
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 08 July 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.703558
This article is part of the Research Topic Redox Potential and Metabolic Behavior in Gastrointestinal Cancers View all 6 articles
 Yusha Yang1†
Yusha Yang1† Zufu Jiang1†
Zufu Jiang1† Weizhou Wu2†
Weizhou Wu2† Libin Ruan1
Libin Ruan1 Chengyang Yu1
Chengyang Yu1 Yuning Xi1
Yuning Xi1 Liling Wang1
Liling Wang1 Kunpeng Wang1
Kunpeng Wang1 Jinggang Mo1
Jinggang Mo1 Shankun Zhao3*
Shankun Zhao3*Mounting studies demonstrated both chronic hepatitis B virus (HBV) and chronic hepatitis C virus (HCV) infection might be associated not only with an increased risk of hepatocellular carcinoma but also extrahepatic malignancies, i.e., gastric cancer (GC). However, a quantitative result addressing the association between HBV/HCV infection and GC development is scarce. A systematic search to identify the eligible studies was performed in four databases, including MEDLINE, EMBASE, Cochrane Library, and the PsychINFO. The relationship between HBV/HCV infection and the risk of GC was quantified by calculating the hazard ratio (HR) with a 95% confidence interval (CI). More methodologies of this study were available in the PROSPERO (ID: CRD42021243719). Thirteen included studies involving 7,027,546 individuals (mean age, 42.6-71.9 years) were enrolled in the pooled analyses. Two articles provided the clinical data of both HBV and HCV infections. The proportion of high methodological quality studies was 76.9% (10/13). Synthetic results from 10 eligible studies of HBV showed that HBV infection was associated with a significantly higher risk of GC when compared with the healthy controls without HBV infection (pooled HR, 1.26; 95% CI, 1.08–1.47; P = 0.003; heterogeneity, I2 = 89.3%; P< 0.001). In line with this finding, the combined effect derived from five included studies of HCV also supported a significant positive association between chronic HBV infection and GC development (pooled HR, 1.88; 95% CI, 1.28–2.76; P = 0.001; heterogeneity, I2 = 74.7%; P = 0.003). In conclusion, both chronic HBV and HCV infections were related to a high risk of GC. The plausible mechanisms underlying such association might be correlated to HBV/HCV infection-induced persistent inflammation, immune dysfunction, and cirrhosis.
Systematic Review Registration: PROSPERO (http://www.crd.york.ac.uk/PROSPERO), identifier (CRD42021243719).
Although the incidence and mortality rates are ongoing declines over the last half century in majority of countries, gastric cancer (GC) remains one of the most frequent and lethal malignancies worldwide and is responsible for over 1,000,000 new cases in 2020 and an estimated 769,000 deaths globally (1). As reported, the overall GC incidence rate is predicted to continue falling in both high-incidence and low-incidence countries (2). For example, the age-standardized incidence rate (ASR) of GC in Japan was recorded at 36 in 2010 and is anticipated to decline to 30 in 2035. With a similar trend, the ASR of GC in Australia was 5.1 in 2010 and is predicted to drop to 4.6 in 2035. However, a high incidence of GC is observed in the younger age groups (younger than 50 years) in most populations (3). This paradoxical increase of GC among younger generations is still under investigation. Till now, an increasing number of risk factors contributing to GC have been identified gradually. Chronic Helicobacter pylori infection is believed to be the leading cause of noncardia GC (4). Known risk factors beyond H. pylori infection for GC include cigarette smoking, alcohol consumption, and high consumption of unhealthy meat (i.e., processed, grilled, and barbecued meat) (1).
In addition to hepatocellular carcinoma, both chronic hepatitis B virus (HBV) and chronic hepatitis C virus (HCV) infection have gradually been recognized to associate with the development and progression of multiple extrahepatic malignancies, including colorectal cancer (5), lung cancer (6), esophageal cancer (7), and lymphoma (8). In recent years, the potential association between chronic hepatitis and GC development has been postulated and achieved special attention. In a large-sample retrospective study, Kocoglu et al. (9) found that the hepatitis B surface antigen (HBsAg) positivity rate of GC was significantly higher than the control group (5.88% vs. 3.3%, P= 0.025). In line with Kocoglu et al.’s findings, a study conducted in Iran demonstrated that HBV DNA was detected in 3.17% of GC individuals (2/63), but no HBV DNA was identified in normal individuals (0.0%, 0/21) (10). As for HCV, a nationwide population-based study indicated that HCV infection was an important risk factor for GC development (P<0.05), showing a significant difference in the cumulative incidence of GC as compared with the control group [HCV-infected sample vs HCV uninfected cohorts: 51/37725 (0.14%) vs 16/30180 (0.05%), P<0.05] (11). Besides, the authors also found a positive association between HCV infection and the mortality of GC (11). Although there is lack of direction of causality, a cross-sectional study developed by Baghbanian et al. (12) also indicated that HBsAg-positive patients have a higher prevalence of GC than HBsAg-negative patients (9/83, 10.8% vs 26/645, 4%).
Although several studies have postulated a close relationship between chronic HBV/HCV infection and GC development, some investigators demonstrated that the incidence rate of chronic hepatitis infected patients was comparable to the non-infected subjects. For example, Sundquist et al. found that chronic HBV infection was not a significant risk factor for GC (standardized incidence ratio, 0.89; 95% confidence interval, 0.35–1.85; P>0.05) (13). Similar to this finding, Kocoglu et al. reported that the anti-HCV positivity rate in the GC group and the control group was 1.57% and 0.84%, respectively, and no statistical difference was detected (P= 0.12) (9).
At present, the underlying connection between chronic HBV/HCV infection and the risk of GC is still controversial among different clinical studies. A number of more recent studies have been struggled to address this association continually (14), whereas a systematic review and meta-analysis are extremely scarce in the literature. In this study, we were intended to summarize all the evidence on the relationship between chronic hepatitis infection and risk of GC to display the risk of GC in HBV/HCV-infected individuals as compared with the healthy general population via a quantitative and pooled analysis, which might better facilitate the clinical understanding with this issue.
The present systematic review and pooled analysis were performed in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA checklist with detail was listed in Supplemental Table 1. This study was registered in the PROSPERO with an access ID of CRD42021243719 (http://www.crd.york.ac.uk/PROSPERO).
We have systematically searched four databases, including MEDLINE (PubMed), EMBASE (OVID), the Cochrane Library, and the PsychINFO, to filter the potential studies prior to March 1, 2021. Only studies representing the English language and human participants were included. The searching strategy employed for screening the relevant studies in PubMed databases was: (“Hepatitis B, chronic”[Mesh]) OR (Chronic Hepatitis B)) OR (Hepatitis B Virus Infection, Chronic)) OR (Chronic Hepatitis B Virus Infection)) OR (HBV)) OR (Hepatitis C, Chronic)) OR (HCV)) AND (“stomach neoplasms”[Mesh]) OR (neoplasm, stomach)) OR (stomach neoplasm)) OR (Neoplasms, stomach)) OR (gastric neoplasms)) OR (gastric neoplasm)) OR (neoplasm, gastric)) OR (neoplasms, gastric)) OR (cancer of stomach)) OR (stomach cancers)) OR (gastric cancer)) OR (cancer, gastric)) OR (cancers, gastric)) OR (gastric cancers)) OR (stomach cancer)) OR (cancer, stomach)) OR (cancers, stomach)) OR (cancer of the stomach)) OR (gastric cancer, familial diffuse)). Moreover, we also reviewed the reference list to identify additional studies by a manual inspection.
Patients with chronic HBV/HCV infection and GC were according to the International Classification of Diseases (ICD) using the corresponding codes and the standard of the World Health Organization (WHO). The presence/positivity of HBsAg was the most primary serum marker for identifying the cases of HBV infection. Patients with chronic HCV infection were diagnosed by anti-HCV seropositivity for 6 months or liver histology. The diagnosis of GC was confirmed by histopathological examination.
Any studies reporting the incidence of GC in chronic HBV/HCV infected patients compared with a non-chronic hepatitis group were considered eligible. Besides, those studies reporting with the association between HBV/HCV infection and risk of GC by calculating with a hazard ratio (HR), odds ratios (OR), or relative risk (RR) with the 95% confidence intervals (CI) were additionally included. Moreover, studies providing sufficient data to generate the effect sizes were also included. The inclusion criteria for this systematic review and pooled analysis were in accordance with the standard of Patient, Intervention, Comparison, Outcome, and Study design (PICOS). The scientific question guiding this cumulative analysis was: Is there a positive relationship between chronic HBV/HCV infection and risk of GC? The components for the PICOS evidence were as follows: chronic HBV/HCV-infected individuals (P); diagnosis of GC or stomach cancer (I); compared with the general healthy population (C); the incidence of GC or the HR of GC (O); study designs (S).
The exclusion criteria in this pooled analysis were as follows: (I) review articles, reader or editorial comments for the published studies, and case reports; (II) duplicated data or previous publications for same samples and the same scientific questions; (III) animal or in-vitro experiments; (IV) those studies reporting the study sample are co-infected with HBV and HCV because the present study is designed for investigating whether HBV or HCV is an independent risk factor for GC development; (V) cross-sectional studies are excluded in this meta-analysis because this kind of studies could not show the direction of causality between chronic hepatitis virus infection and the risk of gastric cancer, as it lacks the information about the temporality of associations. Based on the inclusion and exclusion criteria, the process of study selection was conducted by two authors independently. Any ambiguities were resolved by the corresponding author.
Study characteristics in every single study were extracted by two authors independently. According to a data collection form, the following information was extracted, including the first authors’ names, publication year, study area, study design, age of the participants, GC cases in the HBV/HCV group and the control group, adjusted HR with 95% CI, and variable adjustment in each included study.
The methodological quality of the cohort studies and case-control studies was evaluated by the Newcastle–Ottawa Scale (NOS), in which low quality, moderate quality, and high quality were based on the scores of 0–3, 4–6, and 7–9, respectively.
The pooled analysis was conducted by using STATA version 13.0 software for Windows (Stata Corp LP, College Station, Texas, USA). The overall HR with 95% CI synthesized by all the included studies was used to quantitatively evaluate the strength of the relationship between HBV/HCV infection and the risk of GC. Statistical significance was assumed at a two-tailed P values < 0.05. I2 statistics and the Cochrane Q statistic were used for the heterogeneity test. I2 > 50% was regarded as substantial heterogeneity, whereas the P-value of the Q test < 0.10 was considered to be statistically significant. On account of a high likelihood of between-study variance for differences in a designed study and the demographic characteristics, a random-effects model rather than a fixed-effects model was used in the current study. Subgroup analyses on different important factors were performed to better illustrate the association between HBV/HCV infection and risk of GC and further explore the origin of heterogeneity. Additionally, sensitivity analyses were applied to identify the potential origin of the between-study heterogeneity. The funnel plot, Begg’s rank-correlation test, and Egger’s regression asymmetry test were used to evaluate the publication bias.
The selection process for screening the eligible articles is shown in Figure 1. A total of 623 articles were identified during the initial searching in the four databases. After excluding duplicates, non-clinical studies, studies out of the research question, review articles, comments, and case reports, 562 publications were eliminated, and the remaining 61 potentially related articles were retrieved for the full-text review. Among these studies, 14 studies were eliminated for lacking control group, 11 studies did not meet the inclusion criteria, 12 studies for inappropriate grouping; 10 studies were excluded due to insufficient outcome data, and 1 study was eliminated for its study design was cross-sectional. Finally, 13 studies (11, 13, 15–25) met the aforementioned inclusion criteria and thus were included in this pooled analysis. Of which, two included studies (16, 22) have provided the data of both HBV and HCV. As a result, 10 included studies reporting HBV (13, 15–23) and five included studies (11, 16, 22, 24, 25) reporting HCV were used for pooling the overall HR.
The publication years of the 13 eligible studies ranged from 2014 to 2020. The mean age of the participants ranged from 42.6 ± 12.6 to 71.9 ± 14.59 years. For the study geographical area, 10 studies were conducted in Asia, 2 studies in the United States, and 1 study in Europe. The study design of the eligible studies included cohort and case-control studies. The sample size among these included ranged from 1,160 to 5,332,903, involving a total of 7,027,546 individuals. Among the 13 included studies, two studies (Kamiza et al. and Hong et al.) (16, 22) providing the clinical information of both HBV and HCV. In An et al.’s study (18), the adjusted HR contained both female and male subjects independently. In Lu et al.’s study (19), the adjusted HR was divided into different age groups, including 0–39, 40–59, and ≥ 60 years old. In Song et al.’s study (21), there were three adjusted HR from three cohorts based on the different study areas of China, including Beijing city, Qingdao city, and Guangzhou city. The characteristics of the 15 eligible studies were summarized in Table 1.
Based on the NOS, three included studies (13, 19, 24) were considered to be moderate quality and 10 included studies (11, 15–18, 20–23, 25) were high quality. The proportion of high methodological quality study was 76.9% (10/13). The detail-specific scoring of the study quality was shown in Supplementary Table 2.
As shown in Figure 2, after multivariable adjustment, the pooled effect from 10 included studies reporting HBV supported a significant positive relationship between chronic HBV infection and high risk of GC (overall synthetic HR derived from 15 single HR, 1.26; 95% CI, 1.08–1.47; P=0.003) by using a random-effects model. Statistical heterogeneity was identified during this meta-analysis (I2 = 89.3%, P< 0.001).
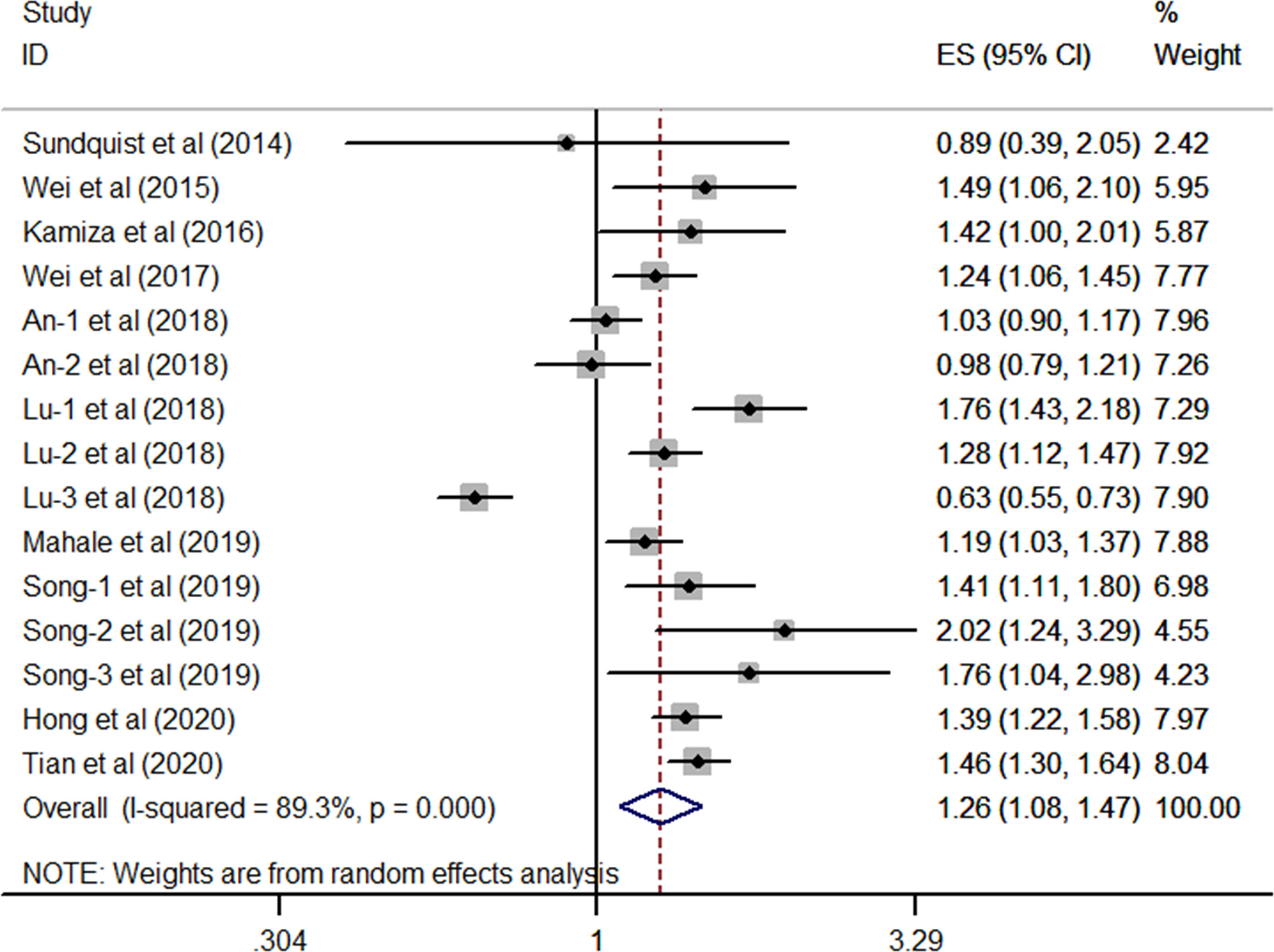
Figure 2 Forest plots of the pooled analysis of the included studies on the association between HBV and risk of gastric cancer.
In the present study, we have conducted subgroup analyses to detect the source of heterogeneity in the relationship between HBV infection and the risk of GC. Notably, the disease duration of HBV infection, antiviral therapy, gender, and mean age among different studies might be the confounding factors. However, only limited included studies have provided detail information on the above factors, so we could not perform a subgroup analysis on these confounding factors.
All the 10 eligible studies reporting the association between HBV infection and GC have provided the study design and the research area; thus, a subgroup analysis was conducted on the two factors. As shown in Figure 3, results from the subgroup analysis on the study design showed that the relationship between HBV infection and high risk of GC existed in the eight cohort studies (HR, 1.33; 95% CI, 1.21–1.45; P <0.001), and no significant heterogeneity was identified (I2 = 18.9%, P = 0.28). However, such association was not found in the seven case-control studies (HR, 1.17; 95% CI, 0.90–1.52; P =0.247), and the substantial heterogeneity was detected (I2 = 94.6%, P <0.001). When focused on the geographical area, this subgroup analysis revealed that the positive association between HBV infection and the risk of GC was existed either in the Europe/US or the Asian countries (all P> 0.05). Furthermore, we have found that such association is stronger in the Asian countries (HR, 1.29; 95% CI, 1.08–1.53; P = 0.004; I2 = 90.8%; P < 0.001) than the Europe/US (HR, 1.18; 95% CI, 1.03–1.36; P = 0.021; I2 = 0.0%, P = 0.5) (Figure 4).
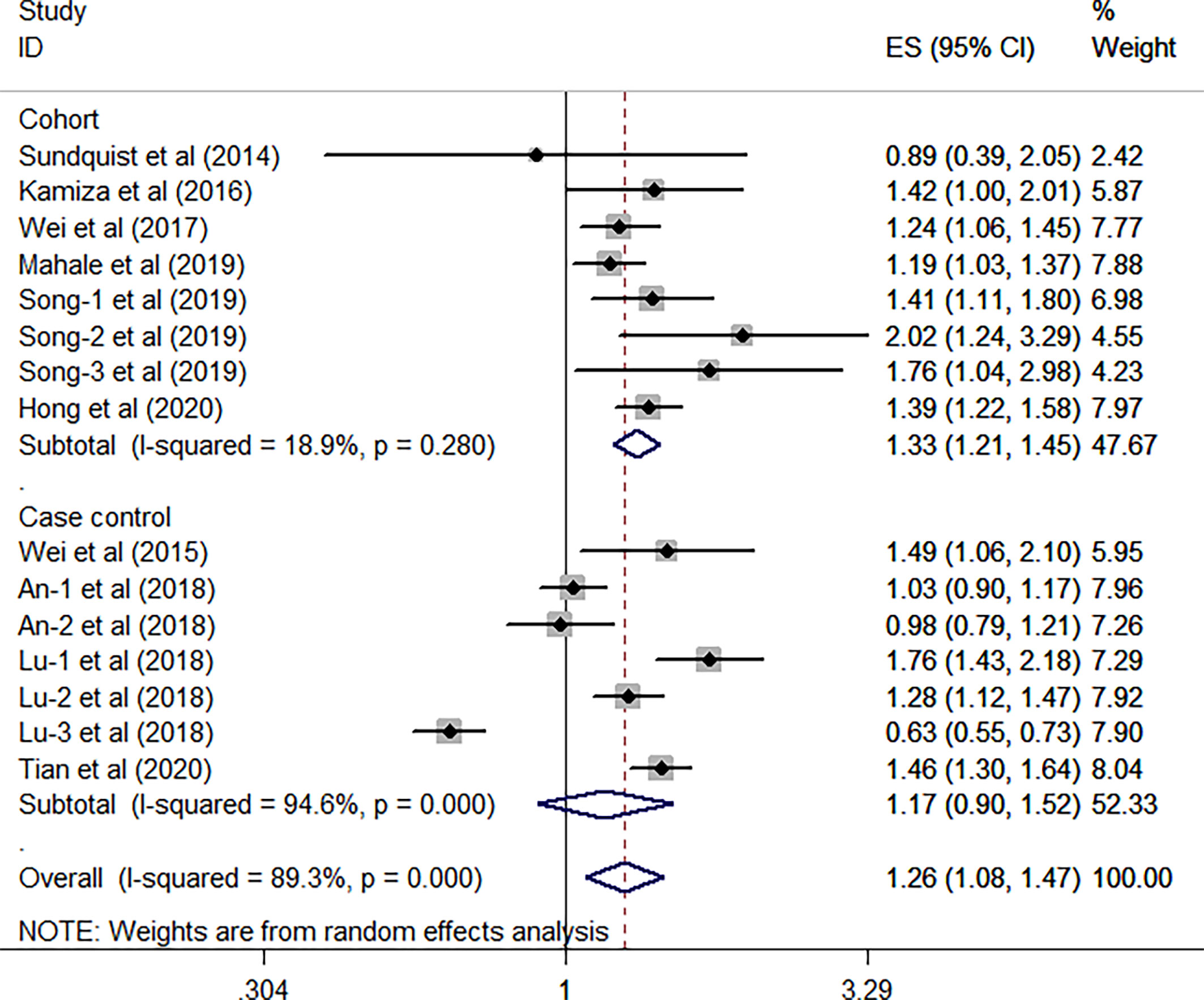
Figure 3 Forest plots of the subgroup analysis (study design) on the association between HBV and gastric cancer.
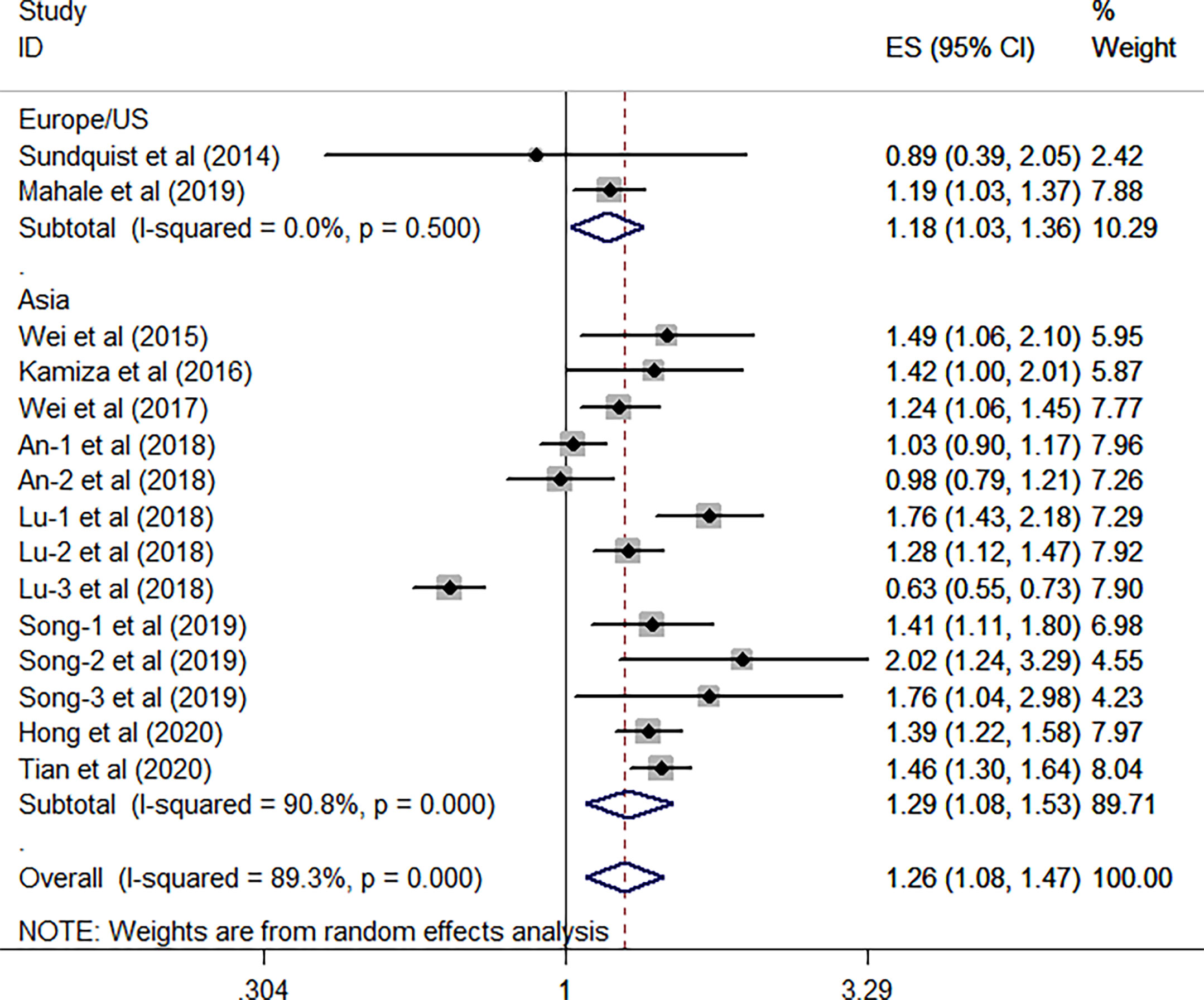
Figure 4 Forest plots of the subgroup analysis (research areas) on the association between HBV and gastric cancer.
As for HCV, the combined results from FIVE independent HR of each included study revealed that HCV infected patients was significantly associated with an increased risk of GC when compared WITH the general population without HCV infection (overall HR of five studies, 1.88; 95% CI, 1.28–2.76; P = 0.001; heterogeneity, I2 = 74.7%; P = 0.003) (Figure 5).
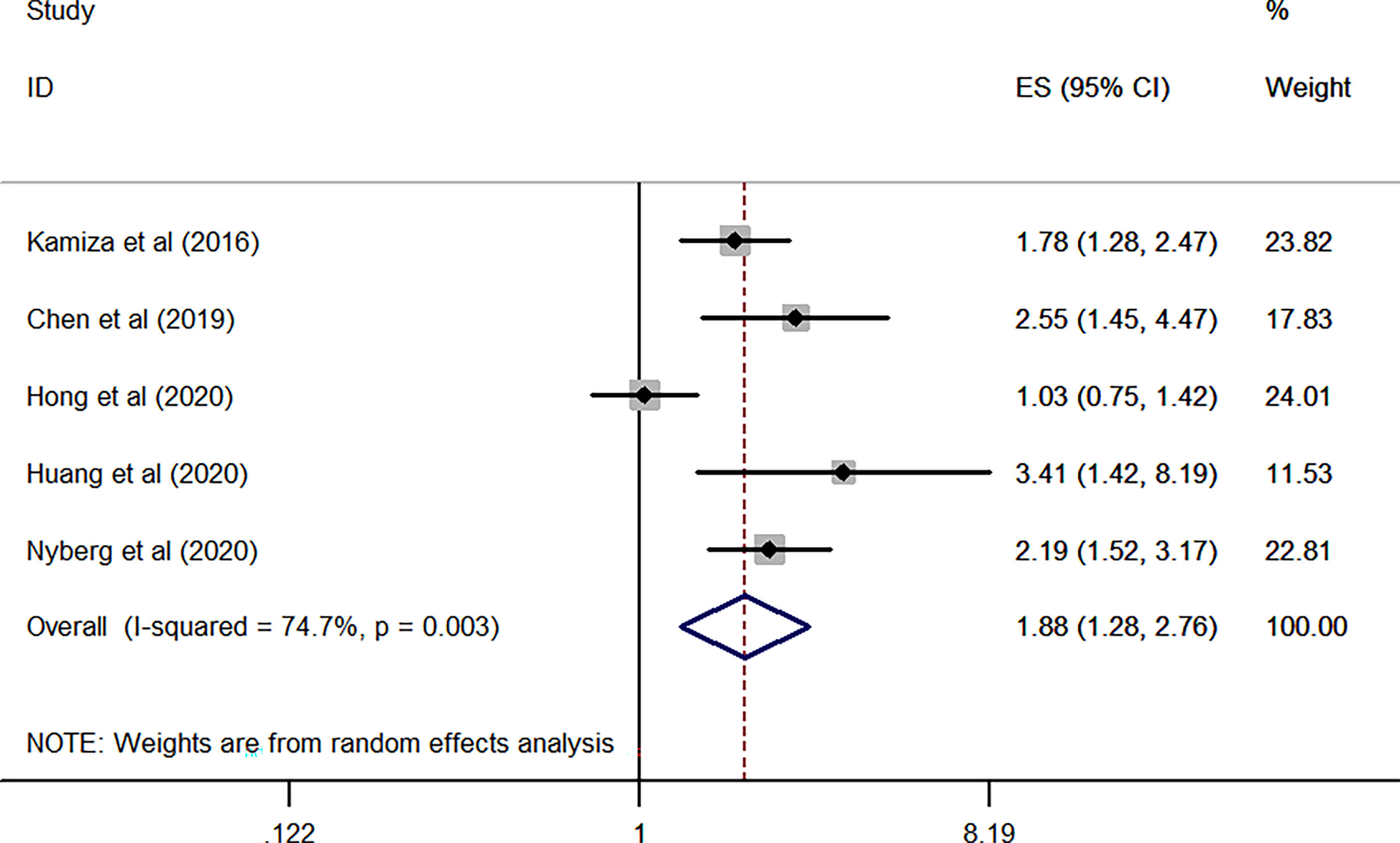
Figure 5 Forest plots of the pooled analysis of the included studies on the association between HCV and risk of gastric cancer.
Based on the above results, the stratified analysis revealed that patients with HCV infection exhibited an increased odds of GC as compared to those with HBV infection (adjusted HR, 1.88 vs 1.26).
In this study, sensitivity analysis from both of the studies of HBV and HCV was conducted to assess the influence of individual study on the newly generated overall HR. As listed in Table 2 and Figure 6A, there was no substantial change in the overall combined HR for those studies reporting the HBV cases, which ranged from 1.23 (95% CI, 1.053–1.437; P= 0.009) to 1.49 (95% CI, 1.059–2.097; P= 0.002) after excluding any of the studies. Besides, similar heterogeneity was observed after each exclusion, the I2 ranged from 67.4% to 90.1% (all P< 0.001). The abovementioned results indicated that no single study dominated the pooled HR and heterogeneity in those studies reporting HBV.
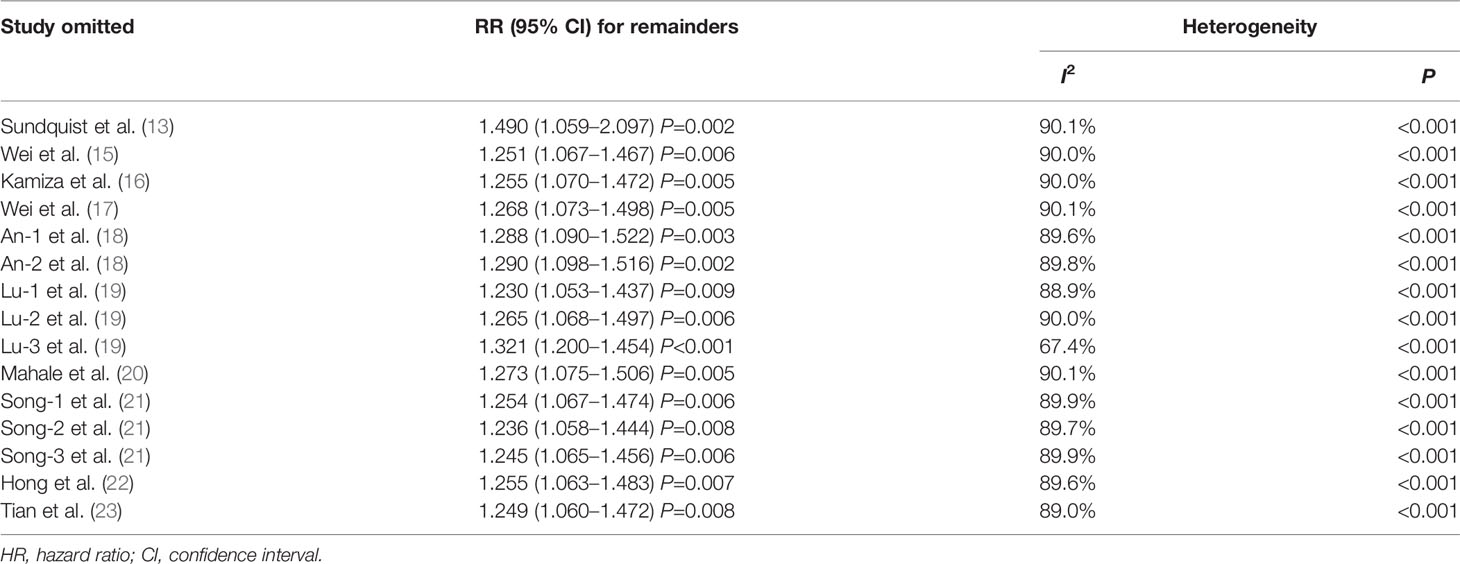
Table 2 Sensitivity analysis after each study was excluded by turns in those studies reporting HBV and risk of gastric cancer.
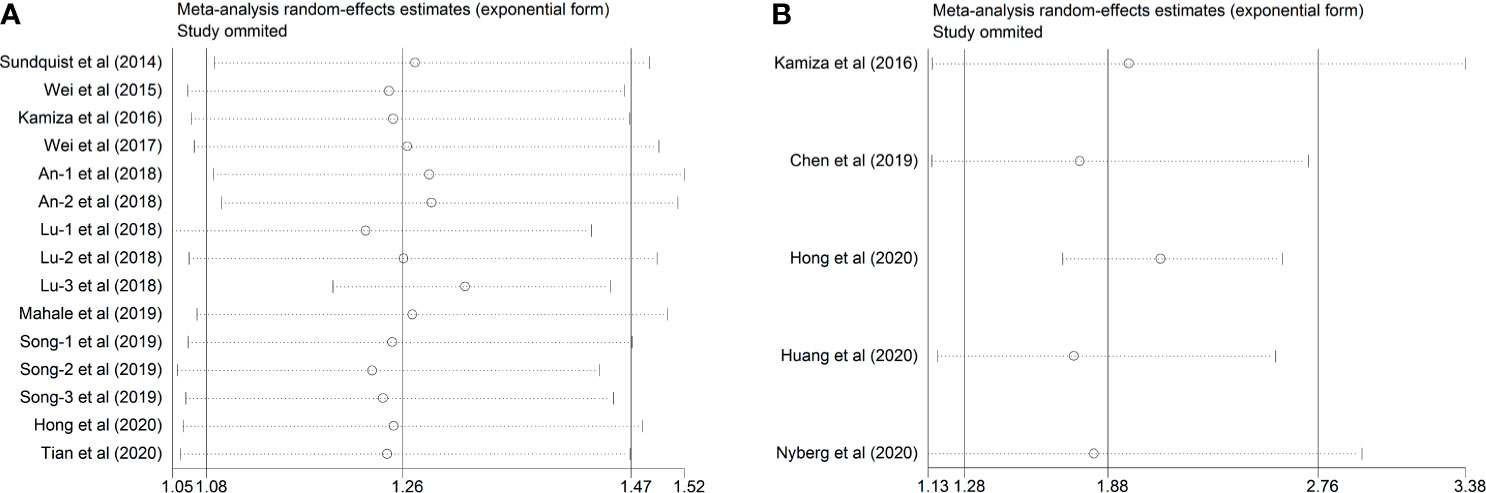
Figure 6 Sensitivity analysis after each study was excluded by turns. (A) Studies of HBV and GC (B) Studies of HCV and GC.
As for the five included studies reporting HCV, sensitivity analysis demonstrated that there was no remarkable variation in the pooled HR after omitting any one of the included studies the new HR ranged from 1.739 (95% CI, 1.169–2.586; P= 0.006) to 2.103 (95% CI, 1.693–2.612; P< 0.001). Intriguingly, the substantial heterogeneity disappeared after omitting the study conducted by Hong et al.’s study (22) (I2 = 0.0%, P= 0.444), suggesting that this study was the origin of the substantial heterogeneity of the combined effect (Table 3 and Figure 6B).

Table 3 Sensitivity analysis after each study was excluded by turns in those studies reporting HCV and risk of gastric cancer.
As displayed in Figure 7, the funnel plots indicated that no significant publication bias was found among the included studies reporting the HBV cases (Begg’s, P > |z| = 1.000; Egger, P > |t| = 0.455; 95% CI, −2.64 to 5.55). Similar to this finding, the funnel plots from five eligible studies reporting HCV infection cases also demonstrated that no significant publication bias existed in the combined HR (Begg’s, P > |z| = 0.221; Egger, P > |t| = 0.228; 95% CI, −4.13 to 11.61) (Figure 8).
Mounting studies have confirmed a significant positive association between virus infection and the development of various malignancies, i.e., Epstein-Barr (EB) virus in nasopharyngeal carcinoma (26), human papillomavirus (HPV) in cervical cancer (27), and human cytomegalovirus (HCMV) in glioblastoma (28). Type B viral hepatitis is one of the most common communicable diseases afflicting humans. It was estimated that 257 million persons worldwide have suffered from chronic HBV infection (29). Chronic HBV infection is the predominant cause of hepatocellular carcinoma (HCC). Besides, increasing evidence has postulated a potential relationship between HBV infection and risk of extrahepatic cancer, i.e., colorectal cancer (5), esophageal cancer (7), and pancreatic cancer (30). Moreover, recent studies also indicated that HBV infection might increase the risk of GC (31). Type C viral hepatitis is also one of the common infectious diseases originating from the liver. As reported, chronic HCV infection can induce the development of both HCC and extrahepatic malignancies (i.e., GC) because of the persistent inflammation caused by HCV (32). Over the past few decades, a growing number of studies have investigated the relationship between HBV/HCV infection and the tumorigenesis of GC but provided inconsistent results, and a quantified result is still scarce. Based on the pooled data from 12 relevant studies exploring the association between HBV infection and GC, the combined effect revealed that that HBV infected patients (HBsAg positive) were at 1.26-fold higher risk of GC than the general population without HBV infection with a statistical significance (RR, 1.26; 95% CI, 1.08−1.47; P = 0.003). This result was consistent with two previous meta-analyses related to this topic, which also supported a significant positive relationship between HBV infection and GC development (31, 33). Up to date, there is no meta-analysis yet that has been published on the issue of the association between HCV infection and the risk of GC. According to the present study with a pooled analysis of five included studies, the risk of GC in HCV-infected patients was increased by 88% compared with those without HCV infection (combined HR, 1.88; 95% CI, 1.28−2.76; P = 0.001). In the present study, sensitivity analyses showed that the quantification of the risk for GC in patients with either HBV or HCV remained prominently higher in all of the included studies (the HR in those studies reporting HBV ranged from 1.23 to 1.49; the HR in five studies reporting HCV ranged from 1.739 to 2.103), indicating that our findings were robust. In this study, all the HRs were adjusted for the common confounders, including the known risk factors for GC (e.g., Helicobacter pylori infection, chronic gastritis, and family history of GC) and some other factors, such as age, sex, geographical region, occupation, and comorbidities. Based on the abovementioned evidence, a significant positive relationship between HBV/HCV infection and GC development was verified. Also, HBV/HCV infection might be an independent risk factor for the tumorigenesis of GC.
In the subgroup analysis of the study design, studies with cohort design have verified the positive association between HBV infection and the high prevalence of GC but such association was eliminated in those studies with a case-control design. According to the epidemiologic investigation, a cohort is defined as studies that sample participants on the basis of exposure and assess the outcome during follow-up. In a cohort study, patients are sampled on the basis of exposure and are followed up over time, and the occurrence of outcomes is assessed. Case-control studies, the common analytical epidemiological studies, are the only practical approach for identifying risk factors for some diseases. Case-control studies lack temporality because they are retrospective designs. Therefore, the methodological quality and the level of evidence are higher in cohort studies as compared with case-control studies. Given this inconsistent outcome, further well-signed cohort studies are still warranted to validate the evidence of HBV infection predisposing to the development of GC. In the subgroup analysis of different research areas, pooled results from studies conducted in the Asian countries had a higher prevalence of GC when compared with the Europe/US. This phenomenon may be attributable to the prevalence and the disease severity of HBV infection, which are higher in Asian countries than those in Europe and the United States. However, few studies have been conducted to investigate the prevalence of GC in HBV infection patients in both Asian and European countries. Therefore, the etiology underlying this observation is still unknown.
The pathophysiological mechanisms of HBV/HCV-associated GC development remain elusive. The underlying mechanisms that existed in the association between HBV/HCV infection and the development of GC might be correlated with multiple etiologies, including chronic inflammation, systemic impairment of immune function, liver cirrhosis, and the direct effects of HBV/HCV proteins on oncogenesis and/or on tumor suppression genes (34, 35).
HBV is one of the most common hepatotropic viruses, which replicate in the hepatocytes. Viral oncogenic hepatitis B virus X proteins (HBX) have been confirmed to play a key role in the development of multiple cancers. Intriguingly, however, some investigators also found that HBX was significantly higher in GC cells than in healthy parts of the specimens (21). In addition, viral antigens and genomes have been identified in extra-hepatic tissues. Therefore, HBV might be not only harbored in the hepatocytes but also in the extrahepatic cells and thus induce local chronic inflammation of this organ subsequently, such as in the stomach. It was suggested that chronic inflammation is a crucial component of cancer progression (36). Local persistent inflammation caused by the HBV might promote the cancerous transformation of gastric epithelial cells. Chronic inflammation might also lead to genetic instability in cells and further elevate the genetic and epigenetic alterations. Supported by this evidence, Cui et al. found that HBV infection in epithelium could induce cellular atypia and generate a prominent lymphocytes’ infiltration in lamina propria, indicating the persistence of long-term inflammation context in the gastric tissues (31). Long-term chronic HBV infection might be concurrent with damage of gastric mucosa epithelial cells, thus promoting the development of GC (15, 37).
It is well known that immune dysregulation facilitates the development of cancer (38). The impaired immune system also plays a vital role in the development of HBV infection-associated GC. Infectious agents increase the risk of GC, which might be via the integration of their genomes into the host’s chromosomes or immune system inhibition (10). Song et al. (21) speculated the potential link between HBV infection and GC might be owing to the specific influence of the virus on the immune system or more vulnerable to a malicious attack on the gastric histiocytes. Chronic immune suppression may also participate in this pathological process. In China, a majority of HBV carriers infected HBV during their childhood, leading to long-term immune suppression, which may contribute to a higher incidence of GC. In contrast, those individuals never exposed to HBV or with antibody-positive hepatitis B surface (anti-HBs+) are immune to HBV, having a decreased risk of GC (23). In addition to the immune mechanisms, some relevant viral proteins and the intracellular cascades pathways function may also play role in the action of HBV infection-induced GC. The integration and mutation of the viral genome into the host cellular DNA contribute to the altered expression of some cellular genes, which can induce direct insertional mutagenesis of various cancer-related genes and thus lead to malignant transformation (17). The stomach is adjacent to the liver and thus may be infected by HBV. Several investigators observed that HBsAg presented in the gastric epithelial cells (15, 39), suggesting that HBV directly increased the risk of GC.
In addition to chronic inflammation and immune dysregulation, liver cirrhosis may also play an important role in the carcinogenesis of GC. According to the WHO, about 20% of the chronically HBV-infected patients will develop cirrhosis (40). HBsAg is proven to be an independent risk factor for liver cirrhosis (41), whereas cirrhosis has been reported to be a risk factor for GC (42). It was suggested that liver cirrhosis might cause hypoxia, which was not only a risk factor for GC but also a poor prognosis for patients with GC (43, 44). Besides, a meta-analysis that included 21 eligible studies showed that there was a significantly high prevalence of H. pylori infection in patients with liver cirrhosis (45). It is known that there is a close association between H. pylori infection and the development of GC (46). Therefore, there might be a chain reaction effect of “HBV–liver cirrhosis–H. pylori infection–GC”. Of note, although liver cirrhosis may play a role in increasing the risk of GC in HBV-infected people, the association between HBV infection and GC persisted among non-cirrhotic subjects (20). Therefore, more studies are still warranted to better illustrate this issue.
The positive association between HCV infection and the risk of GC shares many common pathogenetic risk factors, including chronic inflammation, immune dysfunction, cirrhosis, and the altered expression of the related oncogenes. It was reported that HCV acted as an indirect carcinogen for GC by promoting and maintaining a state of chronic inflammation in infected sites (47). HCV infection-induced chronic inflammation may lead to the progressive rearrangement of gastric tissue structure and thus promote the cancerous transformation (48). Valli De Re et al. (49) found that HCV infection might induce aberrant expression of MHC-I peptides, favoring the NK-mediated cell lysis and producing cytokines and chemokines, resulting in inflammation in the stomach. HCV might be also associated with the progressive development of a tissue necroinflammatory process in the infected-organs (i.e., the stomach), which might evolve toward malignant transformation (48). Immune and/or endocrine dysregulation after HCV infection might partially attribute to the increased risk of GC (11, 50). A previous study (49) demonstrated that a few proteins involved in oxidative stress responses were over-expressed in HCV-positive gastric tissue. The relationship between oxidative stress and cancer has been studied extensively (51), thus it is tempting to speculate that HCV may induce GC development and that oxidative stress involves in this action. Similar to one of the pathological mechanisms laid in HBV and GC, Nyberg et al. (25) found a significant relationship between the presence of cirrhosis and the increased GC risk via the multivariable analyses and suggested that the underlying mechanisms might correlate to the general immune response in cirrhosis. The direct action of some oncogenic viral proteins induced by HCV has also been implicated in tumorigenesis of various extrahepatic cancers (52), whereas whether this theory exists in the development of GC is still under investigation. Based on this evidence, further understanding of the possible mechanism laid in HBV infection and the risk of GC might provide a novel strategy to prevent the tumorigenesis and progression of GC.
In this systematic review and meta-analysis, we have not only addressed the association between HBV infection and GC development but also quantified the association between HCV infection and the risk of GC. However, an inherent limitation within this study should be noted during the interpretation in clinical practice. Statistical heterogeneity has been observed in this study, for either HBV or HCV pooled analysis. Inconsistency of study geographical area, study design, sample size, mean age, HBV/HCV-infected duration and severity, and the different variable adjustments could be partly responsible for such heterogeneity.
In conclusion, the present study demonstrated that both HBV and HCV infection was associated with a significantly higher risk of GC. HCV infection had a higher odds of GC development when compared to HBV infection. A few plausible mechanisms might explain the causality of HBV/HCV infection and the increased risk of GC, i.e., persistent inflammation, immune dysfunction, and cirrhosis. However, such causal association remains inconclusive among studies, thus further investigations are still required.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YY, ZJ, and WW contributed to conceive and design the study. LR and CY performed the systematic searching. YX and KW extracted the data. SZ and JM wrote the manuscript. YY and SZ supervised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the grants from Clinical Research Funding of Zhejiang Medical Association (ID: 2019ZYC-A182), Science and Technology Planning Project of Taizhou City, Zhejiang Province (ID: 1902ky37 and 20ywb40), the Scientific Research Project of Taizhou University (ID: 2017PY047), and the High-level Hospital Construction Research Project of Maoming People’s Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.703558/full#supplementary-material
ASR, age-standardized incidence rate; CI, confidence interval; GC, gastric cancer; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; NOS, Newcastle–Ottawa scale.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is Gastric Cancer Becoming a Rare Disease? A Global Assessment of Predicted Incidence Trends to 2035. Gut (2020) 69(5):823–9. doi: 10.1136/gutjnl-2019-320234
3. Anderson WF, Rabkin CS, Turner N, Fraumeni JJ, Rosenberg PS, Camargo MC. The Changing Face of Noncardia Gastric Cancer Incidence Among Us Non-Hispanic Whites. J Natl Cancer Inst (2018) 110(6):608–15. doi: 10.1093/jnci/djx262
4. Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global Burden of Gastric Cancer Attributable to Helicobacter Pylori. Int J Cancer (2015) 136(2):487–90. doi: 10.1002/ijc.28999
5. Su FH, Bai CH, Le TN, Muo CH, Chang SN, Te A, et al. Patients With Chronic Hepatitis C Virus Infection Are at an Increased Risk of Colorectal Cancer: A Nationwide Population-Based Case-Control Study in Taiwan. Front Oncol (2020) 10:561420. doi: 10.3389/fonc.2020.561420
6. Ponvilawan B, Charoenngam N, Rujirachun P, Wattanachayakul P, Tornsatitkul S, Rittiphairoj T, et al. Chronic Hepatitis C Virus Infection Is Associated With an Increased Risk of Lung Cancer: A Systematic Review and Meta-Analysis. Lung (2020) 198(4):705–14. doi: 10.1007/s00408-020-00365-y
7. Ponvilawan B, Rittiphairoj T, Charoenngam N, Rujirachun P, Wattanachayakul P, Tornsatitkul S, et al. Association Between Chronic Hepatitis C Virus Infection and Esophageal Cancer: A Systematic Review and Meta-Analysis. J Clin Gastroenterol (2021). doi: 10.1097/MCG.0000000000001532
8. Rong X, Wang H, Ma J, Pan S, Wang H, Jing S, et al. Chronic Hepatitis B Virus Infection Is Associated With a Poorer Prognosis in Diffuse Large B-Cell Lymphoma: A Meta-Analysis and Systemic Review. J Cancer (2019) 10(15):3450–8. doi: 10.7150/jca.31033
9. Kocoglu H, Karaca M, Tural D, Hocaoglu E, Okuturlar Y, Fetullahoglu Z, et al. Hepatitis B and C Rates Are Significantly Increased in Certain Solid Tumors: A Large Retrospective Study. J Cancer Res Ther (2018) 14:S774–8. doi: 10.4103/0973-1482.174544
10. Fattahi S, Nikbakhsh N, Taheri H, Ghadami E, Kosari-Monfared M, Amirbozorgi G, et al. Prevalence of Multiple Infections and the Risk of Gastric Adenocarcinoma Development at Earlier Age. Diagn Microbiol Infect Dis (2018) 92(1):62–8. doi: 10.1016/j.diagmicrobio.2018.04.015
11. Chen CW, Cheng JS, Chen TD, Le PH, Ku HP, Chang ML. The Irreversible HCV-Associated Risk of Gastric Cancer Following Interferon-Based Therapy: A Joint Study of Hospital-Based Cases and Nationwide Population-Based Cohorts. Therap Adv Gastroenterol (2019) 12:1756284819855732. doi: 10.1177/1756284819855732
12. Baghbanian M, Hoseini MS, Doosti M, Moghimi M. Association Between Gastric Pathology and Hepatitis B Virus Infection in Patients With or Without Helicobacter Pylori. Asian Pac J Cancer Prev (2019) 20(7):2177–80. doi: 10.31557/APJCP.2019.20.7.2177
13. Sundquist K, Sundquist J, Ji J. Risk of Hepatocellular Carcinoma and Cancers at Other Sites Among Patients Diagnosed With Chronic Hepatitis B Virus Infection in Sweden. J Med Virol (2014) 86(1):18–22. doi: 10.1002/jmv.23754
14. Wang H, Chen XZ, Chen XL, Zhang WH, Liu K, Wang YJ, et al. Associations Between Hepatitis B Virus Exposure and the Risk of Extrahepatic Digestive System Cancers: A Hospital-Based, Case-Control Study (SIGES). Cancer Med (2021) 10(11):3741–55. doi: 10.1002/cam4.3901
15. Wei XL, Qiu MZ, Jin Y, Huang YX, Wang RY, Chen WW, et al. Hepatitis B Virus Infection Is Associated With Gastric Cancer in China: An Endemic Area of Both Diseases. Br J Cancer (2015) 112(7):1283–90. doi: 10.1038/bjc.2014.406
16. Kamiza AB, Su FH, Wang WC, Sung FC, Chang SN, Yeh CC. Chronic Hepatitis Infection Is Associated With Extrahepatic Cancer Development: A Nationwide Population-Based Study in Taiwan. BMC Cancer (2016) 16(1):861. doi: 10.1186/s12885-016-2918-5
17. Wei XL, Luo HY, Li CF, Jin Y, Zeng ZL, Ju HQ, et al. Hepatitis B Virus Infection Is Associated With Younger Median Age at Diagnosis and Death in Cancers. Int J Cancer (2017) 141(1):152–9. doi: 10.1002/ijc.30719
18. An J, Kim JW, Shim JH, Han S, Yu CS, Choe J, et al. Chronic Hepatitis B Infection and Non-Hepatocellular Cancers: A Hospital Registry-Based, Case-Control Study. PloS One (2018) 13(3):e0193232. doi: 10.1371/journal.pone.0193232
19. Lu T, Yang Q, Li M, Zhang J, Zou J, Huang L, et al. HBV Infection and Extra-Hepatic Cancers in Adolescents and 20s: A Retrospective Study in China. Cancer Epidemiol (2018) 55:149–55. doi: 10.1016/j.canep.2018.05.012
20. Mahale P, Engels EA, Koshiol J. Hepatitis B Virus Infection and the Risk of Cancer in the Elderly US Population. Int J Cancer (2019) 144(3):431–9. doi: 10.1002/ijc.31643
21. Song C, Lv J, Liu Y, Chen JG, Ge Z, Zhu J, et al. Associations Between Hepatitis B Virus Infection and Risk of All Cancer Types. JAMA Netw Open (2019) 2(6):e195718. doi: 10.1001/jamanetworkopen.2019.5718
22. Hong CY, Sinn DH, Kang D, Paik SW, Guallar E, Cho J, et al. Incidence of Extrahepatic Cancers Among Individuals With Chronic Hepatitis B or C Virus Infection: A Nationwide Cohort Study. J Viral Hepat (2020) 27(9):896–903. doi: 10.1111/jvh.13304
23. Tian T, Song C, Jiang L, Dai J, Lin Y, Xu X, et al. Hepatitis B Virus Infection and the Risk of Cancer Among the Chinese Population. Int J Cancer (2020) 147(11):3075–84. doi: 10.1002/ijc.33130
24. Huang CF, Lai HC, Chen CY, Tseng KC, Kuo HT, Hung CH, et al. Extrahepatic Malignancy Among Patients With Chronic Hepatitis C After Antiviral Therapy: A Real-World Nationwide Study on Taiwanese Chronic Hepatitis C Cohort (T-COACH). Am J Gastroenterol (2020) 115(8):1226–35. doi: 10.14309/ajg.0000000000000606
25. Nyberg AH, Sadikova E, Cheetham C, Chiang KM, Shi JX, Caparosa S, et al. Increased Cancer Rates in Patients With Chronic Hepatitis C. Liver Int (2020) 40(3):685–93. doi: 10.1111/liv.14305
26. Peng H, Li Z, Long Y, Li J, Liu Z, Zhou R. Clinical Value of a Plasma Epstein-Barr Virus DNA Assay in the Diagnosis of Recurrent or Metastatic Nasopharyngeal Carcinoma: A Meta-Analysis. Biosci Rep (2019) 39(9):BSR20190691. doi: 10.1042/BSR20190691
27. McBride E, Tatar O, Rosberger Z, Rockliffe L, Marlow LM, Moss-Morris R, et al. Emotional Response to Testing Positive for Human Papillomavirus at Cervical Cancer Screening: A Mixed Method Systematic Review With Meta-Analysis. Health Psychol Rev (2020) 1–35. doi: 10.1080/17437199.2020.1762106
28. Foster H, Ulasov IV, Cobbs CS. Human Cytomegalovirus-Mediated Immunomodulation: Effects on Glioblastoma Progression. Biochim Biophys Acta Rev Cancer (2017) 1868(1):273–6. doi: 10.1016/j.bbcan.2017.05.006
29. Young J, Payton C, Walker P, White D, Brandeland M, Kumar GS, et al. Evaluation of a Program to Improve Linkage to and Retention in Care Among Refugees With Hepatitis B Virus Infection - Three U.S. Cities, 2006-2018. MMWR Morb Mortal Wkly Rep (2020) 69(21):647–50. doi: 10.15585/mmwr.mm6921a2
30. Liu X, Zhang ZH, Jiang F. Hepatitis B Virus Infection Increases the Risk of Pancreatic Cancer: A Meta-Analysis. Scand J Gastroenterol (2021) 56(3):252–8. doi: 10.1080/00365521.2020.1868568
31. Cui H, Jin Y, Chen F, Ni H, Hu C, Xu Y, et al. Clinicopathological Evidence of Hepatitis B Virus Infection in the Development of Gastric Adenocarcinoma. J Med Virol (2020) 92(1):71–7. doi: 10.1002/jmv.25584
32. Tang L, Marcell L, Kottilil S. Systemic Manifestations of Hepatitis C Infection. Infect Agent Cancer (2016) 11:29. doi: 10.1186/s13027-016-0076-7
33. Wang H, Chen XL, Liu K, Bai D, Zhang WH, Chen XZ, et al. Associations Between Gastric Cancer Risk and Virus Infection Other Than Epstein-Barr Virus: A Systematic Review and Meta-Analysis Based on Epidemiological Studies. Clin Transl Gastroenterol (2020) 11(7):e00201. doi: 10.14309/ctg.0000000000000201
34. He Y, Mao M, Shi W, He Z, Zhang L, Wang X. Development and Validation of a Prognostic Nomogram in Gastric Cancer With Hepatitis B Virus Infection. J Transl Med (2019) 17(1):98. doi: 10.1186/s12967-019-1841-3
35. Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-Induced IgA+ Cells Dismantle Anti-Liver Cancer Immunity. Nature (2017) 551(7680):340–5. doi: 10.1038/nature24302
36. Coussens LM, Werb Z. Inflammation and Cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
37. Huang J, Magnusson M, Torner A, Ye W, Duberg AS. Risk of Pancreatic Cancer Among Individuals With Hepatitis C or Hepatitis B Virus Infection: A Nationwide Study in Sweden. Br J Cancer (2013) 109(11):2917–23. doi: 10.1038/bjc.2013.689
38. Oh JK, Weiderpass E. Infection and Cancer: Global Distribution and Burden of Diseases. Ann Glob Health (2014) 80(5):384–92. doi: 10.1016/j.aogh.2014.09.013
39. Chen NL, Bai L, Deng T, Zhang C, Kong QY, Chen H. Expression of Hepatitis B Virus Antigen and Helicobacter Pylori Infection in Gastric Mucosa of Patients With Chronic Liver Disease. Hepatob Pancreat Dis Int (2004) 3(2):223–5. doi: 10.1016/j.diagmicrobio.2018.04.015
40. Sulkowski MS. Viral Hepatitis and HIV Coinfection. J Hepatol (2008) 48(2):353–67. doi: 10.1016/j.jhep.2007.11.009
41. Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, et al. Prediction Models of Long-Term Cirrhosis and Hepatocellular Carcinoma Risk in Chronic Hepatitis B Patients: Risk Scores Integrating Host and Virus Profiles. Hepatology (2013) 58(2):546–54. doi: 10.1002/hep.26385
42. Zullo A, Romiti A, Tomao S, Hassan C, Rinaldi V, Giustini M, et al. Gastric Cancer Prevalence in Patients With Liver Cirrhosis. Eur J Cancer Prev (2003) 12(3):179–82. doi: 10.1097/00008469-200306000-00002
43. Lin S, Ma R, Zheng XY, Yu H, Liang X, Lin H, et al. Meta-Analysis of Immunohistochemical Expression of Hypoxia Inducible Factor-1alpha as a Prognostic Role in Gastric Cancer. World J Gastroenterol (2014) 20(4):1107–13. doi: 10.3748/wjg.v20.i4.1107
44. Zheng F, Du F, Zhao J. Clinicopathological Differences and Prognostic Value of Hypoxia-Inducible Factor-2alpha Expression for Gastric Cancer: Evidence From Meta-Analysis. Medicine (Baltimore) (2016) 95(7):e2871. doi: 10.1097/MD.0000000000002871
45. Feng H, Zhou X, Zhang G. Association Between Cirrhosis and Helicobacter Pylori Infection: A Meta-Analysis. Eur J Gastroenterol Hepatol (2014) 26(12):1309–19. doi: 10.1097/MEG.0000000000000220
46. Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter Pylori Infection With Gastric Carcinoma: A Meta-Analysis. Am J Gastroenterol (1999) 94(9):2373–9. doi: 10.1111/j.1572-0241.1999.01360.x
47. Chen CJ, Hsu WL, Yang HI, Lee MH, Chen HC, Chien YC, et al. Epidemiology of Virus Infection and Human Cancer. Recent Results Cancer Res (2014) 193:11–32. doi: 10.1007/978-3-642-38965-8_2
48. Fiorino S, Bacchi-Reggiani L, de Biase D, Fornelli A, Masetti M, Tura A, et al. Possible Association Between Hepatitis C Virus and Malignancies Different From Hepatocellular Carcinoma: A Systematic Review. World J Gastroenterol (2015) 21(45):12896–953. doi: 10.3748/wjg.v21.i45.12896
49. De Re V, Simula MP, Cannizzaro R, Sansonno D, Canzonieri V, Gloghini A, et al. HCV Inhibits Antigen Processing and Presentation and Induces Oxidative Stress Response in Gastric Mucosa. Proteomics Clin Appl (2008) 2(9):1290–9. doi: 10.1002/prca.200800059
50. Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic Morbidity and Mortality of Chronic Hepatitis C. Gastroenterology (2015) 149(6):1345–60. doi: 10.1053/j.gastro.2015.08.035
51. Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell (2020) 38(2):167–97. doi: 10.1016/j.ccell.2020.06.001
Keywords: gastric cancer, hepatitis B virus, hepatitis C virus, cumulative analysis, risk
Citation: Yang Y, Jiang Z, Wu W, Ruan L, Yu C, Xi Y, Wang L, Wang K, Mo J and Zhao S (2021) Chronic Hepatitis Virus Infection Are Associated With High Risk of Gastric Cancer: A Systematic Review and Cumulative Analysis. Front. Oncol. 11:703558. doi: 10.3389/fonc.2021.703558
Received: 30 April 2021; Accepted: 21 June 2021;
Published: 08 July 2021.
Edited by:
Nicola Schiavone, University of Florence, ItalyReviewed by:
Ashraf A. Tabll, National Research Centre, EgyptCopyright © 2021 Yang, Jiang, Wu, Ruan, Yu, Xi, Wang, Wang, Mo and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shankun Zhao, emhhb3NoYW5rdW5AdHpjLmVkdS5jbg==; orcid.org/0000-0002-0305-3912
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.