
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 June 2021
Sec. Radiation Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.706236
 Zijian Tian1,2†
Zijian Tian1,2† Lingfeng Meng1,2†
Lingfeng Meng1,2† Xin Wang1
Xin Wang1 Xuan Wang1
Xuan Wang1 Tianming Ma1,2
Tianming Ma1,2 Miao Wang1,2
Miao Wang1,2 Qiuzi Zhong1*
Qiuzi Zhong1* Yaqun Zhang1,2*
Yaqun Zhang1,2* Ming Liu1,2*
Ming Liu1,2*Objectives: To explore the effectiveness of radiotherapy in mPCa patients with different PSA stratifications based on the cancer database of a large population.
Background: Screening criteria for patients with metastatic prostate cancer, who are candidates for radiotherapy, are rarely reported.
Patients and Methods: We identified 22,604 patients with metastatic prostate cancer in the Surveillance, Epidemiology, and End Results database and divided them into a radiotherapy group and a control group. Patients with metastatic prostate cancer were divided into subgroups according to their levels of prostate-specific antigen to evaluate the efficacy of radiotherapy. They were also divided into six subgroups according to their prostate-specific antigen levels. We used multivariate Cox analysis to evaluate overall survival and cancer-specific survival. After 1:1 propensity score matching, Kaplan-Meier analysis was used to explore the difference in overall survival and cancer-specific survival in the radiotherapy and control group.
Results: In all, 5,505 patients received radiotherapy, compared to 17,099 in the control group. In the multivariate Cox analysis, radiotherapy improved overall survival (hazard ratio [HR]: 0.730, 95% confidence interval [CI]: 0.636–0.838; P<0.001) and cancer-specific survival (HR: 0.764, 95% CI: 0.647–0.903; P=0.002) in patients with a PSA level of 4–10 ng/mL. Similar results were obtained by Kaplan-Meier analysis after 1:1 propensity score matching. In patients with prostate-specific antigen levels between 4–10 ng/mL, the overall survival (P<0.001) and cancer-specific survival (P<0.05) in the radiotherapy group was significantly better than those in the control group.
Conclusion: The result of this large population-based study shows that rigorous selection of appropriate metastatic prostate cancer patients for radiotherapy can benefit prognosis significantly. This can be the basis for future prospective trials.
The incidence of prostate cancer (PCa) has been increasing annually, ranking first among male malignant tumors in the United States. It is estimated that there were 191,930 new cases of PCa in 2020, with 33,330 patients dying from PCa (1). PCa can present in different stages, and metastasis is an important stage adversely affecting prognosis (2, 3). Although the widespread use of prostate-specific antigen (PSA) testing has reduced the incidence of metastatic prostate cancer (mPCa) (4), 6% of PCa patients in the United States and 15.8% in the Netherlands still have metastatic disease at diagnosis (3, 5). The incidence can be as high as 50–64% in Asian countries (6, 7).
Two recent, prominent randomized controlled trials, HORRAD and STAMPEDE, have shown that radiotherapy has no survival benefit in overall unscreened cohorts of mPCa. In the HORRAD trial, 432 mPCa patients with PSA > 20 ng/mL were divided into a radiotherapy and a control group. The results revealed that radiotherapy did not significantly prolong the overall survival (OS) of patients with mPCa [hazard ratio (HR), 0.90; 95% confidence interval (CI): 0.70–1.14; P=0.4] (8). In the STAMPEDE trial of 2,061 mPCa patients with PSA > 30 ng/mL, there were no survival benefits of radiotherapy in the general population (HR: 0.92, 95% CI 0.80–1.06; P=0.27) (9). This was similar to the HORRAD results. However, in the subgroup analysis based on metastatic burden, radiotherapy was found to prolong the OS of patients with oligometastatic PCa. A meta-analysis combining data from the HORRAD and STAMPEDE studies revealed that radiotherapy could increase the absolute value of 3-year OS by 7% if the number of metastases was less than 4 (10). At present, radiotherapy for oligometastatic PCa has become a routine recommendation (11). Patient selection is important for radiotherapy, with clinicians trying to define further clinical parameters for optimal patient selection. However, no study has reported the efficacy of radiotherapy in patients with mPCa under different PSA subgroups.
PSA levels reflect the load of tumor cells. PCa with a higher PSA level is more aggressive and carries a higher risk of death due to cancer. However, recent studies have reported that PSA levels in patients with mPCa are not linearly related to prognosis. Patients with lower PSA levels (≤ 4 ng/mL) exhibited poorer prognoses than patients with higher PSA levels (12). The HORRAD and STAMPEDE studies also included only mPCa patients with PSA levels greater than 20 and 30 ng/mL, respectively. Previous retrospective studies (13–15) did not stratify mPCa patients according to PSA levels to explore the efficacy of radiotherapy. Therefore, the purpose of this study was to explore the effectiveness of radiotherapy in mPCa patients with different PSA stratifications based on the cancer database of a large population.
The Surveillance, Epidemiology, and End Results (SEER) database is the authoritative cancer statistics database in the United States. It covers about 28% of the clinical cancer patients in the United States and records the morbidity, mortality, and illness of these patients among other clinical information. In the SEER database (2004–2015), we identified 22,604 mPCa patients who met our inclusion criteria. The inclusion criteria were as follows: pathological diagnosis of prostate cancer, metastatic disease at the time of diagnosis, age > 18 years, and having received radiotherapy. The exclusion criteria were as follows: those who underwent radical prostatectomy, patients whose radiotherapy details were incomplete, patients whose PSA information was unrecorded, and patients whose survival time information was lacking. Age, race, Gleason score, T stage, N stage, M stage, and PSA level were recorded for patients who met the criteria.
This study’s main outcome index is OS; the secondary outcome index is cancer-specific survival (CSS). Pearson’s chi-square analysis was used to determine the variables between different treatment groups. We stratified the data according to PSA levels (< 4.0, 4.1–10.0, 10.1–20.0, 20.1–40.0, 40.1–80.0, > 80.1 ng/mL). Covariates were included in univariate and multivariate Cox regression analyses to determine the prognostic HR and 95% CI of the treatment group. In the multivariate analysis, PSA (4.1–10.0 ng/mL) was used as a reference to explore the relative HR of other subgroups. To balance covariance and reduce deviation in the evaluation of therapeutic effect, we measured OS and CSS using the Kaplan-Meier (KM) method at 1:1 propensity score matching in the radiotherapy group and control group of each PSA subgroup. We designed the analysis for two-sided tests using IBM SPSS Statistics for Windows, Version 25.0. (IBM Corp., Armonk, NY). Statistical significance was set at P < 0.05.
A total of 22,604 mPCa patients who met the inclusion criteria were identified from the SEER database. There were 5,505 patients in the radiotherapy group and 17,099 participants in the control group. Patient characteristics are shown in Table 1.
Univariate and multivariate analysis were used to determine whether there was a statistical correlation between the PSA subgroups and the prognosis of patients within each subgroup. With each PSA subgroup, we included age, race, Gleason score, treatment groups, T stage, N stage, and M stage in a univariate Cox analysis. The univariate Cox analysis showed that the PSA group with the highest OS was the PSA 4.1–10.0 ng/mL subgroup (P < 0.001), while the PSA groups correlated with CSS were the PSA 4.1–10.0 ng/mL and PSA > 80.1 ng/mL subgroups (P<0.05) (Supplementary Table 1). All significant factors in the univariate Cox analysis (e.g., 4.1–10.0 ng/mL, 40.1–80.0 ng/mL, and > 80.1 ng/mL subgroups) were included in the multivariate Cox regression analysis. The results showed that radiotherapy could significantly improve OS (HR: 0.730, 95% CI: 0.636–0.838; P < 0.001) and CSS (HR: 0.764,95% CI: 0.647–0.903; P=0.002) of mPCa in the PSA 4.1–10.0 ng/mL subgroup (Table 2). In the PSA > 80.1 ng/mL subgroup, the radiotherapy group was associated with worse CSS (HR: 1.065, 95% CI: 1.009–1.124; P=0.023) (Supplementary Table 2).
In the multivariate analysis, using the PSA 4.1–10.0 ng/mL subgroup as the reference, the distribution of OS in the SEER cohort showed a U-shaped distribution relative to PSA (Figure 1), the HRs of the PSA < 4.0, 10.1–20.0, 20.1–40.0, 40.1–80.0, and > 80.1 ng/mL groups were 1.331, 1.136, 1.211, 1.379, and 1.544, respectively (Supplementary Table 3). When CSS was used as the outcome index, the HRs of the PSA < 4.0, 10.1–20.0, 20.1–40.0, 40.1–80.0, and > 80.1 ng/mL groups were 1.436, 1.120, 1.257, 1.459, and 1.679, respectively (Supplementary Table 3). CSS and PSA showed a similar U-shaped distribution (Figure 1).
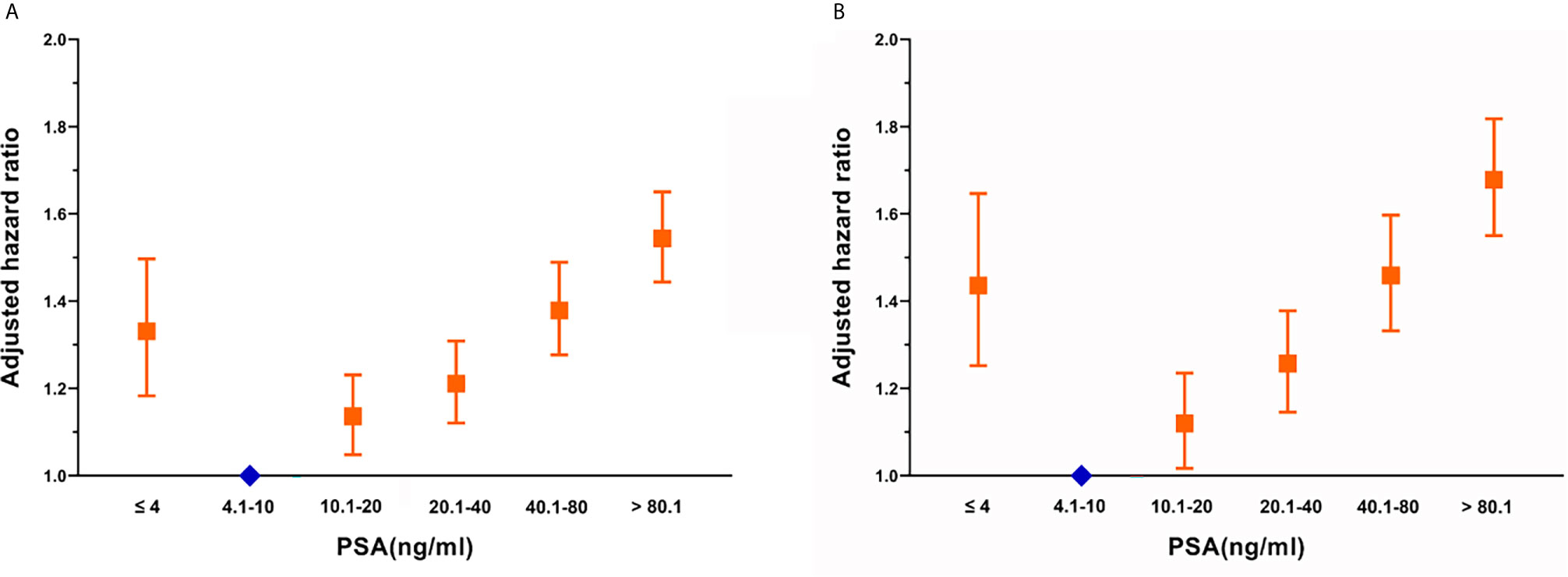
Figure 1 Adjusted hazard ratios with 95% confidence intervals for the association between PSA and (A) overall survival and (B) cancer-specific survival.
After matching with a 1:1 tendency score, there was no significant difference in age, race, Gleason score, T stage, N stage, and M stage between the radiotherapy and control group in each PSA subgroup (Tables 3–8). KM analysis showed that radiotherapy could significantly improve OS only in patients of the PSA 4.1–10.0 ng/mL subgroup (P < 0.001). There was no significant difference in OS between the radiotherapy and control groups in other PSA subgroups (Figure 2). When CSS was used as the outcome index, KM analysis showed that radiotherapy was most beneficial in mPCa patients with PSA levels of 4.1–10.0 ng/mL. In the PSA > 80.1 ng/mL subgroup, the CSS of the radiotherapy group was inferior to that of the control group (P < 0.05), but there was no significant difference in the improvement of prognosis among other PSA subgroups (Figure 3).
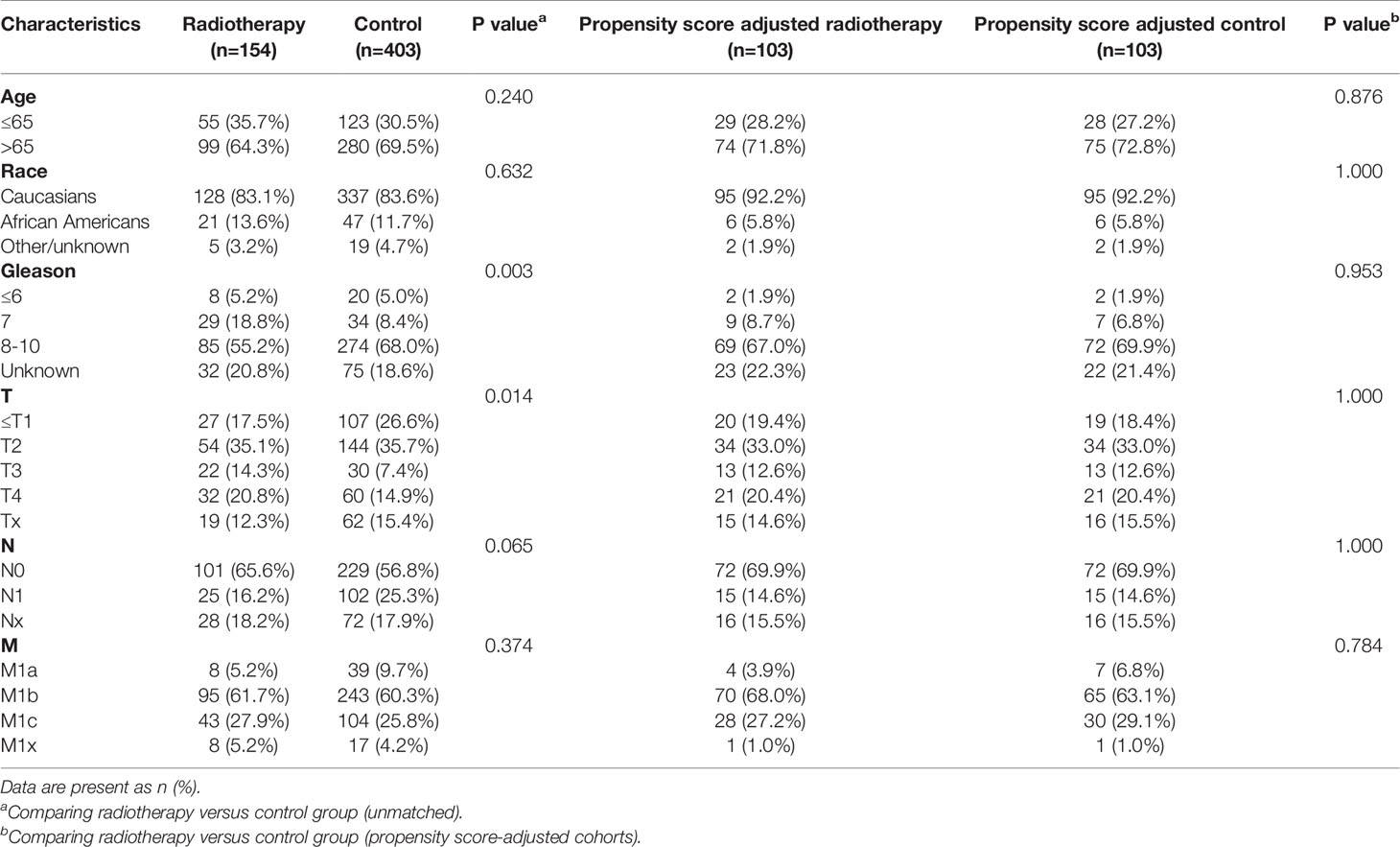
Table 3 Clinicopathological characteristics of the PSA <4 ng/ml subgroup stratified according to treatment modality with and without propensity score matching.
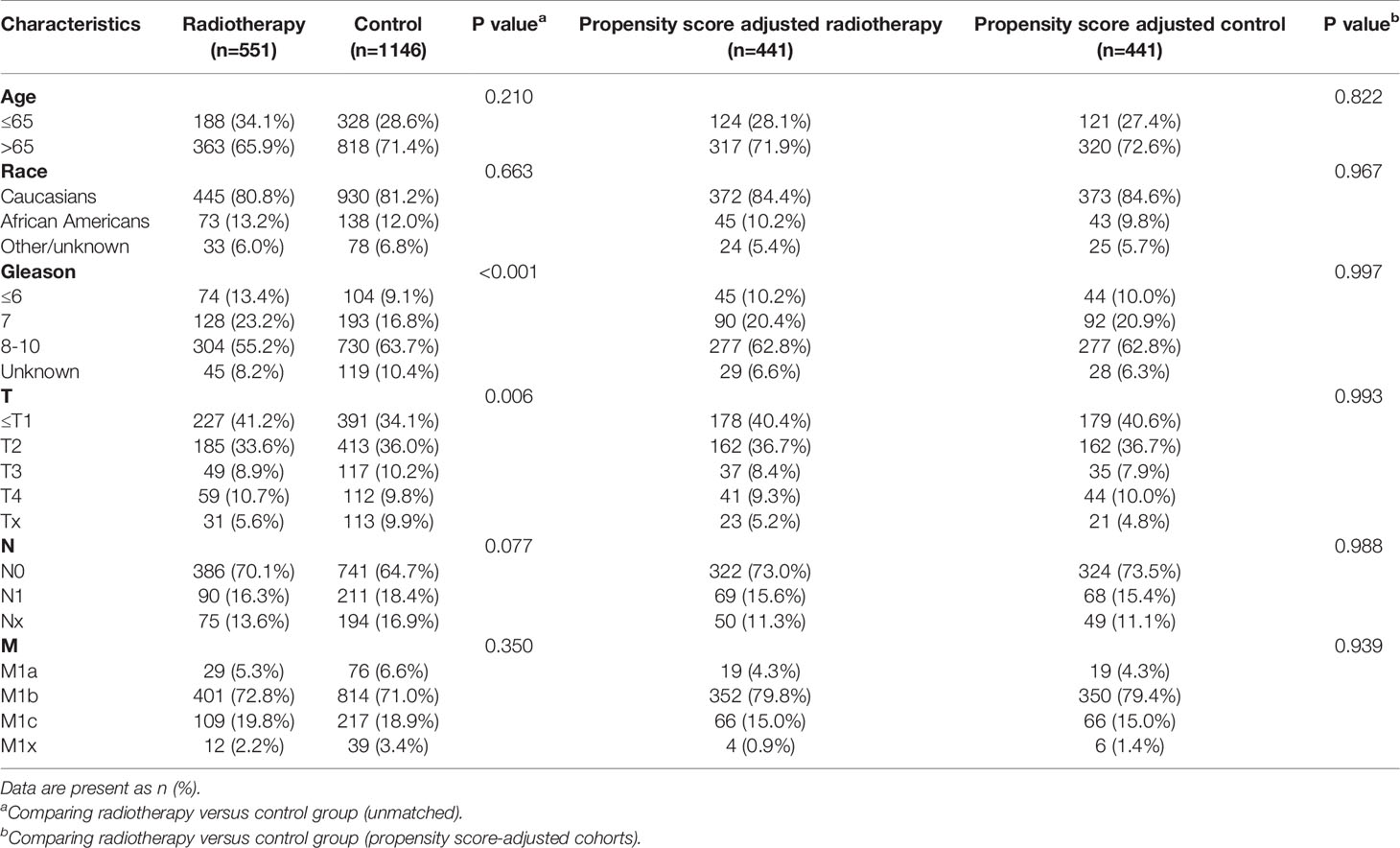
Table 4 Clinicopathological characteristics of the PSA 4.1-10 ng/ml subgroup stratified according to treatment modality with and without propensity score matching.
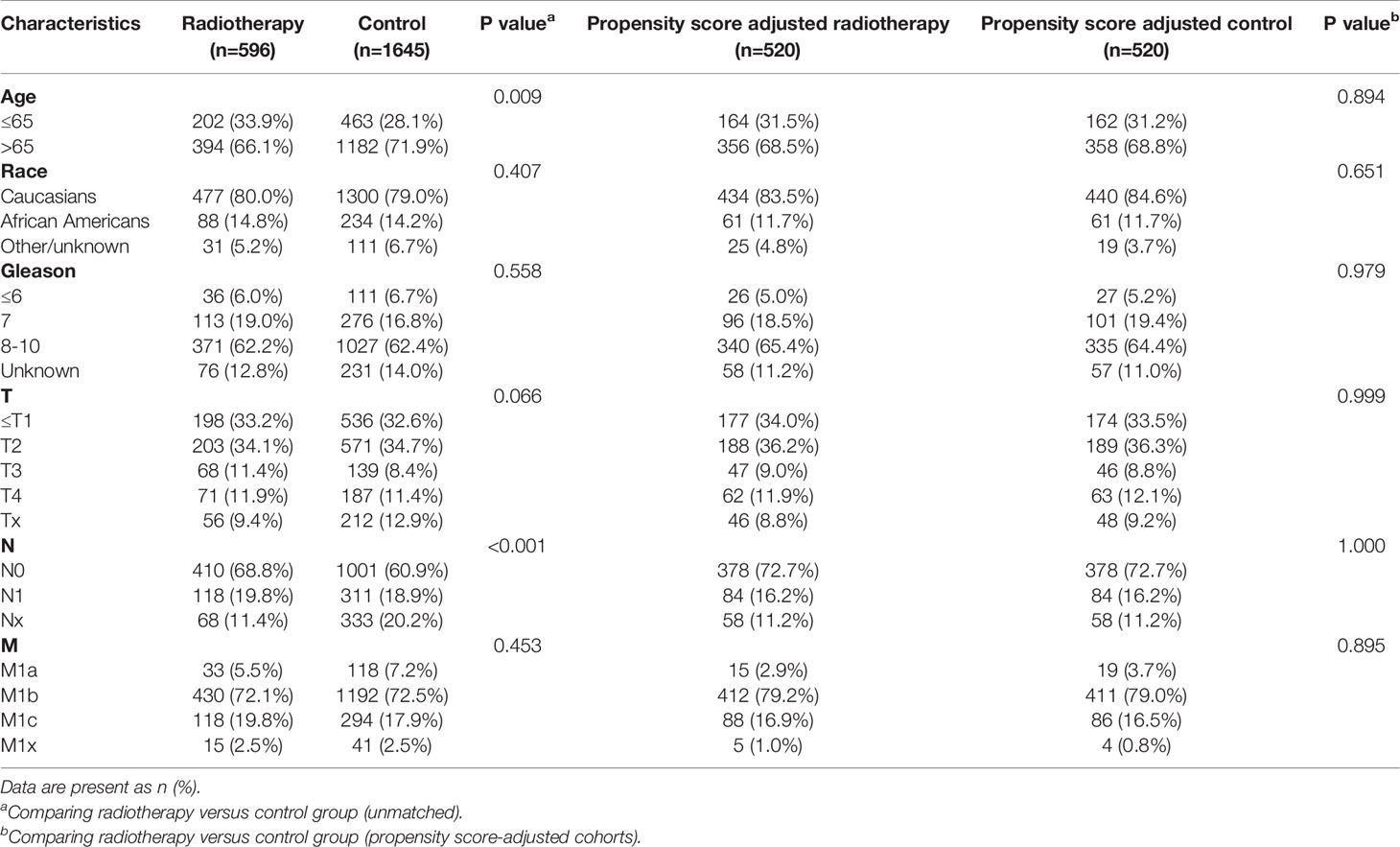
Table 5 Clinicopathological characteristics of the PSA 10.1-20 ng/ml subgroup stratified according to treatment modality with and without propensity score matching.
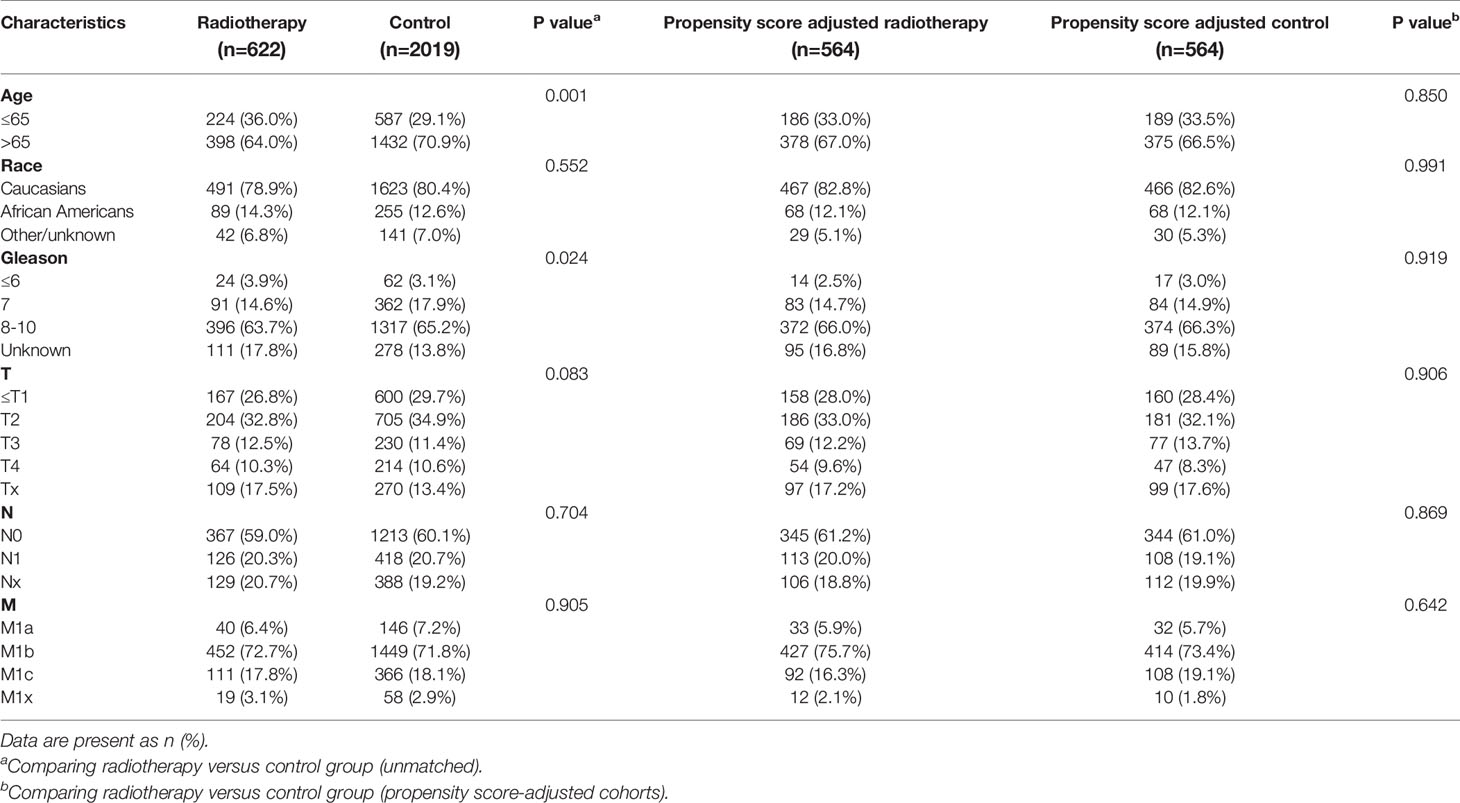
Table 6 Clinicopathological characteristics of the PSA 20.1-40 ng/ml subgroup stratified according to treatment modality with and without propensity score matching.
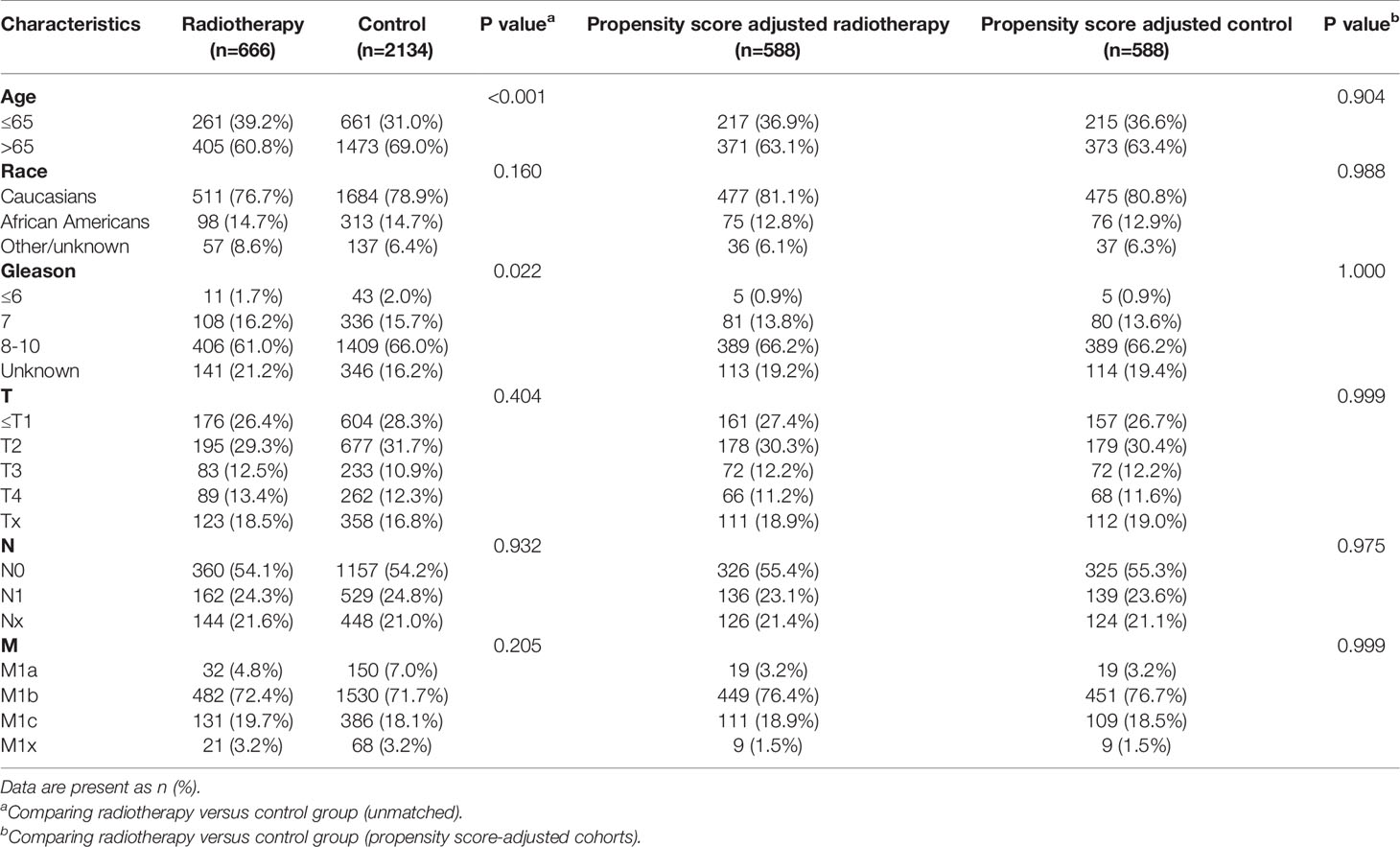
Table 7 Clinicopathological characteristics of the PSA 40.1-80ng/ml subgroup stratified according to treatment modality with and without propensity score matching.
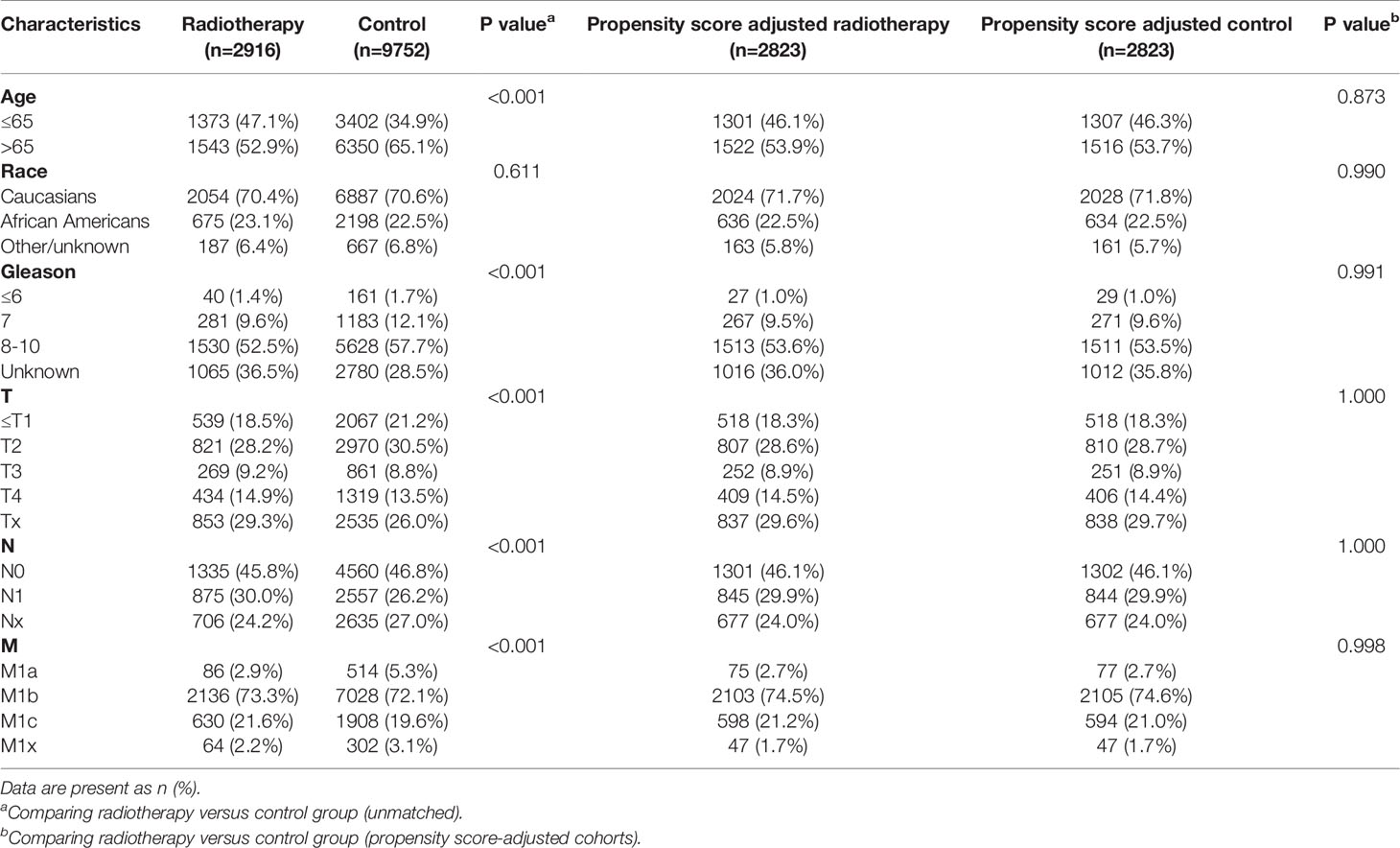
Table 8 Clinicopathological characteristics of the PSA >80.1 ng/ml subgroup stratified according to treatment modality with and without propensity score matching.
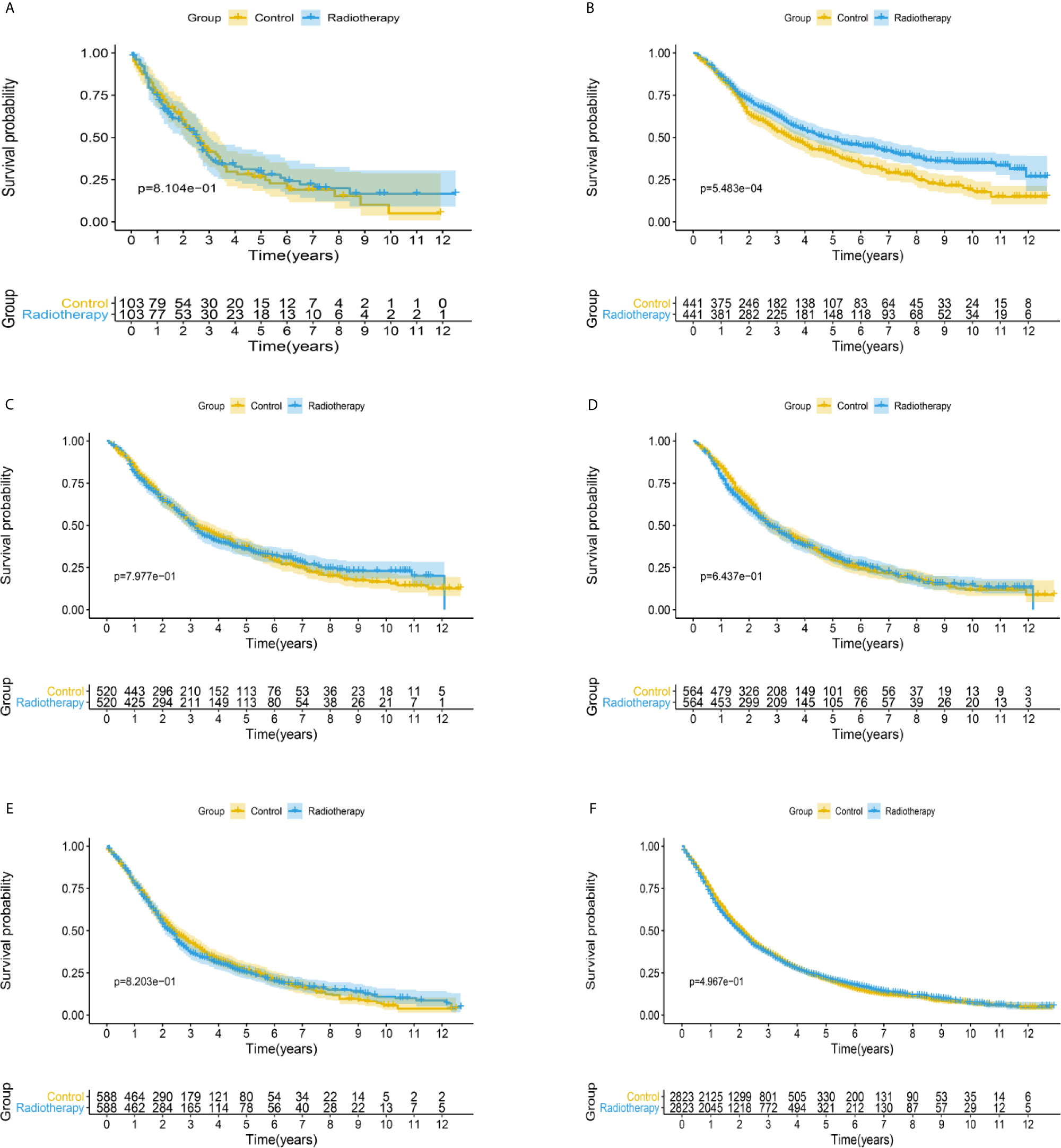
Figure 2 Kaplan-Meier survival analysis of overall survival in (A) PSA <4 ng/mL, (B) PSA 4.1–10 ng/mL, (C) PSA 10.1–20 ng/mL, (D) PSA 20.1–40 ng/mL, (E) PSA 40.1–80 ng/mL, and (F) PSA >80.1 ng/mL subgroups.
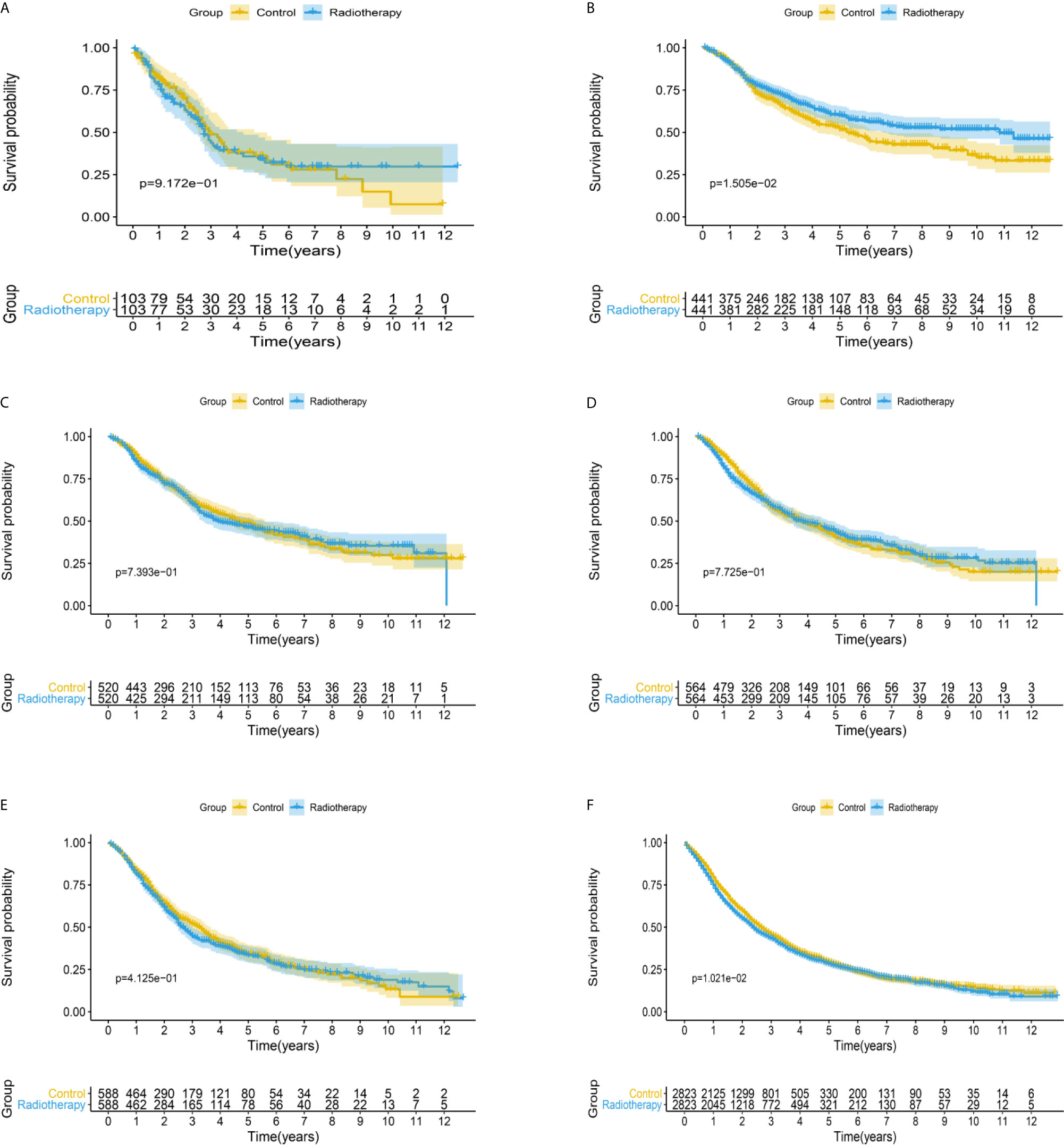
Figure 3 Kaplan-Meier survival analysis of cancer-specific survival in (A) PSA <4 ng/mL, (B) PSA 4.1–10 ng/mL, (C) PSA 10.1–20 ng/mL, (D) PSA 20.1–40 ng/mL, (E) PSA 40.1–80 ng/mL, and (F) PSA >80.1 ng/mL subgroups.
Large-scale population-based cohort analyses of PCa patients can provide important guidelines for clinical treatment and further prospective studies. At present, a stratified analysis of 22,604 patients with mPCa in the SEER database was conducted to determine whether PSA grouping would affect the efficacy of radiotherapy.
The direct cytotoxic effects of local radiotherapy for PCa have been widely reported (16). The biological mechanism of radiotherapy may be related to tumor cell death and changes in the tumor microenvironment (TME) induced by radiation (17). In short, radiation directly damages the TME, triggering the release of immunostimulatory factors, such as heat shock protein 70, enhancing the anti-tumor activity of immune cells (18, 19). Radiotherapy also changes the vascular endothelial cells of the tumor bed and promotes the release of chemokines, thus promoting the recruitment and entry of activated immune cells to tumors (20, 21). These synergistic effects eventually lead to the invasion of CD8+ T cells and other effector cells into the TME, resulting in a strong local anti-tumor immune response (22). The release of immunomodulatory factors, activation of dendritic cells, and increased antigen presentation mediated by MHC I in tumor cells, induced by radiotherapy, can promote tumor-associated antigens’ immune recognition initiating anti-tumor T cells (23–25). Subsequently, tumor-associated antigen-specific cytotoxic T cells can attack tumor cells not only in irradiated local tumors but also in distant metastatic tumors (26).
Interestingly, this study showed that radiotherapy only significantly improved OS in mPCa patients with PSA levels of 4.1–10.0 ng/mL. Radiotherapy for mPCa with PSA < 4.0 ng/mL did not improve the survival rate. In the multivariate analysis, PSA was identified as an independent prognostic factor for mPCa, and the prognosis of the PSA in the < 4.0 ng/mL subgroup was lower than that of the PSA in the 4.1–10.0 ng/mL subgroup, which may explain why the curative effect of radiotherapy in the PSA < 4.0 ng/mL subgroup was not as good as that of the PSA in the 4.1–10.0 ng/mL subgroup. Wang et al. also pointed out that low PSA (< 4 ng/mL) is a unique entity that represents more invasive disease in mPCa and indicates a poor prognosis (12). It is reported that about 5–10% of PCa show low PSA (27); this may be due to the potential biological characteristics of dedifferentiation where epithelial cells lose the expression of PSA-coding genes (28), and patients with this type of PCa may have a higher incidence of non-organ-limiting diseases (29). In a study of 183 patients with mPCa, low PSA secretors’ molecular characteristics and clinical results were described. Compared with normal secretors, RB1 and TP53 gene deletions were more common in low PSA secretors. More importantly, similar to our results, patients with low PSA secretion had a shorter OS (30). Therefore, a low PSA level is an indicator of poorly differentiated aggressive tumor behavior, while a very high PSA level is an indicator of a high tumor burden (31). We believe that poor differentiation and a high tumor load may have a negative impact on the survival rate and efficacy of radiotherapy in patients with mPCa.
Boevé et al. provided important data for evaluating the quality of life in patients with mPCa after radiotherapy. In their recent study, they reported that patients who received radiotherapy had more diarrhea, urinary, and intestinal symptoms than those in the control group. Although the differences in urinary symptoms and diarrhea disappeared, the intestinal symptoms were still higher than those in the control group after two years (5). Therefore, the choice of radiotherapy for patients with mPCa should be carefully considered to avoid unnecessary complications.
There are some limitations to our study. Since it is a retrospective study, it is inevitably affected by some potential biases. Second, the database lacks detailed information on systemic treatments, androgen deprivation therapy, and radiation doses. The variables available in the SEER database may be inadequate to account for all the variation in treatment selection. Third, our data was obtained only from the SEER database since there are very few mPCa patients with low PSA levels in the clinic; consequently, there is no real-world data verification. Our results need to be verified by high-quality prospective randomized trials.
In this assessment of a large, population-based dataset, we found that therapeutic effects in mPCa patients after radiotherapy varied depending on PSA level. This study shows that radiotherapy is most beneficial for mPCa patients with PSA levels of 4.1–10.0 ng/mL. We not only confirmed that radiotherapy provides no added benefit when the tumor load is high, but also proposed for the first time that radiotherapy for poorly differentiated mPCa patients does not significantly improve the prognosis. Our study provides direction to prospective clinical trials and clinical treatment in the future and is expected to change the existing treatment strategies for mPCa.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Study concept & design: ZT and ML. Data acquisition or data analysis/interpretation: LM, QZ, and YZ. Manuscript drafting or manuscript revision for important intellectual content: ZT and XinW. Approval of final version of submitted manuscript: ML and XuanW. Literature research: TM and MW. All authors contributed to the article and approved the submitted version.
This study was financially supported by the Beijing Municipal Science and Technology Project (Z201100005620007), the Beijing Hospital Clinical Research 121 Project (BJ-2018-090), and the Fundamental Research Funds for the Central Universities (3332020069).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The results published here are based on data in the SEER database (https://seer.cancer.gov/data/).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.706236/full#supplementary-material
1. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal Cancer Statistics, 2020. CA Cancer J Clin (2020) 70:145–64. doi: 10.3322/caac.21601
2. Gillessen S, Attard G, Beer TM, Beltran H, Bjartell A, Bossi A, et al. Management of Patients With Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol (2020) 77:508–47. doi: 10.1016/j.eururo.2020.01.012
3. SEER. Cancer Stat Facts: Prostate Cancer . Bethesda, MD: National Cancer Institute. Available at: https://seer.cancer.gov/statfacts/html/prost.html (Accessed March 8, 2021).
4. Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. Eur Urol Focus (2018) 4:121–7. doi: 10.1016/j.euf.2017.10.014
5. Boevé L, Hulshof MCCM, Verhagen PCMS, Twisk JWR, Witjes WPJ, de Vries P, et al. Patient-Reported Quality of Life in Patients With Primary Metastatic Prostate Cancer Treated With Androgen Deprivation Therapy With and Without Concurrent Radiation Therapy to the Prostate in a Prospective Randomised Clinical Trial; Data From the HORRAD Trial. Eur Urol (2021) 79:188–97. doi: 10.1016/j.eururo.2020.08.023
6. Chen R, Ren S, Chinese Prostate Cancer Consortium, Yiu MK, Fai NC, Cheng WS, et al. Prostate Cancer in Asia: A Collaborative Report. Asian J Urol (2014) 1:15–29. doi: 10.1016/j.ajur.2014.08.007
7. Uemura H, Ye D, Kanesvaran R, Chiong E, Lojanapiwat B, Pu YS, et al. United in Fight Against prOstate Cancer (UFO) Registry: First Results From a Large, Multi-Centre, Prospective, Longitudinal Cohort Study of Advanced Prostate Cancer in Asia. BJU Int (2020) 125:541–52. doi: 10.1111/bju.14980
8. Boevé LMS, Hulshof MCCM, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined With Concurrent Radiation Therapy to the Prostate in Patients With Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data From the HORRAD Trial. Eur Urol (2019) 75:410–8. doi: 10.1016/j.eururo.2018.11.030
9. Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the Primary Tumour for Newly Diagnosed, Metastatic Prostate Cancer (STAMPEDE): A Randomised Controlled Phase 3 Trial. Lancet (2018) 392:2353–66. doi: 10.1016/S0140-6736(18)32486-3
10. Burdett S, Boevé LM, Ingleby FC, Fisher DJ, Rydzewska LH, Vale CL, et al. Prostate Radiotherapy for Metastatic Hormone-Sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-Analysis. Eur Urol (2019) 76:115–24. doi: 10.1016/j.eururo.2019.02.003
11. Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. Eau-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part Ii-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur Urol (2021) 79:263–82. doi: 10.1016/j.eururo.2020.09.046
12. Wang J, Abudurexiti M, Shao N, Wei Y, Zhu Y, Ye DW. The U Shape of Prostate-Specific Antigen and Prostate Cancer-Specific Mortality in High-Grade Metastatic Prostate Adenocarcinoma. Eur Urol Focus (2020) 6:53–62. doi: 10.1016/j.euf.2018.08.024
13. Pompe RS, Tilki D, Preisser F, Leyh-Bannurah SR, Bandini M, Marchioni M, et al. Survival Benefit of Local Versus No Local Treatment for Metastatic Prostate cancer-Impact of Baseline PSA and Metastatic Substages. Prostate (2018) 78:753–7. doi: 10.1002/pros.23519
14. Stolzenbach LF, Deuker M, Collà-Ruvolo C, Nocera L, Mansour M, Tian Z, et al. External Beam Radiation Therapy Improves Survival in Low-Volume Metastatic Prostate Cancer Patients: A North American Population-Based Study. Prostate Cancer Prostatic Dis (2020) 24:253–60. doi: 10.1038/s41391-020-00276-2
15. Stolzenbach LF, Rosiello G, Deuker M, Martin T, Knipper S, Tian Z, et al. External Beam Radiation Therapy Improves Survival in Elderly Metastatic Prostate Cancer Patients With Low PSA. Urol Oncol (2021) 39:131.e–7. doi: 10.1016/j.urolonc.2020.10.011
16. Vilalta M, Rafat M, Graves EE. Effects of Radiation on Metastasis and Tumor Cell Migration. Cell Mol Life Sci (2016) 73:2999–3007. doi: 10.1007/s00018-016-2210-5
17. Brooks ED, Chang JY. Time to Abandon Single-Site Irradiation for Inducing Abscopal Effects. Nat Rev Clin Oncol (2019) 16:123–35. doi: 10.1038/s41571-018-0119-7
18. Levy A, Chargari C, Marabelle A, Perfettini JL, Magné N, Deutsch E. Can Immunostimulatory Agents Enhance the Abscopal Effect of Radiotherapy? Eur J Cancer (2016) 62:36–45. doi: 10.1016/j.ejca.2016.03.067
19. Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jäättelä M, et al. Dual Function of Membrane-Bound Heat Shock Protein 70 (Hsp70), Bag-4, and Hsp40: Protection Against Radiation-Induced Effects and Target Structure for Natural Killer Cells. Cell Death Differ (2005) 12:38–51. doi: 10.1038/sj.cdd.4401510
20. Hellevik T, Martinez-Zubiaurre I. Radiotherapy and the Tumor Stroma: The Importance of Dose and Fractionation. Front Oncol (2014) 4:1. doi: 10.3389/fonc.2014.00001
21. Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-Induced CXCL16 Release by Breast Cancer Cells Attracts Effector T Cells. J Immunol (2008) 181:3099–107. doi: 10.4049/jimmunol.181.5.3099
22. Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, et al. Radiation-Induced Equilibrium is a Balance Between Tumor Cell Proliferation and T Cell-Mediated Killing. J Immunol (2013) 190:5874–81. doi: 10.4049/jimmunol.1202612
23. Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-Induced Immunogenic Modulation of Tumor Enhances Antigen Processing and Calreticulin Exposure, Resulting in Enhanced T-cell Killing. Oncotarget (2014) 5:403–16. doi: 10.18632/oncotarget.1719
24. Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation Fosters Dose-Dependent and Chemotherapy-Induced Immunogenic Cell Death. Oncoimmunology (2014) 3:e28518. doi: 10.4161/onci.28518
25. Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn GO, Dutt S, et al. Ablative Tumor Radiation can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res (2015) 21:3727–39. doi: 10.1158/1078-0432.CCR-14-2824
26. Siva S, MacManus MP, Martin RF, Martin OA. Abscopal Effects of Radiation Therapy: A Clinical Review for the Radiobiologist. Cancer Lett (2015) 356:82–90. doi: 10.1016/j.canlet.2013.09.018
27. Genega EM, Hutchinson B, Reuter VE, Gaudin PB. Immunophenotype of High-Grade Prostatic Adenocarcinoma and Urothelial Carcinoma. Mod Pathol (2000) 13:1186–91. doi: 10.1038/modpathol.3880220
28. Weir EG, Partin AW, Epstein JI. Correlation of Serum Prostate Specific Antigen and Quantitative Immunohistochemistry. J Urol (2000) 163:1739–42. doi: 10.1016/S0022-5347(05)67532-5
29. Berglund RK, Stephenson AJ, Cronin AM, Vickers AJ, Eastham JA, Klein EA, et al. Comparison of Observed Biochemical Recurrence-Free Survival in Patients With Low PSA Values Undergoing Radical Prostatectomy and Predictions of Preoperative Nomogram. Urology (2009) 73:1098–103. doi: 10.1016/j.urology.2008.07.052
30. Aggarwal R, Romero GR, Friedl V, Weinstein A, Foye A, Huang J, et al. Clinical and Genomic Characterization of Low Psa Secretors: A Unique Subset of Metastatic Castration Resistant Prostate Cancer. Prostate Cancer Prostatic Dis (2020) 24:81–7. doi: 10.1038/s41391-020-0228-0
Keywords: prostate-specific antigen, metastatic prostate cancer, SEER, survival analysis, radiotherapy
Citation: Tian Z, Meng L, Wang X, Wang X, Ma T, Wang M, Zhong Q, Zhang Y and Liu M (2021) Survival in Patients With Metastatic Prostate Cancer Undergoing Radiotherapy: The Importance of Prostate-Specific Antigen-Based Stratification. Front. Oncol. 11:706236. doi: 10.3389/fonc.2021.706236
Received: 07 May 2021; Accepted: 24 May 2021;
Published: 10 June 2021.
Edited by:
An Liu, City of Hope National Medical Center, United StatesReviewed by:
Chengyu Shi, New York Proton Center, United StatesCopyright © 2021 Tian, Meng, Wang, Wang, Ma, Wang, Zhong, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Liu, bGl1bWluZ2JqeXlAMTI2LmNvbQ==; Yaqun Zhang, emhhbmd5cWJqeXlAMTI2LmNvbQ==; Qiuzi Zhong, emhvbmdxemJqeXlAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.