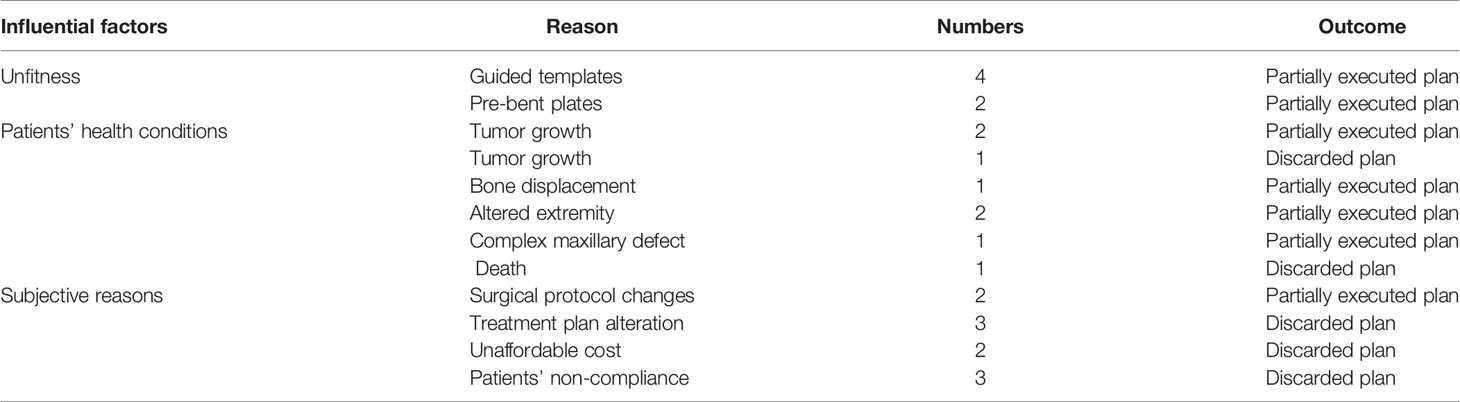
Following inclusion and exclusion criteria, clinical and image data of 136 consecutive patients (58 females, 78 males, mean age: 55.8 ± 18 years) undergoing CAS-based maxillofacial reconstruction were served further analysis. Table 1 describes the patient- and surgery-related characteristics, where the majority of the patients were diagnosed with malignant tumor (n = 72) followed by maxillofacial trauma (n=16), benign tumor or odontogenic keratocyst (n=13), osteoradionecrosis (n=25) and temporomandibular joint ankyloses/congenital maxillofacial defect (n=10). The main reasons for partial abandonment of the planned CAS included unfitness of the cutting guide (n = 4) and pre-bent plates (n = 2), patients health condition (n=7). Figure 4 illustrates an example of a case showing partial CAS compliance. In contrast, the complete discard of CAS was mainly attributed to subjective reasoning (Table 2).
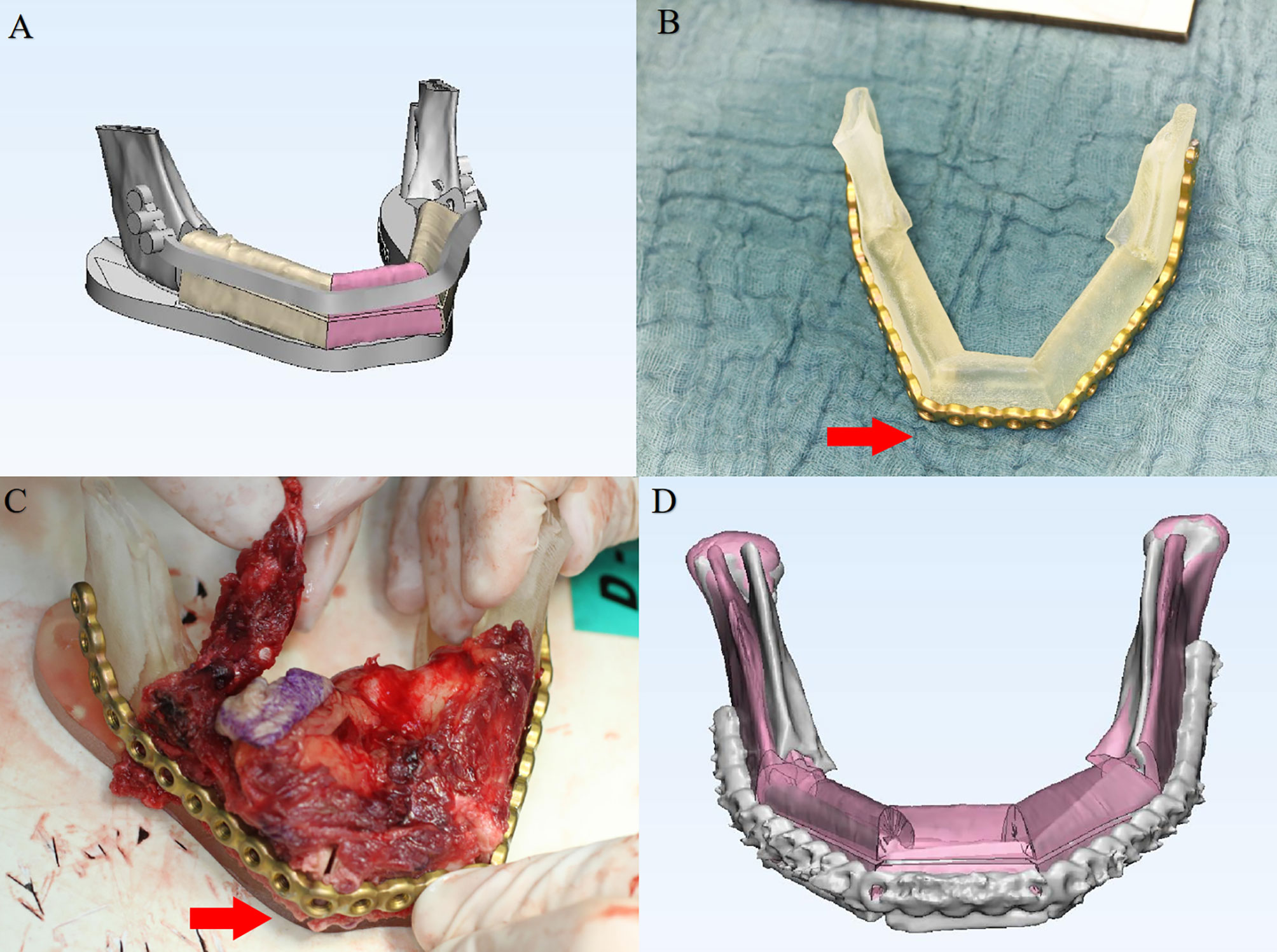
Figure 4 A 56-year-old patient with mandibular squamous cell carcinoma showing partial computer-assisted surgical compliance. (A) Virtual surgical planning for mandibular reconstruction. (B) Plate prebending on the 3D printed model. (C) Intra-operative plate bending modified due to unfitness. (D) Postoperative superimposition verifying the 3-D deviation of the reconstructed region compared with the original virtual surgical plan.
Table 3 describes the factors influencing the compliance to the planned CAS. When evaluating the CAS compliance based on the defect site, patients who underwent mandibular reconstruction showed higher complete adherence (83.9%) compared to the midface reconstruction (72.2%) without any statistically significant difference (p = 0.361). Based on the size of the defect, a significantly higher conformity to the CAS (p = 0.031) was observed for patients with a minor defect (80.6%) compared to the large-sized ones (74.1%). The bone flaps with more than two segments were significantly (p=0.003) prone to observe partial (15.4%) or complete discard of the CAS (12.8%). The malignant tumors showed the lowest conformity to the CAS when compared to other disorders without any significant difference (p=0.1). As for the patients treated with a bone flap, complete adherence was significantly higher (85.3%, p=0.016) when compared with the non-bony flap group (65.0%).
Discussion
The present study explored the conformity to CAS for maxillofacial reconstructive procedures and investigated the influence of the parameters to identify the reasons it was partially executed or wholly discarded.
The present study’s findings suggested that the unfitness of the guided templates and patients’ health condition were most commonly observed in the partially abandoned CAS, whereas complete CAS discard was based on subjective reasoning. The factors which could have attributed to the reduced CAS compliance might include CT data segmentation accuracy, medical engineer proficiency, or precision of the printed stereolithographic model. Any error occurring due to the aforementioned factors would influence the CAS compliance. Besides, a prolonged waiting time for the surgery or an early CT scan in oncology patients caused the further growth of the malignant tumors, thereby requiring partial or complete discard of the plan. It should be kept that the CAS-based surgical planning and implementation only rely on the hard tissue, without considering the intra-operative influence of the soft tissue. The soft tissue and musculature have been known to forcefully position the bone flap in complex reconstructive procedures, which is not considered at the treatment planning phase and might lead to partial or complete discard of the CAS (19). Therefore, a surgeon should be aware of the biomechanical deformation of the soft tissue during CAS, and a patient-specific soft tissue predictive model should be generated based on the CT data, and finite element analysis at the planning phase improved planning (20).
Efanov et al. assessed the adherence to CAS for maxillofacial reconstruction and their findings were consistent with the results of the current study (21). However, their sample mostly involved orthognathic surgery patients, with only six patients requiring free tissue transfer, unlike our study where orthognathic surgical procedures were excluded to reduce the risk of bias. Hanken et al. reported a relationship between surgical accuracy and the number of bone flap segments for the maxillofacial reconstruction, where higher deviations occurred between virtual and real segment position in patients requiring reconstruction with two or three fibular or iliac crest segments compared to a single segment (22). The accuracy of CAS decreases with the increased number of segments, which might explain the partial adherence or complete discard. Previous evidence failed to report whether the defect size decreases the CAS compliance. Our findings suggested that a large-sized defect and increased bone segments were more prone to lower CAS compliance, especially in cases involving condylar region or mandibular angle where unfitness of pre-bent plates was mainly observed.
A variety of approaches can establish the improvement in CAS. Effective and constant communication between the surgeon and medical engineer might significantly improve the planned CAS. As the incomplete adherence not only leads to an increased risk of intra-operative complications but is also associated with higher financial costs if the plan is changed at the pre-operative stage (23). For improving the virtual planning and CAS, it is recommended to utilize a CT image with a slice thickness of less than 1mm and to advocate a professional 3D printing for printing the skull model to improve the contouring of the pre-bent plates (6). Another option could be the 3D printing of the patient-specific titanium plates which offers improved accuracy compared to the traditional pre-bent plates (24). Regarding the cutting guides, patient-specific titanium alloy cutting guides could be an alternative to improve fitness. These guides are thinner than the polyamide guides, allowing easier intraoral placement and decrease the amount of periosteal stripping and cutaneous resection (25).
The study had certain limitations. Firstly, the quantitative accuracy of the CAS was not assessed. Secondly, the retrospective nature of the study could have acted as a medium of bias. Thirdly, sample distribution was heterogeneous, mainly involving reconstruction following resection of the malignant tumors. Future studies should investigate the amount of error induced at each step of the planning to understand better and improve complex reconstructive procedures.
Conclusion
CAS-based maxillofacial reconstructive surgery offered optimal conformity to the initially executed plan. However, large-sized defects and an increased number of bone flap segments led to a higher rate of partial or complete abandonment of CAS. Thereby, a surgeon should be aware of the possibility of non-adherence to the planned CAS for complex reconstructive procedures.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HM and SS: study design, manuscript preparation, statistical analysis, data analysis, and interpretation. RJ and CP: study supervision. JD and YS: data collection. MB, JD, SS, JV, RJ, and CP: contributed to the manuscript review, critical revision for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the statistician Mr. Wim Coucke for his assistance regarding the statistics applied in this research.
References
1. Yu P, Chang DW, Miller MJ, Reece G, Robb GL. Analysis of 49 Cases of Flap Compromise in 1310 Free Flaps for Head and Neck Reconstruction. Head Neck (2009) 31(1):45–51. doi: 10.1002/hed.20927
2. Wijbenga JG, Schepers RH, Werker PM, Witjes MJ, Dijkstra PU. A Systematic Review of Functional Outcome and Quality of Life Following Reconstruction of Maxillofacial Defects Using Vascularized Free Fibula Flaps and Dental Rehabilitation Reveals Poor Data Quality. J Plast Reconstr Aesthet Surg (2016) 69(8):1024–36. doi: 10.1016/j.bjps.2016.05.003
3. Largo RD, Garvey PB. Updates in Head and Neck Reconstruction. Plast Reconstr Surg (2018) 141(2):271e–85e. doi: 10.1097/PRS.0000000000004070
4. Harrison P, Patel A, Cheng A, Bell RB. Three-Dimensional Computer-Assisted Surgical Planning, Manufacturing, and Intraoperative Navigation in Oncologic Surgery. Atlas Oral Maxillofac Surg Clin North Am (2020) 28(2):129–44. doi: 10.1016/j.cxom.2020.06.001
5. Ma H, Shujaat S, Bila M, Sun Y, Vranckx J, Politis C, et al. Computer-Assisted Versus Traditional Freehand Technique for Mandibular Reconstruction With Free Vascularized Fibular Flap: A Matched-Pair Study. J Plast Reconstr Aesthet Surg (2021). doi: 10.1016/j.bjps.2021.03.121. in press.
6. van Baar GJC, Forouzanfar T, Liberton N, Winters HAH, Leusink FKJ. Accuracy of Computer-Assisted Surgery in Mandibular Reconstruction: A Systematic Review. Oral Oncol (2018) 84:52–60. doi: 10.1016/j.oraloncology.2018.07.004
7. van Baar GJC, Schipper K, Forouzanfar T, Leeuwrik L, Winters HAH, Ridwan-Pramana A, et al. Accuracy of Computer-Assisted Surgery in Maxillary Reconstruction: A Systematic Review. J Clin Med (2021) 10(6):1226. doi: 10.3390/jcm10061226
8. D’Haese J, Ackhurst J, Wismeijer D, De Bruyn H, Tahmaseb A. Current State of the Art of Computer-Guided Implant Surgery. Periodontol 2000 (2017) 73(1):121–33. doi: 10.1111/prd.12175
9. Mazzola F, Smithers F, Cheng K, Mukherjee P, Hubert Low TH, Ch’ng S, et al. Time and Cost-Analysis of Virtual Surgical Planning for Head and Neck Reconstruction: A Matched Pair Analysis. Oral Oncol (2020) 100:104491. doi: 10.1016/j.oraloncology.2019.104491
10. Rommel N, Kesting MR, Rohleder NH, Bauer FMJ, Wolff KD, Weitz J. Mandible Reconstruction With Free Fibula Flaps: Outcome of a Cost-Effective Individual Planning Concept Compared With Virtual Surgical Planning. J Craniomaxillofac Surg (2017) 45(8):1246–50. doi: 10.1016/j.jcms.2017.04.010
11. Fatima A, Hackman TG, Wood JS. Cost-Effectiveness Analysis of Virtual Surgical Planning in Mandibular Reconstruction. Plast Reconstr Surg (2019) 143(4):1185–94. doi: 10.1097/PRS.0000000000005418
12. Bosc R, Hersant B, Carloni R, Niddam J, Bouhassira J, De Kermadec H, et al. Mandibular Reconstruction After Cancer: An in-House Approach to Manufacturing Cutting Guides. Int J Oral Maxillofac Surg (2017) 46(1):24–31. doi: 10.1016/j.ijom.2016.10.004
13. Mottini M, Jafari SMS, Shafighi M, Schaller B. New Approach for Virtual Surgical Planning and Mandibular Reconstruction Using a Fibula Free Flap. Oral Oncol (2016) 59:E6–9. doi: 10.1016/j.oraloncology.2016.06.001
14. Graboyes EM, Garrett-Mayer E, Sharma AK, Lentsch EJ, Day TA. Adherence to National Comprehensive Cancer Network Guidelines for Time to Initiation of Postoperative Radiation Therapy for Patients With Head and Neck Cancer. Cancer (2017) 123(14):2651–60. doi: 10.1002/cncr.30651
15. Knitschke M, Bäcker C, Schmermund D, Böttger S, Streckbein P, Howaldt H-P, et al. Impact of Planning Method (Conventional Versus Virtual) on Time to Therapy Initiation and Resection Margins: A Retrospective Analysis of 104 Immediate Jaw Reconstructions. Cancers (2021) 13(12):3013. doi: 10.3390/cancers13123013
16. Tarsitano A, Battaglia S, Ricotta F, Bortolani B, Cercenelli L, Marcelli E, et al. Accuracy of CAD/CAM Mandibular Reconstruction: A Three-Dimensional, Fully Virtual Outcome Evaluation Method. J Craniomaxillofac Surg (2018) 46(7):1121–5. doi: 10.1016/j.jcms.2018.05.010
17. Brown JS, Barry C, Ho M, Shaw R. A New Classification for Mandibular Defects After Oncological Resection. Lancet Oncol (2016) 17(1):e23–30. doi: 10.1016/S1470-2045(15)00310-1
18. Brown JS, Shaw RJ. Reconstruction of the Maxilla and Midface: Introducing a New Classification. Lancet Oncol (2010) 11(10):1001–8. doi: 10.1016/S1470-2045(10)70113-3
19. Kim RY, Bae SS, Feinberg SE. Soft Tissue Engineering. Oral Maxillofac Surg Clin North Am (2017) 29(1):89–104. doi: 10.1016/j.coms.2016.08.007
20. Mollemans W, Schutyser F, Nadjmi N, Maes F, Suetens P. Predicting Soft Tissue Deformations for a Maxillofacial Surgery Planning System: From Computational Strategies to a Complete Clinical Validation. Med Image Anal (2007) 11(3):282–301. doi: 10.1016/j.media.2007.02.003
21. Efanov JI, Roy AA, Huang KN, Borsuk DE. Virtual Surgical Planning: The Pearls and Pitfalls. Plast Reconstr Surg Glob Open (2018) 6(1):e1443. doi: 10.1097/GOX.0000000000001443
22. Hanken H, Schablowsky C, Smeets R, Heiland M, Sehner S, Riecke B, et al. Virtual Planning of Complex Head and Neck Reconstruction Results in Satisfactory Match Between Real Outcomes and Virtual Models. Clin Oral Invest (2015) 19(3):647–56. doi: 10.1007/s00784-014-1291-5
23. Lou Y, Cai L, Wang C, Tang Q, Pan T, Guo X, et al. Comparison of Traditional Surgery and Surgery Assisted by Three Dimensional Printing Technology in the Treatment of Tibial Plateau Fractures. Int Orthop (2017) 41(9):1875–80. doi: 10.1007/s00264-017-3445-y
24. Yang WF, Choi WS, Wong MC, Powcharoen W, Zhu WY, Tsoi JK, et al. Three-Dimensionally Printed Patient-Specific Surgical Plates Increase Accuracy of Oncologic Head and Neck Reconstruction Versus Conventional Surgical Plates: A Comparative Study. Ann Surg Oncol (2021) 28(1):363–75. doi: 10.1245/s10434-020-08732-y
Keywords: computer-assisted surgery (CAS), treatment adherence and compliance, patient-specific model, virtual surgical planning (VSP), 3D printing, oral and maxillofacial reconstruction, head and neck
Citation: Ma H, Shujaat S, Van Dessel J, Sun Y, Bila M, Vranckx J, Politis C and Jacobs R (2021) Adherence to Computer-Assisted Surgical Planning in 136 Maxillofacial Reconstructions. Front. Oncol. 11:713606. doi: 10.3389/fonc.2021.713606
Received: 23 May 2021; Accepted: 06 July 2021;
Published: 16 July 2021.
Edited by:
Florian M. Thieringer, University Hospital Basel, SwitzerlandReviewed by:
Zhi-Gang Cai, Peking University Hospital of Stomatology, ChinaPaolo Scolozzi, Geneva University Hospitals (HUG), Switzerland
Gustaaf Van Baar, Amsterdam University Medical Center, Netherlands
Jochen Weitz, Technical University of Munich, Germany
Copyright © 2021 Ma, Shujaat, Van Dessel, Sun, Bila, Vranckx, Politis and Jacobs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reinhilde Jacobs, cmVpbmhpbGRlLmphY29ic0BrdWxldXZlbi5iZQ==
 Hongyang Ma
Hongyang Ma Sohaib Shujaat
Sohaib Shujaat Jeroen Van Dessel1,2
Jeroen Van Dessel1,2 Michel Bila
Michel Bila Constantinus Politis
Constantinus Politis