- 1Radiation Oncology Department, Dalhousie University, Halifax, NS, Canada
- 2Radiation Oncology Department, Erasmus MC, Rotterdam, Netherlands
- 3Radiation Oncology, 21st Century Oncology, Farmington Hills, MI, United States
Purpose: Secondary lung cancer (SLC) can offset the benefit of adjuvant breast radiotherapy (RT), and risks compound sharply after 25 to 30 years. We hypothesized that SLC risk is mainly an issue for early-stage breast cancer, and that lives could be saved using different RT techniques.
Patients and Methods: The SEER database was used to extract breast patient age, stage survival, and radiotherapy utilization over time and per stage and to assess the factors associated with increased SLC risk with a multivariable competing risk Cox model. The number of SLC was calculated using the BEIR model modified with patient survival, age, and use of RT from the SEER database. Stage distribution and number of new breast cancer cases were obtained from the NAACCR. Mean lung dose for various irradiation techniques was obtained from measurement or literature.
Results: Out of the 765,697 non-metastatic breast cancers in the SEER database from 1988 to 2012, 49.8% received RT. RT significantly increased the SLC risk for longer follow-up (HR=1.58), early stage including DCIS, stage I and IIA (HR = 1.11), and younger age (HR=1.061) (all p<0.001). More advanced stages did not have significantly increased risk. In 2019, 104,743 early-stage breast patients received radiotherapy, and an estimated 3,413 will develop SLC (3.25%) leading to an excess of 2,900 deaths (2.77%). VMAT would reduce this mortality by 9.9%, hypofractionation 26 Gy in five fractions by 38.8%, a prone technique by 70.3%, 3D-CRT APBI by 43.3%, HDR brachytherapy by 71.1%, LDR by 80.7%, and robotic 4π APBI by 85.2%.
Conclusions: SLC after breast RT remains a clinically significant issue for early-stage breast cancers. This mortality could be significantly reduced using a prone technique or APBI.
Introduction
With the generalization of mammography screening, breast cancer can be diagnosed at an early stage (1), and the treatment gold standard includes breast-conserving surgery followed by whole breast radiotherapy. Radiotherapy improves the disease-free survival and local control (2–4), and there are long-term life-threatening complications that can offset the overall survival benefit. The most significant include cardiovascular morbidity and secondary cancers (3, 5).
Cardiac morbidity appears relatively soon after the radiation treatment, generally 5 to 10 years following exposure of the heart (5, 6). It is well documented in long-term reports of randomized trials or meta-analysis (4, 5). It has justified technique changes, including the generalization of breath-hold or gating techniques and the development of constraints for the mean heart dose (7, 8). Conversely, radiation-induced secondary cancers, including lung cancers, have a much delayed occurrence, compounding over time to become clinically significant after two to three decades (9–11). Using a modified version of the BEIR VII model, we previously confirmed a delay of the lifetime attributable risk (LAR) of excess lung cancers, which would be 0.33% 10 years after radiotherapy, 0.7% after 15 years, and 3% after 25 years (11).
It is difficult to use LAR in making the decision to adopt a new radiation technique, or discussing the radiation treatment with a given patient, since this risk assumes the patient would survive for a long time. It is also challenging to get a clear picture from population-based studies of the absolute number of secondary lung cancers for patients diagnosed with breast cancer today. Most meta-analysis or registry studies calculate the risk of secondary lung cancer based on patients treated a long time ago (9, 10, 12). For example, the evaluation of secondary lung cancer risk in the Inskip cohort includes 9,000 patients treated between 1935 and 1971 (10). Similarly, Grantzau meta-analysis includes patients treated between 1935 and 2007 (12). During that time frame, the utilization, techniques, and dose/fractionation of adjuvant radiotherapy have dramatically changed (7, 13). Also, early-stage breast cancer patients diagnosed today would live longer (14), meaning they are more likely to experience secondary lung cancer.
This study aimed first at, confirming that early-stage breast cancers have a higher risk of secondary lung cancer; second, comparing the true number of secondary lung cancers for different radiotherapy techniques; and third, estimating the number of lives that could be spared depending on the technique for early breast cancer patients diagnosed today.
Materials and Method
Patient Cohort for Probability Extraction
The SEER*Stats software version 8.3.4 was used to extract a case-listing from the Surveillance Epidemiology and End Results (SEER) 18 Custom Database with additional treatment information, which includes radiotherapy delivery information (15). This database includes reliable information on radiotherapy delivery, with a sensitivity of 68.6% and a predictive positive value of 92.2%, meaning that when radiotherapy is recorded, it was most likely delivered (16). This information was only available after 1988, so female patients with a breast cancer diagnosis after 1988 were selected. Patients with at least 5 years of follow-up and with known information on the delivery of radiotherapy were extracted. Metastatic patients were excluded, and to avoid bias, patients with a pre-existing lung cancer diagnosed before the breast one, patients who received radiotherapy for a non-breast cancer before the breast cancer, and patients who had breast cancer treated without radiotherapy and a subsequent other non-breast cancer treated with radiotherapy were excluded.
The collected information included the patient’s SEER ID, tumor site, cancer stage, age at diagnosis, month and year of diagnosis, survival in months, vital status at study cut-off, and delivery and type of radiotherapy if any. When patients had multiple breast cancer records, it is either the first one treated with radiotherapy or, if radiotherapy was not delivered, the first one diagnosed that was chosen as date of diagnosis. Patients were deemed to have received radiotherapy when the record indicated “beam radiotherapy”, or “combination of beam with implanted radioisotope”, or “radiotherapy delivered but method not specified”, or “radiotherapy recommended but it is unknown if administrated”. Conversely, patients were deemed not to have received radiotherapy when the record indicated “no/unknown” or “radiotherapy recommended but refused”. The occurrence and date of lung cancer after breast cancer was matched using the patient’s SEER ID.
The final database was stratified in prognostic groups of relatively similar sizes, including DCIS, T1a N0, T1b N0, T1c N0, Stage IIA T2 tumors, Stage IIA N1 tumors, Stage IIB, Stage IIIA, Stage IIIB, or Stage IIIC. Radiotherapy utilization was evaluated per decade for each prognostic group, ranging from 1988 to 1997, 1998 to 2007, and 2008 to 2012.
Survival Analysis
The overall survival and lung cancer–free survival were calculated for each patient stage group using Kaplan Meyer statistics. Univariate regression analysis was used to identify the risk factors of developing secondary lung cancer. A multivariable competing risk Cox proportional hazard model was developed using a stepwise regression (PROC PHREG procedure in SAS) to account for competing risks of all-cause mortality. Independent variables included the breast cancer stage, age at diagnosis, year of diagnosis, overall survival, and the delivery of radiotherapy, as well as the resultant interaction terms between these variables. Statistical analyses were performed using SAS/STAT 14.2 (SAS Institute, Cary, NC, USA) or SPSS 25.0.0.1 (IBM Corporation, New York, NY, USA). Because multiple tests were performed, the level of significance had been set to p<0.001.
Number of Patients at Risk of Developing a Secondary Lung Cancer
To calculate the number of patients at risk of developing a secondary cancer after breast radiotherapy, a modified BEIR VII model for a female breast cancer patient was constructed, accounting for the age and stage distribution at diagnosis, present use of radiotherapy, and survival per stage derived from the SEER cohort (11, 17). Stage distribution and number of new breast cancer was obtained from the NAACCR (18). Using this model, the risks of developing a secondary lung cancer were calculated for various mean lung dose values that were either measured in a phantom of a medium-size breast patient, or simulated using treatment planning system, or extracted from literature (11, 19–21). Techniques included whole breast irradiation (WBI) techniques excluding nodal irradiation treated in supine or prone position, delivering 42,5 Gy in 16 fractions, or 26 Gy in five fractions following the new FAST-Forward regimen (22, 23). Also, various accelerated partial breast irradiation (APBI) including 3D-conformal radiotherapy (CRT) (24, 25), volumetric modulated arc therapy (VMAT) (11), or 4π robotic radiosurgery (21) delivering lower dose in 10 or 5 fractions, high-dose rate (HDR) brachytherapy using either a balloon or multicatheter and delivering 34 Gy in 10 fractions (26), or low-dose rate 106-palladium seeds brachytherapy have been tested (27). The lung cancer mortality was derived using a 0.8 incidence-to-mortality ratio and the 2019 incidence of breast cancers in the USA (18, 28), and the number of lives that could be saved was calculated from the risk of dying of secondary lung cancer using various techniques compared to standard supine radiotherapy.
Results
Patients
A total of 900,085 patients with 1,079,406 cancer records were found in the SEER database. After removing stage IV, unknown stages, unknown dates of event, and patients with confounding factors, the cohort included 765,697 patients. The median age was 60 years, with an interquartile range of 21 years. Overall, 15.2% of patients had two cancer records, 1.9% had three records, and 0.24% had more than three. Breast cancer was the most frequent new cancer event recorded.
Table 1 describes the stage distribution and median survival per stage. The median survival ranged from 22.9 years for DCIS to 6.1 years for stage IIIC. Surprisingly, there was a small number of DCIS, suggesting that in situ breast disease is not appropriately reported to the SEER. To compensate for this potential under-reporting, the final calculation of lung cancer risk and lives saved used the 2012–2016 North American Association of Central Cancer Registries (NAACCR) (18) stage distribution.
Table 2 summarizes the utilization of radiotherapy per stage and over time. There was an equal split between patients receiving adjuvant radiotherapy and surgery alone, 49.8 versus 50.2%, respectively. The usage of radiotherapy changed over time, with a significant increase in the most recent cohorts. While there was 40.3% of patients receiving adjuvant radiotherapy during the 1988–1997 decade, this increased the following decades, 52.0% between 1998 and 2007 and 52.2% between 2008 and 2012 (p<0.001). Of note, the radiotherapy usage decreased from 65 to 53% for the very favorable T1a N0 stage group.
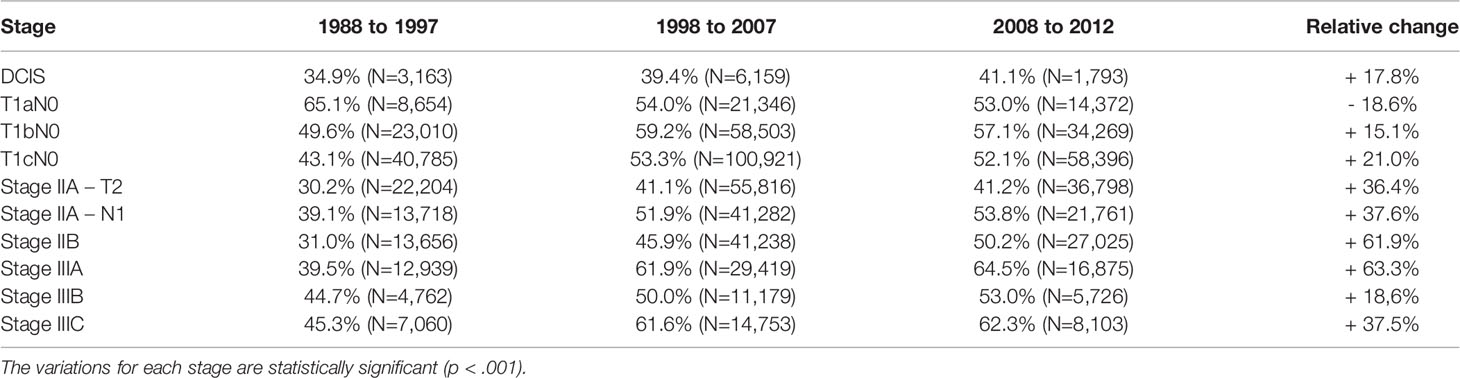
Table 2 Proportion of patients treated with radiotherapy increased over time for various breast cancer stages but for T1aN0 patients.
Factors of Secondary Lung Cancers
A total of 13,689 lung cancers were detected in the cohort. Using a stepwise regression procedure, all factors were highly significantly associated (p<0.001) with an increased risk of developing secondary lung cancer, including the use of radiotherapy, an earlier cancer stage, a younger age, an earlier year of diagnosis, and a longer survival. The interaction factors were also highly significantly correlated (p<0.001). Accounting for the interaction factors, the Hazard Ratio (HR) to develop a secondary cancer was higher for patients receiving radiotherapy and treated between 1988 and 1993 (HR = 1.58, 95% CI = 1.47–1.70), compared to those receiving radiotherapy between 1994 and 1999 (HR = 1.35, 95% CI = 1.28–1.42) and those receiving radiotherapy between 2000 and 2005 (HR = 1.15, 95% CI = 1.11–1.19).
Table 3 shows that the risk of developing a secondary lung cancer is higher for DCIS patients, who also have the longest median of survival, with a HR of 1.66. Figure 1 shows that the excess risk is small during the first 15 years after radiotherapy, to increase between 20 to 25 years. The risk is lower for stage I with HR about 1.2 and loses significance for Stage IIB and III, which confirms our first hypothesis that secondary lung cancer is a clinically significant issue mainly for early-stage breast cancers, and hence preventative measures should target this population.

Table 3 Excess risk developing a lung cancer after breast adjuvant radiotherapy compared to no radiotherapy per cancer stage.
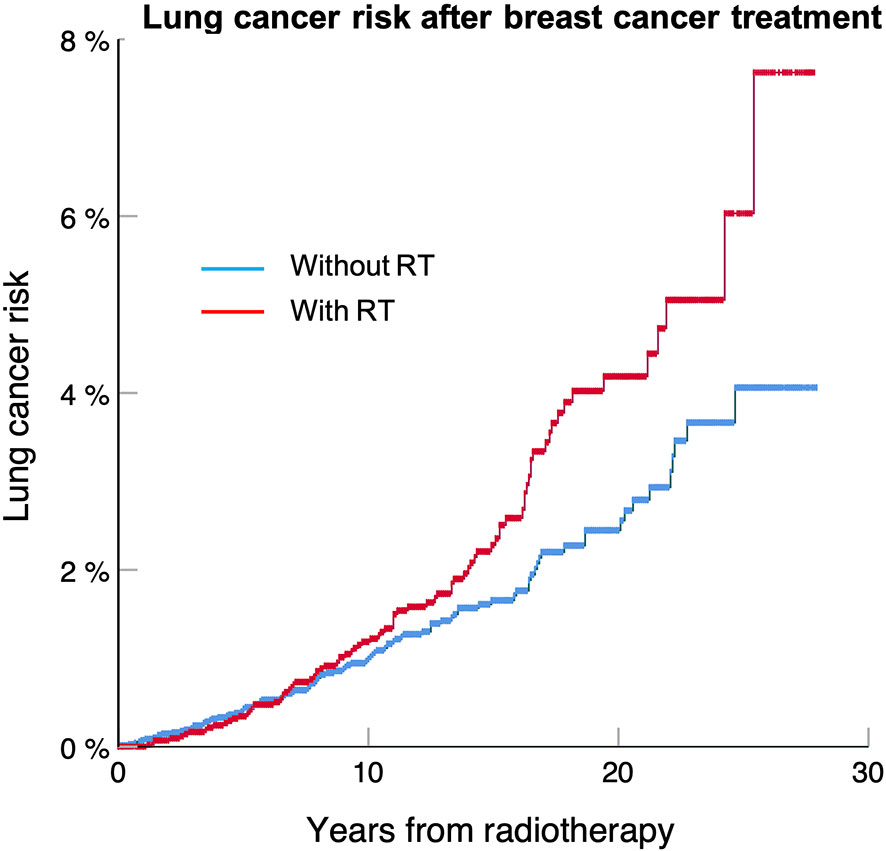
Figure 1 Lung cancer free survival for DCIS treated with or without radiotherapy for patients included in the SEER 18 database between 1988 and 2012. The lung cancer risk at 25 years is 8.2% for patients treated with radiotherapy compared to 4.3% without (p < 0.001).
Number of Lung Cancers Using Various Breast Radiotherapy Techniques
Out of the 268,600 women diagnosed with breast cancer in the USA in 2019, an estimated 205,318 had an early stage including DCIS, stage I, and node negative stage IIA (18). Accounting for modern utilization of radiotherapy, 104,743 patients have received this treatment. We calculated that for women diagnosed with an early-stage breast cancer in 2019, a total of 3,625 of them (3.25%) will develop a radiation-induced lung cancer during their lifetime using a standard breast radiotherapy delivering a dose of 42.5 Gy in 16 fractions. Assuming a 20% lung cancer survival rate, we estimated that an excess of 2,900 patients or an absolute proportion of 2.77% of patients will die of this secondary lung cancer (Table 4).
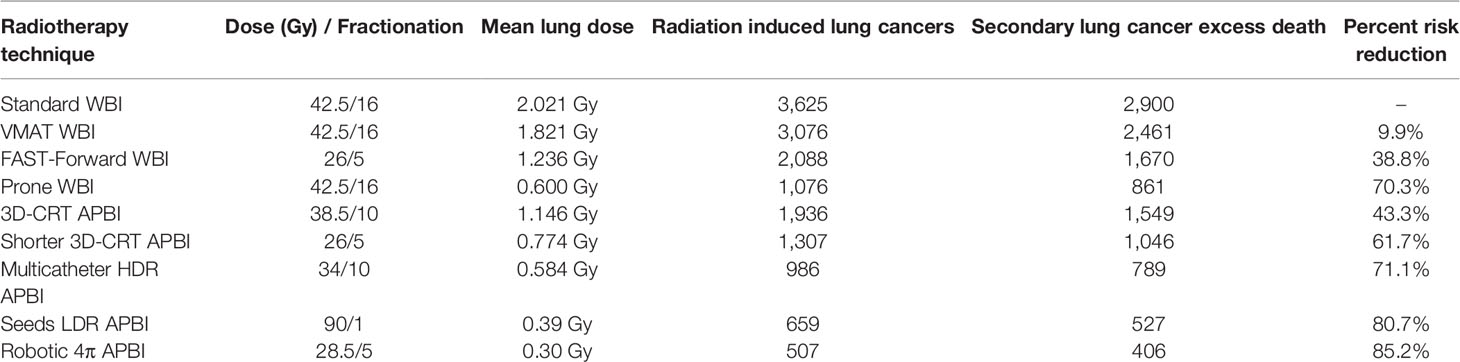
Table 4 Annual number and mortality of secondary lung secondary cancers for patients diagnosed in 2019 with early-stage breast cancers depending on the radiotherapy technique.
We previously reported that using a VMAT technique optimizing the lung protection could slightly reduce the mean lung dose, eventually saving 9.9% of those excess lung cancer deaths. Using a lower total dose, 26 Gy in five fractions, would reduce the mean lung dose and eventually reduce by 38.8% the excess lung cancer mortality. Based on a literature review, Aznar reported that the prone technique enables a significant reduction of the lung exposure with a mean lung dose of about 0.6 Gy (19). This would result in the prevention of 70.3% of the excess mortality.
APBI also induces a lower mean lung dose, though not all techniques are equal. We measured for external beam 3D-CRT APBI a mean lung dose of 1.146 Gy, which would reduce the excess mortality by 43.3%, and for multicatheter HDR brachytherapy a mean lung dose of 0.584 Gy, enabling the reduction of 71.1% of the lung cancer death (11). Using low-energy 106-palladium sources, breast seed implant delivers a low dose to the lung, and 80.7% of lives could be saved (29). Taking full advantage of a 4π non-coplanar irradiation, Hoekstra reported that robotic APBI could generate the lowest mean lung dose, 0.3 Gy (21). Eventually, 85.2% of secondary lung cancers could be prevented, and more than 2,470 lives spared (Table 4).
Discussion
Accounting for changes in radiation usage, protocols and techniques, current survival outcome, and patient’s stage and age characteristics, this study confirms the long-term risk of secondary lung cancer after radiotherapy for breast cancer patients diagnosed today. It adds to knowledge that the issue is statistically significant mainly for early-stage and young patients, who have a higher probability to have 20 to 30 years of survival (1, 14). This persistent risk is noticeable since several publications suggest that improved techniques reduce the risk of body exposure during radiotherapy, with, for example, a threefold reduction using field-in-field breast IMRT (30).
Although they occur late in the patient’s life, the absolute risk of excess lung cancers and deaths calculated for good prognosis cancers in the present work is high and in the same order of magnitude as radiation-induced cardiac morbidity and mortality. So, the same weight should be placed on reducing the mean lung dose when planning breast radiotherapy, as it is to minimize the mean heart dose (5). The good news is that there are several validated techniques for early-stage breast cancer which reduce the lung dose. Our study shows that they are not equal in terms of benefit. For example, VMAT only leads to a small 9.9% risk reduction. A larger benefit could be expected using a prone technique, which would reduce the mortality by as much as 70%. While the prone technique has been advocated to reduce the heart dose and to prevent skin side effects for large-breasted patients (20, 31, 32), our data suggest there is also a survival benefit.
APBI is recommended by various societies for early breast cancer stages, which our study also shows to be the main benefactors for the secondary lung cancer prevention (33–35). In 2019 two large randomized clinical trials comparing APBI to whole breast radiotherapy showed no difference at 8.6 and 10.2 years in the overall survival at the cost of a 0.7% ipsilateral breast recurrence increase (24, 25). Accounting for the survival benefit provided preventing lung cancer death, the 0.7% increase of local recurrence appears well acceptable. Importantly, our data show that the 3D-CRT technique evaluated in the RAPID and NSABP-B39 trials may not be optimal as it leads to a 43.3% reduction of the secondary lung cancer mortality. This is small compared to brachytherapy using HDR or seeds LDR with mortality reduction of, respectively, 71 and 81%. The largest benefit is calculated for the 4π robotic APBI technique with a reduction over 85%. APBI is a safe and effective substitute for whole breast radiotherapy in selected early-stage breast cancer patients. Based on the low reported toxicity (0–6.6%), APBI should be recommended in patients with life expectancies larger than 10 years.
The FAST-Forward regimen has been strongly recommended by experts to minimize travel and potential exposure of frail patients at the hospital (36, 37). As this trial shows local control and long-term side effects equivalent to standard radiotherapy over 3 weeks (23), it is likely that this regimen will be kept in the long term. However, the FAST-Forward regimen still treats the whole breast and hence has a smaller 38.8% mortality risk reduction compared to APBI techniques. To facilitate APBI adoption, single daily fraction regimens like the ACCEL trial delivering 27 Gy in five fractions (38), or the Rotterdam regimen delivering 28.5 Gy in five fractions with 4π robotic APBI, might be helpful (21).
Caution is needed in interpreting clinical outcomes from modeling, but similarly to the evaluation of cardiac morbidity, this might be the only possible strategy to assess very long-term breast radiotherapy toxicities (9). It is neither realistic nor ethical to design randomized clinical trials testing the impact of radiotherapy on the development of secondary lung cancer. It is also challenging to extract very long-term data, beyond 25 years, from population-based studies or meta-analysis because radiotherapy techniques, indications, and protocols have changed. Also, today patients are often diagnosed at an earlier stage and live longer. In the present study, which uses a very large initial cohort from the SEER database, only a small proportion of patients have very long-term follow-up: 5.1% had a follow-up exceeding 20 years, and less than 1% have follow-up between 26 and 30 years.
We used several adjustments in our model. The finding that DCIS might be underreported into the SEER database led us to opt for a hybrid model using the survival and radiotherapy utilization per stage from the SEER database, and the stage distribution from the NAACCR (18). The calculation of secondary cancer risk in various scenarios is based on the mean lung dose, which was either measured in a medium-size breast phantom or extracted from literature. The value of 2.02 Gy mean bilateral lung dose for standard breast radiotherapy is consistent with the one recently reported by Kirby for the ipsilateral lung based on a dosimetry study for 65 patients (20). It is also consistent with the values reported by Jain for the mean ipsilateral lung dose on 25 consecutive patients randomized in the NSABP B-39/RTOG 0413 protocol (39).
Caution is also needed when applying the excess lung cancer risks reported in this work to patients with different anatomy, like a smaller or a larger breast. On one hand a larger-breast patient may experience higher dose to the lung due to the larger radiation scattering volume. On the other hand, a larger body mass index means more shielding tissue and therefore less dose scattered to organs as shown on Woo’s prospective study measuring scatter dose with skin dosimeters (30). This means that the calculation of secondary lung cancer risk for a given patient should come from plan comparison testing various radiotherapy scenarios. It is possible that different anatomies may produce different classifications for the safest technique. Also, other co-factors that could have a supra-additive effect on the carcinogenic effect of radiotherapy, including smoking or the use of certain chemotherapy regimens that may have changed over time, have not been included in the model and should be considered. In a comparable modeling, Taylor used current smoker and non-smoker population mortality rates in 5-year age groups to account for the near 20-fold higher risk of lung cancers linked to tobacco consumption (9). In comparing the excess risk of secondary lung cancer for various techniques, we have used the mean lung dose as primary dose distribution metric. This was guided by the BEIR model that derives the lifetime excess risk of cancer based on the mean dose to a given target organ (40). There could be a need to add other dosimetric constraints for planning purposes. For example, the Quantec guidelines limit the volume of ipsilateral lung receiving 20 Gy or more to 30% and 30 Gy or more to 20%. To reduce the risk of pneumonitis, the volume of lung receiving 5 Gy or more is also a frequently used constraint (41). Finally, in weighing treatment options, one should carefully consider treatment safety and quality of life, and for selected early-stage breast cancer, the need for adjuvant systemic therapy may be debatable (42).
In conclusion, this study confirms that, accounting for the current utilization and techniques of radiotherapy, patient’s characteristics, and outcomes, a significant number of patients diagnosed with early breast cancer will succumb after two to three decades to radiation-induced secondary lung cancer. This could be prevented by reducing the mean lung dose with techniques like the prone technique, brachytherapy, or ultra-hypofractionated 4π robotic APBI.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
J-PP, HD, and DW built the model and did the analysis. J-PP and NH collected experimentally the mean lung doses for the model. J-PP, NH, HD, MN, and FV wrote the first draft of the manuscript. J-PP, NH, HD, DW, MN, and FV finalized the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
Author FV is employed by 21st Century Oncology, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Farwell MF, Foster RS, Costanza MC. Breast Cancer and Earlier Detection Efforts. Realized and Unrealized Impact on Stage. Arch Surg (1993) 128:510–3. doi: 10.1001/archsurg.1993.01420170040005
2. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-Year Follow-Up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy Plus Irradiation for the Treatment of Invasive Breast Cancer. N Engl J Med (2002) 347:1233–41. doi: 10.1056/NEJMoa022152
3. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet (2005) 366:2087–106. doi: 10.1016/S0140-6736(05)67887-7
4. Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of Radiotherapy After Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
5. Darby SC, McGale P, Taylor CW, Peto R. Long-Term Mortality From Heart Disease and Lung Cancer After Radiotherapy for Early Breast Cancer: Prospective Cohort Study of About 300,000 Women in US SEER Cancer Registries. Lancet Oncol (2005) 6:557–65. doi: 10.1016/S1470-2045(05)70251-5
6. Andratschke N, Maurer J, Molls M, Trott KR. Late Radiation-Induced Heart Disease After Radiotherapy. Clinical Importance, Radiobiological Mechanisms and Strategies of Prevention. Radiother Oncol (2011) 100:160–6. doi: 10.1016/j.radonc.2010.08.010
7. Lee LJ, Harris JR. Innovations in Radiation Therapy (RT) for Breast Cancer. Breast (2009) 18:S103–11. doi: 10.1016/S0960-9776(09)70284-X
8. Taylor CW, Kirby AM. Cardiac Side-Effects From Breast Cancer Radiotherapy. Clin Oncol (2015) 27:621–9. doi: 10.1016/j.clon.2015.06.007
9. Taylor C, Correa C, Duane FK, Aznar MC, Anderdson SJ, Bergh J, et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol (2017) 35:1641–9. doi: 10.1200/JCO.2016.72.0722
10. Inskip PD, Stovall M, Flannery JT. Lung Cancer Risk and Radiation Dose Among Women Treated for Breast Cancer. J Natl Cancer Inst (1994) 86:983–8. doi: 10.1093/jnci/86.13.983
11. Hoekstra N, Fleury E, Merino Lara TR, van der Baan P, Bahnerth A, Struik G, et al. Long-Term Risks of Secondary Cancer for Various Whole and Partial Breast Irradiation Techniques. Radiother Oncol (2018) 128:428–33. doi: 10.1016/j.radonc.2018.05.032
12. Grantzau T, Overgaard J. Risk of Second non-Breast Cancer Among Patients Treated With and Without Postoperative Radiotherapy for Primary Breast Cancer: a Systematic Review and Meta-Analysis of Population-Based Studies Including 522,739 Patients. Radiother Oncol (2016) 121:402–13. doi: 10.1016/j.radonc.2016.08.017
13. Pignol JP, Truong P, Rakovitch E, Sattler MG, Whelan TJ, Olivotto IA. Ten Years Results of the Canadian Breast Intensity Modulated Radiation Therapy (IMRT) Randomized Controlled Trial. Radiother Oncol (2016) 121:414–9 2016. doi: 10.1016/j.radonc.2016.08.021
14. Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA Breast Cancer Death Down 25% in Year 2000 at Ages 20-69 Years. Lancet (2000) 335:1822. doi: 10.1016/S0140-6736(00)02277-7
15. Surveillance, Epidemiology, and End Results (SEER) Program (2018). Available at: https:\\www.seer.cancer.gov(Accessed January 10, 2019).
16. Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care (2016) 54:e55–64. doi: 10.1097/MLR.0000000000000073
17. National Research Council. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: National Academies Press (2006). doi: 10.17226/11340
18. North American Association of Central Cancer Registries (NAACCR). Fast Stats 2012-2016 Cancer Incidence Data, Statistics Stratified by Stage (2019). Available at: https://faststats.naaccr.org (Accessed December 15th 2019).
19. Aznar MC, Duane FK, Darby SC, Wang Z, Taylor CW. Exposure of the Lungs in Breast Cancer Radiotherapy: A Systematic Review of Lung Doses Published 2010-2015. Radiother Oncol (2018) 126:148–54. doi: 10.1016/j.radonc.2017.11.022
20. Kirby AM, Evans PM, Donovan EM, Convery HM, Haviland JS, Yarnold JR. Prone Versus Supine Positioning for Whole and Partial-Breast Radiotherapy: A Comparison of non-Target Tissue Dosimetry. Radiother Oncol (2010) 96:178–84. doi: 10.1016/j.radonc.2010.05.014
21. Hoekstra N, Habraken S, Swaak-Kragten A, Breedveld S, Pignol JP, Hoogeman M. Reducing the Risk of Secondary Lung Cancer in Treatment Planning of Accelerated Partial Breast Irradiation. Front Oncol (2020) 10:1445. doi: 10.3389/fonc.2020.01445
22. Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie RM, Parpia S, et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. N Engl J Med (2010) 362:513–20. doi: 10.1056/NEJMoa0906260
23. Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfoeld DJ, et al. Hypofractionated Breast Radiotherapy for 1 Week Versus 3 Weeks (FAST-Forward): 5-Year Efficacy and Late Normal Tissue Effects Results From a Multicentre, non-Inferiority, Randomised, Phase 3 Trial. Lancet (2020) 395:1613–26. doi: 10.1016/S0140-6736(20)30932-6
24. Vicini FA, Cecchini RS, White JR, Arthur DW, Julian TB, Rabinovitch RA, et al. Long-Term Primary Results of Accelerated Partial Breast Irradiation After Breast-Conserving Surgery for Early-Stage Breast Cancer: a Randomised, Phase 3, Equivalence Trial. Lancet (2019) 394:2155–64. doi: 10.1016/S0140-6736(19)32514-0
25. Whelan TJ, Julian JA, Berrang TS, Kim DH, Germain I, Nichol AM, et al. External Beam Accelerated Partial Breast Irradiation Versus Whole Breast Irradiation After Breast Conserving Surgery in Women With Ductal Carcinoma in Situ and Node-Negative Breast Cancer (RAPID): A Randomised Controlled Trial. Lancet (2019) 394:2165–72. doi: 10.1016/S0140-6736(19)32515-2
26. Strnad V, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T, et al. 5-Year Results of Accelerated Partial Breast Irradiation Using Sole Interstitial Multicatheter Brachytherapy Versus Whole-Breast Irradiation With Boost After Breast-Conserving Surgery for Low-Risk Invasive and in-Situ Carcinoma of the Female Breast: a Randomised, Phase 3, non-Inferiority Trial. Lancet (2016) 387:229–38. doi: 10.1016/S0140-6736(15)00471-7
27. Pignol JP, Caudrelier JM, Crook J, McCann C, Truong P, Verkooijen HA. Report on the Clinical Outcomes of Permanent Breast Seed Implant for Early-Stage Breast Cancers. Int J Radiat Oncol Biol Phys (2015) 93:614–21. doi: 10.1016/j.ijrobp.2015.07.2266
28. National Cancer Institute Surveillance Epidemiology and End Results Program. Table 15.20 Cancer of the Lung and Bronchus (Invasive) (2018). Available at: https://seer.cancer.gov/csr/1975_2014/browse_csr.php?sectionSEL=4&pageSEL=sect_04_table.17.html (Accessed December 15th 2019).
29. Pignol JP, Keller BM, Ravi A. Doses to Internal Organs for Various Breast Radiation Techniques–Implications on the Risk of Secondary Cancers and Cardiomyopathy. Radiat Oncol (2011) 6:5. doi: 10.1186/1748-717X-6-5
30. Woo TC, Pignol JP, Rakovitch E, Vu TTT, Hicks D, O’Brien P, et al. Body Radiation Exposure in Breast Cancer Radiotherapy: Impact of Breast IMRT and Virtual Wedge Compensation Techniques. Int J Radiat Oncol Biol Phys (2006) 65:52–8. doi: 10.1016/j.ijrobp.2005.11.023
31. Grann A, McCormick B, Chabner ES, Gollamudi SV, Schupak KD, Mychalczak BR, et al. Prone Breast Radiotherapy in Early-Stage Breast Cancer: A Preliminary Analysis. Int J Radiat Oncol Biol Phys (2000) 47:319–25. doi: 10.1016/s0360-3016(00)00448-x
32. Mulliez T, Veldeman L, van Greveling A, Speleers B, Sadeghi S, Berwouts D, et al. Hypofractionated Whole Breast Irradiation for Patients With Large Breasts: A Randomized Trial Comparing Prone and Supine Positions. Rad Oncol (2013) 108:203–8. doi: 10.1016/j.radonc.2013.08.040
33. Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, et al. Accelerated Partial Breast Irradiation: Executive Summary for the Update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol (2017) 7:73–9. doi: 10.1016/j.prro.2016.09.007
34. Strnad V, Major T, Polgar C, Lotter M, Guinot J-L, Guttierez-Miguelez C, et al. ESTRO-ACROP Guideline: Interstitial Multi-Catheter Breast Brachytherapy as Accelerated Partial Breast Irradiation Alone or as Boost - GEC-ESTRO Breast Cancer Working Group Practical Recommendations. Radiother Oncol (2018) 128:411–20. doi: 10.1016/j.radonc.2018.04.009
35. Shah C, Vicini F, Shaitelman SF, Hepel J, Keisch M, Arthur D, et al. The American Brachytherapy Society Consensus Statement for Accelerated Partial-Breast Irradiation. Brachytherapy (2018) 17:154–70. doi: 10.1016/j.brachy.2017.09.004
36. Levy A, Rivera S. 1-Week Hypofractionated Adjuvant Whole-Breast Radiotherapy: Towards a New Standard? Lancet (2020) 395:1588–9. doi: 10.1016/S0140-6736(20)30978-8
37. Coles CE, Aristei C, Bliss J, Boersma L, Brunt AM, Chatterjee S, et al. International Guidelines on Radiation Therapy for Breast Cancer During the COVID-19 Pandemic. Clin Oncol (R Coll Radiol) (2020) 32:279–81. doi: 10.1016/j.clon.2020.03.006
38. Grendarova P, Roumeliotis M, Quirk S, Lesiuk M, Craighead P, Liu HW, et al. One-Year Cosmesis and Fibrosis From ACCEL: Accelerated Partial Breast Irradiation (APBI) Using 27 Gy in 5 Daily Fractions. Pract Radiat Oncol (2019) 9:e457–64. doi: 10.1016/j.prro.2019.04.002
39. Jain AK, Vallow LA, Gale AA, Buskirk SJ. Does Three-Dimensional External Beam Partial Breast Irradiation Spare Lung Tissue Compared With Standard Whole Breast Irradiation? Int J Radiat Oncol Biol Phys (2009) 75:82–8. doi: 10.1016/j.ijrobp.2008.10.041
40. National Research Council of the National Academies. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press (2006).
41. Lancellotta V, Iacco M, Perrucci E, Falcinelli L, Zucchetti C, de Bari B, et al. Comparing Four Radiotherapy Techniques for Treating the Chest Wall Plus Levels III-IV Draining Nodes After Breast Reconstruction. Br J Radiol (2018) 91:20160874. doi: 10.1259/bjr.20160874
Keywords: breast radiotherapy, secondary cancer, accelerated partial breast irradiation, brachytherapy, SBRT
Citation: Pignol JP, Hoekstra N, Wilke D, Dahn H, Nolan M and Vicini F (2021) Estimation of Annual Secondary Lung Cancer Deaths Using Various Adjuvant Breast Radiotherapy Techniques for Early-Stage Cancers. Front. Oncol. 11:713328. doi: 10.3389/fonc.2021.713328
Received: 22 May 2021; Accepted: 09 July 2021;
Published: 09 August 2021.
Edited by:
Abraham Kuten, Israel Cancer Association, IsraelReviewed by:
Diane Ling, University of Southern California, United StatesValentina Lancellotta, Catholic University of the Sacred Heart, Italy
Copyright © 2021 Pignol, Hoekstra, Wilke, Dahn, Nolan and Vicini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Philippe Pignol, amVhbi1waGlsaXBwZS5waWdub2xAZGFsLmNh
 Jean-Philippe Pignol
Jean-Philippe Pignol Nienke Hoekstra
Nienke Hoekstra Derek Wilke1
Derek Wilke1