
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 16 July 2021
Sec. Breast Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.699323
This article is part of the Research TopicAdvances in Treatment of HER2+ Breast CancerView all 8 articles
 Lili Zhang1
Lili Zhang1 Xiaohong Wu2
Xiaohong Wu2 Jun Zhou3
Jun Zhou3 Mingzhen Zhu4
Mingzhen Zhu4 Hao Yu5
Hao Yu5 Yusong Zhang6
Yusong Zhang6 Yutian Zhao7
Yutian Zhao7 Zhengxiang Han8
Zhengxiang Han8 Yujiang Guo9
Yujiang Guo9 Xiaoqing Guan10
Xiaoqing Guan10 Xufen Wang11
Xufen Wang11 Hong Xu12
Hong Xu12 Li Sun13
Li Sun13 Jiaxin Zhang14
Jiaxin Zhang14 Min Zhuang15
Min Zhuang15 Li Xie16
Li Xie16 Shiyou Yu17
Shiyou Yu17 Ping Chen18
Ping Chen18 Jifeng Feng1*
Jifeng Feng1*Background: HER2-positive breast cancer was aggressive, resulting in a poorer prognosis. This multicenter study analyzed the real-world data of women treated with pyrotinib-based therapy, aiming to describe their characteristics, treatment regimens, and to investigate the clinical outcomes.
Methods: A total of 141 patients with HER2-positive breast cancer were enrolled from February 2019 to April 2020. Last follow-up time was February 2021. All patients were treated with pyrotinib-based therapy in 21-day cycles. The primary endpoint was progression-free survival (PFS).
Results: The median PFS (mPFS) for pyrotinib-based therapy was 12.0 months (95%CI 8.1-17.8) in all patients. Among the patients with liver metastases, mPFS was 8.7 months (95%CI, 6.3-15.4) compared to 12.3 months (95%CI, 8.8-23.3) for patients without liver metastases (P=0.172). In addition, patients receiving pyrotinib-based therapy as their >2 lines treatment had a numerically lower mPFS than those receiving pyrotinib-based therapy as their ≤2 lines treatment [8.4 (95%CI, 5.9-15.4) vs. 15.1 (95%CI, 9.3-22.9) months, P=0.107]. The mPFS was 12.2 months (95%CI, 7.9-18.8) in patients with previous exposure to trastuzumab and 11.8 months (95%CI, 6.8-22.9) in patients without previous exposure to trastuzumab (P=0.732). Moreover, mPFS in patients receiving regimens with and without capecitabine were 15.1 months (95%CI, 10.0-18.8) and 8.4 months (95%CI, 6.7-22.9), respectively (P=0.070). Furthermore, in patients with brain metastases, estimated 6-month PFS rate was 70.0%, and rate at 12 months was 60.0%. Seventy patients with measurable lesions were evaluable for response. The objective response rate was 38.6% and disease control rate was 85.7%. The most common adverse event was diarrhea (85.0%).
Conclusion: Pyrotinib-based therapy showed promising efficacy in patients with HER2-positive breast cancer and was well tolerated, especially in patients treated with pyrotinib as ≤2 lines treatment and receiving regimens with capecitabine. The results of this real-world study further confirmed the intriguing efficacy of pyrotinib.
Breast cancer is one of the most commonly diagnosed malignancy worldwide. Due to lifestyle changes, the incidence of breast cancer is rising in women (1). With the development of early detection and efficient therapies, mortality from breast cancer has decreased. Nevertheless, breast cancer remains an important cause of death (2, 3). Human epidermal growth factor receptor 2 (HER2)-positive is a key oncogenic driver event, with pathogenesis mainly being the activation of the PI3K/Akt and MAPK pathways (4). Overexpression of HER2 occurs in 15%-20% of breast cancer (5).
HER2-positive breast cancer was aggressive, resulting in a poorer prognosis (6, 7). The anti-HER2 agents, such as trastuzumab, pertuzumab, lapatinib, and trastuzumab emtansine (T-DM1), have dramatically improved the survival in patients with HER2-positive breast cancer (8, 9). However, drug resistance remains a major challenge (10, 11). Thus, the continued development of novel therapies is required.
Pyrotinib is a newly-developed irreversible pan-ErbB receptor tyrosine kinase inhibitor inhibiting HER1, HER2, and HER4 (12). In an open-label phase II study, pyrotinib plus capecitabine had significantly longer progression-free survival (PFS) (18.1 months vs. 7.0 months, P<0.001) and higher objective response rate (ORR) (78.5% vs. 57.1%, P<0.05) than lapatinib plus capecitabine in patients with HER2-positive breast cancer (13). In addition, a randomized phase III trial (PHOEBE) enrolled 267 patients previously treated with trastuzumab. The results showed that the median PFS (mPFS) was significantly longer in patients receiving pyrotinib plus capecitabine than in those receiving lapatinib plus capecitabine (12.5 months vs 6.8 months, P <0.0001), and ORR was higher for patients treated with pyrotinib plus capecitabine than those treated with lapatinib plus capecitabine (67.2% vs 51.5%). The most common adverse event was diarrhea which was well manageable (14). Several randomized trials showed the promising efficacy of pyrotinib in HER2-positive breast cancer. However, the efficacy of pyrotinib in patients with different baseline characteristics in the actual clinical practice was rarely reported.
This multicenter study analyzed the real-world data of women treated with pyrotinib-based therapy, aiming to describe their characteristics, treatment regimens, and to investigate the clinical outcomes.
This was a multicenter, prospective, real-world study conducted at 15 hospitals in China. Patients with HER2-positive breast cancer were enrolled from February 2019 to April 2020. Last follow-up time was February 2021. All patients were treated with pyrotinib-based therapy in 21-day cycles. Starting dose and combination therapy with chemotherapeutic drugs and/or HER2-targeted agents and/or radiotherapy were determined by physicians’ choice based on previous clinical trials results, general health status and willing of patients, and collected in the electronic case report form (13–15). This study was registered with Chinese Clinical Trial Registry (ChiCTR1900021819).
Patients were eligible if they were aged ≥18 years and pathologically confirmed HER2-positive advanced breast cancer. HER2 status was evaluated according to the American Society of Clinical Oncology/College of American Pathologists guidelines (16). HER2-positive status was identified when (on observing within an area of tumor that amounts to >10% of contiguous and homogeneous tumor cells) there is evidence of protein overexpression by immunohistochemistry or gene amplification by in situ hybridization (HER2 copy number or HER2/CEP17 ratio based on counting at least 20 cells within the area) (16). Patients were excluded if they were pregnant or lactating; had been previously treated with pyrotinib; lost information of treatment; or received less than one cycle of treatment with pyrotinib. No limits on prior therapy were required.
The primary endpoint was PFS, which was defined as the time from the beginning of treatment with pyrotinib to confirmed disease progression or death, whichever came first. The secondary endpoints included ORR (defined as the proportion of patients with a best overall response of complete or partial response), disease control rate (DCR defined as the proportion of patients with a best overall response of complete response, partial response, or stable disease) and overall survival (OS, defined as the time from the beginning of treatment with pyrotinib to death from any cause). Tumor response assessments were conducted in patients with measurable lesions by the investigator according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Safety assessments were performed using the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
Patient characteristics, treatment regimens and starting dosages of pyrotinib were summarized as frequency count (percentage) or median (range). Median PFS was estimated by the Kaplan–Meier method, and its 95% confidence interval (CI) was calculated using the Brookmeyer-Crowley method. Median PFS between subgroups (brain metastasis, liver metastasis, the lines of pyrotinib-based therapy, prior exposure to trastuzumab, and regimen with capecitabine) were compared using the log-rank test, and the Cox proportional hazard model was used to analyze the hazard ratio (HR) and 95%CI. Analyses were performed using STATA statistical software version 15.1 or MedCalc version 18.2.1. P<0.05 was considered statistically significant.
A total of 141 patients were included from February 2019 to April 2020. Baseline characteristics are showed in Table 1. Median age was 52 (range 29–78 years). One hundred and thirty-six patients (96.5%) had Eastern Cooperative Oncology Group performance status score <2. Hormone receptor-positive was present in 56.0% of the patients. The metastatic sites were in brain (14.9%), liver (31.2%), bone (44.0%) and lung (54.6%). Trastuzumab was previously administered in the (neo)adjuvant setting, metastatic setting, and both (neo)adjuvant setting and metastatic setting to 54 (43.5%), 88 (71.0%), and 18 (14.5%) patients, respectively. Patients with primary trastuzumab-resistant breast cancer was 12.1%.
One hundred and four (73.8%) patients received pyrotinib-based therapy as the second or further line treatment. One hundred (70.9%) patients initiated pyrotinib treatment at 400 mg, 39 (27.7%) patients started with 320 mg, and 2 (1.4%) patients had a starting dose of 160 mg. The patients received the treatment regimens with capecitabine (55.3%), trastuzumab (17.7%), and endocrine therapy, radiotherapy or antiangiogenic drugs (3.5%). Only 11 (7.8%) patients were treated with pyrotinib monotherapy (Table 2).
By the cutoff date in February 2021, the median duration of follow-up was 11.3 months (range, 1.0-26.4 months) and 52 (45.6%) patients were followed up over 1 year. Fifty-eight (41.1%) patients were still receiving pyrotinib. The median PFS (mPFS) for pyrotinib-based therapy was 12.0 months (95%CI, 8.1-17.8) in all patients (Figure 1). Subgroup analyses were conducted according to brain metastasis, liver metastasis, the lines of pyrotinib-based therapy, prior exposure to trastuzumab, and regimen with capecitabine. Among the patients with liver metastases, mPFS was 8.7 months (95%CI, 6.3-15.4) compared to 12.3 months (95%CI, 8.8-23.3) for patients without liver metastases (HR=0.72; 95%CI, 0.44-1.18; P=0.174) (Figure 2 and Table 3). In addition, patients receiving pyrotinib-based therapy as their >2 lines treatment had a numerically lower mPFS than those receiving pyrotinib-based therapy as their ≤2 lines treatment [8.4 (95%CI, 5.9-15.4) vs. 15.1 (95%CI, 9.3-22.9) months; HR=0.69; 95%CI, 0.44-1.10; P=0.109] (Figure 3 and Table 3). The mPFS was 12.2 months (95%CI, 7.9-18.8) in patients with previous exposure to trastuzumab and 11.8 months (95%CI, 6.8-22.9) in patients without previous exposure to trastuzumab (HR=1.12; 95%CI, 0.56-2.25; P=0.732) (Figure 4 and Table 3). Moreover, mPFS in patients receiving regimens with and without capecitabine were 15.1 months (95%CI, 10.0-18.8) and 8.4 months (95%CI, 6.7-22.9), respectively (HR=1.51; 95%CI, 0.95-2.39; P=0.072) (Figure 5 and Table 3). Furthermore, in patients with brain metastases, estimated 6-month PFS rate was 70.0%, and PFS rate at 12 months was 60.0% (Figure 6). By the data cutoff date, the median OS has not yet been reached.
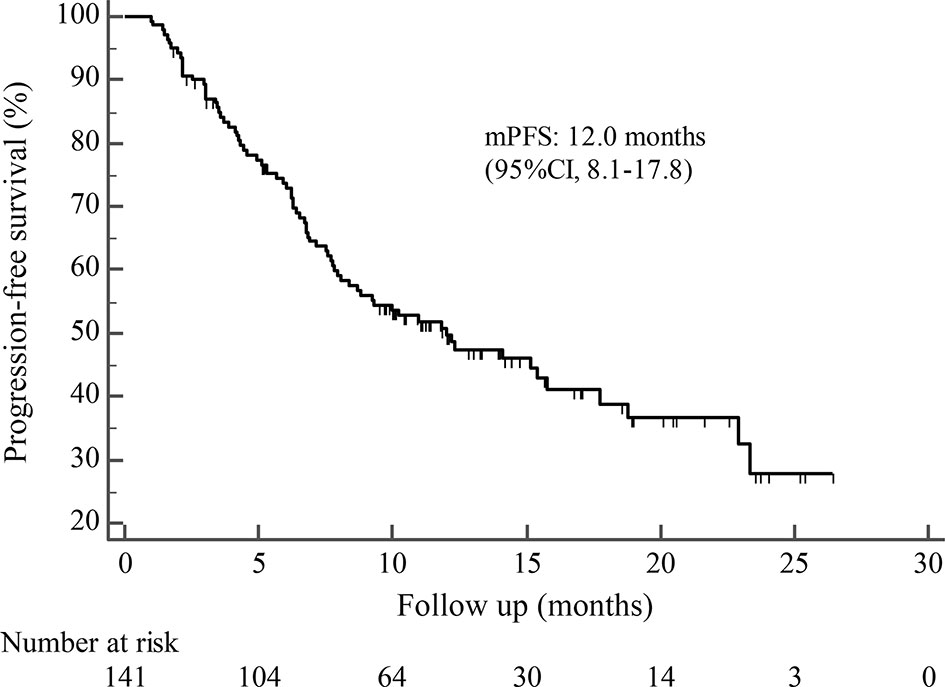
Figure 1 Kaplan-Meier estimates of progression-free survival (PFS) for all patients treated with pyrotinib-based therapy.
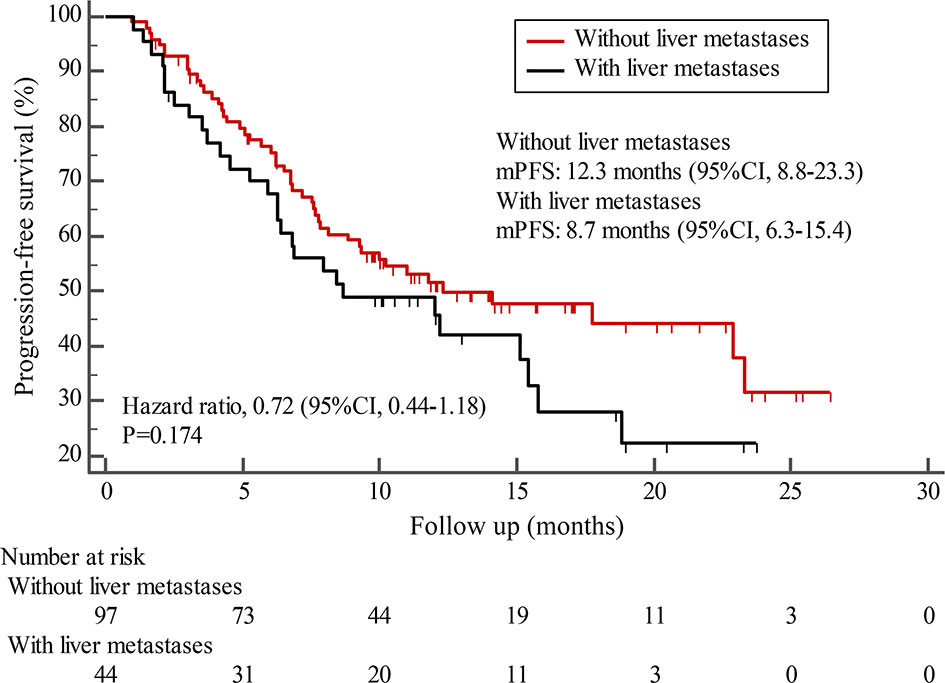
Figure 2 Kaplan-Meier estimates of progression-free survival (PFS) for patients with and without liver metastases.
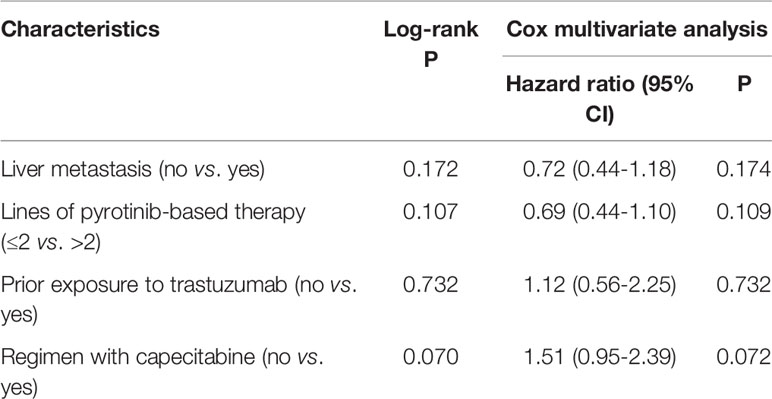
Table 3 Log-rank and Cox multivariate analyses for factors associated with progression-free survival.
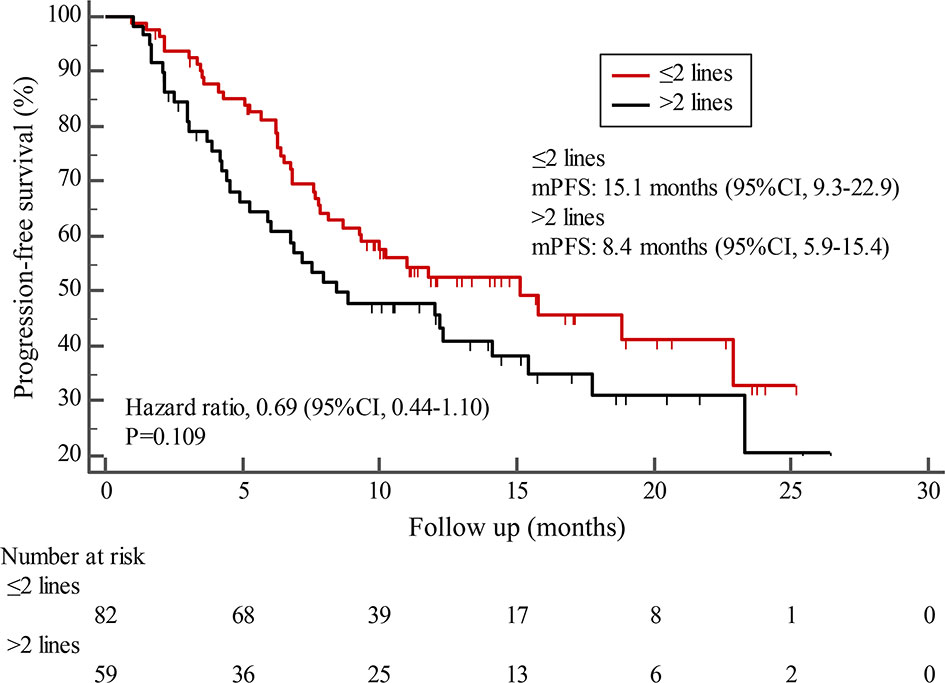
Figure 3 Kaplan-Meier estimates of progression-free survival (PFS) for patients stratified by treatment lines.
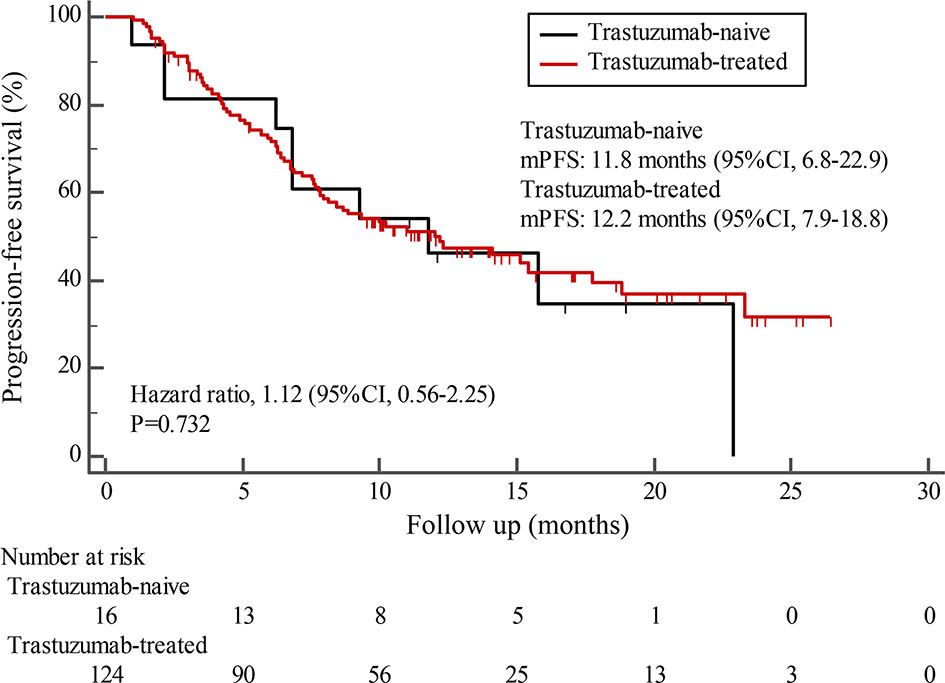
Figure 4 Kaplan-Meier estimates of progression-free survival (PFS) for patients with and without prior trastuzumab exposure.
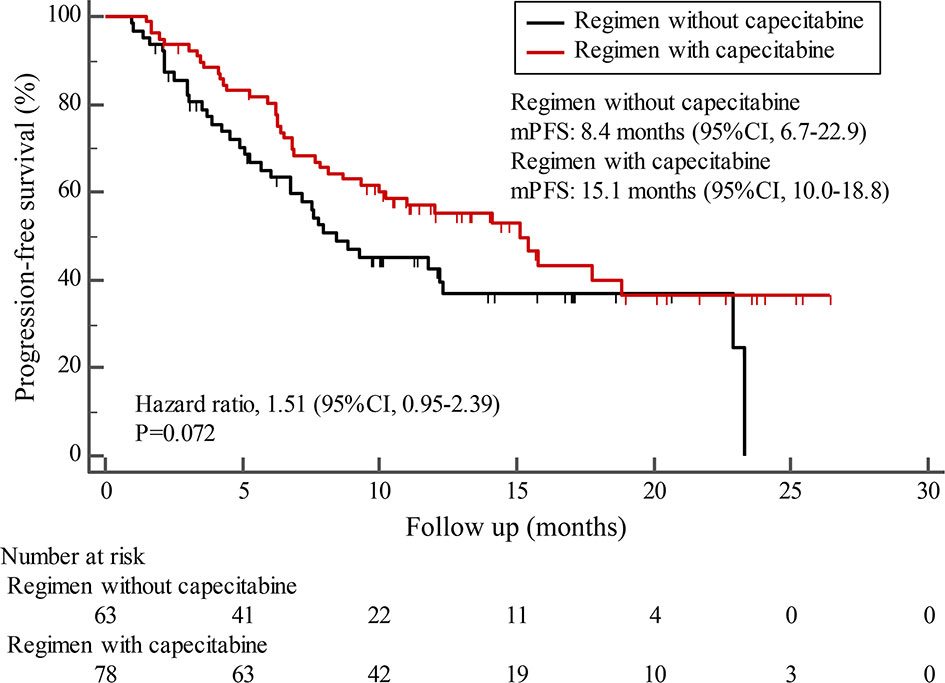
Figure 5 Kaplan-Meier estimates of progression-free survival (PFS) for patients receiving regimen with and without capecitabine.
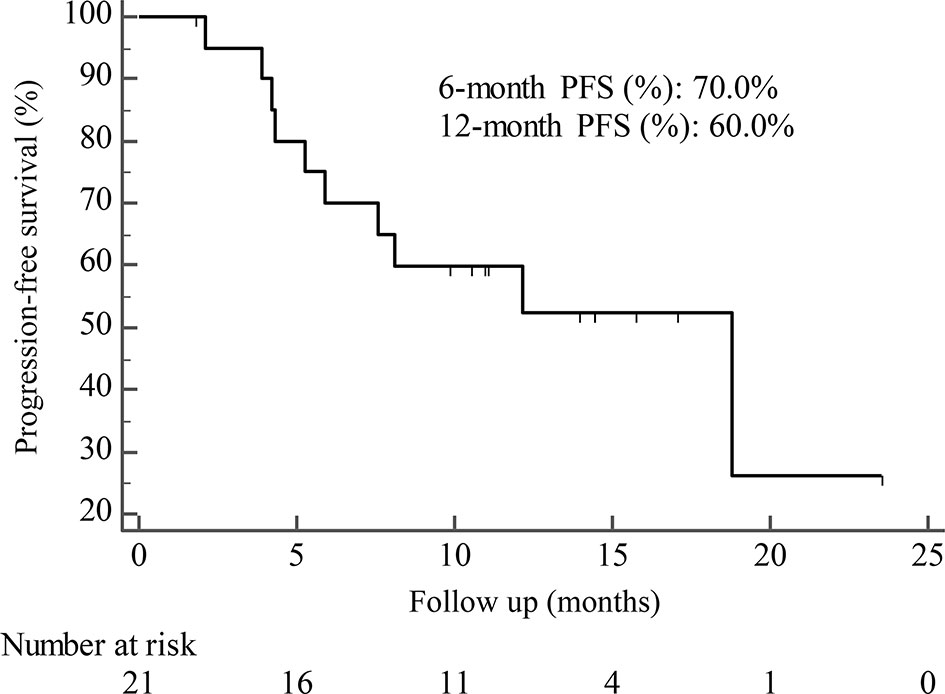
Figure 6 Kaplan-Meier estimates of progression-free survival (PFS) for patients with brain metastases.
Seventy patients with measurable lesions were evaluable for response. The ORR was 38.6% and DCR was 85.7%. One (1.4%) patient achieved complete response and 26 (37.1%) patients had partial response. Thirty-three (47.1%) patients achieved stable disease and 10 (14.3%) patients had progressive disease (Table 4). Subgroup analyses of ORR and DCR based on brain metastasis, liver metastasis, the lines of pyrotinib-based therapy, prior exposure to trastuzumab, and regimen with capecitabine are shown in Table 5.
There were 14 patients without available safety data, leaving 127 patients included in the safety assessments. The adverse events (AEs) of all grades and grade ≥3 were reported in 123 patients (96.9%) and 14 patients (11.0%), respectively (Table 6). The most common AE was diarrhea (85.0%), but only 6 patients (4.7%) reported grade ≥3 diarrhea. Moreover, the AEs of all grades that were documented in ≥15% of patients included anemia (37.0%), leukopenia (24.4%), vomiting (24.4%), neutropenia (22.0%), and hyperbilirubinemia (17.3%). No treatment-related deaths were reported.
HER2-positive breast cancer is a more aggressive phenotype (17). The anti-HER2 agents (trastuzumab, pertuzumab, lapatinib, and T-DM1) have dramatically improved the prognosis in patients with HER2-positive breast cancer (18). However, primary or acquired resistance to anti-HER2 agents remains a major challenge (10). Thus, the novel therapy is required to provide option to patients.
This study was carried out to analyze the efficacy and safety of pyrotinib-based therapy in patients with HER2-positive breast cancer in the real world. The most inspiring result of the study was a mPFS of 12.0 months, higher than that of trastuzumab (10.9 months) and T-DM1 (10.0 months) in the real-world setting (19, 20), and close to the mPFS result (12.5 months) of pyrotinib in the phase III PHOEBE study (14). In addition, the ORR of pyrotinib-based therapy in this study was 38.6% was also superior to that of T-DM1 (20.0%) (15).
Subgroup results confirmed that the mPFS in patients with liver metastases was 8.7 months, which was shorter than the 12.3 months found in patients without liver metastases. This result is concordant with previous report in which liver metastases correlated with worse survival in patients with breast cancer (21, 22). Moreover, the mPFS was 15.1 months in patients receiving pyrotinib as ≤2 lines treatment, similar to the result of trastuzumab as first-line treatment reported in the phase III PUFFIN study (14.5 months) (23). Our study demonstrated the efficacy of pyrotinib, providing evidence for the patients treated with pyrotinib as ≤2 lines treatment. In addition, 87.9% of patients in our study received prior trastuzumab treatment, thus we assessed the benefit of pyrotinib-based therapy in patients progressed on trastuzumab. The results showed that patients could benefit from pyrotinib, regardless of whether they had been previously exposed to trastuzumab or not. Our results also confirmed that pyrotinib-based therapy with capecitabine achieved a numerically higher ORR and longer mPFS than that without capecitabine, which merits further assessment in the future. In addition, among patients with brain metastases, the 6-month and 12-month PFS rates were 70.0% and 60.0%, respectively, numerically better than that of anti-HER2 monoclonal antibodies in patients with brain metastases (19, 24, 25), indicating that pyrotinib is an important treatment option for patients with brain metastases.
Diarrhea was the most common AE (85.0%), but only 4.7% of patients reported grade ≥3 diarrhea which could be well controlled. The incidence of diarrhea in our study was lower than that in previously studies of pyrotinib (14, 26), which may be the result of the relatively low dose of pyrotinib (only 70.92% of patients were treated with pyrotinib at a starting dose of 400 mg/day). The antidiarrhea treatment or dose reduction after diarrhea could well control the occurrence of diarrhea.
This study confirmed the advantages of pyrotinib. However, the data were acquired from an observational study which included potential information bias or incomplete data. The sample size was small, and the safety profile of 14 (9.9%) patients could not be evaluated due to the missing data. In addition, the median OS has not yet been reached, but the follow up is ongoing. Despite these limitations, the results of this study provide evidence for the real-world use of pyrotinib in patients with HER2-positive breast cancer.
In conclusion, pyrotinib-based therapy showed promising efficacy in patients with HER2-positive breast cancer and was well tolerated, especially in patients treated with pyrotinib as ≤2 lines treatment and receiving regimens with capecitabine. The results of the real-world study further confirmed the intriguing efficacy of pyrotinib.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Jiangsu Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
JF and LZ were involved in study conception and design. LZ, XHW, JZ, MZZ, HY, YSZ, YTZ, ZH, YG, XG, XFW, HX, LS, JXZ, MZ, LX, SY, PC, and JF were involved in the acquisition of data. LZ was involved in the analysis and interpretation of data. XHW was involved in drafting the manuscript. LZ was involved in revising the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Chinese Society of Clinical Oncology-Hengrui Oncology research foundation (grant number: Y-HR2019-0348). The funding source had no role in the study design, collection, analysis and interpretation of data, writing of the manuscript, or decision to submit the article for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank all the patients and their families for the participation.
1. Harbeck N, Gnant M. Breast Cancer. Lancet (2017) 389(10074):1134–50. doi: 10.1016/S0140-6736(16)31891-8
2. McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical Diagnosis and Management of Breast Cancer. J Nucl Med (2016) 57 Suppl 1:9S–16S. doi: 10.2967/jnumed.115.157834
3. Abderrahman B, Jordan VC. Successful Targeted Therapies for Breast Cancer: The Worcester Foundation and Future Opportunities in Women’s Health. Endocrinology (2018) 159(8):2980–90. doi: 10.1210/en.2018-00263
4. D’Amato V, Raimondo L, Formisano L, Giuliano M, De Placido S, Rosa R, et al. Mechanisms of Lapatinib Resistance in HER2-driven Breast Cancer. Cancer Treat Rev (2015) 41(10):877–83. doi: 10.1016/j.ctrv.2015.08.001
5. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science (1989) 244(4905):707–12. doi: 10.1126/science.2470152
6. Loibl S, Gianni L. HER2-Positive Breast Cancer. Lancet (2017) 389(10087):2415–29. doi: 10.1016/S0140-6736(16)32417-5
7. Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA (2019) 321(3):288–300. doi: 10.1001/jama.2018.19323
8. Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-positive Metastatic Breast Cancer. N Engl J Med (2015) 372(8):724–34. doi: 10.1056/NEJMoa1413513
9. Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab Emtansine Versus Capecitabine Plus Lapatinib in Patients With Previously Treated HER2-positive Advanced Breast Cancer (EMILIA): A Descriptive Analysis of Final Overall Survival Results From a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(6):732–42. doi: 10.1016/S1470-2045(17)30312-1
10. Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS, et al. Resistance Mechanisms to anti-HER2 Therapies in HER2-positive Breast Cancer: Current Knowledge, New Research Directions and Therapeutic Perspectives. Crit Rev Oncol Hematol (2019) 139:53–66. doi: 10.1016/j.critrevonc.2019.05.001
11. Sung M, Tan X, Lu B, Golas J, Hosselet C, Wang F, et al. Caveolae-Mediated Endocytosis as a Novel Mechanism of Resistance to Trastuzumab Emtansine (T-Dm1). Mol Cancer Ther (2018) 17(1):243–53. doi: 10.1158/1535-7163.MCT-17-0403
12. Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, et al. Discovery and Development of Pyrotinib: A Novel Irreversible EGFR/HER2 Dual Tyrosine Kinase Inhibitor With Favorable Safety Profiles for the Treatment of Breast Cancer. Eur J Pharm Sci (2017) 110:51–61. doi: 10.1016/j.ejps.2017.01.021
13. Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, et al. Pyrotinib or Lapatinib Combined With Capecitabine in HER2-Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase Ii Study. J Clin Oncol (2019) 37(29):2610–9. doi: 10.1200/JCO.19.00108
14. Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib Plus Capecitabine Versus Lapatinib Plus Capecitabine for the Treatment of HER2-positive Metastatic Breast Cancer (PHOEBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2021) 22(3):351–60. doi: 10.1016/S1470-2045(20)30702-6
15. Li F, Xu F, Li J, Wang T, Bian L, Zhang S, et al. Pyrotinib Versus Trastuzumab Emtansine for HER2-positive Metastatic Breast Cancer After Previous Trastuzumab and Lapatinib Treatment: A Real-World Study. Ann Transl Med (2021) 9(2):103. doi: 10.21037/atm-20-4054
16. Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol (2013) 31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984
17. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human Breast Cancer: Correlation of Relapse and Survival With Amplification of the HER-2/neu Oncogene. Science (1987) 235(4785):177–82. doi: 10.1126/science.3798106
18. Cesca MG, Vian L, Cristóvão-Ferreira S, Pondé N, de Azambuja E. HER2-Positive Advanced Breast Cancer Treatment in 2020. Cancer Treat Rev (2020) 88:102033. doi: 10.1016/j.ctrv.2020.102033
19. Hardy-Werbin M, Quiroga V, Cirauqui B, Romeo M, Felip E, Teruel I, et al. Real-World Data on T-DM1 Efficacy - Results of a Single-Center Retrospective Study of HER2-positive Breast Cancer Patients. Sci Rep (2019) 9(1):12760. doi: 10.1038/s41598-019-49251-5
20. Li Y, Gong C, Lu Q, Zhou Z, Luo T, Li W, et al. Real-World Data of Triplet Combination of Trastuzumab, Lapatinib, and Chemotherapy in HER2-Positive Metastatic Breast Cancer: A Multicenter Retrospective Study. Front Oncol (2020) 10:271. doi: 10.3389/fonc.2020.00271
21. Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib Plus Capecitabine in Women With HER-2-positive Advanced Breast Cancer: Final Survival Analysis of a Phase III Randomized Trial. Oncologist (2010) 15(9):924–34. doi: 10.1634/theoncologist.2009-0181
22. Göksu SS, Bozcuk H, Koral L, Çakar B, Gündüz S, Tatlı AM, et al. Factors Predicting Lapatinib Efficacy in HER-2+ Metastatic Breast Carcinoma: Does it Work Better in Different Histologic Subtypes? Indian J Cancer (2015) 52(4):517–9. doi: 10.4103/0019-509X.178382
23. Xu B, Li W, Zhang Q, Shao Z, Li Q, Wang X, et al. Pertuzumab, Trastuzumab, and Docetaxel for Chinese Patients With Previously Untreated HER2-positive Locally Recurrent or Metastatic Breast Cancer (PUFFIN): A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. Breast Cancer Res Treat (2020) 182(3):689–97. doi: 10.1007/s10549-020-05728-w
24. Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab Emtansine (T-DM1) Versus Lapatinib Plus Capecitabine in Patients With HER2-positive Metastatic Breast Cancer and Central Nervous System Metastases: A Retrospective, Exploratory Analysis in EMILIA. Ann Oncol (2015) 26(1):113–9. doi: 10.1093/annonc/mdu486
25. Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J Clin Oncol (2020) 38(23):2610–9. doi: 10.1200/JCO.20.00775
26. Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, et al. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol (2017) 35(27):3105–12. doi: 10.1200/JCO.2016.69.6179
Keywords: pyrotinib, breast cancer, human epidermal growth factor receptor 2, brain metastasis, real-world
Citation: Zhang L, Wu X, Zhou J, Zhu M, Yu H, Zhang Y, Zhao Y, Han Z, Guo Y, Guan X, Wang X, Xu H, Sun L, Zhang J, Zhuang M, Xie L, Yu S, Chen P and Feng J (2021) Pyrotinib in the Treatment of Women With HER2-Positive Advanced Breast Cancer: A Multicenter, Prospective, Real-World Study. Front. Oncol. 11:699323. doi: 10.3389/fonc.2021.699323
Received: 23 April 2021; Accepted: 30 June 2021;
Published: 16 July 2021.
Edited by:
Jian Zhang, Fudan University, ChinaReviewed by:
Francesco Pepe, University of Naples Federico II, ItalyCopyright © 2021 Zhang, Wu, Zhou, Zhu, Yu, Zhang, Zhao, Han, Guo, Guan, Wang, Xu, Sun, Zhang, Zhuang, Xie, Yu, Chen and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jifeng Feng, ZmVuZ0pGX05KQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.