- 1Department of Oncology, Second Xiangya Hospital, Central South University, Changsha, China
- 2Central South University, Changsha, China
- 3Hunan Cancer Mega-Data Intelligent Application and Engineering Research Centre, Changsha, China
- 4Hunan Key Laboratory of Tumor Models and Individualized Medicine, Second Xiangya Hospital, Central South University, Changsha, China
- 5Hunan Key Laboratory of Early Diagnosis and Precise Treatment of Lung Cancer, Second Xiangya Hospital, Central South University, Changsha, China
Anaplastic lymphoma kinase (ALK) is a validated molecular target for non-small-cell lung cancer (NSCLC). The use of tyrosine kinase inhibitors (TKIs) has led to significantly improved survival benefits. However, the clinical benefits of targeting ALK using TKIs are limited due to the emergence of drug resistance. The landscape of resistance mechanisms and treatment decisions has become increasingly complex. Therefore, continued research into new drugs and combinatorial therapies is required to improve outcomes in NSCLC. In this review, we explore the resistance mechanisms of ALK TKIs in advanced NSCLC in order to provide a theoretical basis and research ideas for solving the problem of ALK drug resistance.
1 Background
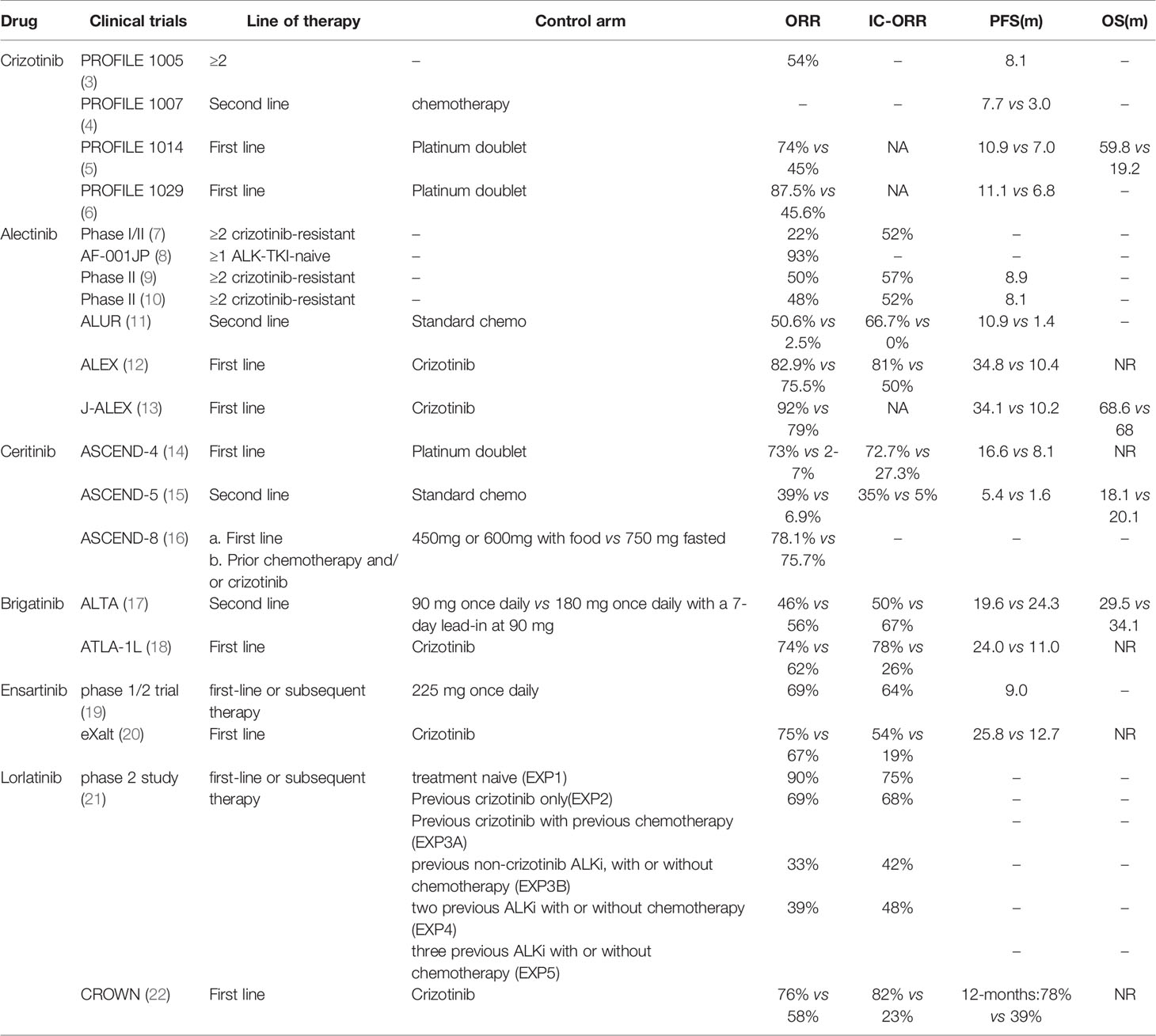
Rearrangement of the anaplastic lymphoma kinase (ALK) gene creates potent oncogenic drivers in patients with non-small cell lung cancer (NSCLC) occurring in approximately 3-7% of all cases. The most common fusion partner is EML4 (echinoderm microtubule associated protein like 4) (1). In addition, at least 20 other types of fusion genes have been discovered and reported, such as TGF-ALK, KIF5B-ALK, and STRN-ALK. ALK+ NSCLC has been associated with the absence of smoking, younger age, and adenocarcinoma histology (2). Tyrosine kinase inhibitors (TKIs) targeting ALK have made significant breakthroughs in recent years such as extending patients’ survival periods with ALK-advanced NSCLC. To date, ALK TKIs have received approval from the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) to treat advanced “ALK-positive” NSCLC. These ALK TKIs include crizotinib (first-generation), ceritinib, alectinib, brigatinib (second-generation), lorlatinib (third-generation). Clinical trials demonstrated remarkable responses within this patient population (Table 1). However, the clinical benefits of ALK inhibitors (ALKi) are almost universally limited by the emergence of multi-drug resistance. In this review, we analyze and summarize the mechanisms of resistance, as well as treatment strategies after resistance, in order to provide better therapeutic strategies for clinicians.
2 Mechanisms of Resistance to ALK TKIs
Resistance is divided into primary and acquired resistance. Primary resistance is defined as the de novo lack of treatment response and can be seen after treatment with a TKI (23). While the mechanism of resistance to ALKi is less well-understood, it can be divided into two categories, on target or ALK dependent alterations and off target or ALK independent alterations.
2.1 ALK Dependent Resistance
2.1.1 Secondary Mutations in the ALK Tyrosine Kinase Domain
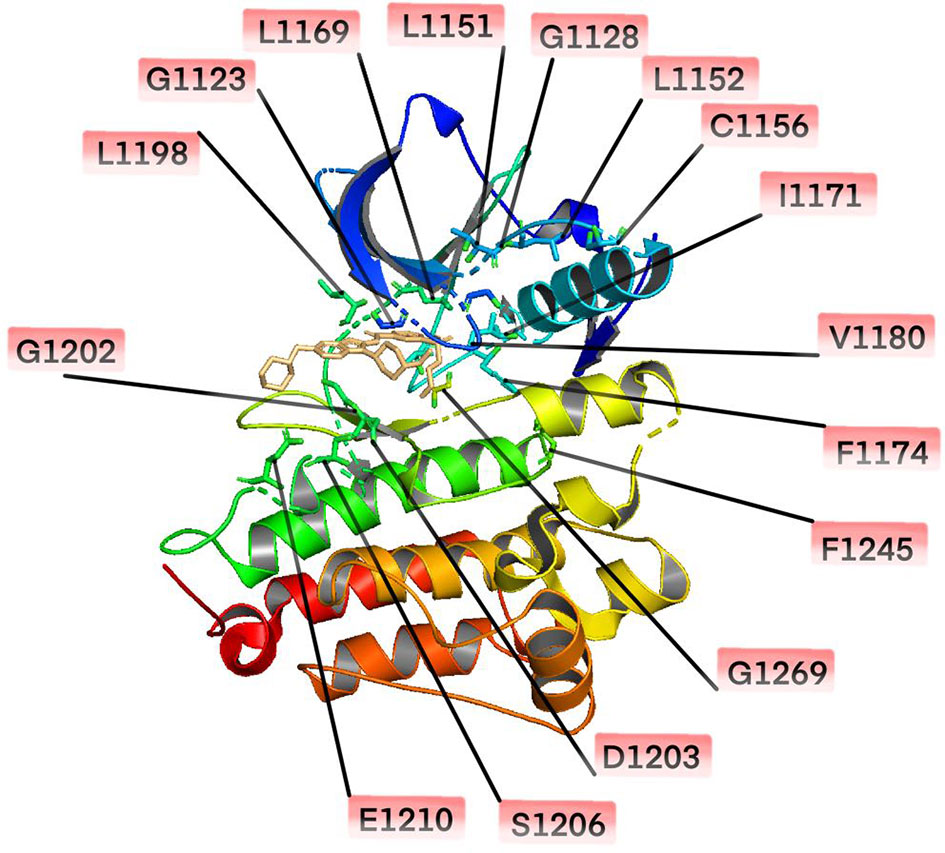
Resistance mutations in the ALKi account for 30-40% of all known resistance mechanisms (24). These resistance mutations lead to structural changes in the kinase domain that interfere with the binding of the drug. A much broader spectrum of on-target mutations has been identified in ALK-positive NSCLC treated with ALK TKIs. Resistant mutations to crizotinib include L1196M、G1269A、C1156Y、G1202R、I1171T/N/S、S1206C/Y、E1210K、L1152P/R、V11180L、I1151T、G1128A、and F1174V (25–28) (Figure 1). The most common ALK mutations are mutations L1196M and G1269A, where the deep binding pocket of ATP. G1202R was found in 2% of the samples following crizotinib resistance and was the primary mechanism of second-generation ALKi resistance (Figures 2A, B).
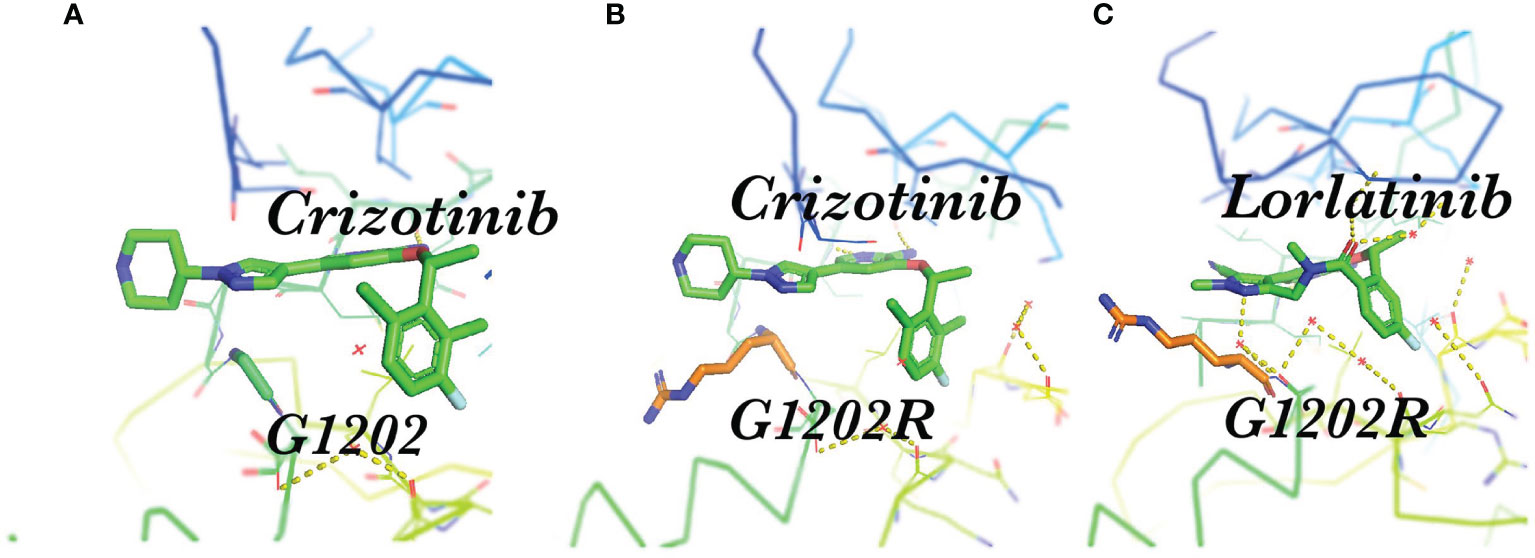
Figure 2 Spatial position of G1202R and ALK-TKI. (A) Structure of the stick representation of crizotinib (green) bound to ALK G1202; (B) Crizotinib bound to ALK the G1202R mutation, showing steric hindrance; (C) Structure of the stick representation of lorlatinib (green) bound to the ALK G1202R mutation.
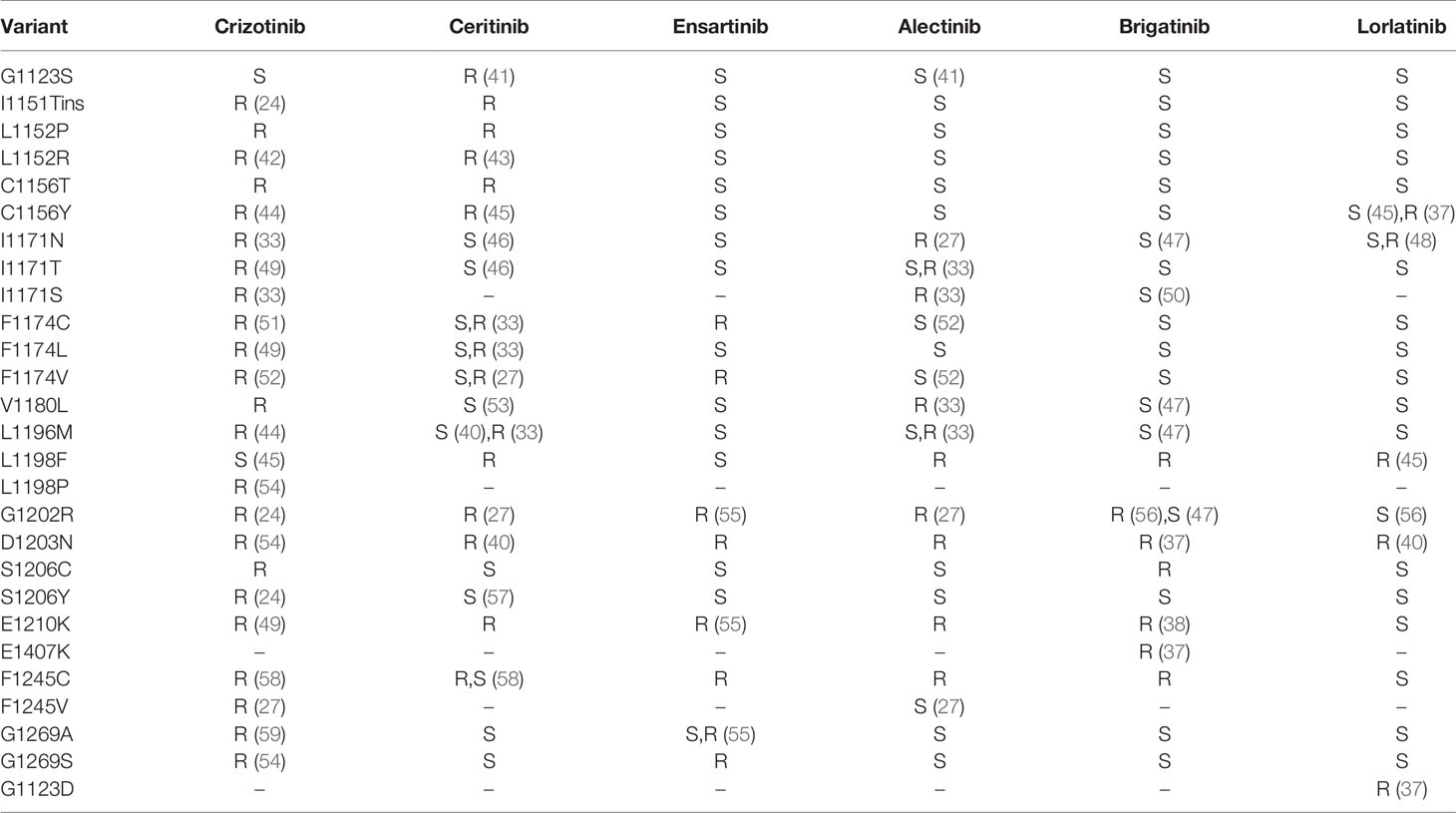
After the first-generation ALK inhibitors exhibit resistance within NSCLC, many studies have shown that the sequential second-generation drugs alectinib, ceritinib, brigatinib, and ensatinib can achieve better curative effects and are superior to chemotherapy (11, 15, 29, 30). Of note, the second-generation TKIs alectinib and brigatinib are currently the preferred first-line therapies in Europe (31), while the third-generation compound lorlatinib is also approved as initial therapy by the FDA and an additional preferred first-line drug according to the current NCCN guidelines (32) (Table 1). The progression free survival (PFS) of alectinib was significantly better than that of crizotinib, response rate (RR) and PFS of 92% and 34.1 months, respectively. The G1202R mutation is the most common secondary resistant ALK mutant in patients post-treatment with second-generation ALK inhibitors, occurring in 21%, 29% and 43% of patients treated with ceritinib, alectinib, and brigatinib (33). It is speculated that although the second-generation ALKi have increased activity, one of the costs was the larger molecular volume of their compounds, which is heavily dependent on the direct binding to the solvent front region such as G1202 in order to increase its activity; thus, “inducing” resistance mutations within this region. Resistant mutations to alectinib include G1202R and I1171N. Tumor mutation burden and heterogeneous tumor evolution might be responsible for the rapid acquisition of alectinib resistance (34). Resistant mutations to ceritinib include G1202R、F1174V、T1151K、and T1151R (27, 35, 36), and to brigatinib include D1203N, and E1210K (37, 38).
Lorlatinib is a reversible third-generation ALK and ROS1 inhibitor that can overcome multiple ALK resistance mutations and penetrate the blood-brain barrier. Lorlatinib has strong activity for common mutations such as L1196M and G1269A. The G1202R mutation is particularly important as it is the primary resistance mechanism to ceritinib, alectinib, and brigatinib, whereas only lorlatinib can inhibit the ALK G1202R mutation (Figure 2C) (39). The whole exome sequencing of compound ALK mutations occurring in several lorlatinib-resistant patients confirms the stepwise accumulation of ALK mutations during sequential treatment. Several of these ALK kinase compound mutations that have been described include the L1196M/D1203N, F1174L/G1202R, and C1156Y/G1269A mutations (40). Absolute IC50 values of crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib on cellular ALK phosphorylation in Ba/F3 cells are depicted (33). In Ba/F3 cells, ALK F1174C and ALK I1171T appear sensitive to ceritinib and alectinib, respectively; however, these mutations may not be susceptible to these agents in vivo based upon prior clinical reports. Therefore, we further combined clinical data at the cellular level in Table 2, which can help with medication selection after resistance. The objective response rate (ORR) was 69% in patients who had received crizotinib or crizotinib plus chemotherapy (21),which means that regardless of the previous use of several first or second-generation ALKi or chemotherapy, the efficacy of lorlatinib as a follow-up treatment is superior. Furthermore, the ORR of lorlatinib and crizotinib as a first-line therapeutic for advanced ALK+ NSCLC is 76% and 58%, revealing that lorlatinib has an advantage in regard to efficacy (22).
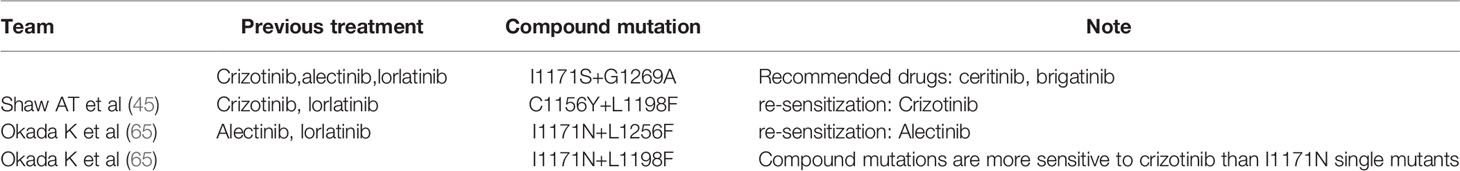
When patients receive sequential ALKi treatment, the cancer cells accumulate new mutations in addition to the previously acquired mutations, making treatment more complex (33, 37, 40, 60). Takahashi, Ken reported a patient who underwent sequential treatment with crizotinib, alectinib and lorlatinib; thus, developing the double mutations I1171S and G1269A. Ceritinib and brigatinib have the potential to become the therapeutic agents to treat this double mutation (61). Geeta G. Sharma’s team reported a case of ALK-positive NSCLC with the dual mutation ALK L1196M/G1202R after brigatinib treatment. Lorlatinib was effective against the G1202R mutation. Interestingly, this patient’s L1196M/G1202R dual mutation also increased primary resistance to lorlatinib, further limiting treatment options (62). ALK D1203N was significantly more common at relapse with lorlatinib than second-generation ALKi’s (63). In one case of ALK-positive NSCLC, after the failure of continuous treatment with crizotinib and alectinib, the mutation of the ALK fusion gene L1196M was detected, and no other acquired drug resistance mechanism was found. The patient developed resistance to alectinib, but remained sensitive to ceritinib (64).
However, not all complex mutations increase the difficulty of treatment (Table 3). Interestingly, some compound mutations that lead to lorlatinib resistance led to re-sensitization of the first or second generation ALKi (65). A patient receiving sequential treatment for ALK-positive NSCLC was resistant to crizotinib due to the mutation C1156Y in the ALK kinase region (Figure 3A). Sequencing revealed the mutation ALK L1198F in addition to C1156Y (Figure 3B). The L1198F mutation developed resistance to lorlatinib through spatial interference with drug binding. However, the L1198F mutation enhanced its binding to crizotinib (Figure 3C), making it sensitive to the C1156Y mutation. The patient was treated again with crizotinib, resulting in the successful treatment of cancer-related symptoms and liver failure (45). Other researchers have also demonstrated that the L1198F mutation leads to conformational changes in the inhibitor site as well as changes in the binding affinity of ALK to crizotinib and lorlatinib (66).
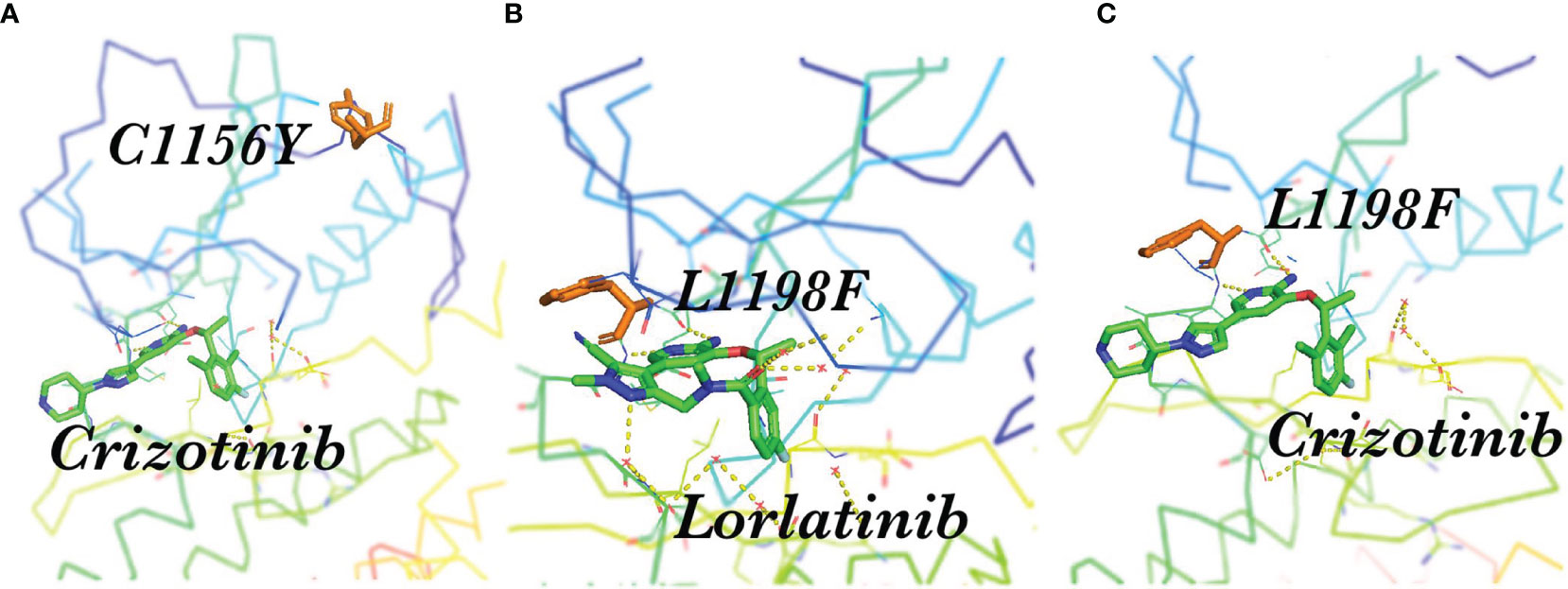
Figure 3 Spatial position of C1156Y, L1198F, and ALK-TKI. (A) Crizotinib and the resistance mutation C1156Y in the ALK kinase region; (B) L1198F developed resistance to lorlatinib through the spatial interference of drug binding; (C) L1198F enhanced ALK kinase domain binding to crizotinib.
For patients with drug resistance after ALK-TKI treatment, a re-biopsy is recommended to provide optimal treatment. Haratake N et al. retrospectively analyzed ALK-TKI treatment patterns and clinical outcomes. Of the 71 patients treated with ALK-TKI for NSCLC, 20 were re-biopsied, and 8 had secondary drug-resistant mutations. The ORR of patients with ALK point mutations receiving ALK-TKI was 88.9%, while patients without the ALK point mutations receiving ALK-TKI or chemotherapy were only 20.0%. However, PFS in patients with secondary drug-resistant mutations are relatively short, and their mechanism needs to be further studied (67).
2.1.2 Amplification of ALK
ALK amplification occurs at a low frequency, but it is responsible for acquired resistance to crizotinib. Katayama R reports a high level of ALK amplification in 15 NSCLC patients with crizotinib resistance (24).
2.2 ALK-Independent Resistance
2.1.1 Activation of Bypass Signaling Pathways
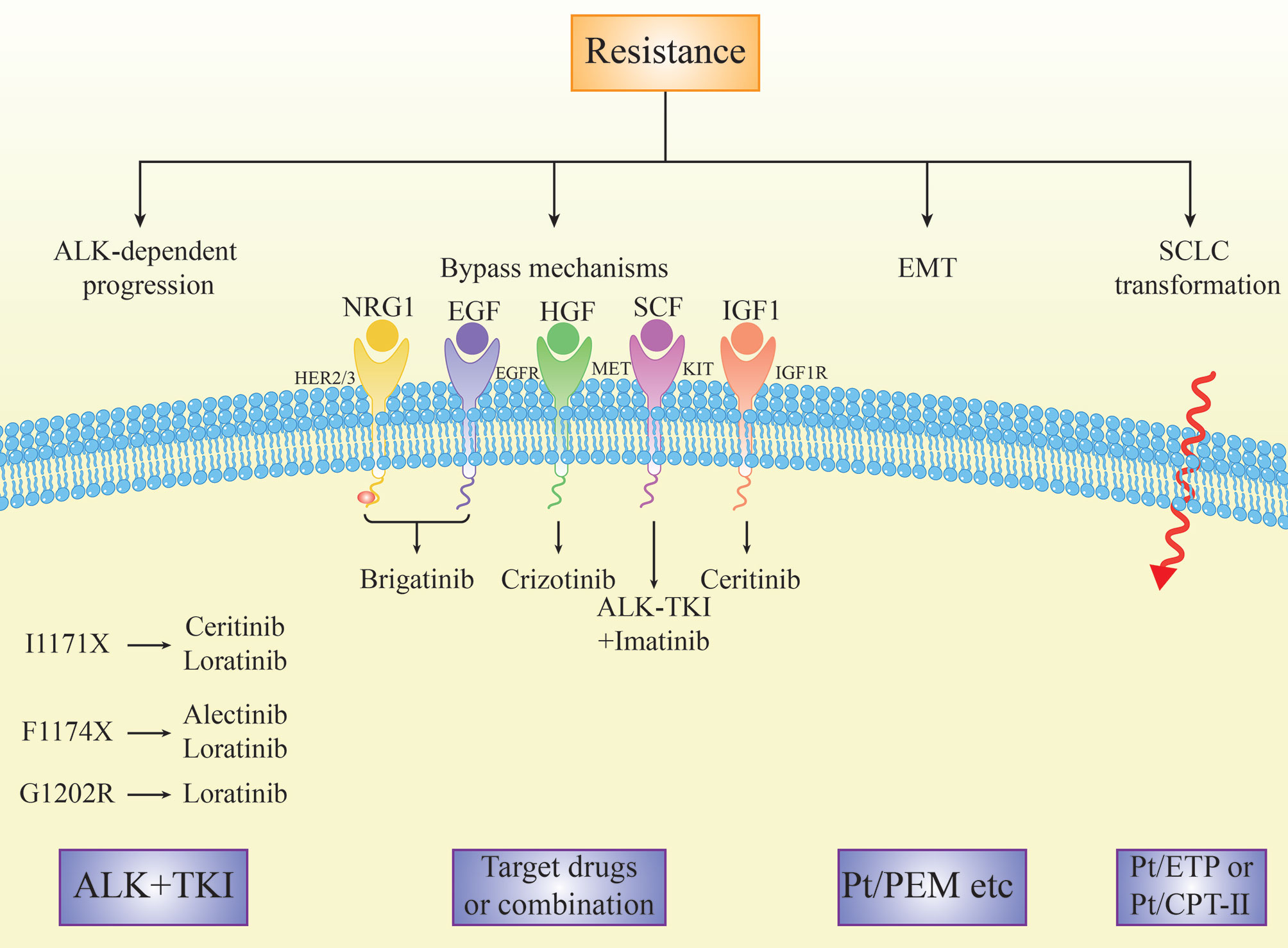
Activation of the bypass signaling pathways is the resistance mechanism of ALK-TKIs, including EGFR signaling (42, 68), amplification of KIT (24), IGF-1R-IRS-1 pathway (69), MAPK (70), MET amplification (71–73), BRAF V600E mutation (73), and the activation of the transcriptional co-regulator YAP (74) (Figure 4). In addition, Recondo G et al. found a new bypass mechanism caused by drug resistance due to NF2 functional deletion mutations, increasing mTOR inhibitor treatment sensitivity (40). Bypass activation is more common in patients with sequential TKI than in patients with crizotinib alone (49).
The activation of the EGFR pathway is one of the mechanisms of ALK-TKI resistance, such as crizotinib and alectinib. Ceritinib and afatinib combinatorial treatment partially restored the sensitivity to ceritinib (68). Afatinib may be a promising treatment for overcoming ceritinib resistance in ALK or ROS1-positive NSCLC cells by inhibiting the neuroregulatory protein (NRG1) signaling pathway (75).
Increased expression of hepatocyte growth factor (HGF) and its physiological receptor tyrosine kinase MET is associated with acquired resistance to various TKIs. MET amplification was detected in 12% and 22% of biopsies of patients using second-generation inhibitors or lorlatinib, respectively. Patients treated with second-generation ALKi during first-line therapy were more likely to have MET amplification than those treated with second-generation ALK inhibitors after crizotinib treatment (76). Gab1 is a key effector in the HGF/MET signaling pathway that mediates alectinib resistance. The antidiabetic drug metformin combined with alectinib overcomes HGF/Met-induced alectinib resistance by blocking the complex formation between MET and Gab1, thus inhibiting Gab1 phosphorylation and activating the downstream signaling pathway. These results suggest that metformin combined with alectinib may help overcome alectinib resistance caused by the HGF/MET signaling pathway activation, improving the efficacy of alectinib (77).
Cerivastatin, a rate-limiting enzyme inhibitor of the mevalonate pathway, showed anticancer activity against ALK-TKI in vitro and in vivo, accompanied by inactivation of the transcription-assisted regulator YAP. Cerivastatin can significantly induce YAP-targeted oncogenes (EGFR, AXL, CYR61 and TGFbetaR2) in drug-resistant cells, providing a theoretical basis using YAP as a potential therapeutic option in patients with acquired drug-resistant ALK-TKI (74).
2.2.2 Drug Efflux Pump
P-glycoproteins (P-gp) are highly conserved ATP-dependent effluents encoded by the multidrug resistance 1(MDR1) gene, also known as the ATP binding box subfamily B member 1(ABCB1) (78). The central nervous system (CNS) is the primary site of failure in most patients with crizotinib resistance. P-gp efflux and limited diffusion of crizotinib results in limited blood-brain barrier penetration (79). In contrast, alectinib is not a P-gp substrate and can achieve higher CNS levels (80, 81).
2.2.3 Lineage Changes
Morphological changes are also one of the mechanisms of ALK-TKI resistance in NSCLC. Many cases have reported drug resistance due to the conversion to small cell lung cancer (SCLC) or squamous cell carcinoma (SCC) after targeted therapy against ALK-positive adenocarcinoma (82–86). Deletion of p53 and retinoblastoma (RB) genes is important for SCLC transformation, although the transformation mechanism is not fully understood (87). Mutations in the TP53 and PTEN genes were also found in a patient with SCLC transformation (88). In addition, a patient who underwent alactinib treatment developed transformed SCLC. The levels of gastrin-releasing peptide precursor and neuron-specific enolase in the patients were increased, indicating SCLC transformation during the drug resistance of ALK-tyrosine kinase inhibitors (83).
In addition to the conversion of adenocarcinoma to SCC or SCLC, Koyama K. et al. reported a rare case of ALK-positive adenocarcinoma that converted to NSCLC with neuroendocrine differentiation. Histopathological examination of the tumor following alectinib resistance revealed a poorly differentiated carcinoma with insulinoma associated protein 1 (INSM1) expression. The expressions of CD133, Bcl-2, and SOX2 were positive when compared with the initial tumor. SOX2 expression was significantly increased compared to that before treatment. Immunohistochemical results of these markers associated with tumor stem-like cells and neuroendocrine differentiation suggest that tumor stem cells play a role in the histological transformation and acquired resistance mechanisms of ALK-reposition-positive tumors (89). HER2 plays an important role in regulating cancer stem cell phenotypes of ALK translocation lung cancer, which is primarily mediated by HER2/HER3 heterodimers (90).
Epithelial-to-mesenchymal transition (EMT) is a morphological change in which epithelial cells lose their polarity and intercellular connections becoming more mobile and invasive. Through EMT, tumor cells acquire mesenchymal morphology and the ability to migrate and invade. There are four pathways associated with EMT: proteoglycan in cancer, HIF-1 signaling, FoxO signaling, and extracellular matrix receptor interactions, related to the drug resistance mechanisms of crizotinib (91) (Figure 4). ALK mutants L1196M and EMT were simultaneously detected in a patient with crizotinib resistance. ALK L1196M primarily existed within the epithelial tumor cells, suggesting that EMT and ALK mutations co-exist as independent mechanisms of drug resistance. EMT was associated with decreased expression of miR-200c and increased expression of ZEB1, leading to cross-resistance of the new generation of ALKi. The histone deacetylase (HDAC) inhibitor overcomes this resistance by reversing EMT in vitro and in vivo, suggesting that adding a new ALKi after pretreatment with an HDAC inhibitor may help overcome the co-occurrence of ALK resistance mutations and EMT (92).
Kang, J. et al. performed next generation gene sequencing (NGS) on 42 crizotinib-resistant NSCLC patients. Two patients were found to have acquired mutations in the DNA mismatch repair gene POLE, leading to a significant increase in the tumor mutation burden, possibly leading to a poor response to crizotinib (93). Lai, Y. et al. investigated the resistance of microRNAs (miRNAs) to the ALK TKIs NSCLC cell lines. It was found that miR-100-5p makes EML4-ALK NSCLC cells resistant to crizotinib and lorlatinib, and maybe a therapeutic target for drug resistance (94).
The expression of the ATP-binding domain C-member 11 (ABCC11) in alectinib-resistant cell lines was significantly higher than that in alectinib-sensitive cell lines. This indicated that ABCC11 expression may be involved in the acquired drug resistance of alectinib (95). In addition, the neuroregulatory peptide U (NMU) may make NSCLC resistant to alectinib (96).
2.3 Primary ALK TKI Resistance
Progression of ALK-TKI within 3 months is considered primary resistance. In theory, any of these mechanisms of acquired resistance that existed before the use of TKI could also lead to primary resistance (23).
BIM is a Bcl-2 (B lymphocytoma-2) -like protein 11 that activates programmed cell death in cells. The study found that patients with BIM with missing polymorphisms had shortened PFS and reduced objective response rate, which was an independent predictor of patients treated with crizotinib and was related to primary drug resistance (97). In addition, the low minimum allele frequency (MAF) of the EML4-ALK rearrangement may also be a mechanism of primary resistance to crizotinib (98).
Rihawi K reported a patient with primary resistance to crizotinib. MYC amplification was a potentially new mechanism of primary ALK-TKI, resistance and proposed as a potential MYC-oriented inhibition strategy to overcome primary resistance of advanced ALK-rearrangement NSCLC (99). Similarly, the results of Pilling AB et al. showed a dual oncogene mechanism, in which ALK positively regulates the MYC signaling axis, providing an additional oncogene target (100).
3 Discussion
The treatment of ALK-rearranged NSCLC with ALK TKIs has significantly changed these patients’ outcome and quality of life. However, all patients will inevitably progress in time. Clinicians use imaging to determine whether a patient is resistant so that, if possible, they can switch to the next generation of ALK-TKI quickly. However, caution should be exercised in judging disease progression, as radiological progression may either be non-tumor cell proliferation and/or accumulation (101). In patients who have received radiation therapy, sequential ALK-TKI should be recognized as radionecrosis of the central nervous system, since treatment with the next generation of ALK-TKI may increase its severity (102).
The brain is the primary site of failure with ALK inhibitors in ALK-positive patients and is considered a sanctuary site owing to the blood–brain barrier (BBB) (103, 104). ALK-rearranged NSCLC patients exhibiting a history of prior ALKi treatment are reported to harbor a high incidence of CNS metastases, i.e., from approximately 45 to 70%, suggesting that brain metastasis is the most common form of failure with ALKi therapy. A limitation of crizotinib is that relapse in the brain after treatment was commonly reported (104). Next-generation ALK inhibitors were designed to pass the BBB. The time to CNS progression was significantly longer with alectinib than with crizotinib (cause-specific hazard ratio, 0.16, 95% CI, 0.10 to 0.28; rate of events of CNS progression, 12% with alectinib and 45% with crizotinib), which is attributed to the expression of P-gp’s on the luminal side of the BBB endothelium (9, 80, 105, 106).
Acquired resistance has become an important issue. Previous investigations additionally presented the in vitro IC50 values for all available ALK TKIs regarding the different mutations. The findings illustrate that lorlatinib has the broadest activity against the G1202R mutation (33, 107). However, whether ALKi is sensitive or resistant is complex within the real world. For example, G1202R has been detected in biopsy specimens from patients with ALK-rearranged NSCLC who relapsed on brigatinib, suggesting that its potency may be compromised with this mutation; however, some cases were effective with brigatinib treatment. This may result from the steric hindrance between the side chain of G1202R and the extended solubilization group of brigatinib (Figure 2)
The fusion variant background should also be taken into consideration when interpreting ALK resistance mutations. Among>15 EML4-ALK variants have been identified to date, the five most common variants are variant 1 (v1; E13, A20), variant 2 (v2; E20, A20), variant 3 (v3; E6, A20), variant 4 (v4; E15, A20), and variant 5 (v5; E2, A20). The two EML4-ALK variants that together account for up to 70-80% of all EML4-ALK variants are v1 and EML4-ALK v3a/b (108). The ALTA-1L analysis by variants was the first validation of the significance of EML4-ALK variants in the context of a prospective randomized phase 3 study (109). Table 4 shows the differences in PFS between variants 1 and 3. That suggests that the ALK fusion variant may affect clinical outcomes. The reason for this difference may be relatively stable in short EML4-ALK variants, which leads to accumulation and stronger carcinogenic signaling, and their better interactions with cell skeletons, which increases the migration capabilities of V3-positive cancer cells (117, 118). However, the molecular basis for this association is unknown. Besides, TP53 mutations and V3 are independently associated with enhanced metastatic spread, shorter TKI responses and inferior overall survival in ALK positive lung adenocarcinoma (115). Furthermore, ALK resistance mutations were significantly more common in variant 3 than in variant 1 (57% v 30%; P = .023). In particular, the ALK G1202R mutation was more common in variant 3 than in variant 1 (32% v 0%; P <.001). Among the patients treated with the third-generation ALK TKI lorlatinib, variant 3 was associated with a significantly longer progression-free survival than variant 1 (hazard ratio, 0.31; 95% CI, 0.12 to 0.79; P = .011) (112, 119). These results suggest that among the EML4-ALK v3 patients, we should consider introducing more aggressive therapies earlier on in the course of the disease (Table 4).
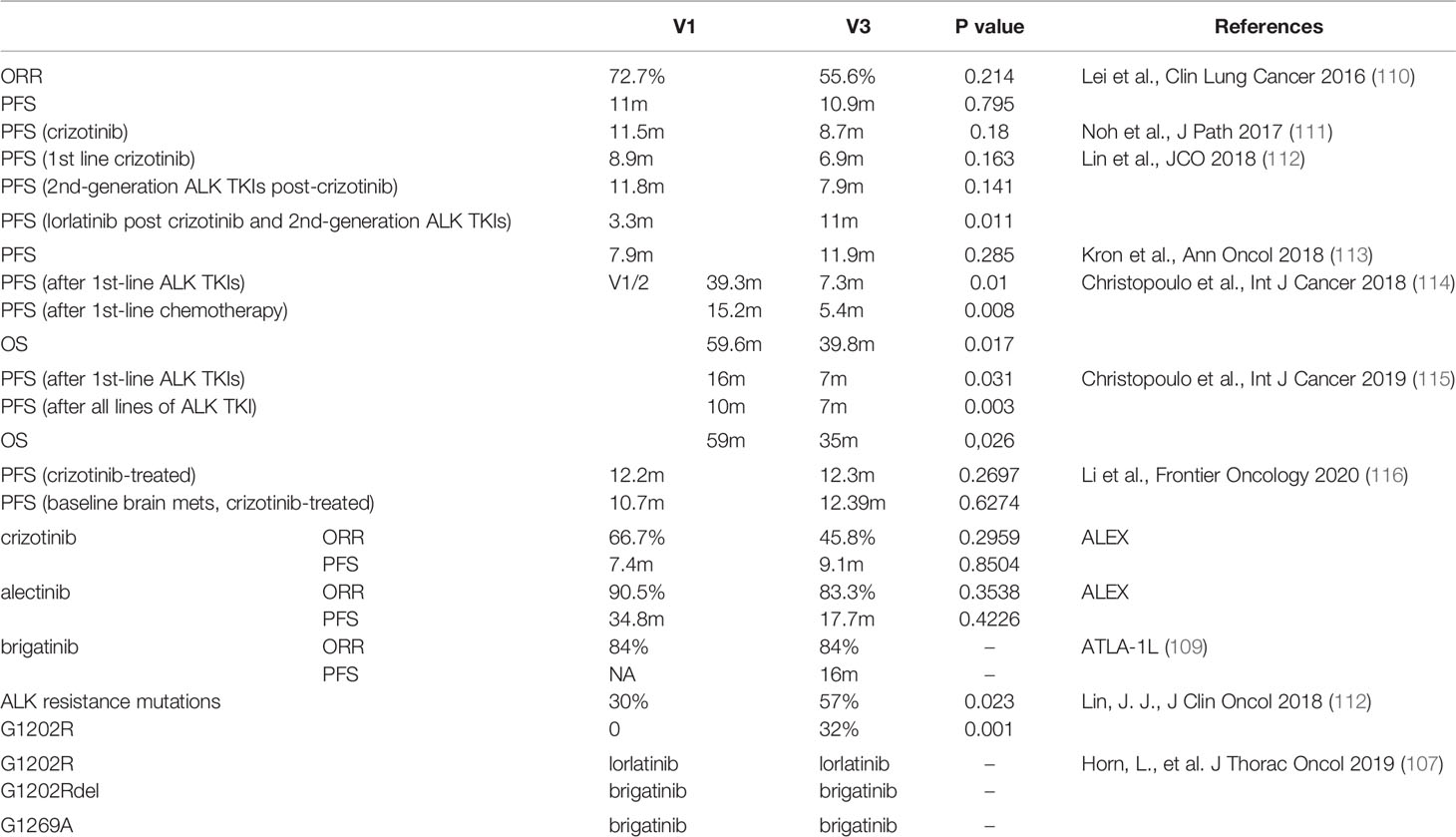
Table 4 List of retrospective analyses comparing clinical efficacy of EML4-ALK variants and ORR and PFS in prospective phase 3 trial of first-line ALK TKIs.
Interestingly, more patients are using second-generation TKIs. However, in this final J-ALEX OS analysis, prolongation of OS in the alectinib arm was not observed compared to the crizotinib arm. This indicates that longer PFS does not translate into longer OS, which gives clinicians something to think about when using ALKi’s. Therefore, there is much debate about whether PFS can be converted to OS, due to the following reasons. First, after the PFS benefit, disease progression may be faster than in the control group (120). Second, in a study that included 14 studies (N = 12567) in patients with advanced NSCLC submitted to the FDA between 2003 and 2013, a logarithmic scale scatter plot of the therapeutic effects showed no association was observed between PFS and OS in all studies (14, including the targeted studies) (R2 = 0.08; 95% CI 0-0.31) (121).
To expand PFS and OS, it is crucial to think about evidence-based treatment sequencing. Every ALK TKI has its own advantages and disadvantages (122). Therefore, a second biopsy is recommended for gene sequencing when the patient is resistant (123). However, repeated tumor biopsies to identify secondary resistance mutations are invasive and in certain cases not feasible. New tools are needed to evaluate tumor heterogeneity better and monitor tumor mutational profiles over time and throughout disease evolution (124, 125). Circulating tumor DNA (ctDNA) can be used as a strategy to identify therapeutic response and drug resistance (107). In contrast to ctDNA, circulating tumor cells (CTC) are either apoptotic or alive, but viable CTCs contain tumorigenic cell clones with high relevance for metastatic progression (126). Besides, copy number variation (CNV) profiling and targeted panel sequencing from cell-free DNA (cfDNA) were also performed to monitor ALK+ NSCLC (127).
Approximately30% of ALK-positive NSCLC patients resistant to crizotinib are related to secondary ALK mutations or amplification. Therefore, the next generation of ALK-TKIs becomes sensitive to some mutations. However, nearly 40% of patients with second-generation TKI resistance are no longer dependent on ALK, so treatment opportunities for these patients are limited. Third-generation ALK-TKI or pemetrexed-based chemotherapy may be beneficial making loratinib more effective in patients with ALK kinase domain point mutation than those without ALK re-mutation (128). An effective long-term strategy may be to pre-treat with third-generation ALK-TKI in order to prevent the emergence of resistance (129). The use of immunotherapies for ALK-TKI is still lacking (130, 131). Although patients with advanced NSCLC showed a good response to immune checkpoint inhibitors, this was associated with high PD-L1 expression levels, a high mutant load, and a history of smoking (132). However, ALK-positive patients tend not to smoke, have a low tumor mutation load (133), and have a poor response to PD-1 inhibition (134). Positive PD-L1 expression was associated with unfavorable clinical outcomes in patients with ALK-positive lung adenocarcinoma receiving crizotinib (135). ALK-positive tumors progressing with ceritinib therapy are not immunogenic enough to respond to immune checkpoint inhibitors (136). However, a successful pembrolizumab treatment case of lung adenocarcinoma after becoming resistant to ALK-TKI treatment due to G1202R mutation was reported (137). Therefore, the potential benefits of adding immunotherapy to ALK TKI therapy remains unclear.
Activation of bypass signals has emerged as another potential strategy for combating ALK-TKI resistance. Leptomeningeal Carcinomatosis (LMC) often occurs in ALK-positive NSCLC. EGFR bypass activation is known to be the drug resistance mechanism against ALK-TKI therapy. EGFR-TKI in vitro resensitizes cells to alectinib and successfully controls the progression of LMC, indicating the therapeutic potential of new therapies targeting both ALK and EGFR for ALK-TKI resistant LMC (138). In addition, apatinib can restore sensitivity to alectinib by inhibiting the downstream ALK and anti-angiogenic signaling pathway. Furthermore, reversing ALK-TKI and inhibiting angiogenesis in combination with alectinib and apatinib, thus inhibits ALK and VEGF R2 controlling the progression of the EML4-ALK fusion gene lung cancers (139). Furthermore, PFS was more severe in patients with TP53 co-mutations than in patients with wild-type TP53, meaning the combination of proteasome inhibitors with alectinib is a promising therapy for NSCLC with ALK rearrangement/TP53 mutations (49, 140).
4 Conclusion
Despite the significant efficacy of ALKi in ALK-positive NSCLC patients, drug resistance is inevitable in some patients. Although the mechanism of drug resistance can be divided into ALK-dependent and non-dependent, the specific mechanisms have not been clarified, so there is urgency in developing strategies to overcome or prevent drug resistance. With a growing understanding of the mechanisms of drug resistance, a new generation of ALKi is expected to be more effective in overcoming and suppressing drug resistance. After drug resistance, it is recommended to biopsy again to identify the mutation site. Moreover, variants should also be of concern. In addition, combination therapy is also an option. However, there may be potential problems of increased toxicity or emergence of new toxicities, so these combinatorial treatment regimens still need to be explored. Furthermore, there is much debate about whether PFS can be converted to OS. In targeted therapy, it depends on the PFS1, 2, and 3. In patients with ALK fusion, the first generation may be followed by second generation therapy, or the second generation is followed by another second generation therapeutic. In short, these new approaches are promising at more effectively overcoming and suppressing drug resistance, translating into more profound and more prolonged responses in patients with ALK-driven cancers.
Author Contributions
YP participated in the analysis, data interpretation, and wrote the manuscript. CD and ZQ polish the language and search the literature. CC analyzed the data and drew diagrams. FW designed the article ideas analyzed the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
References
1. Tsao AS, Scagliotti GV, Bunn PA Jr, Carbone DP, Warren GW, Bai C, et al. Scientific Advances in Lung Cancer 2015. J Thorac Oncol (2016) 11(5):613–38. doi: 10.1016/j.jtho.2016.03.012
2. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical Features and Outcome of Patients With Non-Small-Cell Lung Cancer Who Harbor EML4-ALK. J Clin Oncol (2009) 27(26):4247–53. doi: 10.1200/JCO.2009.22.6993
3. Blackhall F, Ross Camidge D, Shaw AT, Soria J-C, Solomon BJ, Mok T, et al. Final Results of the Large-Scale Multinational Trial PROFILE 1005: Efficacy and Safety of Crizotinib in Previously Treated Patients With Advanced/Metastatic ALK-Positive Non-Small-Cell Lung Cancer. ESMO Open (2017) 2(3):e000219. doi: 10.1136/esmoopen-2017-000219
4. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib Versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N Engl J Med (2013) 368(25):2385–94. doi: 10.1056/NEJMoa1214886
5. Solomon BJ, Mok T, Kim DW, Wu Y-L, Nakagawa K, Mekhail T, et al. First-Line Crizotinib Versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med (2014) 371(23):2167–77. doi: 10.1056/NEJMoa1408440
6. Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib Versus Chemotherapy in East Asian Patients With ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol (2018) 13(10):1539–48. doi: 10.1016/j.jtho.2018.06.012
7. Kodama T, Tsukaguchi T, Yoshida M, Kondoh O, Sakamoto H. Selective ALK Inhibitor Alectinib With Potent Antitumor Activity in Models of Crizotinib Resistance. Cancer Lett (2014) 351(2):215–21. doi: 10.1016/j.canlet.2014.05.020
8. Takeuchi K, Togashi Y, Kamihara Y, Fukuyama Y, Yoshioka T, Inoue H, et al. Prospective and Clinical Validation of ALK Immunohistochemistry: Results From the Phase I/II Study of Alectinib for ALK-Positive Lung Cancer (AF-001JP Study). Ann Oncol (2016) 27(1):185–92. doi: 10.1093/annonc/mdv501
9. Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and Activity of Alectinib Against Systemic Disease and Brain Metastases in Patients With Crizotinib-Resistant ALK-Rearranged Non-Small-Cell Lung Cancer (AF-002JG): Results From the Dose-Finding Portion of a Phase 1/2 Study. Lancet Oncol (2014) 15(10):1119–28. doi: 10.1016/S1470-2045(14)70362-6
10. Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for Patients With ALK-Rearranged Advanced Non-Small-Cell Lung Cancer (AF-001JP Study): A Single-Arm, Open-Label, Phase 1-2 Study. Lancet Oncol (2013) 14(7):590–8. doi: 10.1016/S1470-2045(13)70142-6
11. Novello S, Mazières J, Oh IJ, de Castro J, Migliorino MR, Helland Å, et al. Alectinib Versus Chemotherapy in Crizotinib-Pretreated Anaplastic Lymphoma Kinase (ALK)-Positive Non-Small-Cell Lung Cancer: Results From the Phase III ALUR Study. Ann Oncol (2018) 29(6):1409–16. doi: 10.1093/annonc/mdy121
12. Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac oncology (2019) 14(7):1233–43. doi: 10.1016/j.jtho.2019.03.007
13. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib Versus Crizotinib in Patients With ALK -Positive non-Small-Cell Lung Cancer (J-ALEX): An Open-Label, Randomised Phase 3 Trial. Lancet (2017) 390(10089):29–39. doi: 10.1016/s0140-6736(17)30565-2
14. Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-Line Ceritinib Versus Platinum-Based Chemotherapy in Advanced ALK-Rearranged Non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet (London England) (2017) 389(10072):917–29. doi: 10.1016/s0140-6736(17)30123-x
15. Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al. Ceritinib Versus Chemotherapy in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Given Chemotherapy and Crizotinib (ASCEND-5): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(7):874–86. doi: 10.1016/s1470-2045(17)30339-x
16. Cho BC, Kim DW, Bearz A, Laurie SA, McKeage M, Borra G, et al. ASCEND-8: A Randomized Phase 1 Study of Ceritinib, 450 Mg or 600 Mg, Taken With a Low-Fat Meal Versus 750 Mg in Fasted State in Patients With Anaplastic Lymphoma Kinase (ALK)-Rearranged Metastatic Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol (2017) 12(9):1357–67. doi: 10.1016/j.jtho.2017.07.005
17. Huber RM, Hansen KH, Paz-Ares Rodríguez L, West HL, Reckamp KL, Leighl NB, et al. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: 2-Year Follow-Up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J Thorac Oncol (2020) 15(3):404–15. doi: 10.1016/j.jtho.2019.11.004
18. Camidge DR, Kim HR, Ahn M-J, Yang JCH, Han J-Y, Hochmair MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1l Trial. J Clin Oncol (2020) 38(31):3592–603. doi: 10.1200/JCO.20.00505
19. Horn L, Infante JR, Reckamp KL, Blumenschein GR, Leal TA, Waqar SN, et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results From a First-In-Human Phase I/II, Multicenter Study. Clin Cancer Res (2018) 24(12):2771–9. doi: 10.1158/1078-0432.Ccr-17-2398
20. Selvaggi G, Wakelee HA, Mok T, Wu Y-L, Reck M, Chiappori A, et al. ID:1882 Phase III Randomized Study of Ensartinib vs Crizotinib in Anaplastic Lymphoma Kinase (ALK) POSITIVE NSCLC Patients: Exalt3. J Thorac Oncol (2020) 15:e41–2. doi: 10.1016/j.jtho.2020.08.003
21. Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer: Results From a Global Phase 2 Study. Lancet Oncol (2018) 19(12):1654–67. doi: 10.1016/s1470-2045(18)30649-1
22. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med (2020) 383(21):2018–29. doi: 10.1056/NEJMoa2027187
23. Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov (2017) 7(2):137–55. doi: 10.1158/2159-8290.CD-16-1123
24. Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Sci Trans Med (2012) 4(120):120ra17. doi: 10.1126/scitranslmed.3003316
25. Kim S, Kim TM, Kim D-W, Go H, Keam B, Lee S-H, et al. Heterogeneity of Genetic Changes Associated With Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancer. J Thorac Oncol (2013) 8(4):415–22. doi: 10.1097/JTO.0b013e318283dcc0
26. Ai X, Niu X, Chang L, Chen R, Ou SI, Lu S. Next Generation Sequencing Reveals a Novel ALK G1128A Mutation Resistant to Crizotinib in an ALK-Rearranged NSCLC Patient. Lung Cancer (2018) 123:83–6. doi: 10.1016/j.lungcan.2018.07.004
27. Yanagitani N, Uchibori K, Koike S, Tsukahara M, Kitazono S, Yoshizawa T, et al. Drug Resistance Mechanisms in Japanese Anaplastic Lymphoma Kinase-Positive non-Small Cell Lung Cancer and the Clinical Responses Based on the Resistant Mechanisms. Cancer Sci (2020) 111(3):932–9. doi: 10.1111/cas.14314
28. Dehghanian F, Kay M, Vallian S. F1174V Mutation Alters the ALK Active Conformation in Response to Crizotinib in NSCLC: Insight From Molecular Simulations. J Mol Graphics Modelling (2017) 75:287–93. doi: 10.1016/j.jmgm.2017.06.010
29. Yang Y, Zhou J, Zhou J, Feng J, Zhuang W, Chen J, et al. Efficacy, Safety, and Biomarker Analysis of Ensartinib in Crizotinib-Resistant, ALK-Positive Non-Small-Cell Lung Cancer: A Multicentre, Phase 2 Trial. Lancet Respir Med (2020) 8(1):45–53. doi: 10.1016/s2213-2600(19)30252-8
30. Camidge DR, Kim DW, Tiseo M, Langer CJ, Ahn MJ, Shaw AT, et al. Exploratory Analysis of Brigatinib Activity in Patients With Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer and Brain Metastases in Two Clinical Trials. J Clin Oncol (2018) 36(26):2693–701. doi: 10.1200/jco.2017.77.5841
31. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2018) 29(Suppl 4):iv192–237. doi: 10.1093/annonc/mdy275
33. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov (2016) 6(10):1118–33. doi: 10.1158/2159-8290.CD-16-0596
34. Makimoto G, Ohashi K, Tomida S, Nishii K, Matsubara T, Kayatani H, et al. Rapid Acquisition of Alectinib Resistance in ALK-Positive Lung Cancer With High Tumor Mutation Burden. J Thorac Oncol (2019) 14(11):2009–18. doi: 10.1016/j.jtho.2019.07.017
35. Zhu VW, Cui JJ, Fernandez-Rocha M, Schrock AB, Ali SM, Ou SI. Identification of a Novel T1151K ALK Mutation in a Patient With ALK-Rearranged NSCLC With Prior Exposure to Crizotinib and Ceritinib. Lung Cancer (2017) 110:32–4. doi: 10.1016/j.lungcan.2017.05.018
36. Mehlman C, Chaabane N, Lacave R, Kerrou K, Ruppert AM, Cadranel J, et al. Ceritinib ALK T1151R Resistance Mutation in Lung Cancer With Initial Response to Brigatinib. J Thorac Oncol (2019) 14(5):e95–6. doi: 10.1016/j.jtho.2018.12.036
37. Dagogo-Jack I, Rooney M, Lin JJ, Nagy RJ, Yeap BY, Hubbeling H, et al. Treatment With Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res (2019) 25(22):6662–70. doi: 10.1158/1078-0432.Ccr-19-1436
38. Sabari JK, Santini FC, Schram AM, Bergagnini I, Chen R, Mrad C, et al. The Activity, Safety, and Evolving Role of Brigatinib in Patients With ALK-Rearranged Non-Small Cell Lung Cancers. OncoTargets Ther (2017) 10:1983–92. doi: 10.2147/ott.S109295
39. Zou HY, Friboulet L, Kodack DP, Engstrom LD, Li Q, West M, et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell (2015) 28(1):70–81. doi: 10.1016/j.ccell.2015.05.010
40. Recondo G, Mezquita L, Facchinetti F, Planchard D, Gazzah A, Bigot L, et al. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in ALK-Rearranged Lung Cancer. Clin Cancer Res (2020) 26(1):242–55. doi: 10.1158/1078-0432.Ccr-19-1104
41. Toyokawa G, Inamasu E, Shimamatsu S, Yoshida T, Nosaki K, Hirai F, et al. Identification of a Novel ALK G1123S Mutation in a Patient With ALK-Rearranged Non-Small-Cell Lung Cancer Exhibiting Resistance to Ceritinib. J Thorac Oncol (2015) 10(7):e55–7. doi: 10.1097/JTO.0000000000000509
42. Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A Novel ALK Secondary Mutation and EGFR Signaling Cause Resistance to ALK Kinase Inhibitors. Cancer Res (2011) 71(18):6051–60. doi: 10.1158/0008-5472.CAN-11-1340
43. Tchekmedyian N, Ali SM, Miller VA, Haura EB. Acquired ALK L1152R Mutation Confers Resistance to Ceritinib and Predicts Response to Alectinib. J Thorac Oncol (2016) 11(7):e87–8. doi: 10.1016/j.jtho.2016.03.018
44. Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK Mutations in Lung Cancer That Confer Resistance to ALK Inhibitors. New Engl J Med (2010) 363(18):1734–9. doi: 10.1056/NEJMoa1007478
45. Shaw AT, Friboulet L, Leshchiner I, Gainor JF, Bergqvist S, Brooun A, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med (2016) 374(1):54–61. doi: 10.1056/NEJMoa1508887
46. Ou S-HI, Greenbowe J, Khan ZU, Azada MC, Ross JS, Stevens PJ, et al. I1171 Missense Mutation (Particularly I1171N) Is a Common Resistance Mutation in ALK-Positive NSCLC Patients Who Have Progressive Disease While on Alectinib and is Sensitive to Ceritinib. Lung Cancer (Amsterdam Netherlands) (2015) 88(2):231–4. doi: 10.1016/j.lungcan.2015.02.005
47. Nishio M, Yoshida T, Kumagai T, Hida T, Toyozawa R, Shimokawaji T, et al. Brigatinib in Japanese Patients With ALK-Positive NSCLC Previously Treated With Alectinib and Other Tyrosine Kinase Inhibitors: Outcomes of the Phase 2 J-ALTA Trial. J Thorac Oncol (2021) 16(3):452–63. doi: 10.1016/j.jtho.2020.11.004
48. Mizuta H, Okada K, Araki M, Adachi J, Takemoto A, Kutkowska J, et al. Gilteritinib Overcomes Lorlatinib Resistance in ALK-Rearranged Cancer. Nat Commun (2021) 12(1):1261. doi: 10.1038/s41467-021-21396-w
49. Yu Y, Ou Q, Wu X, Bao H, Ding Y, Shao YW, et al. Concomitant Resistance Mechanisms to Multiple Tyrosine Kinase Inhibitors in ALK-Positive non-Small Cell Lung Cancer. Lung Cancer (2019) 127:19–24. doi: 10.1016/j.lungcan.2018.11.024
50. Sehgal K, Peters MLB, VanderLaan PA, Rangachari D, Kobayashi SS, Costa DB. Activity of Brigatinib in the Setting of Alectinib Resistance Mediated by ALK I1171S in ALK-Rearranged Lung Cancer. J Thorac Oncol (2019) 14(1):e1–3. doi: 10.1016/j.jtho.2018.06.020
51. Noé J, Lovejoy A, Ou SI, Yaung SJ, Bordogna W, Klass DM, et al. ALK Mutation Status Before and After Alectinib Treatment in Locally Advanced or Metastatic ALK-Positive NSCLC: Pooled Analysis of Two Prospective Trials. J Thorac Oncol (2020) 15(4):601–8. doi: 10.1016/j.jtho.2019.10.015
52. Ou S-H, Milliken JC, Azada MC, Miller VA, Ali SM, Klempner SJ. ALK F1174V Mutation Confers Sensitivity While ALK I1171 Mutation Confers Resistance to Alectinib. The Importance of Serial Biopsy Post Progression. Lung Cancer (Amsterdam Netherlands) (2016) 91:70–2. doi: 10.1016/j.lungcan.2015.09.006
53. Lin JJ, Zhu VW, Schoenfeld AJ, Yeap BY, Saxena A, Ferris LA, et al. Brigatinib in Patients With Alectinib-Refractory ALK-Positive NSCLC. J Thorac Oncol (2018) 13(10):1530–8. doi: 10.1016/j.jtho.2018.06.005
54. Heuckmann JM, Hölzel M, Sos ML, Heynck S, Balke-Want H, Koker M, et al. ALK Mutations Conferring Differential Resistance to Structurally Diverse ALK Inhibitors. Clin Cancer Res (2011) 17(23):7394–401. doi: 10.1158/1078-0432.CCR-11-1648
55. Yang Y, Huang J, Wang T, Zhou J, Zheng J, Feng J, et al. Decoding the Evolutionary Response to Ensartinib in ALK-Positive Non-Small-Cell Lung Cancer Patients by Dynamic Circulating Tumor DNA Sequencing. J Thorac Oncol (2021) 16(5):827–39. doi: 10.1016/j.jtho.2021.01.1615
56. Hu J, Zhang B, Yao F, Fu Y, Chen D, Li D, et al. Acquired Multiple Mutations ALK I1171N, L1196M and G1202R Mediate Lorlatinib Resistance in EML4-ALK-Rearranged Malignant Pleural Mesothelioma: A Case Report. Ther Adv Respir Dis (2020) 14:1753466620935770. doi: 10.1177/1753466620935770
57. Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, et al. The ALK Inhibitor Ceritinib Overcomes Crizotinib Resistance in Non-Small Cell Lung Cancer. Cancer Discov (2014) 4(6):662–73. doi: 10.1158/2159-8290.Cd-13-0846
58. Kodityal S, Elvin JA, Squillace R, Agarwal N, Miller VA, Ali SM, et al. A Novel Acquired ALK F1245C Mutation Confers Resistance to Crizotinib in ALK-Positive NSCLC But Is Sensitive to Ceritinib. Lung Cancer (Amsterdam Netherlands) (2016) 92:19–21. doi: 10.1016/j.lungcan.2015.11.023
59. Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of Resistance to Crizotinib in Patients With ALK Gene Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res (2012) 18(5):1472–82. doi: 10.1158/1078-0432.CCR-11-2906
60. Pailler E, Faugeroux V, Oulhen M, Mezquita L, Laporte M, Honoré A, et al. Acquired Resistance Mutations to ALK Inhibitors Identified by Single Circulating Tumor Cell Sequencing in -Rearranged Non-Small-Cell Lung Cancer. Clin Cancer Res (2019) 25(22):6671–82. doi: 10.1158/1078-0432.CCR-19-1176
61. Takahashi K, Seto Y, Okada K, Uematsu S, Uchibori K, Tsukahara M, et al. Overcoming Resistance by ALK Compound Mutation (I1171S + G1269A) After Sequential Treatment of Multiple ALK Inhibitors in Non-Small Cell Lung Cancer. Thorac Cancer (2020) 11(3):581–7. doi: 10.1111/1759-7714.13299
62. Sharma GG, Cortinovis D, Agustoni F, Arosio G, Villa M, Cordani N, et al. A Compound L1196M/G1202R ALK Mutation in a Patient With ALK-Positive Lung Cancer With Acquired Resistance to Brigatinib Also Confers Primary Resistance to Lorlatinib. J Thorac Oncol (2019) 14(11):e257–9. doi: 10.1016/j.jtho.2019.06.028
63. Dagogo-Jack I, Rooney M, Lin JJ, Nagy RJ, Yeap BY, Hubbeling H, et al. Treatment With Next-Generation ALK Inhibitors Fuels Plasma Mutation Diversity. Clin Cancer Res (2019) 25(22):6662–70. doi: 10.1158/1078-0432.CCR-19-1436
64. Makuuchi Y, Hayashi H, Haratani K, Tanizaki J, Tanaka K, Takeda M, et al. A Case of ALK-Rearranged Non-Small Cell Lung Cancer That Responded to Ceritinib After Development of Resistance to Alectinib. Oncotarget (2018) 9(33):23315–9. doi: 10.18632/oncotarget.25143
65. Okada K, Araki M, Sakashita T, Ma B, Kanada R, Yanagitani N, et al. Prediction of ALK Mutations Mediating ALK-TKIs Resistance and Drug Re-Purposing to Overcome the Resistance. EBioMedicine (2019) 41:105–19. doi: 10.1016/j.ebiom.2019.01.019
66. Li J, Sun R, Wu Y, Song M, Li J, Yang Q, et al. L1198F Mutation Resensitizes Crizotinib to ALK by Altering the Conformation of Inhibitor and ATP Binding Sites. Int J Mol Sci (2017) 18(3):482. doi: 10.3390/ijms18030482
67. Haratake N, Seto T, Takamori S, Toyozawa R, Nosaki K, Miura N, et al. Short Progression-Free Survival of ALK Inhibitors Sensitive to Secondary Mutations in ALK-Positive NSCLC Patients. Thorac Cancer (2019) 10(9):1779–87. doi: 10.1111/1759-7714.13143
68. Miyawaki M, Yasuda H, Tani T, Hamamoto J, Arai D, Ishioka K, et al. Overcoming EGFR Bypass Signal-Induced Acquired Resistance to ALK Tyrosine Kinase Inhibitors in ALK-Translocated Lung Cancer. Mol Cancer Research: MCR (2017) 15(1):106–14. doi: 10.1158/1541-7786.Mcr-16-0211
69. Lovly CM, McDonald NT, Chen H, Ortiz-Cuaran S, Heukamp LC, Yan Y, et al. Rationale for Co-Targeting IGF-1R and ALK in ALK Fusion-Positive Lung Cancer. Nat Med (2014) 20(9):1027–34. doi: 10.1038/nm.3667
70. Crystal AS, Shaw AT, Sequist LV, Friboulet L, Niederst MJ, Lockerman EL, et al. Patient-Derived Models of Acquired Resistance can Identify Effective Drug Combinations for Cancer. Science (2014) 346(6216):1480–6. doi: 10.1126/science.1254721
71. Tsuji T, Ozasa H, Aoki W, Aburaya S, Funazo T, Furugaki K, et al. Alectinib Resistance in ALK-Rearranged Lung Cancer by Dual Salvage Signaling in a Clinically Paired Resistance Model. Mol Cancer Research: MCR (2019) 17(1):212–24. doi: 10.1158/1541-7786.Mcr-18-0325
72. Gouji T, Takashi S, Mitsuhiro T, Yukito I. Crizotinib can Overcome Acquired Resistance to CH5424802: Is Amplification of the MET Gene a Key Factor? J Thorac Oncol (2014) 9(3):e27–8. doi: 10.1097/JTO.0000000000000113
73. Shi R, Filho SNM, Li M, Fares A, Weiss J, Pham NA, et al. BRAF V600E Mutation and MET Amplification as Resistance Pathways of the Second-Generation Anaplastic Lymphoma Kinase (ALK) Inhibitor Alectinib in Lung Cancer. Lung Cancer (2020) 146:78–85. doi: 10.1016/j.lungcan.2020.05.018
74. Yun MR, Choi HM, Lee YW, Joo HS, Park CW, Choi JW, et al. Targeting YAP to Overcome Acquired Resistance to ALK Inhibitors in ALK-Rearranged Lung Cancer. EMBO Mol Med (2019) 11(12):e10581. doi: 10.15252/emmm.201910581
75. Chen H, Zhang Q, Zhang Y, Jia B, Zhang B, Wang C. Afatinib Reverses Ceritinib Resistance (CR) in ALK/ROS1-Positive Non-Small-Cell Lung Cancer Cell (NSCLC) via Suppression of NRG1 Pathway. OncoTargets Ther (2018) 11:8201–9. doi: 10.2147/ott.S173008
76. Dagogo-Jack I, Yoda S, Lennerz JK, Langenbucher A, Lin JJ, Rooney MM, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res (2020) 26(11):2535–45. doi: 10.1158/1078-0432.Ccr-19-3906
77. Chen H, Lin C, Peng T, Hu C, Lu C, Li L, et al. Metformin Reduces HGF-Induced Resistance to Alectinib. via inhibition Gab1. Cell Death Dis (2020) 11(2):111. doi: 10.1038/s41419-020-2307-5
78. Gottesman MM, Fojo T, Bates SE. Multidrug Resistance in Cancer: Role of ATP-Dependent Transporters. Nat Rev Cancer (2002) 2(1):48–58. doi: 10.1038/nrc706
79. Zhang I, Zaorsky NG, Palmer JD, Mehra R, Lu B. Targeting Brain Metastases in ALK-Rearranged Non-Small-Cell Lung Cancer. Lancet Oncol (2015) 16(13):e510–21. doi: 10.1016/S1470-2045(15)00013-3
80. Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, et al. P-Glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-Rearranged Non-Small Cell Lung Cancer. EBioMedicine (2016) 3:54–66. doi: 10.1016/j.ebiom.2015.12.009
81. Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor Activity of the Selective ALK Inhibitor Alectinib in Models of Intracranial Metastases. Cancer chemotherapy Pharmacol (2014) 74(5):1023–8. doi: 10.1007/s00280-014-2578-6
82. Zhu YC, Liao XH, Wang WX, Xu CW, Zhuang W, Zhong LH, et al. Patients Harboring ALK Rearrangement Adenocarcinoma After Acquired Resistance to Crizotinib and Transformation to Small-Cell Lung Cancer: A Case Report. OncoTargets Ther (2017) 10:3187–92. doi: 10.2147/ott.S139718
83. Oya Y, Yoshida T, Uemura T, Murakami Y, Inaba Y, Hida T. Serum ProGRP and NSE Levels Predicting Small Cell Lung Cancer Transformation in a Patient With ALK Rearrangement-Positive non-Small Cell Lung Cancer: A Case Report. Oncol Lett (2018) 16(4):4219–22. doi: 10.3892/ol.2018.9158
84. Park S, Han J, Sun JM. Histologic Transformation of ALK-Rearranged Adenocarcinoma to Squamous Cell Carcinoma After Treatment With ALK Inhibitor. Lung Cancer (2019) 127:66–8. doi: 10.1016/j.lungcan.2018.11.027
85. Gong J, Gregg JP, Ma W, Yoneda K, Moore EH, Daly ME, et al. Squamous Cell Transformation of Primary Lung Adenocarcinoma in a Patient With EML4-ALK Fusion Variant 5 Refractory to ALK Inhibitors. J Natl Compr Canc Netw (2019) 17(4):297–301. doi: 10.6004/jnccn.2019.7291
86. Kaiho T, Nakajima T, Iwasawa S, Yonemori Y, Yoshino I. ALK Rearrangement Adenocarcinoma With Histological Transformation to Squamous Cell Carcinoma Resistant to Alectinib and Ceritinib. OncoTargets Ther (2020) 13:1557–60. doi: 10.2147/ott.S236706
87. Ou SI, Lee TK, Young L, Fernandez-Rocha MY, Pavlick D, Schrock AB, et al. Dual Occurrence of ALK G1202R Solvent Front Mutation and Small Cell Lung Cancer Transformation as Resistance Mechanisms to Second Generation ALK Inhibitors Without Prior Exposure to Crizotinib. Pitfall of Solely Relying on Liquid Re-Biopsy? Lung Cancer (2017) 106:110–4. doi: 10.1016/j.lungcan.2017.02.005
88. Levacq D, D’Haene N, de Wind R, Remmelink M, Berghmans T. Histological Transformation of ALK Rearranged Adenocarcinoma Into Small Cell Lung Cancer: A New Mechanism of Resistance to ALK Inhibitors. Lung Cancer (Amsterdam Netherlands) (2016) 102:38–41. doi: 10.1016/j.lungcan.2016.10.012
89. Koyama K, Katsurada N, Jimbo N, Tachihara M, Tamura D, Nakata K, et al. Overexpression of CD 133 and BCL-2 in Non-Small Cell Lung Cancer With Neuroendocrine Differentiation After Transformation in ALK Rearrangement-Positive Adenocarcinoma. Pathol Int (2019) 69(5):294–9. doi: 10.1111/pin.12782
90. Honkanen T, Wilenius E, Koivunen P, Koivunen JP. HER2 Regulates Cancer Stem-Like Cell Phenotype in ALK Translocated NSCLC. Int J Oncol (2017) 51(2):599–606. doi: 10.3892/ijo.2017.4048
91. Wei J, van der Wekken AJ, Saber A, Terpstra MM, Schuuring E, Timens W, et al. Mutations in EMT-Related Genes in ALK Positive Crizotinib Resistant Non-Small Cell Lung Cancers. Cancers (Basel) (2018) 10(1):10. doi: 10.3390/cancers10010010
92. Fukuda K, Takeuchi S, Arai S, Katayama R, Nanjo S, Tanimoto A, et al. Epithelial-To-Mesenchymal Transition Is a Mechanism of ALK Inhibitor Resistance in Lung Cancer Independent of ALK Mutation Status. Cancer Res (2019) 79(7):1658–70. doi: 10.1158/0008-5472.Can-18-2052
93. Kang J, Chen HJ, Zhang XC, Su J, Zhou Q, Tu HY, et al. Heterogeneous Responses and Resistant Mechanisms to Crizotinib in ALK-Positive Advanced non-Small Cell Lung Cancer. Thorac Cancer (2018) 9(9):1093–103. doi: 10.1111/1759-7714.12791
94. Lai Y, Kacal M, Kanony M, Stukan I, Jatta K, Kis L, et al. miR-100-5p Confers Resistance to ALK Tyrosine Kinase Inhibitors Crizotinib and Lorlatinib in EML4-ALK Positive NSCLC. Biochem Biophys Res Commun (2019) 511(2):260–5. doi: 10.1016/j.bbrc.2019.02.016
95. Funazo TY, Tsuji T, Ozasa H, Furugaki K, Yoshimura Y, Oguri T, et al. Acquired Resistance to Alectinib in ALK-Rearranged Lung Cancer Due to ABCC11/MRP8 Overexpression in a Clinically Paired Resistance Model. Mol Cancer Ther (2020) 19(6):1320–7. doi: 10.1158/1535-7163.Mct-19-0649
96. You S, Gao L. Identification of NMU as a Potential Gene Conferring Alectinib Resistance in Non-Small Cell Lung Cancer Based on Bioinformatics Analyses. Gene (2018) 678:137–42. doi: 10.1016/j.gene.2018.08.032
97. Zhang L, Jiang T, Li X, Wang Y, Zhao C, Zhao S, et al. Clinical Features of Bim Deletion Polymorphism and I2ts Relation With Crizotinib Primary Resistance in Chinese Patients With ALK/ROS1 Fusion-Positive Non-Small Cell Lung Cancer. Cancer (2017) 123(15):2927–35. doi: 10.1002/cncr.30677
98. Zhang L, Li Y, Zhang S, Gao C, Nie K, Ji Y. Primary Resistance to Crizotinib Treatment in a Non-Small Cell Lung Cancer Patient With an EML4-ALK Rearrangement: A Case Report. Cancer Biol Med (2018) 15(2):178–81. doi: 10.20892/j.issn.2095-3941.2018.0003
99. Rihawi K, Alfieri R, Fiorentino M, Fontana F, Capizzi E, Cavazzoni A, et al. MYC Amplification as a Potential Mechanism of Primary Resistance to Crizotinib in ALK-Rearranged Non-Small Cell Lung Cancer: A Brief Report. Transl Oncol (2019) 12(1):116–21. doi: 10.1016/j.tranon.2018.09.013
100. Pilling AB, Kim J, Estrada-Bernal A, Zhou Q, Le AT, Singleton KR, et al. ALK Is a Critical Regulator of the MYC-Signaling Axis in ALK Positive Lung Cancer. Oncotarget (2018) 9(10):8823–35. doi: 10.18632/oncotarget.24260
101. Facchinetti F, Gnetti L, Balestra V, Silva M, Silini EM, Ventura L, et al. Sarcoid-Like Reaction Mimicking Disease Progression in an ALK-Positive Lung Cancer Patient Receiving Lorlatinib. Investigational New Drugs (2019) 37(2):360–3. doi: 10.1007/s10637-018-0652-3
102. Zhu VW, Nagasaka M, Kubota T, Raval K, Robinette N, Armas O, et al. Symptomatic CNS Radiation Necrosis Requiring Neurosurgical Resection During Treatment With Lorlatinib in ALK-Rearranged NSCLC: A Report of Two Cases. Lung Cancer (Auckland N.Z.) (2020) 11:13–8. doi: 10.2147/lctt.S224991
103. Toyokawa G, Seto T, Takenoyama M, Ichinose Y. Insights Into Brain Metastasis in Patients With ALK+ Lung Cancer: Is the Brain Truly a Sanctuary? Cancer Metastasis Rev (2015) 34(4):797–805. doi: 10.1007/s10555-015-9592-y
104. Costa DB, Shaw AT, Ou S-HI, Solomon BJ, Riely GJ, Ahn M-J, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol (2015) 33(17):1881–8. doi: 10.1200/JCO.2014.59.0539
105. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim D-W, et al. Alectinib Versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377(9):829–38. doi: 10.1056/NEJMoa1704795
106. Costa DB, Kobayashi S, Pandya SS, Yeo W-L, Shen Z, Tan W, et al. CSF Concentration of the Anaplastic Lymphoma Kinase Inhibitor Crizotinib. J Clin Oncol (2011) 29(15):e443–5. doi: 10.1200/JCO.2010.34.1313
107. Horn L, Whisenant JG, Wakelee H, Reckamp KL, Qiao H, Leal TA, et al. Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients With ALK+ Lung Cancer. J Thorac Oncol (2019) 14(11):1901–11. doi: 10.1016/j.jtho.2019.08.003
108. Zhang SS, Nagasaka M, Zhu VW, Ou S-HI. Going Beneath the Tip of the Iceberg. Identifying and Understanding EML4-ALK Variants and TP53 Mutations to Optimize Treatment of ALK Fusion Positive (ALK+) NSCLC. Lung Cancer (Amsterdam Netherlands) (2021) 158:126–36. doi: 10.1016/j.lungcan.2021.06.012
109. Camidge DR, Niu H, Kim HR, Yang JC-H, Ahn M-J, Li JY-C, et al. Correlation of Baseline Molecular and Clinical Variables With ALK Inhibitor Efficacy in ALTA-1l. J Clin Oncol (2020) 38(15 SUPPL):9517–7. doi: 10.1200/JCO.2020.38.15_suppl.9517
110. Lei Y-Y, Yang J-J, Zhang X-C, Zhong W-Z, Zhou Q, Tu H-Y, et al. Anaplastic Lymphoma Kinase Variants and the Percentage of ALK-Positive Tumor Cells and the Efficacy of Crizotinib in Advanced NSCLC. Clin Lung Cancer (2016) 17(3):223–31. doi: 10.1016/j.cllc.2015.09.002
111. Noh K-W, Lee M-S, Lee SE, Song SE, Shin SE, Kim SE, et al. Molecular Breakdown: A Comprehensive View of Anaplastic Lymphoma Kinase (ALK)-Rearranged Non-Small Cell Lung Cancer. J Pathol (2017) 243(3):307–19. doi: 10.1002/path.4950
112. Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J Clin Oncol (2018) 36(12):1199–206. doi: 10.1200/jco.2017.76.2294
113. Kron A, Alidousty C, Scheffler M, Merkelbach-Bruse S, Seidel D, Riedel R, et al. Impact of TP53 Mutation Status on Systemic Treatment Outcome in ALK-Rearranged Non-Small-Cell Lung Cancer. Ann Oncol (2018) 29(10):2068–75. doi: 10.1093/annonc/mdy333
114. Christopoulos P, Endris V, Bozorgmehr F, Elsayed M, Kirchner M, Ristau J, et al. EML4-ALK Fusion Variant V3 Is a High-Risk Feature Conferring Accelerated Metastatic Spread, Early Treatment Failure and Worse Overall Survival in ALK Non-Small Cell Lung Cancer. Int J Cancer (2018) 142(12):2589–98. doi: 10.1002/ijc.31275
115. Christopoulos P, Kirchner M, Bozorgmehr F, Endris V, Elsayed M, Budczies J, et al. Identification of a Highly Lethal V3 TP53 Subset in ALK Lung Adenocarcinoma. Int J Cancer (2019) 144(1):190–9. doi: 10.1002/ijc.31893
116. Li M, Hou X, Zhou C, Feng W, Jiang G, Long H, et al. Prevalence and Clinical Impact of Concomitant Mutations in Anaplastic Lymphoma Kinase Rearrangement Advanced Non-Small-Cell Lung Cancer (Guangdong Association of Thoracic Oncology Study 1055). Front Oncol (2020) 10:2020.01216. doi: 10.3389/fonc.2020.01216
117. O’Regan L, Barone G, Adib R, Woo CG, Jeong HJ, Richardson EL, et al. EML4-ALK V3 Oncogenic Fusion Proteins Promote Microtubule Stabilization and Accelerated Migration Through NEK9 and NEK7. J Cell Sci (2020) 133(9):jcs241505. doi: 10.1242/jcs.241505
118. Elsayed M, Christopoulos P. Therapeutic Sequencing in ALK NSCLC. Pharmaceuticals (Basel) (2021) 14(2):80. doi: 10.3390/ph14020080
119. Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, Kuroda H, et al. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol (2016) 34(28):3383–9. doi: 10.1200/JCO.2015.65.8732
120. McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell (2017) 168(4):613–28. doi: 10.1016/j.cell.2017.01.018
121. Blumenthal GM, Karuri SW, Zhang H, Zhang L, Khozin S, Kazandjian D, et al. Overall Response Rate, Progression-Free Survival, and Overall Survival With Targeted and Standard Therapies in Advanced Non-Small-Cell Lung Cancer: US Food and Drug Administration Trial-Level and Patient-Level Analyses. J Clin Oncol (2015) 33(9):1008–14. doi: 10.1200/JCO.2014.59.0489
122. Kauffmann-Guerrero D, Kahnert K, Huber RM. Treatment Sequencing for Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer. Drugs (2021) 81(1):87–100. doi: 10.1007/s40265-020-01445-2
123. Sánchez-Herrero E, Blanco Clemente M, Calvo V, Provencio M, Romero A. Next-Generation Sequencing to Dynamically Detect Mechanisms of Resistance to ALK Inhibitors in ALK-Positive NSCLC Patients: A Case Report. Trans Lung Cancer Res (2020) 9(2):366–72. doi: 10.21037/tlcr.2020.02.07
124. Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and Future Perspectives of Liquid Biopsies in Genomics-Driven Oncology. Nat Rev Genet (2019) 20(2):71–88. doi: 10.1038/s41576-018-0071-5
125. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating Liquid Biopsies Into the Management of Cancer. Nat Rev Clin Oncol (2017) 14(9):531–48. doi: 10.1038/nrclinonc.2017.14
126. Alix-Panabières C, Pantel K. Challenges in Circulating Tumour Cell Research. Nat Rev Cancer (2014) 14(9):623–31. doi: 10.1038/nrc3820
127. Dietz S, Christopoulos P, Yuan Z, Angeles AK, Gu L, Volckmar A-L, et al. Longitudinal Therapy Monitoring of ALK-Positive Lung Cancer by Combined Copy Number and Targeted Mutation Profiling of Cell-Free DNA. EBioMedicine (2020) 62:103103. doi: 10.1016/j.ebiom.2020.103103
128. Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin CC, Soo RA, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol (2019) 37(16):1370–9. doi: 10.1200/jco.18.02236
129. Yoda S, Lin JJ, Lawrence MS, Burke BJ, Friboulet L, Langenbucher A, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov (2018) 8(6):714–29. doi: 10.1158/2159-8290.Cd-17-1256
130. Metro G, Tazza M, Matocci R, Chiari R, Crino L. Optimal Management of ALK-Positive NSCLC Progressing on Crizotinib. Lung Cancer (2017) 106:58–66. doi: 10.1016/j.lungcan.2017.02.003
131. Jahanzeb M, Lin HM, Pan X, Yin Y, Baumann P, Langer J. Immunotherapy Treatment Patterns and Outcomes Among ALK-Positive Patients With Non-Small-Cell Lung Cancer. Clin Lung Cancer (2020) 22(1):49–57. doi: 10.1016/j.cllc.2020.08.003
132. Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2016) 34(25):2969–79. doi: 10.1200/JCO.2016.66.9861
133. Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic Landscape of Non-Small Cell Lung Cancer in Smokers and Never-Smokers. Cell (2012) 150(6):1121–34. doi: 10.1016/j.cell.2012.08.024
134. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR Mutations and ALK Rearrangements Are Associated With Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res (2016) 22(18):4585–93. doi: 10.1158/1078-0432.CCR-15-3101
135. Yang C-Y, Liao W-Y, Ho C-C, Chen K-Y, Tsai T-H, Hsu C-L, et al. Association of Programmed Death-Ligand 1 Expression With Fusion Variants and Clinical Outcomes in Patients With Anaplastic Lymphoma Kinase-Positive Lung Adenocarcinoma Receiving Crizotinib. Oncologist (2020) 25(8):702–11. doi: 10.1634/theoncologist.2020-0088
136. Pyo KH, Lim SM, Park CW, Jo HN, Kim JH, Yun M, et al. Comprehensive Analyses of Immunodynamics and Immunoreactivity in Response to Treatment in ALK-Positive Non-Small-Cell Lung Cancer. J Immunother Cancer (2020) 8(2):e000970. doi: 10.1136/jitc-2020-000970
137. Shimada M, Tamura A, Yokosuka K, Kusaka K, Matsui H, Nagai H, et al. A Successful Pembrolizumab Treatment Case of Lung Adenocarcinoma After Becoming Resistant to ALK-TKI Treatment Due to G1202R Mutation. Respir Invest (2018) 56(4):365–8. doi: 10.1016/j.resinv.2018.04.004
138. Arai S, Takeuchi S, Fukuda K, Taniguchi H, Nishiyama A, Tanimoto A, et al. Osimertinib Overcomes Alectinib Resistance Caused by Amphiregulin in a Leptomeningeal Carcinomatosis Model of ALK-Rearranged Lung Cancer. J Thorac Oncol (2020) 15(5):752–65. doi: 10.1016/j.jtho.2020.01.001
139. Chen Y, Ma G, Su C, Wu P, Wang H, Song X, et al. Apatinib Reverses Alectinib Resistance by Targeting Vascular Endothelial Growth Factor Receptor 2 and Attenuating the Oncogenic Signaling Pathway in Echinoderm Microtubule-Associated Protein-Like 4-Anaplastic Lymphoma Kinase Fusion Gene-Positive Lung Cancer Cell Lines. Anti-cancer Drugs (2018) 29(10):935–43. doi: 10.1097/cad.0000000000000667
Keywords: anaplastic lymphoma kinase (ALK), TKI - tyrosine kinase inhibitor, resistance, NSCLC, therapy
Citation: Pan Y, Deng C, Qiu Z, Cao C and Wu F (2021) The Resistance Mechanisms and Treatment Strategies for ALK-Rearranged Non-Small Cell Lung Cancer. Front. Oncol. 11:713530. doi: 10.3389/fonc.2021.713530
Received: 23 May 2021; Accepted: 13 September 2021;
Published: 01 October 2021.
Edited by:
Petros Christopoulos, Heidelberg University Hospital, GermanyReviewed by:
Bengt Hallberg, University of Gothenburg, SwedenAlexander Deneka, Fox Chase Cancer Center, United States
Diego Kauffmann-Guerrero, LMU Munich University Hospital, Germany
Copyright © 2021 Pan, Deng, Qiu, Cao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wu, d3VmYW5nNDQ2MUBjc3UuZWR1LmNu
 Yue Pan1
Yue Pan1 Fang Wu
Fang Wu