
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 January 2022
Sec. Breast Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.706606
This article is part of the Research Topic Advancements in Immunology and Immunotherapy for Breast Cancer View all 16 articles
Introduction: Neoadjuvant trastuzumab plus chemotherapy may affect programmed death-ligand 1 (PD-L1) expression and tumor-infiltrating lymphocytes (TILs) in HER2-positive breast cancer. Discordant results were shown on the correlation between PD-L1 expression or TILs and the effectiveness of neoadjuvant therapy in HER2-positive breast cancer patients. This study aimed to clarify the predictive value of PD-L1 expression and TILs in neoadjuvant therapy in patients with HER2-positive breast cancer.
Methods: HER2-positive breast cancer cases receiving neoadjuvant treatment (NAT; n = 155) were retrospectively collected from July 2013 to November 2018. Histopathologic analysis of TILs was performed on hematoxylin and eosin (H&E)-stained sections from pre- and post-NAT specimens. The TIL score as a categorical variable can be divided into high (≥30%) and low (<30%) categories. The expression of PD-L1 was detected by immunohistochemistry, and the percentage of positive membranous staining (at least 1%) in tumor cells (PD-L1+TC) and TILs (PD-L1+TILs) was scored.
Results: In our study, 87 patients received neoadjuvant chemotherapy alone and 68 received neoadjuvant trastuzumab plus chemotherapy. Multivariate logistic regression analysis confirmed that lymph node metastasis, high TILs, and PD-L1+TILs in pre-neoadjuvant therapy specimens were independent predictors of pathological complete response (pCR) in neoadjuvant therapy (p < 0.05, for all). Among all patients, TILs were increased in breast cancer tissues post-neoadjuvant therapy (p < 0.001). Consistent results were found in the subgroup analysis of the trastuzumab plus chemotherapy group and the chemotherapy alone group (p < 0.05, for both). In 116 non-pCR patients, PD-L1+TC was decreased in breast cancer tissues post-neoadjuvant therapy (p = 0.0219). Consistent results were found in 43 non-pCR patients who received neoadjuvant trastuzumab plus chemotherapy (p = 0.0437). However, in 73 non-pCR patients who received neoadjuvant chemotherapy, there was no significant difference in PD-L1+TC expression in pre- and post-neoadjuvant therapy specimens (p = 0.1465). On the other hand, in the general population, the neoadjuvant trastuzumab plus chemotherapy group, and the neoadjuvant chemotherapy group, PD-L1+TILs decreased after treatment (p < 0.05, for both).
Conclusion: Higher TIL counts and PD-L1+TILs in pre-neoadjuvant therapy specimens and lymph node metastasis are independent predictors of pCR in patients with HER2-positive breast cancer treated with neoadjuvant therapy. TIL counts, PD-L1+TC, and PD-L1+TILs changed before and after neoadjuvant trastuzumab plus chemotherapy for HER2-positive breast cancer, which may suggest that, in HER2-positive breast cancer, neoadjuvant trastuzumab plus chemotherapy may stimulate the antitumor immune effect of the host, thereby preventing tumor immune escape.
Trastuzumab plus pertuzumab and chemotherapy have been confirmed as the neoadjuvant therapy for stage II–III HER2-positive breast cancer (1). Before 2020, since pertuzumab was not included in medical insurance, many patients with HER2-positive breast cancer still choose trastuzumab plus chemotherapy as neoadjuvant treatment. In HER2-positive breast cancer, neoadjuvant trastuzumab plus chemotherapy can dramatically increase effectiveness compared to chemotherapy alone. However, there were still 25% of patients who showed tumor progression after treatment, thus affecting the prognosis (1–4). Therefore, there is an urgent need to find an accurate and reliable biomarker to predict who will benefit from this treatment. Up to now, several clinical factors, such as lymph node metastasis, tumor size, and hormone receptor (HR) expression, have been correlated with the efficacy of neoadjuvant treatment (NAT) (5, 6). However, choosing NAT based on the above factors does not benefit all patients. Therefore, a molecular marker that can reliably and efficiently assess the effectiveness of NAT is critical in HER2-positive breast cancer.
Programmed death-ligand 1 (PD-L1) is a B7 immune molecule transmembrane protein found in several tumor cells and immune cells, which mediates tumor immunosuppression and is linked to tumor cell immune escape (7, 8). Research shows that trastuzumab can affect PD-L1 expression on CD8+ T cells and cancer cells in HER2-positive breast cancer (9–11). However, another study observed that trastuzumab could downregulate the effects of PD-L1 on cancer cells through HER2 inhibition (10). Furthermore, the PANACEA trial also proposed the hypothesis that trastuzumab can reverse tumor-mediated immunosuppression and activate the local antitumor immune effect (12). Chemotherapy can also cause immunogenic cell death and cellular damage (13). However, the impact of PD-L1 expression on cancer cells and lymphocytes in HER2-positive breast cancer remains unknown.
Tumor-infiltrating lymphocytes (TILs), as immune cells that penetrate tumor tissues, may be associated with immune-mediated tumor–host interaction and antibody-dependent cell-mediated cytotoxicity (ADCC) (14–16). Previous research suggested that increased baseline TILs in patients may be related to the benefit of the anti-HER2 monoclonal antibody trastuzumab (17–20). However, another study showed that high levels of TILs were linked to a lack of benefits from trastuzumab therapy (21). Consequently, the effects of TILs on neoadjuvant trastuzumab plus chemotherapy on HER2-positive breast cancer patients remain a mystery.
Recent research has shown that the active HER2 oncogene regulates the mobilization and activation of tumor-infiltrating immune cells and the therapeutic activity of trastuzumab (22, 23). In several trials, elevated levels of TILs were linked to the benefits of trastuzumab plus chemotherapy. However, experiments on the predictive value of PD-L1 and TILs in the effectiveness of NAT for HER2-positive breast cancer patients showed discordant results. They mainly emphasized the correlation between the expression of PD-L1 or TILs and the efficiency of NAT in the tissue; however, there were no differences in the harmonizing tissues prior to and following NAT.
This research aimed to investigate how TILs and PD-L1 expression in paired tissues changed prior to and following NAT, as well as the relationship between these improvements and the effectiveness of neoadjuvant trastuzumab plus chemotherapy in HER2-positive breast cancer patients.
Data were obtained from 155 cases of HER2-positive invasive breast cancer patients at the Shandong Cancer Hospital from July 2013 to November 2018. Diagnosis of patients was confirmed histologically by core needle biopsy, and the stage of the disease was clarified using ultrasonography (US), bone scintigraphy, and computed tomography (CT). The medical and pathology records of these patients were examined through the hospital medical record system. A flowchart summarizing the patient selection process followed is shown in Figure 1. We accessed formalin-fixed, paraffin-embedded tissue samples from NAT patients. A proportion of patients receiving NAT were treated with a taxane-containing regimen along with platinum or an anthracycline. Another proportion of patients received anti-HER2 trastuzumab combined with chemotherapy. The following clinicopathologic variables were acquired: age, tumor dimension, status of the lymph node, initial HR, Ki-67 proliferation index, and neoadjuvant therapy (with and without trastuzumab). Pathological complete response (pCR) was identified as noninvasive breast cancer and axillary lymph nodes remaining after NAT (ypT0 ypN0).
Immunohistochemical staining was performed on 155 paired breast cancer surgery and biopsy samples with 3.7% neutral formaldehyde, the samples were embedded in paraffin, and 4-μm-thick serial parts were fixed to the samples. This was followed by xylene dewaxing, ethanol graded hydration, ethylenediaminetetraacetic acid (EDTA) antigen repair solution, phosphate-buffered saline (PBS) rinsing (1:50 dilution; clone SP142, Ventana, Shanghai Roche Diagnostic Products Limited Company, Shanghai, China) overnight in a 37°C incubator, treatment with goat anti-mouse/rabbit IgG polymer secondary antibody dropwise, and 3,3′-diaminobenzidine (DAB) development. Contrast dyeing of hematoxylin was performed, followed by ethanol dehydration and sealing. Immunophenotyping was carried out using the following antibodies: anti-ER (clone 6F11; Leica Microsystems, Bannockburn, IL, USA), anti-PR (clone 16; Leica Microsystems), anti-HER2/neu (Ventana 4B5; Ventana Medical Systems, Tucson, AZ, USA), and Ki-67 (MIB-1; Ventana Medical Systems). Estrogen receptor (ER) and progesterone receptor (PR) positivity was defined as staining of ≥1% tumor cell nuclei, while HER2 positivity was evaluated following the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) criteria (24). Briefly, sections with a HER2/CEP17 ratio of ≥2.0 and copy number ≥4.0 or a dual-probe HER2/CEP17 ratio of <2.0 with ≥6.0 HER2 signal per nucleus were determined as positive. A HER2-negative status was defined as a HER2/CEP17 ratio ≥2.0 with <4.0 HER2 signal per nucleus, or a HER2/CEP17 ratio <2.0 and ≥4.0 + <6.0 HER2 signal per nucleus, or a HER2/CEP17 ratio <2 and <4.0 HER2 signal per nucleus. The Ki-67 status was determined by analyzing at least 500 cancer cells per patient. Five high-power-field images were used for each section. Patients were categorized into three groups based on the percentages of Ki-67-positive tumor cells: low, <15%; intermediate, 15%–30%; and high, >30% (25).
In this study, a two-step immunohistochemistry method was used. The PD-L1 antibody reagent is a rabbit monoclonal antibody (ZZR3). PD-L1 on the tumor cell (TC) membrane or cytoplasm was recorded as PD-L1+TC, and the expression on TILs was recorded as PD-L1+TILs (Figure 2). The monoclonal antibody was used to stain breast cancer pathological sections using established methods (26, 27). A 5% increase from 0% to 100% was observed in carcinoma cells with direct membrane PD-L1 expression; less than 1% had a negative markup. For each tumor, the mean PD-L1 labeling was calculated across all cells (28). PD-L1 expression (in percentage) by TILs was also documented in 5% increments and scored as none (0), focal (1+; <5%), moderate (2+; 5%–50%), or severe (3+; 51%–100%). If the PD-L1-positive membrane staining percentage scores of TC and TILs in the tissue after treatment were lower than those before NAT, it was defined as a decrease in PD-L1+TC or a decrease in PD-L1+TILs; otherwise, it was defined as an increase in PD-L1+TC or PD-L1+TIL.
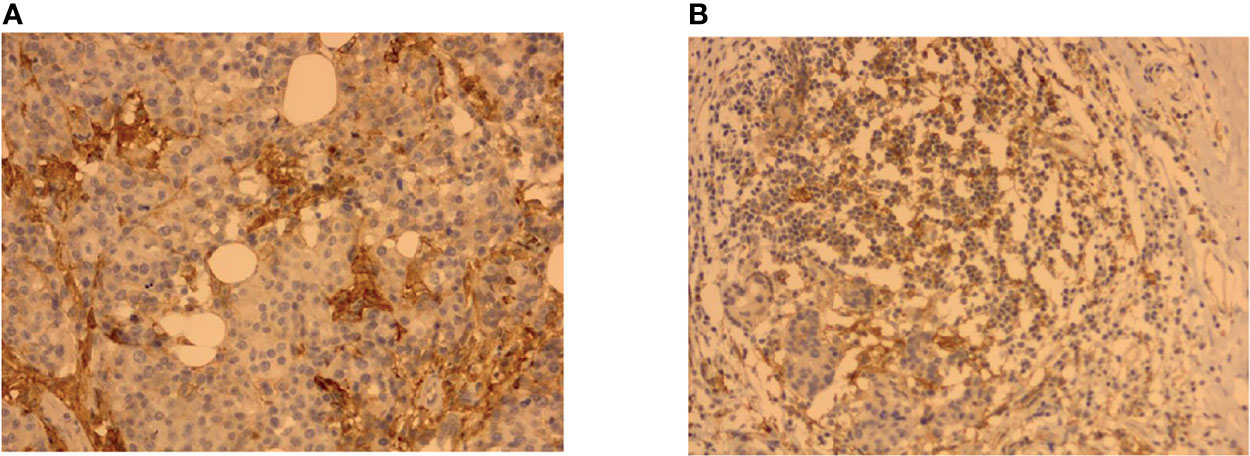
Figure 2 Representative immunohistochemistry (IHC) images showing PD-L1+TC and PD-L1+TILs in breast cancer tissues. (A) PD-L1 immunostaining on tumor cells (TC). (B) PD-L1 immunostaining on tumor-infiltrating lymphocytes (TILs).
TILs were histopathologically examined using H&E-stained portions from 155 breast cancer samples. The tumor bed was tested and graded in those cases that achieved pCR. Two pathologists (YSG and HY) blinded to the clinical criteria and reactions evaluated the TILs separately. All mononuclear cells including lymphocytes and plasma cells were graded, except granulocytes and other polymorphonuclear leukocytes; invasive lesions and inflammatory infiltration in the matrix of normal breast structures were excluded (29). The TIL count was defined as a percentage estimate of the stromal area adjacent to the tumor that contained mononuclear cells (30). When the TIL score was used as a categorical variable, it was divided into two categories: high TILs (≥30%) and low TILs (<30%) (29). The TIL count of post-NAT surgical resection tissue minus the TIL count of pre-neoadjuvant therapy core needle biopsies represents the change in TILs. If the TIL count of the tissue after treatment was increased compared with that before treatment, it was defined as an increase in TILs; otherwise, it was defined as a decrease in TILs.
The chi-square test was employed to assess the relationships between PD-L1+TC, PD-L1+TILs, TILs, and patients’ clinicopathological characteristics. To determine the variables that were substantially correlated with pCR, we applied univariate and multivariate logistic regression analyses. Wilcoxon’s non-parametric test was used to compare the changes between the values of PD-L1+TC, PD-L1+TILs, and TILs before and after neoadjuvant treatment. The relationship between PD-L1+TC, PD-L1+TILs, TILs, and disease-free survival (DFS) was determined using the Kaplan–Meier procedure, and the results were compared using the log-rank test. The Cox regression model was adopted to conduct a multivariate study of the prognostic variables. SPSS version 22 was used for all analyses (IBM Corp., Armonk, NY, USA). A p < 0.05 was considered statistically significant.
One hundred and fifty-five HER2-positive breast cancer patients were included in this study. The characteristic features of the study population are listed in Table 1. The median age of the patients was 50 years (range = 28–74 years). Most of the patients were older than 50 years (55.5%) at the time of diagnosis. Of the patients, 71 (45.8%) were menopausal. There were 126 (81.3%) patients with a clinical tumor diameter larger than 2.0 cm and 148 (93.5%) patients with clinical lymph node metastases. Sixty-eight patients (43.9%) received neoadjuvant trastuzumab plus chemotherapy, while 87 (56.1%) received neoadjuvant chemotherapy. According to the Miller–Payne scoring system, 39 (25.2%) patients realized pCR and 116 (74.8%) patients were non-pCR.
As shown in Tables 2 and 3, in samples before neoadjuvant therapy, TILs were negatively associated with the expression of PR, while PD-L1+TILs were negatively associated with the expressions of ER and PR (p < 0.05, for all). However, in samples before neoadjuvant therapy, no correlation between PD-L1+TC and age, primary tumor (cT), lymph node involvement (cN), ER status, PR status, or Ki-67 index was found (p > 0.05). In samples after neoadjuvant therapy, the expression of PD-L1+TC was negatively correlated with ER (p < 0.05). As for the TILs and PD-L1+TILs in samples after neoadjuvant therapy, there was no correlation with age, cT, cN, ER status, PR status, or Ki-67 index.
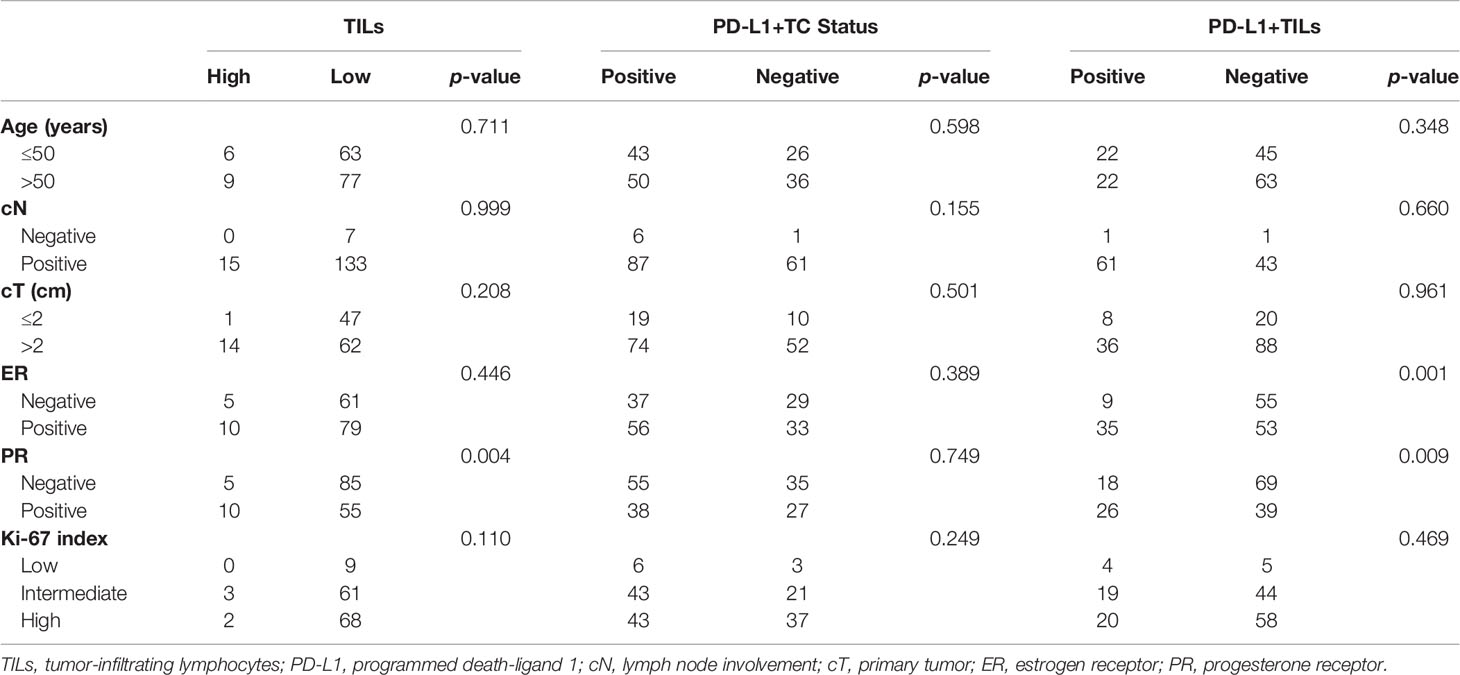
Table 2 Expressions of TILs, PD-L1+TC, and PD-L1+TILs in pre-neoadjuvant therapy specimens and correlations with clinicopathological characteristics.
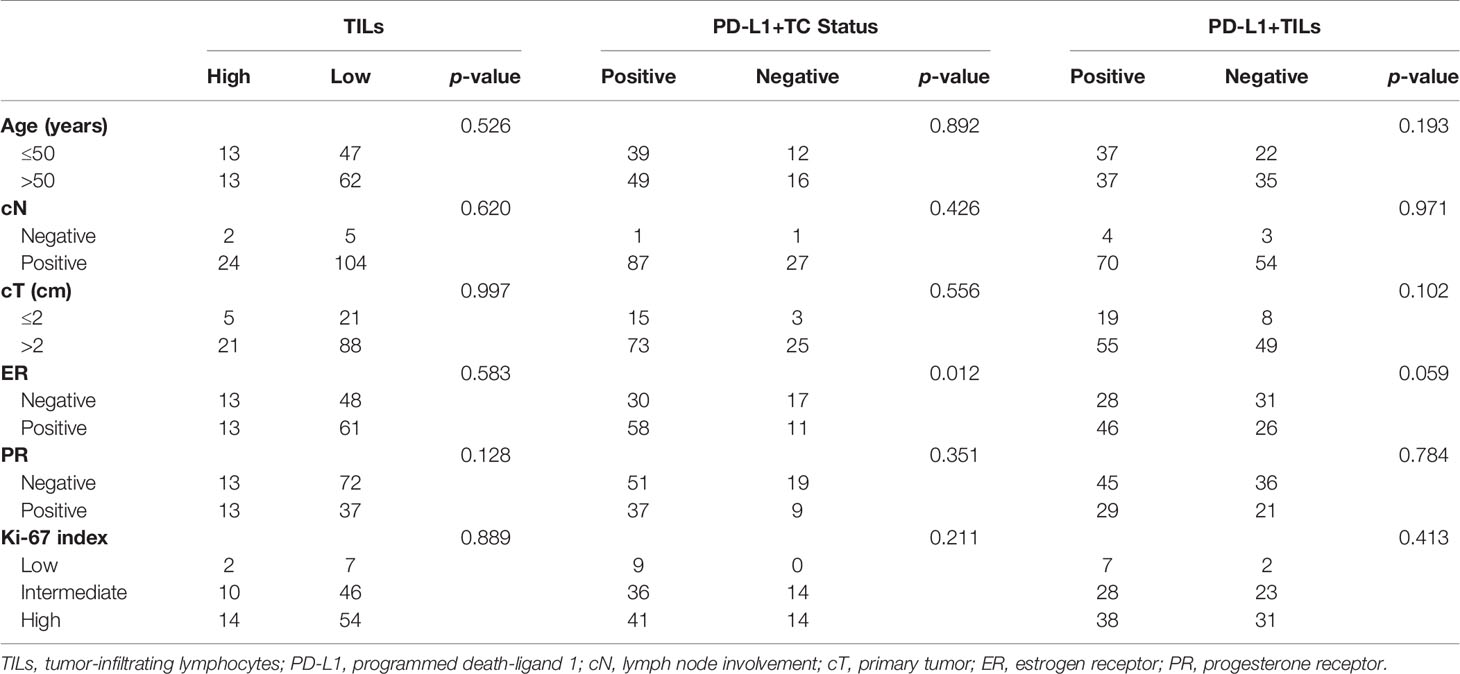
Table 3 Expressions of TILs, PD-L1+TC, and PD-L1+TILs in post-neoadjuvant therapy specimens and correlations with clinicopathological characteristics.
As shown in Table 4, univariate analysis confirmed that pCR had a positive correlation with cN, high TIL counts, and PD-L1+TILs in specimens prior to neoadjuvant therapy (p < 0.05, for all), but not with age, menstruation, cN, cT, ER, PR, and Ki-67 index in PD-L1+TC before neoadjuvant therapy, PD-L1+TILs after neoadjuvant therapy, TIL changes, or PD-L1+TIL changes (p > 0.05, for all). Multivariate logistic regression analysis verified that cN, high TIL counts, and PD-L1+TILs in pre-NAT samples were significantly correlated with pCR (p < 0.05, for all).
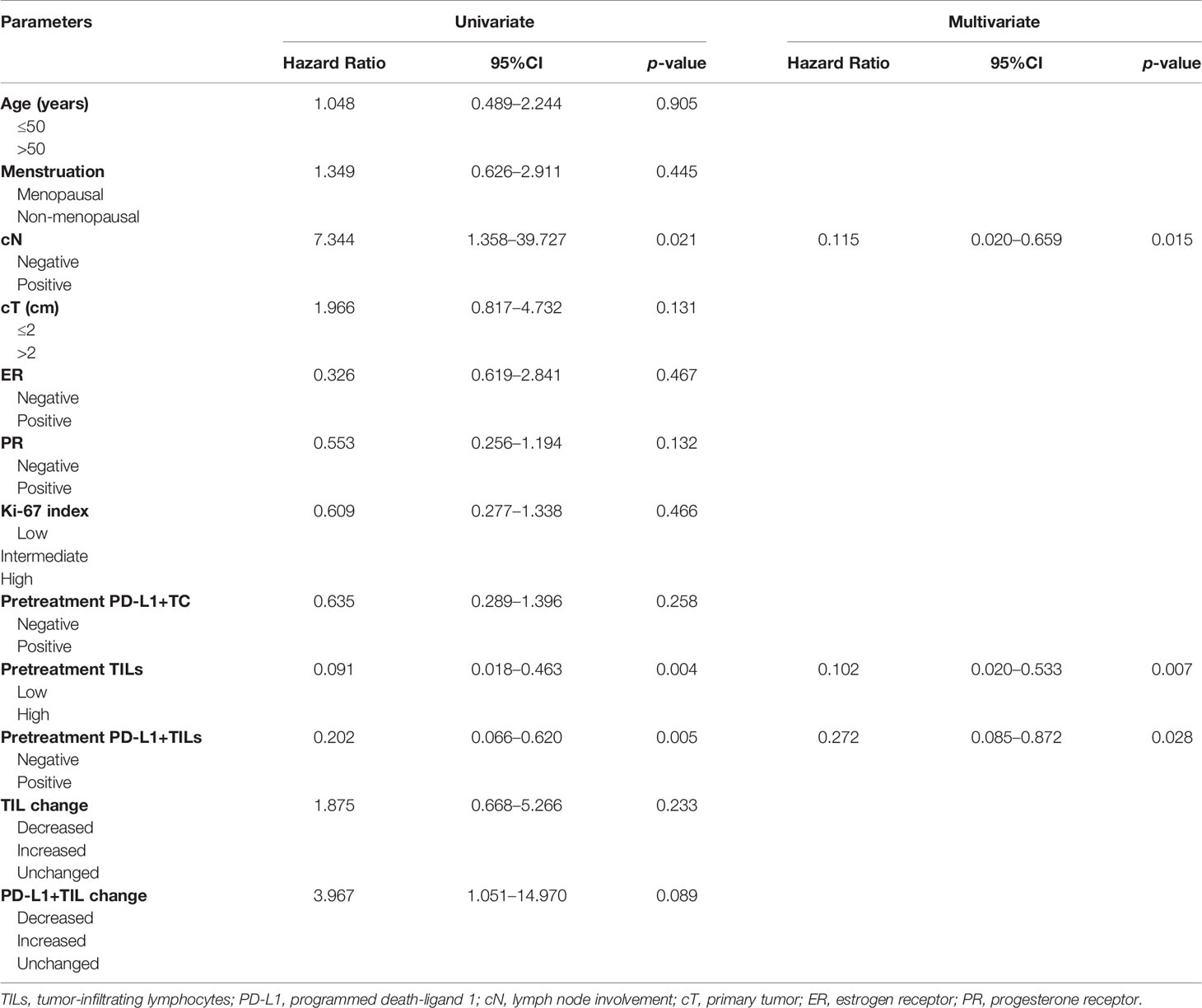
Table 4 Correlation of the expressions of TILs, PD-L1+TC, and PD-L1+TILs with clinicopathological factors including pCR to neoadjuvant therapy.
The TIL counts in breast cancer tissues improved after neoadjuvant therapy in all patients (p < 0.001). Subgroup analysis of the trastuzumab plus chemotherapy group and the chemotherapy alone group revealed consistent findings (p < 0.05). The expressions of PD-L1+TC was reduced in breast cancer tissues after NAT in 116 non-pCR patients (p = 0.0219). In 43 non-pCR patients who received neoadjuvant trastuzumab plus chemotherapy, consistent findings were observed (p = 0.0437). Although neoadjuvant chemotherapy was given to 73 non-pCR patients, there was no substantial difference in PD-L1+TC expression before and after neoadjuvant therapy (p = 0.1465). PD-L1+TILs were downregulated following treatment in the general population, the neoadjuvant trastuzumab plus chemotherapy group, and the neoadjuvant chemotherapy group (p < 0.05; Figure 3).
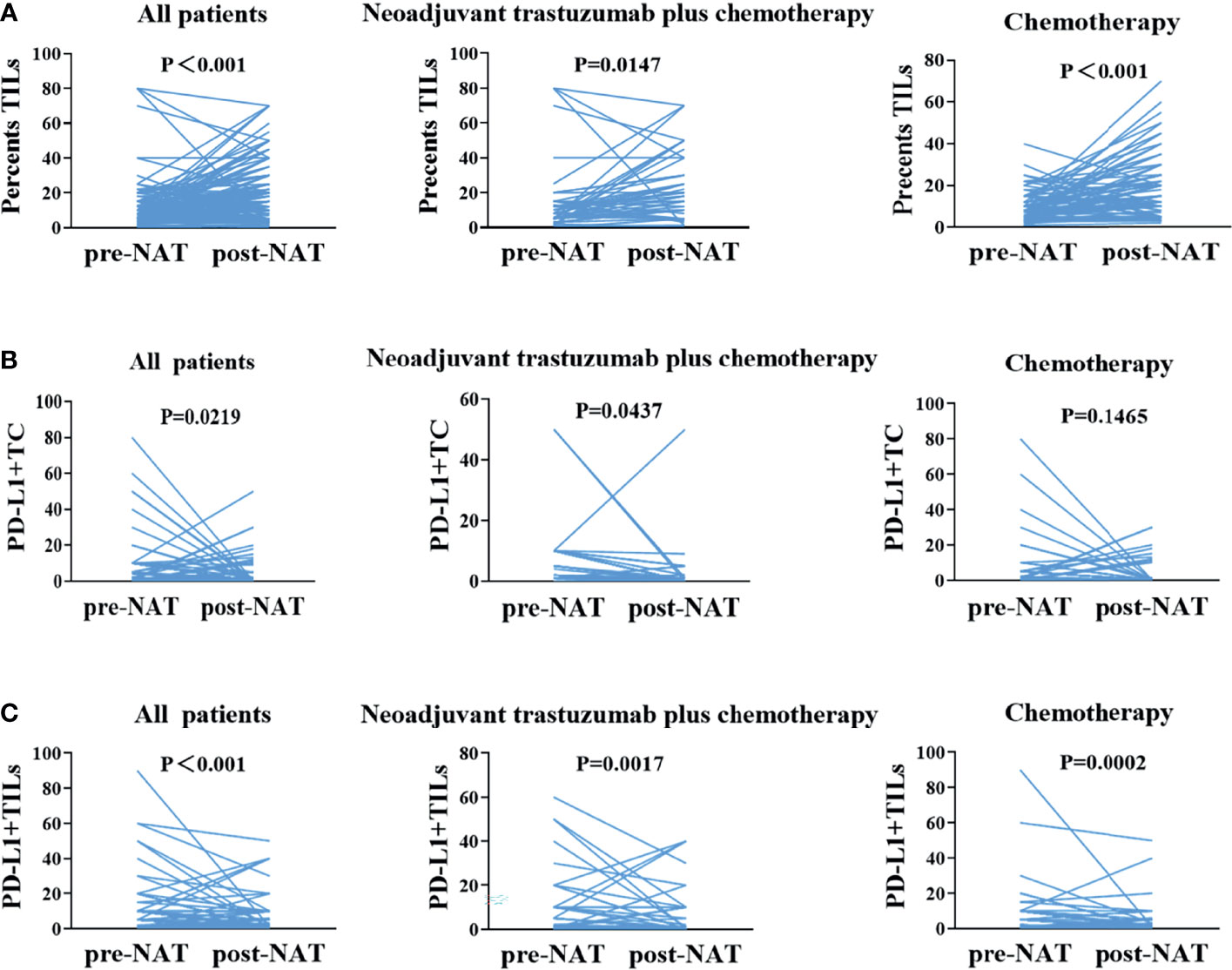
Figure 3 One-to-one correspondence of tumor-infiltrating lymphocytes (TILs), PD-L1+TC, and PD-L1+TILs between pre- and post-neoadjuvant therapy samples for all cases. (A) Changes in TILs between before and after neoadjuvant therapy, before and after neoadjuvant trastuzumab plus chemotherapy, and before and after neoadjuvant chemotherapy. (B) In non-pCR patients, changes in PD-L1+TC between pre- and post-neoadjuvant therapy, before and after neoadjuvant trastuzumab plus chemotherapy, and before and after neoadjuvant chemotherapy. (C) Changes in PD-L1+TILs between pre- and post-neoadjuvant therapy and before and after neoadjuvant trastuzumab plus chemotherapy. pCR, pathological complete response.
According to the Kaplan–Meier study, only the changes in PD-L1+TC before and after neoadjuvant chemotherapy were related to DFS (p = 0.0080). Nevertheless, the transition in TILs and PD-L1+TILs between pre and post-NAT showed no association with DFS (p > 0.05; Figure 4). A multivariate Cox regression study was performed using the significant clinicopathological variables identified by univariate analysis (cN, cT, and PD-L1+TC before treatment). We did not find the above factors to be independent predictors of DFS (p > 0.05, for all; Table 5).
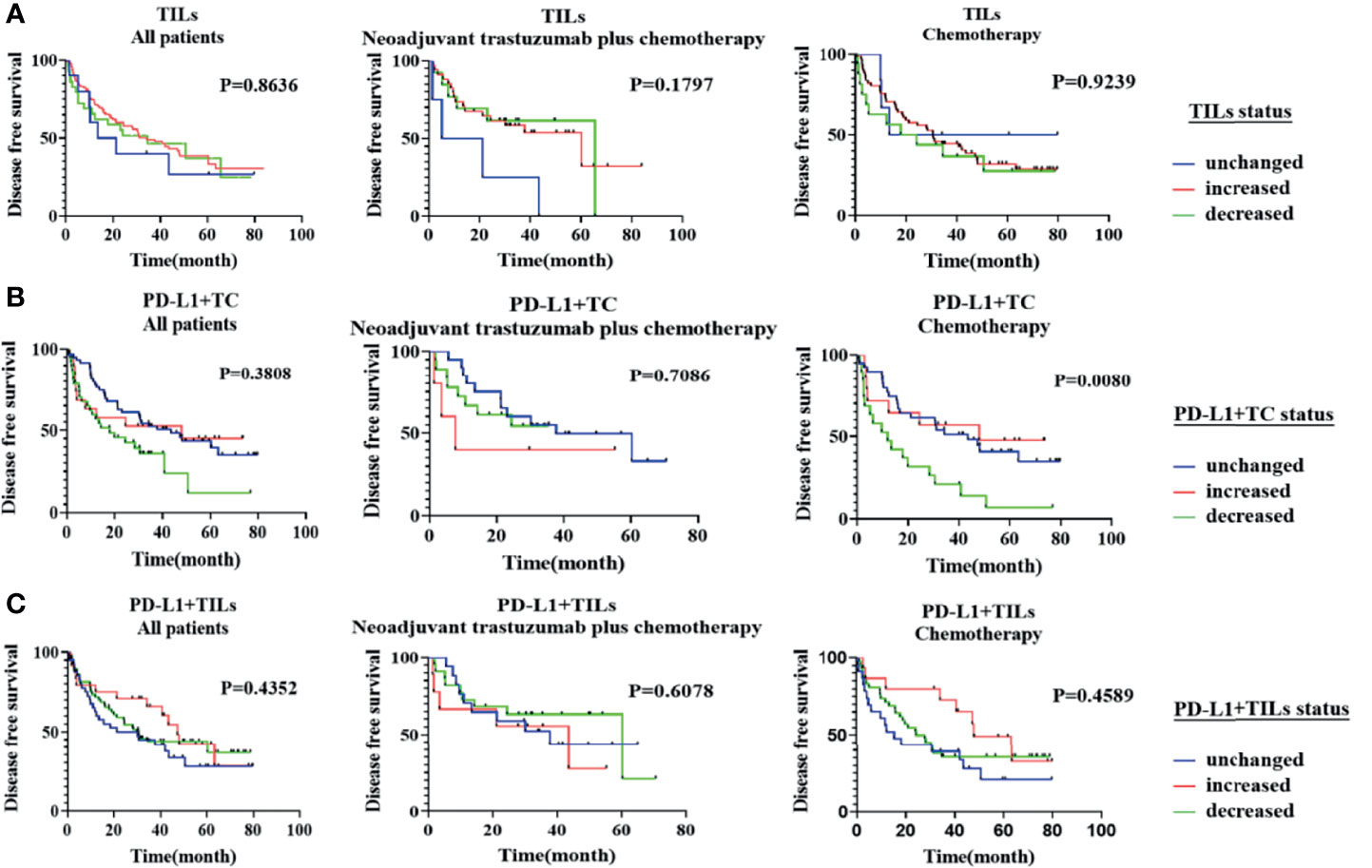
Figure 4 Kaplan–Meier analysis of disease-free survival (DFS) according to the changes in tumor-infiltrating lymphocytes (TILs), PD-L1+TC, and PD-L1+TILs between pre- and post-neoadjuvant treatment. (A) The changes in TILs before and after neoadjuvant treatment were not significantly correlated with patients’ DFS in all populations, the neoadjuvant trastuzumab plus chemotherapy group, and the neoadjuvant chemotherapy group (p > 0.05, for all). (B) The changes in PD-L1+TC before and after neoadjuvant chemotherapy were related to DFS (p = 0.0080), but the changes in PD-L1+TC in all populations and the neoadjuvant trastuzumab plus chemotherapy population were not related to DFS (p > 0.05, for all). (C) The changes in PD-L1+TILs before and after neoadjuvant treatment were not significantly correlated with patients’ DFS in all populations, the neoadjuvant trastuzumab plus chemotherapy group, and the neoadjuvant chemotherapy group (p > 0.05, for all).
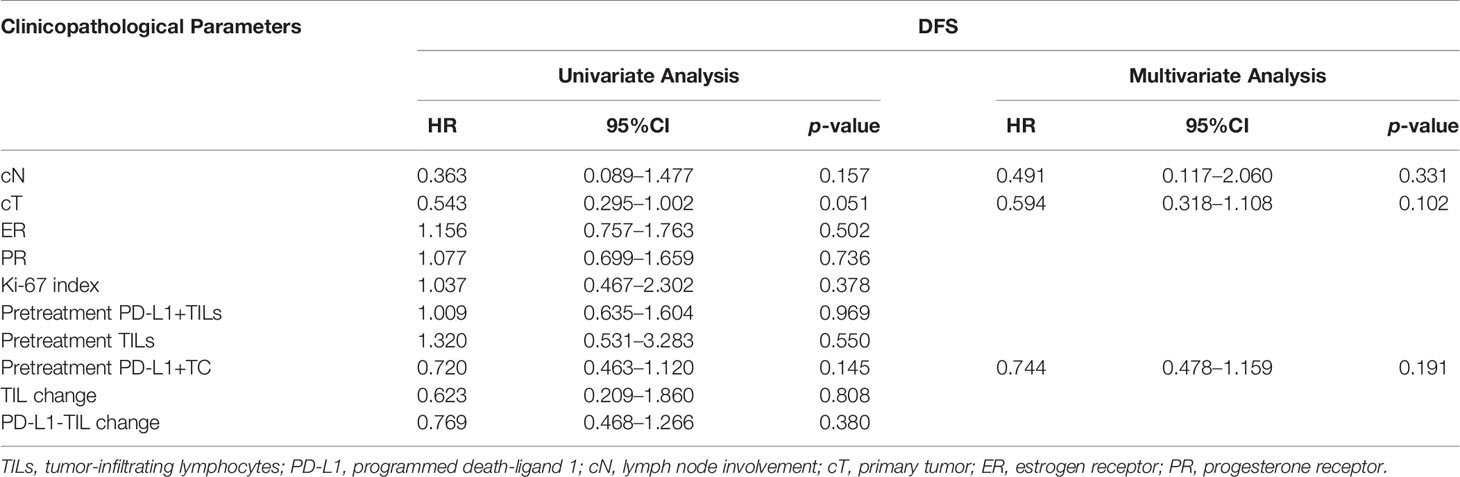
Table 5 Factors correlated with disease-free survival (DFS) in univariate and multivariate analyses.
Numerous experiments have been conducted to investigate the predictors of NAT effectiveness in HER2-positive breast cancer. Until now, no accurate and commonly used biomarker has been discovered, except for a few clinicopathological factors such as HER2. The HER2 oncogene can affect the therapeutic effect of trastuzumab by inducing the expression of PD-L1 and the recruitment and activation of TILs, suggesting that TILs and PD-L1 have been linked to trastuzumab efficacy (9, 21, 23, 31). Several studies have confirmed that TILs and PD-L1 have such predictive values in HER2-positive breast cancer patients, but debate is still ongoing (32–34). Most of the previous studies have concentrated on the expression of PD-L1 or TILs in tissues before NAT in HER2-positive breast cancer to predict the effectiveness of neoadjuvant therapy. There is still lack of information on whether the changes in PD-L1 and TILs in the tissues before and after NAT could predict the efficacy of neoadjuvant treatment.
We first tested whether there was any association between the TIL counts and clinicopathological characteristics before and after neoadjuvant therapy. Previous studies have shown that higher TIL counts pre-NAT were significantly associated with more aggressive clinicopathological features, such as higher cT staging, histological grade, and Ki-67 index (35). In our study, we concluded that the TIL counts in tissues before NAT were significantly higher in PR-negative cases. Consistent with previous research (36), no evidence of an association was found between the TIL counts after NAT and age, postoperative staging, cT, cN, distant metastasis, ER and PR status, or Ki-67 index. We have reached conclusions inconsistent with previous studies regarding the relationship between TILs post-NAT and the clinicopathological characteristics. The different TIL evaluation criteria, including only HER2-positive breast cancer types, and the heterogeneity of the histopathological tissues of HER2-positive breast cancer have likely caused the conflicting results.
According to some studies, cytotoxic agents may release tumor antigens and aid in the recruitment of immune cells to the tumor through mediators such as the pro-inflammatory cytokine interferon-c (37). Moreover, by inducing ADCC through immune cells and immunogenic cell death, trastuzumab can increase the density of CD3+ and CD8+ TILs and enhance the antitumor immune response (38). This laid the theoretical foundation for our research. Our study showed a significant increase in TILs following NAT in all patients, prompting us to speculate that NAT may activate the local antitumor immune status.
Inconsistent with our research, a previous research has shown that, in HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, high grades of TILs in tissues before NAT were associated with a significant improvement in the pCR rate (39). We observed that cN, higher TILs, and PD-L1+TILs in specimens before neoadjuvant therapy, but no other clinicopathological factors, were independent predictors of pCR in NAT. Previous studies have confirmed that PD-L1+TILs are regulated through adaptive mechanisms and reflect preexisting immunity, and their expressions may be caused by an organism’s strong primary cytotoxic immune attack on tumor neoantigens (34, 40, 41). Therein, chemotherapy and targeted therapy-induced cellular damage and immunogenic cell death will cause a cascade of cellular immune responses, the development of new immunogenic epitopes, antigen cross-presentation, cytokine and chemokine secretion, induction of tumor-specific cytotoxic T cells, and activation of dendritic cells. Similarly, chemotherapy and targeted therapy can also cause a cascade of humoral immune responses (13, 36, 42). This supported a previous theory that chemotherapy and targeted therapy could improve treatment efficacy by increasing the immune activity of patients (43). Furthermore, the FinHER Study showed that the high level of stromal TILs at diagnosis predicted the benefits of trastuzumab adjuvant therapy and proposed that the establishment of a HER2 signal might be the reason for the maintenance of the immunosuppressive microenvironment, However, trastuzumab may break the hypothesis of such an immunosuppressive microenvironment (19). Further research is urgently needed to investigate the relationship between neoadjuvant trastuzumab plus chemotherapy and the immune microenvironment of HER2-positive breast cancer and whether this treatment can affect the immune microenvironment of local antitumor. Besides, our study proved that PD-L1+TILs in pre-NAT specimens were also an independent predictor of pCR in neoadjuvant treatment. One possible explanation for such findings is that the expression of PD-L1 by immune cells, especially TILs, reflects a robust primary immune response and shows an adaptive response to an intensive primary cytotoxic immune attack on cancer neoantigens (44). In conclusion, higher TILs and PD-L1+TILs in pre-NAT specimens may also forecast the effectiveness of neoadjuvant trastuzumab with chemotherapy for HER2-positive breast cancer, in accordance with our findings. However, more research is needed to explicate the antitumor immune response mechanism of TILs and PD-L1+TILs and to clarify the role of PD-L1+TIL in neoadjuvant trastuzumab combined with chemotherapy.
A basic experiment confirmed two main ways to regulate PD-L1 expression after trastuzumab treatment (10). Firstly, the cytokines released by trastuzumab through external pathways may activate trastuzumab-mediated ADCC, thereby upregulating PD-L1 expression on breast tumor cells. Secondly, a trastuzumab-mediated intrinsic pathway to inhibit HER2 downstream cell signal transduction downregulates PD-L1 expression on tumor cells. These pointed out that this extrinsic pathway is related to trastuzumab resistance and that the internal pathway is related to the antitumor immune effect of trastuzumab. The results of the basic experiment may explain the following conclusions we have reached. In our subgroup analysis, PD-L1+TC in the neoadjuvant trastuzumab plus chemotherapy subgroup was significantly reduced, and the results were statistically significant. However, no statistically significant reduction in PD-L1+TC was found in the general population and the subgroup of neoadjuvant chemotherapy alone. This result indicates that neoadjuvant trastuzumab plus chemotherapy may affect the expression of PD-L1+TC through the intrinsic pathway mediated by trastuzumab. Moreover, a study has shown that PD-L1+TC can mediate antitumor immune escape (45). In our study, only the subgroup of trastuzumab combined with chemotherapy showed a decrease in PD-L1+TC after treatment, suggesting that PD-L1-TC is related to the efficacy of neoadjuvant trastuzumab plus chemotherapy. However, the relationship between PD-L1+TC and trastuzumab in truncating tumor immune escape needs further confirmation by basic experiments.
Professor Arlene H. Sharpe has shown that PD-L1 on highly immunogenic tumor cells is enough to promote tumor immune escape and constrain the tumor lysing ability of CD8+ T cells (46). Furthermore, chemotherapy can activate the antitumor immune response. This laid the theoretical foundation for our research hypothesis. We found that, in HER2-positive breast cancer, the TIL counts in post-NAT tissues were increased, but PD-L1+TC was decreased, suggesting that neoadjuvant trastuzumab plus chemotherapy may activate the antitumor immune response, thereby inhibiting tumor immune escape.
Our study has several limitations. Firstly, the low sample size hampered conducting statistical analysis on subtype comparisons and adequately powered multivariate analysis. We also did not investigate other immune-oncologic biomarkers such as CTLA-4 and the expressions of other immune checkpoints in tumor and immune cells. Secondly, the PD-L1 status was based on a single antibody. Due to the significant differences in previous studies using different PD-1/PD-L1 antibodies, our results may be limited by the use of a single antibody. Finally, limited by economic factors, this study only included patients receiving single-target chemotherapy, but failed to show a relationship between TILs, PD-L1+TC, and PD-L1+TILs and the efficacy of neoadjuvant dual-target plus chemotherapy in HER2-positive breast cancer patients. To confirm and endorse our results, larger prospective trials with multi-institution cohorts, homogeneous breast cancer tumor subtypes, and several distinct anti-HER2 treatment regimens are required.
High TILs and PD-L1+TILs in samples prior to NAT and lymph node metastasis can predict the pCR for neoadjuvant treatment in HER2-positive breast cancer patients. Both PD-L1+TILs and TILs were changed in pre- and post-NAT samples of HER2-positive breast cancer, suggesting that the immune microenvironment has a crucial role in neoadjuvant treatment. More studies on the mechanism and prospective clinical verification are required.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Shandong Cancer Hospital and Institute. The approval number is SDTHEC 201907003 (Shandong, China). The patients/participants provided written informed consent to participate in this study. Written informed consent was obtained from individual(s) for the publication of any potentially identifiable images or data included in this article.
HL and MS were responsible for the study design and concept. MS, YC, JC, SY, QT, and XM performed data acquisition. MS analyzed and interpreted the data. JZ and HY contributed to pathological section reading. MS and HL prepared and edited the manuscript. All authors have read and approved the final version of the manuscript.
This work was supported by the Natural Science Foundation of China (grant no. 81902713). Financial support was provided by the Natural Science Foundation of Shandong Province (grant nos. ZR2016HM41 and ZR2015HZ004) and the Key Research and Development Program of Shandong Province (grant no. 2018GSF118089).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all individuals who participated in this study and donated samples.
1. Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, et al. Neoadjuvant Trastuzumab, Pertuzumab, and Chemotherapy Versus Trastuzumab Emtansine Plus Pertuzumab in Patients With HER2-Positive Breast Cancer (KRISTINE): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol (2018) 19(1):115–26. doi: 10.1016/s1470-2045(17)30716-7
2. Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant Therapy With Paclitaxel Followed by 5-Fluorouracil, Epirubicin, and Cyclophosphamide Chemotherapy and Concurrent Trastuzumab in Human Epidermal Growth Factor Receptor 2-Positive Operable Breast Cancer: An Update of the Initial Randomized Study Population and Data of Additional Patients Treated With the Same Regimen. Clin Cancer Res (2007) 13(1):228–33. doi: 10.1158/1078-0432.ccr-06-1345
3. Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly Higher Pathologic Complete Remission Rate After Neoadjuvant Therapy With Trastuzumab, Paclitaxel, and Epirubicin Chemotherapy: Results of a Randomized Trial in Human Epidermal Growth Factor Receptor 2-Positive Operable Breast Cancer. J Clin Oncol (2005) 23(16):3676–85. doi: 10.1200/jco.2005.07.032
4. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, et al. 2 Years Versus 1 Year of Adjuvant Trastuzumab for HER2-Positive Breast Cancer (HERA): An Open-Label, Randomised Controlled Trial. Lancet (2013) 382(9897):1021–8. doi: 10.1016/s0140-6736(13)61094-6
5. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of Preoperative Chemotherapy on the Outcome of Women With Operable Breast Cancer. J Clin Oncol (1998) 16(8):2672–85. doi: 10.1200/jco.1998.16.8.2672
6. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol (2008) 26(5):778–85. doi: 10.1200/jco.2007.15.0235
7. Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell (2017) 168(3):487–502.e15. doi: 10.1016/j.cell.2016.12.022
8. Annibali O, Crescenzi A, Tomarchio V, Pagano A, Bianchi A, Grifoni A, et al. PD-1 /PD-L1 Checkpoint in Hematological Malignancies. Leuk Res (2018) 67:45–55. doi: 10.1016/j.leukres.2018.01.014
9. Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, et al. Immune Checkpoint Inhibition Overcomes ADCP-Induced Immunosuppression by Macrophages. Cell (2018) 175(2):442–57.e23. doi: 10.1016/j.cell.2018.09.007
10. Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C, Calin GA, et al. Trastuzumab Upregulates PD-L1 as a Potential Mechanism of Trastuzumab Resistance Through Engagement of Immune Effector Cells and Stimulation of IFNgamma Secretion. Cancer Lett (2018) 430:47–56. doi: 10.1016/j.canlet.2018.05.009
11. Savas P, Caramia F, Teo ZL, Loi S. Oncogene Addiction and Immunity: Clinical Implications of Tumour Infiltrating Lymphocytes in Breast Cancers Overexpressing the HER2/neu Oncogene. Curr Opin Oncol (2014) 26(6):562–7. doi: 10.1097/CCO.0000000000000131
12. Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab Plus Trastuzumab in Trastuzumab-Resistant, Advanced, HER2-Positive Breast Cancer (PANACEA): A Single-Arm, Multicentre, Phase 1b-2 Trial. Lancet Oncol (2019) 20(3):371–82. doi: 10.1016/s1470-2045(18)30812-x
13. Nelson BH. CD20+ B Cells: The Other Tumor-Infiltrating Lymphocytes. J Immunol (Baltimore Md 1950) (2010) 185(9):4977–82. doi: 10.4049/jimmunol.1001323
14. Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J Clin Oncol (2010) 28(1):105–13. doi: 10.1200/jco.2009.23.7370
15. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J Clin Oncol (2011) 29(15):1949–55. doi: 10.1200/jco.2010.30.5037
16. De Angelis C, Nagi C, Hoyt CC, Liu L, Roman K, Wang C, et al. Evaluation of the Predictive Role of Tumor Immune Infiltrate in Patients With HER2-Positive Breast Cancer Treated With Neoadjuvant Anti-HER2 Therapy Without Chemotherapy. Clin Cancer Res (2020) 26(3):738–45. doi: 10.1158/1078-0432.CCR-19-1402
17. Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, et al. Tumor-Infiltrating Lymphocytes: A Predictive and Prognostic Biomarker in Neoadjuvant-Treated HER2-Positive Breast Cancer. Clin Cancer Res (2016) 22(23):5747–54. doi: 10.1158/1078-0432.ccr-15-2338
18. Solinas C, Ceppi M, Lambertini M, Scartozzi M, Buisseret L, Garaud S, et al. Tumor-Infiltrating Lymphocytes in Patients With HER2-Positive Breast Cancer Treated With Neoadjuvant Chemotherapy Plus Trastuzumab, Lapatinib or Their Combination: A Meta-Analysis of Randomized Controlled Trials. Cancer Treat Rev (2017) 57:8–15. doi: 10.1016/j.ctrv.2017.04.005
19. Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor Infiltrating Lymphocytes are Prognostic in Triple Negative Breast Cancer and Predictive for Trastuzumab Benefit in Early Breast Cancer: Results From the FinHER Trial. Ann Oncol (2014) 25(8):1544–50. doi: 10.1093/annonc/mdu112
20. Bianchini G, Gianni L. The Immune System and Response to HER2-Targeted Treatment in Breast Cancer. Lancet Oncol (2014) 15(2):e58–68. doi: 10.1016/s1470-2045(13)70477-7
21. Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, et al. Association of Stromal Tumor-Infiltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA Oncol (2016) 2(1):56–64. doi: 10.1001/jamaoncol.2015.3239
22. Martinez VG, O'Neill S, Salimu J, Breslin S, Clayton A, Crown J, et al. Resistance to HER2-Targeted Anti-Cancer Drugs is Associated With Immune Evasion in Cancer Cells and Their Derived Extracellular Vesicles. Oncoimmunology (2017) 6(12):e1362530. doi: 10.1080/2162402X.2017.1362530
23. Li Y, Opyrchal M, Yao S, Peng X, Yan L, Jabbour H, et al. The Role of Programmed Death Ligand-1 and Tumor-Infiltrating Lymphocytes in Breast Cancer Overexpressing HER2 Gene. Breast Cancer Res Treat (2018) 170(2):293–302. doi: 10.1007/s10549-018-4745-7
24. Mitchell KG, Parra ER, Nelson DB, Zhang J, Wistuba II, Fujimoto J, et al. Tumor Cellular Proliferation is Associated With Enhanced Immune Checkpoint Expression in Stage I non-Small Cell Lung Cancer. J Thorac Cardiovasc Surg (2019) 158(3):911–9.e6. doi: 10.1016/j.jtcvs.2019.04.084
25. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Thresholds for Therapies: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol (2009) 20(8):1319–29. doi: 10.1093/annonc/mdp322
26. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of Inflammatory Response With B7-H1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci Trans Med (2012) 4(127):127ra37–ra37. doi: 10.1126/scitranslmed.3003689
27. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature (2014) 515(7528):568–71. doi: 10.1038/nature13954
28. Ubago JM, Blanco LZ, Shen T, Siziopikou KP. The PD-1/PD-L1 Axis in HER2+ Ductal Carcinoma In Situ (DCIS) of the Breast. Am J Clin Pathol (2019) 152(2):169–76. doi: 10.1093/ajcp/aqz020
29. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol (2015) 26(2):259–71. doi: 10.1093/annonc/mdu450
30. Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L Inhibitory Pathway Affects Both CD4(+) and CD8(+) T Cells and is Overcome by IL-2. Eur J Immunol (2002) 32(3):634–43. doi: 10.1002/1521-4141(200203)32:3<634::aid-immu634>3.0.co;2-9
31. Triulzi T, Forte L, Regondi V, Di Modica M, Ghirelli C, Carcangiu ML, et al. HER2 Signaling Regulates the Tumor Immune Microenvironment and Trastuzumab Efficacy. OncoImmunology (2018) 8(1):e1512942. doi: 10.1080/2162402x.2018.1512942
32. Ochi T, Bianchini G, Ando M, Nozaki F, Kobayashi D, Criscitiello C, et al. Predictive and Prognostic Value of Stromal Tumour-Infiltrating Lymphocytes Before and After Neoadjuvant Therapy in Triple Negative and HER2-Positive Breast Cancer. Eur J Cancer (Oxford Engl 1990) (2019) 118:41–8. doi: 10.1016/j.ejca.2019.05.014
33. Force J, Howie LJ, Abbott SE, Bentley R, Marcom PK, Kimmick G, et al. Early Stage HER2-Positive Breast Cancers Not Achieving a pCR From Neoadjuvant Trastuzumab- or Pertuzumab-Based Regimens Have an Immunosuppressive Phenotype. Clin Breast Cancer (2018) 18(5):410–7. doi: 10.1016/j.clbc.2018.02.010
34. Hou Y, Nitta H, Wei L, Banks PM, Parwani AV, Li Z. Evaluation of Immune Reaction and PD-L1 Expression Using Multiplex Immunohistochemistry in HER2-Positive Breast Cancer: The Association With Response to Anti-HER2 Neoadjuvant Therapy. Clin Breast Cancer (2018) 18(2):e237–44. doi: 10.1016/j.clbc.2017.11.001
35. Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, et al. A Nomogram to Predict Pathologic Complete Response (pCR) and the Value of Tumor-Infiltrating Lymphocytes (TILs) for Prediction of Response to Neoadjuvant Chemotherapy (NAC) in Breast Cancer Patients. Breast Cancer Res Treat (2019) 173(2):255–66. doi: 10.1007/s10549-018-4981-x
36. Pelekanou V, Carvajal-Hausdorf DE, Altan M, Wasserman B, Carvajal-Hausdorf C, Wimberly H, et al. Effect of Neoadjuvant Chemotherapy on Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Breast Cancer and its Clinical Significance. Breast Cancer Res (2017) 19(1):91. doi: 10.1186/s13058-017-0884-8
37. Frey B, Rubner Y, Kulzer L, Werthmöller N, Weiss EM, Fietkau R, et al. Antitumor Immune Responses Induced by Ionizing Irradiation and Further Immune Stimulation. Cancer Immunol Immunother (2014) 63(1):29–36. doi: 10.1007/s00262-013-1474-y
38. Weiner GJ. Monoclonal Antibody Mechanisms of Action in Cancer. Immunol Res (2007) 39(1-3):271–8. doi: 10.1007/s12026-007-0073-4
39. Kurozumi S, Inoue K, Matsumoto H, Fujii T, Horiguchi J, Oyama T, et al. Prognostic Utility of Tumor-Infiltrating Lymphocytes in Residual Tumor After Neoadjuvant Chemotherapy With Trastuzumab for HER2-Positive Breast Cancer. Sci Rep (2019) 9(1):1583. doi: 10.1038/s41598-018-38272-1
40. Kim HR, Ha S-J, Hong MH, Heo SJ, Koh YW, Choi EC, et al. PD-L1 Expression on Immune Cells, But Not on Tumor Cells, is a Favorable Prognostic Factor for Head and Neck Cancer Patients. Sci Rep (2016) 6(1):10. doi: 10.1038/srep36956
41. Bae SB, Cho HD, Oh MH, Lee JH, Jang SH, Hong SA, et al. Expression of Programmed Death Receptor Ligand 1 With High Tumor-Infiltrating Lymphocytes Is Associated With Better Prognosis in Breast Cancer. J Breast Cancer (2016) 19(3):242–51. doi: 10.4048/jbc.2016.19.3.242
42. Arias-Pulido H, Cimino-Mathews A, Chaher N, Qualls C, Joste N, Colpaert C, et al. The Combined Presence of CD20 + B Cells and PD-L1 + tumor-Infiltrating Lymphocytes in Inflammatory Breast Cancer is Prognostic of Improved Patient Outcome. Breast Cancer Res Treat (2018) 171(2):273–82. doi: 10.1007/s10549-018-4834-7
43. Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, et al. Effects of Conventional Therapeutic Interventions on the Number and Function of Regulatory T Cells. Oncoimmunology (2014) 2(10):e27025. doi: 10.4161/onci.27025
44. Kollmann D, Ignatova D, Jedamzik J, Chang YT, Jomrich G, Baierl A, et al. PD-L1 Expression is an Independent Predictor of Favorable Outcome in Patients With Localized Esophageal Adenocarcinoma. Oncoimmunology (2018) 7(6):e1435226. doi: 10.1080/2162402x.2018.1435226
45. Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, et al. PD-L1 (B7-H1) Expression and the Immune Tumor Microenvironment in Primary and Metastatic Breast Carcinomas. Hum Pathol (2016) 47(1):52–63. doi: 10.1016/j.humpath.2015.09.003
Keywords: HER2-positive breast cancer, neoadjuvant trastuzumab plus chemotherapy, therapeutic effect, PD-L1, TILs
Citation: Shang M, Chi Y, Zhang J, Chang J, Yang H, Yin S, Tan Q, Man X and Li H (2022) The Therapeutic Effectiveness of Neoadjuvant Trastuzumab Plus Chemotherapy for HER2-Positive Breast Cancer Can Be Predicted by Tumor-Infiltrating Lymphocytes and PD-L1 Expression. Front. Oncol. 11:706606. doi: 10.3389/fonc.2021.706606
Received: 08 July 2021; Accepted: 06 December 2021;
Published: 05 January 2022.
Edited by:
Tomoharu Sugie, Kansai Medical University Hospital, JapanReviewed by:
Marco Invernizzi, University of Eastern Piedmont, ItalyCopyright © 2022 Shang, Chi, Zhang, Chang, Yang, Yin, Tan, Man and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huihui Li, aHVpaHVpbGk4MkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.