- 1Department of Respiratory Medicine and Infectious Diseases, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
- 2Department of Respiratory Medicine, Niigata Prefectural Shibata Hospital, Niigata, Japan
- 3Department of Respiratory Medicine and Infectious Diseases, Niigata City General Hospital, Niigata, Japan
- 4Department of Internal Medicine, Niigata Cancer Center Hospital, Niigata, Japan
- 5Department of Respiratory Medicine, Saiseikai Niigata Hospital, Niigata, Japan
- 6Department of Respiratory Medicine, Nishi-Niigata Chuo National Hospital, Niigata, Japan
- 7Department of Respiratory Medicine, Niigata Prefectural Central Hospital, Joetsu, Japan
- 8Department of Respiratory Medicine, Nagaoka Chuo General Hospital, Nagaoka, Japan
- 9Department of Respiratory Medicine, Nagaoka Red Cross Hospital, Nagaoka, Japan
- 10Department of Respiratory Medicine, Niigata Medical Center, Niigata, Japan
Objectives: Although immune checkpoint inhibitors (ICIs) have been shown to improve overall survival (OS) in advanced non-small-cell lung cancer (NSCLC) patients, ICIs sometimes cause various types of immune-related adverse events (irAEs), which lead to the interruption of ICI treatment. This study aims to evaluate the clinical significance of the continuation of ICIs in NSCLC patients with irAEs and to assess the safety and efficacy of the readministration of ICIs after their discontinuation due to irAEs.
Methods: We retrospectively identified patients with advanced NSCLC who were treated with first- to third-line anti-programmed cell death-1 (PD-1) therapy from January 2016 through October 2017 at multiple institutions belonging to the Niigata Lung Cancer Treatment Group. Progression-free survival (PFS) and OS from the initiation of ICI treatment were analyzed in patients with and without irAEs, with and without ICI interruption, and with and without ICI readministration. A 6-week landmark analysis of PFS and OS was performed to minimize the lead-time bias associated with time-dependent factors.
Results: Of 231 patients who received anti-PD-1 antibodies, 93 patients (40%) developed irAEs. Of 84 eligible patients with irAEs, 32 patients (14%) continued ICIs, and OS was significantly longer in patients who continued ICIs than that in patients who discontinued ICIs [not reached (95% CI: NE-NE) vs. not reached (95% CI: 22.4–NE); p = 0.025]. Of 52 patients who discontinued ICIs, 14 patients (6.1%) readministered ICIs, and OS in patients with ICI readministration was significantly longer than that in patients without ICI readministration [not reached (95% CI: NE-NE) vs. not reached (95% CI: 8.4–NE); p = 0.031].
Conclusion: The current study demonstrated that both the continuation and readministration of ICIs after irAE occurrence improved OS compared to the permanent interruption of ICIs in NSCLC patients with ICI-related irAEs.
Introduction
Immune checkpoint inhibitors (ICIs), such as anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) antibodies, have achieved durable responses in some patients with non-small-cell lung cancer (NSCLC) (1–6). Anti-PD-1/PD-L1 therapy has become the standard of care for advanced NSCLC patients.
Treatment with ICIs is often accompanied by immune-related adverse events (irAEs), and irAEs can be lethal or the main reason for the discontinuation of ICIs. The decision of whether to continue or discontinue ICIs after the occurrence of irAEs is generally based on the type of irAE and its severity (7). We previously reported that ICI-related interstitial lung disease (ILD), whose appearance was ground-glass opacities (GGOs), was a significant predictor of poor survival outcomes (8). However, recent evidence has demonstrated that the occurrence of irAEs is associated with better survival outcomes in patients with NSCLC (9–13). Several retrospective studies have also shown that there were some cases in which the effects of ICIs were sustained even after the discontinuation of treatment due to ICI-related irAEs (14, 15). In contrast, a retrospective study reported that the interruption of ICIs due to irAEs was associated with a lower overall survival (OS) than continuous ICI treatment (16). Furthermore, the clinical outcomes of the rechallenge of ICIs in patients who recovered from irAEs remain unclear.
This study aims to assess the significance of the continuation of ICIs in NSCLC patients who developed irAEs and to evaluate the safety and efficacy of the readministration of ICIs in patients who discontinued ICI treatment due to ICI-related irAEs.
Materials and Methods
Study Design and Patients
We retrospectively analyzed the medical records of patients with advanced NSCLC who were treated with single-agent anti-PD-1 as first- to third-line therapy at multiple institutions belonging to the Niigata Lung Cancer Treatment Group from January 2016 to October 2017. To prevent selection bias, all consecutive patients who met eligibility were enrolled. This study was approved by the institutional review board of each participating institution.
Study Assessment
The following data were collected retrospectively for all patients: demographics, phenotypes of cancers, types of anti-PD-1 therapies, and irAEs. Treatment responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1. Each irAE was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Progression-free survival (PFS) was measured as the time from the start of anti-PD-1 therapy to progressive disease (PD) or death due to any cause. OS was measured as the time from the first administration of immunotherapy to death due to any cause. The objective response rate (ORR) was defined as the percentage of patients assessed as having complete response (CR) or partial response (PR) of all patients treated with anti-PD-1 therapy. The disease control rate (DCR) was defined as the percentage of patients assessed as having CR, PR, or stable disease (SD) of all patients treated with anti-PD-1 therapy. Treatment interruption was defined as either the delay or cessation of ICI treatment due to irAEs. Patients with ICI readministration were defined as those who were readministered a PD-1 inhibitor at least one time after the interruption of ICI treatment due to irAEs. Patients without ICI readministration were defined as those whose ICI treatment was permanently stopped due to irAEs.
Statistical Analysis
Kaplan–Meier survival curves were constructed for PFS and OS, and differences between groups were identified using the log-rank test. The univariate Cox proportional hazards model was used to assess the effects of the presence of irAEs, the continuation of ICIs, and the readministration of ICIs on PFS and OS. Continuous variables are presented as the median (range) and were compared by two-sided t-tests. Comparisons between groups were performed by Fisher’s exact test or the chi-square test. To minimize the lead-time bias associated with time-dependent factors, we performed a 6-week landmark analysis including only patients who were alive or whose disease was under control at 43 days after the initiation of anti-PD-1 therapy, which is the median time of onset of irAEs, for PFS and OS. For this 6-week landmark analysis, we excluded 66 patients for PFS and 17 patients for OS in the analysis of Figure 2, 13 patients for PFS and three patients for OS in the analysis of Figure 3, and 12 patients for PFS and two patients for OS in the analysis of Figure 4 because these patients had PD or died for any cause within 6 weeks of initiation of anti-PD-1 therapy. Additionally, we excluded seven patients who experienced irAEs after the discontinuation of anti-PD-1 treatment due to PD and two patients who died suddenly for unknown reasons after developing irAEs (Figures 1, 3 and Table 4). All the reported p-values were two-sided, and p < 0.05 was considered significant. Statistical analysis was performed using JMP 14.2.0 statistical software (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
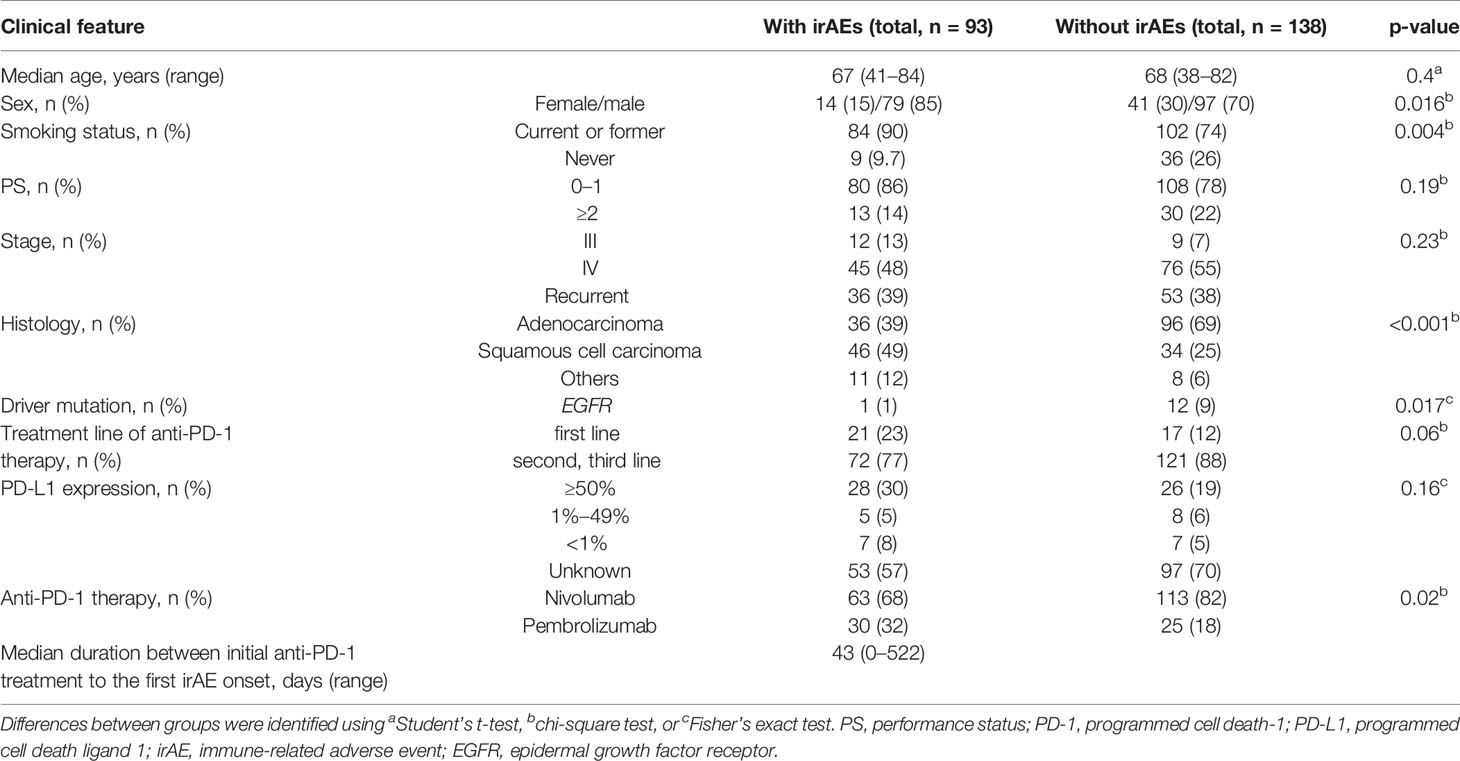
In total, 231 patients were enrolled in this study. Among these patients, 93 patients (40%) developed irAEs (Figure 1). The baseline characteristics at the initiation of anti-PD-1 therapy of patients with and without irAEs are presented in Table 1. The percentages of males, current or former smokers, squamous cell carcinoma, and pembrolizumab use were significantly higher in patients with irAEs than those in patients without irAEs. On the other hand, the percentage of epidermal growth factor receptor (EGFR) mutations was lower in patients with irAEs than that in patients without irAEs. Other clinical features, including age, Eastern Cooperative Oncology Group performance status, treatment line, and PD-L1 expression, were not significantly different.
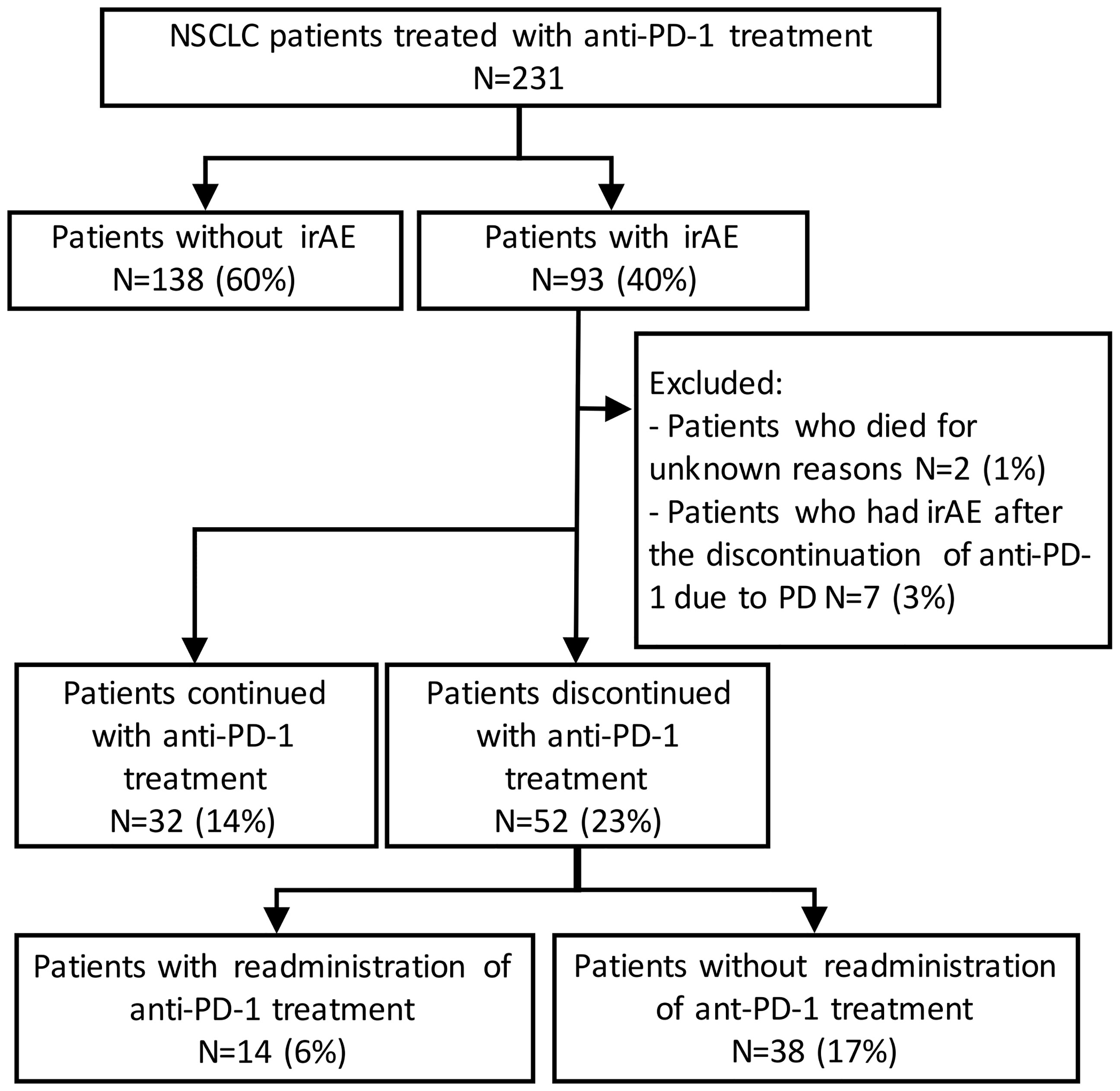
Figure 1 Patient flow diagram. NSCLC, non-small-cell lung cancer; PD-1, programmed cell death-1; irAE, immune-related adverse event.
Association of Immune-Related Adverse Events With Clinical Outcomes
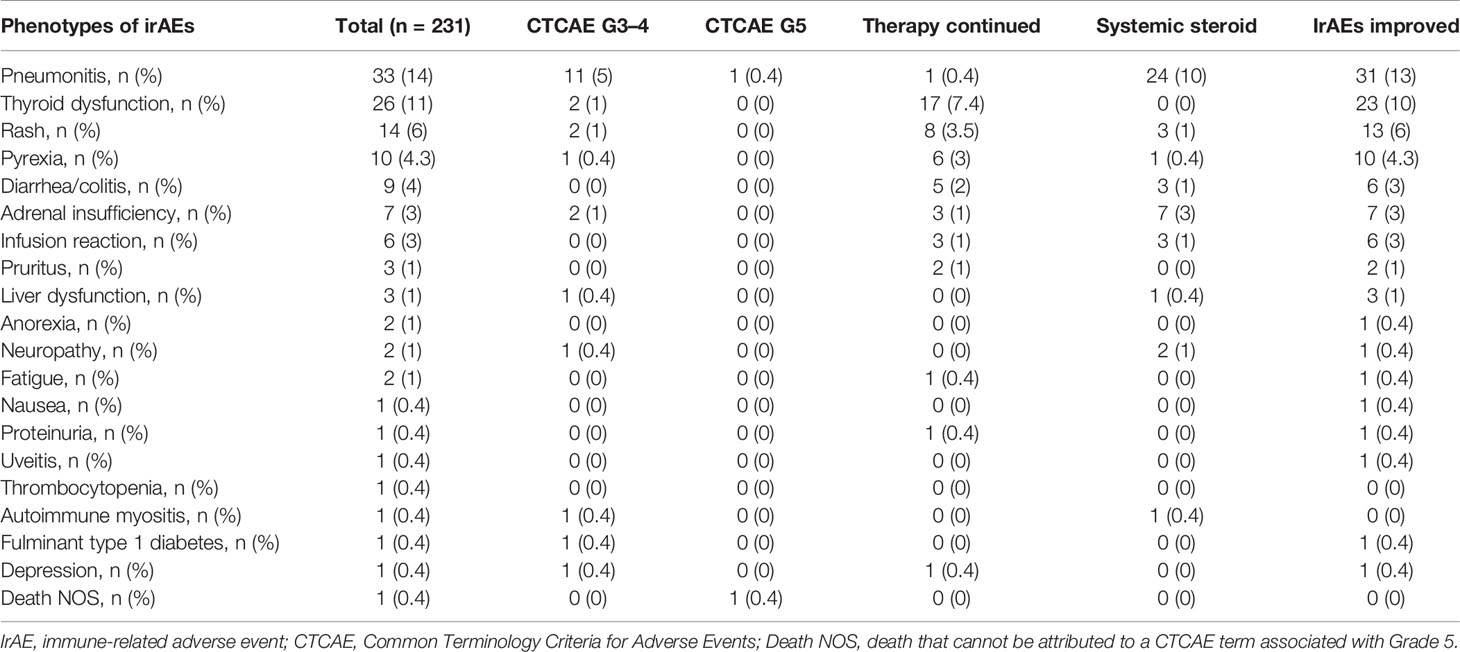
The distribution of irAEs is shown in Table 2. The Kaplan–Meier curves of the 6-week landmark analysis for PFS and OS in patients with and without irAEs are shown in Figure 2. The median PFS was significantly longer in patients with irAEs than that in patients without irAEs [14.3 (95% CI: 9.0–16.5) vs. 4.8 (95% CI: 3.2–7.6); p < 0.001]. The median OS was also significantly longer in patients with irAEs than that in patients without irAEs [not achieved (95% CI: NE–NE) vs. 21.0 (95% CI: 15.1–NE); p = 0.005]. The hazard ratios estimated by the Cox proportional hazards model were as follows: the PFS hazard ratio was 0.51 (95% CI: 0.34–0.75; p < 0.001), and the OS hazard ratio was 0.49 (95% CI: 0.30–0.81; p = 0.005). Furthermore, the ORR and DCR were significantly higher in patients with irAEs than those in patients without irAEs (Table 3).
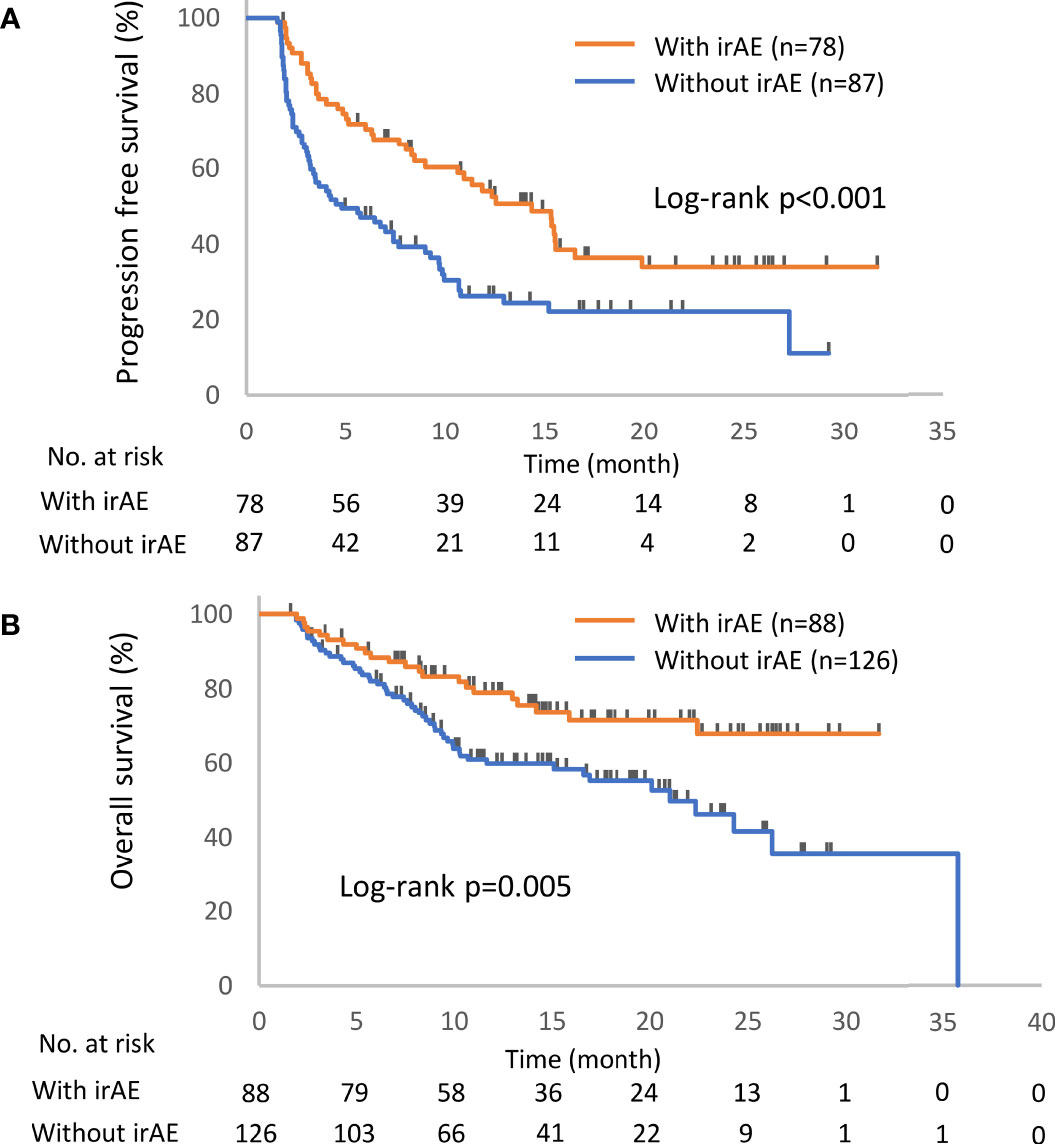
Figure 2 Kaplan–Meier curves for the 6-week landmark analysis of the progression-free survival (A) and overall survival (B) of patients with or without irAEs. IrAE, immune-related adverse event.
Association of Immune Checkpoint Inhibitor Interruption Due to Immune-Related Adverse Events With Clinical Outcomes
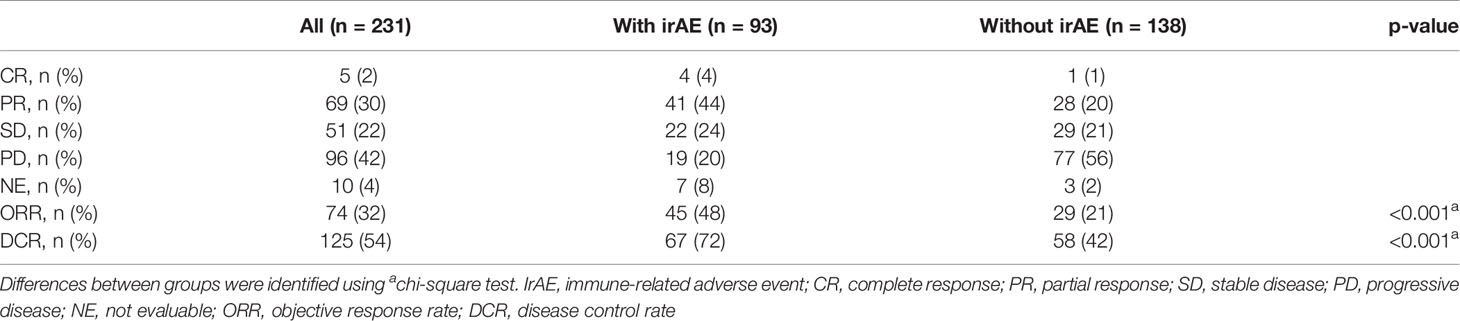
Of the 93 patients with irAEs, 32 patients continued anti-PD-1 treatment, and 52 patients discontinued anti-PD-1 treatment (Supplementary Table S1). Patients who died for unknown reasons (n = 2) and those who had irAEs after the discontinuation of anti-PD-1 treatment due to PD (n = 7) were excluded from the following analysis (Figure 1). The Kaplan–Meier curves of the 6-week landmark analysis for patients who continued and discontinued anti-PD-1 therapy are shown in Figure 3. The median PFS was not different between patients who continued and discontinued anti-PD-1 treatment [15.4 (95% CI: 9.0–NE) vs. 15.3 (95% CI: 8.3–NE); p = 0.76]. However, the median OS was significantly longer in patients who continued ICIs than that in patients who discontinued ICIs [not reached (95% CI: NE–NE) vs. not reached (95% CI: 22.4–NE); p = 0.025]. The hazard ratios estimated by the Cox proportional hazards model were as follows: the PFS hazard ratio was 0.9 (95% CI: 0.47–1.74; p = 0.76), and the OS hazard ratio was 0.27 (95% CI: 0.077–0.92; p = 0.036). In terms of irAE phenotypes, the percentage of patients who experienced immune-related pneumonitis was higher in the anti-PD-1 treatment interruption group than that in the anti-PD-1 continuation group (Table 4). On the other hand, the percentage of patients who experienced immune-related thyroid dysfunction was higher in the anti-PD-1 continuation group than that in the anti-PD-1 interruption group. As expected, the percentage of patients who experienced grade 3 or higher irAEs was higher in the anti-PD-1 interruption group than that in the anti-PD-1 continuation group. Other phenotypes of irAEs, the timing of the first irAE, ORR, and DCR were not different among patients with anti-PD-1 interruption and those with anti-PD-1 continuation.
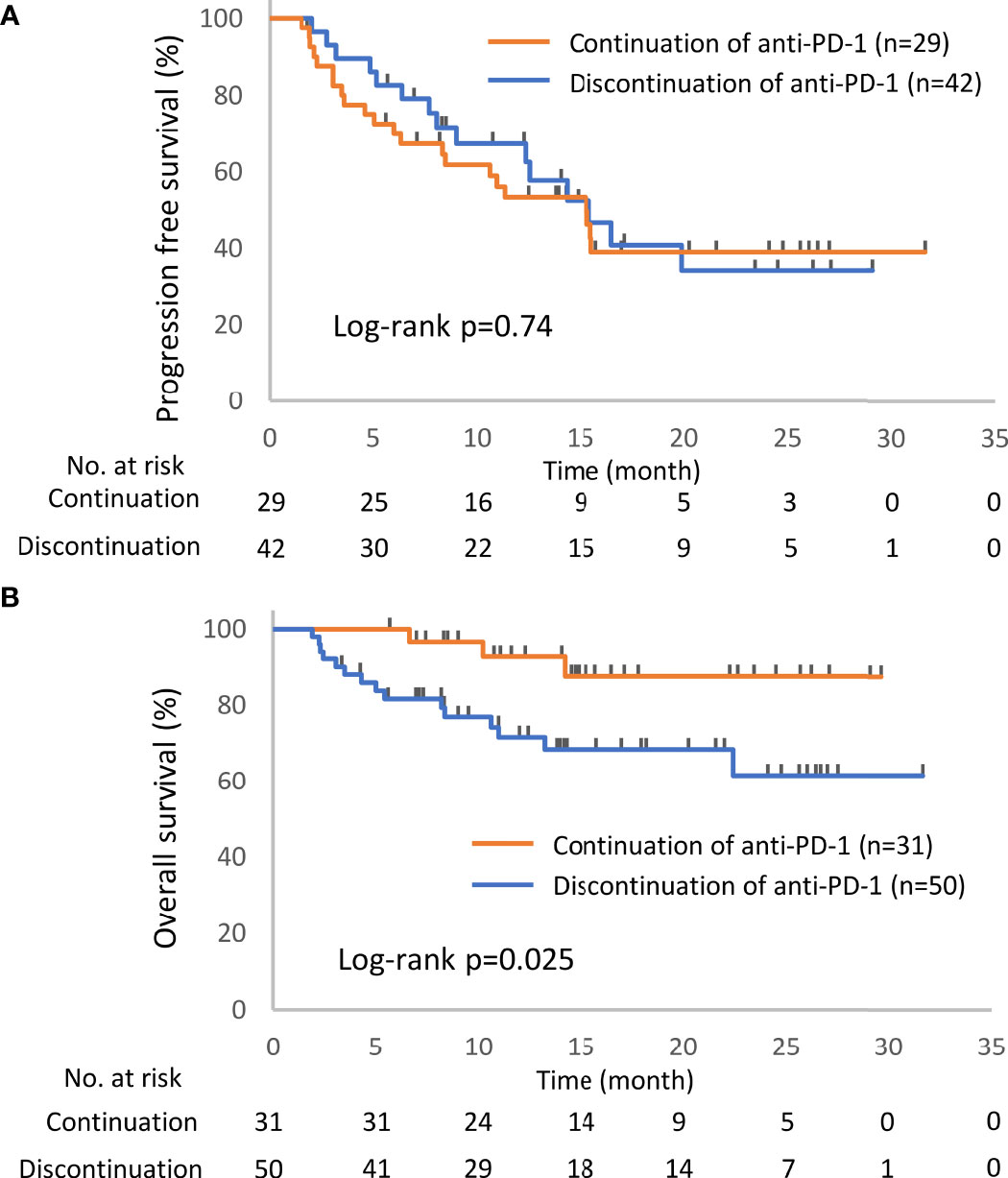
Figure 3 Kaplan–Meier curves for the 6-week landmark analysis of the progression-free survival (A) and overall survival (B) of patients continuing or stopping PD-1 treatment after irAE occurrence. PD-1, programmed cell death-1; irAE, immune-related adverse event.
Association of Immune Checkpoint Inhibitor Readministration With Clinical Outcomes
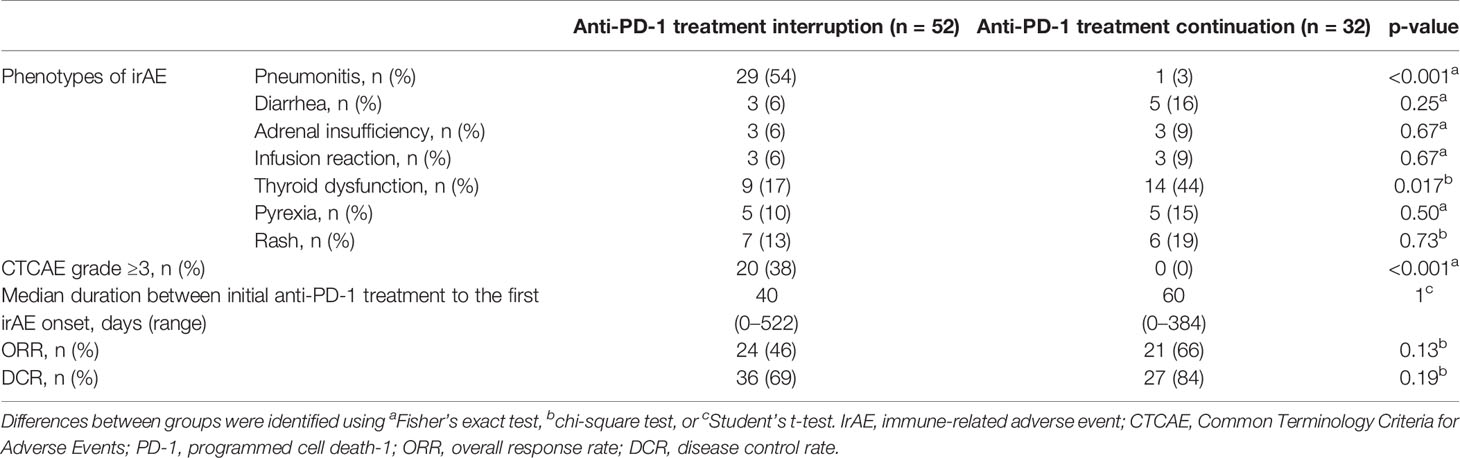
In total, 52 patients discontinued anti-PD-1 treatment, and 14 patients were readministered ICIs (Supplementary Table S2). All patients had received the same type of anti-PD-1 inhibitor prior to discontinuation. The Kaplan–Meier curves of the 6-week landmark analysis for patients readministered and not readministered anti-PD-1 therapy are shown in Figure 4. Two patients who were readministered ICIs after PD were excluded from the PFS analysis. The median PFS was not significantly different between patients with and without the readministration of anti-PD-1 treatment [15.3 (95% CI: 8.3–NE) vs. 11.3 (95% CI: 3.5–NE); p = 0.17]. On the other hand, the median OS was significantly longer in patients with ICI readministration than that in patients without ICI readministration [not reached (95% CI: NE–NE) vs. not reached (95% CI: 8.4–NE); p = 0.031]. The hazard ratios estimated by the Cox proportional hazards model were as follows: the PFS hazard ratio was 0.46 (95% CI: 0.16–1.41; p = 0.18), and the OS hazard ratio was 0.15 (95% CI: 0.019–1.1; p = 0.063). The characteristics of the initial irAEs stratified by readministration are shown in Table 5. The percentage of patients who experienced immune-related pneumonitis at initial ICI treatment was significantly higher in patients who did not receive ICI readministration than that in patients who did receive ICI readministration. The percentage of patients who experienced grade 3 or higher irAEs was not significantly different between patients with ICI readministration and those without ICI readministration. There were no differences between the anti-PD-1 readministration and permanent interruption groups regarding other phenotypes of irAEs or subsequent systemic therapy after anti-PD-1 treatment (Table 5). The recurrent and new irAEs that developed after the readministration of anti-PD-1 treatment are detailed in Table 6. In 14 patients who were readministered ICIs, two had recurrent irAEs (14%), and two developed new irAEs (14%). Only one patient developed a severe recurrent irAE that was CTCAE grade 3. We have summarized initial irAEs, tumor responses to first anti-PD-1 therapy, and clinical outcomes in patients with continuation and those with or without readministration of anti-PD-1 therapy in Supplementary Table S3.
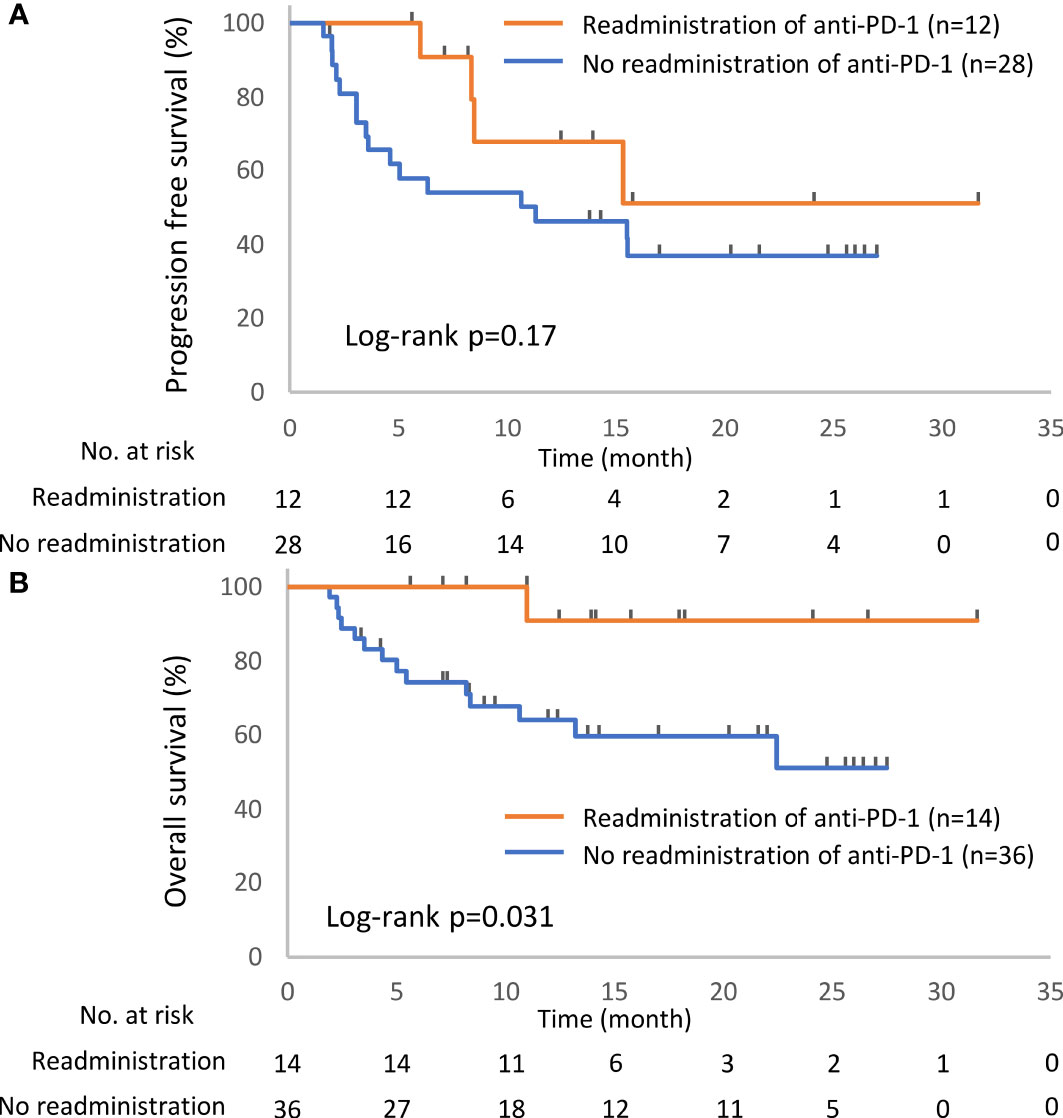
Figure 4 Kaplan–Meier curves for the 6-week landmark analysis of the progression-free survival (A) and overall survival (B) of patients with or without the readministration of anti-PD-1 treatment after the discontinuation of anti-PD-1 treatment due to irAEs. PD-1, programmed cell death-1; irAE, immune-related adverse event.
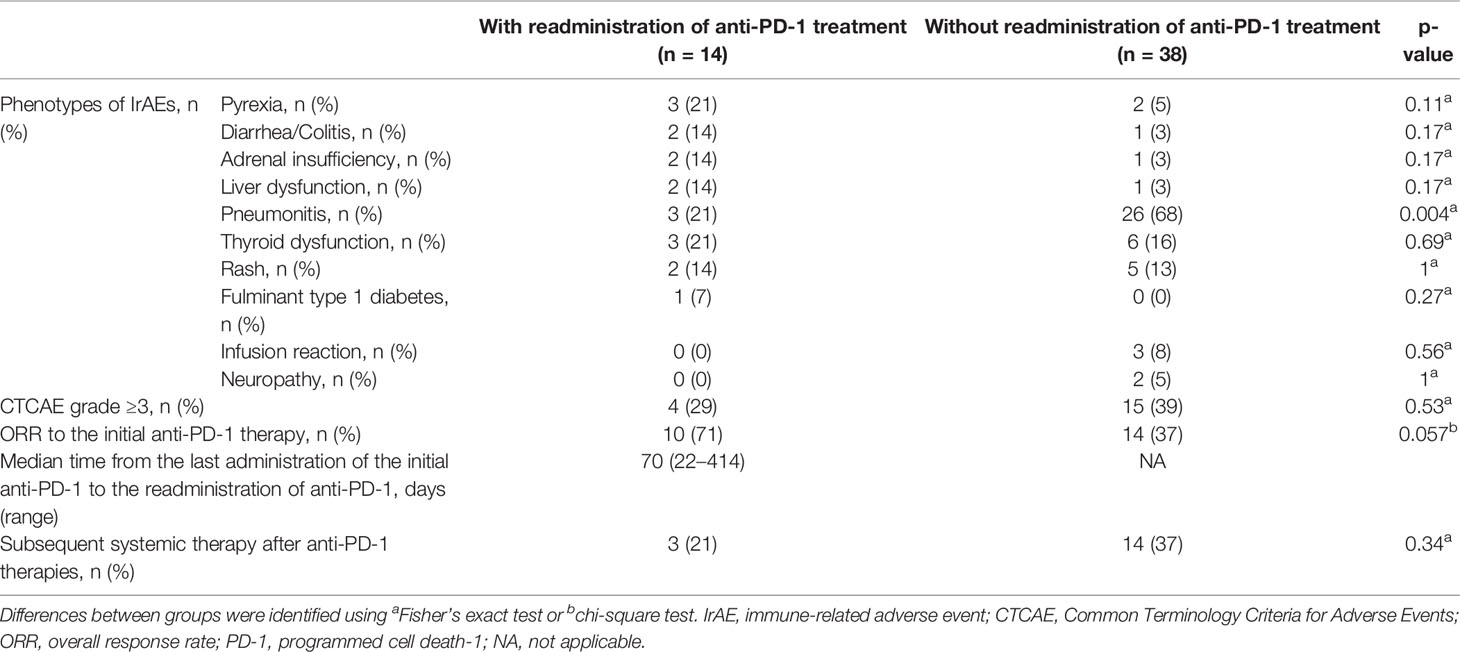
Table 5 Characteristics of the initial irAEs and clinical courses, related readministration of anti-PD-1 treatment.

Table 6 Details of recurrent and new irAEs out of 14 patients with readministered ICI after irAE occurrence.
Discussion
This study demonstrated the prognostic significance of the occurrence of irAEs, ICI continuation after the development of irAEs, and ICI rechallenge after the interruption of ICIs due to irAEs. Patients with irAEs had a better prognosis than those without irAEs (Figure 2), and the continuation or rechallenge of ICIs was associated with better survival times than the permanent interruption of ICIs due to irAEs (Figures 3, 4 and Supplementary Figure S1). Although some patients with ICI readministration experienced the recurrence of the same or new irAEs, most of these recurrent irAEs were controllable (Table 6).
There have been no reports that simultaneously evaluated the significance of ICI continuation and readministration after the occurrence of irAEs in NSCLC patients. In a retrospective study verifying the impact of ICI interruption due to irAEs, the median OS was worse in patients with ICI interruption than that in others with continuous ICI administration (16). Several retrospective studies have examined the safety of ICI rechallenge after initial irAE occurrence in cancer patients and indicated that the safety of ICI rechallenge was acceptable (17–19). A retrospective study of NSCLC demonstrated that among patients with ICI interruption at the time of initial irAE occurrence, ICI rechallenge prolonged OS in patients who had no treatment response before irAE onset (17). While these previous studies focused on only the initial irAEs, our study included all irAEs that occurred during the whole clinical course. The current study showed that among patients with irAEs, although permanent ICI interruption was associated with poor prognosis, ICI readministration and ICI continuation improved prognostic outcomes (Supplementary Figure S1). The better prognosis of patients with ICI continuation or readministration than that in patients with permanent ICI interruption may be biologically plausible. The blocking effect of PD-1/PD-L1 is generally expected to diminish with the interruption of ICIs because the binding of the anti-PD-1 antibody to PD-1-positive tumor-infiltrating CD8+ T cells is transient (20). Although not related to irAEs, a randomized phase 3b/4 study of NSCLC that compared patients who continued nivolumab for more than 1 year with those who discontinued nivolumab after 1 year of treatment demonstrated that continuous ICI therapy had better clinical outcomes (21). Collectively, these findings suggest the significance of continuously administering ICIs as much as possible after irAE occurrence via continuation or readministration.
In the current study, there was no PFS advantage from the continuation or readministration of ICIs in patients with ICI-related irAEs (Figures 3, 4). As a prospective study of patients with nonsquamous NSCLC showed that there was no difference in PFS between nivolumab and docetaxel (1), the efficacy of single-agent ICI therapy might not be able to be evaluated properly by PFS.
Unexpectedly, our study demonstrated no difference in the frequency of irAEs whose CTCAE grade was over 3 between patients with ICI readministration and those with permanent ICI interruption (Tables 4, 5). This result might suggest that clinicians aggressively readministered ICIs to patients whose irAEs had been severe but improved. Indeed, better survival outcomes were observed in patients who had experienced grade 3–4 irAEs and received the readministration of anti-PD-1 therapy (Supplementary Figure S2). Although Johnson et al. (22) suggested that severe or life-threatening toxicity is one of the factors that argues against ICI rechallenge, the readministration of ICIs might be considered in patients whose irAEs had been severe but recovered. However, it is noteworthy in the current study that the frequency of pneumonitis as an irAE was significantly higher in patients who discontinued ICIs and in those who permanently interrupted ICIs (Tables 4, 5). In addition, our study suggests that the readministration of anti-PD-1 therapy had no survival benefit in patients with pneumonitis (Supplementary Figure S2). Several meta-analyses have reported that pneumonitis is one of the most common fatal irAEs in patients treated with ICIs (23, 24). Therefore, although there has been no evidence that the continuous administration or readministration of ICIs tends to lead to fatal irAEs, it should be noted that the continuation or readministration of ICIs to patients who experienced irAEs such as pneumonitis, which could be fatal if exacerbated, should be carefully determined on a patient-by-patient basis.
The limitations of the current study include the relatively small number of patients with ICI readministration and the retrospective nature of the study. Clinicians might have tended to avoid continuing and readministering ICIs to patients with irAEs such as pneumonitis, which could be fatal if exacerbated. There is no detailed analysis for each irAE in this study. In addition, response rate to initial anti-PD-1 therapies in patients with readministration tended to be higher than that in patients without readministration (ORR 71% vs. 37%, p = 0.057; Table 5). There is a possibility that clinicians might have tended to readminister anti-PD-1 therapy to patients with good tumor response to initial ICI even with irAEs.
In summary, we retrospectively investigated the clinical significance of the continuation and readministration of single-agent anti-PD-1 therapy in NSCLC patients with ICI-related irAEs. The continuation and readministration of ICIs significantly prolonged OS, and their safety was acceptable. A future prospective study is needed to establish optimal treatment strategies for patients with irAEs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Niigata University Graduate School of Medical and Dental Sciences, Niigata Prefectural Shibata Hospital, Niigata City General Hospital, Niigata Cancer Center Hospital, Saiseikai Niigata Hospital, Nishi-Niigata Chuo National Hospital, Niigata Prefectural Central Hospital, Nagaoka Chuo General Hospital, Nagaoka Red Cross Hospital, Niigata Medical Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TO devised this study. TO and SW designed the protocol. SW, TO, SS, KN, KI, RK, TM, TA, SM, HT, MO, MTe, NM, TI, AI, KS, HY, MH enrolled patients into the study. TF and SW co-wrote this manuscript, conducted data analysis, and prepared figures and tables. All authors contributed to the article and approved the submitted version.
Funding
The primary author was supported by a grant from the Niigata Medical Association, Japan.
Conflict of Interest
SW reports grants and personal fees from AstraZeneca, personal fees from Chugai Pharma, personal fees from Ono Pharmaceutical, personal fees from Bristol-Myers, grants and personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from MSD, personal fees from Taiho Pharmaceutical, personal fees from Pfizer, personal fees from Novartis, and personal fees from Daiichi Sankyo outside the submitted work. TO reports personal fees from Boehringer Ingelheim, personal fees from MSD, personal fees from Eli Lilly, personal fees from AstraZeneca, personal fees from Chugai-pharm, and personal fees from Bristol-Myers Squibb outside the submitted work. SS reports personal fees from AstraZeneca, personal fees from Chugai Pharma, personal fees from Taiho Pharmaceutical, and personal fees from MSD outside the submitted work. KN reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Taiho Pharmaceutical, and personal fees from MSD outside the submitted work. AI reports personal fees from AstraZeneca, personal fees from Chugai Pharma, personal fees from Bristol-Myers, personal fees from Boehringer Ingelheim, personal fees from Ono Pharmaceutical, personal fees from Taiho Pharmaceutical, personal fees from Novartis International AG, and personal fees from Daiichi Sankyo Company outside the submitted work. SH reports personal fees from GlaxoSmithKline Inc. outside the submitted work. TA reports personal fees from Eli Lilly Japan, personal fees from Chugai Pharmaceutical, personal fees from Taiho Pharmaceutical, personal fees from Ono Pharmaceutical, personal fees from Bristol-Myers Squibb, personal fees from AstraZeneca, and personal fees from Mylan outside the submitted work. SM reports personal fees from Chugai Pharmaceutical, personal fees from ONO Pharmaceutical, personal fees from AstraZeneca, personal fees from Eli Lilly, personal fees from MSD, personal fees from Boehringer Ingelheim, personal fees from Taiho Pharmaceutical, personal fees from Novartis, personal fees from Bristol-Myers Squibb, and personal fees from Kyowa Hakko Kirin outside the submitted work. HT reports grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Eli Lilly, grants and personal fees from MSD, grants and personal fees from Taiho pharmaceutical, grants and personal fees from Pfizer, grants and personal fees from Novartis, grants and personal fees from Chugai pharmaceutical, grants and personal fees from Astra Zeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Ono pharmaceutical, and grants and personal fees from Merck outside the submitted work. MO reports personal fees from AstraZeneca, personal fees from Ono Pharmaceutical, personal fees from Bristol-Myers, personal fees from Boehringer Ingelheim, personal fees from MSD, and personal fees from Taiho Pharmaceutical outside the submitted work. NA reports personal fees from Meiji Seika Pharma, grants from MSD, and personal fees from MSD outside the submitted work. MH reports personal fees from Boehringer-Ingelheim Japan, personal fees from AstraZeneca, personal fees from Taiho Pharmaceutical, and personal fees from Daiichi Sankyo outside the submitted work. YO reports personal fees from Boehringer-Ingelheim Japan and personal fees from Meiji Seika Pharma outside the submitted work. TKo reports personal fees from AstraZeneca, Boehringer-Ingelheim, Sanofi Genzyme, Novartis, Daiichi Sankyo, Kyorin Pharmaceutical, and GlaxoSmithKline outside the submitted work. TKi reports grants and personal fees from Chugai Pharma, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Eli Lilly, grants and personal fees from MSD K.K., personal fees from Astellas Pharma Inc., grants and personal fees from Taiho Pharmaceutical Co., Ltd., personal fees from Bristol-Myers Squibb Company, personal fees from Pfizer Japan Inc., grants and personal fees from Daiichi Sankyo Co., Ltd., personal fees from Taisho Toyama Pharmaceutical Co., Ltd., personal fees from Janssen Pharmaceutical K.K., personal fees from Japan BCG Laboratory, grants and personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Novartis Pharma K.K., personal fees from Mylan N.V., grants and personal fees from AstraZeneca, personal fees from Roche Diagnostics K.K., grants and personal fees from Shionogi & Co., Ltd., grants from TEIJIN PHARMA Ltd., and grants from KYORIN Pharmaceutical Co., Ltd., outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the patients, their families, all study investigators, and Hiroko Aita for their contributions to the study. The primary author was supported by a grant from the Niigata Medical Association, Japan.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.704475/full#supplementary-material
Abbreviations
ICI, immune checkpoint inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; NSCLC, non-small-cell lung cancer; ILD, interstitial lung disease; GGO, ground-glass opacity; PFS, progression-free survival; OS, overall survival; irAE, immune-related adverse event; NOS, not otherwise specified; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
References
1. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
2. Brahmer J, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
3. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (London England) (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
4. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (London England) (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
5. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
6. Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro Carpeño J, Pluzanski A, et al. Four-Year Survival With Nivolumab in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer: A Pooled Analysis. Lancet Oncol (2019) 20(10):1395–408. doi: 10.1016/S1470-2045(19)30407-3
7. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
8. Watanabe S, Ota T, Hayashi M, Ishikawa H, Otsubo A, Shoji S, et al. Prognostic Significance of the Radiologic Features of Pneumonitis Induced by Anti-PD-1 Therapy. Cancer Med (2020) 9(9):3070–7. doi: 10.1002/cam4.2974
9. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol (2018) 4(3):374–8. doi: 10.1001/jamaoncol.2017.2925
10. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early Immune-Related Adverse Events and Association With Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated With Nivolumab: A Prospective Cohort Study. J Thorac Oncol (2017) 12(12):1798–805. doi: 10.1016/j.jtho.2017.08.022
11. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation Between Immune-Related Adverse Events and Efficacy in Non-Small Cell Lung Cancer Treated With Nivolumab. Lung Cancer (2018) 115:71–4. doi: 10.1016/j.lungcan.2017.11.019
12. Baldini E, Lunghi A, Cortesi E, Turci D, Signorelli D, Stati V, et al. Immune-Related Adverse Events Correlate With Clinical Outcomes in NSCLC Patients Treated With Nivolumab: The Italian NSCLC Expanded Access Program. Lung Cancer (2020) 140:59–64. doi: 10.1016/j.lungcan.2019.12.014
13. Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, et al. Association of Immune-Related Adverse Events With Clinical Benefit in Patients With Advanced Non-Small-Cell Lung Cancer Treated With Nivolumab. Oncologist (2018) 23(11):1358–65. doi: 10.1634/theoncologist.2017-0384
14. Tachihara M, Negoro S, Inoue T, Tamiya M, Akazawa Y, Uenami T, et al. Efficacy of Anti-PD-1/PD-L1 Antibodies After Discontinuation Due to Adverse Events in Non-Small Cell Lung Cancer Patients (HANSHIN 0316). BMC Cancer (2018) 18(1):946. doi: 10.1186/s12885-018-4819-2
15. Komiya K, Nakamura T, Abe T, Ogusu S, Nakashima C, Takahashi K, et al. Discontinuation Due to Immune-Related Adverse Events Is a Possible Predictive Factor for Immune Checkpoint Inhibitors in Patients With Non-Small Cell Lung Cancer. Thorac Cancer (2019) 10(9):1798–804. doi: 10.1111/1759-7714.13149
16. Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E, Poonja Z, et al. Efficacy of Nivolumab and Pembrolizumab in Patients With Advanced Non-Small-Cell Lung Cancer Needing Treatment Interruption Because of Adverse Events: A Retrospective Multicenter Analysis. Clin Lung Cancer (2019) 20(1):e97–106. doi: 10.1016/j.cllc.2018.09.005
17. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and Efficacy of Re-Treating With Immunotherapy After Immune-Related Adverse Events in Patients With NSCLC. Cancer Immunol Res (2018) 6(9):1093–9. doi: 10.1158/2326-6066.CIR-17-0755
18. Abou Alaiwi S, Xie W, Nassar AH, Dudani S, Martini D, Bakouny Z, et al. Safety and Efficacy of Restarting Immune Checkpoint Inhibitors After Clinically Significant Immune-Related Adverse Events in Metastatic Renal Cell Carcinoma. J Immunother Cancer (2020) 8(1). doi: 10.1136/jitc-2019-000144
19. Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol (2019) 5(9):1310–7. doi: 10.1001/jamaoncol.2019.1022
20. Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, et al. In Vivo Imaging Reveals a Tumor-Associated Macrophage-Mediated Resistance Pathway in Anti-PD-1 Therapy. Sci Transl Med (2017) 9(389). doi: 10.1126/scitranslmed.aal3604
21. Waterhouse DM, Garon EB, Chandler J, McCleod M, Hussein M, Jotte R, et al. Continuous Versus 1-Year Fixed-Duration Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: CheckMate 153. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(33):3863–73. doi: 10.1200/JCO.20.00131
22. Johnson DB, Jakubovic BD, Sibaud V, Sise ME. Balancing Cancer Immunotherapy Efficacy and Toxicity. J Allergy Clin Immunol Pract (2020) 8(9):2898–906. doi: 10.1016/j.jaip.2020.06.028
23. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/jamaoncol.2018.3923
24. Sun X, Roudi R, Dai T, Chen S, Fan B, Li H, et al. Immune-Related Adverse Events Associated With Programmed Cell Death Protein-1 and Programmed Cell Death Ligand 1 Inhibitors for Non-Small Cell Lung Cancer: A PRISMA Systematic Review and Meta-Analysis. BMC Cancer (2019) 19(1):558. doi: 10.1186/s12885-019-5701-6
Keywords: drug therapy, immune-related adverse event, immunology, NSCLC, PD-1
Citation: Fujisaki T, Watanabe S, Ota T, Kushiro K, Sato Y, Takahashi M, Ohtsubo A, Shoji S, Nozaki K, Ichikawa K, Hokari S, Kondo R, Miyabayashi T, Abe T, Miura S, Tanaka H, Okajima M, Terada M, Matsumoto N, Ishida T, Iwashima A, Sato K, Yoshizawa H, Aoki N, Hayashi M, Ohshima Y, Koya T and Kikuchi T (2021) The Prognostic Significance of the Continuous Administration of Anti-PD-1 Antibody via Continuation or Rechallenge After the Occurrence of Immune-Related Adverse Events. Front. Oncol. 11:704475. doi: 10.3389/fonc.2021.704475
Received: 03 May 2021; Accepted: 01 September 2021;
Published: 24 September 2021.
Edited by:
Tao Jiang, Shanghai Pulmonary Hospital, ChinaReviewed by:
Bruna Pellini, Moffitt Cancer Center, United StatesKoichi Saruwatari, Kumamoto University Hospital, Japan
Copyright © 2021 Fujisaki, Watanabe, Ota, Kushiro, Sato, Takahashi, Ohtsubo, Shoji, Nozaki, Ichikawa, Hokari, Kondo, Miyabayashi, Abe, Miura, Tanaka, Okajima, Terada, Matsumoto, Ishida, Iwashima, Sato, Yoshizawa, Aoki, Hayashi, Ohshima, Koya and Kikuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Watanabe, c2F0b3NoaTdAbWVkLm5paWdhdGEtdS5hYy5qcA==
 Toshiya Fujisaki
Toshiya Fujisaki Satoshi Watanabe
Satoshi Watanabe Takeshi Ota2
Takeshi Ota2 Toshiyuki Koya
Toshiyuki Koya Toshiaki Kikuchi
Toshiaki Kikuchi