
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 13 July 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.709070
This article is part of the Research Topic Advanced Approaches on Multidisciplinary Management of Rectal Cancer View all 21 articles
Background: Magnetic resonance imaging (MRI)-based lymph node staging remains a significant challenge in the treatment of rectal cancer. Pretreatment evaluation of lymph node metastasis guides the formulation of treatment plans. This systematic review aimed to evaluate the diagnostic performance of MRI in lymph node staging using various morphological criteria.
Methods: A systematic search of the EMBASE, Medline, and Cochrane databases was performed. Original articles published between 2000 and January 2021 that used MRI for lymph node staging in rectal cancer were eligible. The included studies were assessed using the QUADAS-2 tool. A bivariate random-effects model was used to conduct a meta-analysis of diagnostic test accuracy.
Results: Thirty-seven studies were eligible for this meta-analysis. The pooled sensitivity, specificity, and diagnostic odds ratio of preoperative MRI for the lymph node stage were 0.73 (95% confidence interval [CI], 0.68–0.77), 0.74 (95% CI, 0.68–0.80), and 7.85 (95% CI, 5.78–10.66), respectively. Criteria for positive mesorectal lymph node metastasis included (A) a short-axis diameter of 5 mm, (B) morphological standard, including an irregular border and mixed-signal intensity within the lymph node, (C) a short-axis diameter of 5 mm with the morphological standard, (D) a short-axis diameter of 8 mm with the morphological standard, and (E) a short-axis diameter of 10 mm with the morphological standard. The pooled sensitivity/specificity for these criteria were 75%/64%, 81%/67%, 74%/79%, 72%/66%, and 62%/91%, respectively. There was no significant difference among the criteria in sensitivity/specificity. The area under the receiver operating characteristic (ROC) curve values of the fitted summary ROC indicated a diagnostic accuracy rate of 0.75–0.81.
Conclusion: MRI scans have minimal accuracy as a reference index for pretreatment staging of various lymph node staging criteria in rectal cancer. Multiple types of evidence should be used in clinical decision-making.
Rectal cancer has become the leading cause of cancer-related deaths in China and worldwide. By 2030, it is estimated that there will be approximately 2.2 million cases (1, 2). The determination of lymph node staging remains a significant challenge in rectal cancer treatment. Lymph nodes at a risk of metastasis in rectal cancer are mainly located in the mesentery and usually range in size from 1 to 10 mm. Lymph node status is the most important determinant of local recurrence and overall survival (3).
According to the National Comprehensive Cancer Network (NCCN) and American Joint Committee on Cancer (AJCC) staging standards (4, 5), lymph node invasion should be evaluated before treatment to guide the formulation of treatment plans. Patients with lymph node involvement can benefit from preoperative neoadjuvant therapy, considerably reducing the local recurrence rate. However, over-treatment of the lymph node stage may lead to genitourinary system damage and other consequences (6, 7). Therefore, accurate preoperative staging is essential for providing patients with the optimal treatment.
The diagnostic methods currently used for preoperative lymph node staging include magnetic resonance imaging (MRI), computed tomography (CT), and endoscopic ultrasound (EUS). MRI can accurately display the mesorectal fascia, the depth of tumor invasion, circumferential resection margin (CRM), and extramural venous invasion (EMVI), and it has now become the gold standard for preoperative staging and re-staging in local areas (8).
Unfortunately, the results of previous studies have shown that MRI has a poor performance in detecting metastatic lymph nodes (9, 10). At present, there are various diagnostic criteria for metastatic lymph nodes, including size, shape, and boundaries, that have been widely discussed. However, there is no consensus on the accurate diagnosis of metastatic lymph nodes (11–13).
Four previous meta-analyses assessed the accuracy of MRI for lymph node staging of rectal cancer but did not differentiate the lymph nodes defined by different morphological standards (14–17). Additionally, the included studies only used histological results to assess the lymph node status indirectly and did not directly assess lymph nodes on MRI scans. The studies did not perform a histological examination of each lymph node in the specimen so that the position of each lymph node was accurately matched with its corresponding MRI scan, allowing for the node-by-node comparison of MRI scans and histological results to accurately analyze the status of each lymph node.
To the best of our knowledge, this study is the first systematic review and meta-analysis of the accuracy of various lymph node staging criteria in rectal cancer with MRI and includes the literature that contained the node-by-node correspondence between MRI scans and histopathologic results for analysis. To more accurately evaluate the accuracy of MRI in the pretreatment staging of rectal cancer lymph nodes, we hope to obtain more detailed results by synthesizing a large number of published studies.
A comprehensive search of Medline (January 2000–January 2021), Embase (January 2000–January 2021), and the Cochrane Database (2000–January 2021) was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (18) by two investigators (ZZX and ZY), using index terms “((((((((N-stage) OR (Nodal staging)) OR (Lymph node)) OR (Diagnostic imaging)) OR (mesorectal lymph nodes)) OR (Neoplasm Staging)) OR (Lymphatic Metastasis))) AND (((“Magnetic Resonance Imaging”[Mesh]) AND (“Rectal Neoplasms”[Mesh])) AND (sensitiv*[Title/Abstract] OR sensitivity and specificity[Mesh Terms] OR (predictive[Title/Abstract] AND value*[Title/Abstract]) OR predictive value of tests[Mesh Term] OR accuracy*[Title/Abstract])) as text words. The last search was on January 10, 2021.
Studies were included based on the following criteria: 1) original articles on the diagnostic performance of MRI in the staging of rectal cancer, 2) a phased-array MRI coil was used for imaging, 3) histopathologic findings were used as reference standards, 4) the reference criteria for assessing metastatic lymph nodes were clearly mentioned, and 5) sufficient data were available to calculate true-positive, false-positive, false-negative, and true-negative values.
The exclusion criteria were as follows: 1) inclusion of patients with non-rectal cancer, 2) research using other less common MRI types, 3) assessment of staging according to a non-Tumor–Node–Metastasis (TNM) staging system, 4) inclusion of patients who received preoperative chemoradiotherapy, 5) articles that were not original research articles, such as reviews, letters, or case reports, 6) repeated publications.
Titles and abstracts identified by the search strategy were independently reviewed by two reviewers. For all abstracts that met the inclusion criteria or were potentially eligible, full articles were retrieved and independently reviewed by two reviewers. Disagreements were resolved by consensus or by discussion with a third reviewer. All included studies followed the PICOS criteria.
Two reviewers independently extracted the data. The following data was collected: (year of publication, sample size, country), study design (prospective or retrospective), MRI protocol (field strength and resolution parameters), reference criteria for assessing metastatic lymph nodes, and blinding procedure.
The diagnostic results were calculated on a lesion level for each outcome: Patients/lymph nodes with histologically confirmed lymph node metastasis are classified as node-positive (pN+), regardless of the number of metastatic lymph nodes. Patients/lymph nodes without any metastatic lymph nodes are classified as node-negative (pN-).
The QUADAS-2 evaluation tool was used to evaluate the quality of all studies in the systematic review.
Meta-analysis and the associated I2 statistic were evaluated with Meta-Disc 1.4(Ramón y Cajal Hospital, Madrid, Spain) and Stata 16.0(STATA Corporation, College Station, TX, USA) (19).
The threshold effect was evaluated using Spearman’s correlation coefficient of the logit of sensitivity and logit of 1-specificity.
A bivariate random-effects model was used to summarize diagnostic statistics and displayed using summary receiver operating characteristics (SROC) plots.
Meta-regression and subgroup analyses were performed to detect heterogeneity. Additionally, a sensitivity analysis was conducted (20).
Publication bias was evaluated with an asymmetry test and a Deek’s funnel plot assessment using Stata 16.0 (21).
A preliminary database search yielded 1,970 articles, of which 163 were considered relevant for a full test assessment. After screening and data extraction to evaluate whether the articles were suitable for inclusion, 37 eligible items were included in this meta-analysis (9, 11, 22–56). The research selection flowchart is presented in Figure 1. The characteristics of the studies are presented in Table 1. The reference standards were divided into the following five categories according to different morphological criteria: (A) a short-axis diameter of 5 mm (22–34), (B) morphological standard, including an irregular border and mixed-signal intensity within the lymph node (35–40), (C) a short-axis diameter of 5 mm with the morphological standard (11, 41–48), (D) a short-axis diameter of 8 mm with the morphological standard (49–52), and (E) a short-axis diameter of 10 mm with the morphological standard (11, 45, 53, 54). In all of the included articles, 36 indirectly evaluated the lymph node stage of patients through histopathology and 5 (9, 41, 42, 55, 56) identified the node-by-node correspondence between lymph node MRI scans and histopathologic results. Across all studies analyzed, 2,875 patients and 983 lymph nodes were included. Table 2 shows the details of the quality assessment. Figure 2 gives a graphical display for QUADAS-2 results regarding the distribution of the risk of bias.
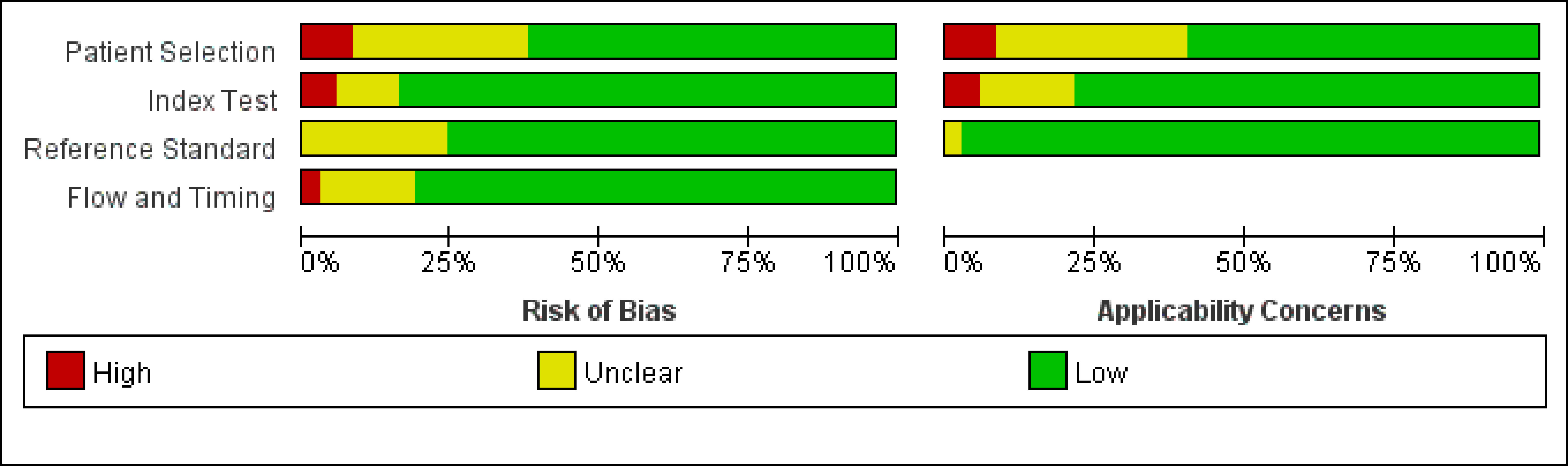
Figure 2 Graphical display for Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) results regarding the proportion of studies with low, high, or unclear risk of bias.
The pooled sensitivity and specificity of MRI in the comprehensive diagnosis of metastatic lymph nodes were 0.73 (95% confidence interval [CI], 0.68–0.77) and 0.74 (95% CI, 0.68–0.80), respectively. The pooled sensitivity, specificity, diagnostic odds ratio, positive likelihood ratio, and negative likelihood ratio with corresponding 95% CIs are listed in Table 3. The area under the ROC curve (AUC) value of the fitted summary ROC was 0.7877 (Figure 3).
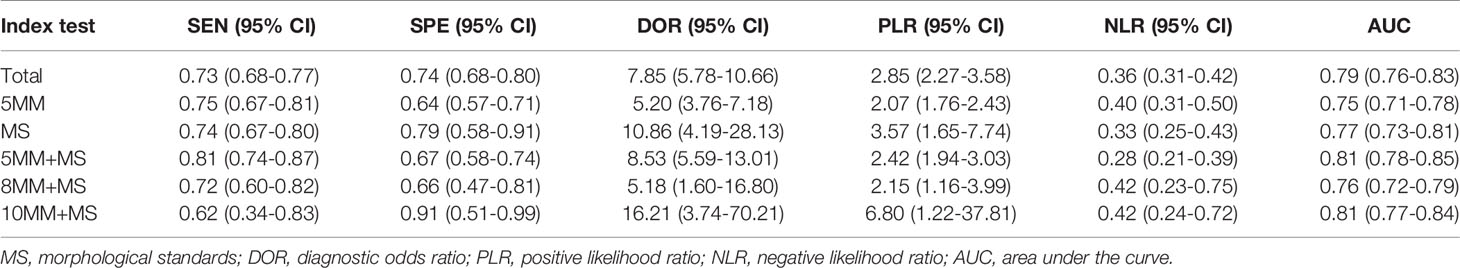
Table 3 The pooled sensitivity, specificity, PLR, and NLR with corresponding 95% CIs for each included study under different morphological standards.
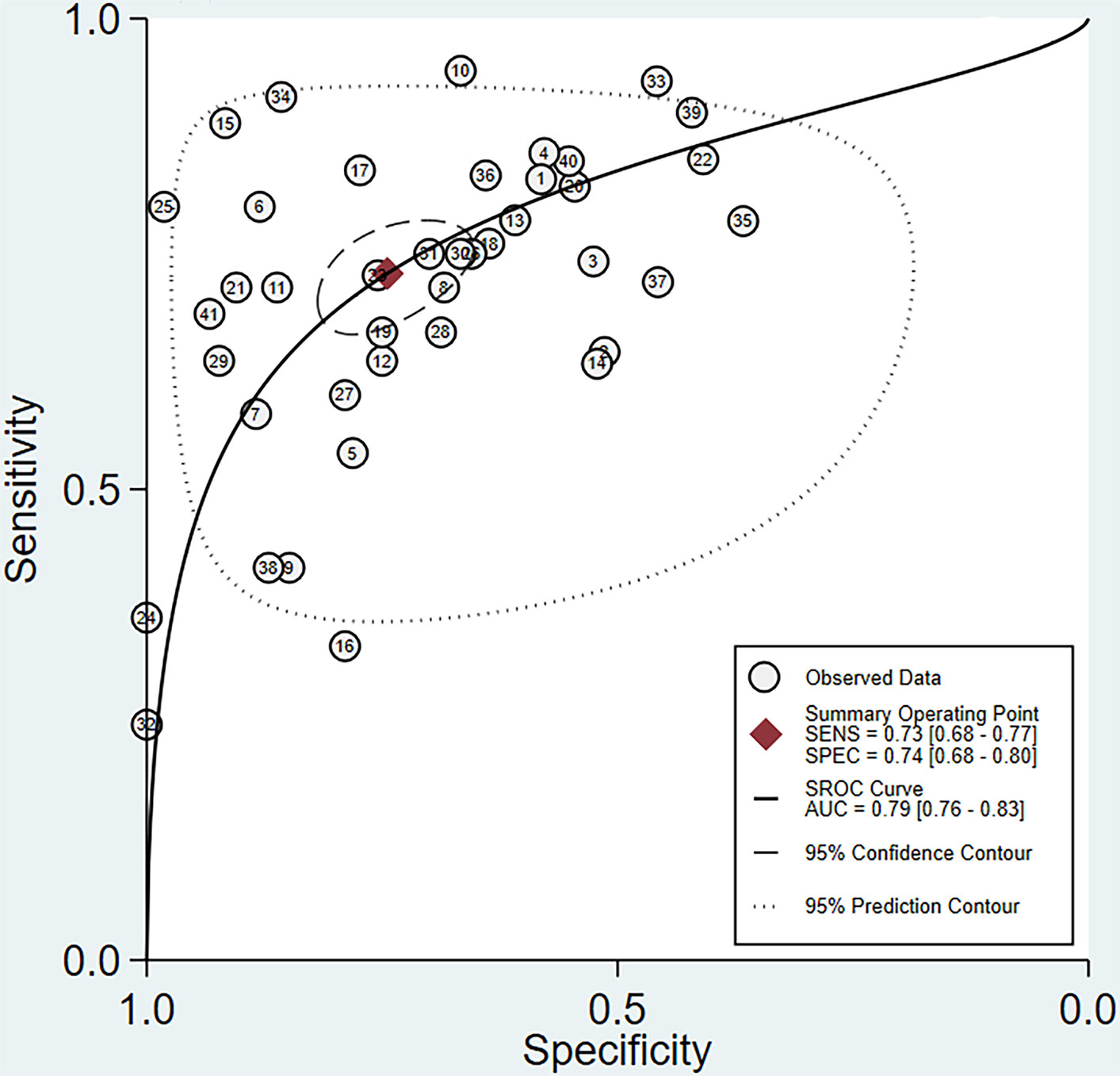
Figure 3 Summary receiver operating characteristic (SROC) curve for MRI assessment of lymph node metastasis in rectal cancer.
Among the different morphological criteria, “a short-axis diameter of 5 mm with the morphological standard” revealed the highest sensitivity of 0.81 (95% CI, 0.74–0.87), and “a short-axis diameter of 10 mm with the morphological standard” revealed the highest specificity of 0.91 (95% CI, 0.51–0.99) (Table 3). The AUCs indicated a diagnostic accuracy rate of 0.75–0.81. The morphological standards with the highest accuracy were “a short-axis diameter of 5 mm with the morphological standard” and “a short-axis diameter of 10 mm with the morphological standard” (Figure 4).
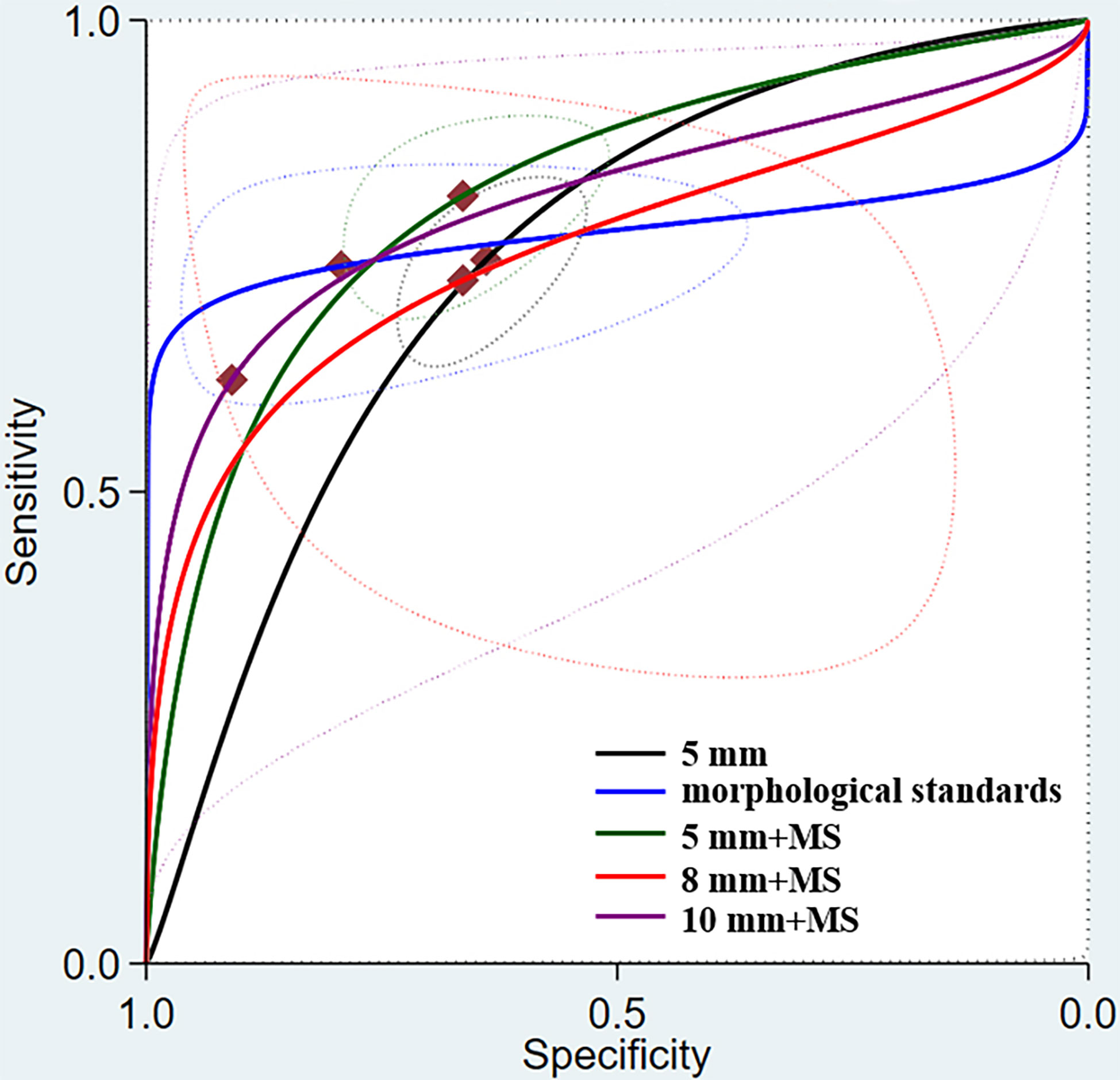
Figure 4 Summary receiver operating characteristic (SROC) curve for MRI assessment of lymph node metastasis under different morphological standards.
The heterogeneity tests showed that the Spearman’s correlation coefficient was 0.446 (p = 0.004), indicating the presence of a threshold effect. This means that different evaluation criteria have led to a significant heterogeneity. Under different morphological standards, there is considerable heterogeneity among 1) the morphological standard, 2) a short-axis diameter of 8 mm with the morphological standard, and 3) a short-axis diameter of 10 mm with the morphological standard (all p < 0.05, i2 > 50%). Therefore, in addition to the threshold effect, there must be other factors that cause significant heterogeneity. A single-factor meta-regression analysis was performed on all the elements. The results showed that the blinding procedure had a particular impact on the heterogeneity of the research (Table 4).
Subgroup analyses were performed for the different study characteristics. By comparing references with or without node-by-node correspondence, we found that a lower sensitivity of 0.55 (95% CI, 0.40–0.69) and higher specificity of 0.89 (95% CI, 0.79–0.95) were yielded. When considering different MRI types, both 3.0T and high-resolution MRI yielded a higher sensitivity and specificity. Through a subgroup analysis of the study design, read approach, and blinding procedure, studies that used double blinding yielded a higher sensitivity of 73% (95% CI, 0.67–0.78) and specificity of 78% (95% CI, 0.70–0.84), whereas prospective studies yielded a higher specificity of 77% (95% CI, 0.75–0.79). The results of the subgroup analysis are shown in Table 4.
A sensitivity analysis of all the studies revealed that five original studies had a strong sensitivity (Figure 5), whereas the other original studies did not strongly affect the calculation results. After excluding the literature mentioned above, the other 36 sub-datasets still had threshold effects. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 0.74 (95% CI, 0.71–0.78), 0.70 (95% CI, 0.64–0.75), 2.45 (95% CI, 2.08–2.89), 0.37 (95% CI, 0.32–0.42), and 6.67 (95% CI, 5.23–8.48), respectively. Further, the AUC was 0.7750.

Figure 5 Sensitivity analysis results of all studies: (A) goodness of fit, (B) bivariate normality, (C) influence analysis, and (D) outlier detection.
For all studies, the p-value of the bias on the Deek’s funnel plot asymmetry test was 0.55, indicating that these studies did not have significant publication bias (Figure 6).
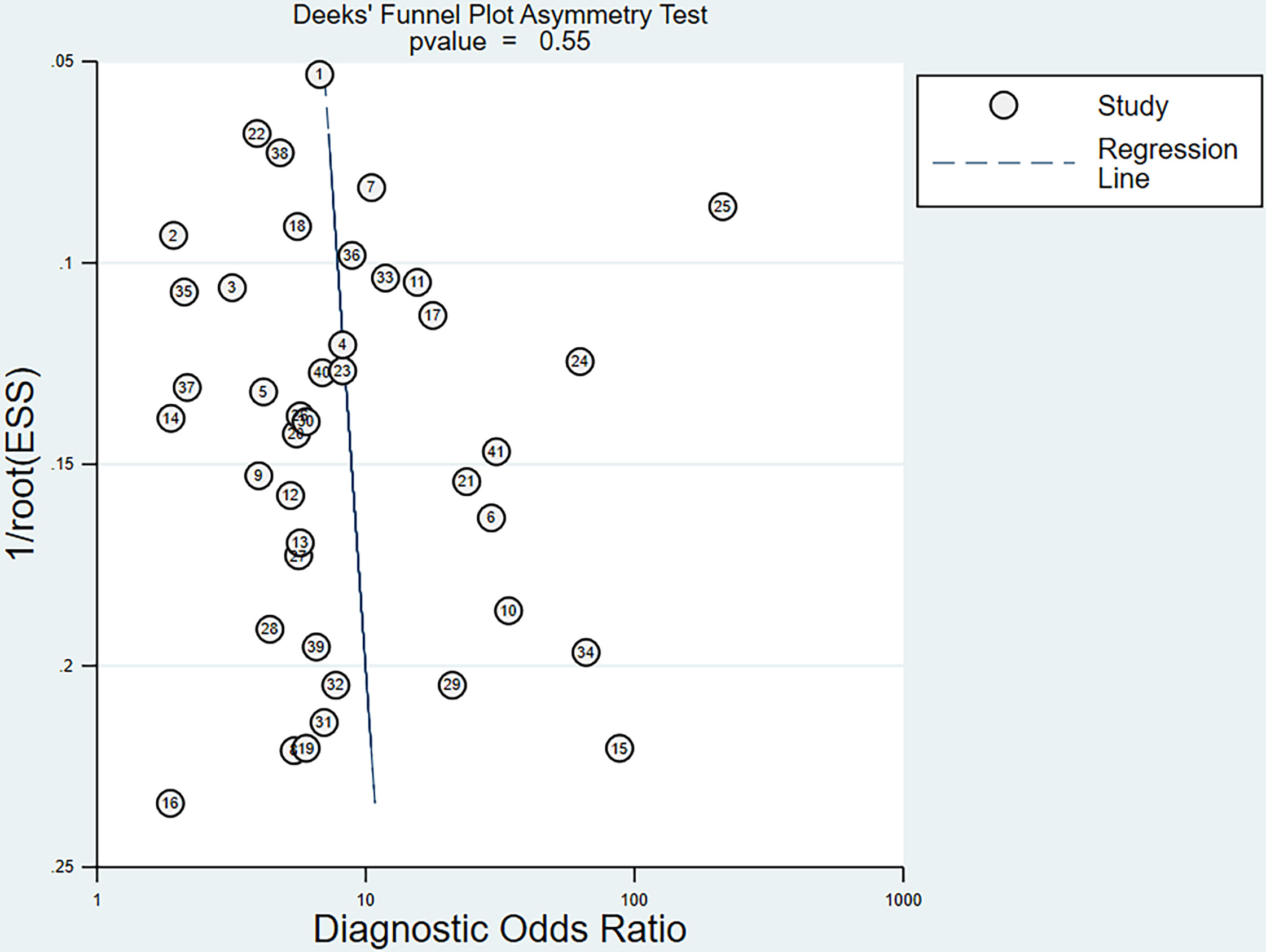
Figure 6 Funnel plot of the reciprocal of effective sample size (ESS) plotted on the y-axis against the diagnostic odds ratio plotted on the x-axis. The regression line is used as a measure of asymmetry. The circles represent included studies.
Lymph node status plays a vital role in selecting treatment strategies for colorectal cancer, with the presence or absence of regional lymph node metastasis being the key to treatment selection. The advantage of MRI is that it can identify the mesorectal fascia, enabling accurate preoperative identification of patients with lymph nodes that cannot be entirely surgically removed. Therefore, in the context of neoadjuvant therapy, preoperative MRI must provide an accurate diagnosis of regional lymph nodes, avoid overestimation and underestimation before treatment, and provide the optimal treatment decision for individual patients. In this study, we evaluated the ability of MRI to determine the lymph node stage of rectal cancer. The results showed that the value of MRI in diagnosing metastatic lymph nodes was low (57–59).
These findings are similar to those reported by Al-Sukhni et al. (15–17), who concluded that MRI only moderates the diagnostic ability for lymph node metastasis. It is worth noting that the previous meta-analysis found significant heterogeneity in the assessment of lymph node metastasis and speculated that the threshold effect is the primary source of heterogeneity. Therefore, we corrected for some of the limitations recognized by previous studies by including more original articles and classifying lymph nodes for statistical analysis based on different morphological standards.
Most MRI studies on colorectal cancer published have used lymph node size as a standard criterion for predicting lymph node involvement. However, previous studies demonstrated that using only the size of lymph nodes as a criterion does not improve the accuracy of lymph node staging of colorectal cancer (9–11), which is consistent with our results. We found that there was no significant difference in the accuracy of MRI diagnosis when using different standards. It is worth mentioning that under the same morphological standard, as the shorter diameter of the lymph node increases, the sensitivity gradually decreases and the specificity gradually increases (Table 3). This may be because although malignant lymph nodes usually have a larger short-axis diameter than benign lymph nodes, there is a considerable size overlap between benign and malignant lymph nodes, with approximately 30% of metastatic lymph nodes having a diameter of ≤4 mm (12). In addition, benign lymph nodes may appear to increase in size with the development of fibrosis (60).
Compared with the size standard alone, different morphological features have been previously considered as good criteria for judging metastatic lymph nodes. Brown et al. first described the use of MRI to improve the correct diagnosis of lymph node involvement in rectal cancer when boundary contours and signal intensity features were used instead of size standards alone (9). Kim et al. demonstrated that in addition to size, new criteria, such as burr-like or inconspicuous borders and uneven appearance, can be used to predict regional lymph node involvement (11). Their results were better than our findings. We found that after adding morphological features, the pooled sensitivity and specificity of lymph node diagnosis improved. However, the diagnostic performance did not improve significantly (Table 3), possibly because the morphological characteristics are more subjective among different observers.
We found that both high-field strength (3.0 Tesla) and high-resolution MRI yielded a higher sensitivity and specificity than low-field strength (1.5 Tesla) according to a subgroup analysis (Table 4). Due to the retrospective design of the research, patient selection, and MRI plan, the diagnostic performance of prospectively designed research was slightly better in the subgroup analysis. In addition, double-blind studies had a higher specificity than single-blind studies (0.78, 95% CI 0.70–0.84). As with other diagnostic meta-analyses, heterogeneity is a vital limitation among studies, including study design, MRI protocols, blinding procedures, and reference standards. In the regression analysis, we found that the blinding procedure (single-blind/double-blind) helps assess heterogeneity, leading to differences among research conclusions.
In most previous studies, the assessment of the lymph node staging of patients mainly relied on the number of positive lymph nodes found in the mesorectum after the overall sampling of rectal specimens, which does not have a high accuracy and reliability. Thus far, few studies have reported that the individual lymph nodes seen on MRI scans match the exact pathological correspondence after rectal resection. We included five references as subgroups. Our analysis found that the sensitivity of MRI for the diagnosis of a single lymph node decreased, the specificity significantly improved, and the accuracy of the assessment was lower than expected. The possible reasons for the inconsistent diagnostic accuracy could be due to small number of references, a lack of consistency in the threshold, and the difference in the realization of node-by-node correspondence.
Currently, new technologies are being explored to improve preoperative staging. The chemical shift effect is a reliable indicator for identifying benign and malignant lymph nodes (61), and Farshchian first proposed that it has the potential to diagnose benign lymph nodes (62). Grovik et al. showed that a low Ktrans of the primary tumor can predict the presence of nodal metastasis (63), which can be achieved by dynamic contrast-enhanced MRI (DCE-MRI) (64, 65). In addition to DCE-MRI, special diffusion-weighted MRI parameters are helpful in differentiating metastatic lymph nodes (45, 66).
The use of lymphatic contrast agents is considered a method for improving the staging of lymph nodes. USPIO is the most widely used contrast agent (67, 68). This technology allows for the differentiation of malignant and benign lymph nodes according to the contrast-enhanced pattern. Although MRI with USPIO has achieved some success in characterizing small lymph nodes, further research is needed regarding its clinical applicability (55, 69–71).
Radiomics is a rapidly developing discipline that uses computer algorithms to extract quantitative features from MRI scans (72–74). These algorithms capture the image texture and morphology of tumors based on their gray values. Since 2018, many reports on radiological methods for rectal cancer lymph node assessment have been published (75–78). However, when analyzing imaging information and building predictive models, all these parameters require time-consuming calculations. In the future, artificial intelligence is expected to become the optimal option for determining lymph node staging and treatments options for patients with locally advanced rectal cancer.
Recently, the importance of lymph node metastasis in the process of tumor recurrence has begun to be questioned, i.e., the indications of neoadjuvant therapy are not based on clinical TNM staging. Additionally, determining whether there are other prognostic markers detected by MRI, such as extra-mural venous invasion (EMVI) and circumferential resection margin (CRM), is more important (79–81). The MERCURY study showed that lymph node involvement is not an independent predictor of local recurrence, and using CRM was recommended for evaluating neoadjuvant therapy (82). In this case, clinical lymph node assessment for rectal cancer may only play a secondary role in guiding future treatment decisions (83, 84).
This study has some limitations. First, our meta-analysis included 37 studies and 2,875 patients. Although this is a comprehensive literature search, more studies may provide more accurate estimates and comparisons of results. Second, the content of some reports is insufficient, limiting our quality assessment and individual analysis of more subgroups. Finally, heterogeneity is still an essential issue in meta-analyses. In future studies, the definition of critical staging elements and MRI protocols should be standardized to reduce heterogeneity. Therefore, considering the limitations of diagnostic meta-analysis, the results should be interpreted prudently.
In summary, the performance of MRI in the detection of lymph node metastasis is inadequate, and either through using more morphological characteristics or shorter diameter, is not significantly improved. At present, when making preoperative neoadjuvant treatment decisions, evidence from a variety of imaging methods should be combined to determine the optimal treatment strategy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
ZZ contributed the most to this article. ZZ designed the project, developed the search strategy and wrote the manuscript. ZZ and YZ checked the search, and reviewed the manuscript. MW performed literature screening and data extraction, conduct the quality assessment of the included studies. XY carried out the data analysis. ZW reviewed the manuscript and finally approved the version to be published. All authors contributed to the article and approved the submitted version.
The work is supported by the Department of Science and Technology of Sichuan Province (2019YFS0375; 2018RZ0091; 2018SZ0242; 2021YFS0025)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MRI, magnetic resonance imaging; RC, rectal cancer; LN, lymph nodes; CRM, circumferential resection margin; EMVI, extramural venous invasion; AJCC, American Joint Commission on Cancer; NCCN, National Comprehensive Cancer Network; QUADAS-2, Quality assessment of Diagnostic accuracy studies-2; CI, confidence interval; AUC, area under the ROC curve; SROC, summary receiver operating characteristics; USPIO, ultrasmall particles of iron oxide; DCE-MRI, dynamic contrast-enhanced MRI.
1. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal Cancer Statistics, 2017. CA Cancer J Clin (2017) 67(3):177–93. doi: 10.3322/caac.21395
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Koh DM, Brown G, Temple L, Blake H, Raja A, Toomey P, et al. Distribution of Mesorectal Lymph Nodes in Rectal Cancer: In Vivo MR Imaging Compared With Histopathological Examination. Initial observations Eur Radiol (2005) 15(8):1650–7. doi: 10.1007/s00330-005-2751-8
4. Benson AB III, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2018) 16:874–901. doi :10.6004/jnccn.2018.0061
5. Nicholls RJ, Zinicola R, Haboubi N. Extramural Spread of Rectal Cancer and the AJCC Cancer Staging Manual 8th Edition, 2017. Ann Oncol (2019) 30(8):1394–5. doi: 10.1093/annonc/mdz147
6. Pollack J, Holm T, Cedermark B, Holmström B, Mellgren A. Long-Term Effect of Preoperative Radiation Therapy on Anorectal Function. Dis Colon Rectum (2006) 49:345–52. doi: 10.1007/s10350-005-0296-1
7. Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, et al. Impact of Short-Term Preoperative Radiotherapy on Health-Related Quality of Life and Sexual Functioning in Primary Rectal Cancer: Report of a Multicenter Randomized Trial. J Clin Oncol (2005) 23(9):1847–58. doi: 10.1200/JCO.2005.05.256
8. Beets-Tan R, Lambregts D, Maas M, Bipat S, Barbaro B, Curvo-Semedo, et al. Magnetic Resonance Imaging for Clinical Management of Rectal Cancer: Updated Recommendations From the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Consensus Meeting. Eur Radiol (2018) 28(4):1465–75. doi: 10.1007/s00330-017-5026-2
9. Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic Predictors of Lymph Node Status in Rectal Cancer With Use of High-Spatial-Resolution MR Im-Aging With Histopathologic Comparison. Radiology (2003) 227:371–7. doi: 10.1148/radiol.2272011747
10. Vogl TJ, Pegios W, Mack MG, Hünerbein M, Hintze R, Adler A, et al. Accuracy of Staging Rectal Tumors With Contrast-Enhanced Transrectal MR Imaging. AJR Am J Roentgenol (1997) 168:1427–34. doi: 10.2214/ajr.168.6.9168702
11. Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-Resolution MR Imaging for Nodal Staging in Rectal Cancer: Are There Any Criteria in Addition to the Size? Eur J Radiol (2004) 52(1):78–83. doi: 10.1016/j.ejrad.2003.12.005
12. Langman G, Patel A, Bowley DM. Size and Distribution of Lymph Nodes in Rectal Cancer Resection Specimens. Dis Colon Rectum (2015) 58(4):406–14. doi: 10.1097/DCR.0000000000000321
13. Kotanagi H, Fukuoka T, Shibata Y, Yoshioka T, Aizawa O, Saito Y, et al. The Size of Regional Lymph Nodes Does Not Correlate With the Presence or Absence of Metastasis in Lymph Nodes in Rectal Cancer. J Surg Oncol (1993) 54:252–4. doi: 10.1002/jso.2930540414
14. Gao Y, Li J, Ma X, Wang J, Wang B, Tian J, et al. The Value of Four Imaging Modalities in Diagnosing Lymph Node Involvement in Rectal Cancer: An Overview and Adjusted Indirect Comparison. Clin Exp Med (2019) 19(2):225–34. doi: 10.1007/s10238-019-00552-z
15. Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, et al. Diagnostic Accuracy of MRI for Assessment of T Category, Lymph Node Metastases, and Circumferential Resection Margin Involvement in Patients With Rectal Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol (2012) 19(7):2212–23. doi: 10.1245/s10434-011-2210-5
16. Li XT, Sun YS, Tang L, Cao K, Zhang XY. Evaluating Local Lymph Node Metastasis With Magnetic Resonance Imaging, Endoluminal Ultrasound and Computed Tomography in Rectal Cancer: A Meta-Analysis. Colorectal Dis (2015) 17(6):O129–35. doi: 10.1111/codi.12909
17. Chan BPH, Patel R, Mbuagbaw L, Thabane L, Yaghoobi M. EUS Versus Magnetic Resonance Imaging in Staging Rectal Adenocarcinoma: A Diagnostic Test Accuracy Meta-Analysis. Gastrointest Endosc (2019) 90(2):196–203.e1. doi: 10.1016/j.gie.2019.04.217
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Dinnes J, Deeks J, Kirby J, Roderick P. A Methodological Review of How Heterogeneity has Been Examined in Systematic Reviews of Diagnostic Test Accuracy. Health Technol Assess (2005) 9:1–113, iii. doi: 10.3310/hta9120
20. Leeflang MM, Deeks JJ, Gatsonis C. Bossuyt Pm; Cochrane Diagnostic Test Accuracy Working Group. Systematic Reviews of Diagnostic Test Accuracy. Ann Intern Med (2008) 149:889–97. doi: 10.7326/0003-4819-149-12-200812160-00008
21. Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric Funnel Plots and Publication Bias in Meta-Analyses of Diagnostic Accuracy. Int J Epidemiol (2002) 31(1):88–95. doi: 10.1093/ije/31.1.88
22. Xu L, Zhang Z, Qin Q, Zhang C, Sun X. Assessment of T and N Staging With MRI3T in Lower and Middle Rectal Cancer and Impact on Clinical Strategy. J Int Med Res (2020) 48(6):300060520928685. doi: 10.1177/0300060520928685
23. Tersteeg JJC, Crolla RMPH, Gobardhan PD, Kint PAM, Niers-Stobbe I, Boonman-de Winter L, et al. MRI-Based Guidelines for Selective Neoadjuvant Treatment in Rectal Cancer: Does MRI Adequately Predict the Indication for Radiotherapy in Daily Practice in a Large Teaching Hospital. Eur J Cancer Care (Engl) (2020) 29(2):e13190. doi: 10.1111/ecc.13190
24. Iannicelli E, Di Renzo S, Ferri M, Pilozzi E, Di Girolamo M, Sapori A, et al. Accuracy of High-Resolution MRI With Lumen Distention in Rectal Cancer Staging and Circumferential Margin Involvement Prediction. Korean J Radiol (2014) 15(1):37–44. doi: 10.3348/kjr.2014.15.1.37
25. White R, Ung KA, Mathlum M. Accuracy of Magnetic Resonance Imaging in the Pre-Operative Staging of Rectal Adenocarcinoma: Experience From a Regional Australian Cancer Center. Asia Pac J Clin Oncol (2013) 9(4):318–23. doi: 10.1111/ajco.12033
26. Winter L, Bruhn H, Langrehr J, Neuhaus P, Felix R, Hänninen LE. Magnetic Resonance Imaging in Suspected Rectal Cancer: Determining Tumor Localization, Stage, and Sphincter-Saving Resectability at 3-Tesla-Sustained High Resolution. Acta Radiol (2007) 48(4):379–87. doi: 10.1080/02841850701196914
27. Tatli S, Mortele KJ, Breen EL, Bleday R, Silverman SG. Local Staging of Rectal Cancer Using Combined Pelvic Phased-Array and Endorectal Coil MRI. J MagnReson Imaging (2006) 23(4):534–40. doi: 10.1002/jmri.20533
28. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal Ultrasonography and Magnetic Resonance Imaging in the Staging of Rectal Cancer. Effect experience Scand J Gastroenterol (2008) 43(4):440–6. doi: 10.1080/00365520701745842
29. Matsuoka H, Nakamura A, Masaki T, Sugiyama M, Takahara T, Hachiya J, et al. A Prospective Comparison Between Multidetector-Row Computed Tomography and Magnetic Resonance Imaging in the Preoperative Evaluation of Rectal Carcinoma. Am J Surg (2003) 185(6):556–9. doi: 10.1016/S0002-9610(03)00067-9
30. Kocaman O, Baysal B, Şentürk H, İnce AT, Müslümanoğlu M, Kocakoç E, et al. Staging of Rectal Carcinoma: MDCT, MRI or EUS. Single Center Experience. Turk J Gastroenterol (2014) 25(6):669–73. doi: 10.5152/tjg.2014.6214
31. Halefoglu AM, Yildirim S, Avlanmis O, Sakiz D, Baykan A. Endorectal Ultrasonography Versus Phased-Array Magnetic Resonance Imaging for Preoperative Staging of Rectal Cancer. World J Gastroenterol (2008) 14(22):3504–10. doi: 10.3748/wjg.14.3504
32. Gagliardi G, Bayar S, Smith R, Salem RR. Preoperative Staging of Rectal Cancer Using Magnetic Resonance Imaging With External Phase-Arrayed Coils. Arch Surg (2002) 137(4):447–51. doi: 10.1001/archsurg.137.4.447
33. Gröne J, Loch FN, Taupitz M, Schmidt C, Kreis ME. Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer With Magnetic Resonance Imaging. J Gastrointest Surg (2018) 22(1):146–53. doi: 10.1007/s11605-017-3568-x
34. Ferri M, Laghi A, Mingazzini P, Iafrate F, Meli L, Ricci F, et al. Pre-Operative Assessment of Extramural Invasion and Sphincteral Involvement in Rectal Cancer by Magnetic Resonance Imaging With Phased-Array Coil. Colorectal Dis (2005) 7(4):387–93. doi: 10.1111/j.1463-1318.2005.00787.x
35. Fernández-Esparrach G, Ayuso-Colella JR, Sendino O, Pagés M, Cuatrecasas M, Pellisé M, et al. EUS and Magnetic Resonance Imaging in the Staging of Rectal Cancer: A Prospective and Comparative Study. Gastrointest Endosc (2011) 74(2):347–54. doi: 10.1016/j.gie.2011.03.1257
36. Jao SY, Yang BY, Weng HH, Yeh CH, Lee LW. Evaluation of Gadolinium-Enhanced T1-Weighted Magnetic Resonance Imaging in the Preoperative Assessment of Local Staging in Rectal Cancer. Colorectal Dis (2010) 12(11):1139–48. doi: 10.1111/j.1463-1318.2009.01959.x
37. Chun HK, Choi D, Kim MJ, Lee J, Yun SH, Kim SH, et al. Preoperative Staging of Rectal Cancer: Comparison of 3-T High-Field MRI and Endorectal Sonography. AJR Am J Roentgenol (2006) 187(6):1557–62. doi: 10.2214/AJR.05.1234
38. Kim CK, Kim SH, Choi D, Kim MJ, Chun HK, Lee SJ, et al. Comparison Between 3-T Magnetic Resonance Imaging and Multi-Detector Row Computed Tomography for the Preoperative Evaluation of Rectal Cancer. J Comput Assist Tomogr (2007) 31(6):853–9. doi: 10.1097/RCT.0b013e318038fc84
39. Bogach J, Tsai S, Zbuk K, Wong R, Grubac V, Coates A, et al. Quality of Preoperative Pelvic Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) for Rectal Cancer in a Region in Ontario: A Retrospective Population-Based Study. J Surg Oncol (2018) 117(5):1038–42. doi: 10.1002/jso.25000
40. Kim YW, Cha SW, Pyo J, Kim NK, Min BS, Kim MJ, et al. Factors Related to Preoperative Assessment of the Circumferential Resection Margin and the Extent of Mesorectal Invasion by Magnetic Resonance Imaging in Rectal Cancer: A Prospective Comparison Study. World J Surg (2009) 33(9):1952–60. doi: 10.1007/s00268-009-0126-z
41. Park JS, Jang YJ, Choi GS, Park SY, Kim HJ, Kang H, et al. Accuracy of Preoperative MRI in Predicting Pathology Stage in Rectal Cancers: Node-for-Node Matched Histopathology Validation of MRI Features. Dis Colon Rectum (2014) 57(1):32–8. doi: 10.1097/DCR.0000000000000004
42. Lambregts DM, Beets GL, Maas M, Kessels AG, Bakers FC, Cappendijk VC, et al. Accuracy of Gadofosveset-Enhanced MRI for Nodal Staging and Restaging in Rectal Cancer. Ann Surg (2011) 253(3):539–45. doi: 10.1097/SLA.0b013e31820b01f1
43. Jiang JB, Dai Y, Zhang XM, Li CF, Jin ZT, Bi DS, et al. Accuracy of Preoperative Magnetic Resonance Imaging in Prediction of Pathological Stage and Circumferential Resection Margin in Rectal Cancer. Zhonghua Yi Xue Za Zhi (2006) 86(14):961–4.
44. Algebally AM, Mohey N, Szmigielski W, Yousef RR, Kohla S. The Value of High-Resolution MRI Technique in Patients With Rectal Carcinoma: Pre-Operative Assessment of Mesorectal Fascia Involvement, Circumferential Resection Margin and Local Staging. Pol J Radiol (2015) 80:115–21. doi: 10.12659/PJR.892583
45. Armbruster M, D’Anastasi M, Holzner V, Kreis ME, Dietrich O, Brandlhuber B, et al. Improved Detection of a Tumorous Involvement of the Mesorectal Fascia and Locoregional Lymph Nodes in Locally Advanced Rectal Cancer Using DCE-MRI. Int J Colorectal Dis (2018) 33(7):901–9. doi: 10.1007/s00384-018-3083-x
46. Halefoglu AM, Atasoy ST, Sakiz D, Baykan A. Accuracy of Thin-Section Magnetic Resonance Imaging With a Pelvic Phased-Array Coil in the Local Staging of Rectal Cancer. J Comput Assist Tomogr (2013) 37(1):58–64. doi: 10.1097/RCT.0b013e3182772ec5
47. Akasu T, Iinuma G, Takawa M, Yamamoto S, Muramatsu Y, Moriyama N. Accuracy of High-Resolution Magnetic Resonance Imaging in Preoperative Staging of Rectal Cancer. Ann Surg Oncol (2009) 16(10):2787–94. doi: 10.1245/s10434-009-0613-3
48. Kim MJ, Lim JS, Oh YT, Kim JH, Chung JJ, Joo SH, et al. Preoperative MRI of Rectal Cancer With and Without Rectal Water Filling: An Intraindividual Comparison. AJR Am J Roentgenol (2004) 182(6):1469–76. doi: 10.2214/ajr.182.6.1821469
49. Xu H, Zhao W, Guo W, Cao S, Gao C, Song T, et al. Prediction Model Combining Clinical and MR Data for Diagnosis of Lymph Node Metastasis in Patients With Rectal Cancer. J Magn Reson Imaging (2021) 53(3):874–83. doi: 10.1002/jmri.27369
50. Kim DJ, Kim JH, Ryu YH, Jeon TJ, Yu JS, Chung JJ. Nodal Staging of Rectal Cancer: High-Resolution Pelvic MRI Versus ¹⁸F-FDGPET/CT. J Comput Assist Tomogr (2011) 35(5):531–4. doi: 10.1097/RCT.0b013e318225720f
51. Zhang S, Peng WJ, Cai SJ, Tang F, Mao J, Qian M, et al. Value of High-Spatial-Resolution MRI Imaging in Preoperative Staging of Rectal Carcinoma. Chin J Cancer Prev Treat (2007) 14(8):617–20. doi :10.16073/j.cnki.cjcpt.2007.08.017
52. Kim SH, Lee JM, Lee MW, Kim GH, Han JK, Choi BI. Diagnostic Accuracy of 3.0-Tesla Rectal Magnetic Resonance Imaging in Preoperative Local Staging of Primary Rectal Cancer. Invest Radiol (2008) 43(8):587–93. doi: 10.1097/RLI.0b013e31817e9083
53. Song Y. Value of High Resolution Magnetic Resonance Imaging in Preoperative Staging of Rectal Cancer. World Chin J Digestology (2018) 26(8):530–6. doi: 10.11569/wcjd.v26.i8.530
54. Kim NK, Kim MJ, Park JK, Park SI, Min JS. Preoperative Staging of Rectal Cancer With MRI: Accuracy and Clinical Usefulness. Ann Surg Oncol (2000) 7(10):732–7. doi: 10.1007/s10434-000-0732-3
55. Koh DM, George C, Temple L, Collins DJ, Toomey P, Raja A, et al. Diagnostic Accuracy of Nodal Enhancement Pattern of Rectal Cancer at MRI Enhanced With Ultrasmall Superparamagnetic Iron Oxide: Findings in Pathologically Matched Mesorectal Lymph Nodes. AJR Am J Roentgenol (2010) 194(6):W505–13. doi: 10.2214/AJR.08.1819
56. Kim CK, Kim SH, Chun HK, Lee WY, Yun SH, Song SY, et al. Preoperative Staging of Rectal Cancer: Accuracy of 3-Tesla Magnetic Resonance Imaging. Eur Radiol (2006) 16(5):972–80. doi: 10.1007/s00330-005-0084-2
57. Garcia-Aguilar J, Shi Q, Thomas CR Jr, Chan E, Cataldo P, Marcet J, et al. A Phase II Trial of Neoadjuvant Chemoradiation and Local Excision for T2N0 Rectal Cancer: Preliminary Results of the ACOSOG Z6041 Trial. Ann Surg Oncol (2012) 19(2):384–91. doi: 10.1245/s10434-011-1933-7
58. Gaertner WB, Kwaan MR, Madoff RD, Melton GB. Rectal Cancer: An Evidence-Based Update for Primary Care Providers. World J Gastroenterol (2015) 21:7659–71. doi: 10.3748/wjg.v21.i25.7659
59. Wolmark N, Fisher B, Wieand HS. The Prognostic Value of the Modifications of the Dukes’ C Class of Colorectal Cancer: An Analysis of the NSABP Clinical Trials. Ann Surg (1986) 203:115–22. doi: 10.1097/00000658-198602000-00001
60. Nahas SC, Nahas CSR, Cama GM, de Azambuja RL, Horvat N, Marques CFS, et al. Diagnostic Performance of Magnetic Resonance to Assess Treatment Response After Neoadjuvant Therapy in Patients With Locally Advanced Rectal Cancer. Abdom Radiol (NY) (2019) 44(11):3632–40. doi: 10.1007/s00261-019-01894-8
61. Zhang H, Zhang C, Zheng Z, Ye F, Liu Y, Zou S, et al. Chemical Shift Effect Predicting Lymph Node Status in Rectal Cancer Using High-Resolution MR Imaging With Node-for-Node Matched Histopathological Validation. Eur Radiol (2017) 27(9):3845–55. doi: 10.1007/s00330-017-4738-7
62. Farshchian N, Tamari S, Farshchian N, Madani H, Rezaie M, Mohammadi-Motlagh HR. Diagnostic Value of Chemical Shift Artifact in Distinguishing Benign Lymphadenopathy. Eur J Radiol (2011) 80(2):594–7. doi: 10.1016/j.ejrad.2010.10.005
63. Grovik E, Redalen KR, Storås TH, Negård A, Holmedal SH, Ree AH, et al. Dynamic Multi-Echo DCE- and DSC-MRI in Rectal Cancer: Low Primary Tumor Ktrans and DeltaR2* Peak Are Significantly Associated With Lymph Node Metastasis. J Magn Reson Imaging (2017) 46(1):194–206. doi: 10.1002/jmri.25566
64. Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic Parameters in CNS Gd-DTPA Enhanced MR Imaging. J Comput Assist Tomogr (1991) 15(4):621–8. doi: 10.1097/00004728-199107000-00018
65. Yang X, Chen Y, Wen Z, Lu B, Shen B, Xiao X, et al. Role of Quantitative Dynamic Contrast-Enhanced MRI in Evaluating Regional Lymph Nodes With a Short-Axis Diameter of Less Than 5 Mm in Rectal Cancer. AJR Am J Roentgenol (2019) 212(1):77–83. doi: 10.2214/AJR.18.19866
66. Yu XP, Wen L, Hou J, Bi F, Hu P, Wang H, et al. Discrimination Between Metastatic and Nonmetastatic Mesorectal Lymph Nodes in Rectal Cancer Using Intravoxel Incoherent Motion Diffusion-Weighted Magnetic Resonance Imaging. Acad Radiol (2016) 23(4):479–85. doi: 10.1016/j.acra.2015.12.013
67. Mack MG, Balzer JO, Straub R, Eichler K, Vogl TJ. Superparamagnetic Iron Oxide-Enhanced MR Imaging of Head and Neck Lymph Nodes. Radiology (2002) 222:239–44. doi: 10.1148/radiol.2221010225
68. Harisinghani MG, Saini S, Hahn PF, Weissleder R, Mueller PR. MR Imaging of Lymph Nodes in Patients With Primary Abdominal and Pelvic Malignancies Using Ultrasmall Superparamagnetic Iron Oxide (Combidex). Acad Radiol (1998) 5 Suppl 1:S167–9, discussion S183–4. doi: 10.1016/S1076-6332(98)80095-0
69. Koh DM, Brown G, Temple L, Raja A, Toomey P, Bett N, et al. Rectal Cancer: Mesorectal Lymph Nodes at MR Imaging With USPIO Versus Histopathologic Findings–Initial Observations. Radiology (2004) 231(1):91–9. doi: 10.1148/radiol.2311030142
70. Stijns RCH, Philips BWJ, Nagtegaal ID, Polat F, de Wilt JHW, Wauters CAP, et al. USPIO-Enhanced MRI of Lymph Nodes in Rectal Cancer: A Node-to-Node Comparison With Histopathology. Eur J Radiol (2021) 138:109636. doi: 10.1016/j.ejrad.2021.109636
71. Scheenen TWJ, Zamecnik P. The Role of Magnetic Resonance Imaging in (Future)Cancer Staging: Note the Nodes. Invest Radiol (2021) 56(1):42–9. doi: 10.1097/RLI.0000000000000741
72. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: Extracting More Information From Medical Images Using Advanced Feature Analysis. Eur J Cancer (2012) 48(4):441–6. doi: 10.1016/j.ejca.2011.11.036
73. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More Than Pictures, They Are Data. Radiology (2016) 278(2):563–77. doi: 10.1148/radiol.2015151169
74. Bibault JE, Xing L, Giraud P, El Ayachy R, Giraud N, Decazes P, et al. Radiomics: A Primer for the Radiation Oncologist. Cancer Radiother (2020) 24(5):403–10. doi: 10.1016/j.canrad.2020.01.011
75. Liu X, Yang Q, Zhang C, Sun J, He K, Xie Y, et al. Multiregional-Based Magnetic Resonance Imaging Radiomics Combined With Clinical Data Improves Efficacy in Predicting Lymph Node Metastasis of Rectal Cancer. Front Oncol (2021) 10:585767. doi: 10.3389/fonc.2020.585767
76. Tse DM, Joshi N, Anderson EM, Brady M, Gleeson FV. A Computer-Aided Algorithm to Quantitatively Predict Lymph Node Status on MRI in Rectal Cancer. Br J Radiol (2012) 85(1017):1272–8. doi: 10.1259/bjr/13374146
77. Vag T, Slotta-Huspenina J, Rosenberg R, Bader FG, Nitsche U, Drecoll E, et al. Computerized Analysis of Enhancement Kinetics for Preoperative Lymph Node Staging in Rectal Cancer Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Clin Imaging (2014) 38(6):845–9. doi: 10.1016/j.clinimag.2014.06.011
78. Li J, Zhou Y, Wang X, Zhou M, Chen X, Luan K. An MRI-based Multi-Objective Radiomics Model Predicts Lymph Node Status in Patients With Rectal Cancer. Abdom Radiol (NY) (2020) 46(5):1816–24. doi: 10.1007/s00261-020-02863-2
79. Knijn N, van Erning FN, Overbeek LI, Punt CJ, Lemmens VE, Hugen N, et al. Limited Effect of Lymph Node Status on the Metastatic Pattern in Colorectal Cancer. Oncotarget (2016) 7(22):31699–707. doi: 10.18632/oncotarget.9064
80. Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, et al. Origins of Lymphatic and Distant Metastases in Human Colorectal Cancer. Science (2017) 357(6346):55–60. doi: 10.1126/science.aai8515
81. Nagtegaal ID, Schmoll HJ. Colorectal Cancer: What Is the Role of Lymph Node Metastases in the Progression of Colorectal Cancer? Nat Rev Gastroenterol Hepatol (2017) 14(11):633–4. doi: 10.1038/nrgastro.2017.122
82. Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative Magnetic Resonance Imaging Assessment of Circumferential Resection Margin Predicts Disease-Free Survival and Local Recurrence: 5-Year Follow-Up Results of the MERCURY Study. J Clin Oncol (2014) 32(1):34–43. doi: 10.1200/JCO.2012.45.3258
83. Kreis ME, Maurer CA, Ruppert R, Ptok H, Strassburg J, Junginger T, et al. Lymph Node Dissection After Primary Surgery and Neoadjuvant Radiochemotherapy of Rectal Cancer. Interim Analysis of a Multicenter Prospective Observational Study (OCUM). Chirurg (2015) 86(12):1132–7. doi: 10.1007/s00104-015-0062-4
Keywords: rectal cancer, magnetic resonance imaging, metastasis, lymph node, lymph node staging, node-by-node
Citation: Zhuang Z, Zhang Y, Wei M, Yang X and Wang Z (2021) Magnetic Resonance Imaging Evaluation of the Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 11:709070. doi: 10.3389/fonc.2021.709070
Received: 13 May 2021; Accepted: 22 June 2021;
Published: 13 July 2021.
Edited by:
Francesca De Felice, Sapienza University of Rome, ItalyReviewed by:
Stepan Tucek, Masaryk Memorial Cancer Institute (MMCI), CzechiaCopyright © 2021 Zhuang, Zhang, Wei, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziqiang Wang, d2FuZ3ppcWlhbmdAc2N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.