- 1Department of Respiratory and Critical Care Medicine, Jiangsu Provincial Hospital of Traditional Chinese Medicine, Nanjing, China
- 2Department of Research and Development, Nanjing Geneseeq Technology Inc., Nanjing, China
- 3Department of Radiology, Jiangsu Provincial Hospital of Traditional Chinese Medicine, Nanjing, China
For advanced lung adenocarcinoma patients with common epidermal growth factor receptor (EGFR) mutations (exon 19 deletions or the exon 21 L858R mutation), tyrosine kinase inhibitors (TKIs) are the standard therapies, and achieve favorable responses. However, for the rare EGFR deletion-insertion mutation of exon 18, there is no evidence of the efficacy of EGFR TKIs. Herein, we report a lung adenocarcinoma patient harboring a rare EGFR E709_T710delinsD mutation who was treated with afatinib as the first-line therapy and achieved a progression-free survival of 23 months. After the disease progressed, the patient received almonertinib treatment and exhibited a stable disease. This case indicated that non-small cell lung cancer patients harboring the EGFR E709_T710delinsD mutation could benefit from afatinib treatment, followed with almonertinib treatment, as a potential therapeutic strategy.
Highlights
● A lung adenocarcinoma patient harbored a rare exon 18 deletion-insertion mutation of in EGFR (E709_T710delinsD) at with a mutant allele frequency of 74.8%.
● The patient was treated with afatinib as the first-line therapy and achieved a progression-free survival of 23 months.
● After the disease progressed, almonertinib was administered as the second-line therapy for the NSCLC patient, and led to a stable disease.
Introduction
Lung cancer, of which 80% - 85% is classified as non-small-cell lung cancer (NSCLC), has the highest death rate of all cancers worldwide (1, 2). Somatic activating mutations in the epidermal growth factor receptor (EGFR) are the most common oncogenic driver mutations in Asian NSCLC patients, with a prevalence of 47% (3). Such mutations typically occur within exons 18 - 21. The most common EGFR mutations (nearly 85% - 90%) in NSCLC patients are deletions in exon 19 (19Del) and the L858R point mutation in exon 21, which are defined as classical mutations. The remaining 10% - 15% of EGFR mutations are non-classical mutations, including point mutations and deletions in exon 18, and point mutations and insertion mutations in exon 20 (4).
For NSCLC patients with EGFR mutations, it has been well documented that patients with EGFR 19Del and L858R mutations exhibit good clinical responses to EGFR tyrosine kinase inhibitors (TKIs). Clinically, first-line therapy with EGFR TKIs is recommended and significantly improves the survival of NSCLC patients with EGFR variations (5, 6). Although the T790M mutation in exon 20 is resistant to first- and second-generation EGFR TKIs, it is responsive to the third-generation EGFR TKI, osimertinib (7). However, there is insufficient clinical evidence to confirm the sensitivity of exon 18 mutations to EGFR TKIs. In this case, we report a lung adenocarcinoma patient with a rare EGFR exon 18 deletion-insertion mutation (E709_T710delinsD) that responded well to afatinib and achieved a progressive-free survival of 23 months. Following the development of afatinib resistance, the patient then benefited from almonertinib treatment.
Case Presentation
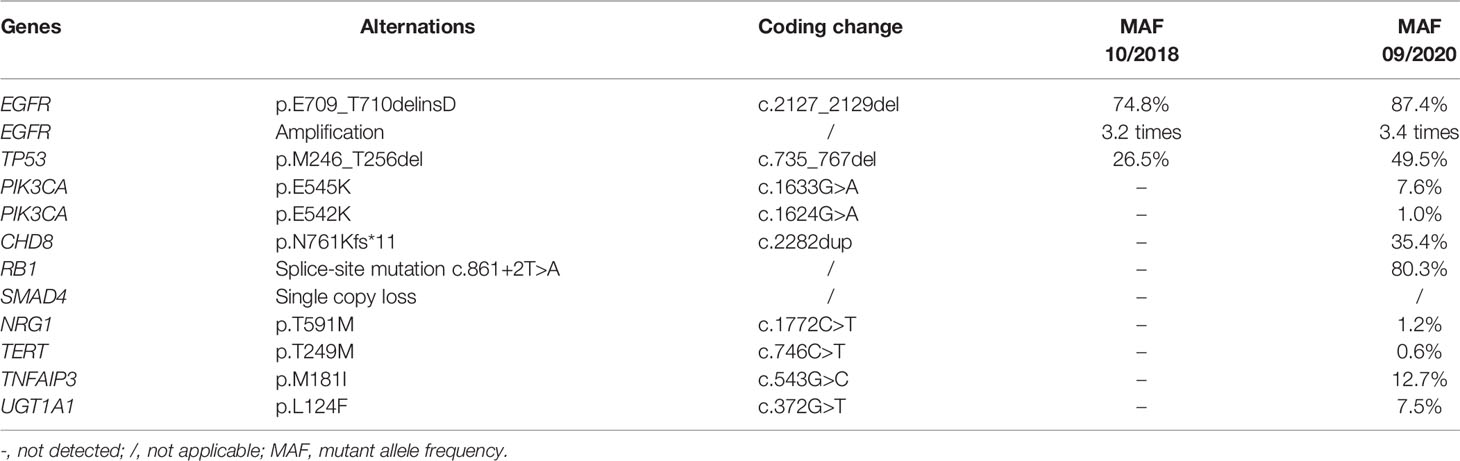
A 70-year-old Chinese female non-smoker with no family history of cancer suffered from repeated coughing for more than 10 days in October 2018. Computed tomography (CT) of the chest revealed a 5.1 × 3.6 cm density mass in the dorsal segment of the lower left lung (Figure 1A). Magnetic resonance imaging (MRI) scans showed that there were no metastases in the brain (Figure 1B). The level of the serum tumor biomarker, carcinoembryonic antigen (CEA), was 16.27 ng/mL, which was much higher than the normal range of < 5.0 ng/mL (Figure 1C). Immunohistochemical analyses revealed that the tumor cells of the left lung were positive for thyroid transcription factor-1 (TTF-1) and Napsin A, whereas focal staining was positive for CK5/6 (data not shown). Based on those data, the patient was diagnosed with stage II lung adenocarcinoma (cT3N0M0 of TNM staging system), and was recommended surgical treatment according to the National Comprehensive Cancer Network Guidelines. However, the lung lesion was tightly connected to great vessels, and thus, could not be completely removed by surgery. To identify a more effective treatment, a left lung biopsy was subjected to genetic testing of fourteen lung cancer-related genes using next-generation sequencing (NGS). A rare EGFR exon 18 mutation (p.E709_T710delinsD) was identified with a mutant allele frequency (MAF) of 74.8%, and accompanied by EGFR amplification and a TP53 p.M246_T256del mutation (Table 1 and Figure 1D).
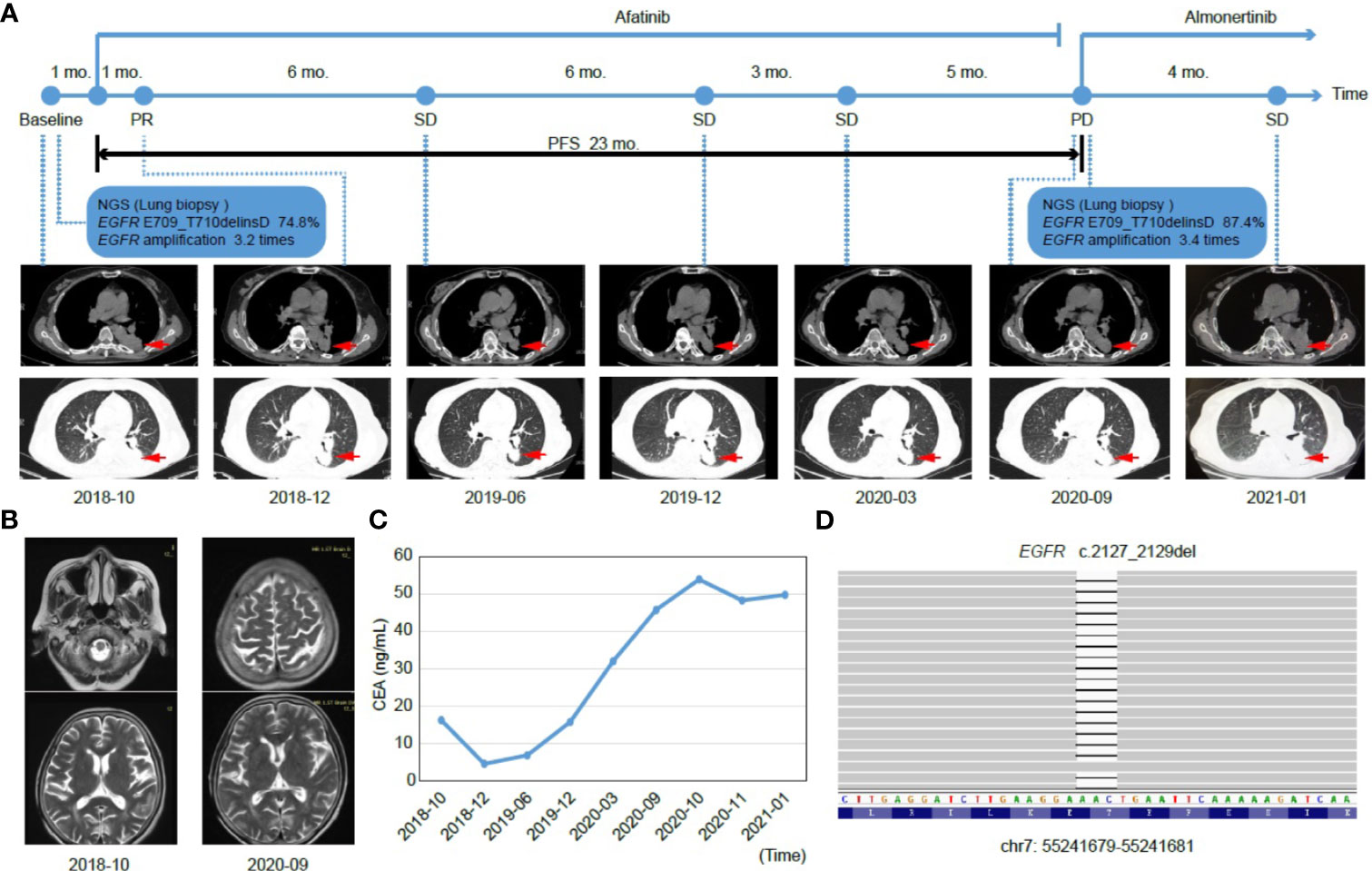
Figure 1 Tumor progression of the patient before and after treatment. (A) The timeline of therapies and tumor progression are indicated (Top). CT images revealed lesions in lower left lung. The tumor is indicated by red arrows. PFS, progression-free survival; PR, partial response; SD, stable disease; PD, progressive disease; NGS, next-generation sequencing; mo., months. (B) Brain MRI scans revealed no metastasis before (October 2018) and after (September 2020) treatment. (C) Line chart showing changes in the levels of the serum tumor biomarker, CEA (carcinoembryonic antigen) during the course of treatment. (D) The EGFR p.E709_T710delinsD mutation was visualized by IGV software. Deletions are indicated with a black dash (–).
In consideration of the patient’s age and physical condition, the patient and her family refused radiotherapy and chemotherapy. Rather, the second-generation EGFR TKI, afatinib, was administered as a once-daily oral dose of 30 mg in November 2018. One month post treatment, a partial response (PR) was achieved with lesion shrinkage in the left lung according to the RECIST response criteria, and the serum CEA level returned to normal (4.58 ng/mL, Figures 1A, C). Seven months after afatinib treatment, the left lung tumor volume was further reduced, accompanied by a stable CEA level (6.87 ng/mL, Figures 1A, C). The lung lesion was re-evaluated using CT scans in December 2019 and revealed an increased tumor size (Figure 1A). The serum CEA level (15.80 ng/mL) was also elevated at that time (Figure 1C). However, the patient exhibited a stable disease (SD) according to RECIST response criteria. In March 2020, CT scans revealed no marked enlargement of the lesion, while the CEA level was markedly elevated (32.05 ng/mL, Figures 1A, C). In spite of the SD that was maintained during this period, the possibility of slow progress was not ruled out. In the case of remission and with no obvious adverse reactions, the patient continued to be treated with afatinib until September 2020. At that time, there was no metastasis in the brain, but the target lesion in the lower left lung increased with a concomitant increase in CEA levels (45.80 ng/mL), thus, indicating progressive disease (PD) (Figure 1A–C).
After the development of afatinib-resistance, another tumor biopsy was collected and subjected to NGS-based genetic testing of 425 cancer-related genes. The results revealed that the MAF of the EGFR E709_T710delinsD mutation increased from 74.8% to 87.4% (Table 1). The patient was then switched to a third-generation EGFR TKI, almonertinib, with a once-daily oral dose of 80 mg, as it was thought to impart fewer adverse effects than osimertinib. In January 2021, although the lesion in the left lung was slightly enlarged and serum CEA levels (49.81 ng/mL) were higher than those in September 2020 (Figures 1A, C), the patient was considered as SD under the RECIST response criteria. Until February 2021, the patient was still receiving almonertinib treatment.
Discussion
Patients with EGFR mutations exhibit various responses to TKI treatment, and the classical mutations in exons 19 and 20 exhibit good responses to such treatments. However, the sensitivity of exon 18 mutations to TKIs has not been determined. According to the latest update of the Catalogue of Somatic Mutations in Cancer (COSMIC) database, the E709_T710delinsD mutation accounts for only 0.064% of EGFR mutations (17/26499), and thus, its clinical significance is unclear.
In recent years, studies have reported the responses of lung cancer patients with E709_T710delinsD mutations to EGFR TKIs (Table 2) (2, 8–16). Among patients receiving gefitinib or erlotinib, one achieved a PR, two achieved a SD, and nine others were non-responders (i.e., PD). These patients achieved PD and SD experienced a short PFS from 0.9 to 8 months, with a median of 3 months (2, 8–13).
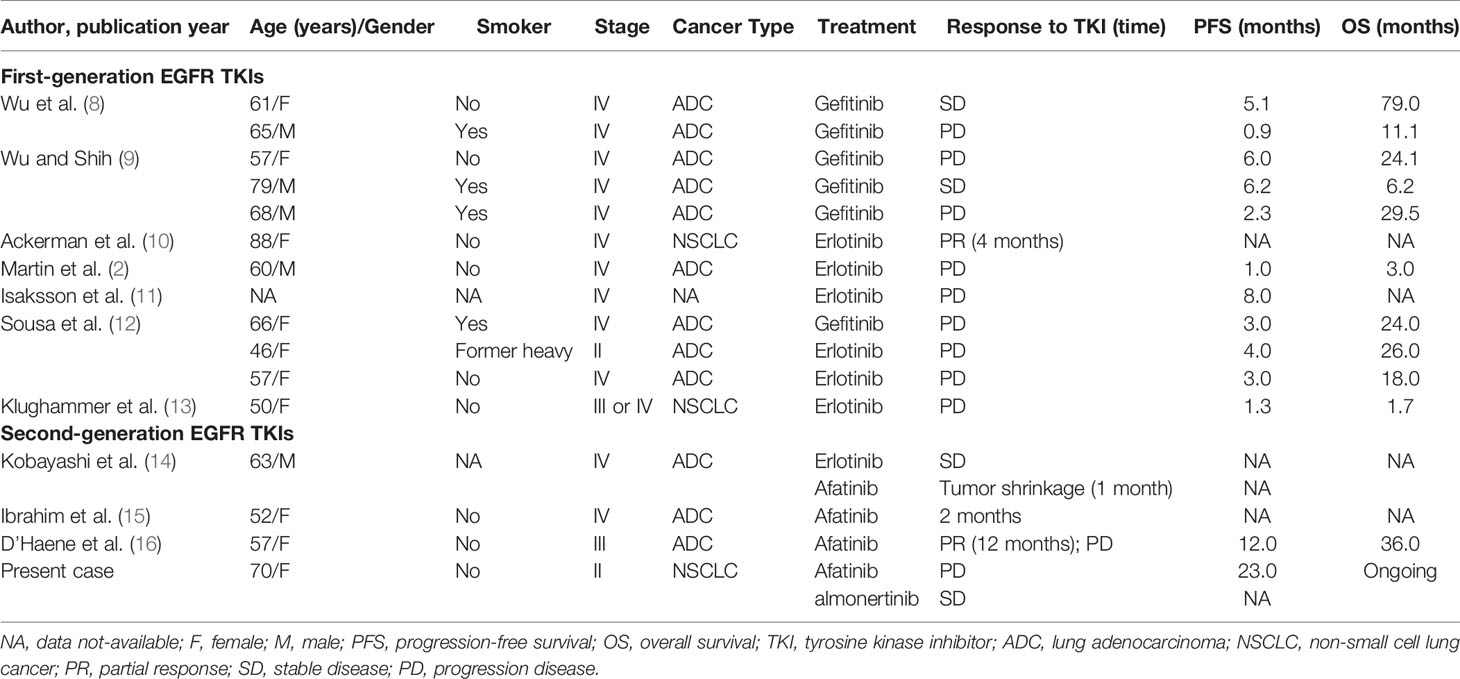
Table 2 All reported cases of the EGFR E709_T710delinsD mutation in lung adenocarcinoma patients treated with EGFR TKIs.
Another group compared the efficacy of first-generation (gefitinib and erlotinib), second-generation (afatinib and dacomitinib, and neratinib), and third-generation TKIs (osimertinib and CO1686) in vitro (14), and identified that cells transfected with the E709_T710delinsD mutation were more sensitive to second-generation TKIs, and especially afatinib. That study also showed that a lung adenocarcinoma patient who acquired the E709_T710delinsD mutation benefited from afatinib after erlotinib treatment failed. Ibrahim et al. also reported a patient who had reduced lung nodules after 2 months of afatinib treatment (15). Similarly, a 57-year-old female with lung adenocarcinoma was treated with afatinib after the disease progressed following chemotherapy and maintained a PR for one year (16). In the current case, the patient was treated with afatinib and achieved a PFS of 23 months. After the development of afatinib-resistance, almonertinib was then administered and the patient achieved SD.
Otherwise, Zeng et al. found that the E709_T710delinsD mutation was an acquired drug resistance mechanism and not sensitivity to afatinib in an advanced lung adenocarcinoma patient with an EGFR exon 18 E709H mutation (17). Thus, additional evidence of the clinical significance of the E709_T710delins mutation needs to be explored.
For uncommon EGFR mutations, although the data from prospective clinical trials are insufficient because of the low frequency and diversity of such mutations, some cases harboring uncommon EGFR mutations have been reported with effective treatment by EGFR TKIs. It has been reported that the major uncommon EGFR mutations, including G719X, S768I, and L861Q are more sensitive to afatinib and osimertinib, compared to first-generation EGFR TKIs (18). Therefore, afatinib or osimertinib have been suggested as possible first-line treatment options for major uncommon EGFR mutations (19). However, patients with uncommon mutations that co-occurred with common EGFR mutations exhibited responses to first-generation EGFR TKIs (20). Limited clinical data and our analyses suggest that other rare EGFR mutations, including E709X, L747P/S, Del18 mutations, and some exon 19 insertion-deletions are more sensitive to afatinib or osimertinib than gefitinib or erlotinib (Table 2). In particular, NSCLC patients with compound EGFR mutations involving T790M exhibit good responses to osimertinib compared to NSCLC patients with other mutations (19).
In summary, this study reported a lung adenocarcinoma patient who harbored an EGFR E709_T710delinsD mutation and received the second-generation EGFR TKI, afatinib, as the first-line therapy. Unexpectedly, lung lesion shrinkage lasted 7 months and the patient achieved a PFS of up to 23 months. Following the development of afatinib-resistance, almonertinib was administered and achieved a SD. Thus, this case detailed a reliable treatment option for NSCLC patients harboring a rare EGFR exon 18 deletion-insertion mutation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary files. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical committee of Jiangsu Province Hospital of Traditional Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conception and design: LZ, LL, and YW. Collection and assembly of data: YW, YG, and YC. Manuscript writing and revising: All authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
YC and YG are employees of Nanjing Geneseeq Technology Inc., China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the patient for providing written informed consent for publication, as well as the research staff involved in the study.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Martin J, Lehmann A, Klauschen F, Hummel M, Lenze D, Grohé C, et al. Clinical Impact of Rare and Compound Mutations of Epidermal Growth Factor Receptor in Patients With Non-Small-Cell Lung Cancer. Clin Lung Cancer (2019) 20:350–62.e354. doi: 10.1016/j.cllc.2019.04.012
3. Midha A, Dearden S, McCormack R. EGFR Mutation Incidence in non-Small-Cell Lung Cancer of Adenocarcinoma Histology: A Systematic Review and Global Map by Ethnicity (Mutmapii). Am J Cancer Res (2015) 5:2892–911.
4. Shen YC, Tseng GC, Tu CY, Chen WC, Liao WC, Chen WC, et al. Comparing the Effects of Afatinib With Gefitinib or Erlotinib in Patients With Advanced-Stage Lung Adenocarcinoma Harboring Non-Classical Epidermal Growth Factor Receptor Mutations. Lung Cancer (2017) 110:56–62. doi: 10.1016/j.lungcan.2017.06.007
5. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
6. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal Growth Factor Receptor Mutations in Lung Cancer. Nat Rev Cancer (2007) 7:169–81. doi: 10.1038/nrc2088
7. Gristina V, Malapelle U, Galvano A, Pisapia P, Pepe F, Rolfo C, et al. The Significance of Epidermal Growth Factor Receptor Uncommon Mutations in Non-Small Cell Lung Cancer: A Systematic Review and Critical Appraisal. Cancer Treat Rev (2020) 85:101994. doi: 10.1016/j.ctrv.2020.101994
8. Wu J-Y, Yu C-J, Chang Y-C, Yang C-H, Shih J-Y, Yang P-C. Effectiveness of Tyrosine Kinase Inhibitors on “Uncommon” Epidermal Growth Factor Receptor Mutations of Unknown Clinical Significance in Non–Small Cell Lung Cancer. Clin Cancer Res (2011) 17:3812–21. doi: 10.1158/1078-0432.ccr-10-3408
9. Wu JY, Shih JY. Effectiveness of Tyrosine Kinase Inhibitors on Uncommon E709X Epidermal Growth Factor Receptor Mutations in non-Small-Cell Lung Cancer. Onco Targets Ther (2016) 9:6137–45. doi: 10.2147/OTT.S118071
10. Ackerman A, Goldstein MA, Kobayashi S, Costa DB. Egfr delE709_T710insD: A Rare But Potentially EGFR Inhibitor Responsive Mutation in Non-Small-Cell Lung Cancer. J Thorac Oncol (2012) 7:e19–20. doi: 10.1097/JTO.0b013e3182635ab4
11. Isaksson S, Hazem B, Jönsson M, Reuterswärd C, Karlsson A, Griph H, et al. Clinical Utility of Targeted Sequencing in Lung Cancer: Experience From an Autonomous Swedish Health Care Center. JTO Clin Res Rep (2020) 1:100013. doi: 10.1016/j.jtocrr.2020.100013
12. Sousa AC, Silveira C, Janeiro A, Malveiro S, Oliveira AR, Felizardo M, et al. Detection of Rare and Novel EGFR Mutations in NSCLC Patients: Implications for Treatment-Decision. Lung Cancer (2020) 139:35–40. doi: 10.1016/j.lungcan.2019.10.030
13. Klughammer B, Brugger W, Cappuzzo F, Ciuleanu T, Mok T, Reck M, et al. Examining Treatment Outcomes With Erlotinib in Patients With Advanced non-Small Cell Lung Cancer Whose Tumors Harbor Uncommon Egfr Mutations. J Thorac Oncol (2016) 11:545–55. doi: 10.1016/j.jtho.2015.12.107
14. Kobayashi Y, Togashi Y, Yatabe Y, Mizuuchi H, Jangchul P, Kondo C, et al. Egfr Exon 18 Mutations in Lung Cancer: Molecular Predictors of Augmented Sensitivity to Afatinib or Neratinib as Compared With First- or Third-Generation Tkis. Clin Cancer Res (2015) 21:5305–13. doi: 10.1158/1078-0432.CCR-15-1046
15. Ibrahim U, Saqib A, Atallah JP. EGFR Exon 18 delE709_T710insD Mutated Stage IV Lung Adenocarcinoma With Response to Afatinib. Lung Cancer (2017) 108:45–7. doi: 10.1016/j.lungcan.2017.02.023
16. D’Haene N, Le Mercier M, Salmon I, Mekinda Z, Remmelink M, Berghmans T. Smad4 Mutation in Small Cell Transformation of Epidermal Growth Factor Receptor Mutated Lung Adenocarcinoma. Oncologist (2019) 24:9–13. doi: 10.1634/theoncologist.2018-0016
17. Zeng L, Zhang Y, Yang N. EGFR Exon 18 DelE709_T710insD as an Acquired Resistance Mechanism to Afatinib in an Advanced EGFR Exon 18 E709H Lung Adenocarcinoma. J Thorac Oncol (2018) 13:e93–5. doi: 10.1016/j.jtho.2018.01.006
18. Kobayashi Y, Mitsudomi T. Not All Epidermal Growth Factor Receptor Mutations in Lung Cancer Are Created Equal: Perspectives for Individualized Treatment Strategy. Cancer Sci (2016) 107:1179–86. doi: 10.1111/cas.12996
19. Passaro A, Mok T, Peters S, Popat S, Ahn MJ, de Marinis F. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, non Exon 20 Insertions, Egfr Mutations. J Thorac Oncol (2021) 16:764–73. doi: 10.1016/j.jtho.2020.12.002
Keywords: NSCLC, EGFR mutation, exon 18, E709_T710insdelD, afatinib
Citation: Wei Y, Cui Y, Guo Y, Li L and Zeng L (2021) A Lung Adenocarcinoma Patient With a Rare EGFR E709_T710delinsD Mutation Showed a Good Response to Afatinib Treatment: A Case Report and Literature Review. Front. Oncol. 11:700345. doi: 10.3389/fonc.2021.700345
Received: 26 April 2021; Accepted: 24 May 2021;
Published: 11 June 2021.
Edited by:
Kenneth K. W. To, The Chinese University of Hong Kong, ChinaReviewed by:
Antonio Passaro, European Institute of Oncology (IEO), ItalyAlessandro Morabito, Istituto Nazionale Tumori Fondazione G. Pascale (IRCCS), Italy
Copyright © 2021 Wei, Cui, Guo, Li and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Zeng, WmVuZ0xAMTI2LmNvbQ==; Lei Li, MTM3NzY2MTIxMzRAMTYzLmNvbQ==
 Yu Wei
Yu Wei Yueli Cui2
Yueli Cui2