- 1Department of Gynecology Oncology, Tianjin Central Hospital of Obstetrics and Gynecology, Tianjin, China
- 23D Medicines Inc., Shanghai, China
- 3Department of Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing, China
- 4Department of Gynecologic Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 5Department of Gynecology, Yunnan Tumor Hospital, The Third Affiliated Hospital of Kunming Medical University, Kunming, China
- 6Key Laboratory for Major Obstetric Diseases of Guangdong Province, Gynecology Department of the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 7Gynecology Department, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
Epithelial ovarian carcinoma (EOC) is one of the most common gynecologic malignancies with a high mortality rate. Serum biomarkers and imaging approaches are insufficient in identifying EOC patients at an early stage. This study is to set up a combination of proteins from serum small extracellular vesicles (sEVs) for the diagnosis of early-stage EOC and to determine its performance. A biomarker for early-stage ovarian cancer (BESOC) cohort was used as a Chinese multi-center population-based biomarker study and registered as a Chinese Clinical Trial ChiCTR2000040136. The sEV protein levels of CA125, HE4, and C5a were measured in 299 subjects. Logistic regression was exploited to calculate the odds ratio and to create the sEV protein model for the predicted probability and subsequently receiver-operating characteristic (ROC) analysis. The combined sEV marker panel of CA125, HE4, and C5a as a sEV model obtained an area under curve (AUC) of 0.912, which was greater than the serum model (0.809), by ROC analysis to identify EOC patients from the whole cohort. With the cutoff of 0.370, the sensitivity and specificity of the sEV model were 0.80 and 0.89, which were much better performance than the serum markers (sensitivity: 0.55~0.66; specificity: 0.59~0.68) and the risk of ovarian malignancy algorithm (ROMA) index approved by the U.S. Food and Drug Administration (sensitivity: 0.65; specificity: 0.61), to identify EOC patients from patients with benign ovarian diseases or other controls. The sEV levels of CA125 significantly differed among early-stage and late-stage EOC (p < 0.001). Moreover, the AUC of ROC to identify early-stage EOC patients was 0.888. Further investigation revealed that the sEV levels of these 3 proteins significantly decreased after cytoreductive surgery (CA125, p = 0.008; HE4, p = 0.025; C5a, p = 0.044). In summary, our study showed that CA125, HE4, and C5a levels in serum sEVs can identify EOC patients at the early stage, elucidating the possibility of using a sEV model for the diagnosis of early-stage EOC.
Introduction
Ovarian cancer is the leading gynecologic malignancy and the most common cause of gynecologic cancer death (1). Approximately 95% of primary ovarian malignancies originate from epithelial cells (2, 3). The prognosis of early-stage patients is satisfactory (the five-year survival rates of International Federation of Gynecology and Obstetrics (FIGO) stage I and II patients are 81.3% and 66.9%, respectively, and the five-year survival rates of FIGO stage III and IV patients are only 41.3% and 31.3%, respectively) (4). However, more than 60% of patients with ovarian cancer are diagnosed at advanced stages. The high mortality rate of epithelial ovarian carcinoma (EOC) can be attributed to the fact that the majority of patients are diagnosed with advanced disease (5). Therefore, an approach to identify EOC at an early, localized, and curable stage can significantly reduce mortality rate.
Unfortunately, attempts to detect EOC at an earlier stage using serum CA-125 and/or transvaginal ultrasonography (TVUS) have not been successful (6–8). Multiple studies have utilized serum CA-125 levels as a screening marker for ovarian cancer. Fifty to ninety percent of early EOC patients showed elevated CA-125 levels (9), but numerous other conditions can increase CA-125 levels (10, 11). The serum level of human epididymis protein 4 (HE4) showed higher sensitivity than CA-125 when identifying patients with ovarian cancer patients from patients with benign gynecologic disease (12). However, HE4 appears to differ due to multiple non-ovarian conditions such as pregnancy, menopause status, and rake (13–15). The secreted proteins of malignant cancer cells might be detected earlier; however, the signals of which are often masked by various proteins (e.g., albumin, immunoglobulin) in blood (16).
Small extracellular vesicles (sEVs) are bilayer membrane vesicles that contain proteins and nucleic acids, thus reflecting the contents of the cell from which these originate (17). Several studies have demonstrated the prominent roles of sEV in the progression of various cancers (18). Furthermore, sEVs can be isolated from plasma and serum to reduce the interference of other abundant plasma proteins (16). Therefore, the protein in or on top of sEVs can be a potential source of biomarkers for the detection of early-stage diseases. For example, Kalluri et al. reported that sEV covered with the proteoglycan glypican-1 may be a potential diagnostic tool for early-stage pancreatic cancer, with a sensitivity of 100% and specificity of 100% (19).
In a previous sEV proteomics study, the complement system is reported as one of the most over-expressed pathways in the sEVs of EOC patients (20). Complement component 5a (C5a), a core protein of the complement system, has been associated with the pathological status of EOC (21, 22). Having been detected in a sEV-proteomics study, we hypothesize that sEV levels of C5a may be potential utilized as a marker for EOC patients (20). In this study, we examined protein levels of sEV-derived CA125, HE4, and C5a as candidates of potential biomarkers for EOC, particularly at the early stages, in a larger and more complex Chinese cohort.
Materials and Methods
Study Design and Participants
The patients with ovarian cancer and benign ovarian disease in this study were enrolled in the Biomarker for Early Stage Ovarian Cancer (BESOC) cohort between 2016 and 2018. BESOC is a multi-center (n = 5, Tianjin Central Hospital of Gynecology Obstetrics, Fudan University Shanghai Cancer Center, the Third Affiliated Hospital of Kunming Medical University, the Third Affiliated Hospital of Guangzhou Medical University, and the First Affiliated Hospital of Chengdu Medical College) cohort (2016–2021) registered as a Chinese Clinical Trial ChiCTR2000040136 (http://www.chictr.org.cn/showproj.aspx?proj=63907) and enrolled subjects (over 20 years old) who presented an ovarian adnexal mass and went through surgery afterwards. To diversify the control group, gastric cancer or colorectal cancer patients were enrolled. The female patients with gastric cancer or colorectal cancer in this study were enrolled in the Gastrointestinal Cancer Cohort in 2017, which is a prospective, single-center (Changhai Hospital of Second Military Medical University) cohort that enrolled subjects (over 20 years old) who were diagnosed with gastrointestinal cancer. Blood samples and clinical information were collected on admission before cancer-related therapies (surgery, radiotherapy, or chemotherapy). All blood samples were collected in serum tubes and spun at 4,000 g for 10 min at 4°C to isolate the serum.
The healthy subject group consisted of age-matched healthy female volunteers (no diagnosis of any cancer, no family history of breast cancer or ovarian cancer, and ovarian-related-disease-free at least six months after sample collection), undergoing routine gynecologic examinations. The post-operation samples of 20 stage IIb or IIIc patients were collected 6 days after surgery (e.g., salpingo-oophorectomy, omentectomy, and pelvic and paraaortic lymph node dissection). All serum samples were stored at -80°C until analysis.
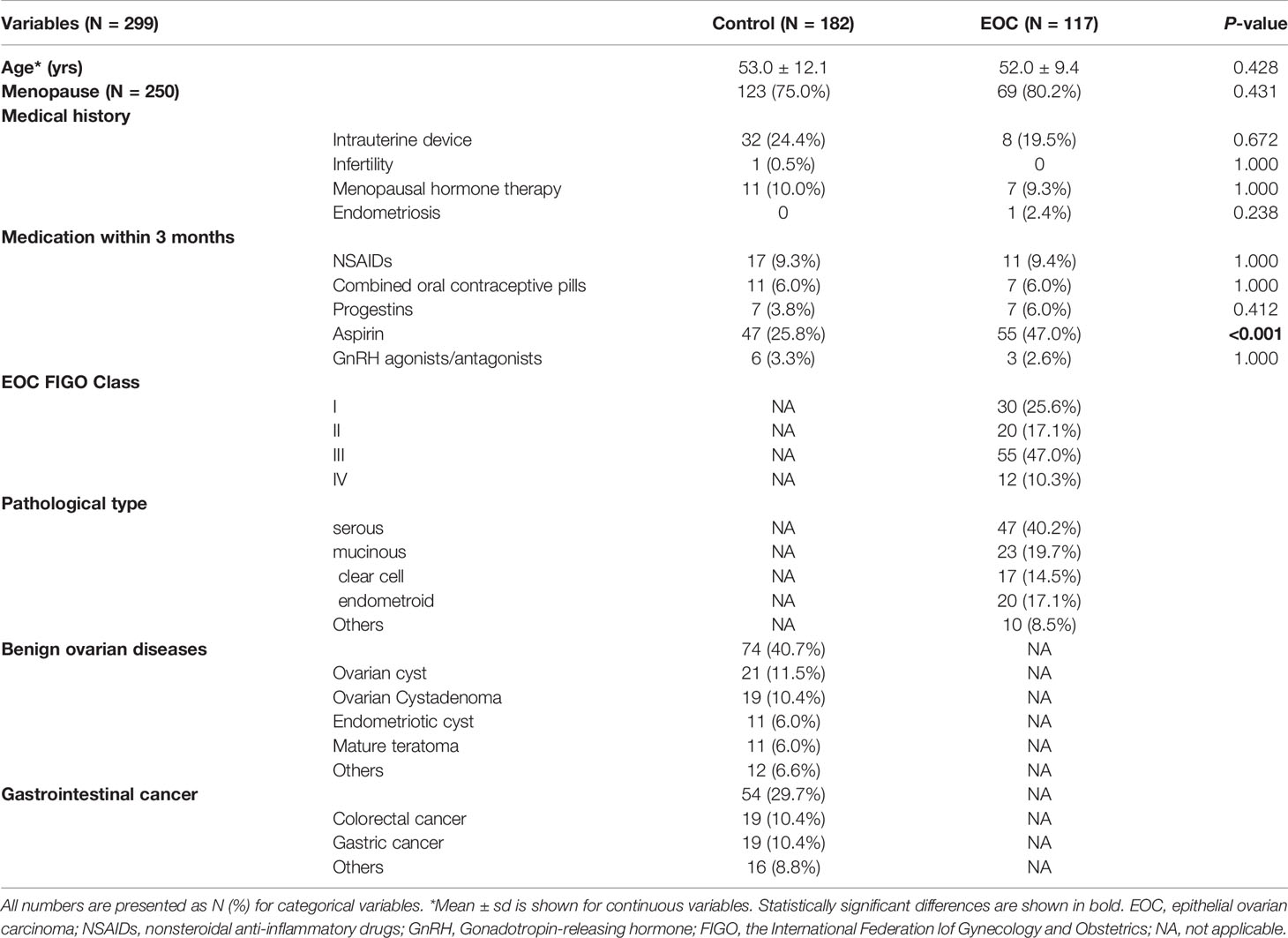
The selection of each subject was reviewed by two dedicated gynecologic pathologists, who were blinded to each other’s diagnosis and serum marker levels. The diagnosis and staging were decided based on the post-operation histopathology reports. Tumors were classified and divided into pathological subtypes: serous, mucinous, endometrioid, clear cell, and others. According to the FIGO classification criteria, all EOC patients were diagnosed as stage I to stage IV (23). In this study, early stages included stages I and II, and advanced stage included stages III and IV. A flowchart of this study is shown in Figure S1.
Isolation of Serum sEVs
The sEV isolation method was previously reported in detail (24) and strictly followed in this study. Briefly, 3D-EVN kit (3DMed Co., Ltd., #3DEVN3525, China Food and Drug Administration Ref. No.# SHMHMD20170019, v/v, 1:4) was added to 1 mL of serum and mixed until cloudy. The mixture was spun at 4,700 g at 4°C for 10 min. The pellet was lysed in 100 µL of a 3D-EVL lysis kit (3DMed Co., Ltd., #3DEVL0409) and used as 10-fold concentration to meet the detection range of the subsequent immunoassay.
Characterization of Serum sEVs
To characterize of the serum sEVs, western blotting (WB), nanoparticle tracking analysis (NTA), and electron microscopy (EM) were performed in this study. To carry out the EM analysis, the isolated sEVs were resuspended in PBS and fixed in 5% glutaraldehyde. After washing with PBS for 5 min, the sEVs were immobilized in 1% OsO4 in PBS and dehydrated with a series of ethanol concentrations (40%, 60%, 80%, and 96–98%). After the ethanol was evaporated, the samples were allowed to dry at ambient temperature for 24 h on Si substrate and then analyzed via EM (Hitachi High-Technologies, Tokyo, Japan) after gold-palladium sputtering. To perform the WB, the extraction of sEV proteins was done using the 3D-EVN kit (3DEVN3525; 3DMed, Shanghai, China) and sEVs were homogenized in RIPA lysis buffer with proteinase inhibitors (P0013B; Beyotime, Shanghai, China) on ice for 30 min. Then the lysed samples were centrifuged at 12,000 × g for 10 min at 4°C, and the protein concentration of the supernatant was measured using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, USA). The anti-Alix antibody (diluted 1:1000; cat. no. 2171; Cell Signaling Technology, Danvers, MA, USA), anti-CD9 antibody (diluted 1:500; cat. no.13,174; Cell Signaling Technology, Danvers, MA, USA) and anti-TSG101 polyclonal antibody (diluted 1:500; cat. no. abs115706; Absin Bioscience Inc., Shanghai, China) were used as the primary antibodies. Horseradish peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG antibodies (Beyotime Biotechnology, China) were used as the secondary antibodies. The antibody binding was detected using an enhanced chemiluminescence system according to the manufacturer’s protocol (Tanon-5200 Multi; Tanon Science & Technology Co. Ltd., Shanghai, China). For the particle size and concentration of serum sEVs, a Nanosight NS 300 system (NanoSight Technology, Malvern, UK) was used. Each sample was configured with a 488-nm laser and a high-sensitivity scientific complementary metal-oxide semiconductor camera, and measurement were performed in triplicate at camera setting 13 with an acquisition time of 30 s and a detection threshold setting of 7. At least 200 completed tracks were analyzed and obtained per video. Finally, the NTA analytical software (version 2.3) was used to analyze the nanoparticle tracking data of serum sEV samples.
Human Protein Level Measurement and ROMA Calculation
The levels of CA125 II and HE4 were measured by Cobas e 602 analyzer (Roche Diagnostics) with corresponding assays (Roche Diagnostics # 11776223 for CA125 II in U/mL and # 05950929 for HE4 in pmol/L) based on standard protocols (ISO15189:2012). The level of EV C5a levels were measured with ELISA (R&D Systems #DY2037, in ng/mL). All tests were run in duplicates.
In this study, the cut-offs of serum levels of CA125, HE4, and the risk of ovarian malignancy algorithm (ROMA) were adapted for the Chinese population based on the results of the clinical trial of Tian et al. (15). The cutoff of serum CA125 II levels was 35 U/mL (15). The cutoff for serum HE4 levels was 105.10 pmol/L for the overall Chinese population, 68.96 pmol/L for the premenopausal population, and 114.90 pmol/L for the postmenopausal population (15).
Based on the clearance of Roche Diagnostics from the Food and Drug Administration (FDA 510(k) #K153607), ROMA was calculated using the following algorithms:
Premenopausal: PI = -12.0 + 2.38 × LN(HE4) + 0.0626 × LN(CA125); and
Postmenopausal: PI = -8.09 + 1.04 × LN(HE4) + 0.732 × LN(CA125).
Menopause was defined as 12 months without a menstrual period.
ROMA Calculation Tool using Elecsys® assays value = Exp(PI)/(1 + exp(PI)) × 10.
The index of ROMA ≥ 1.14 and ≥ 2.99 indicates a high likelihood for the presence of epithelial ovarian cancer in premenopausal and postmenopausal women, respectively (25).
Statistical Analyses
Differences among groups (p-values) were analyzed using the Chi-square test for categorical variables, T-tests for normally distributed continuous variables, and Mann-Whitney-U-tests for continuous variables that were not normally distributed. The pre-/post operation comparison was conducted with using a paired t-test. To calculate the odds ratio (OR) and enable the direct comparison among variables, protein levels were converted into standard deviation units or z-scores using the observed value minus the mean value and divided by the standard deviation. Natural logarithm transformed values were used to reduce the effect of skewness in the distribution of protein levels. Differences with a p-value less than 0.05 were deemed statistically significant.
To assess the sensitivity and specificity of the serum sEV model, pathological diagnosis is regards as gold standard. In the EOC diagnosis: sensitivity = true positive/(true positive + false negative); specificity = true negative/(true negative + false positive); positive predictive value (PPV) = true positive/(true positive + false positive)*100%; negative predictive value (NPV) = true negative/(true negative + false negative) *100%.
Logistic regression was exploited to calculate the odds ratio and to create the sEV protein model for the predicted probability and subsequently receiver-operating characteristic (ROC) analysis. Both combined serum maker model and combined sEV protein model were built by entering the corresponding variables. All of the statistics were conducted with SPSS® (IBM®, version 24.0.0.0).
Results
Baseline Characteristics
In this study, 299 subjects were enrolled (Figure S1). In the event group, 117 patients were diagnosed as EOC without other gynecologic complications, and serous carcinoma was the most often diagnosed type (Table 1). A total of 50 (42.7%) patients were at stage I or II and 67 (57.3%) were at stage III or IV. Seventy-four patients with benign ovarian diseases, 54 apparently healthy subjects, and 54 patients with gastrointestinal cancer were used as controls. Patients in the EOC group were significantly more often taking aspirin (47% vs. 26%) within 3 months prior to sample collection.
Characterization of Serum sEVs
EM, WB, and NTA results to qualify the minimum information for studies of extracellular vesicles 2018 (MISEV2018) (26) are shown (Figure S2). EM analysis of representative sample showed that serum small EVs isolated in this study were bowl-shaped (Figure S2A). In addition, the sEV protein markers Alix, TSG101 and CD9 were present in the six representative samples using WB (Figure S2B). The size distribution of serum sEVs showed a main peak around ~60 nm by NTA analysis (Figure S2C).
Serum sEV and Serum Protein Levels Between the EOC and Control Groups
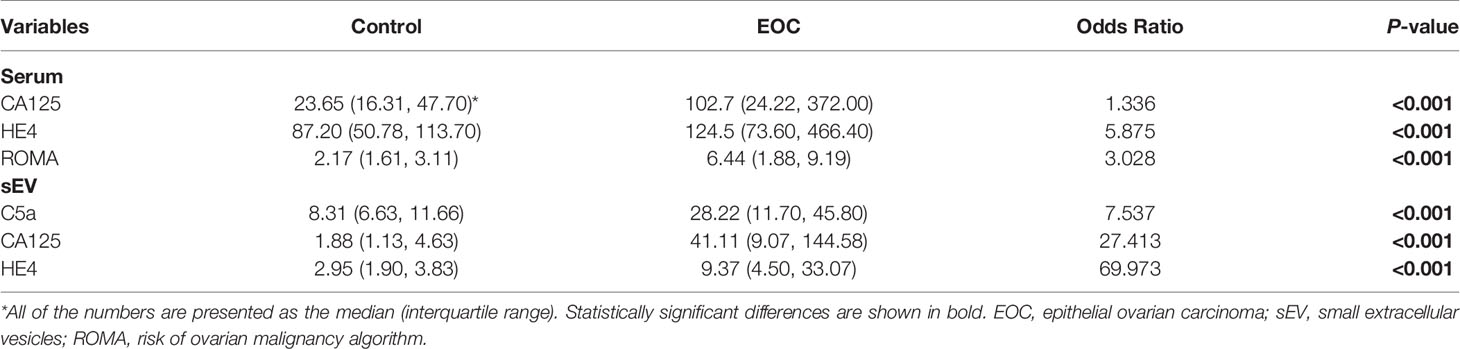
All of the measured protein levels in both serum sEVs and serum were significantly different between the EOC and control groups. The levels of C5a (OR 7.537, p < 0.001), CA125 (OR 27.413, p < 0.001), and HE4 (OR 69.973, p < 0.001) in the EVs were significantly higher in EOC patients compared to controls (Table 2). Although the serum level of CA125 and HE4 as well as ROMA index were also significantly higher in the EOC group (Table 2), at the corresponding cutoff points, the sensitivity of these 2 markers was 0.66 and 0.56, and specificity was 0.68 and 0.68, respectively (Table 3). Moreover, the sensitivity and specificity of the ROMA index were 0.65 and 0.61, respectively (Table 3). Notably, the odds ratios of serum sEV proteins were obviously higher than the serum markers.
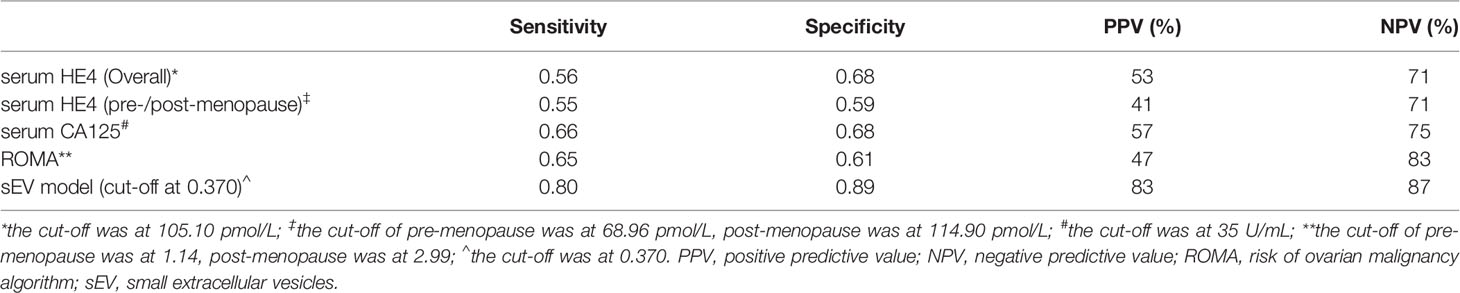
Table 3 The sensitivity and specificity of serum markers and sEV model to identify the EOC patients from the whole cohort.
Serum sEV Proteins to Identify Early-Stage EOC Patients From Other Groups
After establishing that serum sEV protein levels are related to EOC, we then examined the potential of identifying early-stage patients. All of the protein levels were significantly higher in the late-stage group compared with the early-stage group (p < 0.05). However, serum CA125 levels or ROMA indices showed no difference among the early-stage, benign ovarian disease, and other cancer groups. Serum HE4 levels were significantly higher in the early-stage group than in the healthy and benign ovarian disease groups (Table 4). Serum sEV C5a levels were significantly lower in the healthy group compared to the early-stage group (p = 0.007), and almost showed a significant difference between the early-stage group and the benign ovarian disease and other cancer groups (p = 0.085 and 0.061, respectively). Meanwhile, serum sEV CA125 and HE4 levels significantly differed between the early-stage and any other group (Table 4). This implies the potential of serum sEV proteins to discriminate early-stage EOC patients from healthy subjects, benign ovarian disease patients, and other gastrointestinal cancer patients.
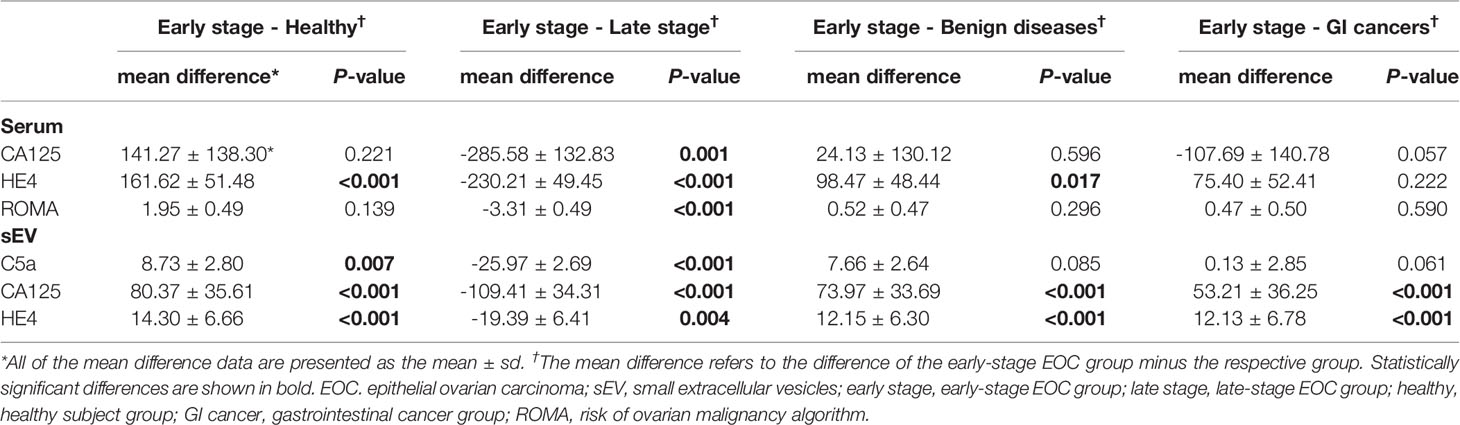
Table 4 Comparison of protein levels from serum sEVs and serum in early-stage EOC patients versus healthy subjects, late-stage EOC, benign ovarian disease, and gastrointestinal cancer patients.
Serum sEV Protein Levels Differ With EOC FIGO Stage
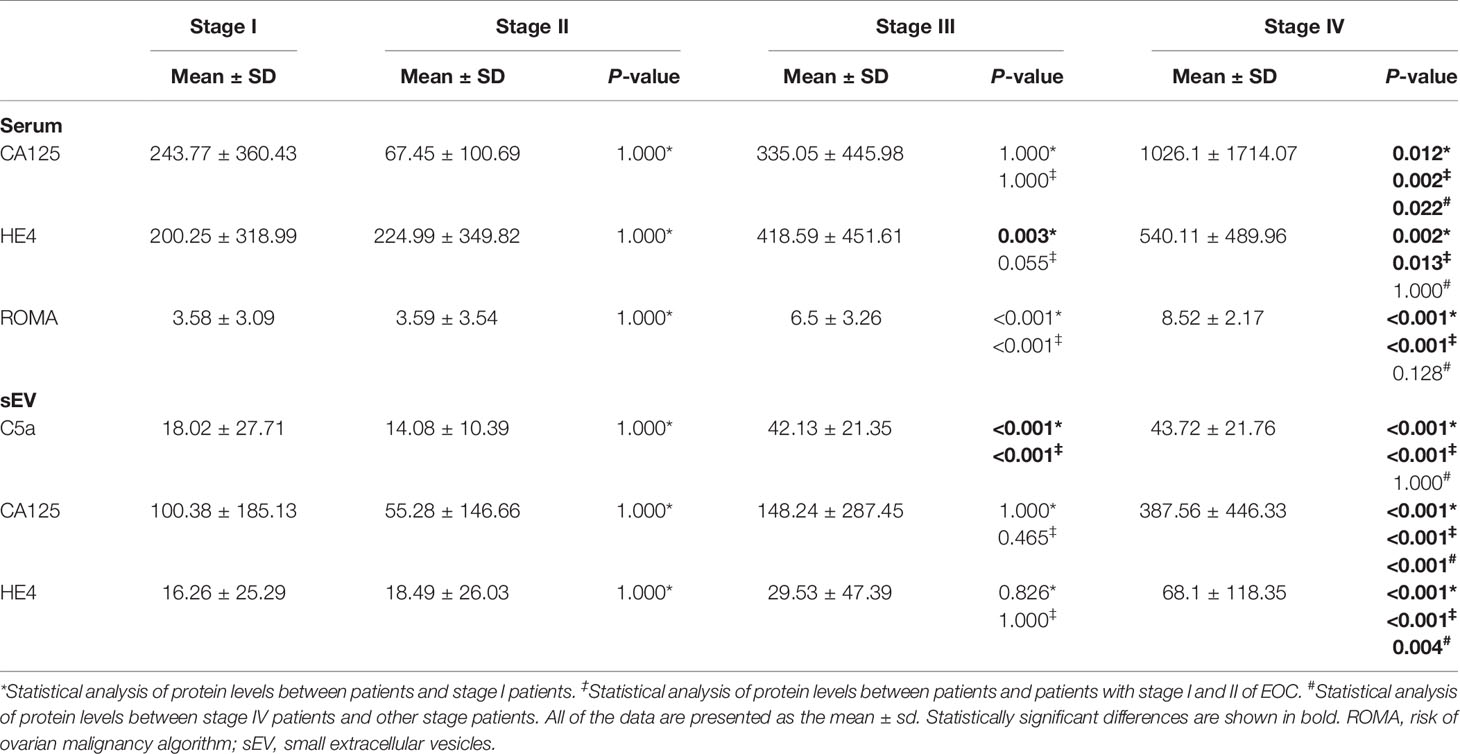
With the observation of the difference of serum sEV proteins between early- and late-stage groups, we further examined serum sEV protein levels among EOC FIGO stages. No protein levels differed between c I and II patient groups (Table 5). Serum HE4 levels, ROMA indices, and serum sEV C5a levels were significantly higher in stage III patients compared to stage I and II patients (p < 0.001). All of the protein levels in stage IV patients significantly increased compared to other stages, except that ROMA indices, serum HE4 levels, and serum sEV C5a levels showed no difference between stage III and stage IV patients.
Diagnosis of EOC Using Serum sEV Markers
The predicted probability of combined serum sEV levels of CA125, HE4, and C5a (serum sEV model) as well as combined serum CA125 and HE4 (serum model) were used for ROC analysis. The area under curve (AUC) of ROC analysis of the serum sEV model (0.912) was greater than the serum model (0.809) (Figure 1). This indicated that the serum sEV model has better diagnosis performance compared to the combined serum model. The algorithm of serum sEV model is presented below.

Figure 1 Receiver operating characteristic (ROC) analysis for identifying EOC using serum sEV or serum marker panel models. The serum sEV model consists of serum sEV levels of C5a, CA125, and HE4. Serum model includes serum level of CA125 and HE4 levels.
When the cutoff point was 0.370, the corresponding sensitivity was 0.80 and specificity was 0.89 (Table 3). The serum sEV model demonstrated much better diagnosis performance than serum marker alone or ROMA index.
The potential of serum sEV markers to identify early-stage EOC patients was further evaluated. The population was further narrowed down to early-stage EOC patients, healthy subjects, and benign ovarian cancer patients. With the calculated predicted probability above, the AUC of ROC to distinguish EOC patients was 0.888 (Figure S3). When the cutoff was set at 0.154, the sensitivity and specificity were 0.88 and 0.74, respectively (Table 6). The performance of the serum sEV model was better than ROMA, serum CA125, and serum HE4 at the set cutoff point. Even when the cutoff of serum sEV model remained at 0.370, the sensitivity and specificity were 0.58 and 0.68 (Table 6), respectively, which were still better than the ROMA index and serum markers.

Table 6 The sensitivity and specificity of serum markers and sEV model to identify the early stage EOC patients from controls.
Serum sEV Protein Levels Decrease After Operation
To assess the correlation between serum sEV protein markers and tumor burden, we compared the serum sEV levels of C5a, CA125, and HE4 of 20 patient samples prior to surgery and postoperation. All three serum sEV markers significantly decreased after cytoreductive surgery (Table 7). This is suggestive of the positive correlation between tumor burden and serum sEV markers.
Discussion
The survival rate of EOC is closely related to the stage at diagnosis, with those diagnosed at the earlier stage with a greater chance of survival (23). In this study, we have established a model using the levels of CA125, HE4, and C5a in serum sEVs to identify EOC patients and early-stage EOC patients from subjects with benign ovarian diseases or other diseases. This novel serum sEV model has been compared with the current serum protein marker HE4 and CA125, as well as ROMA, which was approved by the U.S. FDA (510(k) Number: k103358). The results have demonstrated that serum sEV model is much better diagnosis performance than serum marker alone or ROMA index in the diagnosis of EOC patients, including early-stage EOC. This provides the possibility of the levels of serum sEV proteins for the diagnosis of early-stage EOC.
Several studies have explored the potential of serum sEV content (e.g., mRNA, miRNA, protein) as biomarkers for ovarian cancer (27, 28). These studies mainly employed ultracentrifugation to isolate serum sEVs, which may be regarded as the “gold standard” of serum sEV isolation (26). However, ultracentrifugation often involves a large volume of plasma or serum, hours of spinning in a vacuum and strict temperature control, costly equipment, and maintenance. This is thus challenging to implement for clinical utilization. On the contrary, in this study, we adapted a polyethylene glycol (PEG)-based approach to isolate serum sEVs (24), which has advantages of being high-throughput, with better reproducibility, and lower cost.
Over the years, several tests have been developed to identify malignant ovarian cancer, including serologic markers (e.g., CA125 and HE4), ultrasonography, imaging, and combined multimodalities. Transvaginal ultrasonography (TVUS) (29) and computed tomography (CT) (30) have been proven to be inefficient in screening early-stage EOC, which may further be underutilized in less developed clinical circumstances such as poor staffing and equipment availability (31). Both the Prostate, Lung, Colorectal, and Ovarian (PLCO) (32) Cancer Screening Trial and the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOS) (29) showed that screening ovarian cancer in asymptomatic women using serum CA125 levels and/or TVUS has brought little benefit. Based on its cost-effectiveness and demographic coverage, biomarkers have the highest potential for the early diagnosis and screening of EOC (31).
Serum levels of CA125 and HE4 are both well-established biomarkers for ovarian cancer. So far, both markers are only intended to monitor the progress and estimate the treatment efficacy of EOC. However, the levels of these two proteins in serum sEVs showed better capability of distinguishing EOC patients from non-EOC patients. The increase in serum sEV CA125 and HE4 levels in EOC patients is coincided with the findings of previous proteomics studies (33).
The discovery of novel serological protein biomarkers mainly relies on mass spectrometry and proteomics. During the discovery phase of proteomics, the bona fide candidate markers are masked by high-abundance proteins (e.g., albumin and immunoglobulins), which account for over 99% of the total proteins. However, the percentage of these abundant proteins is significantly reduced in extracellular vesicles (16), which makes serum sEVs a potential source for biomarker discovery. In our previous study, complementary-, coagulation-, apoptosis-related pathways were discovered to be significantly overexpressed in serum sEVs of EOC patients (20).
Our results on ROMA indices are discordant to those of previous studies (34) and may be attributed to three causes. First, the Chinese population showed nearly 30% lower serum HE4 levels (overall cut-off: 105.10 pmol/L) compared to Caucasians (overall cutoff: 140.00 pmol/L) (15). In addition, neither the algorithm nor the cutoff for the ROMA index has been adjusted for the Chinese population. Second, the U.S. Food and Drug Administration (FDA) clearances of serum CA125 II (510(k) Number K143534) (35) and HE4 (510(k) Number: k112624) (36) are employed to aid the detection of residual or recurrent ovarian carcinoma and to monitor disease progression or response to therapy. ROMA (510(k) Number: k103358) (37) was approved by the US FDA for “assessing whether a premenopausal or postmenopausal woman who presents with an ovarian adnexal mass is at high or low likelihood of finding malignancy on surgery”. However, owing to lack of evidence of clinical trials on the Chinese population, this intended use has not been approved by the National Medical Products Administration. Finally, the cohort composition of the present study is different. The Roche ROMA cohort consists of approximately 80% benign ovarian diseased patients and 10% EOC (about 20% early stage and 80% late stage), but this cohort comprised approximately 40% EOC (about 40% early stage and 60% late stage) and 25% benign ovarian diseased patients (34, 37).
The mechanism of the progression of EOC remains unclear. With the concurrence of multiple foci and carcinomatosis after ovary-removal surgery (38), detection of EOC or postoperative recurrence at the early stage is challenging using non-invasive imaging tools. Although this serum sEV protein marker panel can detect EOC better at the early stages, there is still a need to establish a marker panel for early diagnosis or even as a screening tool for EOC patients. Therefore, more markers need to be discovered and validated for better marker panels not only to screen EOC but also to reveal the underlying mechanism of EOC.
Conclusion
In summary, our study disclosed that CA125, HE4, and C5a levels in serum sEVs can identify EOC at the early stage. In spite of the complexity of clinical diseases, comparison of serum protein marker alone or ROMA index using a multi-center population-based study in the Chinese population confirmed that serum sEV model has relatively high performance for the diagnosis of early-stage EOC. Further test this model in an even larger and more complex population to determine its performance and limitations is required.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
This study has received approval from the Ethics Committee of Tianjin Central Hospital of Gynecology Obstetrics (IRB#2018KY002), Fudan University Shanghai Cancer Center (IRB#1909182-4), the Third Affiliated Hospital of Kunming Medical University (IRB#2016NS089), the Third Affiliated Hospital of Guangzhou Medical University (IRB#2018CYFYHEC-021-02), and the First Affiliated Hospital of Chengdu Medical College [IRB#201807(3)]. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YH, YaZ, HW, and DZ conceived and designed the study. PL, YB, BS, WZ, ZL, YiZ, XX, QC, XS, XD, and ZG performed the experiments. PL, YB, BS, YaZ, and DZ contributed to the data analysis. PL, BS, DZ, YaZ, HW, and YH wrote and revised the manuscript All authors contributed to the article and approved the submitted version.
Conflict of Interest
Authors YB, ZL, XX, DZ, and YaZ were employed by 3D Medicines.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate the support and participation of the physicians and patients in this study. We would like to thank Peng Zeng and Min Yang for their help to this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.707658/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian Cancer: Changes in Patterns at Diagnosis and Relative Survival Over the Last Three Decades. Am J Obstet Gynecol (2003) 189:1120–7. doi: 10.1067/S0002-9378(03)00579-9
3. Robboy SJ, Russell P, Anderson MC, Prat J, Mutter GL. Robboy’s Pathology of the Female Reproductive Tract. New York, NY: Elsevier Health Sciences (2009).
4. Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. (eds) SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD (2017) p. 25. https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
5. Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal Primary Surgical Treatment for Advanced Epithelial Ovarian Cancer. Cochrane Database Syst Rev (2011) 2011(8):CD007565. doi: 10.1002/14651858.CD007565.pub2
6. Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, et al. A Randomized Study of Screening for Ovarian Cancer: A Multicenter Study in Japan. Int J Gynecol Cancer (2008) 18:414–20. doi: 10.1111/j.1525-1438.2007.01035.x
7. Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and Specificity of Multimodal and Ultrasound Screening for Ovarian Cancer, and Stage Distribution of Detected Cancers: Results of the Prevalence Screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol (2009) 10:327–40. doi: 10.1016/S1470-2045(09)70026-9
8. Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clin Trials (2000) 21:273S–309S. doi: 10.1016/S0197-2456(00)00098-2
9. Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of Screening on Ovarian Cancer Mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA (2011) 305:2295–303. doi: 10.1001/jama.2011.766
10. Mol BW, Bayram N, Lijmer JG, Wiegerinck MA, Bongers MY, van der Veen F, et al. The Performance of CA-125 Measurement in the Detection of Endometriosis: A Meta-Analysis. Fertil Steril (1998) 70:1101–8. doi: 10.1016/S0015-0282(98)00355-0
11. Topalak O, Saygili U, Soyturk M, Karaca N, Batur Y, Uslu T, et al. Serum, Pleural Effusion, and Ascites CA-125 Levels in Ovarian Cancer and Nonovarian Benign and Malignant Diseases: A Comparative Study. Gynecol Oncol (2002) 85:108–13. doi: 10.1006/gyno.2001.6575
12. Shah CA, Lowe KA, Paley P, Wallace E, Anderson GL, McIntosh MW, et al. Influence of Ovarian Cancer Risk Status on the Diagnostic Performance of the Serum Biomarkers Mesothelin, HE4, and CA125. Cancer Epidemiol Prev Biomarkers (2009) 18:1365–72. doi: 10.1158/1055-9965.EPI-08-1034
13. Moore RG, Miller MC, Eklund EE, Lu KH, Bast RC Jr, Lambert-Messerlian G. Serum Levels of the Ovarian Cancer Biomarker HE4 Are Decreased in Pregnancy and Increase With Age. Am J Obstet Gynecol (2012) 206:349. e1–349. e7. doi: 10.1016/j.ajog.2011.12.028
14. Chang X, Ye X, Dong L, Cheng H, Cheng Y, Zhu L, et al. Human Epididymis Protein 4 (HE4) as a Serum Tumor Biomarker in Patients With Ovarian Carcinoma. Int J Gynecol Cancer (2011) 21:852–8. doi: 10.1097/IGC.0b013e31821a3726
15. Tian Y, Wang C, Cheng L, Zhang A, Liu W, Guo L, et al. Determination of Reference Intervals of Serum Levels of Human Epididymis Protein 4 (HE4) in Chinese Women. J Ovarian Res (2015) 8:72. doi: 10.1186/s13048-015-0201-z
16. Boukouris S, Mathivanan S. Exosomes in Bodily Fluids Are a Highly Stable Resource of Disease Biomarkers. Proteomics Clin Appl (2015) 9:358–67. doi: 10.1002/prca.201400114
17. Zhang Y, Vernooij F, Ibrahim I, Ooi S, Gijsberts CM, Schoneveld AH, et al. Extracellular Vesicle Proteins Associated With Systemic Vascular Events Correlate With Heart Failure: An Observational Study in a Dyspnoea Cohort. PloS One (2016) 11:e0148073. doi: 10.1371/journal.pone.0148073
18. Colombo M, Raposo G, Thery C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Biol (2014) 30:255–89. doi: 10.1146/annurev-cellbio-101512-122326
19. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 Identifies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature (2015) 523:177. doi: 10.1038/nature14581
20. Zhang W, Ou X, Wu X. Proteomics Profiling of Plasma Exosomes in Epithelial Ovarian Cancer: A Potential Role in the Coagulation Cascade, Diagnosis and Prognosis. Int J Oncol (2019) 54:1719–33. doi: 10.3892/ijo.2019.4742
21. Cho MS, Vasquez HG, Rupaimoole R, Pradeep S, Wu S, Zand B, et al. Autocrine Effects of Tumor-Derived Complement. Cell Rep (2014) 6:1085–95. doi: 10.1016/j.celrep.2014.02.014
22. Nunez-Cruz S, Gimotty PA, Guerra MW, Connolly DC, Wu Y-Q, DeAngelis RA, et al. Genetic and Pharmacologic Inhibition of Complement Impairs Endothelial Cell Function and Ablates Ovarian Cancer Neovascularization. Neoplasia (New York NY) (2012) 14:994. doi: 10.1593/neo.121262
23. Gray HJ, Garcia RL. Cancer of the Ovary, Fallopian Tube, and Peritoneum: Staging and Surgical Management. Waltham, MA: UpToDate (2019). (cited 2019-03-24): UpToDate.
24. Zhang J, Qin H, Man Cheung FK, Su J, Zhang D, Liu S-Y, et al. Plasma Extracellular Vesicle microRNAs for Pulmonary Ground-Glass Nodules. J Extracell Vesicles (2019) 8:1663666. doi: 10.1080/20013078.2019.1663666
25. Cradic KW, Lasho MA, Algeciras-Schimnich A. Validation of the Cut-Points Recommended for ROMA Using the Roche Elecsys CA125 and HE4 Assays. Ann Clin Lab Sci (2018) 48:90–3.
26. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell Vesicles (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
27. Giannopoulou L, Zavridou M, Kasimir-Bauer S, Lianidou ES. Liquid Biopsy in Ovarian Cancer: The Potential of Circulating miRNAs and Exosomes. Trans Res (2018) 205:77–91. doi: 10.1016/j.trsl.2018.10.003
28. Sharma S, Zuñiga F, Rice GE, Perrin LC, Hooper JD, Salomon C. Tumor-Derived Exosomes in Ovarian Cancer - Liquid Biopsies for Early Detection and Real-Time Monitoring of Cancer Progression. Oncotarget (2017) 8:104687–703. doi: 10.18632/oncotarget.22191
29. Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian Cancer Screening and Mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A Randomised Controlled Trial. Lancet (2016) 387:945–56. doi: 10.1016/S0140-6736(15)01224-6
30. Pickhardt PJ, Hanson ME. Incidental Adnexal Masses Detected at Low-Dose Unenhanced CT in Asymptomatic Women Age 50 and Older: Implications for Clinical Management and Ovarian Cancer Screening. Radiology (2010) 257:144–50. doi: 10.1148/radiol.10100511
31. World-Health-Organization. Guide to Cancer Early Diagnosis. Geneva, Switzerland: World Health Organization (2017).
32. Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, et al. Ovarian Cancer Screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: Findings From the Initial Screen of a Randomized Trial. Am J Obstet Gynecol (2005) 193:1630–9. doi: 10.1016/j.ajog.2005.05.005
33. Shender VO, Pavlyukov MS, Ziganshin RH, Arapidi GP, Kovalchuk SI, Anikanov NA, et al. Proteome–Metabolome Profiling of Ovarian Cancer Ascites Reveals Novel Components Involved in Intercellular Communication. Mol Cell Proteomics (2014) 13:3558–71. doi: 10.1074/mcp.M114.041194
34. Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A Novel Multiple Marker Bioassay Utilizing HE4 and CA125 for the Prediction of Ovarian Cancer in Patients With a Pelvic Mass. Gynecol Oncol (2009) 112:40–6. doi: 10.1016/j.ygyno.2008.08.031
35. FDA. (2019). Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K143534.
36. FDA. (2019). Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K112624.
37. FDA. (2019). ROMA. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K103358.
Keywords: early diagnosis, epithelial ovarian carcinoma, multi-center population-based study, protein contents, serum, small extracellular vesicle
Citation: Li P, Bai Y, Shan B, Zhang W, Liu Z, Zhu Y, Xu X, Chen Q, Sheng X, Deng X, Guo Z, Zhang D, Wang H, Zhang Y and Hu Y (2021) Exploration of Potential Diagnostic Value of Protein Content in Serum Small Extracellular Vesicles for Early-Stage Epithelial Ovarian Carcinoma. Front. Oncol. 11:707658. doi: 10.3389/fonc.2021.707658
Received: 10 May 2021; Accepted: 19 August 2021;
Published: 15 September 2021.
Edited by:
Jian-Jun Wei, Northwestern University, United StatesReviewed by:
Antoni Llueca Abella, University of Jaume I, SpainStephanie M. McGregor, University of Wisconsin-Madison, United States
Irinel Popescu, Fundeni Clinical Institute, Romania
Copyright © 2021 Li, Bai, Shan, Zhang, Liu, Zhu, Xu, Chen, Sheng, Deng, Guo, Zhang, Wang, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanjing Hu, SnVsaWFubmFfaHVAMTYzLmNvbQ==; Yanan Zhang, eWFuYW56aGFuZ0AzZG1lZGNhcmUuY29t; Huaying Wang, d2FuZ2h1YXlpbmcyNzBAMTYzLmNvbQ==; Dadong Zhang, ZGFkb25nLnpoYW5nQDNkbWVkY2FyZS5jb20=
†These authors have contributed equally to this work and share first authorship
 Pu Li1†
Pu Li1† Boer Shan
Boer Shan Xiaoya Xu
Xiaoya Xu Yanan Zhang
Yanan Zhang Yuanjing Hu
Yuanjing Hu