- 1Department of Ultrasound Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Biomedicine, Key Laboratory of Pulsed Power Translational Medicine of Zhejiang Province, Hangzhou, China
Emerging studies have showed irreversible electroporation (IRE) focused on pancreatic cancer (PC). However, the effects of IRE treatment on the immune response of PC remain unknown. Moreover, there are few studies on the therapeutic effect of IRE combining with immunotherapy on PC. Thus, we review recent advances in our understanding of IRE alone and its working with immunotherapy towards the immune response of PC, discussing potential opportunities for exploring future treatment strategies.
Introduction
Pancreatic cancer (PC) is one of the most lethal diseases, which has a rapid progression and a poor prognosis. The 5-year overall survival rate for PC patients is nearly 3% (1). About 80% of patients lose the chance of surgery due to tumor metastasis or local progression when PC is diagnosed (2). The adjuvant treatment of pancreatic cancer mainly includes chemoradiotherapy and local ablation. Chemoradiotherapy as a local treatment option for PC has many side effects. The traditional local ablation including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation have significant curative effects in the solid organ tumors such as liver, kidney, and breast. However, due to the thermal damage, heat sink effects, and high risky region, their application in the treatment of pancreatic cancer is limited (3). Irreversible electroporation (IRE) is a newly developed non-thermal ablation technique for the treatment of tumors, which could induce tumor cell death along with permanent membrane lysis or loss of homeostasis by generating an extremely high electric field across cells (4–6). In particular, it has advantages for lesions located in large vessels, the hilar region, bile duct, and ureter (7). Our center previously successfully treated the lesions located in the liver (8), portal vein tumor thrombus (9), and retroperitoneum (10). The follow-up of the patient after IRE showed that the lesions were completely ablated. In addition, other center studies showed that after IRE treatment for the lesions located in the liver (11, 12), pancreas (13, 14), kidney (15, 16), and prostate (17, 18), the results show that the treatment was effective. The advantage of IRE over traditional ablation is to achieve complete ablation while reducing damage of the surrounding vessel, duct system, and peripheral nerves.
In recent years, emerging studies have evaluated immunomodulatory effect of IRE alone and its working with immunotherapy in PC. We searched PubMed, EMBASE, Web of Science, Scopus, Chinese National Knowledge Infrastructure (CNKI), Wanfang data, EBSCO, and Cochrane Library up to April 2021 for eligible studies using wide search terms: pulsed electric field, irreversible electroporation, IRE, nanoknife, nanosecond, nano-pulse, and pancreas. The screened publications were appraised by two individuals. Other literature was assessed from references in review papers. It seemed that IRE may be an important approach inducing the inflammatory immune response and host defense mechanisms. However, there are few studies on the relationship between IRE and PC immunity, and it has not been fully elucidated (19–21). Therefore, the present study aims to explore the molecular mechanisms of IRE in PC, focusing on the illustrating immunomodulatory effect of IRE alone and its cooperating with immunotherapy in PC.
Signaling Pathway
Studies of IRE against PC have brought a greater understanding of their immunological and molecular mechanisms. Following IRE for PC, signaling downstream of epithelial growth factor receptor (EGFR) and K-RAS was decreased. AKT (22), Janus kinase (JAK), nuclear factor kappa B (NF-κB), vascular endothelial growth factor (VEGF), and signal transducers and activators of transcription 1/3 (STAT1/3) signaling downstream of EGFR, and mitogen-activated protein kinase kinase 1/2 (MEK1/2), c-Jun N-terminal kinase (JNK), and extracellular regulated protein kinases (ERK1/2) signaling downstream of K-RAS, were significantly decreased (23). Interestingly, in the rabbit VX-2 breast cancer model, it was found that IRE enhanced the antitumor immune response by reducing the plasma levels of soluble interleukin-2 receptor (sIL-2R) and transforming growth factor-β1 (TGF-β1) (24). Although TGF-β signaling was upstream of many vital signaling pathways in pancreatic cancer, there was no significant impact of IRE treatment on TGF-β signaling (23). Previous study showed that nanosecond pulsed electric field (nsPEF) could reduce antiapoptosis B-cell lymphocyte/leukemia-2 (Bcl-2) family proteins expressions [phosphorylated Bcl-2 protein (p-Bcl-2), Bcl-xL, and myeloid leukemia-1 (Mcl-1)] and increase proapoptosis Bcl-2 family proteins expressions (Bax, Bim, and BID). NsPEF caused apoptosis of human pancreatic carcinoma cell line (PANC-1) cells through the mitochondria intrinsic apoptosis pathway, which was induced by proportion disorder of anti- or proapoptosis Bcl-2 family proteins on the mitochondrial membrane (25). A study reported that nsPEF in PANC-1 cells might reduce NF-κB pathway proteins [inhibitor of kappaB kinase- alpha (IKK-α), IKK-β, inhibitor of NF-κB alpha (IκB-α), NF-κB p-65 and phosphorylated p65 (p-p65)] and cyclin proteins (cyclin D1 and cyclin A) expressions. It suggested that nsPEF restrained cell proliferation by restricting NF-κB signaling pathway for downregulating cyclin proteins expressions and inhibiting phase G1 of cell cycle (25). A study found that nsPEF in PANC-1 cells could reduce Wnt/β-catenin signaling pathway proteins [human Dapper1 (hDpr1), β-Catenin and c-Myc] and VEGF and matrix metalloproteinases (MMPs) family proteins (MMP1, MMP2, MMP9, MMP11, MMP12, MMP14, and MMP21) expressions at different degrees with different intensities of nsPEF. It indicated that nsPEF may inhibit invasion and metastasis of tumor cells by restricting Wnt/β-catenin signaling pathway to reduce expressions of VEGF and MMPs family proteins (25). Thus, the effect of IRE on KRAS, EGFR, mitochondria intrinsic apoptosis, NF-κB, and Wnt/β-catenin signaling may provide important treatment strategies. These pathways are often dysregulated in PC patients.
The Biological Activity of IRE Alone Against PC
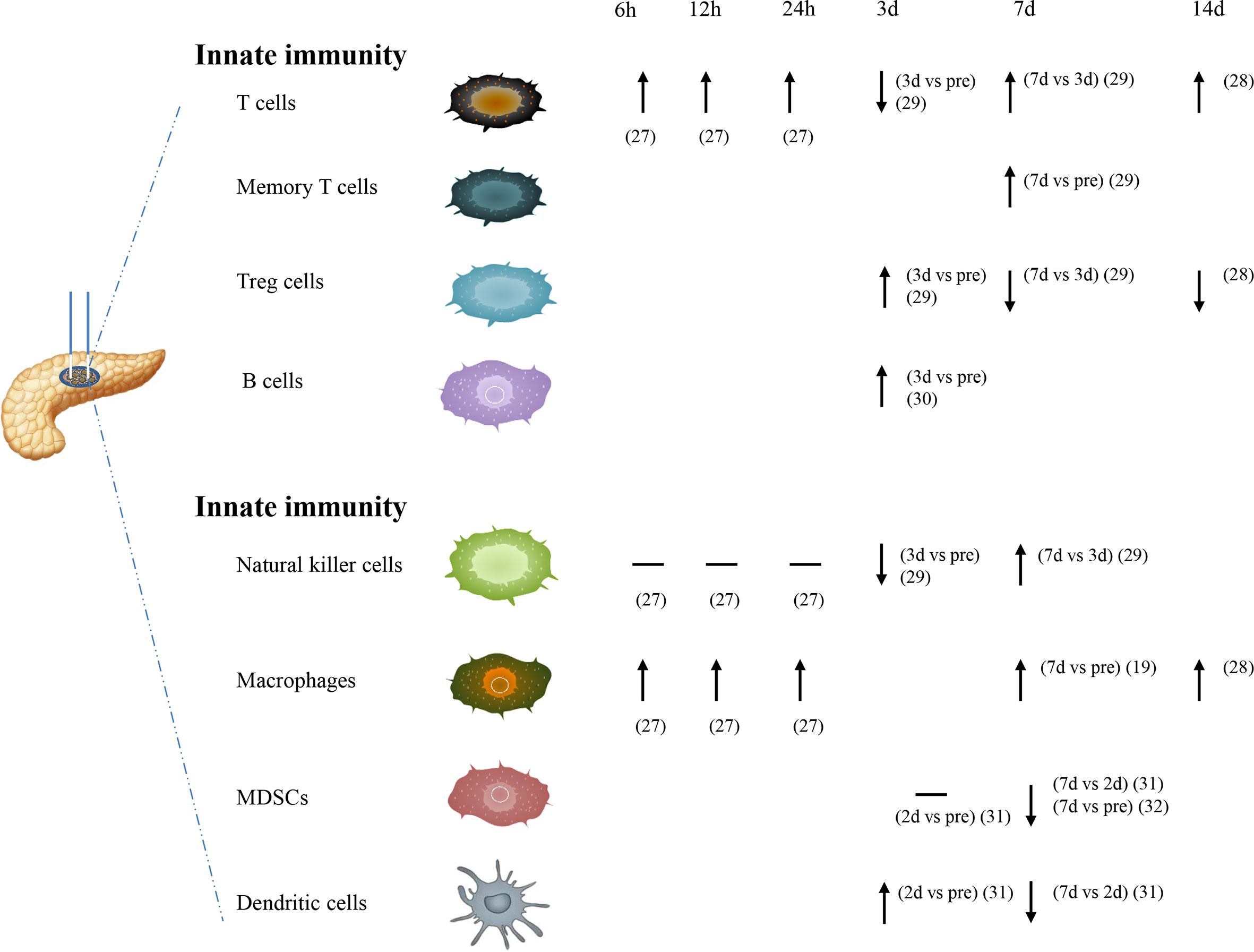
IRE, as a potent trigger in immune responses, has direct effects on both innate and adaptive immunity (Figure 1). IRE could induce apoptosis in the early stages and decrease immune-suppressive cells. IRE may enhance immunotherapy efficacy because it causes a transformation from the inherently immunosuppressive microenvironment to another that was proinflammatory and antitumorigenic (26). For cancer hallmarks, cellular injury and regeneration were mainly affected by treatment. Before IRE treatment, cellular injury signaling was increased in the patient-derived xenograft (PDX) tumors but reduced after the therapy. It showed an increase in regeneration and repaired signaling with increased IRE dosage (23).
At the 6-, 12-, and 24-h time point, the number of macrophages and T cells could be more significantly increased in the IRE group. But for natural killer (NK) cells, no significant differences were found in the IRE group at these three time points. NK cells seemed to have a downward trend compared to before surgery within 24 h after IRE (27). Macrophage cells in the tumor showed a significant increase on day 7 after IRE (19). Memory T cells were also increased significantly on day 7 after IRE in tumor and lymph node (20). The significantly higher number of macrophages and T cells are detected on day 14 after IRE (28). However, a transient decrease in regulatory T cells (Treg) occurred on day 14 after the IRE procedure (21). It was reported that CD4+ T cell, CD8+ T cell, NK cell, IL-2, C3, C4, and IgG have a transitory decrease on day 3 after IRE, then a steady increase on day 7 after IRE, but Treg cell, IL-6, and IL10 have a reverse trend (29). Unlike these findings, in a total of 92 local advanced pancreatic carcinoma (LAPC) patients using IRE alone, on day 3 after treatment, the total T cell count, CD4+ T cell count, CD8+ T cell count, NK cell count, and B-cell count were obviously raised (30). Myeloid-derived suppressor cells (MDSCs) have the same level on day 2 posttreatment compared with that on pretreatment, but they were significantly reduced on day 7 posttreatment (31). Similar to another study by Jayanth Shankara Narayanan et al., it was found that MDSCs were obviously reduced on day 7 after nsPEF (32). Dendritic cells (DCs) have a transitory increase on day 2 posttreatment compare with that on pretreatment, but then were significantly reduced on day 7 posttreatment (31).
The Immune Effect of IRE in the Treatment of PC on Spleen
In orthotopic nude-mouse models, the transformation rate of splenic lymphocytes in the IRE group was higher than that in the control group (p < 0.05). IRE may enhance the activity of splenic B lymphocytes, stimulate the body’s cellular immune function, and achieve the effect of inhibiting tumor cells (33). In a syngeneic mouse with Pan02 pancreatic cancer, it seemed that Treg cells increased to a peak in the tumor microenvironment (TME) and in the spleen 2 days after IRE treatment (31). It was reported that there was a decreased Treg cell infiltration in the spleen on day 7 and a slightly increased macrophage cell infiltration in the spleen on day 7 (19). Memory CD8+ T cells both in the spleen and lymph node increased significantly after IRE. Furthermore, the ratios of effector CD8+ T cells elevated obviously in the spleen and lymph node with the increasing strength of electroporation (20). These showed that not only in the tumor or tumor-draining lymph nodes but also in spleen that IRE increased the systematic infiltration of immune-activated cells. Further analysis was needed to check whether IRE could have similar immunomodulatory function in other organs.
The Effect of IRE Combined With Immunotherapy on PC
It is urgent to have novel therapies and techniques to prolong the survival of PC. Pancreatic cancer lacks response to many individually applied immunotherapy (34). In recent years, electrochemotherapy (ECT), the first application of electroporation in oncology, could temporarily enhance membrane permeability via reversible electroporation to accelerate the transportation of bleomycin or cisplatin into tumor cells, then increase their cytotoxicity (7). ECT has been established as an efficient way for the treatment of cutaneous tumors (35). For PC, ECT seemed be promising, but it was still unclear due to the small number of studies (36–38). ECT could induce systemic antitumor T-cell responses (7). However, it may be that the antitumor immune responses raised by ECT alone were not strong enough to kill fully established distant tumors (39, 40). It seemed that the combination of ECT with immunotherapy, such as immune checkpoint inhibitors or strategies based on electrogenetherapy, could be an efficient approach for the ECT-treated lesions and distant lesions (40). Similarly, some studies showed that the immune effects of IRE alone are inadequate to clear all distant micrometastatic disease in PC patients (32, 41, 42). In some centers, they indicated that local control rates could be >90% after IRE, but many patients experienced distant recurrence (41, 43, 44). Thus, it is necessary to focus on better methods to treat micrometastasis. IRE caused by heterogeneous electric field magnitude could result in inadequate ablation and tumor recurrence. Focusing on the chemoresistance of the tumor microenvironment and the resistance of pancreatic cancer to therapy with immune checkpoint inhibitors, many efforts have been tried to improve systemic therapeutic efficacy (45). Therefore, we elaborated immunomodulatory effect of IRE cooperating with immunotherapy in PC in the following paragraphs (Figure 2).
IRE Combined With Checkpoint Inhibitor Therapy on PC
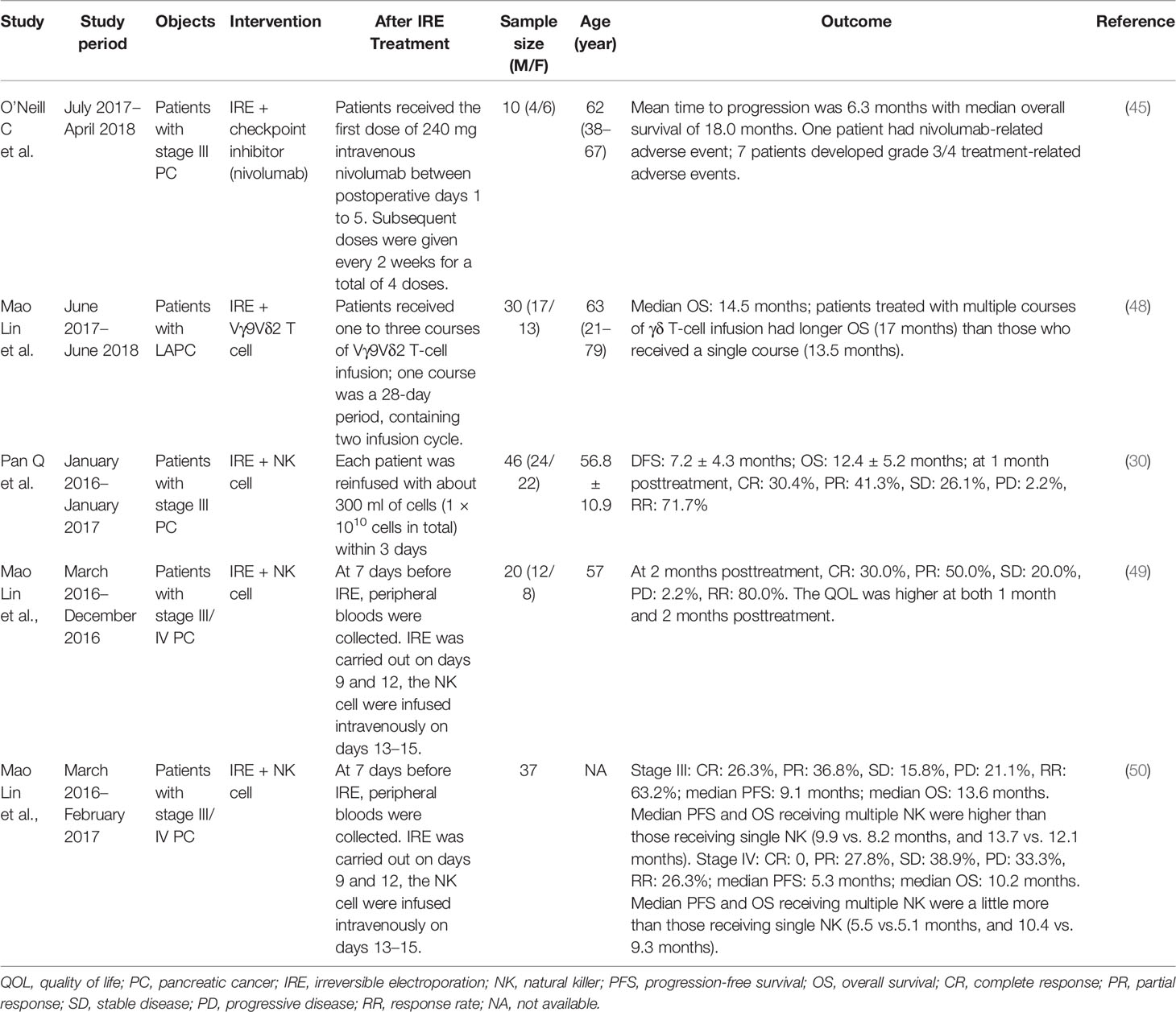
Emerging studies showed that PC could not make response to immune checkpoint blockade due to limited neo-antigen expression and a poor local immunological tumor microenvironment (46). Although combination regimens including chemotherapy indicated initial promise, most combination regimens did not show favorable prognosis in survival compared with standard of care (47). Interestingly, O’Neill et al. indicated that combination therapy of IRE and programmed death ligand-1 (PD-L1) expression in murine models of pancreatic cancer was well tolerated. Effector memory T cells were increased by 1.96 times on day 90 posttreatment. There were no obvious changes among CD4+ T cells, naive T cells, or T-central memory cells (45). During this phase 1b clinical trial of 10 cases with stage III PC, IRE was performed followed by nivolumab. The result showed that mean time to progression was 6.3 months with median overall survival (OS) of 18.0 months. One patient had nivolumab-related adverse event, and seven patients underwent grade 3/4 treatment-related adverse events (45) (Table 1). Combining IRE with intratumoral toll-like receptor-7 (TLR7) agonist (1V270) and systemic antiprogrammed death-1 receptor (PD)-1 checkpoint blockade caused more than fourfold interferon (IFN)-secreting CD8+ T cells than IRE alone (32). This combination elevated the M1/M2 ratio, sand CD8+ DCs, which enhanced tumor antigen presentation to the CD8+ T cells. Survival in this mice model was significantly prolonged. Furthermore, when the cooperation improved the local effects of IRE, they could cause therapeutic abscopal effects against small secondary tumors, providing the potential for the eradication of distant micrometastatic disease (32). However, further studies are still required to identify these possibilities.
IRE Combined With T Cell on PC
The clinical responses of immune checkpoint inhibitors (ICI) for pancreatic cancer were poor. The efficacy of ICIs in PC was prone to immunosuppressive stroma (26). The immunosuppressive microenvironment could restrain the activity of tumor infiltrating lymphocytes (51). Recently, immunotherapy has been used for tumor therapy. In the TME, T cells played an important role, and treatment of ICIs or adoptive cell infusion was promising in cancer therapy (52). Targeting both αβ T cells (CD4+, CD8+ T cells) and γδ T cells was vital in cancer immunity, which has similar features like cytotoxic effector functions and proinflammatory cytokine secretion. However, their dependence on major histocompatibility complex (MHC) molecules varied. γδ T cells consist of 0.5%–16% of CD3+ cells in the peripheral blood, which may be activated by MHC (53). In recent years, the Vγ9Vδ2 T cells have been applied against many types of cancers (54, 55). Previous study showed that IRE and allogenic Vγ9Vδ2 T cells could enhance antitumor effect for PC patients. In addition, there was significant elevation about the αβ T cell and NK cell levels after allogenic Vγ9Vδ2 T-cell infusion, and more infusion courses induced more immune cells. The median OS of LAPC patients receiving IRE and allogenic Vγ9Vδ2 T cells was 14.5 months. These patients receiving IRE plus multiple Vγ9Vδ2 T cells have longer OS (17 months) than those who received IRE plus a single course (13.5 months) (48).
IRE Combined With NK Cell on PC
NK cells are a vital member of the innate immune system against cancers (56). In vitro amplification and reinfusion of NK cells indicated satisfactory prognosis in the solid malignancies treatment of the kidney (57) and breast (58). In patients with stage III PC, Pan et al. found that IRE combined with NK cells had a synergistic impact on strengthening the immune function and could decrease CA19-9 level. In IRE-NK group, it showed that at 1 month posttreatment, the rates of complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and response rate (RR) in 46 patients were 30.4%, 41.3%, 26.1%, 2.2%, and 71.7%, respectively. The mean disease-free survival (DFS) and OS in this group reached 7.2 ± 4.3 months and 12.4 ± 5.2 months. No severe complications during IRE for PC were observed in patients intraoperatively and postoperatively (30) (Table 1). NK cells could identify and break down cells like tumor cells without MHC class I molecules by activating receptors (59). It seemed that more activating killer cell immunoglobulin-like receptors (KIRs) could lead to more NK activation and caused a greater antitumor effect (60). Some studies were focusing on allograft NK cells rather than autologous NK cells for immunotherapy of tumors. In the study of patients with stage III/IV PC, Lin et al. showed that IRE plus allogeneic NK cell therapy had a synergistic effect. Some lymphocyte subsets (CD8 + T cell, CD4 + T cell, NK cell, and B cell) levels and cytokine [IL-2, tumor necrosis factor beta (TNF-β), IFN-γ, and IL-6] levels were significantly higher after the treatment, which might enhance the immune function and reduce CA19-9 and CA242 level. In the IRE-NK group of 20 PC cases, a 2-month follow-up posttreatment indicated 6 cases of CR (30.0%), 10 cases of PR (50.0%), 4 cases of SD (20.0%), 0 case of PD (0%), and 16 cases of RR (80.0%). The quality of life (QOL) was better at both 1 and 2 months posttreatment (Table 1). Moreover, the combined IRE and NK cell treatment was well-tolerated, and the incidences of adverse reactions in the IRE-NK group were low (49), which was similar to the results of another study conducted by this team (50) (Table 1). These supported this combination in a promising way.
IRE Combined With DC Vaccine on PC
Immunotherapy clears cancer cells by reducing patient tolerance to tumor-associated antigens and triggering endogenous antitumor immunity. DC therapy was a powerful immunotherapeutic method (61). However, DC immunotherapy showed limited improved prognosis in PC patients because of the immunosuppressive TME, which limited the infiltration and function of T cells. DC vaccines were obtained by culturing ex vivo DCs that were from patients with a specific antigen. Following maturation and activation, DCs were injected back into the patient. It showed that therapy was promising with the most common side effects like fatigue and/or flu-like symptoms. MHC class I tetramer analysis before and after vaccination indicated effective generation of antigen-specific T cells in three PC patients with stable disease (62). Yang et al. reported that IRE would overcome tumor microenvironment immunosuppression to improve the efficacy of DC cell vaccine in a mice model of PC. Their combination may cause immunogenic cell death and relieve immunosuppressive components in PC microenvironment, including increased tumor infiltration of CD8+ T cells and B+ cells (63), which well indicated that this combination exerted a synergistic effect to enhance the therapeutic efficacy of patients.
IRE Combined With M1 Oncolytic Virus on PC
Oncolytic virotherapy meant that oncolytic viruses selectively disrupted tumor tissues by directly lysing cells, causing systemic antitumor immunity, or regulating tumor vasculature (64). M1 virus could kill residual cancer cells following IRE. Electroporation caused by IRE could offer a non-receptor-dependent membrane channel for M1 virus. IRE may regulate the tumor stroma by elevating microvessel density and tumor vessel permeability. This combined treatment could show more local concentration of M1 virus (26). The combination of IRE and M1 oncolytic virus turned immune-silent tumors into immune-inflamed tumors characterized through T-cell activation, which obviously prolonged the survival of orthotopic PC-bearing immunocompetent mice (22).
Conclusion
In conclusion, studies of IRE on PC immunotherapy indicated new strategies by which IRE could enhance antitumor immune responses. IRE alone has direct effects on both innate and adaptive immunity in PC. IRE cooperating with immunotherapy may play an important role in further prolonging survival of PC patients. However, many questions were still urgent about the properties and functions of IRE in PC. For example, less is known about how to measure the metabolic switch of immune cells during IRE in PC, which is an essential issue for understanding immunometabolic regulations in immune cells. The exciting area of immuno-oncology could be meaningful for prolonging the survival of PC patients. Large-scale prospective randomized controlled trials will be necessary to identify these findings, thus offering references for the options of treatment protocols for PC patients.
Author Contributions
Study concept and design: GT. Acquisition of data: GT, JG, YC, QZ, and TJ. Analysis and interpretation of data: GT and QZ. Drafting of the manuscript: GT. Critical revision of the manuscript for important intellectual content: TJ. Statistical analysis: JG and YC. Obtaining of funding: TJ and QZ. Technical or material support: TJ. Study supervision: TJ. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Development Project of National Major Scientific Research Instrument (82027803), National Natural Science Foundation of China (81971623 and 82171937), Key Project of Natural Science Foundation of Zhejiang Province (LZ20H180001), and Zhejiang Provincial Association Project for Mathematical Medicine (LSY19H180015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lafranceschina S, Brunetti O, Delvecchio A, Conticchio M, Memeo R. Systematic Review of Irreversible Electroporation Role in Management of Locally Advanced Pancreatic Cancer. Cancers (2019) 11:1718. doi: 10.3390/cancers11111718
2. Al Efishat M, Wolfgang CL, Weiss MJ. Stage III Pancreatic Cancer and the Role of Irreversible Electroporation. BMJ (2015) 350:h521. doi: 10.1136/bmj.h521
3. Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic Review of Novel Ablative Methods in Locally Advanced Pancreatic Cancer. World J Gastroenterol (2014) 20:2267–78. doi: 10.3748/wjg.v20.i9.2267
4. Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In Vivo Results of a New Focal Tissue Ablation Technique: Irreversible Electroporation. IEEE Trans BioMed Eng (2006) 53:1409–15. doi: 10.1109/TBME.2006.873745
5. Davalos RV, Mir IL, Rubinsky B. Tissue Ablation With Irreversible Electroporation. Ann BioMed Eng (2005) 33:223–31. doi: 10.1007/s10439-005-8981-8
6. Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, et al. Tumor Ablation With Irreversible Electroporation. PLoS One (2007) 2:e1135. doi: 10.1371/journal.pone.0001135
7. Geboers B, Scheffer HJ, Graybill PM, Ruarus AH, Nieuwenhuizen S, Puijk RS, et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiol (2020) 295:254–72. doi: 10.1148/radiol.2020192190
8. Chai W, Xie L, Zhao Q, Cheng C, Tian G, Jiang T, et al. Ultrasound and Contrast-Enhanced Ultrasound Findings After Percutaneous Irreversible Electroporation of Hepatic Malignant Tumors. Ultrasound Med Biol (2020) 46:620–29. doi: 10.1016/j.ultrasmedbio.2019.12.012
9. Chai W, Tian G, Jiang T. Percutaneous Irreversible Electroporation for Portal Vein Tumor Thrombus: A Case Report. Ultrasound Q (2017) 33:296–99. doi: 10.1097/RUQ.0000000000000305
10. Jiang T, Zhao Q, Tian G, Chen X, Wu L. Irreversible Electroporation Ablation of End-Stage Metastatic Retroperitoneal Lesions: Report on Three Cases and Literature Review. Exp Ther Med (2019) 18:2243–49. doi: 10.3892/etm.2019.7780
11. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria–a 10-Year Update. Radiol (2014) 273:241–60. doi: 10.1148/radiol.14132958
12. Hosein PJ, Echenique A, Loaiza-Bonilla A, Froud T, Barbery K, Rocha Lima CM, et al. Percutaneous Irreversible Electroporation for the Treatment of Colorectal Cancer Liver Metastases With a Proposal for a New Response Evaluation System. J Vasc Interv Radiol (2014) 25:1233–39.e2. doi: 10.1016/j.jvir.2014.04.007
13. Kluger MD, Epelboym I, Schrope BA, Mahendraraj K, Hecht EM, Susman J, et al. Single-Institution Experience With Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann Surg Oncol (2016) 23:1736–43. doi: 10.1245/s10434-015-5034-x
14. Leen E, Picard J, Stebbing J, Abel M, Dhillon T, Wasan H. Percutaneous Irreversible Electroporation With Systemic Treatment for Locally Advanced Pancreatic Adenocarcinoma. J Gastrointest Oncol (2018) 9:275–81. doi: 10.21037/jgo.2018.01.14
15. Canvasser NE, Sorokin I, Lay AH, Morgan MSC, Ozayar A, Trimmer C, et al. Irreversible Electroporation of Small Renal Masses: Suboptimal Oncologic Efficacy in an Early Series. World J Urol (2017) 35:1549–55. doi: 10.1007/s00345-017-2025-5
16. Trimmer CK, Khosla A, Morgan M, Stephenson SL, Ozayar A, Cadeddu JA. Minimally Invasive Percutaneous Treatment of Small Renal Tumors With Irreversible Electroporation: A Single-Center Experience. J Vasc Interv Radiol (2015) 26:1465–71. doi: 10.1016/j.jvir.2015.06.028
17. van den Bos W, Scheltema MJ, Siriwardana AR, Kalsbeek AMF, Thompson JE, Ting F, et al. Focal Irreversible Electroporation as Primary Treatment for Localized Prostate Cancer. BJU Int (2018) 121:716–24. doi: 10.1111/bju.13983
18. Guenther E, Klein N, Zapf S, Weil S, Schlosser C, Rubinsky B, et al. Prostate Cancer Treatment With Irreversible Electroporation (IRE): Safety, Efficacy and Clinical Experience in 471 Treatments. PLoS One (2019) 14:e0215093. doi: 10.1371/journal.pone.0215093
19. Zhao J, Chen S, Zhu L, Zhang L, Liu J, Xu D, et al. Antitumor Effect and Immune Response of Nanosecond Pulsed Electric Fields in Pancreatic Cancer. Front Oncol (2020) 10:621092. doi: 10.3389/fonc.2020.621092
20. He C, Huang X, Zhang Y, Lin X, Li S. T-Cell Activation and Immune Memory Enhancement Induced by Irreversible Electroporation in Pancreatic Cancer. Clin Transl Med (2020) 10:e39. doi: 10.1002/ctm2.39
21. Scheffer HJ, Stam AGM, Geboers B, Vroomen L, Ruarus A, de Bruijn B, et al. Irreversible Electroporation of Locally Advanced Pancreatic Cancer Transiently Alleviates Immune Suppression and Creates a Window for Antitumor T Cell Activation. Oncoimmunology (2019) 8:1652532. doi: 10.1080/2162402X.2019.1652532
22. Sun S, Liu Y, He C, Hu W, Liu W, Huang X, et al. Combining NanoKnife With M1 Oncolytic Virus Enhances Anticancer Activity in Pancreatic Cancer. Cancer Lett (2021) 502:9–24. doi: 10.1016/j.canlet.2020.12.018
23. Brock RM, Beitel-White N, Coutermarsh-Ott S, Grider DJ, Lorenzo MF, Ringel-Scaia VM, et al. Patient Derived Xenografts Expand Human Primary Pancreatic Tumor Tissue Availability for Ex Vivo Irreversible Electroporation Testing. Front Oncol (2020) 10:843. doi: 10.3389/fonc.2020.00843
24. Zhang W, Bi N. The Safety, Effectiveness and Tumor Immunity of Irreversible Electroporation in the Treatment of Rabbit Breast Cancer [Thesis]. Jilin, China: Jilin University (2018).
25. Ren Z, Chen X, Cui G, Yin S, Chen L, Jiang J, et al. Nanosecond Pulsed Electric Field Inhibits Cancer Growth Followed by Alteration in Expressions of NF-κb and Wnt/β-Catenin Signaling Molecules. PLoS One (2013) 8:e74322. doi: 10.1371/journal.pone.0074322
26. Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, et al. Irreversible Electroporation Reverses Resistance to Immune Checkpoint Blockade in Pancreatic Cancer. Nat Commun (2019) 10:899. doi: 10.1038/s41467-019-08782-1
27. White SB, Zhang Z, Chen J, Gogineni VR, Larson AC. Early Immunologic Response of Irreversible Electroporation Versus Cryoablation in a Rodent Model of Pancreatic Cancer. J Vasc Interv Radiol (2018) 29:1764–69. doi: 10.1016/j.jvir.2018.07.009
28. Figini M, Wang X, Lyu T, Su Z, Wang B, Sun C, et al. Diffusion MRI Biomarkers Predict the Outcome of Irreversible Electroporation in a Pancreatic Tumor Mouse Model. Am J Cancer Res (2018) 8:1615–23.
29. He C, Wang J, Sun S, Zhang Y, Li S. Immunomodulatory Effect After Irreversible Electroporation in Patients With Locally Advanced Pancreatic Cancer. J Oncol (2019) 2019:9346017. doi: 10.1155/2019/9346017
30. Pan Q, Hu C, Fan Y, Wang Y, Li R, Hu X. Efficacy of Irreversible Electroporation Ablation Combined With Natural Killer Cells in Treating Locally Advanced Pancreatic Cancer. J buon (2020) 25:1643–49.
31. Guo S, Burcus NI, Hornef J, Jing Y, Jiang C, Heller R, et al. Nano-Pulse Stimulation for the Treatment of Pancreatic Cancer and the Changes in Immune Profile. Cancers (Basel) (2018) 10:217. doi: 10.3390/cancers10070217
32. Narayanan JSS, Ray P, Hayashi T, Whisenant TC, Vicente D, Carson DA, et al. Irreversible Electroporation Combined With Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol Res (2019) 7:1714–26. doi: 10.1158/2326-6066.CIR-19-0101
33. Su J, Chen Y. Clinical Application of Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Cancer [Thesis]. Beijing, China: Medical College of Chinese People's Liberation Army (2019).
34. Zhang J, Wolfgang CL, Zheng L. Precision Immuno-Oncology: Prospects of Individualized Immunotherapy for Pancreatic Cancer. Cancers (Basel) (2018) 10:39. doi: 10.3390/cancers10020039
35. Di Monta G, Caracò C, Simeone E, Grimaldi AM, Marone U, Di Marzo M, et al. Electrochemotherapy Efficacy Evaluation for Treatment of Locally Advanced Stage III Cutaneous Squamous Cell Carcinoma: A 22-Cases Retrospective Analysis. J Transl Med (2017) 15:82. doi: 10.1186/s12967-017-1186-8
36. Granata V, Fusco R, Piccirillo M, Palaia R, Petrillo A, Lastoria S, et al. Electrochemotherapy in Locally Advanced Pancreatic Cancer: Preliminary Results. Int J Surg (2015) 18:230–6. doi: 10.1016/j.ijsu.2015.04.055
37. Rudno-Rudzińska J, Kielan W, Guziński M, Płochocki M, Antończyk A, Kulbacka J. New Therapeutic Strategy: Personalization of Pancreatic Cancer Treatment-Irreversible Electroporation (IRE), Electrochemotherapy (ECT) and Calcium Electroporation (CaEP) - A Pilot Preclinical Study. Surg Oncol (2021) 38:101634. doi: 10.1016/j.suronc.2021.101634
38. Girelli R, Prejanò S, Cataldo I, Corbo V, Martini L, Scarpa A, et al. Feasibility and Safety of Electrochemotherapy (ECT) in the Pancreas: A Pre-Clinical Investigation. Radiol Oncol (2015) 49:147–54. doi: 10.1515/raon-2015-0013
39. Mir LM, Roth C, Orlowski S, Quintin-Colonna F, Fradelizi D, Belehradek J Jr., et al. Systemic Antitumor Effects of Electrochemotherapy Combined With Histoincompatible Cells Secreting Interleukin-2. J Immunother Emphasis Tumor Immunol (1995) 17:30–8. doi: 10.1097/00002371-199501000-00004
40. Calvet CY, Mir LM. The Promising Alliance of Anti-Cancer Electrochemotherapy With Immunotherapy. Cancer Metastasis Rev (2016) 35:165–77. doi: 10.1007/s10555-016-9615-3
41. Huang KW, Yang PC, Pua U, Kim MD, Li SP, Qiu YD, et al. The Efficacy of Combination of Induction Chemotherapy and Irreversible Electroporation Ablation for Patients With Locally Advanced Pancreatic Adenocarcinoma. J Surg Oncol (2018) 118:31–6. doi: 10.1002/jso.25110
42. Holland MM, Bhutiani N, Kruse EJ, Weiss MJ, Christein JD, White RR, et al. A Prospective, Multi-Institution Assessment of Irreversible Electroporation for Treatment of Locally Advanced Pancreatic Adenocarcinoma: Initial Outcomes From the AHPBA Pancreatic Registry. HPB (Oxford) (2019) 21:1024–31. doi: 10.1016/j.hpb.2018.12.004
43. Narayanan G, Hosein PJ, Beulaygue IC, Froud T, Scheffer HJ, Venkat SR, et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J Vasc Interv Radiol (2017) 28:342–48. doi: 10.1016/j.jvir.2016.10.023
44. Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, et al. Treatment of 200 Locally Advanced (Stage III) Pancreatic Adenocarcinoma Patients With Irreversible Electroporation: Safety and Efficacy. Ann Surg (2015) 262:486–94. doi: 10.1097/SLA.0000000000001441
45. O'Neill C, Hayat T, Hamm J, Healey M, Zheng Q, Li Y, et al. A Phase 1b Trial of Concurrent Immunotherapy and Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Adenocarcinoma. Surgery (2020) 168:610–16. doi: 10.1016/j.surg.2020.04.057
46. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature (2013) 499:214–18. doi: 10.1038/nature12213
47. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint Inhibitors in Pancreatic Cancer. Cancer Treat Rev (2019) 78:17–30. doi: 10.1016/j.ctrv.2019.06.005
48. Lin M, Zhang X, Liang S, Luo H, Alnaggar M, Liu A, et al. Irreversible Electroporation Plus Allogenic Vγ9vδ2 T Cells Enhances Antitumor Effect for Locally Advanced Pancreatic Cancer Patients. Signal Transduct Target Ther (2020) 5:215. doi: 10.1038/s41392-020-00260-1
49. Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, et al. Short-Term Clinical Efficacy of Percutaneous Irreversible Electroporation Combined With Allogeneic Natural Killer Cell for Treating Metastatic Pancreatic Cancer. Immunol Lett (2017) 186:20–7. doi: 10.1016/j.imlet.2017.03.018
50. Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, et al. Percutaneous Irreversible Electroporation Combined With Allogeneic Natural Killer Cell Immunotherapy for Patients With Unresectable (Stage III/IV) Pancreatic Cancer: A Promising Treatment. J Cancer Res Clin Oncol (2017) 143:2607–18. doi: 10.1007/s00432-017-2513-4
51. Martinez-Bosch N, Vinaixa J, Navarro P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers (Basel) (2018) 10:6. doi: 10.3390/cancers10010006
52. Silva-Santos B, Mensurado S, Coffelt SB. γδ T Cells: Pleiotropic Immune Effectors With Therapeutic Potential in Cancer. Nat Rev Cancer (2019) 19:392–404. doi: 10.1038/s41568-019-0153-5
53. Hu Y, Liu T, Li J, Mai F, Li J, Chen Y, et al. Selenium Nanoparticles as New Strategy to Potentiate γδ T Cell Anti-Tumor Cytotoxicity Through Upregulation of Tubulin-α Acetylation. Biomaterials (2019) 222:119397. doi: 10.1016/j.biomaterials.2019.119397
54. Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li J, He J, et al. Allogeneic Vγ9vδ2 T-Cell Immunotherapy Exhibits Promising Clinical Safety and Prolongs the Survival of Patients With Late-Stage Lung or Liver Cancer. Cell Mol Immunol (2021) 18:427–39. doi: 10.1038/s41423-020-0515-7
55. Alnaggar M, Xu Y, Li J, He J, Chen J, Li M, et al. Allogenic Vγ9vδ2 T Cell as New Potential Immunotherapy Drug for Solid Tumor: A Case Study for Cholangiocarcinoma. J Immunother Cancer (2019) 7:36. doi: 10.1186/s40425-019-0501-8
56. Zhao Y, Hu J, Li R, Song J, Kang Y, Liu S, et al. Enhanced NK Cell Adoptive Antitumor Effects Against Breast Cancer In Vitro via Blockade of the Transforming Growth Factor-β Signaling Pathway. Onco Targets Ther (2015) 8:1553–9. doi: 10.2147/OTT.S82616
57. Wang D, Zhang B, Gao H, Ding G, Wu Q, Zhang J, et al. Clinical Research of Genetically Modified Dendritic Cells in Combination With Cytokine-Induced Killer Cell Treatment in Advanced Renal Cancer. BMC Cancer (2014) 14:251. doi: 10.1186/1471-2407-14-251
58. Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, et al. Clinical Activity of Adjuvant Cytokine-Induced Killer Cell Immunotherapy in Patients With Post-Mastectomy Triple-Negative Breast Cancer. Clin Cancer Res (2014) 20:3003–11. doi: 10.1158/1078-0432.CCR-14-0082
59. Ljunggren HG, Kärre K. In Search of the 'Missing Self': MHC Molecules and NK Cell Recognition. Immunol Today (1990) 11:237–44. doi: 10.1016/0167-5699(90)90097-S
60. Stringaris K, Barrett AJ. The Importance of Natural Killer Cell Killer Immunoglobulin-Like Receptor-Mismatch in Transplant Outcomes. Curr Opin Hematol (2017) 24:489–95. doi: 10.1097/MOH.0000000000000384
61. Palucka K, Banchereau J. Cancer Immunotherapy via Dendritic Cells. Nat Rev Cancer (2012) 12:265–77. doi: 10.1038/nrc3258
62. Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M, et al. Vaccination With Poly(IC:LC) and Peptide-Pulsed Autologous Dendritic Cells in Patients With Pancreatic Cancer. J Hematol Oncol (2017) 10:82. doi: 10.1186/s13045-017-0459-2
63. Yang J, Eresen A, Shangguan J, Ma Q, Yaghmai V, Zhang Z. Irreversible Electroporation Ablation Overcomes Tumor-Associated Immunosuppression to Improve the Efficacy of DC Vaccination in a Mice Model of Pancreatic Cancer. Oncoimmunol (2021) 10:1875638. doi: 10.1080/2162402X.2021.1875638
Keywords: irreversible electroporation, immunity, pancreatic cancer, immunotherapy, ablation
Citation: Tian G, Guan J, Chu Y, Zhao Q and Jiang T (2021) Immunomodulatory Effect of Irreversible Electroporation Alone and Its Cooperating With Immunotherapy in Pancreatic Cancer. Front. Oncol. 11:712042. doi: 10.3389/fonc.2021.712042
Received: 03 June 2021; Accepted: 19 August 2021;
Published: 10 September 2021.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Martina Ferioli, (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, ItalyLuca Tagliaferri, Fondazione Policlinico A. Gemelli (IRCCS), Italy
Copyright © 2021 Tian, Guan, Chu, Zhao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian’an Jiang, dGlhbmFuamlhbmdAemp1LmVkdS5jbg==
 Guo Tian
Guo Tian Jiajia Guan
Jiajia Guan Yanhua Chu1
Yanhua Chu1 Qiyu Zhao
Qiyu Zhao Tian’an Jiang
Tian’an Jiang