
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 09 November 2021
Sec. Radiation Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.700710
 Ping Jiang1†
Ping Jiang1† Lijuan Zou2†
Lijuan Zou2† Lichun Wei3†
Lichun Wei3† Guanghui Cheng4
Guanghui Cheng4 Baosheng Sun5
Baosheng Sun5 Fuquan Zhang6
Fuquan Zhang6 Ruoyu Wang7
Ruoyu Wang7 Tiejun Wang8
Tiejun Wang8 Ang Qu1
Ang Qu1 Xiangkun Yuan9
Xiangkun Yuan9 Bin Qiu1
Bin Qiu1 Shuhua Wei1
Shuhua Wei1 Zi Liu10*
Zi Liu10* Yunyan Zhang11*
Yunyan Zhang11* Junjie Wang1*
Junjie Wang1*The treatment modality for recurrent cervical cancer (rCC) is limited, and the prognosis of these patients is poor. Seed implantation could be an important component of rCC management in the context of dose boost or salvage therapy after surgery or radiotherapy, which is characterized by a minimally invasive, high local dose, and rapidly does fall, sparing normal tissue. For patients with good performance status and lateral pelvic wall recurrence with an available puncture path, seed implantation was recommended, as well as for selected central pelvic recurrence and extra-pelvic recurrence. The combination of brachytherapy treatment planning system and CT guidance was needed, and three-dimensional printing templates could greatly improve the accuracy, efficiency, and quality of seed implantation to achieve a potential ablative effect and provide an efficient treatment for rCC. However, the recommendations of seed implantation were mainly based on retrospective articles and lack high-quality evidence, and multicenter prospective randomized studies are needed. In this consensus on iodine125 seed implantation for rCC, indication selection, technical process and requirements, dosimetry criteria, radiation protection, combined systemic therapy, and outcomes of seed implantation for rCC are discussed.
Cervical cancer (CC) is the fourth most common female malignancy worldwide. The management for CC includes radical resection (with or without adjuvant radiotherapy) and concurrent chemoradiotherapy (1, 2). The prognosis of patients with complete resection or complete remission after surgery or radiotherapy is excellent, with a 5-year survival rate of about 90% for early-stage and 70% for advanced-stage CC (1). However, recurrent CC (rCC) is still reported in 10–20% of patients with stage IB–IIA and in 40–70% locally advanced disease after initial treatment (3–5). It was found that 31% of the patients relapsed between 18 and 24 months, 58% within 1 year, and 76% within 2 years, and only 6% of these patients with recurrence survived for 3 years (6). Patients with positive pelvic lymph nodes, parametrium invasion, and positive surgical margin were associated with a high risk of postoperative recurrence (7). The percentages of pelvic recurrences fluctuate from 10–74%, depending on different risk factors (3). Recurrences that were distant or detected at multiple sites occurred in 15–61% of patients (4). Most of the patients who developed rCC within 2 years after treatment are associated with a poor prognosis, and most of those patients died of uncontrolled tumors.
The presentation of rCC after initial treatment included local/regional recurrence, distant metastasis, or both, which can be divided into two types: intra-pelvic (IPR) and extra-pelvic recurrence (EPR). The therapy varies from rCC type, mainly including pelvic exenteration, concurrent chemo-radiotherapy (referring to external beam radiotherapy, EBRT), and brachytherapy (BT), while it is still lacking optimal salvage solutions for inoperable and irradiated rCC. Pelvic exenteration is only recommended for select patients yielding a 5-year survival rate of 21%–73%, but the resectable rate is less than 20% and the incidence of postoperative complications is high, with a median survival time of only 7–9 months and a 5-year survival rate of <10% (3, 8).
BT could be an important component of rCC management, with the advantages of highly focused and conformal dose distribution and few damages to the surrounding normal tissues. According to the dose rate, BT could be divided into high-dose-rate BT (HDR-BT) and low-dose-rate BT (LDR-BT); the comparison of HDR-BT and LDR-BT is shown in Table 1. At present, HDR-BT with after-loading is mainly used for the primary treatment of CC (9), endometrial cancer (10), breast cancer (11, 12), skin cancer (13), and prostate cancer (14). LDR-BT (mainly referred to as seed implantation) is commonly used for the treatment of various recurrent cancers, such as recurrent head and neck cancers (15, 16), lung cancer (17), rectal cancer (18), and rCC (19, 20). This Chinese expert consensus was focused on iodine125 seed implantation for rCC.
The members of the Brachytherapy and Intelligent Radiotherapy Branch of Chinese Nuclear Society, Chinese Society of Radiation Oncology, Chinese Medical Doctor Association Brachytherapy Professional Committee, and Chinese Northern Radioactive-Seed Brachytherapy Group carried out a literature search. The literature search was using the keywords “brachytherapy” or “seed implantation” and “cervical cancer” and included studies from 1990 to January 2021 as published in PubMed, Embase, ScienceDirect, and Chinese databases. The evidence was then analyzed, and the opinions and suggestions of the experts were formed. The leader organized the experts to write the primary draft, then sent it to all the members for extensive soliciting of opinions, and finally formed a consensus through centralized discussion.
The clinical diagnosis of rCC is indicated by symptoms such as weight loss, lower extremity edema, pelvic/lower extremity pain, vaginal bleeding, or routine examination after initial treatment. Image evaluation included ultrasound, CT/MRI scan, and PET-CT in certain circumstances. The final diagnosis relied on pathological confirmation by biopsy under ultrasound or CT guidance. Given the very low proportion of operable patients and recent advances in three-dimensional printing template (3D-PT) and BT technology, individualized and precise treatments are available. IPR may be further subdivided into central pelvic recurrence (CPR) and lateral pelvic wall recurrence (LPR) (21), as shown in Table 2. CPR is defined as a recurrent tumor located in the center or midline of the pelvis that may invade anterior (bladder), posterior (rectum), or lateral (vaginal vault) structures, but not the pelvic wall. LPR is defined as invasion of the pelvic wall by the recurrent tumor or adhesion to the pelvic wall or direct invasion of the pelvic wall. EPR includes recurrence in the retroperitoneal lymphatic drainage area, supraclavicular and axillary lymphatic drainage area, mediastinum lymph drainage area, and inguinal lymph node and distance organ metastasis.
For patients with rCC after radical resection who have not previously undergone radiation therapy, the treatment options include pelvic exenteration and concurrent chemoradiotherapy ± BT. Clinical studies comparing the two treatment modalities are absent, and a multidisciplinary discussion is recommended. Pelvic exenteration is usually indicated for selected patients with CPR (2). The 5-year survival rates for patients with CPR are ranging from 6 to 77%, and patients with CPR seem to have a better prognosis compared to those with LPR (3). However, it is difficult to radically remove LPR by surgery when the tumor is invading the pelvic wall. The effect of neoadjuvant therapy is not defined, and evidence for intraoperative radiotherapy is lacking and not routinely recommended. Concurrent chemoradiotherapy (grade IIIC recommendation) was also recommended for rCC (CPR/LPR) (2). Image-guided radiotherapy may be recommended to ensure accurate irradiation as well as dose-boost of the target area while minimizing the dose to the intestine and other organs at risk (OAR). However, EBRT is still limited by the dose tolerated by normal tissues and the anatomical change after resection, making it usually difficult to meet the curative intent, especially for LPR. Despite the very good results obtained with EBRT and the fact that there has been a trend toward reducing the use of BT, the absence of BT resulted in a high-risk rate of local recurrence (14). Evidence indicated that EBRT seemed not better than BT for dose boost in locally advanced CC, usually with a local lesion boost of 10–20 Gy after 45–50 Gy EBRT (22). Iodine125 seed implantation has obvious advantages in therapeutic dose boost. Iodine125 seed implantation could be used alone or combined with EBRT for LPR but not encouraged for CPR (only used for selected patients with mass that has a boundary from the vagina).
The National Comprehensive Cancer Network (NCCN) guidelines provide treatment recommendations for patients with different types of rCC after radiotherapy (2). Pelvic exenteration ± intraoperative radiotherapy could be considered for CPR patients, and BT could be selected for patients with smaller CPR lesions, which is a highly complex procedure and should be performed in high-volume centers (2). As most of the patients who developed rCC after radiotherapy is in the primary locally advanced stage, the usage of pelvic exenteration is limited. Among strictly screened patients, the 5-year survival rate was 30–60% for pelvic exenteration, while the incidence of complications was high, the perioperative mortality rate was 1–10%, and the quality of life decreased significantly (23–25). No preferred treatment is recommended for LPR after EBRT with a 5-year overall survival (OS) rate of <10% and median OS of 7–9 months in general (26). Pelvic exenteration is usually not suitable (27). Given the difficulty in increasing the dose of the clinical target volume (CTV) and dose limitation to OAR for rCC patients after pelvic radiotherapy, re-EBRT and dose boost are difficult due to the dose limitation of OAR (28, 29), while BT is expected to benefit these patients. Seed implantation is characterized by a minimally invasive, high local dose and rapidly does fall. With the advancement of 3D technology, the CTV is accurately determined. 3D-PT is applied in many Chinese BT centers to assist seed implantation with high accuracy (15). It is reasonable to recommend seed implementation only for residual tumors after EBRT.
Iodine125 seed implantation has become one of the standard therapies for early prostate cancer, which is comparable to surgery and EBRT, and has been recommended by the NCCN guidelines (30–32). However, iodine125 seed implantation is previously rarely reported for rCC. Until 2002, Chinese scholars initialed seed implantation to manage rCC, while several disadvantages shadowed the wide clinical practice: (1) owing to the poor controllability of the needle angle and direction and seed distribution, the learning curve of the clinician was quite long, (2) interference of OAR—radiation distributions to CTV were frequently hard to meet the pre-planning, and (3) frequent repeated CT scan during the implantation increased patient radiation exposure, while ultrasound guidance is two-dimensional imaging with poor accuracy (33–36). Since 2015, individualized 3D-PT was successfully developed to facilitate seed implantation in China (37). 3D-PT was divided into 3D printing co-planar template (3D-PCT) and 3D printing non-co-planar template (3D-PNCT) (Figure 1). 3D-PCT applies to the BT with all parallel needle track implants; 3D-PNCT applies to the seed implantation with non-coplanar needle track implants. The technical characteristics of 3D-PT are presented in Table 3. The combination of 3D-PT with CT guidance could greatly improve the accuracy, efficiency, and quality of seed implantation to achieve an ablative effect and provide a new and efficient salvage treatment for rCC (15, 37, 38). Until now, the technical process, criteria, and clinical application of 3D-PT-assisted seed implantation for rCC have been discussed in this consensus (20, 39).
(1) Age 18–80 years old with Karnofsky Performance Status ≥80;
(2) Pathologically confirmed rCC or residual tumor in patients who are intolerant or refuse surgery [with a diameter of ≤7 cm (35)];
(3) No systemic metastasis or with stable metastasis after systematic treatment (number of lesions ≤3);
(4) Expected survival ≥3 months;
(5) Puncture path is available, and the estimated pre-plan could meet the prescription dose requirements; and
(6) Patients tolerated anesthesia and the seed implantation procedure.
(1) Severe coagulation disorders with a bleeding tendency (platelets ≤50 × 109/L, prothrombin time >18 s, or prothrombin activity <40%);
(2) Anticoagulant therapy and/or antiplatelet aggregation drugs are taken within 1 week before seed implantation;
(3) Serious complications: severe diabetes, hypertension, heart, lung, renal function insufficiency, infectious period, or immunocompromise;
(4) Patients in a compulsive position, unable to coordinate, and unable to tolerate anesthesia and puncture; and
(5) Tumor invading the rectum or with fistula formation or invading the skin or with skin ulceration.
(1) Complicated with extensive systemic metastasis or significant local pain;
(2) Allergy to iodine contrast agents; and
(3) Paralysis due to local compression of the spinal cord by the tumor.
(1) Prescription dose of seed implantation alone: gross tumor volume (GTV), 110–130 Gy; clinical tumor volume (CTV), 90–110 Gy. The prescription dose of seed implantation combined with/after EBRT: 90–110 Gy for the GTV and 70–90 Gy for the CTV;
(2) Image fusion is recommended for enhanced CT, MRI, and/or PET-CT, with a scanning slice thickness of 5 mm; CTV is formed with a 5- to 6-mm security margin to the GTV; seed activity, 0.4–0.5 mCi.
(3) OAR dose limitation: the dose parameters of OAR during seed implantation for prostate cancer may be used for reference. Bowel D2cc (maximum doses that covered 2-cm3 volume) < 100% of prescribed dose, D0.1cc (maximum doses that covered 0.1 cm3 volume) <200 Gy (35). Rectum D2cc <100% of the prescription doses; D0.1cc <200 Gy (35). Urethral D10% (maximum dose received by 10% of urethral volume) <150% of prescribed dose, D30% (maximum dose received by 30% of urethral volume) <130% of prescribed dose (35). When the recurrent tumor is adjacent to the spine or invades the spinal cord, attention should be paid to prevent nerve injury. At present, the specific dose threshold for nerve injury is still unclear, while the quantitative analyses of normal tissue effects in the clinic dose limitation for the spinal cord is recommended, with the estimated risk of myelopathy being <1 and <10% at 54 and 61 Gy, respectively (40). It is recommended to maintain a 1-cm distance away from the spinal cord during seed implantation and control the seed activity below 0.5 mCi when the spinal cord is nearby.
The work flow includes the following steps, each of which requires strict quality control to ensure that the seeds are accurately implanted (39, 41) (the seed implantation requirements for rCC at different locations are recommended in Table 4 and Figure 2):
(1) Preoperative preparation: preoperative evaluation, practicing for the position needed during seed implantation, skin preparation, bowel preparation (EPR/LPR/residue), indwelling catheter (LPR/residue), indwelling vaginal dilator (LPR/residue), etc.;
(2) Positioning and fixation: supine or prone position fixation using vacuum pad, enhanced CT scan (slice thickness, 5 mm); setting the tumor center according to the laser rays and mark on the body surface of the patient and vacuum pad;
(3) Preplan: transmit the CT scan images to the brachytherapy treatment planning system (BT-TPS) for atlas of GTV/CTV and definition of OAR; preplan by the physicist according to prescription dose and the dose limitation of OAR given by the clinician;
(4) 3D-PT assistance (optional): the preplan data in the BT-TPS was then transferred into 3D imaging and reverse engineering software for simulation of individualized 3D-PT with a coordinate system, locking needles (used to fix the template)/seed needle pathway (15), and the body surface information of the treatment area; print the 3D-PT according to the simulation using 3D light-cured rapid-forming printer (39);
(5) Patient reposition with the 3D-PT using CT scan to verify the position of locking needles according to the preplan, if the error of the needle tip distance between preoperative CT and intraoperative CT is ≤4 mm, continue to insert other seed needles; if the error is >4 mm, adjust the template position and repeat the above-mentioned steps (39);
(6) Needle insertion: verify the seed needle position by CT scanning after the seed needles are inserted until the error is ≤4 mm; an intraoperative replan may be conducted, if necessary, according to the position of the inserted seed needles: intraoperative real-time CT scan, transmit the image to the BT-TPS, intraoperative needle track verification, real-time planning, and optimization compared with the preplan;
(7) Seed implantation: implant the seed one by one according to the preplan, and the seed needle is removed from the body after seed implantation;
(8) Post-plan: post-operative CT scan: perform CT scan immediately after the seed implantation and transmit the CT images to the BT-TPS for dosimetry evaluation after the seed needle is completely removed (35);
(9) Postoperative care: compress hemostasis and bandaging after removing the insertion needle and template, and send the patient back to the observation room; then, patients with spinal anesthesia should return to the ward with electrocardiography and blood pressure monitoring; and
(10) Follow-up: follow-up is started from the time of seed implantation, and tumor response was first evaluated at 4 weeks and then every 3–6 months thereafter with CT/MRI.
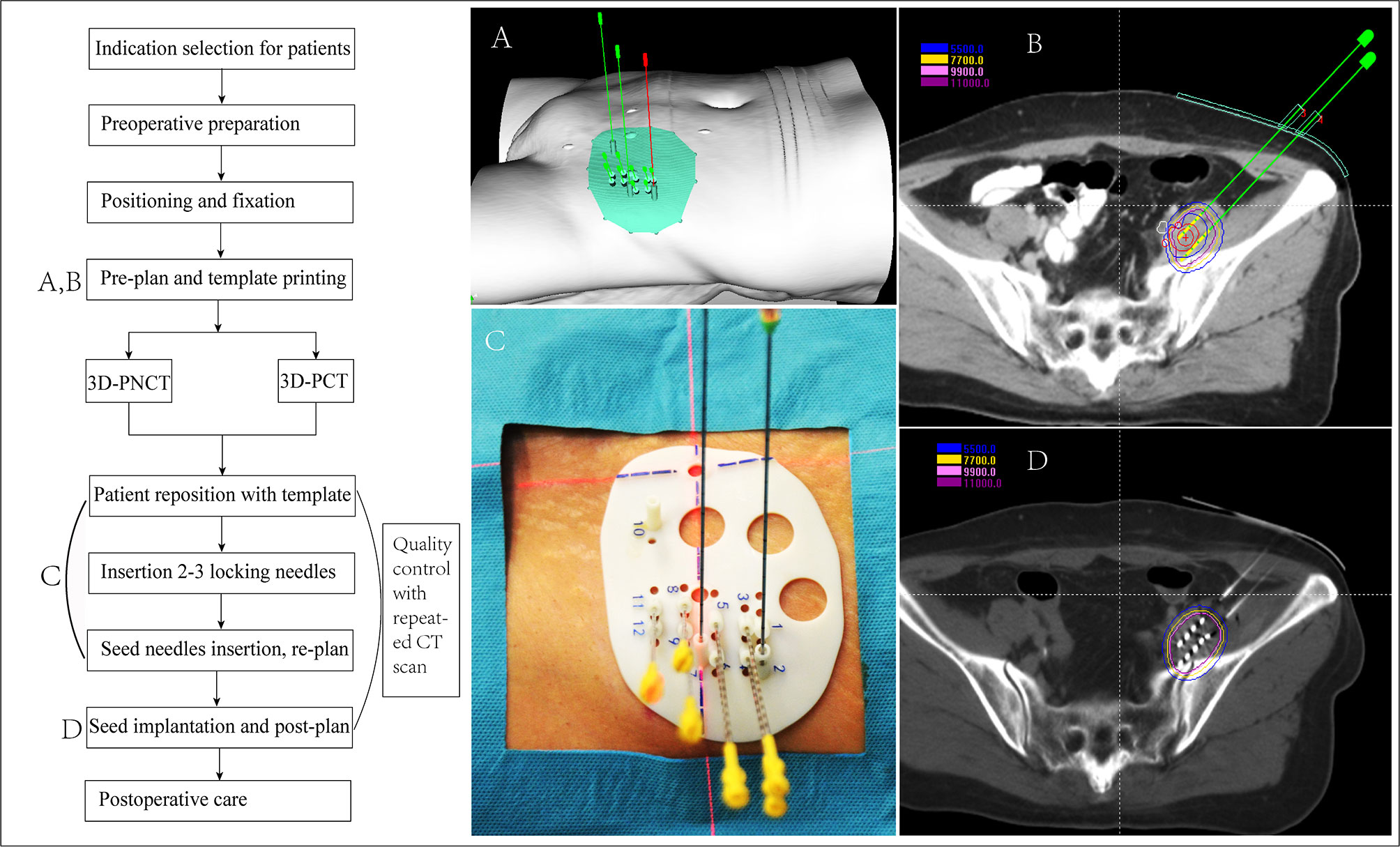
Figure 2 Flow chart of 3D printing template-assisted CT-guided iodine125 seed implantation for recurrent cervical cancer. 3D-PCT, 3D printing co-planar template; 3D-PNCT, 3D printing non-co-planar template.
EPR includes recurrence in lymph nodes and distant organ metastasis. The recurrence rate after concurrent chemoradiotherapy is about 2–12%, and the prognosis is poor (42). For patients with oligometastatic EPR (curative intent) or symptomatic disseminated EPR (palliative intent) for which EBRT was not suitable, seed implantation should be recommended and evaluated. Seed implantation is feasible in patients with EPR after chemoradiotherapy, especially for patients with symptomatic compression by the tumor.
The patients are in supine position with local anesthesia. A 3D-PNCT/3D-PCT may be used. The seed distribution was 1 cm away from the great vessels and spinal cord, and the activity of the seed should be controlled at 0.4–0.5 mCi. The prescription dose was recommended with 110–160 Gy.
The patients are in prone position with local anesthesia. 3D-PNCT should be used. The seed distribution was 1 cm away from the great vessels and spinal cord, and the activity of the seed should be controlled at 0.4–0.5 mCi. The prescription dose was recommended with 110–160 Gy.
The patients were in supine position, and local anesthesia was performed. Both head and body fixation techniques were used with 3D-PNCT. The iodine125 seed distribution was 1 cm away from the great vessels and brachial plexus, and the activity was controlled at 0.4–0.5 mCi. Pre-operative MRI may be used to atlas OAR such as the brachial plexus.
The half-life of the seed is 59.6 days. The energy is reduced to half of the initial value after 60 days, 10% of the initial value after 6 months, and negligible after 1 year. After seed implantation, the patient should wear the lead vest, collar, or abdominal belt of 0.25-mm-lead equivalent at the implantation site. It is recommended that the discharged patient should maintain a 1-m distance during prolonged contact with their attendant or visitor for 2 months. The patients should not live in a room with children and pregnant women and should not contact or hug children during the first year after seed implantation.
Platinum-based chemotherapy combined with local therapy is recommended as the first-line treatment for rCC, and the response rate is about 17–30% (43). The response rate was highest for cisplatin in combination with paclitaxel. The survival time of this regimen is reported to be about 13 months, while only 10.0–10.3 months for other combination regimens (44). For those who have previously used cisplatin, carboplatin combined with paclitaxel is recommended; generally, four to six cycles are appropriate. The order of seed implantation and chemotherapy was not well defined, while chemotherapy was usually conducted before (after) seed implantation for patients with relatively diffused (limited) lesions. Targeted therapy was reported in the treatment of CC, and GOG240 III phase clinical study is a landmark advance. Bevacizumab or topotecan combined with paclitaxel improved the survival time from 13.3 to 17 months, with mild adverse reactions [84]. After the local treatment of rCC, first-line chemotherapy combined with bevacizumab is recommended. In case of failure after the first-line chemotherapy, a combination with a second-line chemotherapy regimen is recommended.
The outcomes of seed implantation for rCC are largely obtained from a single-center retrospective study. The only prospective study (45) by Wang et al. was conducted on 62 patients with rCC after surgery. The patients were randomized into iodine125 seed implantation (n = 30) and concurrent chemoradiation with EBRT (n = 32). The local control rats at 1, 3, 6, and 12 months were higher for iodine125 seed implantation than concurrent chemoradiation with EBRT (76.7 vs. 65.6%, 80.0 vs. 65.5%, 83.3 vs. 62.5%, and 86.7 vs. 71.9%, respectively). The median OS was 4.34 vs. 3.59 years, and the 1-, 2-, 3-, 4-, and 5-year OS were 96.7 vs. 81.3%, 93.3 vs. 71.9%, 86.7 vs. 62.5%, 71.9 vs. 56.3%, and 65.6 vs. 53.1%, respectively. Han et al. (46) reported 17 patients with rCC who received CT-guided iodine125 seed implantation with a median follow-up time of 9.5 months, six patients of whom had a complete response, four patients had a partial response, and seven patients had a progressive disease. The clinical efficacy rate as 58% (10/17). No patient had complications of radiation injury. The rate of 6-month and 1-year survival period was 74.8 and 18.3%, respectively. Compared to patients who responded ineffectively to radioactive seed implantation, patients who responded effectively to radioactive seed implantation had a longer survival period (median 7.2 vs. median 10.4), in which the difference was statistically significant (P = 0.038). Tong et al. (47) evaluated iodine125 seed implantation for 35 patients with rCC after EBRT with a median follow-up of 16 months. The 1-, 3-, 6-, 12-, and 18-month local control rates were 84.5, 74.2, 60.0, 55.5, and 33.3%, respectively. The symptoms significantly improved after implantation. The median local tumor progression-free survival and OS times were 7 months (range, 1–19 months) and 12 months (range, 2–42 months), respectively. The 1- and 2-year OS rates were 65.5 and 43.6%, respectively. Two patients showed grade 3 and 4 toxicity, one patient had a rectovaginal fistula, one patient had incomplete intestinal obstruction, and three cases showed seed migration. No grade 5 event occurred.
3D-PT-assisted CT-guided iodine125 seed implantation is accurate, and it was feasible to obtain favorable dosimetry. Yuan et al. (48) reported 21 patients with postoperative rCC, and they were randomly divided into two groups. One group with 11 patients received 3D-PT, and the other 10 patients received free-hand seed implantation. The D2cm3 value of the bladder, rectum, sigmoid colon, and bowel was significantly decreased in the 3D-PT group compared with free-hand seed implantation. 3D-PT guidance has obvious dosimetry advantages in the treatment of rCC and is associated with shorter treatment duration and better repeatability. Qu et al. (39) have investigated the accuracy of needle distribution and dosimetric parameter differences of 3D-PNCT-assisted CT-guided iodine125 seed implantation in 38 recurrent gynecological cancer patients with non-central pelvic recurrence between pre-operative plan and post-operative plan. All patients had successfully received 3D-PNCT-assisted seed implantation. No significant differences were shown in D90, D100, V100, V150, V200, and the homogeneity index between pre-operative and post-operative plans. Only a few patients suffered from ≤grade 2 toxicities. Liu et al. (20) reported 103 patients with rCC after EBRT who underwent 3D-PT-assisted CT-guided iodine125 seed implantation. The median prescription dose was 120 Gy. The median OS was 17 months, and the 3-year local control rate was 75.1%. Grade 2 adverse events of acute nausea, diarrhea, and pollakiuria occurred in one, two, and one patient, respectively. One patient suffered from grade 3 acute proctitis. Late toxicity was observed in two patients with rectovaginal fistula. No grade 5 toxicity occurred.
According to the experience of many BT centers in China over the past 20 years, seed implantation is recommended as salvage therapy for LPR; the seed implantation for LPR seems more common with better outcomes compared with CPR. In the above-mentioned study by Liu et al. (20), only eight lesions were CPR, 75 lesions were LPR, and 28 lesions were EPR. Studies directly comparing seed implantation for CPR and LPR were lacking, while the outcome differences between these two groups of populations were observed from a sub-group analysis. Qu et al. (19) reported 36 patients with rCC (15 CPR and 21 LPR) who received CT-guided iodine125 seed implantation after EBRT. With a median follow-up of 11.4 months, the 1- and 2-year local progression-free survival (LPFS) rate was 34.9 and 20%, respectively. The 1- and 2-year OS rate was 52.0 and 19.6%, respectively. The multivariate analysis indicated that recurrence sites (CPR or LPR) were the independent factors for both LPFS and OS (hazard ratio = 0.294 and 0.358, respectively). Qu et al. (28) reported 39 patients with rCC who were treated by image-guided seed implantation. The OS of patients with CPR and LPR was 6 and 12 months, respectively, and the 1-year progression-free survival rate was 26.7 and 41.6%, respectively, suggesting that the prognosis of seed implantation for LPR was superior to that of CPR, with a low incidence of side effects.
The studies on seed implantation of EPR were mainly reported for lymphatic metastasis from CC, but along with other cancers, the complications were acceptable, while the survival outcomes may be biased by the mixed analysis with other cancers. 3D-PT-assisted CT-guided iodine125 seed implantation is also feasible. Jiang et al. (38) reported 15 patients with 17 retroperitoneal recurrent carcinomas after EBRT (26.7% from CC). All patients received CT-guided iodine125 seed implantation assisted by 3D-PNCT. No ≥grade 3 adverse reactions were observed. The preliminary clinical study showed that CT-guided iodine125 seed implantation assisted by 3D-PNCT was a safe, accurate, and feasible strategy for recurrent carcinomas located in the retroperitoneal regions. Chen et al. (49) reported 32 patients with retroperitoneal recurrent lymphatic metastasis carcinomas after EBRT who successfully underwent 3D-PNCT-assisted seed implantation. A total of 81.3% of the patients achieved pain relief, and 71.9% were improved. The overall response rate and the local control rate were 85.3 and 94.1%, respectively. The local control rates reached 66.2 and 43.2% in 1 and 2 years, respectively, with a median local control time of 15.8 months. The 1- and 2-year OS rates were 74.1 and 28.1%, respectively, with a median OS of 17.6 months. Except for two patients developing grade 1 retroperitoneal hematomas, no other severe adverse events were observed. Guo (50) reported 14 patients with supraclavicular metastatic tumor (15 lesions) who received 3D-PT-assisted CT-guided iodine125 seed implantation. The difference in D90, V100, V150, V200 (percentage of GTV receiving 100 or 150 or 200% of the prescription dose, respectively), matched peripheral dose, and conformal index between pre- and post-operation was not statistically significant (P > 0.05). The external volume index (defined as the ratio of the non-target volume received dose ≥ prescribed dose to target volume) of pre-operation was significantly higher than that of post-operation (55.8 vs. 33.4, P = 0.02). It was concluded that personalized 3D-PT-assisted CT-guided iodine125 seed implantation for supraclavicular metastatic tumor is accurate and feasible. Further efficacy study focusing on EPR from CC only is warranted.
PJ wrote the manuscript draft. JW, YZ, LZ, and BQ reviewed and revised it. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of 413 China (grant no. 2019YFB1311300) and the National Natural Science Foundation of China (grant no. 82073335) to JW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical Cancer. Lancet (2019) 393(10167):169–82. doi: 10.1016/S0140-6736(18)32470-X
2. Nadeem R, Abu-Rustum CMY. Cervical Cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) 2021; Version 1.2021. (2020).
3. Peiretti M, Zapardiel I, Zanagnolo V, Landoni F, Morrow CP, Maggioni A. Management of Recurrent Cervical Cancer: A Review of the Literature. Surg Oncol (2012) 21(2):e59–66. doi: 10.1016/j.suronc.2011.12.008
4. Elit L, Fyles AW, Oliver TK, Devries-Aboud MC, Fung-Kee-Fung M. Members of the Gynecology Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-Based C. Follow-Up for Women After Treatment for Cervical Cancer. Curr Oncol (2010) 17(3):65–9.
5. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent Chemotherapy and Pelvic Radiation Therapy Compared With Pelvic Radiation Therapy Alone as Adjuvant Therapy After Radical Surgery in High-Risk Early-Stage Cancer of the Cervix. J Clin Oncol (2000) 18(8):1606–13. doi: 10.1200/JCO.2000.18.8.1606
6. Friedlander M, Grogan M, Force USPST. Guidelines for the Treatment of Recurrent and Metastatic Cervical Cancer. Oncologist (2002) 7(4):342–7. doi: 10.1634/theoncologist.2002-0342
7. Perez CA, Grigsby PW, Camel HM, Galakatos AE, Mutch D, Lockett MA. Irradiation Alone or Combined With Surgery in Stage IB, IIA, and IIB Carcinoma of Uterine Cervix: Update of a Nonrandomized Comparison. Int J Radiat Oncol Biol Phys (1995) 31(4):703–16. doi: 10.1016/0360-3016(94)00523-0
8. Jurado M, Alcazar JL, Martinez-Monge R. Resectability Rates of Previously Irradiated Recurrent Cervical Cancer (PIRCC) Treated With Pelvic Exenteration: Is Still the Clinical Involvement of the Pelvis Wall a Real Contraindication? A Twenty-Year Experience. Gynecol Oncol (2010) 116(1):38–43. doi: 10.1016/j.ygyno.2009.09.035
9. Viswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, et al. American Brachytherapy Society Consensus Guidelines for Locally Advanced Carcinoma of the Cervix. Part II: High-Dose-Rate Brachytherapy. Brachytherapy (2012) 11(1):47–52.
10. Harkenrider MM, Block AM, Alektiar KM, Gaffney DK, Jones E, Klopp A, et al. American Brachytherapy Task Group Report: Adjuvant Vaginal Brachytherapy for Early-Stage Endometrial Cancer: A Comprehensive Review. Brachytherapy (2017) 16(1):95–108. doi: 10.1016/j.brachy.2016.04.005
11. Strnad V, Major T, Polgar C, Lotter M, Guinot JL, Gutierrez-Miguelez C, et al. ESTRO-ACROP Guideline: Interstitial Multi-Catheter Breast Brachytherapy as Accelerated Partial Breast Irradiation Alone or as Boost - GEC-ESTRO Breast Cancer Working Group Practical Recommendations. Radiother Oncol (2018) 128(3):411–20. doi: 10.1016/j.radonc.2018.04.009
12. Quero L, Guillerm S, Taright N, Michaud S, Teixeira L, Cahen-Doidy L, et al. 10-Year Follow-Up of 621 Patients Treated Using High-Dose Rate Brachytherapy as Ambulatory Boost Technique in Conservative Breast Cancer Treatment. Radiother Oncol (2017) 122(1):11–6. doi: 10.1016/j.radonc.2016.06.014
13. Shah C, Ouhib Z, Kamrava M, Koyfman SA, Campbell SR, Bhatnagar A, et al. The American Brachytherapy Society Consensus Statement for Skin Brachytherapy. Brachytherapy (2020) 19(4):415–26. doi: 10.1016/j.brachy.2020.04.004
14. Chargari C, Deutsch E, Blanchard P, Gouy S, Martelli H, Guerin F, et al. Brachytherapy: An Overview for Clinicians. CA Cancer J Clin (2019) 69(5):386–401. doi: 10.3322/caac.21578
15. Qiu B, Jiang Y, Ji Z, Sun H, Fan J, Li W, et al. The Accuracy of Individualized 3D-Printing Template-Assisted I(125) Radioactive Seed Implantation for Recurrent/Metastatic Head and Neck Cancer. Front Oncol (2021) 11:664996. doi: 10.3389/fonc.2021.664996
16. Wang H, Wang L, Jiang Y, Ji Z, Guo F, Jiang P, et al. Long-Term Outcomes and Prognostic Analysis of Computed Tomography-Guided Radioactive (125)I Seed Implantation for Locally Recurrent Rectal Cancer After External Beam Radiotherapy or Surgery. Front Oncol (2020) 10:540096. doi: 10.3389/fonc.2020.540096
17. Li W, Dan G, Jiang J, Zheng Y, Zheng X, Deng D. Repeated Iodine-125 Seed Implantations Combined With External Beam Radiotherapy for the Treatment of Locally Recurrent or Metastatic Stage III/IV non-Small Cell Lung Cancer: A Retrospective Study. Radiat Oncol (2016) 11(1):119. doi: 10.1186/s13014-016-0688-5
18. Wang L, Wang H, Jiang Y, Ji Z, Guo F, Jiang P, et al. The Efficacy and Dosimetry Analysis of CT-Guided (125)I Seed Implantation Assisted With 3D-Printing non-Co-Planar Template in Locally Recurrent Rectal Cancer. Radiat Oncol (2020) 15(1):179.
19. Qu A, Jiang P, Sun H, Jiang W, Jiang Y, Tian S, et al. Efficacy and Dosimetry Analysis of Image-Guided Radioactive (1)(2)(5)I Seed Implantation as Salvage Treatment for Pelvic Recurrent Cervical Cancer After External Beam Radiotherapy. J Gynecol Oncol (2019) 30(1):e9. doi: 10.3802/jgo.2019.30.e9
20. Liu Y, Jiang P, Zhang H, Wang J. Safety and Efficacy of 3D-Printed Templates Assisted CT-Guided Radioactive Iodine-125 Seed Implantation for the Treatment of Recurrent Cervical Carcinoma After External Beam Radiotherapy. J Gynecol Oncol (2021) 32(2):e15.
21. Ijaz T, Eifel PJ, Burke T, Oswald MJ. Radiation Therapy of Pelvic Recurrence After Radical Hysterectomy for Cervical Carcinoma. Gynecol Oncol (1998) 70(2):241–6. doi: 10.1006/gyno.1998.5093
22. Campitelli M, Lazzari R, Piccolo F, Ferrazza P, Marsella AR, Macchia G, et al. Brachytherapy or External Beam Radiotherapy as a Boost in Locally Advanced Cervical Cancer: A Gynaecology Study Group in the Italian Association of Radiation and Clinical Oncology (AIRO) Review. Int J Gynecol Cancer (2021). doi: 10.1136/ijgc-2020-002310
23. Barber HR. Relative Prognostic Significance of Preoperative and Operative Findings in Pelvic Exenteration. Surg Clin North Am (1969) 49(2):431–47. doi: 10.1016/S0039-6109(16)38800-4
24. Hockel M, Horn LC, Einenkel J. (Laterally) Extended Endopelvic Resection: Surgical Treatment of Locally Advanced and Recurrent Cancer of the Uterine Cervix and Vagina Based on Ontogenetic Anatomy. Gynecol Oncol (2012) 127(2):297–302. doi: 10.1016/j.ygyno.2012.07.120
25. Hockel M. Long-Term Experience With (Laterally) Extended Endopelvic Resection (LEER) in Relapsed Pelvic Malignancies. Curr Oncol Rep (2015) 17(3):435. doi: 10.1007/s11912-014-0435-8
26. Hockel M, Dornhofer N. Pelvic Exenteration for Gynaecological Tumours: Achievements and Unanswered Questions. Lancet Oncol (2006) 7(10):837–47. doi: 10.1016/S1470-2045(06)70903-2
27. Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic Exenteration for Recurrent Gynecologic Malignancy: Survival and Morbidity Analysis of the 45-Year Experience at UCLA. Gynecol Oncol (2005) 99(1):153–9. doi: 10.1016/j.ygyno.2005.05.034
28. Huang EY, Lin H, Hsu HC, Wang CJ, Chen HC, Sun LM, et al. High External Parametrial Dose can Increase the Probability of Radiation Proctitis in Patients With Uterine Cervix Cancer. Gynecol Oncol (2000) 79(3):406–10. doi: 10.1006/gyno.2000.5997
29. Fenkell L, Assenholt M, Nielsen SK, Haie-Meder C, Potter R, Lindegaard J, et al. Parametrial Boost Using Midline Shielding Results in an Unpredictable Dose to Tumor and Organs at Risk in Combined External Beam Radiotherapy and Brachytherapy for Locally Advanced Cervical Cancer. Int J Radiat Oncol Biol Phys (2011) 79(5):1572–9. doi: 10.1016/j.ijrobp.2010.05.031
30. Bittner NH, Orio PF 3rd, Merrick GS, Prestidge BR, Hartford AC, Rosenthal SA. The American College of Radiology and the American Brachytherapy Society Practice Parameter for Transperineal Permanent Brachytherapy of Prostate Cancer. Brachytherapy (2017) 16(1):59–67. doi: 10.1016/j.brachy.2016.06.003
31. Rosenthal SA, Bittner NH, Beyer DC, Demanes DJ, Goldsmith BJ, Horwitz EM, et al. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) Practice Guideline for the Transperineal Permanent Brachytherapy of Prostate Cancer. Int J Radiat Oncol Biol Phys (2011) 79(2):335–41. doi: 10.1016/j.ijrobp.2010.08.045
32. Rivard MJ, Butler WM, Devlin PM, Hayes JK Jr., Hearn RA, Lief EP, et al. American Brachytherapy Society Recommends No Change for Prostate Permanent Implant Dose Prescriptions Using Iodine-125 or Palladium-103. Brachytherapy (2007) 6(1):34–7. doi: 10.1016/j.brachy.2006.11.001
33. Jiang YL, Meng N, Wang JJ, Jiang P, Yuan H, Liu C, et al. CT-Guided Iodine-125 Seed Permanent Implantation for Recurrent Head and Neck Cancers. Radiat Oncol (2010) 5:68. doi: 10.1186/1748-717X-5-68
34. Jiang P, Liu C, Wang J, Yang R, Jiang Y, Tian S. Computed Tomography (CT)-Guided Interstitial Permanent Implantation of (125)I Seeds for Refractory Chest Wall Metastasis or Recurrence. Technol Cancer Res Treat (2015) 14(1):11–8. doi: 10.7785/tcrt.2012.500402
35. Wang J, Chai S, Zheng G, Jiang Y, Ji Z, Guo F, et al. Expert Consensus Statement on Computed Tomography-Guided (125)I Radioactive Seeds Permanent Interstitial Brachytherapy. J Cancer Res Ther (2018) 14(1):12–7. doi: 10.4103/jcrt.JCRT_888_17
36. Jiang P, Wang J, Ran W, Jiang Y, Tian S, Sun H. Five-Year Outcome of Ultrasound-Guided Interstitial Permanent (125)I Seeds Implantation for Local Head and Neck Recurrent Tumors: A Single Center Retrospective Study. J Contemp Brachytherapy (2019) 11(1):28–34. doi: 10.5114/jcb.2019.83336
37. Ji Z, Jiang Y, Guo F, Sun H, Fan J, Zhang L, et al. Dosimetry Verification of Radioactive Seed Implantation for Malignant Tumors Assisted by 3D Printing Individual Templates and CT Guidance. Appl Radiat Isot (2017) 124:68–74. doi: 10.1016/j.apradiso.2016.12.009
38. Jiang W, Jiang P, Wei S, Jiang Y, Ji Z, Sun H, et al. The Accuracy and Safety of CT-Guided Iodine-125 Seed Implantation Assisted by 3D non-Coplanar Template for Retroperitoneal Recurrent Carcinoma. World J Surg Oncol (2020) 18(1):307.
39. Qu A, Jiang P, Wei S, Jiang Y, Ji Z, Sun H, et al. Accuracy and Dosimetric Parameters Comparison of 3D-Printed non-Coplanar Template-Assisted Computed Tomography-Guided Iodine-125 Seed Ablative Brachytherapy in Pelvic Lateral Recurrence of Gynecological Carcinomas. J Contemp Brachytherapy (2021) 13(1):39–45. doi: 10.5114/jcb.2021.103585
40. Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation Dose-Volume Effects in the Spinal Cord. Int J Radiat Oncol Biol Phys (2010) 76(3 Suppl):S42–49. doi: 10.1016/j.ijrobp.2009.04.095
41. Wang J, Zhang F, Guo J, Chai S, Zheng G, Zhang K, et al. Expert Consensus Workshop Report: Guideline for Three-Dimensional Printing Template-Assisted Computed Tomography-Guided (125)I Seeds Interstitial Implantation Brachytherapy. J Cancer Res Ther (2017) 13(4):607–12. doi: 10.4103/jcrt.JCRT_412_17
42. Singh AK, Grigsby PW, Rader JS, Mutch DG, Powell MA. Cervix Carcinoma, Concurrent Chemoradiotherapy, and Salvage of Isolated Paraaortic Lymph Node Recurrence. Int J Radiat Oncol Biol Phys (2005) 61(2):450–5. doi: 10.1016/j.ijrobp.2004.06.207
43. Boussios S, Seraj E, Zarkavelis G, Petrakis D, Kollas A, Kafantari A, et al. Management of Patients With Recurrent/Advanced Cervical Cancer Beyond First Line Platinum Regimens: Where do We Stand? A Literature Review. Crit Rev Oncol Hematol (2016) 108:164–74. doi: 10.1016/j.critrevonc.2016.11.006
44. Cella D, Huang HQ, Monk BJ, Wenzel L, Benda J, McMeekin DS, et al. Health-Related Quality of Life Outcomes Associated With Four Cisplatin-Based Doublet Chemotherapy Regimens for Stage IVB Recurrent or Persistent Cervical Cancer: A Gynecologic Oncology Group Study. Gynecol Oncol (2010) 119(3):531–7. doi: 10.1016/j.ygyno.2010.08.020
45. Wang R, Zhu J, Yang S, Chen X, Gu C, Liang T, et al. Therapeutic Effects and Prognostic Factors of (125)I Brachytherapy for Pelvic Recurrence After Early Cervical Cancer Surgery. Sci Rep (2021) 11(1):11356.
46. Han L, Li C, Wang J, He X, Zhang X, Yang J, et al. Iodine-125 Radioactive Seed Tissue Implantation as a Remedy Treatment for Recurrent Cervical Cancer. J Cancer Res Ther (2016) 12(Supplement):C176–80.
47. Tong L, Liu P, Huo B, Guo Z, Ni H. CT-Guided (125)I Interstitial Brachytherapy for Pelvic Recurrent Cervical Carcinoma After Radiotherapy. Onco Targets Ther (2017) 10:4081–8. doi: 10.2147/OTT.S139571
48. Yuan X, Zhang Y, Cui M, Miao J, Gao L, Hu J, et al. Dosimetry Comparison Between a 3D Printed Minimally Invasive Guidance Template and Free Implantation in the Brachytherapy Treatment of Postoperative Recurrent Cervical Carcinoma. Cancer Manag Res (2019) 11:5013–8. doi: 10.2147/CMAR.S195829
49. Chen Y, Jiang Y, Ji Z, Jiang P, Xu F, Zhang Y, et al. Dosimetry, Efficacy, and Safety of Three-Dimensional Printing Noncoplanar Template-Assisted and CT-Guided (125)I Seed Implantation for Recurrent Retroperitoneal Lymphatic Metastasis After External Beam Radiotherapy. Brachytherapy (2020) 19(3):380–8. doi: 10.1016/j.brachy.2020.02.009
Keywords: radiotherapy, recurrent cervical cancer, brachytherapy, expert consensus, three-dimensional printing template
Citation: Jiang P, Zou L, Wei L, Cheng G, Sun B, Zhang F, Wang R, Wang T, Qu A, Yuan X, Qiu B, Wei S, Liu Z, Zhang Y and Wang J (2021) Chinese Expert Consensus on Iodine125 Seed Implantation for Recurrent Cervical Cancer in 2021. Front. Oncol. 11:700710. doi: 10.3389/fonc.2021.700710
Received: 26 April 2021; Accepted: 14 October 2021;
Published: 09 November 2021.
Edited by:
Charles A. Kunos, Investigational Drug Branch, National Cancer Institute (NIH), United StatesReviewed by:
Jun Itami, Shinmatsudo Central General Hospital, JapanCopyright © 2021 Jiang, Zou, Wei, Cheng, Sun, Zhang, Wang, Wang, Qu, Yuan, Qiu, Wei, Liu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zi Liu, bGl1em1haWxAMTYzLmNvbQ==; Yunyan Zhang, Wmhhbmd5dW55YW5fMTk3MkAxNjMuY29t; Junjie Wang, anVuamlld2FuZ19lZHVAc2luYS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.