- 1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2School of Basic Medical Science, Chongqing Medical University, Chongqing, China
- 3Department of Pathology, Chongqing Medical University, Chongqing, China
Background: Recently, it has been reported that angiotensin II receptor-associated protein (AGTRAP) plays a substantial role in tumor progression. Nevertheless, the possible role of AGTRAP in hepatocellular carcinoma (HCC) remains unrecognized.
Methods: The metabolic gene rapid visualizer, Cancer Cell Line Encyclopedia, Human Protein Atlas, and Hepatocellular Carcinoma Database were used to analyze the expression of AGTRAP in HCC tissues and normal liver tissues or adjacent tissues. Kaplan-Meier plotter and UALCAN analysis were used to assess the prognostic and diagnostic value of AGTRAP. LinkedOmics and cBioPortal were used to explore the genes co-expressed with AGTRAP in HCC. To further understand the potential mechanism of AGTRAP in HCC, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment pathway analyses were performed using R software, the protein-protein interaction (PPI) network was established using the STRING database, and the immune infiltration and T-cell exhaustion related to AGTRAP were explored via Timer and GEPIA. In addition, immunohistochemistry was used to detect the expression of AGTRAP protein in HCC tissues and paired adjacent tissues from clinical specimens.
Results: This study found that the mRNA and protein levels of AGTRAP in HCC tissues were higher than those in normal liver tissues and adjacent tissues, and higher mRNA levels of AGTRAP were associated with higher histological grade and a poor overall survival in HCC patients. The area under the receiver operating characteristic curve (AUC) of AGTRAP was 0.856, suggesting that it could be a diagnostic marker for HCC. Moreover, the alteration rate of AGTRAP in HCC was 8%, and AGTRAP was involved in HCC probably through the NF-κB and MAPK signaling pathways. Furthermore, AGTRAP was positively correlated with the infiltration of CD8+ T cells, CD4+ T cells, B cells, macrophages, dendritic cells, and neutrophils, and the levels of AGTRAP were significantly correlated with T-cell exhaustion biomarkers. The immunohistochemistry results confirmed that the protein levels of AGTRAP were consistently higher in HCC tissues than in paired adjacent tissues.
Conclusion: The clinical value of AGTRAP and its correlation with immune infiltration in HCC was effectively identified in clinical data from multiple recognized databases. These findings indicate that AGTRAP could serve as a potential biomarker in the treatment of HCC, thereby informing its prognosis, diagnosis, and even immunotherapy.
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common malignant tumor worldwide (1) with low overall survival (OS) of patients and poor prognosis (2). The majority of patients are diagnosed with HCC at an advanced stage, usually accompanied by intra- and extra-hepatic invasion and metastasis, with no specific symptoms at the early stage (3). Although an increasing number of adjuvant therapeutic methods have been found to improve the treatment efficacy of HCC, the recurrence and metastasis of HCC remain difficult obstacles. Therefore, there is an urgent need to identify novel specific biomarkers to improve the clinical diagnosis and prognosis in HCC patients.
Angiotensin II receptor-associated protein (AGTRAP) is localized in the perinuclear vesicle structure and the plasma membrane (4). AGTRAP, which inhibits the activation of angiotensin II, has been broadly studied in cardiovascular diseases and metabolic disorders (5, 6). Recently, Sanz-Pamplona et al. (7) found that AGTRAP is overexpressed in colon cancer and positively correlates with a poor prognosis. Figueiredo et al. (8) reported that AGTRAP-BRAF gene fusion was detected in gastric cancer, indicating a possible role of AGTRAP in tumor progression. However, little is known about the function of AGTRAP in HCC and the oncogenic mechanisms involved.
Bioinformatics is a combination of life science and computer science that aims to identify the sequence, structure, and function of biological macromolecules and their interrelationships. In the last several years, bioinformatics has proved to be a valuable tool in screening tumor biomarkers (9, 10). In this study, we used bioinformatics to explore the expression of AGTRAP in HCC tissues and normal tissues or adjacent tissues, the value of AGTRAP in HCC patient prognosis and diagnosis, and its correlation with clinicopathological parameters, and performed enrichment analyses of AGTRAP alterations and its co-expressed genes using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). Finally, immunohistochemistry (IHC) was used to verify the levels of AGTRAP in HCC tissues and paired adjacent tissues. Our study aimed at defining the potential role of AGTRAP in the occurrence and progression of HCC and whether it could be used as a diagnostic and prognostic biomarker.
Materials And Methods
TCGA Database
The gene expression RNA-Seq (HTSeq-FPKM), clinical data, and survival data were downloaded from the The Cancer Genome Atlas (TCGA) database from UCSC Xena (https://xenabro.wser.net/datapages/), which are processed uniformly by the TOIL process, free of computational batch effects (11).
Metabolic Gene Rapid Visualizer and Cancer Cell Line Encyclopedia
The Metabolic Gene Rapid Visualizer (MERAV, http://merav.wi.mit.edu) database was used to investigate the expression of AGTRAP (12). The gene expression data are normalized across these arrays to provide a means of consistent comparison. The Cancer Cell Line Encyclopedia (CCLE, https://portals.broadinstitute.org/ccle) integrates genetic information, including chromosome copy number, gene expression, and DNA mutations. CCLE can be applied to the evaluation of cellular targets, small molecules, genetic variations, and therapeutic approaches, allowing the recognition of new biomarker drivers of tumor dependence. The CCLE database provides a resource for cancer studies using models of cancer cell lines in vitro (13).
Hepatocellular Carcinoma Database and the Human Protein Atlas
In addition, the expression of AGTRAP in different liver tissues was studied using the Hepatocellular Carcinoma Database (HCCDB, http://lifeome.net/database/hccdb) (14). Simultaneously, co-expression networks of adjacent tissues and HCC tissues were assessed through HCCDB. Datas were normalized by log2 FC, and P-values were adjusted with Benjamini & Hochberg correction.
The protein expression of AGTRAP in both LIHC and normal tissues was obtained from the Human Protein Atlas database (HPA, version 20.1) (https://www.proteinatlas.org/), which is a program with the aim to map all the human proteins in cells, tissues. Next, the HPA was used to analyze the expression of AGTRAP in human tissues and cells (15).
Kaplan-Meier Plotter and UALCAN
The Kaplan-Meier plotter (http://kmplot.com/analysis/) (16) was used to analyze the prognostic value of AGTRAP in HCC samples based on TCGA (https://www.cancer.gov/) datasets.
UALCAN (ualcan.path.uab.edu) (17) is an open database for the analysis of gene expression data (TPM) from TCGA, which has been normalized. We used UALCAN to analyze the difference in AGTRAP expression in normal and HCC samples, the relationships between AGTRAP expression and the clinicopathological parameters of each sample (tumor grade, tumor stage, TP53 mutant, weight, age, sex, nodal metastasis), and its potential prognostic value.
LinkedOmics and cBioPortal
LinkedOmics (https://linkedomics.org/) is an online analysis tool that can be used to compare multi-omic cancer datasets from the Clinical Proteomic Tumor Analysis Consortium (CPTAC) and TCGA with an array of tumor types (32 cancers and 11,158 patients) and proteomics data (18). LinkedOmics involves three important analysis modules (LinkCompare, LinkFinder, and LinkInterpreter) and plots data as heat maps, scatter plots, and volcano plots. We selected RNAseq data from the TCGA-LIHC dataset for analysis, which are RSEM format data that has been converted by log2, using the Pearson correlation test, and they corrected for false positives (False Discovery Rate, FDR) using the Benjamini-Hochberg (BH) correction.
The cBio Cancer Genomics Portal (cBioPortal, version 3.7.1, http://cbioportal.org) was principally applied to explore multidimensional cancer genomics datasets with resources from 20 cancer studies including more than 5,000 tumor samples (19). We selected the log RNA Seq V2 RSEM data from TCGA-LIHC on this website for mutation analysis and co-expression gene analysis, which has been normalized.
STRING
The Search Tool for the Retrieval of Interacting Genes (STRING, version 11.5, http://string-db.org) provides references for studying the mechanism of disease occurrence or progression and provides a basis for exploring the functional interactions between proteins (20). STRING was used to demonstrate the functional networks of AGTRAP in HCC.
TIMER and GEPIA
The Tumor Immune Estimation Resource (TIMER, version 1.0, https://cistrome.shinyapps.io/timer/) was applied to analyze the association between AGTRAP gene expression and immune infiltration in HCC, as well as T-cell exhaustion (21). The relevant immune cells include B Cells, CD8+ T Cells, CD4+ T Cells, macrophages, neutrophils, and dendritic cells. The scatterplots will be generated and displayed after inputs are submitted successfully, showing the purity-corrected partial Spearman’s rho value and statistical significance.
The Gene Expression Profiling Interactive Analysis (GEPIA, version 1.0, http://gepia.cancer-pku.cn/) is a newly developed interactive web server for analyzing the RNA sequencing expression data of 9,736 tumors and 8,587 normal samples from the TCGA and the GTEx projects, using a standard processing pipeline. The RNA-Seq datasets GEPIA used is based on the UCSC Xena project (http://xena.ucsc.edu), which are computed by a standard pipeline. The database provides a customizable and interactive function, including differential expression analysis, correlation analysis, profiling plots, gene analysis, and survival analysis. The associations between AGTRAP levels and T-cell exhaustion biomarkers (PD-1 (PDCD1), GZMB, LAG-3, CTLA-4, and HAVCR2 (TIM-3)) were explored using the GEPIA database (22). The data uses Log2 (TPM+1) for normalization. We performed a survival analysis of the TCGA-LIHC dataset with a median cutoff, Kaplan–Meier (KM) plots are presented with the hazard ratio (HR), the 95% confidence interval (CI), the log-rank p-value (p). Pearson correlation was chosen for gene correlation analysis.
Immunohistochemistry Analysis
The study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. All participants provided informed consent in writing. The pathological sections of 10 HCC patients were re-analyzed by professional pathologists via IHC staining of AGTRAP in paired tumors and adjacent tissues using the streptavidin-peroxidase (SP) method. Paraffin-embedded tumor tissues were collected and sliced into 3 mm sections. The slices were baked at 60°C for 2 h to be fully dewaxed and then rehydrated through a graded alcohol series and washed with phosphate buffered saline. Hydrogen peroxide (3%) was used to block endogenous peroxidase activity for 10 min at 25°C. Antigen retrieval was performed by microwaving in 10 mM citrate buffer (pH = 6.0) for 15 min. The slides were then blocked and sealed with 5% normal goat serum for 30 min at room temperature. The rest of the procedures are in accordance with the kit instructions (SP-9000, OriGene, China). Fifty microliters of anti-AGTRAP antibody (1:100, Sangon Biotech, China) was added to these tissue sections, which were then incubated at 4°C overnight. The sections were then incubated with the corresponding secondary antibody, OriGene, for 30 min at room temperature. Finally, the IHC score for each sample was calculated as the intensity of immunostaining (0, colorless; 1, light-yellow; 2, brownish-yellow; 3, dark brown) multiplied by the percentage of positive cells (0: 0%, 1: 1%–10%, 2: 11%–50%, 3: 51%–75%, and 4: 76%–100%).
Western Blotting
Radioimmunoprecipitation assay buffer (RIPA) buffer purchased from Beyotime Biotechnology (China) was used to extract total protein from tissues, and the supernatant was collected after centrifugation (12,000×g, 15 min, 4°C). BCA Protein Assay Kit (Biyuntian, China) was used to quantify the protein concentration. Equal protein quantities were separated by 12.5% SDS-PAGE gel and then transferred to PVDF membranes (0.22 um, PVDF membrane, GE healthcare life science, Germany). Membranes were blocked with 5% skimmed milk in Tris-buffered saline (TBS) for 1 h and was incubated with primary antibodies against AGTRAP (1:2000, ABclonal, China) and β-actin (1:2000, ABclonal, China) at 4°C overnight. The membrane was then incubated with secondary antibodies (1:2,000, Proteintech, China) for 1 h the next day. Finally, the signals were developed by an ECL detection system (Enhanced chemiluminescence system kit). Subsequently, gray scale quantifications of bands were performed using Image J software (National Institutes of Health, USA).
Statistical Analysis
All statistical analyses were performed using the SPSS software (version 26.0, IBM, USA) and the GraphPad Prism software (version 7.0, USA, www.graphpad.com) was used for graphing the heatmap and analyze the different grayscale values between adjacent tissues and tumor tissues of western blot bands. The Receiver operating characteristic curve (ROC) was analyzed using the “pROC” package (version 1.17.0.1) and “ggplot2” (version 3.3.3) in R Studio (version 3.6.3). The GO and KEGG enrichment analyses were visualized using the R packages “ggplot2” (version 3.3.3) and “clusterProfiler” (version 3.15.3), the threshold for statistically significant differences was set at p adjust ≤0.05. A chi-square test was used to assess the correlation between AGTRAP expression and clinicopathological parameters. Logistic regression and Spearman correlation analyses were used to analyze the correlation between clinical parameters and AGTRAP expression. Univariate and multivariate Cox regression analyses were performed to determine the association between AGTRAP expression and OS. Differences were considered statistically significant at p < 0.05. False discover rate (FDR) < 0.05 was regarded statistically credible.
Results
The mRNA and Protein Expression of AGTRAP in Different Databases
First, we explored the different expression levels of AGTRAP mRNA in HCC cells and HCC tissues as well as normal tissues in the MERAV (Figure 1A). AGTRAP expression was significantly higher in HCC cells than in normal tissue samples. The CCLE database was then used to visualize the gene expression data. As assessed through an array of cancer cell lines, AGTRAP was highly expressed in HCC cells (Figure 1B). Next, we reviewed the HCCDB to identify whether there were significant differences in AGTRAP mRNA expression levels between HCC datasets. As shown in Figure 1C, the AGTRAP level in HCC tissues was higher than in adjacent tissues (HCC/adjacent: logFC = 0.30). Subsequently, we compared AGTRAP levels in 12 different HCC datasets of which 9 supported our results (Figure 1D).
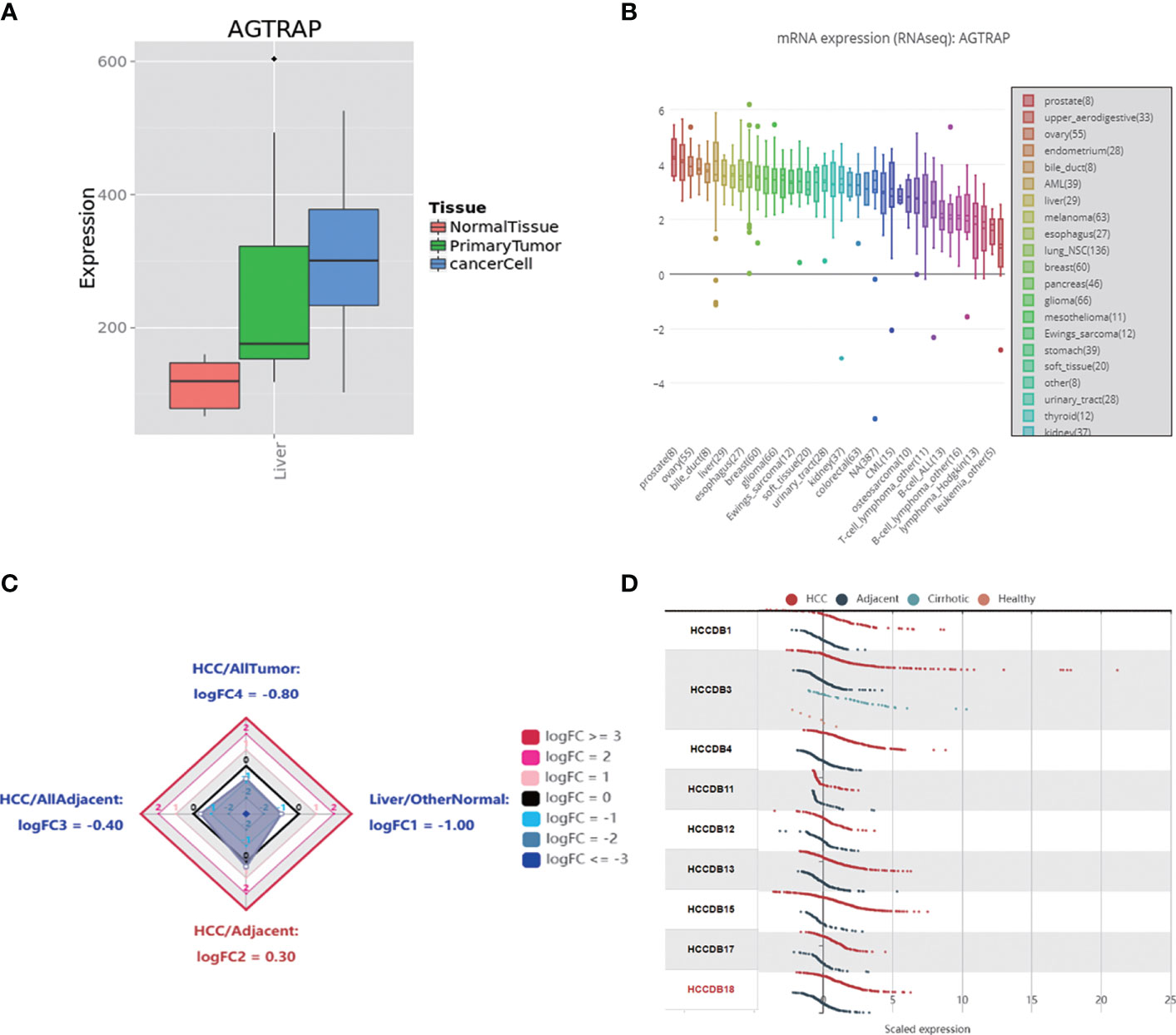
Figure 1 The mRNA expression of AGTRAP in different datasets. (A) The mRNA expression level of AGTRAP was significantly higher in HCC cells and Primary liver tissues than that in normal tissues in the MERAV. (B) Expression of AGTRAP in HCC cell lines using the CCLE database. (C) Radar map of AGTRAP overall expression among different types of tissues. (D) The expression of AGTRAP in different HCC datasets.
AGTRAP expression in normal liver tissues was lower compared to majority of human tissues in HPA (Figure 2A). Additionally, the level of AGTRAP in HCC tissues was the lowest among the cancer samples (Figure 2B). However, AGTRAP levels were higher in HCC cell lines (Hep G2) than in most cancer cell lines (Figure 2C). Intriguingly, the expression of AGTRAP in liver cells was mainly concentrated in Kupffer cells, and its expression was also high in T cells (Figure 2D). Although the expression of AGTRAP was relatively low in normal human liver tissues (Figure 2E), the HPA database showed that AGTRAP protein was moderately expressed in HCC tissues by IHC, mainly localized in cell plasma (Figure 2F).
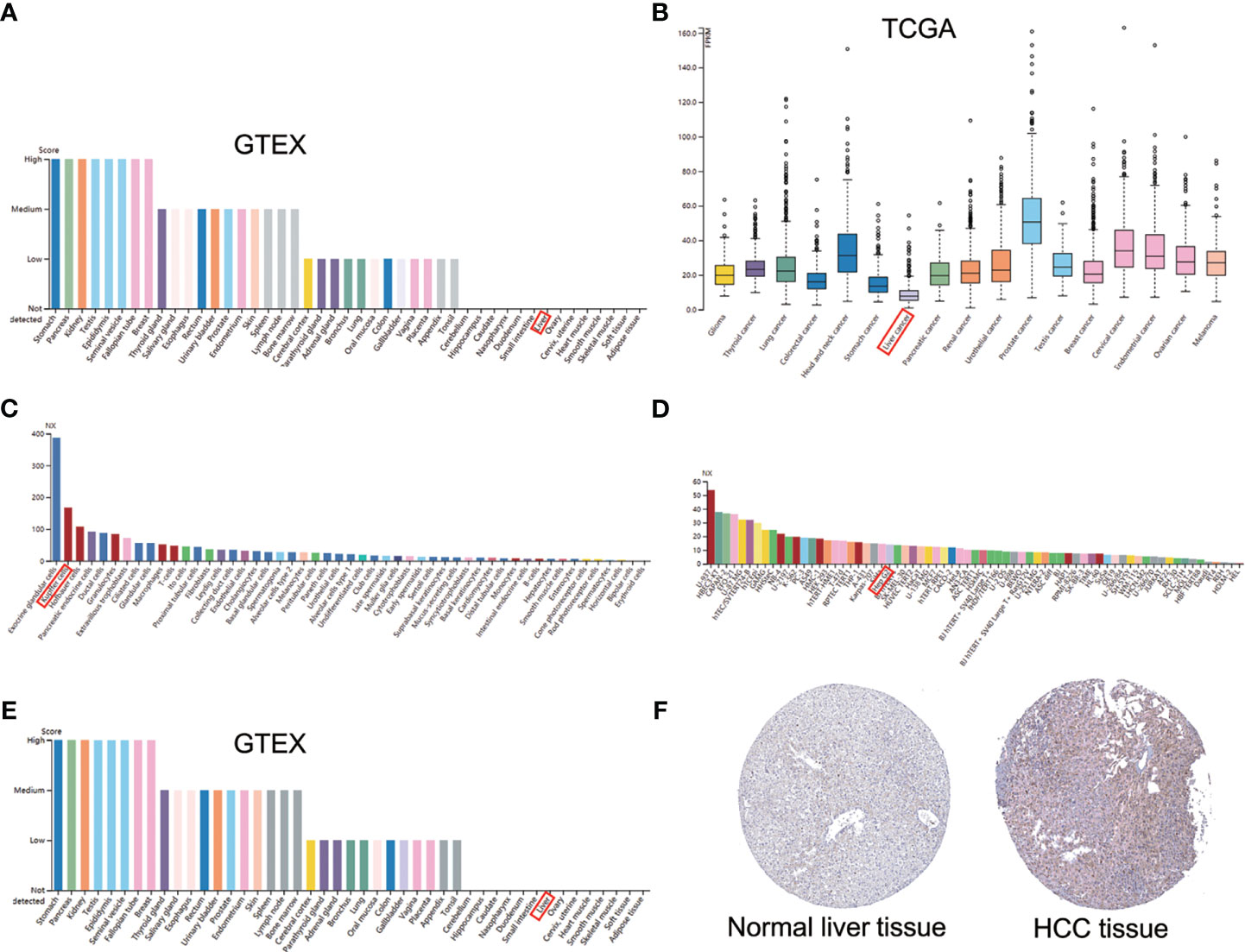
Figure 2 The mRNA and protein expression of AGTRAP in human normal tissues, cancer tissues and cell lines in HPA database. (A) histogram of AGTRAP mRNA expression in kinds of normal tissues from the GTEx project. (B) AGTRAP mRNA expression in different tumor tissues. (C) mRNA expression of AGTRAP in HCC cell lines (HEP G2). (D) The expression of AGTRAP in single cells. (E) Protein expression of AGTRAP in normal tissues from different organs. (F) IHC images with the AGTRAP antibody: HPA044120, HCC tissues had higher staining than normal liver tissues.
High AGTRAP Expression Is Correlated With Poor OS in HCC Patients and May Serve as a Diagnostic Biomarker Based on TCGA Datasets
To explore the association between AGTRAP level and OS in HCC patients, we used the Kaplan-Meier plotter tool and found a significant correlation between high expression of AGTRAP and poor prognosis (Figure 3A, hazard ratio (HR) = 2.13, 95% CI = 1.45–3.03, p = 7.8e-5). Next, we conducted a correlation analysis in the GEPIA database. The resultant violin plots showed that expression of AGTRAP was significantly correlated with the pathological stage of patients (Figure 3B). These findings compare well to HCC patient OS data from the HPA indicating that the high expression of AGTRAP was linked to poor prognosis (Figure 3C). Based on the TCGA dataset, we constructed an ROC using the pROC package (23) and found an AUC of 0.856 (Figure 3D).

Figure 3 The correlation between the expression of AGTRAP mRNA and protein with overall survival and tumor stage in HCC. (A) Higher AGTRAP mRNA expression indicated a poor OS in HCC patients from the Kaplan-Meier plotter. (B) Expression violin plots of AGTRAP based on patient pathological stage [log2(TPM + 1)]. (C) Prognostic value of AGTRAP expression in HCC patients according to HPA. (D) ROC curves of AGTRAP in HCC.
AGTRAP mRNA Expression Is Associated With Clinicopathological Characteristics in HCC Patients Based on TCGA Datasets
The UALCAN database was used to explore whether AGTRAP mRNA expression is associated with clinicopathological parameters. As illustrated in Figure 4A, a positive trend was found between expression of AGTRAP in HCC and the progression of tumor grade. The analysis of additional parameters found AGTRAP mRNA levels were positively correlated with tumor stage, patient weight, and TP53 mutation (Figures 4B–D). Furthermore, the expression of AGTRAP mRNA in cancer tissues was higher than that in normal tissues (Supplementary Figure 1A). There were no statistical differences between the expression of AGTRAP and different age periods, gender, and lymph node metastasis status (Figures 4E, F and Supplementary Figure 1B).
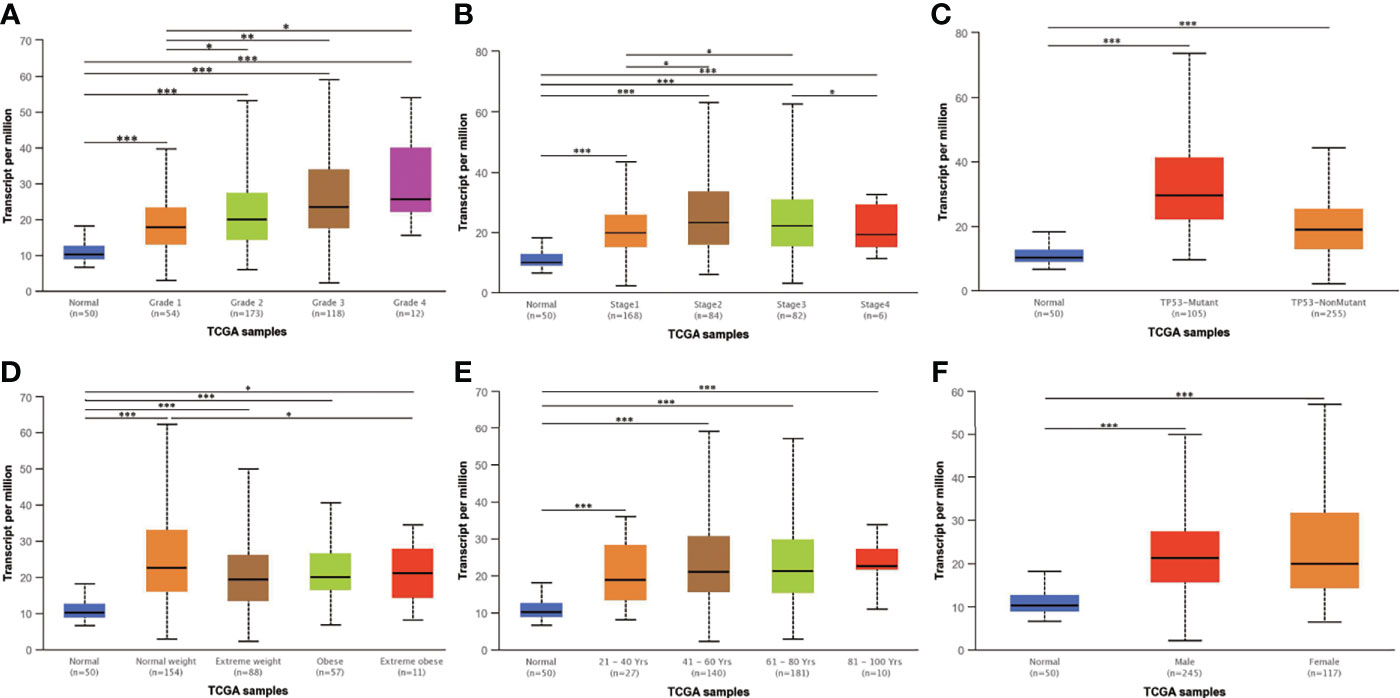
Figure 4 The expression of AGTRAP mRNA in the UALCAN database. AGTRAP expression was positively correlated with (A) tumor grade, (B) tumor stage, (C) TP53 mutation status, and (D) patient weight. No significant differences were found in (E) age and (F) gender. * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001.
The Correlation Between Clinical Features and AGTRAP Expression in HCC From TCGA Data
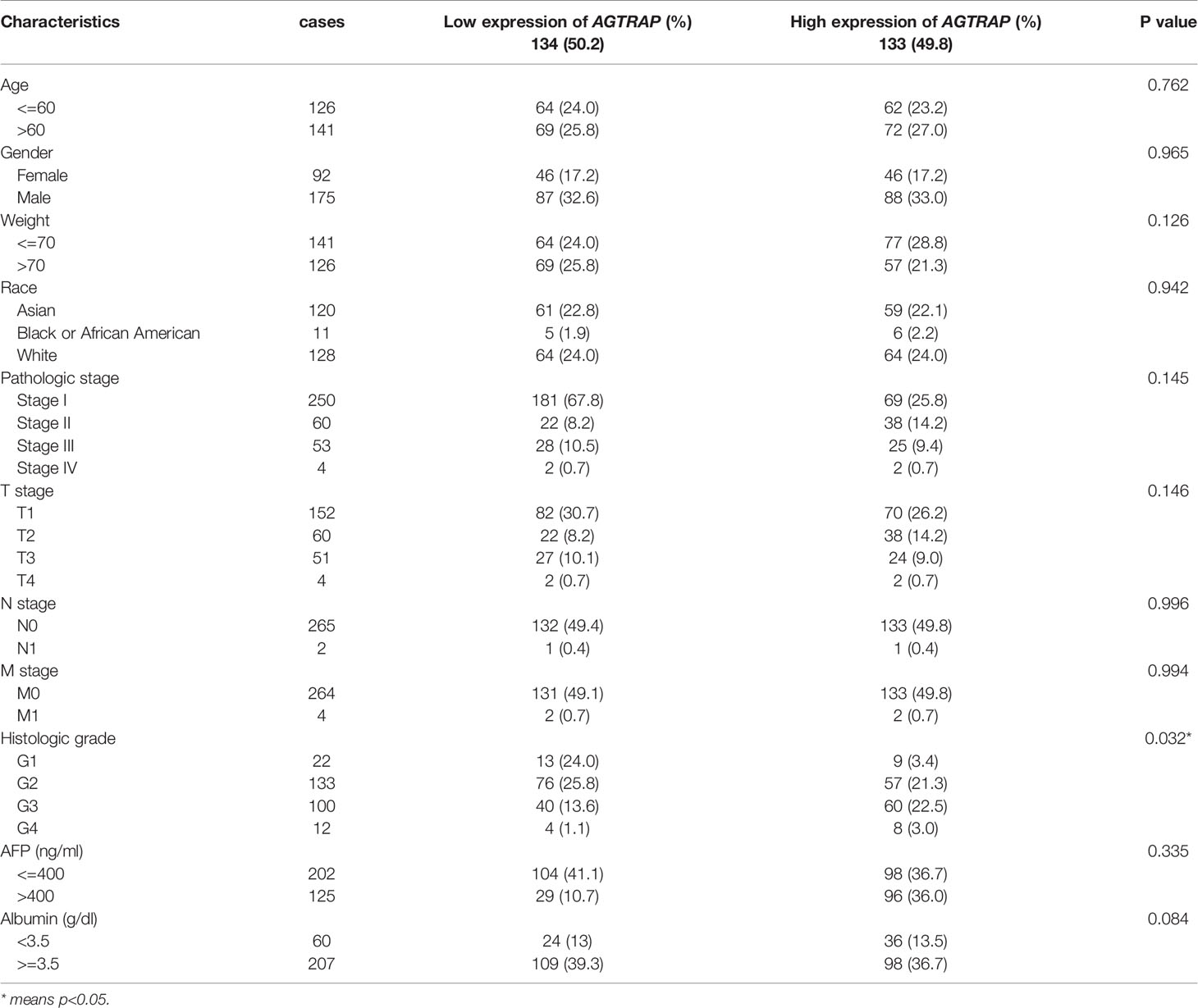
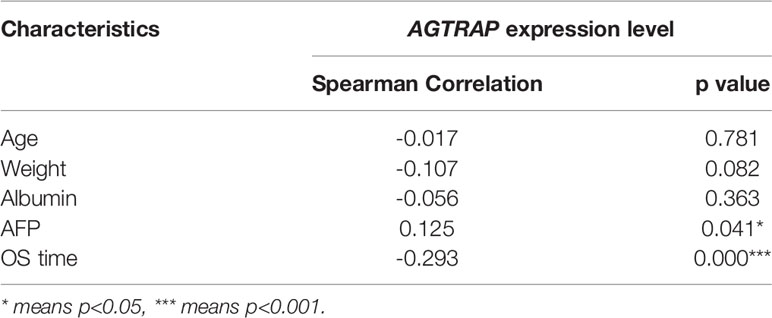
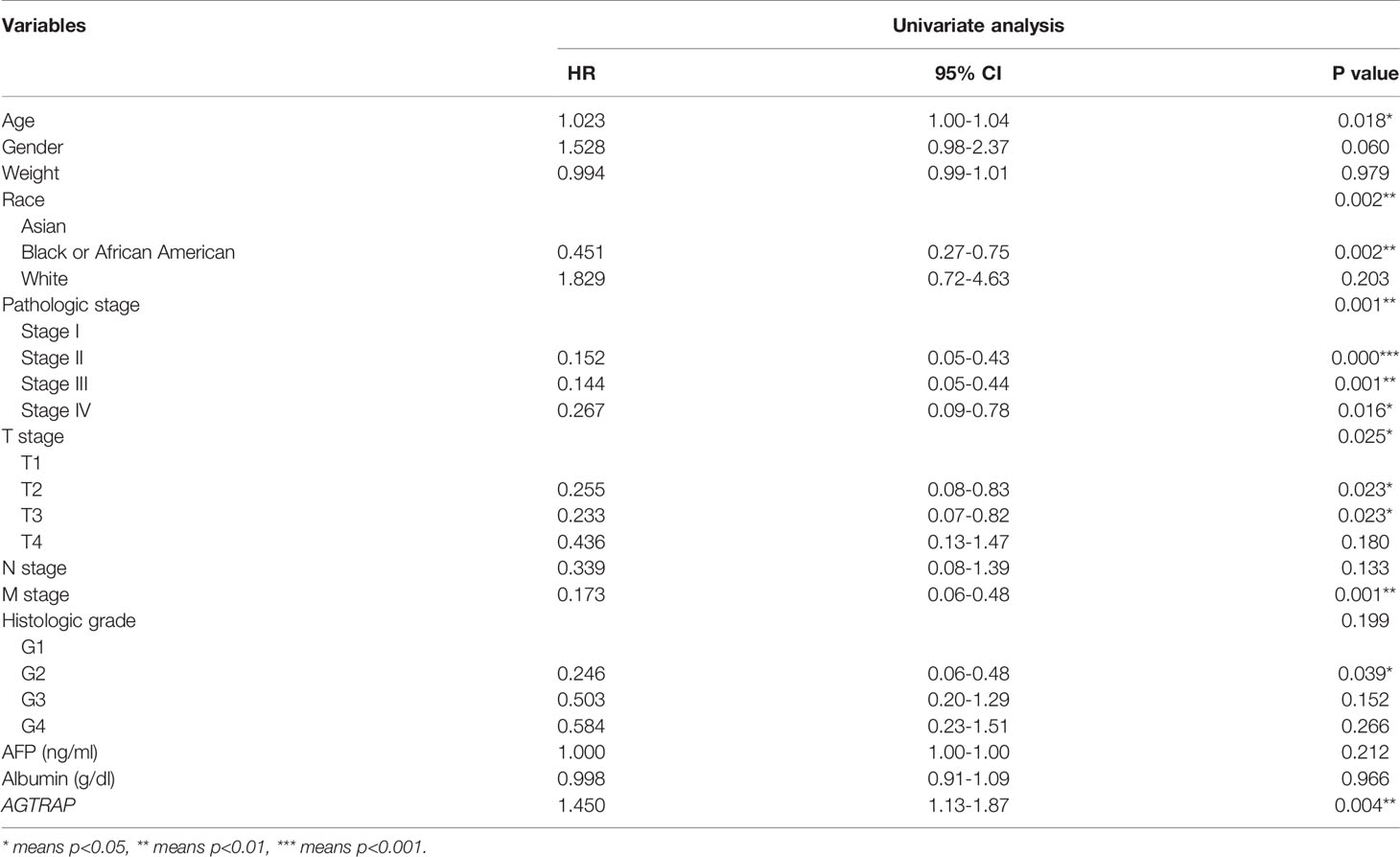
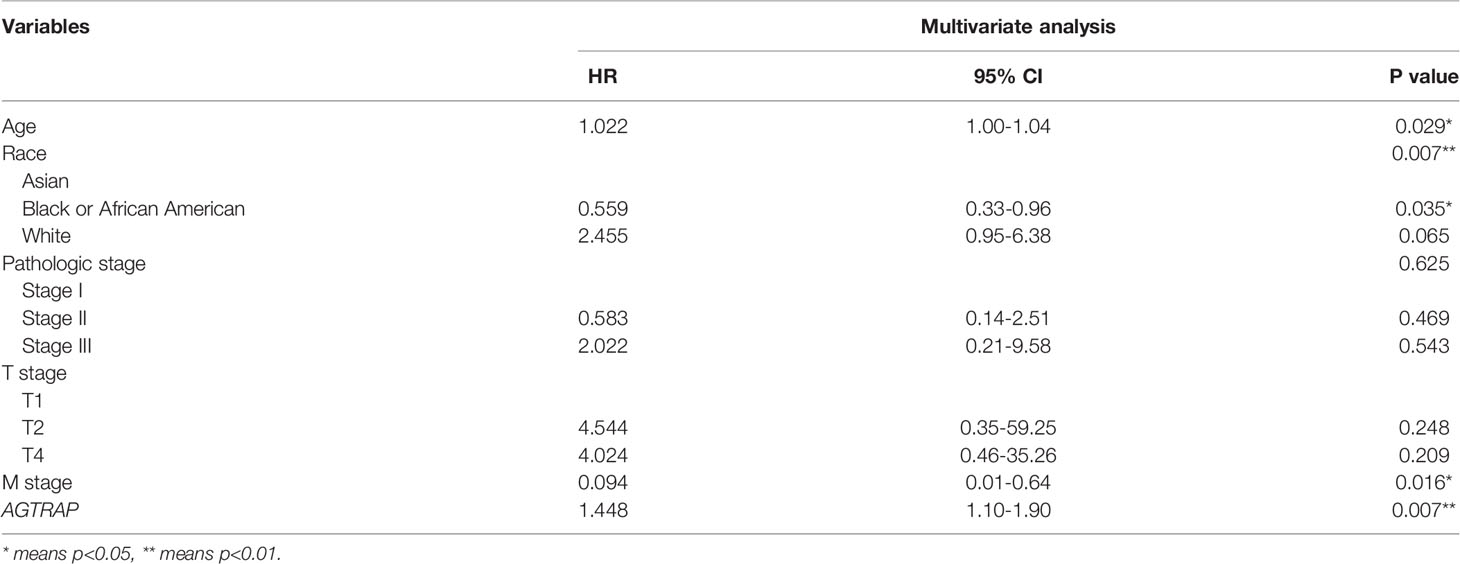
To further analyze the clinical relevance of AGTRAP expression in HCC, we used TCGA clinical parameter data related to AGTRAP expression and deleted cases with missing data. The data was divided into two patient groups based on the median AGTRAP expression level (high or low) and the differences in group clinical features were analyzed using a chi-square test (Table 1). We found that histological grade varied significantly between the low and high expression groups. Logistic regression analysis and Spearman correlation analysis were used to analyze the correlation between these clinical parameters and the expression level of AGTRAP (Tables 2, 3) and indicated a strong correlation between OS time and AGTRAP level. Univariate Cox regression analyses showed that age, race, pathologic stage, histologic grade, and T stage were related to OS. Finally, significant factors were analyzed by multivariate Cox regression which indicated that AGTRAP was an independent risk factor affecting the OS of patients with HCC (Tables 4, 5).
GO and KEGG Analyses of AGTRAP and Its Co-Expressed Genes in HCC
To better understand the biological role of AGTRAP, the LinkedOmics database was used to identify co-expressed genes that are both positively and negatively correlated with AGTRAP (Figure 5A). Heat maps were used to visualize the 50 genes with the strongest correlation to AGTRAP (Figures 5B, C). Next, using the HCCDB, we analyzed the co-expression networks of AGTRAP and found significant differences between HCC tissues and adjacent liver tissues (Figures 5D, E). In addition, we performed GO and KEGG enrichment analyses of the co-expressed genes in HCC tissues and adjacent liver tissues (24). The main biological processes these genes were involved in were neutrophil degranulation, neutrophil activation involved in immune response, and neutrophil activation. These genes were located in the primary lysosome, azurophil granule, and tertiary granule. Additionally, they also play crucial roles in cadherin binding and cell adhesion molecule binding. Lastly, KEGG pathway analyses indicated that these genes were mainly enriched in glycolysis/gluconeogenesis, bacterial invasion of epithelial cells, and endocytosis (Figure 5F).
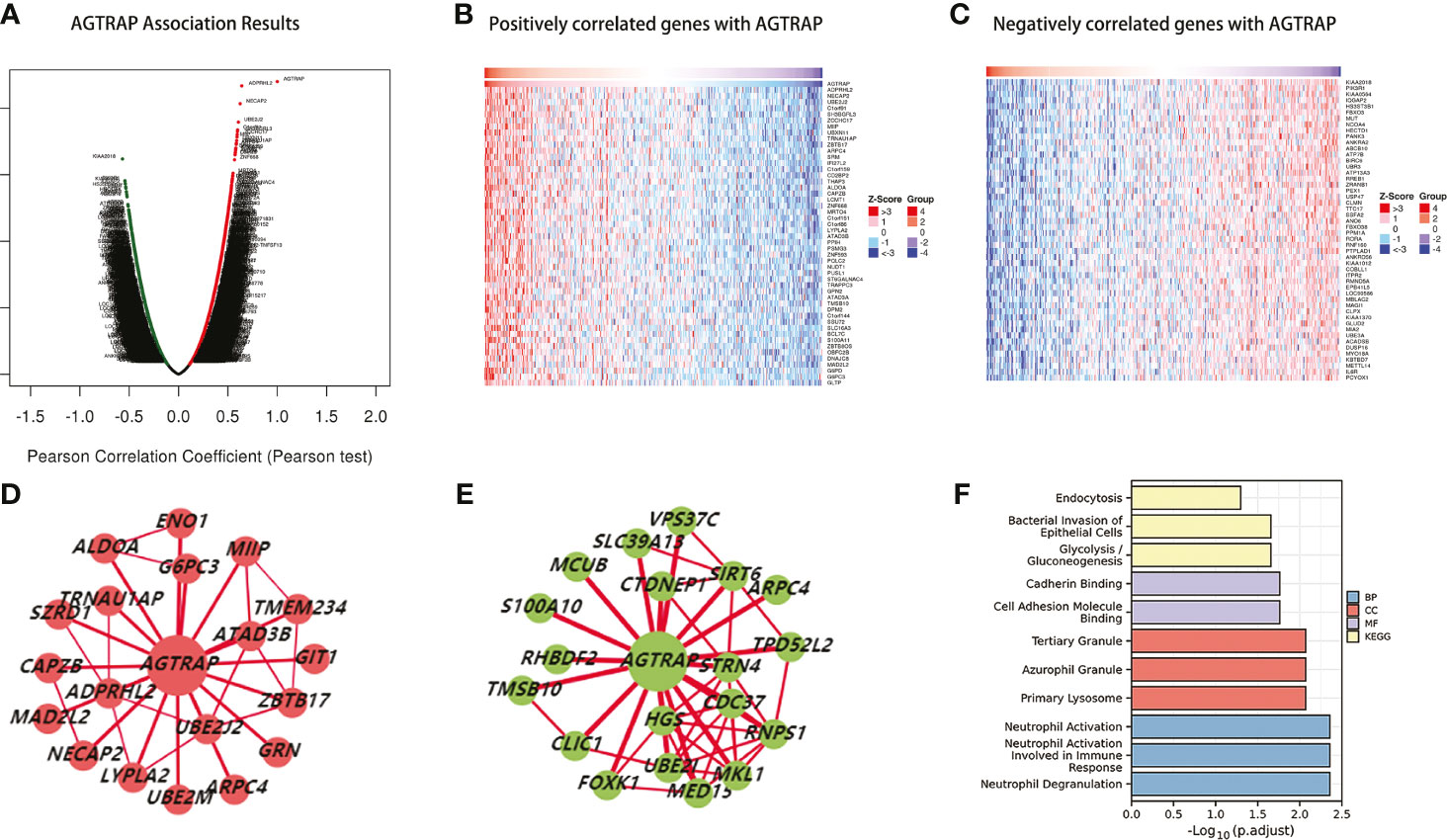
Figure 5 Genes in correlation with AGTRAP in HCC (LinkedOmics). (A) Genes correlated with AGTRAP showing in volcano plot; red (positive), green (negative). (B, C) Heat maps showing the top 50 genes positively and negatively correlated with AGTRAP in HCC. (D) The co-expression networks of AGTRAP in HCC tissues. (E) The co-expression networks of AGTRAP in adjacent liver tissues. (F) GO and KEGG enrichment analysis of genes co-expressed in HCC tissues and adjacent liver tissues.
GO and KEGG Analyses of AGTRAP and Its Neighboring Genes Associated With AGTRAP Alterations in HCC
Considering that genomic alterations of AGTRAP are pathogenic, the CBioPortal database was used to investigate genetic alterations of AGTRAP based on TCGA sequencing data. AGTRAP was altered in 28 of the 360 (8%) patients. From the results there was 1 patient with high AGTRAP mRNA expression (0.29%), 1 patient with amplification (0.29%), 6 patients with deep deletion (1.67%), 1 patient with missense mutation (0.29%), 17 patients with high mRNA mutation (4.72%), and only one patient with two alterations (0.29%) (Figure 6A).

Figure 6 Genetic alterations of AGTRAP (cBioPortal) and the GO and KEGG analyses of the neighboring genes associated with AGTRAP alterations in HCC. (A) The mutation rate of AGTRAP was 8%. The significantly enriched GO annotations: (B) biological processes, (C) cellular components, (D) molecular functions, and (E) KEGG pathways of AGTRAP neighboring genes.
To further analyze the biological function of the AGTRAP gene and its neighboring genes, we performed GO and KEGG analyses on the genes that were positively correlated with AGTRAP gene alteration. The results showed that these genes were mainly enriched in Salmonella infection, pathogenic Escherichia coli infection, regulation of actin cytoskeleton, tight junction, and Yersinia validated infection (Figures 6B–E).
Establishment of the PPI Network
To further understand the mechanisms of this gene, we used the STRING database to conduct a protein-protein interaction network analysis and found the strongest interactions with MAPK3, B-RAF, ARRB2, CLCN6, GNB2L1, and AKAP9 proteins (Figure 7A). We then performed enrichment analysis of the genes associated with these interacting proteins. The results showed that they were mainly enriched in parathyroid hormone synthesis, secretion and action, chemokine signaling pathway, and MAPK signaling pathway (Figure 7B).
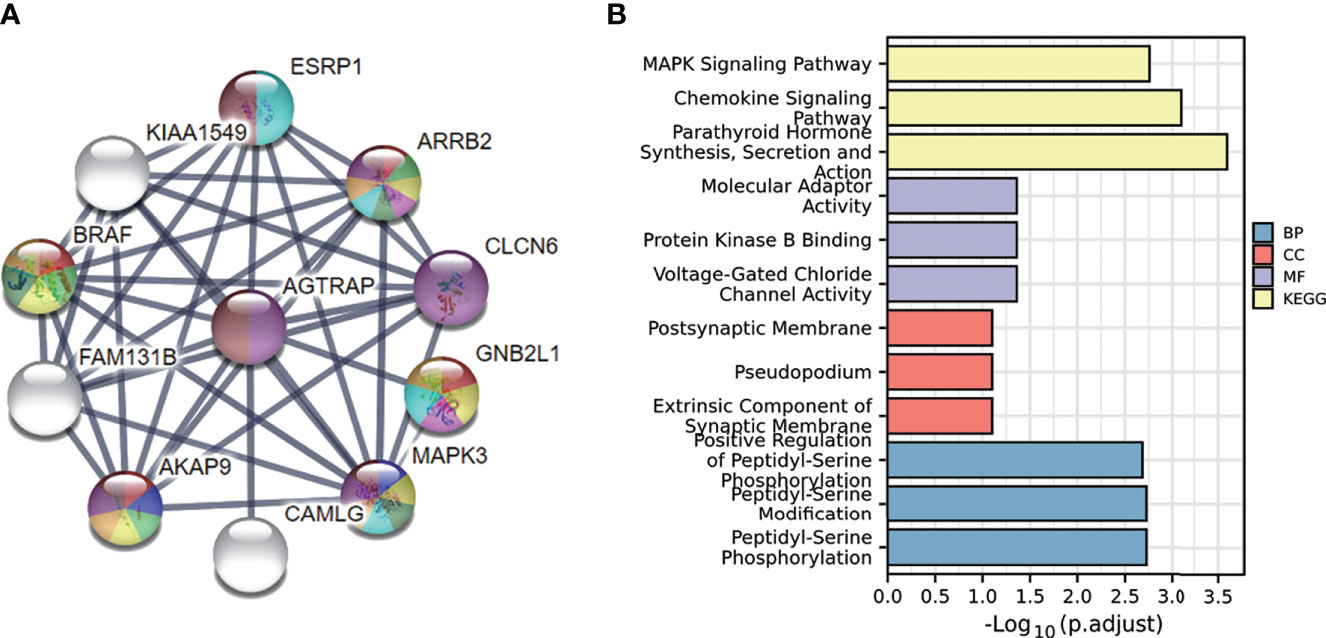
Figure 7 PPI of AGTRAP in HCC patients and the GO and KEGG analyses of these genes in HCC. (A) PPI of AGTRAP and other interacting proteins. (B) GO and KEGG enrichment analyses of genes interacted with AGTRAP in the String database.
AGTRAP Overexpression Is Associated With Immune Infiltration and T-Cell Exhaustion
As shown in the HPA database, AGTRAP was highly expressed in Kupffer cells and T cells. We suggested that this was related to immune infiltration and T-cell exhaustion. The relationship between AGTRAP level and immune infiltration and T-cell exhaustion in HCC was explored using the TIMER online tool based on TCGA data. AGTRAP expression was positively correlated with the infiltration of B cells (correlation = 0.225, p = 2.43e-05), CD8+ T cells (correlation = 0.321, p = 1.29e-09), CD4+ T cells (correlation = 0.292, p = 3.52e-08), macrophages (correlation = 0.417, p = 8.18e-16), neutrophils (correlation = 0.345, p = 4.22e-11), and dendrites (correlation = 0.406, p = 6.14e-15) (Figure 8A). It also correlated with the 3-year (p = 0.007) and 5-year survival rates (p = 0.001) (Figures 8B, C).
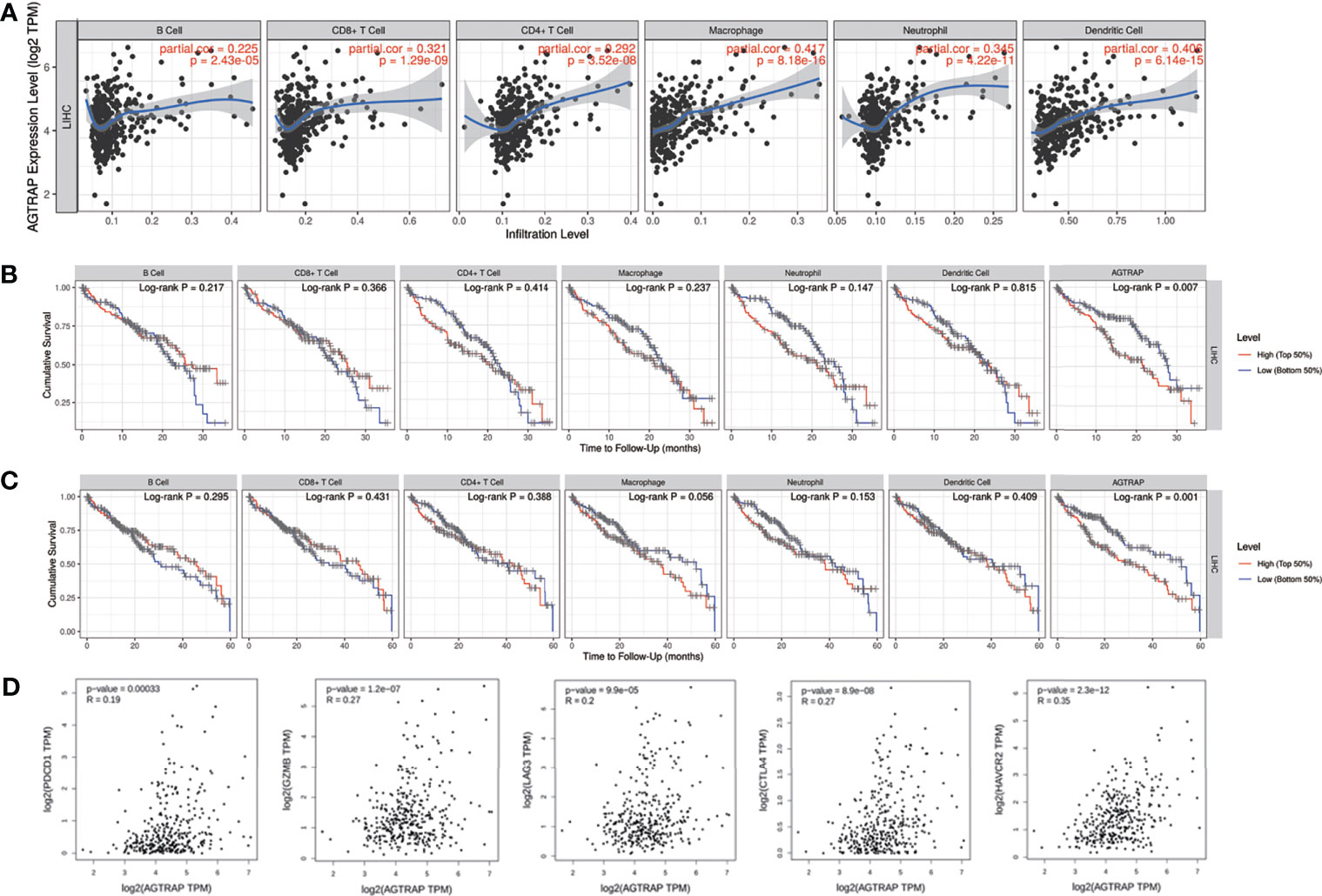
Figure 8 Association between AGTRAP and immune infiltration and T cell exhaustion in HCC. (A) AGTRAP is correlated with immune infiltrations in HCC. (B, C) Analysis of 3-year and 5-year survival of immune infiltration and AGTRAP expression in patients with HCC. (D) The correlation between AGTRAP expression and T cell exhaustion in HCC analyzed by the GEPIA database.
Moreover, the correlations between AGTRAP and T-cell exhaustion markers, such as PD-1 (PDCD1), GZMB, LAG-3, CTLA-4, and HAVCR2 (TIM-3) in HCC tissues were explored using the GEPIA database. The results demonstrated a positive relationship between AGTRAP expression and the five biomarkers of T-cell exhaustion (Figure 8D, all p < 0.001).
Expression of AGTRAP Protein in HCC Tumor Tissues and Adjacent Tissues From Clinical Samples
We assessed the expression of AGTRAP protein in HCC tumor tissues (T) and paired adjacent tissues (A). The protein expression of AGTRAP was higher in tumor tissues than in adjacent tissues (Figure 9A) and was mainly localized in cell plasma. Moreover, the heat map showed that AGTRAP expression was higher in HCC tumor tissues than in adjacent tissues (p < 0.001) (Figure 9B). Western blot were also performed to validate the different expression of AGTRAP between adjacent tissues and tumor tissues in eight couple of paired samples, and the results (Figures 9C, D) showed that AGTRAP has a higher expression in tumor tissues than that in adjacent tissues (p < 0.001), which is consistent with our bioinformatic analysis and IHC results.
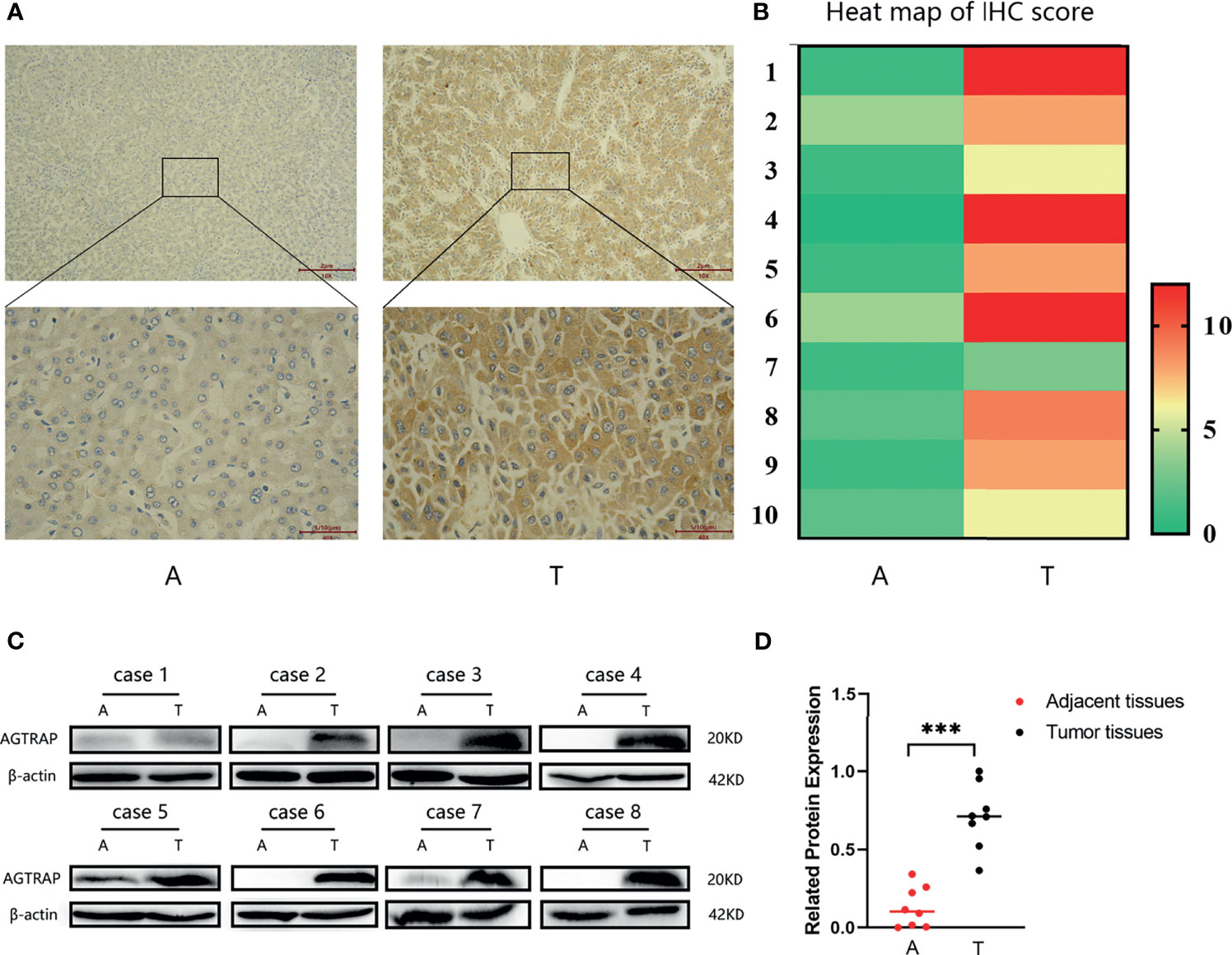
Figure 9 The protein expression of AGTRAP from HCC patients. (A) AGTRAP expression in HCC adjacent tissues and tumor tissues tested by IHC. (B) The heatmap of IHC score. (C) AGTRAP protein level was increased in HCC tumor tissues compared with paired adjacent tissues as measured by western blot. (D) A semi-quantitative analysis of expression differences between adjacent tissues and tumor tissues. (***p < 0.001, paired samples nonparametric test). A, adjacent tissues; T, tumor tissues.
Discussion
HCC continues to cause a serious public health burden worldwide. Most patients with HCC are diagnosed at an advanced stage when the tumor is unresectable (25). A need exists for the development of reliable biomarkers that would allow for early diagnosis of HCC and the accurate prognosis of long-term outcomes. In this study, we identified the diagnostic and prognostic value of AGTRAP in HCC, the mechanisms that might affect the development of HCC, and its association with immune infiltration and T-cell exhaustion.
A growing amount of research suggests that AGTRAP may play an important role in tumor progression (7, 26). Sanz-Pamplona et al. (7) performed an exome sequence of 42 cases of colon tumors and their paired mucosa and discovered that AGTRAP was overexpressed in colon cancer with poor prognosis. Zeng et al. (26) used weighted gene co-expression network analysis (WGCNA) and found that AGTRAP was overexpressed and was related to poor prognosis in tongue squamous cell carcinoma. Palanisamy et al. (27), through paired-end transcriptome sequencing, detected AGTRAP-BRAF gene fusion in gastric cancer. In concurrence with these studies, we found that mRNA and protein levels of AGTRAP were higher in HCC tissues compared to normal tissues, and our results were verified using clinical samples. In addition, our results showed that high expression of AGTRAP mRNA was associated with poor OS. Furthermore, the ROC curve indicated that AGTRAP is a sensitive diagnostic marker. Xiong et al. (28) identified AGTRAP as one of the 10 most notable prognosis genes in lower-grade glioma by WGCNA and survival analysis. Given these results, AGTRAP likely plays an important role in the occurrence and development of HCC.
Next, to better understand whether AGTRAP could affect the progression of HCC, we explored the association between AGTRAP and clinicopathological parameters in HCC. Consistent with our expectations, we found that patients with higher tumor grades had higher AGTRAP levels, accompanied by worse survival outcomes. However, this result was based on a small sample size of patients in stage T4 (n = 6). Additionally, our results demonstrated that the level of AGTRAP was significantly correlated with patient weight, especially BMI ≥ 30 kg/m2, suggesting that obese patients may have a higher risk of HCC. Indeed, obesity has already been associated with several types of cancer (29). Additionally, our results showed that patients with TP 53 mutation also displayed higher AGTRAP levels in HCC. Unexpectedly, there was no statistical difference in lymph node metastasis. This could be attributed to the small number of cases and therefore increased sample size would benefit future research. Although current knowledge suggests that AGTRAP plays an important role in tumor occurrence and development, more research is required to elucidate its effects on tumors.
Genetic alterations and dysregulated amplification are critical for the development of many tumors (30). There was an 8% genetic alteration rate of AGTRAP in HCC patients in the cBioPortal database, and its related genes were mainly involved in ubiquitin-like protein binding and the regulation of the actin cytoskeleton. Protein ubiquitination, which is a type of post-translational protein modification, has been shown to be widely involved in the invasion and metastasis of malignant tumors and that multiple ubiquitin-activating enzymes regulate the expression of key factors in various tumor metastasis-related pathways (31–33). However, changes in the cytoskeleton could affect the activity of NF-κB, which is known to affect the immune response and carcinogenic response (34, 35). Interestingly, our PPI results showed that BRAF and MAPK3 interact with AGTRAP, suggesting that AGTRAP may be related to the MAPK signaling pathway. This is also in accordance with AGTRAP-BRAF gene fusion detected in gastric cancer (8).
Immune infiltration is known to correlate with the prognosis of numerous cancers (36). Our KEGG analysis of genes co-expressed with AGTRAP in HCC tissues and adjacent tissues also showed that its co-expressed genes were mainly enriched in neutrophil degranulation and neutrophil activation. Our analyses, based on datasets from multiple recognized databases, found a positive correlation between AGTRAP expression and all immune cells as well as T-cell exhaustion in HCC patients based on TCGA data. Similarly, Xiong et al. (28) found that AGTRAP displayed potential as an immunotherapy target in brain lower-grade glioma because of its association with different immune infiltrating cells. Here, we found that AGTRAP might affect the tumor microenvironment by regulating immune cells, especially neutrophils and T cells, and may serve as an immunotherapy target in HCC.
Based on our bioinformatics analysis, it suggested that AGTRAP could be involved in the immune microenvironment of hepatocellular carcinoma, and related to MAPK signaling pathway. Many studies have implicated the MAPK pathway in both the development and progression of HCC and indicated that activation of the MAPK pathway correlates with a poor prognosis in human HCC (37, 38). In addition, Liao et al. (38)reported that CXCL8 secreted by HCC cells makes a tumorigenic inflammatory microenvironment to promote epithelial-mesenchymal transition and HCC invasion by MAPK pathway. Better understanding the immune subtypes of HCC could guide personalized immunotherapy and clinical management of HCC (39). And a recent study published in the journal of GUT has been identified that administration of p38 MAPK inhibitor could restrain monocytic myeloid-derived suppressor cell to suppress the proliferation of HCC, indicating that MAPK signaling pathway was closely related to immune microenvironment of HCC (40). Therefore, we speculate that AGTRAP may regulate immune microenvironment of hepatocellular carcinoma through MAPK signaling pathway. Besides, MAPK signaling pathway was known to affect the immune microenvironment of tumor by affecting macrophage polarization (41, 42). Yang et al. (43) illustrated that compound kushen injection activates macrophages form M2 to M1 via triggering TNFR1 and its downstream MAPK p38 signaling cascades, which induces HCC cells apoptosis and suppress HCC tumor growth and recurrence. Furtherly, we also design further mechanism research to verify whether AGTRAP could be involved in immune microenvironment of hepatocellular carcinoma by regulating MAPK signal pathway-induced macrophage polarization form M2 to M1.
In this study, we described the preliminary expression and possible mechanisms of action of AGTRAP in HCC. Further studies on the molecular mechanism of AGTRAP in HCC would be designed both in vivo and in vitro, as well as verified in a large number of clinical samples.
Conclusion
The mRNA and protein levels of AGTRAP in HCC tissues were higher than those in normal liver tissues or adjacent tissues, and higher mRNA levels of AGTRAP were associated with higher histological grade and poor overall survival (OS) time in HCC patients. AGTRAP is involved in HCC, probably through the NF-κB and MAPK signaling pathways. Furthermore, AGTRAP positively correlated with immune infiltration and T-cell exhaustion. According to these results, AGTRAP could serve as a potential biomarker for prognostic, diagnostic, and even immunotherapy target for HCC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SL and NJ conceived and designed this study. SL and WZ collected and analyzed the relative data. XL collected the clinical samples. LZ and YG performed the IHC experiments. SL wrote the paper. QP and CD revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by National Science Foundation of China (No. 97081252) and Science and Technology Research Foundation of Chongqing Municipal Education Commission (No. KJQN201900425).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the LinkedOmics, cBioPortal, TIMER, GEPIA, HPA, HCCDB, CCLE, TCGA databases, etc. for the availability of the data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.713017/full#supplementary-material
Supplementary Figure 1 | (A) The mRNA expression level of AGTRAP was significantly higher in HCC tissues than in normal tissues. (B) The expression level of AGTRAP was positively correlated with nodal metastasis in HCC patients.
References
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat Rev Gastroenterol Hepatol (2019) 16(10):589–604. doi: 10.1038/s41575-019-0186-y
2. Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin (2013) 63(1):11–30. doi: 10.3322/caac.21166
3. Forner A, Reig M, Bruix J. Hepatocellular Carcinoma. Lancet (2018) 391(10127):1301–14. doi: 10.1016/S0140-6736(18)30010-2
4. Li R, Faden DL, Fakhry C, Langelier C, Jiao Y, Wang Y, et al. Clinical, Genomic, and Metagenomic Characterization of Oral Tongue Squamous Cell Carcinoma in Patients Who Do Not Smoke. Head Neck (2015) 37(11):1642–9. doi: 10.1002/hed.23807
5. Wakui H, Dejima T, Tamura K, Uneda K, Azuma K, Maeda A, et al. Activation of Angiotensin II Type 1 Receptor-Associated Protein Exerts an Inhibitory Effect on Vascular Hypertrophy and Oxidative Stress in Angiotensin II-Mediated Hypertension. Cardiovasc Res (2013) 100(3):511–9. doi: 10.1093/cvr/cvt225
6. Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, et al. Angiotensin II Type 1 Receptor Antibodies and Increased Angiotensin II Sensitivity in Pregnant Rats. Hypertension (2011) 58(1):77–84. doi: 10.1161/HYPERTENSIONAHA.111.171348
7. Sanz-Pamplona R, Gil-Hoyos R, López-Doriga A, Alonso MH, Aussó S, Molleví DG, et al. Mutanome and Expression of Immune Response Genes in Microsatellite Stable Colon Cancer. Oncotarget (2016) 7(14):17711–25. doi: 10.18632/oncotarget.7293
8. Figueiredo C, Camargo MC, Leite M, Fuentes-Pananá EM, Rabkin CS, Machado JC. Pathogenesis of Gastric Cancer: Genetics and Molecular Classification. Curr Top Microbiol Immunol (2017) 400:277–304. doi: 10.1007/978-3-319-50520-6_12
9. Hou L, Zhang X, Jiao Y, Li Y, Zhao Y, Guan Y, et al. ATP Binding Cassette Subfamily B Member 9 (ABCB9) Is a Prognostic Indicator of Overall Survival in Ovarian Cancer. Med (Baltimore) (2019) 98(19):e15698. doi: 10.1097/MD.0000000000015698
10. Jiao Y, Fu Z, Li Y, Meng L, Liu Y. High EIF2B5 mRNA Expression and Its Prognostic Significance in Liver Cancer: A Study Based on the TCGA and GEO Database. Cancer Manag Res (2018) 10:6003–14. doi: 10.2147/CMAR.S185459
11. Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, et al. Toil Enables Reproducible, Open Source, Big Biomedical Data Analyses. Nat Biotechnol (2017) 35(4):314–6. doi: 10.1038/nbt.3772
12. Shaul YD, Yuan B, Thiru P, Nutter-Upham A, McCallum S, Lanzkron C, et al. MERAV: A Tool for Comparing Gene Expression Across Human Tissues and Cell Types. Nucleic Acids Res (2016) 44(D1):D560–6. doi: 10.1093/nar/gkv1337
13. Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER 3rd, et al. Next-Generation Characterization of the Cancer Cell Line Encyclopedia. Nature (2019) 569(7757):503–8. doi: 10.1038/s41586-019-1186-3
14. Lian Q, Wang S, Zhang G, Wang D, Luo G, Tang J, et al. HCCDB: A Database of Hepatocellular Carcinoma Expression Atlas. Genomics Proteomics Bioinf (2018) 16(4):269–75. doi: 10.1016/j.gpb.2018.07.003
15. Asplund A, Edqvist PH, Schwenk JM, Pontén F. Antibodies for Profiling the Human Proteome-The Human Protein Atlas as a Resource for Cancer Research. Proteomics (2012) 12(13):2067–77. doi: 10.1002/pmic.201100504
16. Nagy Á, Munkácsy G, Győrffy B. Pancancer Survival Analysis of Cancer Hallmark Genes. Sci Rep (2021) 11(1):6047. doi: 10.1038/s41598-021-84787-5
17. Sighoko D, Curado MP, Bourgeois D, Mendy M, Hainaut P, Bah E. Increase in Female Liver Cancer in the Gambia, West Africa: Evidence From 19 Years of Population-Based Cancer Registration (1988-2006). PloS One (2011) 6(4):e18415. doi: 10.1371/journal.pone.0018415
18. Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: Analyzing Multi-Omics Data Within and Across 32 Cancer Types. Nucleic Acids Res (2018) 46(D1):D956–63. doi: 10.1093/nar/gkx1090
19. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The Cbio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov (2012) 2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095
20. Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING V11: Protein-Protein Association Networks With Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res (2019) 47(D1):D607–13. doi: 10.1093/nar/gky1131
21. Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res (2017) 77(21):e108–10. doi: 10.1158/0008-5472.CAN-17-0307
22. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res (2017) 45(W1):W98–102. doi: 10.1093/nar/gkx247
23. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinf (2011) 12:77. doi: 10.1186/1471-2105-12-77
24. Yu G, Wang LG, Han Y, He QY. Clusterprofiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics (2012) 16(5):284–7. doi: 10.1089/omi.2011.0118
25. Llovet JM, Montal R, Sia D, Finn RS. Molecular Therapies and Precision Medicine for Hepatocellular Carcinoma. Nat Rev Clin Oncol (2018) 15(10):599–616. doi: 10.1038/s41571-018-0073-4
26. Zeng H, Li H, Zhao Y, Chen L, Ma X. Transcripto-Based Network Analysis Reveals a Model of Gene Activation in Tongue Squamous Cell Carcinomas. Head Neck (2019) 41(12):4098–110. doi: 10.1002/hed.25952
27. Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, et al. Rearrangements of the RAF Kinase Pathway in Prostate Cancer, Gastric Cancer and Melanoma. Nat Med (2010) 16(7):793–8. doi: 10.1038/nm.2166
28. Xiong Z, Xiong Y, Liu H, Li C, Li X. Identification of Purity and Prognosis-Related Gene Signature by Network Analysis and Survival Analysis in Brain Lower Grade Glioma. J Cell Mol Med (2020) 24(19):11607–12. doi: 10.1111/jcmm.15805
29. Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and Cancer: Local and Systemic Mechanisms. Annu Rev Med (2015) 66:297–309. doi: 10.1146/annurev-med-050913-022228
30. Wei F, Lin CC, Joon A, Feng Z, Troche G, Lira ME, et al. Noninvasive Saliva-Based EGFR Gene Mutation Detection in Patients With Lung Cancer. Am J Respir Crit Care Med (2014) 190(10):1117–26. doi: 10.1164/rccm.201406-1003OC
31. Zhu R, Liu Y, Zhou H, Li L, Li Y, Ding F, et al. Deubiquitinating Enzyme PSMD14 Promotes Tumor Metastasis Through Stabilizing SNAIL in Human Esophageal Squamous Cell Carcinoma. Cancer Lett (2018) 418:125–34. doi: 10.1016/j.canlet.2018.01.025
32. Duhamel S, Goyette MA, Thibault MP, Filion D, Gaboury L, Côté JF. The E3 Ubiquitin Ligase HectD1 Suppresses EMT and Metastasis by Targeting the +TIP ACF7 for Degradation. Cell Rep (2018) 22(4):1016–30. doi: 10.1016/j.celrep.2017.12.096
33. Shen T, Cai LD, Liu YH, Li S, Gan WJ, Li XM, et al. Ube2v1-Mediated Ubiquitination and Degradation of Sirt1 Promotes Metastasis of Colorectal Cancer by Epigenetically Suppressing Autophagy. J Hematol Oncol (2018) 11(1):95. doi: 10.1186/s13045-018-0638-9
34. Kim JY, Lee YG, Kim MY, Byeon SE, Rhee MH, Park J, et al. Src-Mediated Regulation of Inflammatory Responses by Actin Polymerization. Biochem Pharmacol (2010) 79(3):431–43. doi: 10.1016/j.bcp.2009.09.016
35. Vallabhapurapu S, Karin M. Regulation and Function of NF-kappaB Transcription Factors in the Immune System. Annu Rev Immunol (2009) 27:693–733. doi: 10.1146/annurev.immunol.021908.132641
36. Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-Infiltrating Lymphocyte Grade Is an Independent Predictor of Sentinel Lymph Node Status and Survival in Patients With Cutaneous Melanoma. J Clin Oncol (2012) 30(21):2678–83. doi: 10.1200/JCO.2011.37.8539
37. Liao X, Bu Y, Jiang S, Chang F, Jia F, Xiao X, et al. CCN2-MAPK-Id-1 Loop Feedback Amplification Is Involved in Maintaining Stemness in Oxaliplatin-Resistant Hepatocellular Carcinoma. Hepatol Int (2019) 13(4):440–53. doi: 10.1007/s12072-019-09960-5
38. Huang M, Wang H, Hu X, Cao X. lncRNA MALAT1 Binds Chromatin Remodeling Subunit BRG1 to Epigenetically Promote Inflammation-Related Hepatocellular Carcinoma Progression. Oncoimmunology (2019) 8(1):e1518628. doi: 10.1080/2162402X.2018.1518628
39. Liu Z, Zhang Y, Shi C, Zhou X, Xu K, Jiao D, et al. A Novel Immune Classification Reveals Distinct Immune Escape Mechanism and Genomic Alterations: Implications for Immunotherapy in Hepatocellular Carcinoma. J Transl Med (2021) 19(1):5. doi: 10.1186/s12967-020-02697-y
40. Liu M, Zhou J, Liu X, Feng Y, Yang W, Wu F, et al. Targeting Monocyte-Intrinsic Enhancer Reprogramming Improves Immunotherapy Efficacy in Hepatocellular Carcinoma. Gut (2020) 69(2):365–79. doi: 10.1136/gutjnl-2018-317257
41. He S, Hu Q, Xu X, Niu Y, Chen Y, Lu Y, et al. Advanced Glycation End Products Enhance M1 Macrophage Polarization by Activating the MAPK Pathway. Biochem Biophys Res Commun (2020) 525(2):334–40. doi: 10.1016/j.bbrc.2020.02.053
42. Mendaza S, Fernández-Irigoyen J, Santamaría E, Zudaire T, Guarch R, Guerrero-Setas D, et al. Absence of Nuclear P16 Is a Diagnostic and Independent Prognostic Biomarker in Squamous Cell Carcinoma of the Cervix. Int J Mol Sci (2020) 21(6):2125. doi: 10.3390/ijms21062125
Keywords: hepatocellular carcinoma, AGTRAP, prognosis, immune infiltration, biomarker
Citation: Liu S, Zhao W, Li X, Zhang L, Gao Y, Peng Q, Du C and Jiang N (2021) AGTRAP Is a Prognostic Biomarker Correlated With Immune Infiltration in Hepatocellular Carcinoma. Front. Oncol. 11:713017. doi: 10.3389/fonc.2021.713017
Received: 21 May 2021; Accepted: 30 August 2021;
Published: 14 September 2021.
Edited by:
Weijia Liao, Affiliated Hospital of Guilin Medical University, ChinaCopyright © 2021 Liu, Zhao, Li, Zhang, Gao, Peng, Du and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengyou Du, ZHVjaGVuZ3lvdUAxMjYuY29t; Ning Jiang, amlhbmduaW5nQGNxbXUuZWR1LmNu
 Shanshan Liu
Shanshan Liu Wei Zhao2
Wei Zhao2 Qiling Peng
Qiling Peng Chengyou Du
Chengyou Du Ning Jiang
Ning Jiang