- 1Department of Hepatobiliary Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital (People’s Hospital of Hangzhou Medical College), Hangzhou, China
- 2School of Clinical Medicine, Hangzhou Medical College, Hangzhou, China
- 3Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Navy Medical University), Shanghai, China
- 4Department of General Surgery, Liuyang People’s Hospital, Hunan, China
- 5Department of Hepatobiliary Surgery, The First Affiliated Hospital of Harbin Medical University, Heilongjiang, China
- 6Department of Hepatobiliary Surgery, Pu’er People’s Hospital, Yunnan, China
- 7The First Department of General Surgery, The Fourth Hospital of Harbin, Heilongjiang, China
- 8Department of General Surgery, Pingxiang Mining Group General Hospital, Jiangxi, China
- 9Department of Hepatic Surgery, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
- 10Department of General Surgery, Ziyang First People’s Hospital, Sichuan, China
- 11Department of Hepatobiliary Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 12Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, China
- 13Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
- 14Department of Surgery, Ohio State University, Wexner Medical Center, Columbus, OH, United States
Background: Hepatocellular carcinoma (HCC) is one of the most serious consequences of chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. This study sought to investigate long-term outcomes after liver resection for HCC among patients with HBV/HCV co-infection (HBV/HCV-HCC) compared with patients with HBV infection (HBV-HCC).
Methods: Patients who underwent curative-intent liver resection for HCC were identified from a multicenter Chinese database. Using propensity score matching (PSM), patients with HBV/HCV-HCC were matched one-to-one to patients with HBV-HCC. Overall survival (OS) and recurrence-free survival (RFS) were compared between the two groups before and after PSM.
Results: Among 2,467 patients identified, 93 (3.8%) and 2,374 (96.2%) patients had HBV/HCV-HCC and HBV-HCC, respectively. Compared with patients with HBV-HCC, patients with HBV/HCV-HCC were older, have poorer liver-related characteristics but better tumor-related characteristics. PSM created 88 pairs of patients with comparable liver- and tumor-related characteristics (all P > 0.2). In the PSM cohort, the 3- and 5-year RFS rates in patients with HBV/HCV-HCC were 48.3% and 38.9%, which were significantly poorer than patients with HBV-HCC (61.8% and 49.2%, P = 0.037). Meanwhile, the 3- and 5-year OS rates in patients with HBV/HCV-HCC were also poorer than patients with HBV-HCC (65.4% and 51.1% vs. 73.7% and 63.0%), with a difference close to be significant between them (P = 0.081).
Conclusion: Comparing to patients with HBV-HCC, liver resection resulted in relatively poorer long-term surgical outcomes in patients with HBV/HCV-HCC.
Introduction
Approximately 700,000 persons die of hepatocellular carcinoma (HCC) every year worldwide making it the third leading cause of cancer death (1, 2). Cirrhosis of the liver is the biggest risk factor associated with developing HCC (3). Overall, up to 80% of HCC cases are attributable to cirrhosis secondary to persistent viral infection, mainly hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection (4, 5). In fact, concurrent infection of HBV and HCV is not uncommon, especially in areas where both viruses are highly prevalent as the mode of transmission is the same (6, 7). Co-infection with HBV and HCV has been observed in China, Spain, Italy, Japan, and Iran with a reported prevalence of 5~40%, while 2~25% of patients with chronic HCV infection are HBsAg-positive (7). HBV-HCC accounts for approximately 75~80% of patients with HCC in China, and the proportion of Chinese patients with HCV-associated HCC has rapidly increased over the last two decades. The proportion of HCC patients with HBV/HCV co-infection has, however, likely been underestimated in China (8).
Liver resection is widely accepted as first-line curative treatment for HCC (9). Numerous studies have reported long-term outcomes after curative liver resection for patients with HBV-HCC or HCV-HCC. In virtually all previous studies, patients with HCC and HBV/HCV co-infection (i.e., HBV/HCV-HCC) were excluded from the analytic cohorts due to small case numbers. In fact, to our knowledge, there have been no studies which focused on patients with HBV/HCV-HCC. As such, the long-term surgical outcomes of patients with HBV/HCV-HCC versus HBV-HCC who underwent hepatic resection were compared in this study using a large multicenter database.
Material and Methods
Study Population
Data from a Chinese multi-institutional database of patients who underwent curative-intent liver resection for HCC between January 2005 and December 2017 at 11 hospitals were retrospectively analyzed (Zhejiang Provincial People’s Hospital, Eastern Hepatobiliary Surgery Hospital, Liuyang People’s Hospital, First Affiliated Hospital of Harbin Medical University, Pu’er People’s Hospital, Fourth Hospital of Harbin, Pingxiang Mining Group General Hospital, Tongji Hospital, Ziyang First People’s Hospital, Southwest Hospital, and Affiliated Tumor Hospital of Guangxi Medical University). The diagnosis of HCC was confirmed by histopathological examination of resected specimens. Patients less than 18 years old, as well as individuals with recurrent HCC or who underwent antitumor treatment before surgery were excluded. Curative-intent liver resection was an R0 resection, which was defined as complete resection of all microscopic and macroscopic tumors. Patients who had microscopically positive (R1 resection) or grossly positive (R2 resection) margins were excluded. As the primary aim of the study was to compare surgical outcomes between patients with HBV/HCV-HCC and HBV-related HCC, patients with HCV-HCC or with non-HBV/HCV-HCC were also excluded.
Data were collected using a standardized form both in a prospective fashion and retrospective fashion depending on the data field. The process of pre-operative work-up and evaluation among participating hospitals was virtually identical. The selection criteria for liver resection for HCC were constant over the study period and included location and number of tumors, liver functional reserve and volume of future liver remnant as reported previously (10, 11). The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Boards of all the participating hospitals. Individual consent for this retrospective analysis was waived.
Clinical Characteristics, Operative Variables, and Short-Term Outcomes
Patient demographic characteristics included age, sex, diabetes mellitus, body mass index (BMI), and American Society of Anesthesiologists (ASA) score. The clinicopathologic characteristics included Child-Pugh grading, presence/absence of cirrhosis and/or portal hypertension, serum anti-HCV antibody (anti-HCV) positivity, serum hepatitis B surface antigen (HBsAg) positivity, preoperative anti-HBV therapy, preoperative HBV-DNA level, preoperative bilirubin and albumin levels, preoperative alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, preoperative alpha-fetoprotein (AFP) level, largest tumor size, tumor number, macroscopic or microscopic vascular invasion, satellites nodules, tumor differentiation, and tumor encapsulation were recorded. Cirrhosis was diagnosed by histopathological examination, and portal hypertension was defined as presence of either esophageal varices, or splenomegaly with a decrease in platelet count (≤ 100×109/L). Operative variables included intraoperative blood loss, requirement of blood transfusion, extent of hepatectomy (major or minor), type of liver resection (anatomical or non-anatomical), and resection margin. Major hepatectomy was defined as resection of three or more Couinaud liver segments, and minor hepatectomy as resection of fewer than three segments. Anatomical resection was defined using the Brisbane 2000 Nomenclature of Liver Anatomy and Resections, while non-anatomical resections included wedge resection or limited resection (12). Short-term surgical outcomes included postoperative 30-day mortality and morbidity. Postoperative morbidity included the occurrence of postoperative acute hepatic failure, biliary complications, sepsis, pulmonary, renal, cardiac and wound complications within 30 days of surgery (11, 13, 14). Morbidity was graded according to the Clavien-Dindo classification, and major morbidity was defined as Clavien-Dindo grade ≥ 3 (15).
Patient Follow-up
After hospital discharge, patients were followed-up in each hospital according to a relatively standardized recurrence monitoring program. In the first 6 months of operation, patients were monitored once every 1~2 months, then once every 2-3 months for the next 18 months, and thereafter once every 3~6 months (10). Patients were monitored for recurrence using physical examination, serum AFP, ultrasound or CT or MRI. When recurrence or distant metastasis was suspected, additional investigations were performed, including angiography, bone scan or positron emission tomography. Tumor recurrence was defined as emergence of intrahepatic or extrahepatic nodules with typical imaging features consistent with HCC on enhanced CT or MRI imaging regardless of serum AFP levels. Management of recurrence was based on patterns of recurrent tumors, residual liver functional reserve, and general condition of patients. Treatment included re-resection, local ablation therapy, liver transplantation, transcatheter arterial chemoembolization (TACE), radiotherapy, systematic therapy, or supportive therapy. The site of initial recurrence, the causes of mortality, dates of tumor recurrence, death and last follow-up were recorded.
Study Endpoints and Propensity Score Matching (PSM)
For the purposes of this study, patients with HBV/HCC-HCC and HBV-HCC were compared. HBV/HCV-HCC was defined as pathologically confirmed HCC with serum anti-HCV and HBsAg positivity, while HBV-HCC was defined as pathologically confirmed HCC with serum HBsAg positivity and anti-HCV negativity. The study endpoints were long-term oncological outcomes including overall survival (OS) and recurrence-free survival (RFS). OS was defined as the time from surgery to death from any cause, while RFS was defined as the time from surgery to tumor recurrence or occurrence of a new HCC, or death with evidence of recurrence.
After excluding patients with postoperative 30-day death, patients with HBV/HCV-HCC were matched with patients with HBV-HCC using the propensity score matching (PSM) method as previously described by Rubin and Rosenbaum (16). Covariates entered into the PSM model included age, sex, diabetes mellitus, overweight (BMI > 24kg/m2), ASA score, cirrhosis, portal hypertension, Child-Pugh grading, preoperative AST, ALT, and AFP levels, largest tumor size, tumor number, macrovascular and microvascular invasion, satellite nodules, and tumor differentiation. The model was used to provide a one-to-one match between the two groups; details of the matching procedure have been described previously (11, 17, 18).
Statistical Analysis
Statistical analysis was performed using the IBM SPSS Statistics version 25.0. Continuous variables were expressed as mean ± standard deviations or medians (range) as appropriate after testing for normal distribution using the Kolmogorov-Smirnov test. Categorical variables were reported as numbers (n) and proportions (%). Continuous variables were compared using the Student t test and categorical variables were compared using the Fisher’s exact test or the χ2 test, as appropriate. OS and RFS were calculated by the Kaplan-Meier method and patients with HBV/HCV-HCC were compared with those with HBV-HCC using the log-rank test before and after PSM. Univariable and multivariable Cox proportional hazard regression analyses were performed to identify risk factors associated with OS and RFS after curative liver resection for HCC among patients with HBV/HCV co-infection. All the statistical tests were two-tailed and a P value of less than 0.05 was considered statistically significant.
Results
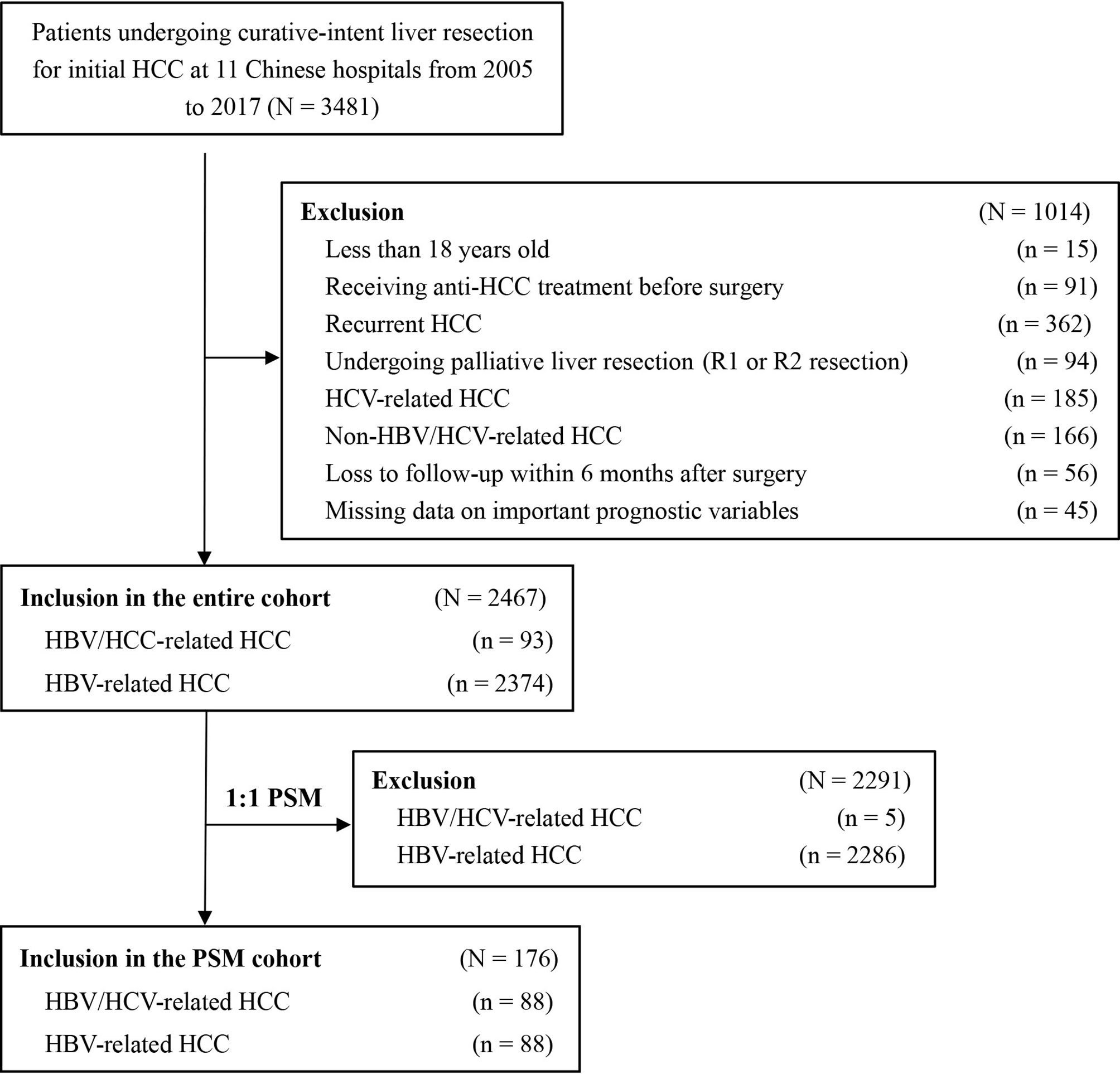
Among the 3,481 patients initially identified, 1014 patients did not fit the inclusion criteria and were excluded. The analytic cohort consisted of 2,467 patients with HBV/HCV-HCC or HBV-HCC who underwent curative liver resection (Figure 1). There were 93 patients with HBV/HCV-HCC and 2,374 with HBV-HCC. PSM was used to create 88 matched pairs of patients who had HBV/HCV-HCC or HBV-HCC.
Comparisons of Clinicopathological Variables and Short-term Outcomes
Patient baseline characteristics, operative variables, and short-term outcomes between patients with HBV/HCV-HCC and HBV-HCC before and after PSM are shown in Table 1. Compared with patients who had HBV-HCC, patients with HBV/HCV-HCC were older (Age > 60 years: 44.1% vs. 19.0%), more often had cirrhosis (92.5% vs. 77.7%) and portal hypertension (43.1% vs. 27.3%) (all P < 0.05). Patients with HBV/HCV-HCC were less often to have larger tumors (largest tumor size > 5 cm: 31.2% vs. 47.7%), multiple tumors (10.8% vs. 20.7%) or satellite nodules (12.9% vs. 23.1%) (all P < 0.05). There were no significant differences in other baseline characteristics and operative variables between the two groups. Patients with HBV/HCV-HCC had higher postoperative mortality and morbidity rates than patients with HBV-HCC (4.3% and 50.5% vs. 1.3% and 33.1%, both P < 0.05).
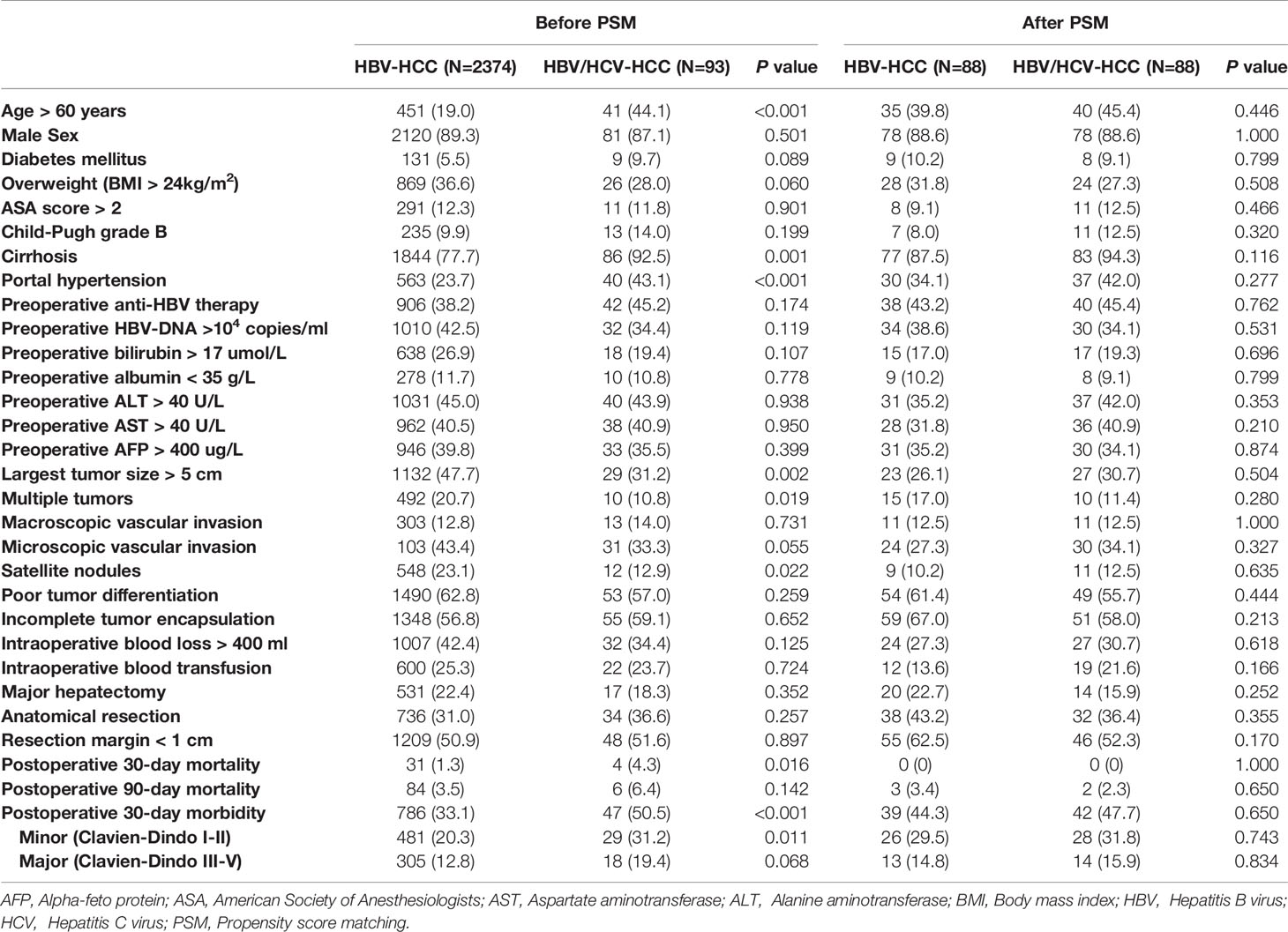
Table 1 Comparisons of patients’ clinicopathologic characteristics, operative variables, and short-term outcomes between patients with HBV/HCV-HCC and HBV-HCC before and after propensity score matching.
After PSM, all the clinicopathological and operative variables were balanced among the two groups of patients with HBV/HCC-HCC and HBV-HCC (all P > 0.02). After PSM, postoperative overall morbidity (47.7% vs. 44.3%, P = 0.650), minor morbidity (31.8% vs. 29.5%, P = 0.743) and major morbidity (15.9% vs. 14.8%, P = 0.834) rates were comparable between the two groups.
Comparisons of Long-term Outcomes
Table 2 demonstrates long-term outcomes of patients with HBV/HCV-HCC versus HBV-HCC before and after PSM. With a median follow-up of 59.8 months, mortality and recurrence were observed in 47.2% and 52.8% of patients with HBV/HCV-HCC, respectively; in contrast, mortality and recurrence occurred in 52.4% and 61.9% of patients with HBV-HCC, respectively (P = 0.333 and 0.084, respectively). In analyzing the entire cohort, the 5-year OS and RFS rates after curative liver resection among patients with HBV/HCV-HCC were 51.9% and 39.8%, respectively, when compared with 54.5% and 37.9%, respectively in patients with HBV-HCC (P = 0.998 and 0.694, respectively) (Figures 2A, B).
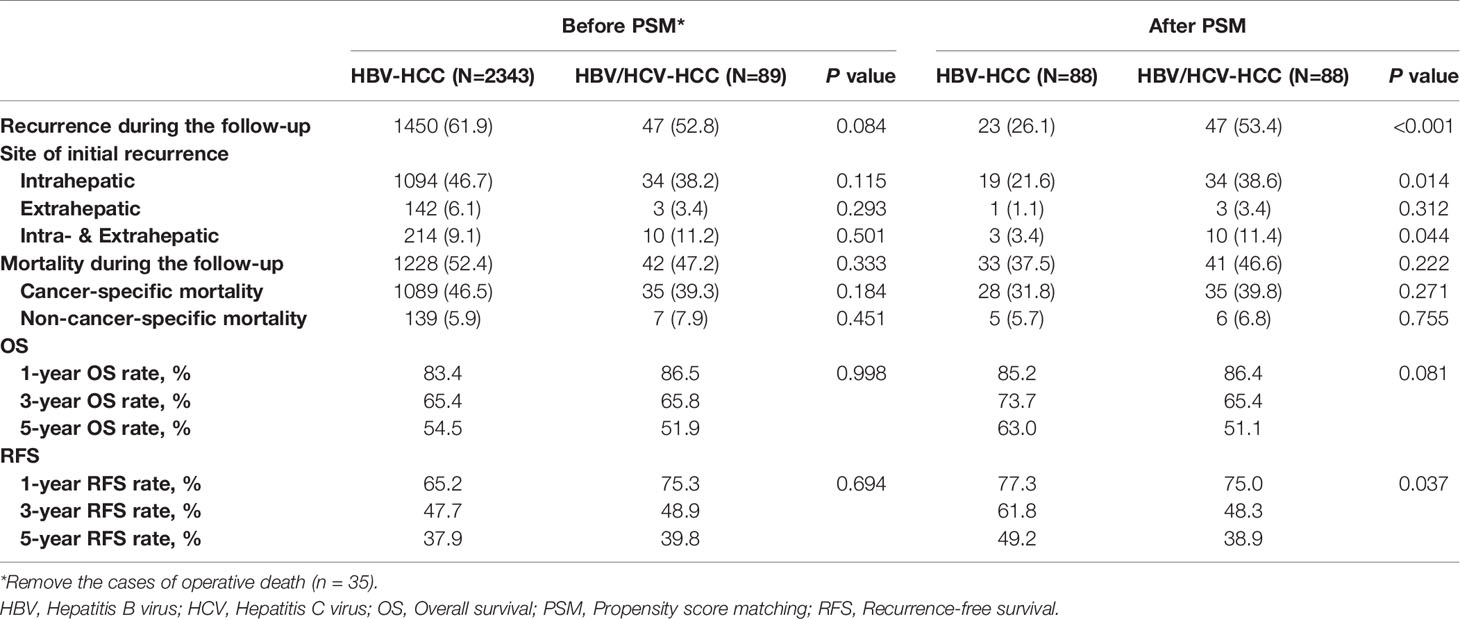
Table 2 Comparisons of long-term outcomes between patients with HBV/HCV-HCC and HBV-HCC before and after propensity score matching.
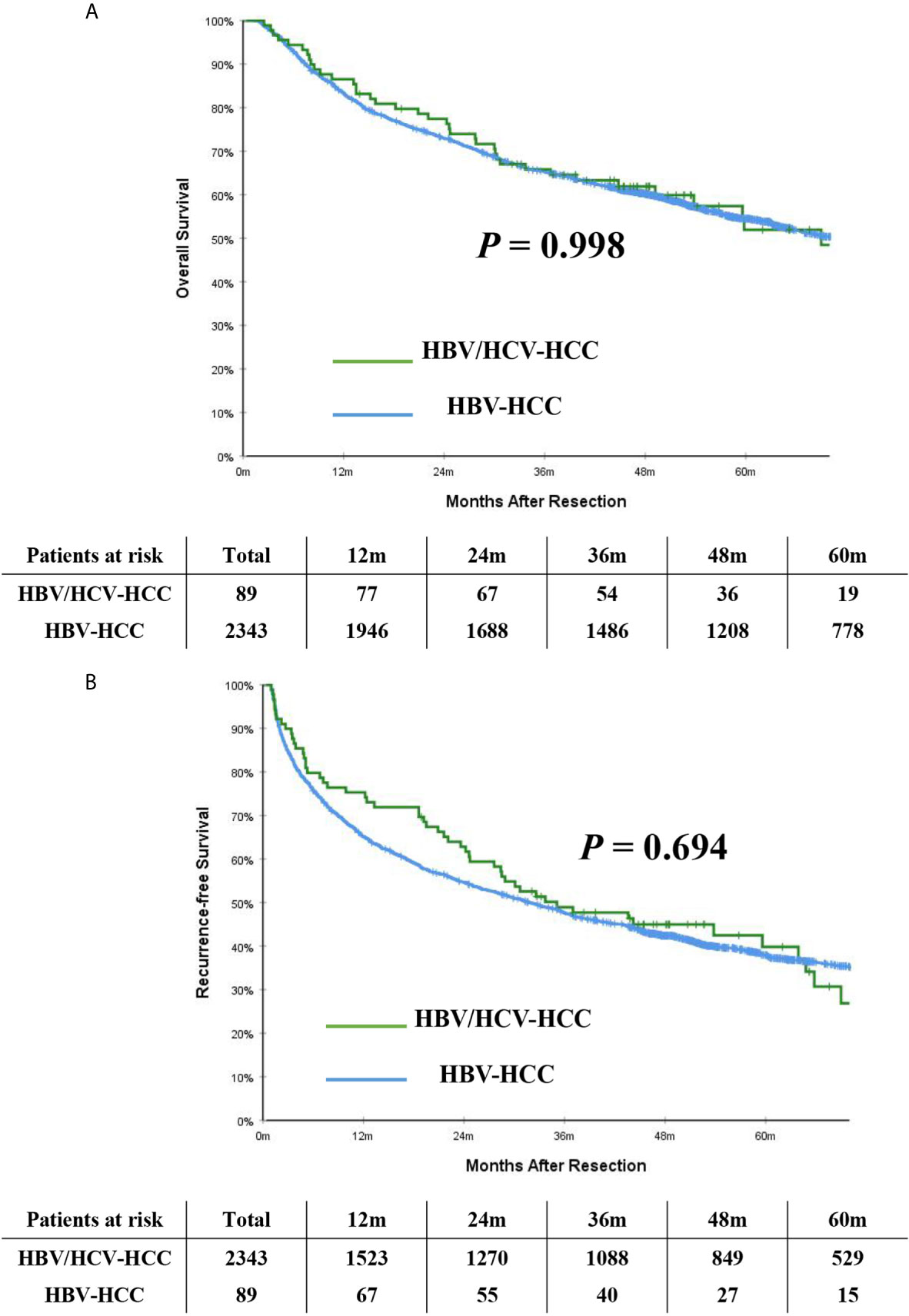
Figure 2 Kaplan-Meier curves of overall survival (A) and recurrence-free survival (B) between patients with HBV/HCV-HCC and HBV-HCC in the entire cohort.
After PSM, the incidence of mortality among the 88 patients with HBV/HCV-HCC was still comparable to the matched 88 patients who had HBV-HCC (46.6% vs. 37.5%, P = 0.222); however, the incidence of recurrence among the HBV/HCV-HCC group was higher than the HBV-HCC group (53.4% vs. 26.1%, P < 0.001). On PSM, the 5-year OS and RFS rates among patients with HBV/HCV-HCC were 51.1% and 38.9%, which were worse than patients who had HBV-HCC (63.0% and 49.2%, P= 0.081 and 0.037, respectively). In the PSM cohort, OS and RFS among patients with HBV/HCV-HCC versus patients with HBV-HCC are demonstrated in Figures 3A, B.
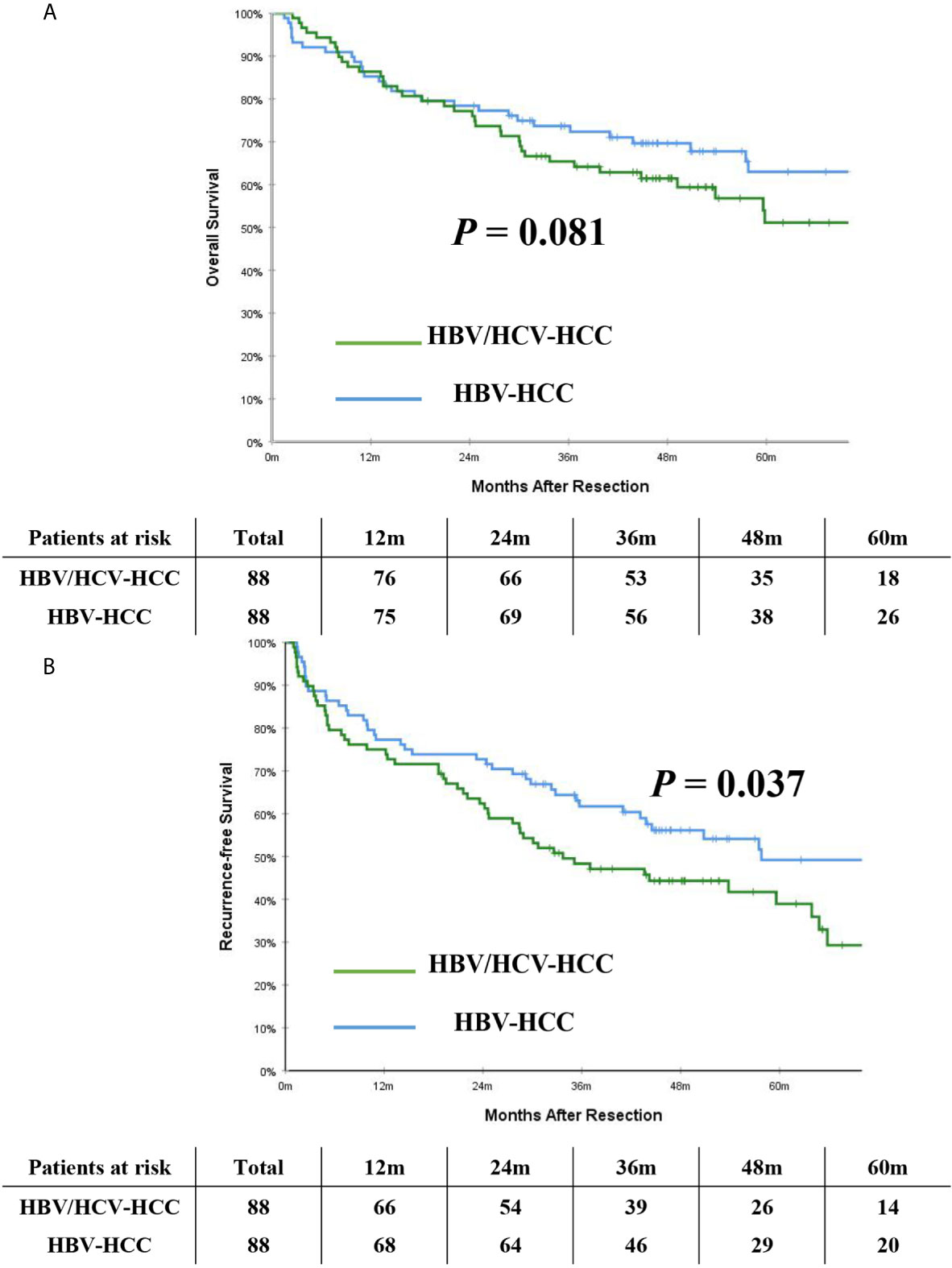
Figure 3 Kaplan-Meier curves of overall survival (A) and recurrence-free survival (B) between patients with HBV/HCV-HCC and HBV-HCC in the propensity score matching cohort.
Prognostic Analysis for Patients With HBV/HCV-HCC
On multivariable analysis after controlling for competing risk factors (Table 3), Child-Pugh grade B, largest tumor size > 5cm, multiple tumors, and macroscopic vascular invasion were independent risk factors associated with poorer OS, while largest tumor size > 5cm, multiple tumors, and macroscopic and microscopic vascular invasion were independent risk factors associated with worse RFS among patients undergoing curative liver resection for HBV/HCV-HCC. Compared with independent risk factors associated with OS and RFS after curative resection for HBV-HCC (Table 4), there were some common independent risk factors between patients with HBV-HCV-HCC and patients with HBV-HCC, including largest tumor size > 5cm, multiple tumors, and macroscopic and microscopic vascular invasion.
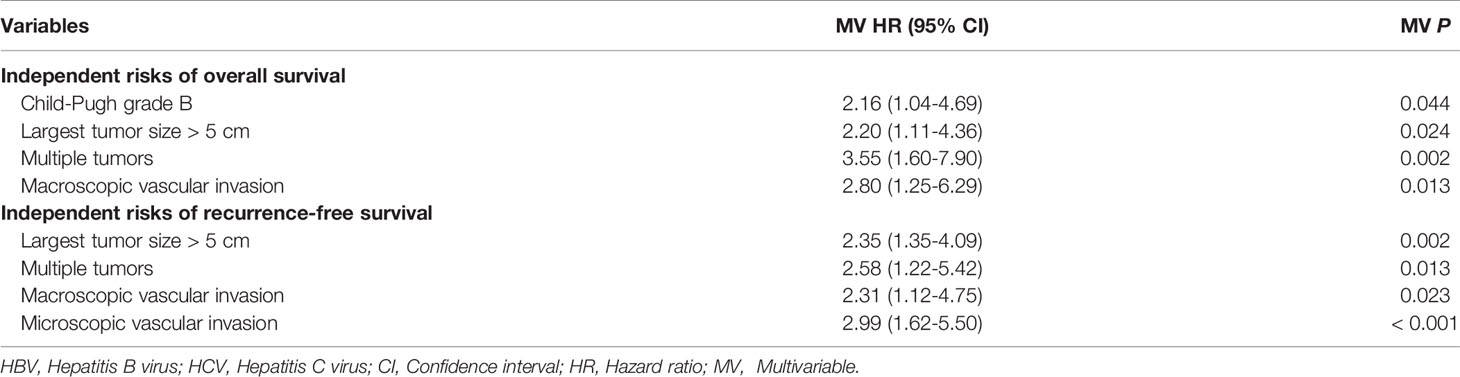
Table 3 Independent prognostic factors associated with overall survival and recurrence-free survival after liver resection for hepatocellular carcinoma among patients with HBV/HCV co-infection by multivariable Cox-regression analysis.
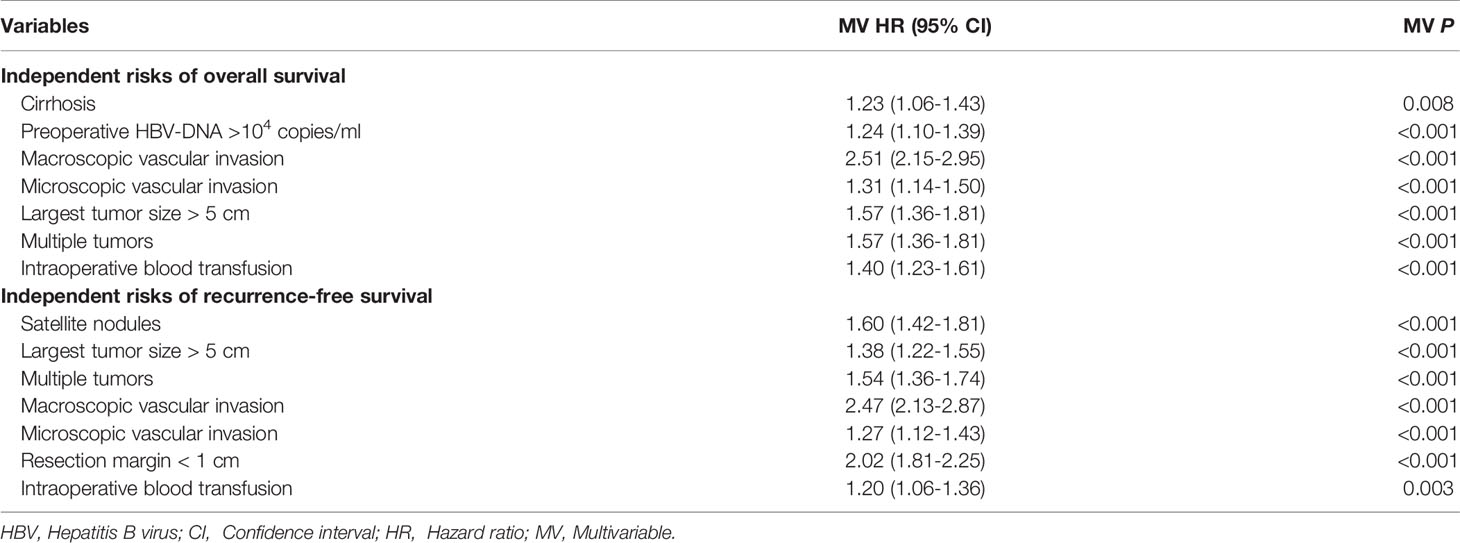
Table 4 Independent prognostic factors associated with overall survival and recurrence-free survival after liver resection for hepatocellular carcinoma among patients with only HBV infection by multivariable Cox-regression analysis.
Discussion
Multiple studies have reported that patients with HBV/HCV co-infection progress faster, have more severe liver disease, and have a higher risk of liver cirrhosis and HCC versus patients with HBV or HCV infection alone (6–8, 19–25). Comparisons of clinical patterns and prognosis, including morbidity and mortality, as well as survival and recurrence, after liver resection among patients with HBV/HCV-HCC versus patients with HBV-HCC have not been well studied. In the current study, data from a large multicenter observational cohort was utilized to identify patients with co-infection with HBV/HCV-HCC. Of note, patients with HBV/HCV-HCC were older, more often had cirrhosis and portal hypertension, yet less often had larger tumors, multiple tumors, or satellite nodules than patients with HBV-HCC. In the entire cohort, although patients with HBV/HCV-HCC had a reasonably lower postoperative mortality rate and an acceptable postoperative morbidity rate after liver resection, the mortality and morbidity rates were higher than patients with HBV-HCC. After balancing baseline and clinicopathological characteristics using PSM, patients with HBV/HCV-HCC had lower 5-year OS and RFS rates after surgery (51.1% and 38.9%, respectively) versus patients with HBV-HCC (63.0% and 49.2%, respectively). In addition, largest tumor size > 5cm, multiple tumors, and macroscopic vascular invasion were independent risk factors associated with worse OS and RFS after liver resection for HBV/HCV-HCC. To our knowledge, this is the first study to evaluate surgical outcomes of liver resection for HBV/HCV-HCC. The results provided useful information that may help inform preoperative planning and postoperative surveillance for patients with HBV/HCV co-infection undergoing liver resection for HCC.
The reasons attributing to the poor short-term and long-term outcomes among patients with HCC who have HBV/HCV co-infection are possibly be related to several factors. Previous studies have suggested a higher risk of developing advanced liver damage among patients with HBV/HCV co-infection versus individuals with either infection alone, including the degree of liver fibrosis or cirrhosis, or even the proportion of patients with portal hypertension (26). This may explain the higher postoperative mortality and morbidity rates noted among patients with HBV/HCV-HCC than patients with HBV-HCC. In the current study, almost all (> 90%) patients with HBV/HCV-HCC failed to receive anti-HCV treatment before liver resection due to a low awareness of chronic HCV infection in China where serum HCV test is not routinely obtained until prior to or after surgery. Therefore, although most of the 93 patients had known HBV infection status, less than 30% of patients had a known history of HCV infection until just prior to or after surgery. Furthermore, patients who underwent resection before 2017 were not able to be treated for HCV, as development of effective drugs against HCV infection was after that time. As such, HCC patients with HBV/HCV co-infection may have poorer long-term survival outcomes after liver resection for HCC because many of these patients had not been treated for HCV. The pathogenesis of both HBV and HCV infections is generally immune mediated, yet these viruses have evolved multiple mechanisms to escape immune elimination and continue replicating in an infected host for many years. Multiple carcinogenesis pathways induced by HBV/HCV co-infection, have been postulated to increase severity of liver disease, increase inflammation specifically and increase oncogenicity (8), which can lead to an increased likelihood to develop tumor recurrence or de novo tumors than tumors only induced by HBV infection (26). Understanding the common mechanisms of HBV and HCV pathogenesis, including changes of DNA methylation, miRNA expression profile and constitutive activation of many signal transduction pathways will help researchers to focus efforts on therapeutic targets that may be used to develop new treatment approaches for patients with HCC (7, 23, 27, 28).
There are several well-known clinicopathological variables which are associated with long-term prognosis with worse OS and RFS among patients undergoing liver resection for HBV/HCV-HCC. These factors include largest tumor size > 5 cm, multiple tumors, and macroscopic vascular invasion, and patients with these risk factors are particularly need of more intense surveillance and adjuvant therapy following liver resection for HBV/HCV-HCC. Recent guidelines recommend surveillance for tumor recurrence after HCC resection at 4-month intervals for patients with a history of HCV infection. The results of this study suggested standard postoperative anti-HBV and anti-HCV therapies and postoperative surveillance once every 3 months should be recommended for patients undergoing curative liver resection for HBV/HCV-HCC.
The present study had several limitations. The study was retrospective with its inherent proneness to potential biases. There were no unified diagnostic criteria for HBV/HCV-HCC (19), which may result in an underestimation of 40%-50% of unrecognized HCV-infected patients with occult HBV infection [defined as absence of hepatitis B surface antigen and HBV-DNA in serum, but detectable HBV-DNA in liver (6, 8)]. Information of viral genotype, baseline viral load, viral load changes over time, as well as presence or absence of regular anti-hepatitis therapies were also not evaluated. Since patients in this study had resectable tumors with compensated liver function, the data cannot be generalized to all patients with HBV/HCV-HCC. Although the PSM method was used to help balance factors between groups, the relatively small number of patients with HBV/HCV-HCC (n = 93) limited the statistical power. To our knowledge, this study, however, is the first to compare surgical outcomes among patients with HBV/HCC-HCC and patients with HBV-HCC. In addition, patients in the current study were also treated exclusively in China. As such, data from the current study should be externally validated in Western patients to ensure generalizability to a broader population of patients.
Conclusion
In conclusion, postoperative morbidity and mortality rates, and long-term prognosis among patients with HBV/HCV-HCC were worse compared with patients who underwent resection for HBV-HCC. While several risk factors, including large tumor size, multiple tumors, and macroscopic vascular invasion were already known to be associated with long-term prognosis, the results of this study served to highlight the differences in short- and long-term outcomes among patients undergoing curative liver resection for HBV/HCV-HCC, especially when compared with patients with HBV-HCC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception: H-DJ, LL, TY, and D-SH. Study design: H-DJ, LL, CL, HWu, HWa, FS, C-WZ, and TY. Administrative support: FS, D-SH, C-WZ, and TY. Data collection and acquisition: H-DJ, LL, CL, HWu, HWa, Y-JL, Y-HZ, W-MG, X-PF, W-GZ, T-HC, Z-YC, J-HZ, Y-KD, and Q-RX. Data analysis: H-DJ, LL, and TY. Manuscript preparation: H-DJ, LL, CL, HWu, and TY. Critical revision: WL, TP, C-WZ, FS, and D-SH. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by the National Natural Science Foundation of China (No. 81972726, TY). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HCC, Hepatocellular carcinoma; HBV, Hepatitis B virus; HCV, Hepatitis C virus; OS, Overall survival; RFS, Recurrence-free survival; ASA, American Society of Anesthesiologists; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; BMI, Body mass index; HBsAg, Hepatitis B surface antigen; AFP, Alpha-feto protein; SD, Standard deviation; HR, Hazard ratio; CI, Confidence interval; DAAs, Direct-acting antiviral agents; IFN, Interferon; US, Ultrasonography; PSM, Propensity score matching; MV, Multivariable.
References
1. Forner A, Reig M, Bruix J. Hepatocellular Carcinoma. Lancet (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
2. Gerbes A, Zoulim F, Tilg H, Dufour JF, Bruix J, Paradis V, et al. Gut Roundtable Meeting Paper: Selected Recent Advances in Hepatocellular Carcinoma. Gut (2018) 67:380–8. doi: 10.1136/gutjnl-2017-315068
3. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology (2018) 67:358–80. doi: 10.1002/hep.29086
4. Franssen B, Alshebeeb K, Tabrizian P, Marti J, Pierobon ES, Lubezky N, et al. Differences in Surgical Outcomes Between Hepatitis B- and Hepatitis C-Related Hepatocellular Carcinoma: A Retrospective Analysis of a Single North American Center. Ann Surg (2014) 260:650–8. doi: 10.1097/SLA.0000000000000917
5. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (2018) 68:723–50. doi: 10.1002/hep.29913
6. Jamma S, Hussain G, Lau DT. Current Concepts of HBV/HCV Coinfection: Coexistence, But Not Necessarily in Harmony. Curr Hepat Rep (2010) 9:260–9. doi: 10.1007/s11901-010-0060-4
7. Sagnelli E, Sagnelli C, Macera M, Pisaturo M, Coppola N. An Update on the Treatment Options for HBV/HCV Coinfection. Expert Opin Pharmacother. (2017) 18:1691–702. doi: 10.1080/14656566.2017.1398233
8. Mavilia MG, Wu GY. HBV-HCV Coinfection: Viral Interactions, Management, and Viral Reactivation. J Clin Transl Hepatol (2018) 6:296–305. doi: 10.14218/JCTH.2018.00016
9. Kauffmann R, Fong Y. Post-Hepatectomy Liver Failure. Hepatobiliary Surg Nutr (2014) 3:238–46. doi: 10.3978/j.issn.2304-3881.2014.09.01
10. Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg (2019) 154:209–17. doi: 10.1001/jamasurg.2018.4334
11. Yang T, Lu JH, Lau WY, Zhang TY, Zhang H, Shen YN, et al. Perioperative Blood Transfusion Does Not Influence Recurrence-Free and Overall Survivals After Curative Resection for Hepatocellular Carcinoma: A Propensity Score Matching Analysis. J Hepatol (2016) 64:583–93. doi: 10.1016/j.jhep.2015.10.012
12. Strasberg SM, Phillips C. Use and Dissemination of the Brisbane 2000 Nomenclature of Liver Anatomy and Resections. Ann Surg (2013) 257:377–82. doi: 10.1097/SLA.0b013e31825a01f6
13. Yang T, He H, Yuan J, Zhang J, Lu J, Lau WY, et al. Surgery for Hepatocellular Carcinoma Presenting With Variceal Bleeding: The Eastern Experience. J Surg Oncol (2016) 113:165–74. doi: 10.1002/jso.24106
14. Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk Factors Influencing Postoperative Outcomes of Major Hepatic Resection of Hepatocellular Carcinoma for Patients With Underlying Liver Diseases. World J Surg (2011) 35:2073–82. doi: 10.1007/s00268-011-1161-0
15. Dindo D, Demartines N, Clavi P-A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
16. Thomas N, Rubin DB. Matching Using Estimated Propensity Scores - Relating Theory to Practice. Biometrics: J Biometric Society: Int Soc Devoted to Math Stat Aspects Biol (1996) 52:249–64. doi: 10.2307/2533160
17. Xing H, Sun LY, Yan WT, Quan B, Liang L, Li C, et al. Repeat Hepatectomy for Patients With Early and Late Recurrence of Hepatocellular Carcinoma: A Multicenter Propensity Score Matching Analysis. Surgery (2021) 169:911–20. doi: 10.1016/j.surg.2019.11.005
18. Yang T, Tabrizian P, Zhang H, Lau WY, Han J, Li ZL, et al. Comparison of Patterns and Outcomes of Liver Resection for Hepatocellular Carcinoma: East vs West. Clin Gastroenterol Hepatol (2017) 15:1972–4. doi: 10.1016/j.cgh.2017.06.025
19. Abdelaal R, Yanny B, El Kabany M. HBV/HCV Coinfection in the Era of HCV-DAAs. Clin Liver Dis (2019) 23:463–72. doi: 10.1016/j.cld.2019.04.003
20. Cho LY, Yang JJ, Ko KP, Park B, Shin A, Lim MK, et al. Coinfection of Hepatitis B and C Viruses and Risk of Hepatocellular Carcinoma: Systematic Review and Meta-Analysis. Int J Cancer. (2011) 128:176–84. doi: 10.1002/ijc.25321
21. Chu CJ, Lee SD. Hepatitis B Virus/Hepatitis C Virus Coinfection: Epidemiology, Clinical Features, Viral Interactions and Treatment. J Gastroenterol Hepatol (2008) 23:512–20. doi: 10.1111/j.1440-1746.2008.05384.x
22. European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: Management of Chronic Hepatitis B Virus Infection. J Hepatol (2012) 57:167–85. doi: 10.1016/j.jhep.2012.02.010
23. Donato F, Boffetta P, Puoti M. A Meta-Analysis of Epidemiological Studies on the Combined Effect of Hepatitis B and C Virus Infections in Causing Hepatocellular Carcinoma. Int J Cancer. (1998) 75:347–54. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2
24. Fattovich G, Tagger A, Brollo L, Giustina G, Pontisso P, Realdi G, et al. Hepatitis C Virus Infection in Chronic Hepatitis B Virus Carriers. J Infect Dis (1991) 163:400–2. doi: 10.1093/infdis/163.2.400
25. Pol S, Haour G, Fontaine H, Dorival C, Petrov-Sanchez V, Bourliere M, et al. The Negative Impact of HBV/HCV Coinfection on Cirrhosis and Its Consequences. Aliment Pharmacol Ther (2017) 46:1054–60. doi: 10.1111/apt.14352
26. Arzumanyan A, Reis HM, Feitelson MA. Pathogenic Mechanisms in HBV- and HCV-Associated Hepatocellular Carcinoma. Nat Rev Cancer. (2013) 13:123–35. doi: 10.1038/nrc3449
27. Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis Accelerates the Progression of Liver Damage of Chronic Hepatitis C Patients and Correlates With Specific HCV Genotype and Visceral Obesity. Hepatology (2001) 33:1358–64. doi: 10.1053/jhep.2001.24432
Keywords: hepatocellular carcinoma, hepatectomy, hepatitis B virus, hepatitis C virus, overall survival, recurrence-free survival
Citation: Jia H-D, Liang L, Li C, Wu H, Wang H, Liang Y-J, Zhou Y-H, Gu W-M, Fan X-P, Zhang W-G, Chen T-H, Chen Z-Y, Zhong J-H, Lau WY, Pawlik TM, Diao Y-K, Xu Q-R, Shen F, Zhang C-W, Huang D-S and Yang T (2021) Long-Term Surgical Outcomes of Liver Resection for Hepatocellular Carcinoma in Patients With HBV and HCV Co-Infection: A Multicenter Observational Study. Front. Oncol. 11:700228. doi: 10.3389/fonc.2021.700228
Received: 25 April 2021; Accepted: 15 July 2021;
Published: 29 July 2021.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Chuan Li, Sichuan University, ChinaAlejandro Serrablo, Hospital Universitario Miguel Servet, Spain
Copyright © 2021 Jia, Liang, Li, Wu, Wang, Liang, Zhou, Gu, Fan, Zhang, Chen, Chen, Zhong, Lau, Pawlik, Diao, Xu, Shen, Zhang, Huang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Yang, eWFuZ3RpYW56cnlAaG90bWFpbC5jb20=; Dong-Sheng Huang, aHVhbmdkb25nc2hlbmd6akBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Hang-Dong Jia
Hang-Dong Jia Lei Liang1,2†
Lei Liang1,2† Han Wu
Han Wu Tian Yang
Tian Yang