
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 July 2021
Sec. Surgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.706543
Background: The appropriate surgical procedure for early-stage retroperitoneal sarcoma (RPS) is unclear. Thus, we used a national database to compare the outcomes of radical and non-radical resection in patients with early stage RPS.
Methods: This retrospective study included 886 stage I RPS patients from 2004 to 2015 in the SEER database. Outcomes were compared using the multivariate Cox proportional hazards models and the results were presented as adjusted hazards ratio (AHR) with corresponding 95% confidence intervals (95%CIs). Propensity score-matched analyses were also performed for sensitive analyses.
Results: For the 886 stage I RPS patients, 316 underwent radical resection, and 570 underwent non-radical resection, with a median follow-up of 4.58 (2.73-8.35) years. No difference was observed in overall mortality (AHR 0.84, 95%CI 0.62-1.15; P = 0.28) or RPS-specific mortality (AHR 0.88, 95%CI 0.57-1.36; P = 0.56) between groups. The results were similar in propensity score-matching analyses. However, subgroup analysis revealed that radical resection was associated with significantly decreased risks of overall mortality in male (AHR 0.61, 95%CI 0.38-0.98; P = 0.04) and in patients with radiotherapy (AHR 0.56, 95%CI 0.32-0.98; P = 0.04).
Conclusion: Radical resection did not improve midterm survival outcomes compared with non-radical resection in overall patients with early stage RPS. However, male patients or patients who received radiotherapy might benefit from radical resection with improved overall survival.
Retroperitoneal sarcomas (RPSs) are rare types of sarcomas arising from the retroperitoneum, which represent approximately 15% of all soft tissue sarcomas (1). The estimated incidence less than 1 case per 100,000 inhabitants/year, with nearly 4,500 new cases diagnosed yearly in Europe and 1785 in the United States (2–4). The long-term prognosis of RPSs varies the between subtypes but is always relatively poor, with a 5-year overall survival of approximately 50% (5). Due to poor responses to systemic therapies, surgery has long been the mainstay of treatment for RPS (5–7). However, the large size of the tumors, their adjacent relationship to vital structures, and the propensity of local recurrence render surgical procedures challenging and complicated.
Over the recent decade, many centers proposed a radical surgical strategy involving en bloc resections of the tumors with adjacent organs or structures to achieve maximum R0 resection and minimize the risk of local recurrence (8, 9). In practice, surrounding structures (e.g. psoas, kidney, or part of the colon), were also excised with the mass even when not infiltrated. However, these radical resections may increase the risk of major postoperative morbidity and affect the quality of life in high-risk patients (10). Additionally, a recent study reported that the number of organs excised could independently predict worse long-term overall survival (11). Therefore, the Transatlantic RPS Working Group has advocated establishing a stage/histological-specific and data-driven standard of surgical strategy for a selective organ resection in PRS patients (12). Specifically, the impact of radical resection in patients with early-stage RPS stratified by different histology has not been investigated before. To fully address the confounding factors that may affect survival outcomes, we performed a propensity score matching analysis using the Surveillance, Epidemiology, and End Results (SEER) registry to evaluate the role of radical resection in early-stage RPS.
The SEER program collected cancer data from population-based cancer registries covering about 35% (https://seer.cancer.gov/about/overview.html) of the United States population including various races since January 1, 1973. We extracted the dataset from the SEER*Stat Database ‘Incidence -SEER 18 Regs Custom Data (with additional treatment fields) based on the November 2017 submission (1973-2015 varying)’. We signed a data-use agreement and accessed the SEER dataset through the ID 10672-Nov2018. The study used de-identified data and did not require ethical approval. The data-use agreement is in the Supplementary materials_1.
Our retrospective cohort study included patients with American Joint Committee on Cancer (AJCC) Stage I retroperitoneal sarcomas who underwent surgery between January 2004 and December 2015. The patients were identified by cross-referencing anatomical sites and histology. They were included when they displayed AJCC Stage I tumors originating primarily in the retroperitoneum (ICD-O-3 Site code:C480) and with the ‘common sarcomas of retroperitoneum’ histology code as defined by the AJCC Cancer Staging Manual, Eighth Edition (13). We considered the patients displaying both 6th and 7th AJCC staging and included patients with 7th AJCC staging when we found differences between the 6th and 7th editions. The patients who underwent cancer-directed surgery were selected and divided into a radical resection group and a non-radical resection group according to the extent of surgery.
Generally, retroperitoneal sarcomas are quite large, present multiple structures, and are prone to recurrence. To maximize the achievement of R0 resections, the current guidelines recommend a radical surgical strategy involving the resection of the tumor with its adjacent organs or structures to minimize the risk of local recurrence (14). In the SEER database, a radical resection was defined as a ‘partial or total removal of the primary site WITH an en bloc resection (partial or total removal) of other organs (code 60 from both 1983 to 1997 and 1998 +)’. Partial removal of the primary site can be considered as a radical surgery if vital organs are involved. Moreover, it was difficult to obtain a reliable microscopic evaluation of the margins even with postoperative pathology due to an incomplete margin sampling and difficulties to determine the primary site in the clinical practice. The other surgery procedures were defined as non-radical resection, including the surgeries considered as “debulking”. The following patients were excluded: patients with incompetent information on tumor size and grade and with recurrent tumors or multiple primary tumors.
The primary endpoint of our study was overall mortality after surgery and the secondary outcome was RPS-specific death after surgery. The survival time was defined as the time between the date of RPS diagnosis and death or it was right-censored at the follow-up cutoff (November 2017). Notably, we found crosses in the survival curves around 8 years in our exploratory analyses. Landmark analyses were performed to estimate outcomes within and beyond 8 years (i.e., the landmark point).
The primary independent variable of interest was surgical procedures (radical vs non-radical). The other covariates of interest, including the age at diagnosis, gender, histology, tumor size, tumor grade, and radiotherapy, are described in Supplementary materials_2.
The distributions of the baseline characteristics were described as medians with interquartile ranges (IQRs) for the continuous variables or as percentages for the categorical variables. We compared the different groups using logistic regression models for all variables.
The effect of radical resection compared with non-radical resection was calculated using the Cox proportional hazards regression model. Age, gender, histology, tumor grade, and treatment with radiotherapy were adjusted because these variables were considered clinically significant.
Other variables that changed the hazards ratio by at least 10 percent when added to the univariate model or deleted from the model, were also used in the final model. The results are presented as adjusted hazards ratios (AHR) with a corresponding 95% confidence interval (95%CI).
Propensity score matching analyses were used for sensitivity analysis. First, we used a propensity score matching was used to minimize the differences between the groups at baseline. The propensity score for the surgery procedure was evaluated using logistic regression based on the following variables: age, gender, histology, tumor size, tumor grade, radiotherapy, and chemotherapy. Matching ratios of 1:1 was were used in the final analysis. We performed The Cox proportional hazards regression models adjusted by the propensity scores using the matched cohort were performed to re-evaluated the effect of radical resection and further control the potential confounders.
TIn order to explore the potential heterogeneity among between different populations, we performed multivariate subgroup analyses were performed stratified by age (≤ 60 and > 60 years), gender, tumor size (≤150mm and >150mm) (15), tumor grade (Grade I and Grade II-IV), and radiotherapy. We calculated the P values to evaluate of the interactions between the surgery procedure and each subgroup variable were evaluated using likelihood ratio tests by including the interaction terms in the Cox regression model.
TAll he statistical analyses were performed using R studio Version 1.2.1335. TAllhe P values were 2-sided, with a significance level of 0.05.
We identified a total of 886 patients who underwent effective surgical resection. The patient selection process is shown in Figure 1. A total of 316 (35.7%) patients underwent radical resections and the other 570 (64.3%) underwent non-radical resections. The patients who underwent radical resections of retroperitoneal sarcoma were younger than those who received non-radical resections [60(51-69) vs 63(53-72) years; P = 0.01]. They also displayed a larger tumor size [222/316(70.3%) displayed tumors >150 mm compared with 283/570(49.6%); P < 0.001]. Additionally, the patients diagnosed with stage IA [59(7.1%)] retroperitoneal sarcomas were rare in this study. The detailed baseline characteristics for the overall population are shown in Table 1.
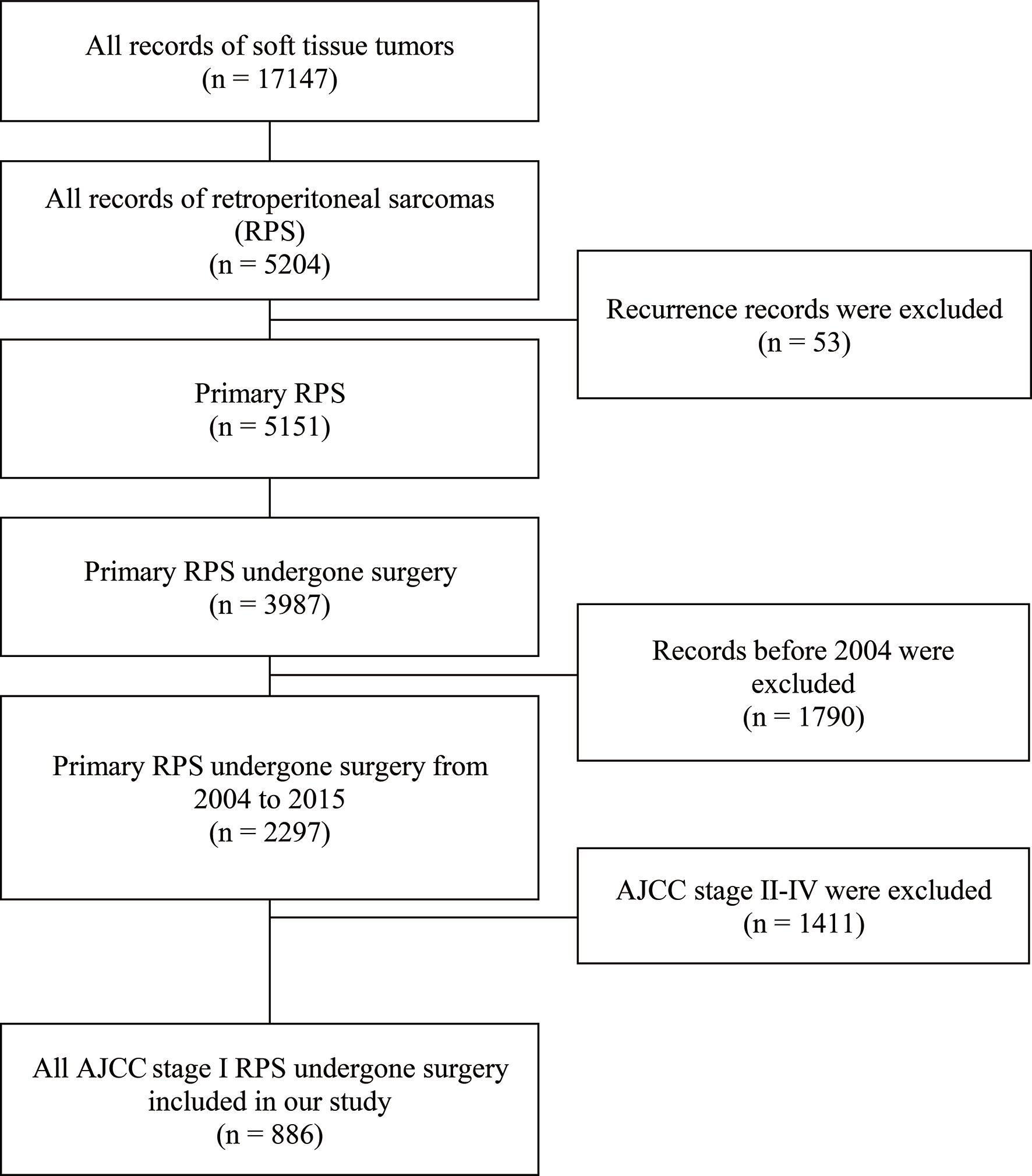
Figure 1 Flow diagram of patient selection from the Surveillance, Epidemiology, and End Results (SEER) database.
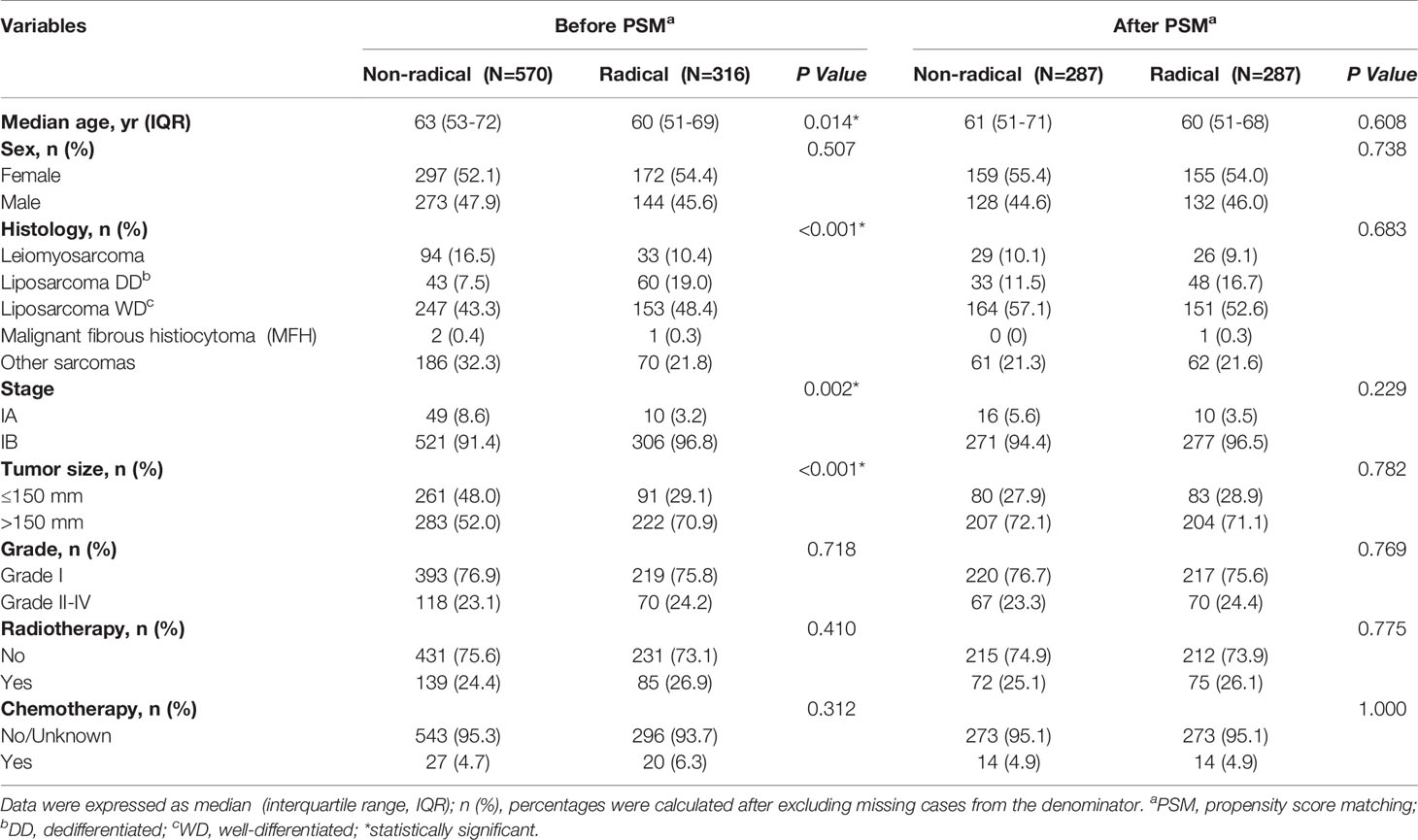
Table 1 Baseline characteristics of patients who received radical resection versus non-radical resection before and after propensity score matching (PSM).
For the 886 patients identified with stage I RPS, the median follow-up evaluated by the reverse Kaplan Meier method was 4.58 years (interquartile range 2.73-8.35 years). During the follow-up period, a total of 73 (23.1%) deaths were observed in the radical resection group and 158 (27.7%) in the non-radical resection group. The 1, 3, 5 and 10-year overall survival rates were 93.6%, 85.9%, 75.4%, and 50.9% in the radical resection group versus 94.2%, 82.1%, 71.0%, and 52.1% in the non-radical resection group, respectively. RPS-specific deaths occurred in 38 (12.0%) patients in the radical resection group and 76 (13.3%) in the non-radical resection group. The 1, 3, 5, and 10-year RPS-specific survival rates were 97.3%, 91.3%, 85.3%, and 85.3% in the radical resection group versus 97.2%, 91.3%, 84.7%, and 69.3% in the non-radical resection group.
The Kaplan-Meier curves of the overall and RPS-specific mortality in the overall and PSM-matched cohorts are shown in Figure 2. In our landmark analysis, we found that the overall (P = 0.23) and the RPS-specific mortality (P = .98) did not differ significantly between the groups within 8 years after surgery. However, 8 years after surgery, the RPS-specific mortality was higher in the non-radical resection group than the radical resection group numerically but non-significantly (P = 0.05). The details are shown in Supplementary Figure 1.
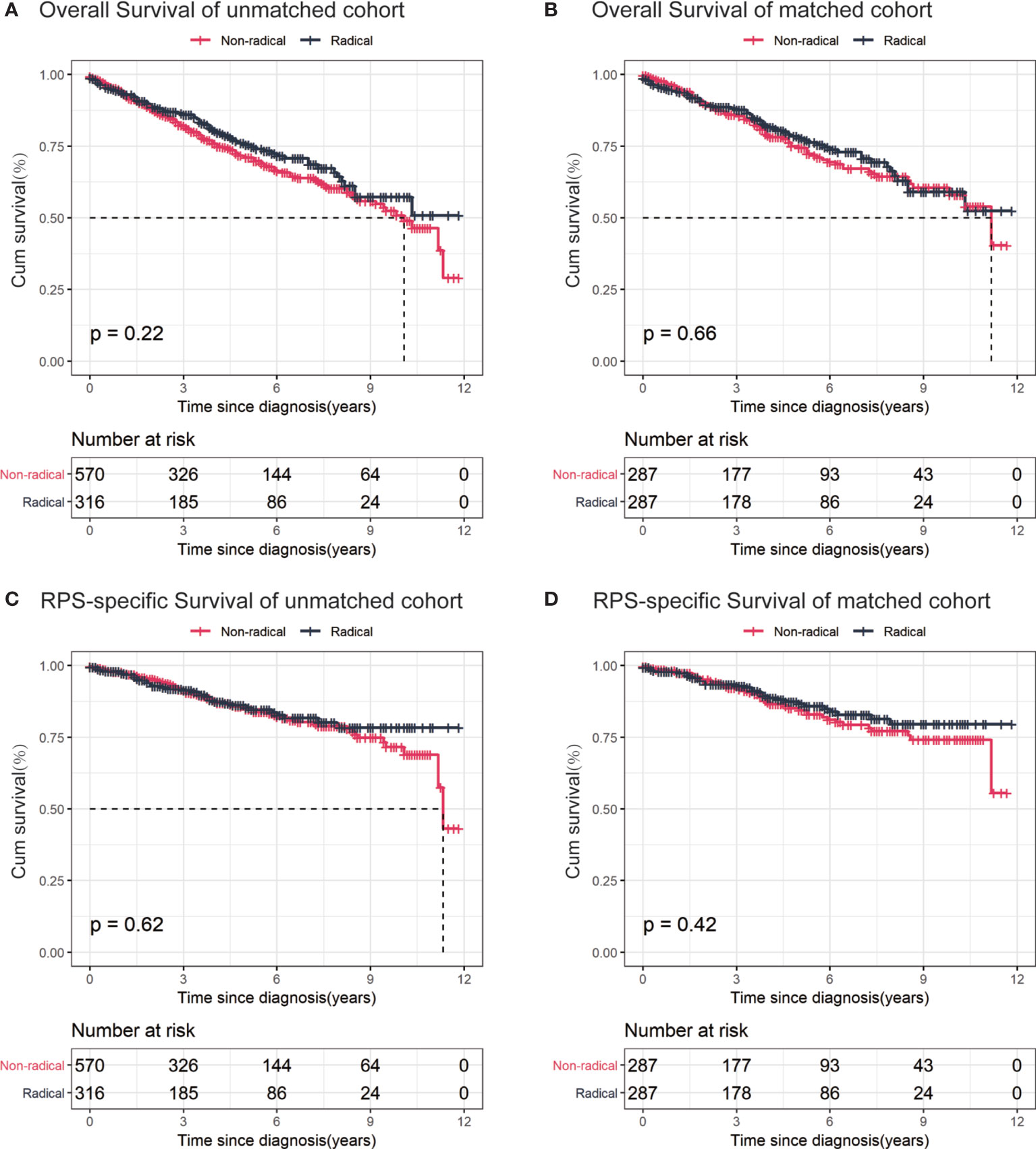
Figure 2 Kaplan–Meier curves for stage I retroperitoneal sarcoma (RPS) patients. (A) overall survival of unmatched cohort; (B) overall survival of propensity score-matched cohort; (C) RPS-specific survival of unmatched cohort; (D) RPS-specific survival of propensity score-matched cohort.
To estimate the effect of the surgery modification on the overall and RPS-specific mortality, we performed univariate and multivariate-adjusted Cox analyses in the unmatched cohort. We did not observe any significant difference between radical and non-radical resections in terms of overall mortality in the univariate analysis (HR 0.79, 95%CI 0.59-1.06; P = 0.12). The results were similar in the basic and fully adjusted models (Table 2). As for the RPS-specific mortality, we also did not observe any significant difference between radical and non-radical resections in both the univariate analysis (HR 0.86, 95%CI 0.57-1.30; P = 0.48) and the multivariate analysis (AHR 0.88, 95%CI 0.57-1.36; P = 0.56).
The propensity-score analyses generated 287 matched pairs, i.e., a total of 574 patients with similar baseline characteristics and propensity to receive radical and non-radical resections (Table 1).
In the matched cohort, a total of 64 (22.3%) deaths were observed in the radical resection group and 74 (25.8%) in the non-radical resection group. RPS-specific deaths occurred in 33 (11.5%) patients in the radical resection group and 42 (14.6%) in the non-radical resection group. We did not observe any differences in overall (HR 0.93, 95%CI 0.66-1.30; P = 0.66) or RPS-specific mortality (HR 0.83, 95%CI 0.53-1.31; P = 0.42) between the groups in the matched cohort. After adjusting by propensity score, the effect of surgery modification on overall (AHR 0.94, 95%CI 0.67–1.31; P = 0.70) and RPS-specific mortality (AHR 0.83, 95%CI 0.52-1.31; P = 0.42) remained similar between the two groups, which was consistent with the results of the multivariate models (Table 2).
To further address whether the effect of surgery modification differed in different groups of the population, we performed subgroup analyses according to age, gender, histology, tumor grade, tumor size, and radiotherapy. The results are summarized in Figure 3 and Supplementary Figure 2.
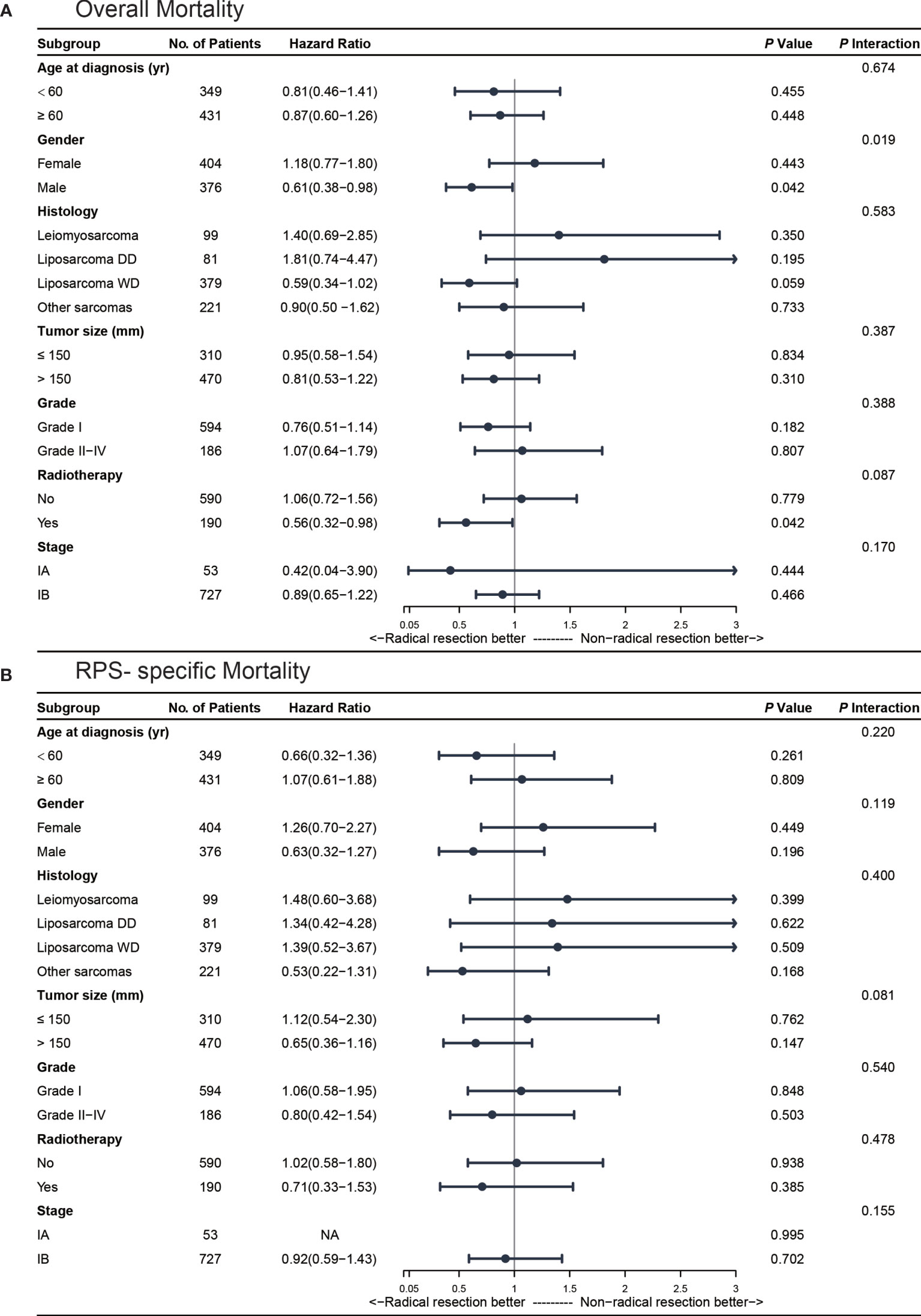
Figure 3 Multivariate subgroup analyses for stage I retroperitoneal sarcoma (RPS) patients. (A) subgroup analysis for overall mortality; (B) subgroup analysis for RPS-specific mortality.
The Kaplan-Meier curves of the overall survival stratified by gender are shown in Supplementary Figure 3A. In the subgroup of male patients (n = 376, 48.2%), radical resections were associated with a significantly decreased overall mortality (AHR 0.61, 95%CI 0.38-0.98, P = 0.04). After propensity score matching, the result was similar in male patients (HR 0.60, 95%CI 0.37-0.97; P = 0.04). Additionally, we found a significant association between gender and surgical procedure after adjusting for age, gender, histology, tumor size, tumor grade, and radiotherapy (Pinteraction= 0.02). As for RPS-specific mortality, we did not find any significant difference in the male patients who received radical or non-radical resections (AHR 0.63, 95%CI 0.32-1.27, P = 0.20) in our multivariate model or after propensity score matching (HR 0.63, 95%CI 0.32-1.25; P = 0.19).
A total of 190 (24.4%) stage I RPS patients received radiotherapy in the perioperative period. In our univariate model, radical resections were associated with a significantly decreased overall mortality (HR 0.52, 95%CI 0.30-0.91; P = 0.02) in the patients who received perioperative radiotherapy (Supplementary Figure 3B). After adjusting by confounding factors, radical resections with radiotherapy were also associated with a significantly decreased overall mortality (AHR 0.56, 95%CI 0.32-0.98; P = 0.04) and the results were similar after propensity score matching (HR 0.53, 95%CI 0.29-0.97; P = 0.04). As for the RPS-specific mortality, we did not find any significant difference between radical and non-radical resections in the patients who receive perioperative radiotherapy in our multivariate model (AHR 0.71, 95%CI 0.33-1.53, P = 0.39).
The patients were divided into four subgroups according to their histology as previously described. Nearly half of the patients [379 (48.6%)] were diagnosed with well-differentiated liposarcoma and 99 (12.7%) with leiomyosarcoma. The Kaplan-Meier curves of overall survival stratified by major histological patterns are shown in Supplementary Figure 3C. In our univariate model, radical resections were not associated with reduced overall mortality (HR 0.64, 95%CI 0.37-1.09; P = 0.10) or RPS-specific mortality (HR 1.49, 95%CI 0.59-3.75; P = 0.40) in the well-differentiated liposarcoma cohort. We found similar results in patients with leiomyosarcoma. For the patients with well-differentiated liposarcoma who received radical resections, the rate of overall mortality during the follow-up period was reduced numerically but not significantly with an absolute risk reduction of 7.1% (AHR 0.59, 95%CI 0.34-1.02; P = 0.06) after adjusting in the multivariate model.
The role of radical resections in RPS has been largely discussed in the recent decade and most studies advocate radical resections as the frontline surgical strategy due to a better local control (8–10). However, due to the limited sample size, only a few studies focused on the necessity of radical resections exclusively in stage I RPS. In this study, we reviewed stage I RPS patients who underwent radical or non-radical resections between 2004 and 2015 in the SEER national database. We found that the proportion of radical resections versus non-radical resections was close to 1:2, namely in most cases, the surgeons performed non-radical resections in early-stage RPS. Our results provided clinical evidence showing that radical resections did not improve survival outcomes compared with non-radical resections in overall stage I RPS patients in the mid-term follow-up. However, we also found that some groups of patients might benefit from radical resections in terms of overall survival, for instance, for male patients or patients who underwent perioperative radiotherapy.
Compared to previous studies assessing the role of radical resections, our study had two major improvements in methodology. First, nearly all the published studies failed to fully adjust for patient characteristics, tumor behavior, and adjuvant therapeutic strategy. Confounding factors, such as age, tumor histology, size, grade, radiotherapy, and chemotherapy, can affect long-term survival outcomes and cause bias. To fully address this issue, we also performed propensity score matching and conducted multivariate analysis adjusted for propensity scores. Secondly, as most of RPSs are initially diagnosed at an advanced stage, the evidence on the management of early-stage RPS was scarce and mainly deduced from advanced-stage tumors or subgroup analysis with a small sample size (5). Our study reviewed the SEER national database and retrieved 886 eligible patients, which may represent the largest cohort for stage I RPS patients. With these two improvements, we identified several noteworthy findings.
First, our subgroup analysis by gender in the two cohorts both suggested that male patients with stage I RPS may benefit from radical resections to improve their overall mortality. Furthermore, radical resections were still advantageous in male patients after accounting for the effects of age, histology, grade, tumor size, and radiotherapy. The improved overall mortality in male patients was also noted in a European study including RPS of all stages (15). A possible explanation for this gender-based disparity might be attributed to innate pelvic anatomic differences between males and females. In most circumstances, radical resections would include more organs in female patients than in males. Additionally, previous evidence revealed that the number of resected organs was adversely associated with long-term overall survival, which might explain the better overall survival in male patients after radical surgery (12). Besides gender differences, other factors, such as social characteristics (16), might also affect the rate of mortality and need more exploration in the future.
Second, our stratification analysis using different histological patterns did not reveal any significant difference in survival outcomes between the two groups. However, we noticed some discrepancies. We observed a numerically higher overall mortality in leiomyosarcoma patients after radical resections but it was lower in well-differentiated liposarcoma patients. To our knowledge, retroperitoneal sarcomas contain various pathologies, each characterized by distinct biological behaviors. It was reported (17) that well-differentiated liposarcomas tend to display local recurrence, while leiomyosarcomas show higher risks of distant recurrence. This might explain the different effects of radical resections in these two types of RPS. Future studies are warranted to further investigate this issue.
Third, we found that stage I RPS patients with perioperative radiotherapy were associated with reduced overall mortality after radical resections. About one-fourth (190/780) of the patients received radiotherapy in our study cohort and only 39.4% (75/190) received radical resections. National data showed that radical resections combined with radiotherapy were only adopted in a minority of stage I RPS patients. Due to a potential increase in overall mortality and long-term dysfunctions (18), determining the impact of radical resections and radiotherapy in combination is crucial (19). Given its potential toxicity and poor evidence, radiotherapy shows a narrow indication in RPS-related guidelines (20). Recently, Nussbaum (21) suggested that both pre- and post-operative radiotherapy could improve overall survival in RPS patients compared with surgery alone using the National Cancer Data Base. Though radiotherapy and radical resections were both associated with good local control effects (22, 23), there were concerns about the adverse quality of life outcomes when using both strategies. Our study showed that using radical resections and radiotherapy in early-stage RPS patients was relatively safe.
Fourth, we did not observe survival benefits after radical resections in stage I RPS patients, however, we visually observed a landmark point in the Kaplan-Meier curve approximately 8 years after surgery. Therefore, we performed a landmark analysis to further explore the presence of a time-point that could distinguish survival differences. Our analyses showed the superiority of radical resections compared with non-radical resections in overall and disease-specific survival after 8 years. However, the difference was only numerical but non-significant, which might be due to insufficient sample size and limited follow-up time. It is possible that the potential benefit of radical resections might emerge after 8 years or later. The French Sarcoma Group reported that 9.3% of patients with RPS experienced delayed (later than 5 years after surgery) local recurrences (24). Therefore, the traditional 5-year primary endpoint assessment for RPS patients might be insufficient to determine which therapeutic strategy could achieve the best long-term prognosis. Additionally, several pathological studies also observed histopathologic organ invasion in more than one-fourth of adherent organs even when it was not suspected intraoperatively (12). Although these pathological findings need to be confirmed in early-stage RPS, our study showed that clinicians should include 10-year long-term prognosis for early-stage RPS patients.
Despite several improvements in the methodology, our study was not devoid of limitations. First, the SEER database did not provide information on postoperative comorbidities and tumor recurrence. Hence, we were unable to systemically evaluate the safety of radical resections in early-stage RPS patients. Instead, our results on disease-specific deaths might shed light on tumor-related adverse outcomes. Second, as the onset of RPS is usually insidious, only a few patients diagnosed with RPS display a tumor size lower than 5cm. Hence, it is difficult to analyze the survival outcomes for patients with stage IA RPS. The surgery of patients with large tumors is usually associated with higher difficulties and risks. Successful R0/R1 resections during the initial treatment seem to influence survival outcomes. The resection margins and numbers of resected organs were, however, not available in the SEER database. Tumor size alone might only act as a moderate predictor of adverse outcomes in RPS but tumor grades and resection margins are believed to be stronger predicted factors (25). Additionally, the anatomic constraints and the high vascularization in the retroperitoneal space limit the possibility to achieve R0/R1 resections in many patients. These variables should also be considered as confounding factors for prognosis in future studies. Moreover, as various resected organs entail different risks of morbidity, a weighed resected organ score system would help to standardize and adjust the details of radical resection. Third, the SEER database provided only a little information about the specific RPS classification system (e.g., French Federation of Cancer Centers Sarcoma (FNCLCC) or National Cancer Institute (NCI) grade), hence, we used the AJCC staging system to identify the patients with stage I RPS and the SEER database to find the tumor grades, which may omit biological information on mitotic activity and necrosis. Fourth, the details of the radiotherapy treatments were limited in the SEER database and the use of radiotherapy to treat RPS patients varied between the different centers. To date, it is still debated whether radiotherapy impacts the survival of patients with RPS and future randomized controlled trials are needed to provide a more precise conclusion. We await the results of the EORTC (European Organization for Research and Treatment of Cancer) study which compared the impact of surgery alone versus preoperative radiotherapy followed by surgery. Fifth, our study was retrospective in nature and subjective to potential innate bias similar to previously published studies.
In the overall cohort of patients with stage I RPS, radical resections were not associated with improved midterm survival outcomes. However, radical resections might improve the overall survival of several subgroups of early-stage RPS patients (e.g., male patients or patients who received radiotherapy. Additionally, given the potential survival difference that we observed after eight years during the follow-up, more studies involving long-term surveillance are warranted in the future.
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) Program (https://seer.cancer.gov/about/overview.html).
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
CW & JW: Concept and design; data acquisition and analysis, interpretation of data; writing initial draft. JZ: Concept and design; data acquisition and analysis, interpretation of data; critical revision of the article. BH: Statistical analysis; interpretation of data; critical revision of the article. DY: Statistical analysis; interpretation of data; writing initial draft; and critical revision of the article. TW: Acquisition, analysis; interpretation of data; writing initial draft. All authors contributed to the article and approved the submitted version.
This work was supported by the Sichuan Foundation of Science and Technology [grant number: 2020YFS0247].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.706543/full#supplementary-material
1. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons Learned From the Study of 10,000 Patients With Soft Tissue Sarcoma. Ann Surg (2014) 260:412–6. doi: 10.1097/SLA.0000000000000869
2. Porter GA, Baxter NN, Pisters PWT. Retroperitoneal Sarcoma: A Population-Based Analysis of Epidemiology, Surgery, and Radiotherapy. Cancer (2006) 106:1610–6. doi: 10.1002/cncr.21761
3. ESMO/European Sarcoma Network Working Group. Soft Tissue and Visceral Sarcomas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol Off J Eur Soc Med Oncol (2014) 25 Suppl 3:iii102–12. doi: 10.1093/annonc/mdu254
4. Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere A-V, Péoc’h M, Istier L, et al. Incidence of Sarcoma Histotypes and Molecular Subtypes in a Prospective Epidemiological Study With Central Pathology Review and Molecular Testing. PloS One (2011) 6:e20294. doi: 10.1371/journal.pone.0020294
5. van Houdt WJ, Zaidi S, Messiou C, Thway K, Strauss DC, Jones RL. Treatment of Retroperitoneal Sarcoma: Current Standards and New Developments. Curr Opin Oncol (2017) 29:260–7. doi: 10.1097/CCO.0000000000000377
6. Gronchi A, Miceli R, Colombo C, Stacchiotti S, Collini P, Mariani L, et al. Frontline Extended Surgery Is Associated With Improved Survival in Retroperitoneal Low- to Intermediate-Grade Soft Tissue Sarcomas. Ann Oncol Off J Eur Soc Med Oncol (2012) 23:1067–73. doi: 10.1093/annonc/mdr323
7. Trans-Atlantic RPS Working Group. Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: A Consensus Approach From the Trans-Atlantic Rps Working Group. Ann Surg Oncol (2015) 22:256–63. doi: 10.1245/s10434-014-3965-2
8. Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, Collini P, et al. Aggressive Surgical Policies in a Retrospectively Reviewed Single-Institution Case Series of Retroperitoneal Soft Tissue Sarcoma Patients. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27:24–30. doi: 10.1200/JCO.2008.17.8871
9. Pisters PWT. Resection of Some – But Not All – Clinically Uninvolved Adjacent Viscera as Part of Surgery for Retroperitoneal Soft Tissue Sarcomas. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27:6–8. doi: 10.1200/JCO.2008.18.7138
10. MacNeill AJ, Gronchi A, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Postoperative Morbidity After Radical Resection of Primary Retroperitoneal Sarcoma: A Report From the Transatlantic Rps Working Group. Ann Surg (2018) 267:959–64. doi: 10.1097/SLA.0000000000002250
11. Tan MCB, Brennan MF, Kuk D, Agaram NP, Antonescu CR, Qin L-X, et al. Histology-Based Classification Predicts Pattern of Recurrence and Improves Risk Stratification in Primary Retroperitoneal Sarcoma. Ann Surg (2016) 263:593–600. doi: 10.1097/SLA.0000000000001149
12. Fairweather M, Wang J, Jo VY, Baldini EH, Bertagnolli MM, Raut CP. Surgical Management of Primary Retroperitoneal Sarcomas: Rationale for Selective Organ Resection. Ann Surg Oncol (2018) 25:98–106. doi: 10.1245/s10434-017-6136-4
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J Clin (2017) 67:93–9. doi: 10.3322/caac.21388
14. Kirane A, Crago AM. The Importance of Surgical Margins in Retroperitoneal Sarcoma. J Surg Oncol (2016) 113:270–6. doi: 10.1002/jso.24135
15. Mussi C, Colombo P, Bertuzzi A, Coladonato M, Bagnoli P, Secondino S, et al. Retroperitoneal Sarcoma: Is it Time to Change the Surgical Policy? Ann Surg Oncol (2011) 18:2136–42. doi: 10.1245/s10434-011-1742-z
16. Kluth LA, Rieken M, Xylinas E, Kent M, Rink M, Rouprêt M, et al. Gender-Specific Differences in Clinicopathologic Outcomes Following Radical Cystectomy: An International Multi-Institutional Study of More Than 8000 Patients. Eur Urol (2014) 66:913–9. doi: 10.1016/j.eururo.2013.11.040
17. Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (Rps): A Report on 1007 Patients From the Multi-Institutional Collaborative RPS Working Group. Ann Surg (2016) 263:1002–9. doi: 10.1097/SLA.0000000000001447
18. Fairweather M, Gonzalez RJ, Strauss D, Raut CP. Current Principles of Surgery for Retroperitoneal Sarcomas. J Surg Oncol (2018) 117:33–41. doi: 10.1002/jso.24919
19. Haas RL, Baldini EH, Chung PW, van Coevorden F, DeLaney TF. Radiation Therapy in Retroperitoneal Sarcoma Management. J Surg Oncol (2018) 117:93–8. doi: 10.1002/jso.24892
20. Baldini EH, Wang D, Haas RLM, Catton CN, Indelicato DJ, Kirsch DG, et al. Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys (2015) 92:602–12. doi: 10.1016/j.ijrobp.2015.02.013
21. Nussbaum DP, Rushing CN, Lane WO, Cardona DM, Kirsch DG, Peterson BL. Blazer DG 3rd. Preoperative or Postoperative Radiotherapy Versus Surgery Alone for Retroperitoneal Sarcoma: A Case-Control, Propensity Score-Matched Analysis of a Nationwide Clinical Oncology Database. Lancet Oncol (2016) 17:966–75. doi: 10.1016/S1470-2045(16)30050-X
22. Stucky C-CH, Wasif N, Ashman JB, Pockaj BA, Gunderson LL, Gray RJ. Excellent Local Control With Preoperative Radiation Therapy, Surgical Resection, and Intra-Operative Electron Radiation Therapy for Retroperitoneal Sarcoma. J Surg Oncol (2014) 109:798–803. doi: 10.1002/jso.23576
23. Bonvalot S, Miceli R, Berselli M, Causeret S, Colombo C, Mariani L, et al. Aggressive Surgery in Retroperitoneal Soft Tissue Sarcoma Carried Out at High-Volume Centers Is Safe and Is Associated With Improved Local Control. Ann Surg Oncol (2010) 17:1507–14. doi: 10.1245/s10434-010-1057-5
24. Toulmonde M, Le Cesne A, Mendiboure J, Blay J-Y, Piperno-Neumann S, Chevreau C, et al. Long-Term Recurrence of Soft Tissue Sarcomas: Prognostic Factors and Implications for Prolonged Follow-Up. Cancer (2014) 120:3003–6. doi: 10.1002/cncr.28836
25. Fisher SB, Chiang YJ, Feig BW, Cormier JN, Hunt KK, Torres KE, et al. An Evaluation of the Eighth Edition of the American Joint Committee on Cancer (Ajcc) Staging System for Retroperitoneal Sarcomas Using the National Cancer Data Base (NCDB). Am J Clin Oncol Cancer Clin Trials (2019) 42:160–5. doi: 10.1097/COC.0000000000000486
Keywords: retroperitoneal sarcoma, radical resection, propensity score, radiotherapy, early stage
Citation: Weng C, Wang J, Zhao J, Yuan D, Huang B and Wang T (2021) Radical Versus Non-Radical Resection for Early-Stage Retroperitoneal Sarcoma: A Propensity Score-Matched Analysis. Front. Oncol. 11:706543. doi: 10.3389/fonc.2021.706543
Received: 07 May 2021; Accepted: 30 June 2021;
Published: 14 July 2021.
Edited by:
Manuel Maglione, Innsbruck Medical University, AustriaReviewed by:
Paolo Del Fiore, Melanoma and Sarcoma Surgical Oncology Unit, Veneto Institute of Oncology (IRCCS), ItalyCopyright © 2021 Weng, Wang, Zhao, Yuan, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ding Yuan, eXVhbmRpbmdAd2Noc2N1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.