- 1Department of Urology, Osaka University Graduate School of Medicine, Suita, Japan
- 2Department of Urology, Osaka General Medical Center, Osaka, Japan
- 3Department of Urology, Kindai University Faculty of Medicine, Osakasayama, Japan
- 4Department of Pathology, University of Alabama at Birmingham, Birmingham, AL, United States
Urothelial carcinoma (UC) is a common urological malignancy with a high rate of disease recurrence. Telomerase activity, a hallmark of cancer characterized by overcoming the replicative senescence, is upregulated in over 90% of patients with UC. Somatic mutations in the promoter region of telomerase reverse transcriptase (TERT) are frequently detected in UC, and drive telomerase activity. Recent studies have demonstrated a strong association between TERT promoter mutation and tumorigenesis of UC. Also, TERT promoter mutation has great potential for diagnosis, as well as prognosis in UC treatment, and this is also applicable for the liquid biopsy techniques. In this review, we discuss the progress in these areas and highlight the challenges, clinical potential, and future direction for developing UC treatment methods.
Introduction
Approximately 95% of cases of urothelial carcinoma (UC) are reported in the bladder (UBC: urothelial bladder carcinoma), and upper tract urothelial carcinoma (UTUC) that occurs in the renal pelvis or ureter corresponds to a small subset of UC (1). UBC is a globally common disease, with the incidence of 570,000 cases and 210,000 deaths reported in 2020 worldwide (2), and cigarette smoking is thought to be the most important risk factor for UC formation (3). Additionally, the exposure to aristolochic acid increased the risk of developing UTUC (4). Comprehensive tumor genome analysis provides a deeper understanding of the molecular biology of both UBC (5) and UTUC (6). Although there are some differences between UTUC and UBC in terms of embryological aspects and their association with the environmental exposure, these two types of carcinoma exhibit a close relationship in clinical practice, as they share some of the pathological and mutational signatures (7). In addition to FGFR3 or TP53 mutations, the promoter of telomerase reverse transcriptase (TERT) is among the most frequently mutated genomic region in UC tissue (8, 9). Because of the high recurrence rate and the need for definitive diagnosis, many patients with UBC and UTUC are often required to perform periodic invasive endoscopy tests, such as cystoscopy or ureteroscopy (10, 11). Although there are few reliable biomarkers of UC, liquid biopsy technique targeting the frequently mutated genes in UC tissue would help resolve this issue in a non-invasive manner. The growing evidences suggest that TERT promoter mutation, telomere length, and telomerase activity play crucial roles in UC tumorigenesis through inducing genomic instability (12–14), and act as clinically useful biomarkers tested using liquid biopsy technique (15). In this review, we summarize the current biological insights on TERT promoter mutation and telomerase activity in UC, and their clinical application in patients with UC.
Role of Telomeres in Cancer
Human telomeres existing at the chromosomal ends play an essential role in protecting the chromosomes from being mistaken as the sites of DNA damage (15). Human telomeres are composed of a long double-stranded “TTAGGG” canonical repetitive sequence comprising thousands of bases (16). The telomeric ends are concealed and protected from DNA damage response through forming a T-loop structure. Telomeres are shortened by less than 50 base pairs in each cell division, which is attributed to the inability of DNA polymerase to duplicate the ends of DNA molecules. This issue of end-replication, and short dysfunctional telomeres eventually result in cell cycle arrest or senescence once the replication limit of cell division, known as the Hayflick limit, is reached (17). Therefore, telomere shortening-induced cellular senescence acts as a strong barrier against tumorigenesis. However, in cancer cells or cancer progenitor cells that acquire the ability to escape the cell cycle arrest pathway or cellular senescence, dysfunctional telomeres act as an origin of genomic instability, and accumulation of somatic mutations in a state referred to as telomere crisis (13). Meeker et al. demonstrated that telomere shortening was widespread in various cancer types using direct telomere-fluorescence in situ hybridization technique, whereas telomere length variability was prevalent in bladder cells with either short or long telomeres (18).
Telomerase and Tert
In 1985, terminal telomere transferase, later referred to as telomerase, was first discovered by Carol Greider and Elizabeth Blackburn (19). They reported the enzymatic activity in T. thermophila extracts that synthesized and elongated telomeres for the first time, and were awarded the Nobel Prize in Physiology or Medicine in 2009. Later, telomerase activity was discovered in a number of species, including humans (16). Telomerase is a telomere-specific ribonucleoprotein polymerase composed of reverse transcriptase (TERT) and telomerase RNA component (TERC). TERC contains a CCCUAA telomere template in its core domain (20). TERT synthesizes TTAGGG telomeric repeats and elongates telomeres where TERC binds to the 3’ end of telomere (16). Telomerase activity was downregulated through silencing TERT in most human adult non-germline tissues, except for a few stem or progenitor cells. The absence of telomerase activity leads to cellular senescence in normal cells during aging. Conversely, almost all human cancer cells maintain telomere length over many cell divisions through activating either DNA recombination alternative lengthening of telomeres (ALT) or upregulating TERT (16). Although the ALT pathway occurs in only about 10% to 15% of cancers, about 85% to 90% of all human cancers exhibit high rate of telomerase activation (16). For these reasons, the expression of telomerase or TERT is considered as a hallmark of cancer, which enables replicative immortality (16, 20).
Two Hotspot Mutations in Tert Promoter Region
In 2013, two cancer-specific hotspot somatic mutations in TERT promoter that activate TERT transcription were first identified in melanoma (21, 22). Thereafter, TERT promoter mutations were found to be one of the most frequently mutated genomic regions in several tumor tissues (23). These mutations are located 124 and 146 bp upstream of the translation start site (TSS) of TERT gene and are referred to as C228T (g.1295228 C>T in GRCh37), and C250T (g.1295250 C>T in GRCh37). These two hotspot C to T transitions are typically heterozygous and form the same 11 base pair sequences of “CCCCTTCCGGG” (20) (Figure 2A). Interestingly, TERT promoter mutations are frequently detected in tumors originating from normal cells with low rates of self-renewal, such as glioblastoma (83%), melanoma (71%), UBC (67%), UTUC (47%), and hepatocellular carcinoma (HCC) (44%) (23). Conversely, some of the prevalent malignant tumors, including breast cancer, prostate cancer, thyroid cancer, colon cancer, stomach cancer, and leukemia, exhibit occasional TERT promoter mutations (23). Although the C228T and C250T hotspot mutations generate identical binding motifs for the transcription factors, there seems to be differences between these two mutations. We selected previous reports with a large number of cases or with seminal initial significance and analyzed the association of frequency of TERT C228T mutation in entire TERT promoter mutation and that of TERT promoter mutation in various types of tumor specimens [melanoma (21, 22, 24–28), glioblastoma (29–35), HCC (23, 36–41), papillary thyroid carcinoma (42–48), UBC (23, 49–58), and UTUC (23, 59)] (Figure 1). The size of the circles indicated the number of samples analyzed in each study. These data have demonstrated that TERT C228T mutation is frequently detected in multiple types of malignant tumors, including glioblastoma, hepatocellular carcinoma, thyroid carcinoma, and UC compared with C250T TERT mutation, except for skin melanoma (Figure 1). UC and glioblastoma exhibit a high frequency of TERT C228T mutation. Conversely, the ratio of TERT C228T mutation per TERT promoter mutations was reported to be high in thyroid cancer, but the frequency of TERT promoter mutations was lower than that in other types of cancer (Figure 1). The frequency of TERT promoter mutation in melanoma varied widely among several reports, and the ratio of TERT C228T mutation was lower than that in other types of cancer (Figure 1). In addition to the difference in this frequency, Li et al. demonstrated that to upregulate TERT or telomerase expression, it is necessary for tumor with C250T mutation, unlike C228T mutation, to introduce ETS1/2 transcription factor in cooperation with p52 downstream of the non-canonical NF-κB signaling pathway (60). Conversely, although the detailed biological mechanism of C228T mutation remains unclear, several reports suggest that C228T mutation acts more aggressively for a malignant phenotype than C250T mutation. In an experiment using genetically engineered hESCs, among a few types of TERT promoter mutations, only C228T mutation increased TERT mRNA expression, but not C250T or other types of mutation (61). Borah et al. demonstrated that TERT promoter mutation, mainly C228T, was associated with high levels of TERT mRNA expression, TERT protein expression, telomerase activity, and telomere length in bladder cancer cell lines (12). Furthermore, we found that C228T mutation detected in urinary cell-free DNA (cfDNA) was associated with bladder tumor recurrence in patients after transurethral surgery for NMIBC or radical nephroureterectomy for localized UTUC (57, 62). We also reported that TERT C228T mutation, but not C250T mutation, in the non-malignant urothelium of patients with NMIBC, was significantly associated with intravesical recurrence after transurethral surgery (58). As mentioned above, TERT C228T mutation was associated with worse clinical outcomes in some tumor types than TERT C250T mutation. These biological differences between the two hotspot mutations may be simply attributed to the difference in frequency, or due to differences in the transcription factor accessibility or epigenetic state; however, further investigation is required to be performed in the future.
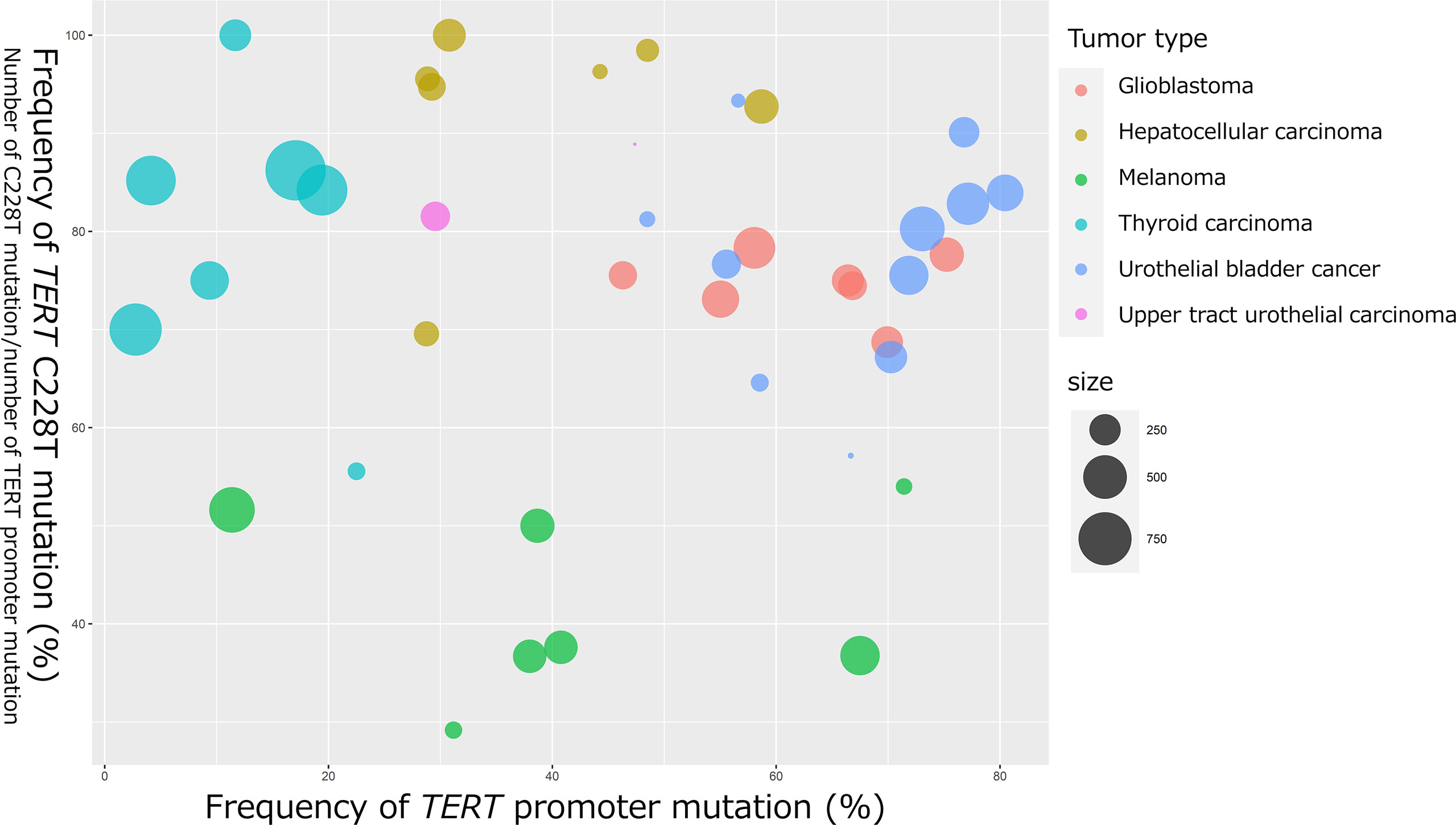
Figure 1 Bubble chart indicating the frequency of TERT promoter mutation and TERT C228T mutation in glioblastoma, HCC, melanoma, papillary thyroid cancer, UBC, and UTUC. The horizontal axis represents the frequency of TERT promoter mutations (number of TERT promoter mutations per number of all cases). The vertical axis represents the frequency of TERT C228T mutation (number of TERT C228T mutations per number of TERT promoter mutations). The bubble size represents the number of samples analyzed.
Potential Mechanism of Tert Promoter Mutation-Induced Tumorigenesis
Promoter mutations of TERT gene (C228T and C250T) creates a de novo binding site of GA-binding protein A (GABPA), an E-twenty-six (ETS) family transcription factor, in close proximity to a native ETS binding site in the wild-type TERT promoter (Figure 2A) (63). Akincilar et al. reported that TERT promoter mutation upregulates TERT expression via long-range chromatin interactions, acquisition of active histone methylation (H3K4Me3) and acetylation (H3K9Ac), and promotes GABPA binding to this region (Figure 2A) (64). Chiba et al. conducted a long-term experiment using cultured cells to determine the role of TERT promoter mutations in telomere maintenance during tumorigenesis (Figure 2B) (13). The authors showed that TERT promoter contributes in tumorigenesis in two steps: immortalization of cells and promotion of genomic instability. In the first step, the point mutations in the promoter region of TERT gene do not prevent the bulk telomere shortening, but preferentially act on critically short telomeres, thereby delaying the replicative senescence and extending the growth limit through maintaining the length of telomere. In the second step, the genomic instability is increased through the fusion of extremely short telomeres, and at the same time, telomerase expression is gradually increased, ensuring further proliferation for cell immortalization (13). A number of studies have showed evidences that TERT promoter mutation, coupled with telomerase activation were associated with early genetic event during tumorigenesis. The TERT promoter mutation was identified in benign follicular thyroid adenoma (65), hepatocellular adenoma (66), benign nevi (67), as well as non-malignant urothelium (58). Furthermore, benign follicular thyroid adenoma with TERT promoter mutation express TERT mRNA and telomerase activity. Taking the fact that TERT promoter mutation was detected in various types of pre-cancerous lesion including benign lesions, and the fact that genomic instability was induced by TERT promoter mutation into consideration, TERT promoter mutation thought to associated with various type of tumorigenesis.
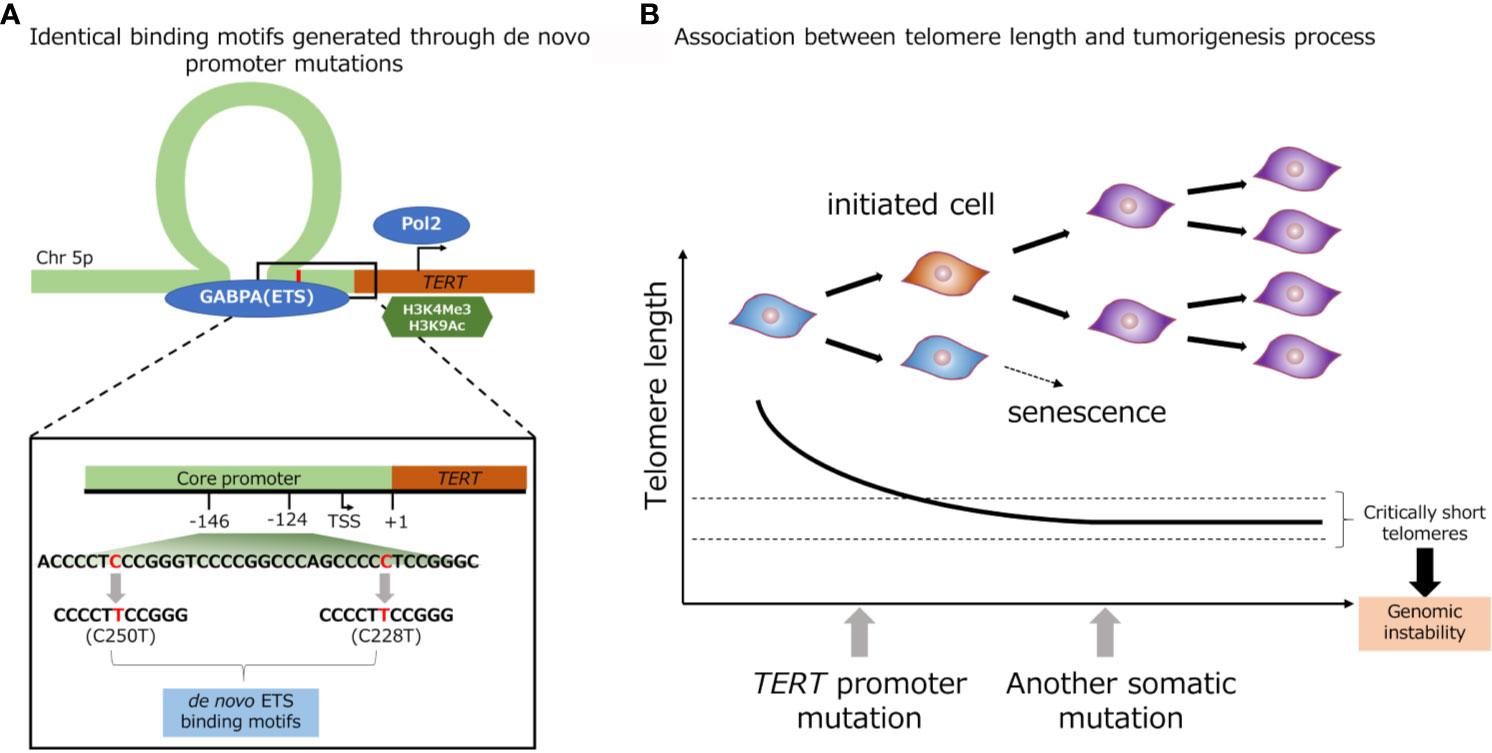
Figure 2 Identical de novo binding motifs generated through promoter mutations (A). Schematic representation of TERT promoter mutation. Association between telomere length and tumorigenesis process (B).
Tert Promoter Mutation in UC and Tumorigenesis
Based on histopathological and molecular observations, there is potential multistep model in UC tumorigenesis. In UC tissue, TERT promoter mutations are frequently detected in pre-cancerous lesions and high grade/stage UBC, or rare variant pathologies with an aggressive phenotype (Table 1) (58, 68–73). Although TERT promoter mutations were detected in the pathologically normal urothelium of patients with NMIBC (58), no mutation was detected in the benign proliferative bladder urothelium, including cystitis, nephrogenic adenoma, or inverted papilloma (74). These results indicate that TERT promoter mutation might occur during the trunk event of cancer clonal evolution and is thought to play a crucial role in UBC tumorigenesis. However, the collection of longitudinal samples from a single patient with malignant tumor over a long period of tumorigenesis poses a major challenge in studying tumor evolution. Therefore, mathematical algorithmic methods to infer tumorigenesis using cross-sectional data of tumor specimens from multiple patients have been proposed to solve this problem. We have demonstrated that this theory of tumorigenesis with TERT promoter as the axis of genome evolution of UBC is supported by phylogenetic inference using CAPRI model based on custom heuristic optimization of cross-sectional genomic data of tumor specimens from multiple patients with UBC (75). Although the biological mechanism of TERT promoter mutation-induced tumorigenesis is still unknown, these data support that genomic instability caused by TERT promoter mutations are essential for bladder cells with a low rate of self-renewal to acquire multiple somatic mutations and undergo malignant transformation in multistep tumor formation in UC.
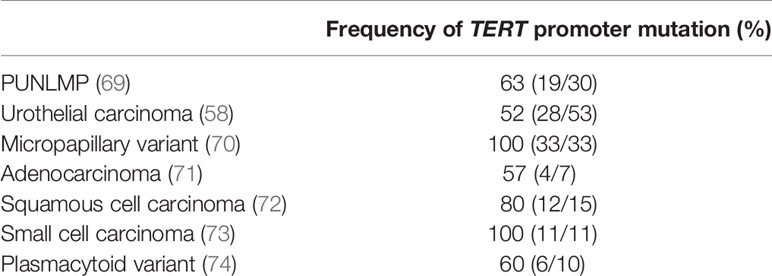
Table 1 Frequency of TERT promoter mutation in different types of bladder tumor, including those with rare variant histology.
Liquid Biopsy
Liquid biopsy allows the analysis in a less invasive, rapid, and sequential manner, and also to overcome the heterogeneity of tumors. Conventional next generation sequencing encountered sequencing error and cannot detect the mutation less than 5% due to error during PCR or reading process. By adding unique molecular identifiers, SafeSeqs technology could overcome these problems, enabling to distinguish between errors and “true mutations” by analyzing each read for each unique molecular identifier (76). This SafeSeqs technology can offer high sensitivity with a mutant allele frequency of 0.05%. droplet digital PCR can also offer highly sensitive analysis for molecular analysis. Droplet digital PCR technology can detect mutant alleles as few as 0.01% by analyzing ~20,000 of partitioned droplets using microfluidics technology (77). These technological advances in unique molecular identifiers, droplet digital PCR, and bioinformatics have made it possible to detect genetic mutations with greater sensitivity in body fluids.
Therefore, the genetic analysis using these techniques can provide useful molecular information and offer various advantages not only as a non-invasive biomarker, but also as an early indicator of minimal residual disease, recurrence, drug resistance, or metastasis. Therefore, it is believed to replace the tissue needle biopsy technique in the near future (15). Liquid biopsy is indispensable for the realization of precision medicine, as the target samples, including plasma cell-free DNA (cfDNA), urinary cfDNA, or urine pellet DNA are frequently analyzed in the UC field. Plasma cfDNA is released into the blood from both cancerous and non-cancerous tissues. The usefulness of tumor-derived cfDNA in plasma in various types of advanced carcinomas has been widely reported as a biomarker, but there is little evidence for the usefulness and efficacy of plasma cfDNA in localized cancer because the absolute amount of circulating tumor-derived cfDNA is low. Conversely, for the patients with UC, tumor-derived DNA can be detected in urine even in localized, tiny, or early-stage carcinoma, since unlike other carcinomas, UC is in constant contact with the urine (78, 79). Therefore, urinary DNA (urine pellet DNA and urinary cfDNA) can provide useful genetic analysis as a non-invasive tool for UC. There is growing evidence regarding the clinical application of liquid biopsy analysis for the patients with UC. Although there are few reports examining only TERT promoter mutations as target genomic region, TERT promoter mutations are frequently used in combination with other oncogenic mutations as a comprehensive panel analysis in liquid biopsy for the patients with UC, because of their high mutation frequency (76). UROSEEK has shown clinical potential for the detection of UC, which detects mutations in TERT promoter combined with FGFR3, TP53, CDKN2A, ERBB2, HRAS, KRAS, PIK3CA, MET, VHL, and MLL (8). We demonstrated clinical potential of TERT promoter in combination with FGFR3 mutation as a simple droplet digital PCR assay (57, 62). Since the promoter region of TERT gene has a high GC content (over 80%), amplification of this region using PCR is more challenging than that of the genomic regions in the exome. To amplify these regions efficiently, some additives are used to prevent non-specific amplification of products during PCR.
Clinical Potential of Tert Promoter Mutations in Urine Liquid Biopsy for UC
Figure 3 shows the association of frequency of TERT promoter mutation in plasma or urine specimens from patients with UTUC or UBC. The sensitivity of TERT promoter mutation assessed using urine liquid biopsy was relatively high, ranging from approximately 50% to 70% for UBC and 30–50% for UTUC (Figure 3A) (8, 57, 62, 80–87), which was consistent with the frequency of TERT promoter mutations in UC tissues (Figure 1). Springer et al. demonstrated the clinical application of urinary pellet analysis using targeted sequence panel “UROSEEK”, including TERT promoter mutations in the patients with UC (8). Several researchers reported that the overall concordance in TERT promoter mutation between urine and tumor tissue was high in both UBC and UTUC (92%, cfDNA in UBC; 89–94%, urine pellet in UTUC; 73–90%, urine pellet in UBC) (83), suggesting that urine liquid biopsy is sufficient for analyzing TERT promoter mutational status in both UBC and UTUC, and exhibits clinical potential for UC diagnosis. Several studies have shown that TERT promoter mutations, mainly C228T, were detectable up to 10 years prior to UBC formation in urine liquid biopsy of patients without visible tumor (86). Additionally, we reported that TERT C228T mutation in urinary cfDNA was associated with bladder tumor recurrence after transurethral surgery for NMIBC or radical nephroureterectomy for UTUC (57, 62). For these reasons, the detection of TERT C228T mutation in urine samples may act as a potential prognostic factor, as well as a diagnostic biomarker, in the clinical setting for UC treatment.
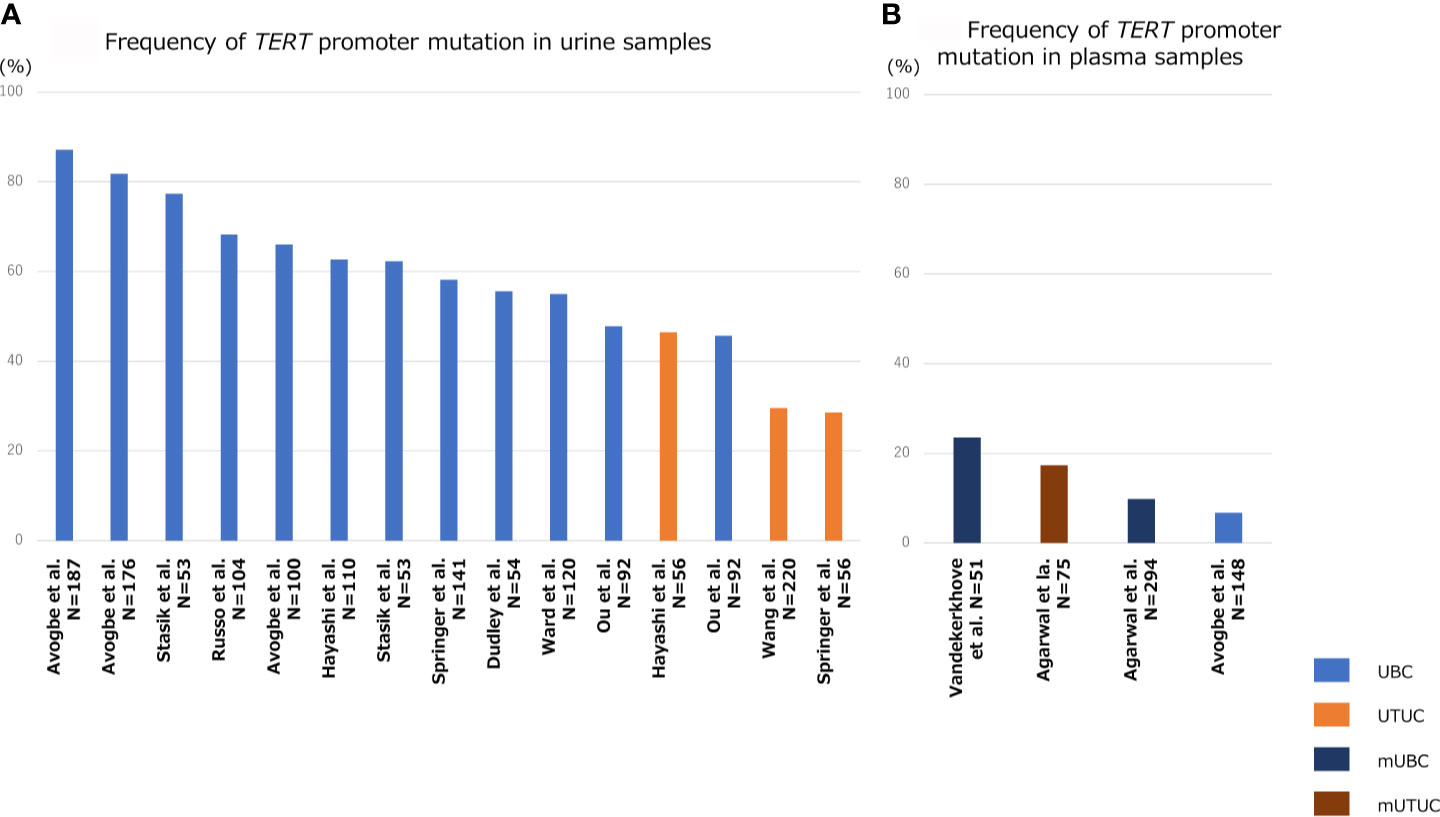
Figure 3 Bar diagram indicating the frequency TERT promoter mutation in liquid biopsy specimens of patients with UBC, UTUC, mUBC (metastatic UBC), and mUTUC (metastatic UTUC). Frequency of TERT promoter mutation in urine samples (A), and that in plasma samples (B).
Clinical Potential of Tert Promoter Mutations in Plasma Liquid Biopsy for Advanced UC
Since the quantity of plasma cfDNA is low in localized or non-muscle invasive tumors, it is difficult to detect tumor-derived mutations in blood samples from patients with early-stage UC. Therefore, the majority of previous studies on plasma cfDNA have focused mainly on metastatic UC. The positive rate of TERT promoter mutation in plasma cfDNA ranged from 10–23% in metastatic UBC and 17% in metastatic UTUC (Figure 3B) (87–89). Avogbe et al. reported that TERT promoter mutation was detected in 6.7% of plasma samples from patients with localized UBC (Figure 3B) (87).
Challenges and Future Directions of Tert as a Potential Therapeutic Target
Researchers are now paying more attention to the regulation of telomerase or TERT because of their unique characteristic of low or no expression in normal somatic cells, but high expression in cancer cells. Many studies have been conducted on TERT-regulating factors, such as epigenetic modifications or telomerase inhibitors. With regard to methylation, there are two regions of TERT promoter: the unmethylated proximal TERT core promoter, and the hypermethylated region called THOR the TERT hypermethylated oncologic region (THOR) located at the distal region of the promoter. This interesting distinct methylation pattern indicates that the core promoter region and THOR are functionally different loci regulating TERT (90). Co-occurrence of THOR methylation and TERT promoter mutation was associated with a high risk of disease recurrence and progression in UBC (54). Although the exact biological mechanism is still unclear, THOR methylation might play an important role in regulating TERT expression and may be a potential target for cancer treatment.
A few telomerase peptide vaccines based on a novel antigen generated by T cells have been used in clinical trials for various types of cancers. In a phase I/II study, INO-5401 plus INO-9012 was administered to the patients with advanced or metastatic UC in combination with atezolizumab (NCT03502785). IN-5401 and INO-9012 are both mixtures of synthetic plasmids, including TERT antigen. Peptide vaccines have been used in various clinical trials with limited success. Since various reports indicate that TERT promoter mutations occur at an early stage of tumorigenesis in UC, these telomerase-targeted therapies might be effective for early-stage UC or prevention of UC recurrence, but not for advanced UC.
Conclusion
The biological function of TERT promoter mutations in UC is gradually being well defined. There are growing evidences that TERT promoter mutation followed by genomic instability is a key step in the tumorigenesis of UC. Furthermore, the detection of TERT promoter mutations in bodily fluids using liquid biopsy is a potential biomarker for UC diagnosis as well as prognosis. Although larger prospective studies are required, liquid biopsy targeting TERT promoter mutation might make a significant contribution to improve the prognosis and quality of life of patients with UC in the near future.
Author Contributions
Conception and design: all authors. Administrative support: KF. Provision of study materials or patients: all authors. Collection and assembly of data: YH. Data analysis and interpretation: YH. Manuscript writing: YH. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Osaka University Grant, and JSPS KAKENHI Grant Number JP 20K18139.
Conflict of Interest
GN: Equity or royalty from the licensed technologies from Johns Hopkins that are related to the work described in this paper.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
cfDNA, cell-free DNA; HCC, hepatocellular carcinoma; NMIBC, non-muscle invasive bladder cancer; TERC, telomerase RNA component; TERT, telomerase reverse transcriptase; UBC, urothelial bladder cancer; UC, urothelial carcinoma; UTUC, upper tract urothelial carcinoma.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
3. Hayashi T, Fujita K, Hayashi Y, Hatano K, Kawashima A, McConkey DJ, et al. Mutational Landscape and Environmental Effects in Bladder Cancer. Int J Mol Sci (2020) 21:E6072. doi: 10.3390/ijms21176072
4. Grollman AP. Aristolochic Acid Nephropathy: Harbinger of a Global Iatrogenic Disease. Environ Mol Mutagen (2013) 54:1–7. doi: 10.1002/em.21756
5. Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, et al. A Consensus Molecular Classification of Muscle-Invasive Bladder Cancer. Eur Urol (2020) 77:420–33. doi: 10.1016/j.eururo.2019.11.011
6. Hassler MR, Bray F, Catto JWF, Grollman AP, Hartmann A, Margulis V, et al. Molecular Characterization of Upper Tract Urothelial Carcinoma in the Era of Next-Generation Sequencing: A Systematic Review of the Current Literature. Eur Urol (2020) 78:209–20. doi: 10.1016/j.eururo.2020.05.039
7. van Doeveren T, Nakauma-Gonzalez JA, Mason AS, van Leenders GJLH, Zuiverloon TCM, Zwarthoff EC, et al. The Clonal Relation of Primary Upper Urinary Tract Urothelial Carcinoma and Paired Urothelial Carcinoma of the Bladder. Int J Cancer (2021) 148:981–7. doi: 10.1002/ijc.33327
8. Springer SU, Chen CH, Rodriguez Pena MDC, Li L, Douville C, Wang Y, et al. Non-Invasive Detection of Urothelial Cancer Through the Analysis of Driver Gene Mutations and Aneuploidy. Elife (2018) 7:e32143. doi: 10.7554/eLife.32143
9. Pietzak EJ, Bagrodia A, Cha EK, Drill EN, Iyer G, Isharwal S, et al. Next-Generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur Urol (2017) 72:952–9. doi: 10.1016/j.eururo.2017.05.032
10. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol (2018) 73:111–22. doi: 10.1016/j.eururo.2017.07.036
11. Babjuk M, Burger M, Compérat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol (2019) 76:639–57. doi: 10.1016/j.eururo.2019.08.016
12. Borah S, Xi L, Zaug AJ, Powell NM, Dancik GM, Cohen SB, et al. TERT Promoter Mutations and Telomerase Reactivation in Urothelial Cancer. Science (2015) 347:1006–10. doi: 10.1126/science.1260200
13. Chiba K, Lorbeer FK, Shain AH, McSwiggen DT, Schruf E, Oh A, et al. Mutations in the Promoter of the Telomerase Gene TERT Contribute to Tumorigenesis by a Two-Step Mechanism. Science (2017) 357:1416–20. doi: 10.1126/science.aao0535
14. Rachakonda PS, Hosen I, de Verdier PJ, Fallah M, Heidenreich B, Ryk C, et al. TERT Promoter Mutations in Bladder Cancer Affect Patient Survival and Disease Recurrence Through Modification by a Common Polymorphism. Proc Natl Acad Sci USA (2013) 110:17426–31. doi: 10.1073/pnas.1310522110
15. Hayashi Y, Fujita K. Toward Urinary Cell-Free DNA-Based Treatment of Urothelial Carcinoma: Review Article. Transl Androl Urol (2021) 10:1865–77. doi: 10.21037/tau-20-1259
16. Maciejowski J, de Lange T. Telomeres in Cancer: Tumour Suppression and Genome Instability. Nat Rev Mol Cell Biol (2017) 18:175–86. doi: 10.1038/nrm.2016.171
17. Shay JW, Wright WE. Telomeres and Telomerase: Three Decades of Progress. Nat Rev Genet (2019) 20:299–309. doi: 10.1038/s41576-019-0099-1
18. Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, et al. Telomere Length Abnormalities Occur Early in the Initiation of Epithelial Carcinogenesis. Clin Cancer Res (2004) 10:3317–26. doi: 10.1158/1078-0432.CCR-0984-03
19. Greider CW, Blackburn EH. Identification of a Specific Telomere Terminal Transferase Activity in Tetrahymena Extracts. Cell (1985) 43:405–13. doi: 10.1016/0092-8674(85)90170-9
20. Bell RJ, Rube HT, Xavier-Magalhães A, Costa BM, Mancini A, Song JS, et al. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol Cancer Res (2016) 14:315–23. doi: 10.1158/1541-7786.MCR-16-0003
21. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA, et al. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science (2013) 339:957–9. doi: 10.1126/science.1229259
22. Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science (2013) 339:959–61. doi: 10.1126/science.1230062
23. Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, et al. TERT Promoter Mutations Occur Frequently in Gliomas and a Subset of Tumors Derived From Cells With Low Rates of Self-Renewal. Proc Natl Acad Sci USA (2013) 110:6021–6. doi: 10.1073/pnas.1303607110
24. Bai X, Kong Y, Chi Z, Sheng X, Cui C, Wang X, et al. MAPK Pathway and TERT Promoter Gene Mutation Pattern and Its Prognostic Value in Melanoma Patients: A Retrospective Study of 2,793 Cases. Clin Cancer Res (2017) 23:6120–7. doi: 10.1158/1078-0432.CCR-17-0980
25. Manrique-Silva E, Rachakonda S, Millán-Esteban D. Clinical, Environmental and Histological Distribution of BRAF, NRAS and TERT Promoter Mutations Among Patients With Cutaneous Melanoma: A Retrospective Study of 563 Patients. Br J Dermatol (2020) 184:504–13. doi: 10.1111/bjd.19297
26. Nagore E, Heidenreich B, Rachakonda S, Garcia-Casado Z, Requena C, Soriano V, et al. TERT Promoter Mutations in Melanoma Survival. Int J Cancer (2016) 139:75–84. doi: 10.1002/ijc.30042
27. Andrés-Lencina JJ, Rachakonda S, García-Casado Z, Srinivas N, Skorokhod A, Requena C, et al. TERT Promoter Mutation Subtypes and Survival in Stage I and II Melanoma Patients. Int J Cancer (2019) 144:1027–36. doi: 10.1002/ijc.31780
28. Heidenreich B, Nagore E, Rachakonda PS, Garcia-Casado Z, Requena C, Traves V, et al. Telomerase Reverse Transcriptase Promoter Mutations in Primary Cutaneous Melanoma. Nat Commun (2014) 5:3401. doi: 10.1038/ncomms4401
29. Nonoguchi N, Ohta T, Oh JE, Kim YH, Kleihues P, Ohgaki H, et al. TERT Promoter Mutations in Primary and Secondary Glioblastomas. Acta Neuropathol (2013) 126:931–7. doi: 10.1007/s00401-013-1163-0
30. Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, et al. Upregulating Mutations in the TERT Promoter Commonly Occur in Adult Malignant Gliomas and Are Strongly Associated With Total 1p19q Loss. Acta Neuropathol (2013) 126:267–76. doi: 10.1007/s00401-013-1141-6
31. Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H, et al. A Combination of TERT Promoter Mutation and MGMT Methylation Status Predicts Clinically Relevant Subgroups of Newly Diagnosed Glioblastomas. Acta Neuropathol Commun (2016) 4:79. doi: 10.1186/s40478-016-0351-2
32. Nguyen HN, Lie A, Li T, Chowdhury R, Liu F, Ozer B, et al. Human TERT Promoter Mutation Enables Survival Advantage From MGMT Promoter Methylation in IDH1 Wild-Type Primary Glioblastoma Treated by Standard Chemoradiotherapy. Neuro Oncol (2017) 19:394–404. doi: 10.1093/neuonc/now189
33. Shu C, Wang Q, Yan X, Wang J. The TERT Promoter Mutation Status and MGMT Promoter Methylation Status, Combined With Dichotomized MRI-Derived and Clinical Features, Predict Adult Primary Glioblastoma Survival. Cancer Med (2018) 7:3704–12. doi: 10.1002/cam4.1666
34. Batista R, Cruvinel-Carloni A, Vinagre J, Peixoto J, Catarino TA, Campanella NC, et al. The Prognostic Impact of TERT Promoter Mutations in Glioblastomas Is Modified by the Rs2853669 Single Nucleotide Polymorphism. Int J Cancer (2016) 139:414–23. doi: 10.1002/ijc.30057
35. Fan X, Wang Y, Liu Y, Liu X, Zhang C, Wang L, et al. Brain Regions Associated With Telomerase Reverse Transcriptase Promoter Mutations in Primary Glioblastomas. J Neurooncol (2016) 128:455–62. doi: 10.1007/s11060-016-2132-y
36. Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, et al. High Frequency of Telomerase Reverse-Transcriptase Promoter Somatic Mutations in Hepatocellular Carcinoma and Preneoplastic Lesions. Nat Commun (2013) 4:2218. doi: 10.1038/ncomms3218
37. Yang X, Guo X, Chen Y, Chen G, Ma Y, Huang K, et al. Telomerase Reverse Transcriptase Promoter Mutations in Hepatitis B Virus-Associated Hepatocellular Carcinoma. Oncotarget (2016) 7:27838–47. doi: 10.18632/oncotarget.8539
38. Chen YL, Jeng YM, Chang CN, Lee HJ, Hsu HC, Lai PL, et al. TERT Promoter Mutation in Resectable Hepatocellular Carcinomas: A Strong Association With Hepatitis C Infection and Absence of Hepatitis B Infection. Int J Surg (2014) 12:659–65. doi: 10.1016/j.ijsu.2014.05.066
39. Lee HW, Park TI, Jang SY, Park SY, Park WJ, Jung SJ, et al. Clinicopathological Characteristics of TERT Promoter Mutation and Telomere Length in Hepatocellular Carcinoma. Med (Baltimore) (2017) 96:e5766. doi: 10.1097/MD.0000000000005766. e5766.
40. Lee SE, Chang SH, Kim WY, Lim SD, Kim WS, Hwang TS, et al. Frequent Somatic TERT Promoter Mutations and CTNNB1 Mutations in Hepatocellular Carcinoma. Oncotarget (2016) 7:69267–75. doi: 10.18632/oncotarget.12121
41. Pezzuto F, Izzo F, Buonaguro L, Annunziata C, Tatangelo F, Botti G, et al. Tumor Specific Mutations in TERT Promoter and CTNNB1 Gene in Hepatitis B and Hepatitis C Related Hepatocellular Carcinoma. Oncotarget (2016) 7:54253–62. doi: 10.18632/oncotarget.9801
42. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al-Rasheed M, et al. Telomerase Reverse Transcriptase Mutations are Independent Predictor of Disease-Free Survival in Middle Eastern Papillary Thyroid Cancer. Int J Cancer (2018) 142:2028–39. doi: 10.1002/ijc.31225
43. Kim SY, Kim T, Kim K, Bae JS, Kim JS, Jung CK, et al. Highly Prevalent BRAF V600E and Low-Frequency TERT Promoter Mutations Underlie Papillary Thyroid Carcinoma in Koreans. J Pathol Transl Med (2020) 54:310–7. doi: 10.4132/jptm.2020.05.12
44. Ebina A, Togashi Y, Baba S, Sato Y, Sakata S, Ishikawa M, et al. TERT Promoter Mutation and Extent of Thyroidectomy in Patients With 1-4 Cm Intrathyroidal Papillary Carcinoma. Cancers (Basel) (2020) 12(8):2115. doi: 10.3390/cancers12082115
45. Jin L, Chen E, Dong S, Cai Y, Zhang X, Zhou Y, et al. BRAF and TERT Promoter Mutations in the Aggressiveness of Papillary Thyroid Carcinoma: A Study of 653 Patients. Oncotarget (2016) 7:18346–55. doi: 10.18632/oncotarget.7811
46. Chien MN, Yang PS, Hsu YC, Liu TP, Lee JJ, Cheng SP, et al. Transcriptome Analysis of Papillary Thyroid Cancer Harboring Telomerase Reverse Transcriptase Promoter Mutation. Head Neck (2018) 40:2528–37. doi: 10.1002/hed.25385
47. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly Prevalent TERT Promoter Mutations in Aggressive Thyroid Cancers. Endocr Relat Cancer (2013) 20:603–10. doi: 10.1530/ERC-13-0210
48. Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, et al. Frequent Somatic TERT Promoter Mutations in Thyroid Cancer: Higher Prevalence in Advanced Forms of the Disease. J Clin Endocrinol Metab (2013) 98:E1562–6. doi: 10.1210/jc.2013-2383
49. Eich ML, Rodriguez Pena MDC, Springer SU, Taheri D, Tregnago AC, Salles DC, et al. Incidence and Distribution of UroSEEK Gene Panel in a Multi-Institutional Cohort of Bladder Urothelial Carcinoma. Mod Pathol (2019) 32:1544–50. doi: 10.1038/s41379-019-0276-y
50. Hurst CD, Platt FM, Knowles MA. Comprehensive Mutation Analysis of the TERT Promoter in Bladder Cancer and Detection of Mutations in Voided Urine. Eur Urol (2014) 65:367–9. doi: 10.1016/j.eururo.2013.08.057
51. Isharwal S, Audenet F, Drill E, Pietzak EJ, Iyer G, Ostrovnaya I, et al. Prognostic Value of TERT Alterations, Mutational and Copy Number Alterations Burden in Urothelial Carcinoma. Eur Urol Focus (2019) 5:201–4. doi: 10.1016/j.euf.2017.07.004
52. Descotes F, Kara N, Decaussin-Petrucci M, Piaton E, Geiguer F, Rodriguez-Lafrasse C, et al. Non-Invasive Prediction of Recurrence in Bladder Cancer by Detecting Somatic TERT Promoter Mutations in Urine. Br J Cancer (2017) 117:583–7. doi: 10.1038/bjc.2017.210
53. Giedl J, Rogler A, Wild A, Riener MO, Filbeck T, Burger M, et al. TERT Core Promotor Mutations in Early-Onset Bladder Cancer. J Cancer (2016) 7:915–20. doi: 10.7150/jca.15006
54. Leão R, Lee D, Figueiredo A, Hermanns T, Wild P, Komosa M, et al. Combined Genetic and Epigenetic Alterations of the TERT Promoter Affect Clinical and Biological Behavior of Bladder Cancer. Int J Cancer (2019) 144:1676–84. doi: 10.1002/ijc.31935
55. Wu S, Huang P, Li C, Huang Y, Li X, Wang Y, et al. Telomerase Reverse Transcriptase Gene Promoter Mutations Help Discern the Origin of Urogenital Tumors: A Genomic and Molecular Study. Eur Urol (2014) 65:274–7. doi: 10.1016/j.eururo.2013.10.038
56. Vinagre J, Almeida A, Pópulo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT Promoter Mutations in Human Cancers. Nat Commun (2013) 4:2185. doi: 10.1038/ncomms3185
57. Hayashi Y, Fujita K, Matsuzaki K, Eich ML, Tomiyama E, Matsushita M, et al. Clinical Significance of Hotspot Mutation Analysis of Urinary Cell-Free DNA in Urothelial Bladder Cancer. Front Oncol (2020) 10:755. doi: 10.3389/fonc.2020.00755
58. Hayashi Y, Fujita K, Nojima S, Tomiyama E, Matsushita M, Koh Y, et al. TERT C228T Mutation in Non-Malignant Bladder Urothelium is Associated With Intravesical Recurrence for Patients With Non-Muscle Invasive Bladder Cancer. Mol Oncol (2020) 14:2375–83. doi: 10.1002/1878-0261.12746
59. Wang K, Liu T, Ge N, Liu L, Yuan X, Liu J, et al. TERT Promoter Mutations are Associated With Distant Metastases in Upper Tract Urothelial Carcinomas and Serve as Urinary Biomarkers Detected by a Sensitive castPCR. Oncotarget (2014) 5:12428–39. doi: 10.18632/oncotarget.2660
60. Li Y, Zhou QL, Sun W, Chandrasekharan P, Cheng HS, Ying Z, et al. Non-Canonical NF-κb Signalling and ETS1/2 Cooperatively Drive C250T Mutant TERT Promoter Activation. Nat Cell Biol (2015) 17:1327–38. doi: 10.1038/ncb3240
61. Chiba K, Johnson JZ, Vogan JM, Wagner T, Boyle JM, Hockemeyer D, et al. Cancer-Associated TERT Promoter Mutations Abrogate Telomerase Silencing. Elife (2015) 21:e07918. doi: 10.7554/eLife.07918
62. Hayashi Y, Fujita K, Matsuzaki K, Matsushita M, Kawamura N, Koh Y, et al. Diagnostic Potential of TERT Promoter and FGFR3 Mutations in Urinary Cell-Free DNA in Upper Tract Urothelial Carcinoma. Cancer Sci (2019) 110:1771–9. doi: 10.1111/cas.14000
63. Stern JL, Theodorescu D, Vogelstein B, Papadopoulos N, Cech TR. Mutation of the TERT Promoter, Switch to Active Chromatin, and Monoallelic TERT Expression in Multiple Cancers. Genes Dev (2015) 29:2219–24. doi: 10.1101/gad.269498.115
64. Akıncılar SC, Khattar E, Boon PL, Unal B, Fullwood MJ, Tergaonkar V, et al. Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discov (2016) 6:1276–91. doi: 10.1158/2159-8290.CD-16-0177
65. Wang N, Liu T, Sofiadis A, Juhlin CC, Zedenius J, Höög A, et al. TERT Promoter Mutation as an Early Genetic Event Activating Telomerase in Follicular Thyroid Adenoma (FTA) and Atypical FTA. Cancer (2014) 120:2965–79. doi: 10.1002/cncr.28800
66. Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, et al. Telomerase Reverse Transcriptase Promoter Mutation is an Early Somatic Genetic Alteration in the Transformation of Premalignant Nodules in Hepatocellular Carcinoma on Cirrhosis. Hepatology (2014) 60:1983–92. doi: 10.1002/hep.27372
67. Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, et al. The Genetic Evolution of Melanoma From Precursor Lesions. N Engl J Med (2015) 373:1926–36. doi: 10.1056/NEJMoa1502583
68. Rodriguez Pena MDC, Tregnago AC, Eich ML, Springer S, Wang Y, Taheri D, et al. Spectrum of Genetic Mutations in De Novo PUNLMP of the Urinary Bladder. Virchows Arch (2017) 471:761–7. doi: 10.1007/s00428-017-2164-5
69. Nguyen D, Taheri D, Springer S, Cowan M, Guner G, Mendoza Rodriguez MA, et al. High Prevalence of TERT Promoter Mutations in Micropapillary Urothelial Carcinoma. Virchows Arch (2016) 469:427–34. doi: 10.1007/s00428-016-2001-2
70. Cowan ML, Springer S, Nguyen D, Taheri D, Guner G, Mendoza Rodriguez MA, et al. Detection of TERT Promoter Mutations in Primary Adenocarcinoma of the Urinary Bladder. Hum Pathol (2016) 8–13. doi: 10.1016/j.humpath.2016.02.009
71. Cowan M, Springer S, Nguyen D, Taheri D, Guner G, Rodriguez MA, et al. High Prevalence of TERT Promoter Mutations in Primary Squamous Cell Carcinoma of the Urinary Bladder. Mod Pathol (2016) 29:511–5. doi: 10.1038/modpathol.2016.53
72. Zheng X, Zhuge J, Bezerra SM, Faraj SF, Munari E, Fallon JT 3rd, et al. High Frequency of TERT Promoter Mutation in Small Cell Carcinoma of Bladder, But Not in Small Cell Carcinoma of Other Origins. J Hematol Oncol (2014) 7:47. doi: 10.1186/s13045-014-0047-7
73. Palsgrove DN, Taheri D, Springer SU, Cowan M, Guner G, Mendoza Rodriguez MA, et al. Targeted Sequencing of Plasmacytoid Urothelial Carcinoma Reveals Frequent TERT Promoter Mutations. Hum Pathol (2019) 85:1–9. doi: 10.1016/j.humpath.2018.10.033
74. Kurtis B, Zhuge J, Ojaimi C, Ye F, Cai D, Zhang D, et al. Recurrent TERT Promoter Mutations in Urothelial Carcinoma and Potential Clinical Applications. Ann Diagn Pathol (2016) 21:7–11. doi: 10.1016/j.anndiagpath.2015.12.002
75. Hayashi Y, Fujita K, Banno E, Eich M-L, Netto GJ, Nonomura N. TERT Promoter Mutation in Tumorigenesis of Bladder Cancer: Evolutionary Trajectory by Algorithmic Inference From Cross-Sectional Data. Int J Urol (2021) 28:774–6. doi: 10.1111/iju.14574
76. Kinde I, Munari E, Faraj SF, Hruban RH, Schoenberg M, Bivalacqua T, et al. TERT Promoter Mutations Occur Early in Urothelial Neoplasia and are Biomarkers of Early Disease and Disease Recurrence in Urine. Cancer Res (2013) 73:7162–7. doi: 10.1158/0008-5472.CAN-13-2498
77. Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci USA (1999) 96:9236–41. doi: 10.1073/pnas.96.16.9236
78. Hayashi Y, Fujita K. A New Era in the Detection of Urothelial Carcinoma by Sequencing Cell-Free DNA. Transl Androl Urol (2019) 8:S497–501. doi: 10.21037/tau.2019.08.26
79. Yoshida T, Kates M, Fujita K, Bivalacqua TJ, McConkey DJ, et al. Predictive Biomarkers for Drug Response in Bladder Cancer. Int J Urol (2019) 26:1044–53. doi: 10.1111/iju.14082
80. Dudley JC, Schroers-Martin J, Lazzareschi DV, Shi WY, Chen SB, Esfahani MS, et al. Detection and Surveillance of Bladder Cancer Using Urine Tumor DNA. Cancer Discov (2019) 9:500–9. doi: 10.1158/2159-8290.CD-18-0825
81. Ward DG, Baxter L, Gordon NS, Ott S, Savage RS, Beggs AD, et al. Multiplex PCR and Next Generation Sequencing for the Non-Invasive Detection of Bladder Cancer. PloS One (2016) 11:e0149756. doi: 10.1371/journal.pone.0149756
82. Stasik S, Salomo K, Heberling U, Froehner M, Sommer U, Baretton GB, et al. Evaluation of TERT Promoter Mutations in Urinary Cell-Free DNA and Sediment DNA for Detection of Bladder Cancer. Clin Biochem (2019) 60–3. doi: 10.1016/j.clinbiochem.2018.11.009
83. Ou Z, Li K, Yang T, Dai Y, Chandra M, Ning J, et al. Detection of Bladder Cancer Using Urinary Cell-Free DNA and Cellular DNA. Clin Transl Med (2020) 9:4. doi: 10.1186/s40169-020-0257-2
84. Russo IJ, Ju Y, Gordon NS, Zeegers MP, Cheng KK, James ND, et al. Toward Personalised Liquid Biopsies for Urothelial Carcinoma: Characterisation of ddPCR and Urinary cfDNA for the Detection of the TERT 228 G>A/T Mutation. Bladder Cancer (2018) 4:41–8. doi: 10.3233/BLC-170152
85. Lu H, Liang Y, Guan B, Shi Y, Gong Y, Li J, et al. Aristolochic Acid Mutational Signature Defines the Low-Risk Subtype in Upper Tract Urothelial Carcinoma. Theranostics (2020) 10:4323–33. doi: 10.7150/thno.43251
86. Hosen MI, Sheikh M, Zvereva M, Scelo G, Forey N, Durand G, et al. Urinary TERT Promoter Mutations Are Detectable Up to 10 Years Prior to Clinical Diagnosis of Bladder Cancer: Evidence From the Golestan Cohort Study. EBioMedicine (2020) 53:102643. doi: 10.1016/j.ebiom.2020.102643
87. Avogbe PH, Manel A, Vian E, Durand G, Forey N, Voegele C, et al. Urinary TERT Promoter Mutations as Non-Invasive Biomarkers for the Comprehensive Detection of Urothelial Cancer. EBioMedicine (2019) 431–8. doi: 10.1016/j.ebiom.2019.05.004
88. Vandekerkhove G, Todenhöfer T, Annala M, Struss WJ, Wong A, Beja K, et al. Circulating Tumor DNA Reveals Clinically Actionable Somatic Genome of Metastatic Bladder Cancer. Clin Cancer Res (2017) 23:6487–97. doi: 10.1158/1078-0432.CCR-17-1140
89. Agarwal N, Pal SK, Hahn AW, Nussenzveig RH, Pond GR, Gupta SV, et al. Characterization of Metastatic Urothelial Carcinoma via Comprehensive Genomic Profiling of Circulating Tumor DNA. Cancer (2018) 124:2115–24. doi: 10.1002/cncr.31314
Keywords: TERT promoter, telomere, urothelial carcinoma, bladder cancer, upper tract urothelial carcinoma, tumorigenesis, liquid biopsy, cell-free DNA
Citation: Hayashi Y, Fujita K, Netto GJ and Nonomura N (2021) Clinical Application of TERT Promoter Mutations in Urothelial Carcinoma. Front. Oncol. 11:705440. doi: 10.3389/fonc.2021.705440
Received: 05 May 2021; Accepted: 02 July 2021;
Published: 29 July 2021.
Edited by:
Masaki Shiota, Kyushu University, JapanReviewed by:
Avaniyapuram Kannan Murugan, King Faisal Specialist Hospital and Research Centre, Saudi ArabiaTakashi Matsumoto, Kyushu University, Japan
Copyright © 2021 Hayashi, Fujita, Netto and Nonomura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazutoshi Fujita, a2F6dWZ1aml0YTJAZ21haWwuY29t
 Yujiro Hayashi
Yujiro Hayashi Kazutoshi Fujita3*
Kazutoshi Fujita3*