
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 25 November 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.698866
This article is part of the Research TopicAdvanced Approaches on Multidisciplinary Management of Rectal CancerView all 21 articles
Background: The recurrence rate of T3N0 rectal cancer after total mesorectal excision (TME) is relatively low, meaning that not all patients need adjuvant therapy (AT) (radiotherapy, chemotherapy, or chemoradiotherapy).
Methods: Patients diagnosed with pT3N0M0 rectal cancer after TME were analyzed using the SEER database, of which 4367 did not receive AT and 2794 received AT. Propensity score matching was used to balance the two groups in terms of confounding factors. Cox proportional hazards regression analysis was used to screen independent prognostic factors, which were then used to establish a nomogram. The patients were then divided into three groups with X-tile software according to their risk scores. We enrolled 334 patients as external validation.
Results: The C-index of the model was 0.725 (95% confidence interval: 0.694–0.756). We divided the patients into three different risk layers based on the nomogram prediction scores, and found that AT did not improve the prognosis of low- and moderate-risk patients, while high-risk patients benefited from AT. External validation data also support the above conclusions.
Conclusion: This study developed a nomogram that effectively and comprehensively evaluates the prognosis of T3N0 rectal cancer patients after TME. After using the nomogram, we recommend AT for high-risk patients, but not for low- and moderate-risk patients.
Colorectal cancer is the third most common cancer and the second leading cause of cancer-related deaths worldwide, among which 2/3 of cases are colon cancer and 1/3 are rectal cancer (1, 2). Current guidelines recommend neoadjuvant chemoradiotherapy (NCRT) combined with total mesorectal excision (TME) and adjuvant therapy (AT) for locally advanced rectal cancer (RC); however, the treatment for patients with early-stage RC (T3NO) is controversial.
Although NCRT can bring better survival prognosis to RC patients, it also increases the incidence of late adverse events and postoperative complications (3, 4). Willem et al. (4) found that while NCRT reduced the local recurrence rate for resectable RC, it had no effect on overall survival (OS). Frasson et al. (5) found that T3NO RC patients did not benefit from NCRT. The local recurrence rate of T3NO RC patients is only approximately 10%. Therefore, it is now considered potentially more suitable to provide direct surgery combined with AT for patients with such low recurrence risk, thereby avoiding the side effects of overtreatment (6–10). The 5-year OS of T3N0 patients after surgery is 74–84%, and such a high survival rate means that not all patients (especially those who underwent complete radical resection) will benefit from AT. T3N0 patients’ local recurrence rate is only 3.6% after TME (9, 11). Due to the lack of randomized controlled trials (RCTs) of AT in T3N0 patients, this study focused on the clinical effect of AT in T3N0 patients at high-risk of recurrence after TME without NCRT.
This study analyzed clinicopathological factors from the SEER database and evaluated the prognosis of patients with T3N0 RC. Furthermore, the patients were divided into low-, moderate-, and high-risk groups according to a novel nomogram score to select the population that could most benefit from AT.
SEER*Stat (version 8.3.6) software was used to search 7161 patients with pT3N0M0 RC diagnosed from 2004 to 2016. The inclusion criteria were (1): pathologically diagnosed RC (ICD-O-3: C19.9, C20.9) (2); complete follow-up and survival data (3); no NCRT (4); underwent TME; and (5) primary RC. Finally, the patients were divided into two groups according to whether they received AT: the non-AT group (n=4367) and the AT group (n=2794). The included clinicopathological variables were: age, sex, race, marital status, tumor grade, size, primary site, histology, lymph nodes retrieved, carcinoembryonic antigen (CEA) level, tumor deposits, perineural invasion, radiotherapy and chemotherapy information, and survival information. Patients were further excluded if information for the above variables was unknown.
The external validation group include 334 pT3N0M0 RC patients at our center between 2008 and 2013. The inclusion criteria and clinicopathological variables were the same as for the SEER group.
Associations of clinicopathological factors with the two groups were analyzed by the chi-square test. To balance potential confounding biases of the included cases, only significant clinicopathological factors according to the chi-square test were included in the propensity score matching (PSM). The non-AT group and the AT group were subjected to nearest neighbor matching according to 1:1 (12). Survival analysis was performed by the Kaplan–Meier method and the log-rank test.
First, univariate and multivariate COX analyses were performed to find correlations between the clinicopathological variables and OS in the non-AT group. Next, significant variables according to Cox multivariate analysis (P<0.05) were included to establish a nomogram. The effectiveness of the nomogram was tested by determining it discriminatory ability by the concordance index (C-index) (13); we also compared the C-index of the nomogram, lymph nodes retrieved, and CEA to evaluate the clinical effectiveness of the model. The calibration curve intuitively displays the consistency between the predicted survival rate and the actual survival data. Decision curve analysis (DCA) was used to evaluate the net clinical benefit as compared with lymph nodes retrieved and CEA. According to the risk score of the nomogram, all cases from the two groups were divided into three groups (high-, moderate-, and low-risk) by X-tile software (14). SPSS 24.0 (IBM, Armonk, NY, USA) and R software (version 3.5.1) were used for the statistical analyses conducted in this study, with P<0.05 used to denote that the difference was statistically significant.
Before PSM, a total of 7161 pT3N0M0 patients who completed TME were included, including 4,367 patients without AT and 2,794 patients with AT (Figure 1). The median survival was 59 months (range: 0–155) and the number of deaths was 2,632 (36.8%). Chi-square analysis showed that patients with AT were significantly correlated with age, sex, marital status, grade, tumor size, primary site, histology, lymphatic invasion, CEA, tumor deposits, and perineural invasion (all P<0.05). After including variables related to AT for the PSM, the final patient number was 5588, including 2794 patients in the non-AT group and 2794 patients in the AT group (Table 1). The median survival was 64 months (range: 0–155) and there were 1,859 deaths (33.3%).
The prognosis of patients who received AT was better than that of the non-AT group (5-year survival rate: 79.9% vs. 66.8%, P<0.05, Figure 2A). After PSM, the prognosis of patients who received AT was still higher than that of the non-AT group (5-year survival rate: 79.9% vs. 71.0%, P<0.05, Figure 2B).
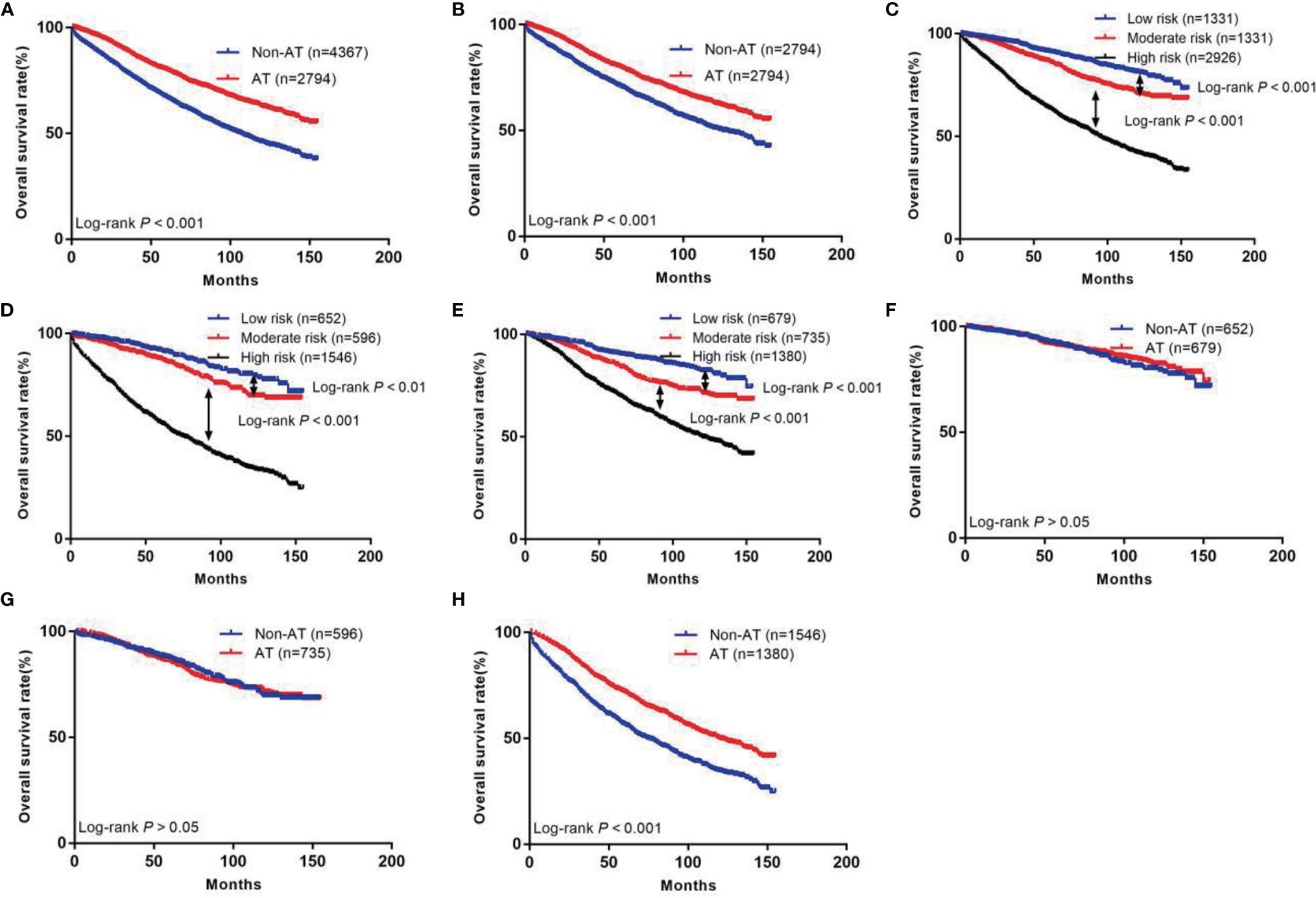
Figure 2 The Kaplan-Meier curves of OS for patients in our study. (A) All patients; (B) Patients after PSM; (C) OS in different subgroups of all patients; (D) OS in different subgroups of non-AT group; (E) OS in different subgroups of AT group; (F) OS for patients with or without AT in low risk group; (G) OS for patients with or without AT in moderate risk group; (H) OS for patients with or without AT in high risk group.
A COX hazards ratio model for patients without AT was constructed (Table 2), and univariate analysis showed that age, sex, race, marital status, tumor grade, size, primary site, histology, lymph nodes retrieved, CEA, tumor deposits, and perineural invasion were correlated with OS (all P<0.05). Next, these variables were included in the multivariate analysis, which showed that age, sex, race, marital status, tumor grade, size, primary site, lymph nodes retrieved, and CEA were independent prognostic factors (P<0.05). Based on these results, a nomogram was constructed to predict the 3- and 5-year survival rates of T3N0 RC after TME (Figure 3).
A nomogram incorporating the above nine risk factors was constructed to judge the prognosis T3N0 RC and had a C-index of 0.725 [95% confidence interval (CI): 0.694–0.756], which is significantly higher than the C-index of prognosis judged by lymph nodes retrieved and CEA [0.581 (95% CI: 0.550–0.612) and 0.547 (95% CI: 0.514–0.580), respectively]. The calibration curve of the 3- and 5-year OS nomogram showed that the predicted survival probability was consistent with the actual survival probability. The net benefits of the nomogram for different decision thresholds were higher than those of the lymph nodes retrieved and CEA system (Figure 4).
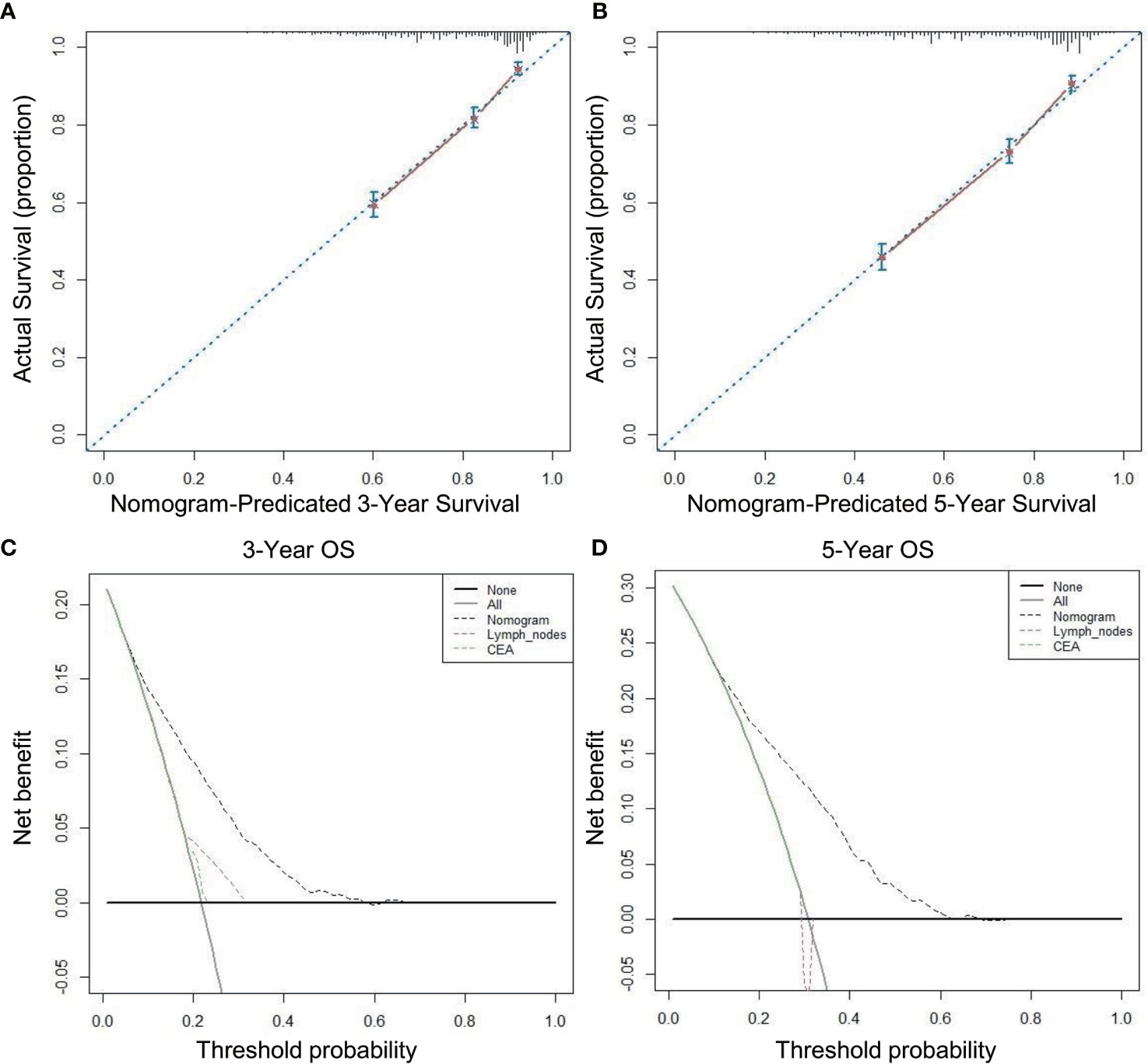
Figure 4 Calibration curves and decision curve for OS prediction: (A) 3-year OS calibration curve in our cohort; (B) 5-year OS calibration curve in our cohort; (C) Nomogram were compared to the lymph nodes retrieved and CEA in terms of 3-year OS in our decision curve analysis; (D) Nomogram were compared to the lymph nodes retrieved and CEA in terms of 5-year OS in our decision curve analysis.
Next, we calculated risk scores for each patient in the two groups with the nomogram (Table 3) and used X-tile software to take two cut-off values that divided the patients into three risk groups (Figure 5), a low-risk group (score ≤146, n=1331), a moderate-risk group (score 147–177, n=1331), and a high risk-group (score ≥178, n=2926). Five-year survival rates for the low-, moderate-, and high-risk groups were 91.2%, 86.6%, and 63.8%, respectively, which were statistically significant differences (P<0.001, Figure 2C).
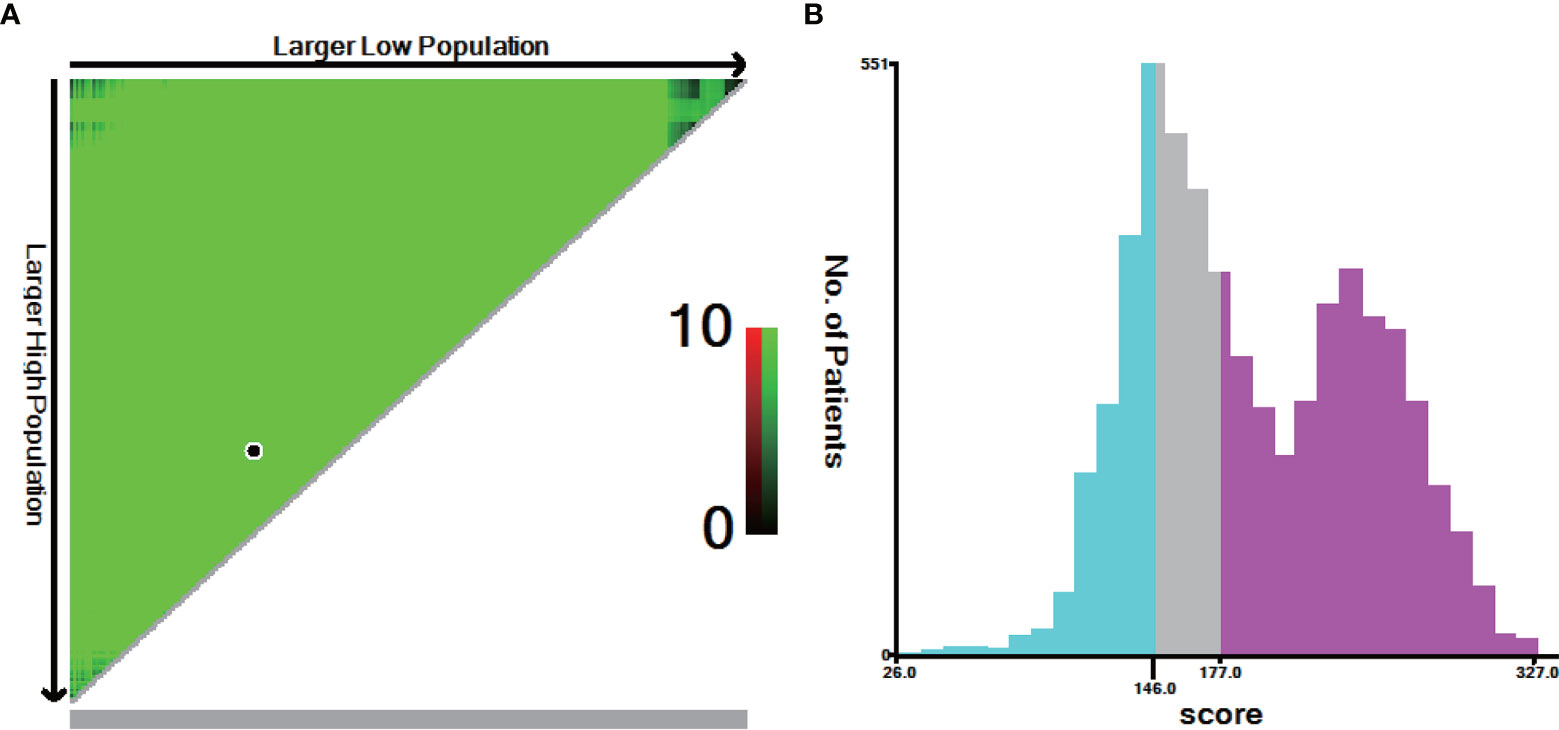
Figure 5 X-tile analysis for risk stratification: (A) The optimal cut-off value; (B) Numbers of patients in low, moderate and high risk subgroups.
We also divided the non-AT group into three groups using the current scoring system, a low-risk group (n=652), moderate-risk group (n=596), and high-risk group (n=1546). Five-year survival rates of these groups were 91.8%, 87.5%, and 56.4%, respectively, which were statistically significant differences (P<0.01, Figure 2D). In the AT group, the 5-year survival rates of the low- (n=679), moderate- (n=735), and high-risk (n=1380) groups were 90.7%, 85.9%, and 71.8%, respectively, which were statistically significant differences (P<0.01, Figure 2E).
We further investigated the benefit of AT in patients with different risk stratification (Table 4). The results showed that patients in the low-risk group did not benefit from AT (hazard ratio [HR]: 0.89, 95% CI: 0.65–1.21, P>0.05, Figure 2F). Patients in the moderate-risk group also did not benefit from AT (HR: 1.04, 95% CI: 0.81–1.32, P>0.05, Figure 2G). In contrast, patients in the high-risk group benefited from AT (HR: 0.61, 95% CI: 0.54–0.67, P<0.001, Figure 2H).
The external validation group included 216 patients without AT and 118 patients with AT. The median survival was 83 months (range: 0–396) and the number of deaths was 192 (57.5%). The prognosis of patients who received AT was better than that of the non-AT group (5-year survival: 61.6% vs. 75.3%; P<0.001) (Figure S1A). According to the above scoring system, the external validation group were also divided into low, moderate, and high-risk groups. Five-year survival rates of all patients for the low-, moderate-, and high-risk groups were 92.1%, 87.4%, and 55.9%, respectively (Figure S1B). Five-year survival rates of non-AT group for the low-, moderate-, and high-risk groups were 83.3%, 86.5%, and 52.6%, respectively (Figure S1C). Five-year survival rates of AT group for the low-, moderate-, and high-risk groups were 100.0%, 88.8%, and 63.2%, respectively (Figure S1D).
The results showed that external validation group in the low-risk group did not benefit from AT (hazard ratio [HR]: 0.45, 95% CI: 0.05–4.33, P>0.05, Figure S1E). Patients in the moderate-risk group also did not benefit from AT (HR: 0.47, 95% CI: 0.19–1.16, P>0.05, Figure S1F). In contrast, patients in the high-risk group benefited from AT (HR: 0.63, 95% CI: 0.46–0.88, P=0.01, Figure S1G).
The postoperative recurrence rate of RC is as high as 40%, and the 5-year survival rate is not greater than 50% (15). TME reduces the local recurrence rate to less than 10% and increases the cancer-free survival rate to more than 70% (16, 17). Although NCRT significantly reduces the local recurrence rate to less than 7%, patients’ 5-year distant metastasis rate still exceeds 20%. The adverse reactions of NCRT may lead to a decline in quality of life and a financial burden, and delay follow-up treatment, which may lead to shorter life expectancy (18–21). For T3N0 patients with relatively low recurrence rates, the application of NCRT is controversial. A German study showed that there was no significant difference in 10-year OS (59.6% vs. 59.9%, P=0.85) between the NCRT group and the adjuvant radiotherapy group, and there was no difference in the distant metastasis rate between the two groups (29.8% vs. 29.6%, P=0.9) (22). Thus, NCRT may not be the best treatment; direct TME surgery can obtain a good prognosis and a lower postoperative recurrence rate, and TME plus AT may be an ideal treatment for patients with a higher risk of recurrence. Our study used a large sample database to screen factors that were associated with the prognosis of T3N0 patients, and then developed a nomogram to evaluate the risk score of patients and guide their AT accurately and individually.
There is still no unified view of the prognosis of young RC patients. Some studies have shown that young patients have histopathological features such as late onset, aggressive disease, and worse prognosis than older patients (23–28). But there is also a view that older patients have poor prognosis (29–31), which suggests that age is a controversial prognostic factor in RC. Our study found that patients ≥65-years old had a poor prognosis (HR: 3.42, 95% CI: 2.97–3.94, P<0.001). One possible reason is that older patients are less sensitive to sensation, which makes the clinical manifestations of RC in the elderly more atypical and easier to ignore, resulting in later staged disease in many older patients. In addition, the proportion of the elderly who have received radical surgery is low, due to the poor tolerance to surgery and a large number of comorbidities, leading to a higher incidence and mortality of perioperative diseases in the elderly and a poor prognosis.
Studies have shown that colorectal cancer incidence is higher in men than in women, which may be associated with estrogen levels. Young and middle-aged women with higher estrogen levels have a decreased risk of colorectal cancer risk, and the cumulative protection of higher estrogen levels can be extended up to 20 to 25 years after menopause (32–34). Our study was consistent with the literature in that female patients had a better prognosis (HR: 0.75, 95% CI: 0.66–0.86, P<0.001) (35).
Pulte et al. (36) found that blacks and Indians had worse outcomes than whites, which is consistent with our results. Our study found that the prognosis of unmarried patients was worse than that of married patients, consistent with previous studies (37–39), which may be related to the lower proportion of unmarried patients participating in RC screening, lower enthusiasm for treatment, and lower proportion of patients receiving surgery and AT.
Currently, serum CEA is the most important tumor marker applied in clinical colorectal cancer management. Serum CEA levels can predict the prognosis and recurrence of colorectal cancer, and the later the disease stage is, the higher serum CEA levels are, and increased CEA is correlated with poor tumor differentiation (40–42). Our study also found that CEA (HR: 1.40, 95% CI: 1.19–1.64, P<0.001) and poor tumor differentiation (HR: 1.27, 95% CI: 1.07–1.51, P=0.006) are poor prognostic factors for T3N0 RC.
Tumor size is related to the time of tumor existence, invasion, and distant metastasis, and therefore, also to poor prognosis (43). Our study found that the prognosis of tumors ≥3 cm was worse (HR: 1.48, 95% CI: 1.20–1.82, P<0.001). As the boundary between the colon and rectum, rectosigmoid junction cancer may be different from RC and colon cancer in terms of pathogenesis, treatment, and prognosis. It is generally believed that the prognosis of diploid DNA tumors is better than that of aneuploid tumors. Diploid status was more common in proximal colorectal cancer than in distal colorectal cancer. The benefit of 5-FU treatment is greater for proximal colorectal cancer, but less for distal colorectal cancer. Therefore, from proximal colorectal cancer to distal colorectal cancer to RC, the prognosis of patients is gradually worse (44). Our study also confirmed that the prognosis of RC was worse than that of rectosigmoid junction cancer (HR: 1.19, 95% CI: 1.05–1.36, P=0.007).
The number of lymph nodes retrieved after surgery is closely related to the postoperative pathological stage of RC patients. Retrieving few lymph nodes may be related to an insufficient degree of lymph node dissection during surgery, and even lead to lymph nodes that are positive in the surgical area are not dissected, which affects prognosis. It is suggested in the guidelines that at least 12 lymph nodes should be detected to ensure that there is no bias in staging (45, 46). There is already evidence that in patients with lymph node-negative colorectal cancer, a higher number of lymph nodes retrieved is associated with prognosis (47). Many studies have found that in stage II/III colorectal cancer, the prognosis of patients with <12 lymph nodes retrieved is worse than that of patients with >12 lymph nodes retrieved (48–51). Our study also found that the prognosis of patients with >12 lymph nodes retrieved was relatively better (HR: 0.64, 95% CI: 0.56–0.72, P<0.001). New lymph node staging indicators such as metastatic lymph nodes ratio, log odds of positive lymph nodes, negative lymph node count, and lymph node micrometastasis may predict prognosis more accurately (52–56).
Our nomogram of multiple prognostic factors was established through large sample data and more comprehensively incorporates factors that affect the prognosis of T3N0 than the number of lymph nodes retrieved [C-index: 0.581 (95% CI: 0.550–0.612)], and CEA [C-index 0.547 (95% CI: 0.514–0.580)], and our nomogram [C-index: 0.725 (95% CI: 0.694–0.756)] better predicts the 3- and 5-year survival rates of T3N0 RC. We applied DCA to further confirm that the nomogram was superior to the number of lymph nodes retrieved and CEA in predicting the OS of T3N0 RC patients after TME.
Whether T3N0 RC can benefit from AT is controversial, and so far, no RCT has studied whether T3N0 RC can benefit from AT. Paula et al. (10) found that postoperative adjuvant chemotherapy had no survival benefit compared with patients without chemotherapy (HR: 0.88, 95% CI: 0.52–1.56, P=0.66). Kim et al. (57) found that the 5-year OS of patients receiving postoperative adjuvant chemotherapy was lower than that of patients without chemotherapy (79.3 vs. 83.0, P=0.92). However, Quinn et al. (9) found that postoperative chemotherapy (HR: 0.74, 95% CI: 0.62–0.89, P=0.001) and chemoradiotherapy (HR: 0.57, 95% CI: 0.50–0.65, P<0.001) improved the prognosis of patients compared with surgery alone. Our findings suggest that AT improved patient survival both before and after PSM. Because AT is often used in patients with poor prognostic factors, they benefit more from AT, resulting in the overall results showing that AT improves prognosis. However, this does not mean that all patients need AT, which requires us to select those who will really benefit from AT for precise and individualized treatment. Our nomogram comprehensively analyzed factors that influence the prognosis and recurrence of T3N0 RC, and scored the impact of each risk subgroup: low, moderate, and high. Moreover, in our non-AT and AT groups, there were obvious survival differences in the low-, moderate-, and high-risk subgroups, indicating that our risk stratification was reasonable and effective. To determine which subgroups of patients benefit from AT, we found that the 5-year survival rate of low-risk patients receiving AT was lower than that of patients without AT (90.7% vs. 91.7%, P>0.05), so we do not recommend AT for low-risk patients. The 5-year survival rate of patients with moderate risk who received AT was lower than that of patients without AT (85.9% vs. 87.5%, P>0.05). We also do not recommend AT for such patients because the harm of AT for low and moderate risk patients exceeds the benefit. The 5-year survival rate of high-risk patients who received AT was higher than that of patients without AT (71.8% vs. 56.4%, P<0.001), we suggest that high-risk patients receive AT. Our external validation data also showed that low and moderate -risk patients did not benefit from AT (P>0.05), while high-risk patients did (P<0.05).
This study has several limitations. This was a retrospective study, and some patients fail to be included in this study due to missing data, which may cause bias. Currently, there is no large-scale RCT study on whether T3N0 RC benefits from AT, and there is no prognostic survival nomogram that incorporates the above clinical pathological factors. The most important thing is that we use this nomogram to stratify the risk of patients, which is of great significance for individualized guidance of clinical AT, as was the goal of this work.
Age, sex, race, marital status, tumor grade, size, primary site, lymph nodes retrieved, and CEA are independent prognostic factors for T3N0 RC patients after TME. Through our innovative risk score stratification, we recommend high-risk patients receive AT, while AT is not recommended for low- and moderate-risk patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. The ethics committee waived the requirement of written informed consent for participation.
XW designed the research. SZ took part in designing the research. SZ collected the data, analyzed the date. CZ analyzed the date and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Department of Finance of Jilin Province (No 2020SCZT031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.698866/full#supplementary-material
Supplementary Figure 1 | The Kaplan-Meier curves of OS for patients in external validation group. (A) All patients; (B) OS in different risk subgroups of all patients; (C) OS in different risk subgroups of non-AT group; (D) OS in different risk subgroups of AT group; (E) OS for patients with or without AT in low risk group; (F) OS for patients with or without AT in moderate risk group; (G) OS for patients with or without AT in high risk group.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA: Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Ikoma N, You YN, Bednarski BK, Rodriguez-Bigas MA, Eng C, Das P, et al. Impact of Recurrence and Salvage Surgery on Survival After Multidisciplinary Treatment of Rectal Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(23):2631–38. doi: 10.1200/jco.2016.72.1464
3. Ansari N, Solomon MJ, Fisher RJ, Mackay J, Burmeister B, Ackland S, et al. Acute Adverse Events and Postoperative Complications in a Randomized Trial of Preoperative Short-Course Radiotherapy Versus Long-Course Chemoradiotherapy for T3 Adenocarcinoma of the Rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04). Ann Surg (2017) 265(5):882–88. doi: 10.1097/sla.0000000000001987
4. van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative Radiotherapy Combined With Total Mesorectal Excision for Resectable Rectal Cancer: 12-Year Follow-Up of the Multicentre, Randomised Controlled TME Trial. Lancet Oncol (2011) 12(6):575–82. doi: 10.1016/s1470-2045(11)70097-3
5. Frasson M, Garcia-Granero E, Roda D, Flor-Lorente B, Roselló S, Esclapez P, et al. Preoperative Chemoradiation May Not Always Be Needed for Patients With T3 and T2N+ Rectal Cancer. Cancer (2011) 117(14):3118–25. doi: 10.1002/cncr.25866
6. Zhu J, Xu Y, Gu W, Peng J, Cai G, Cai G, et al. Adjuvant Therapy for T3N0 Rectal Cancer in the Total Mesorectal Excision Era- Identification of the High Risk Patients. Radiat Oncol (London England) (2010) 5(118):1–8. doi: 10.1186/1748-717x-5-118
7. Zhang Q, Tey J, Yang Z, Li P, Peng L, Jiang R, et al. Intraoperative Radiotherapy in the Combination of Adjuvant Chemotherapy for the Treatment of Pt3n0m0 Rectal Cancer After Radical Surgery. Am J Clin Oncol (2014) 37(1):8–12. doi: 10.1097/COC.0b013e31825eb75c
8. Wo JY, Mamon HJ, Ryan DP, Hong TS. T3N0 Rectal Cancer: Radiation for All? Semin Radiat Oncol (2011) 21(3):212–9. doi: 10.1016/j.semradonc.2011.02.007
9. Quinn TJ, Rajagopalan MS, Gill B, Mehdiabadi SM, Kabolizadeh P. Patterns of Care and Outcomes for Adjuvant Treatment of Pt3n0 Rectal Cancer Using the National Cancer Database. J Gastrointestinal Oncol (2020) 11(1):1–12. doi: 10.21037/jgo.2019.10.02
10. de Paula TR, Gorroochurn P, Kiran RP, Keller DS. Does Adjuvant Chemotherapy Improve Survival in T3N0 Rectal Cancer? An Evaluation of Use and Outcomes From the National Cancer Database (NCDB). J gastrointestinal Surg Off J Soc Surg Aliment Tract (2020) 24(5):1188–91. doi: 10.1007/s11605-020-04541-6
11. Park IJ, Kim HC, Yu CS, Kim TW, Jang SJ, Kim JC. Effect of Adjuvant Radiotherapy on Local Recurrence in Stage II Rectal Cancer. Ann Surg Oncol (2008) 15(2):519–25. doi: 10.1245/s10434-007-9643-x
12. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res (2011) 46(3):399–424. doi: 10.1080/00273171.2011.568786
13. Hanley JA, McNeil BJ. A Method of Comparing the Areas Under Receiver Operating Characteristic Curves Derived From the Same Cases. Radiology (1983) 148(3):839–43. doi: 10.1148/radiology.148.3.6878708
14. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res an Off J Am Assoc Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.Ccr-04-0713
15. Hughes ES. Results of Treatment of Carcinoma of Colon and Rectum. Br Med J (1963) 2(5348):9–12. doi: 10.1136/bmj.2.5348.9
16. Kearney DE, Coffey JC. A Randomized Trial of Laparoscopic Versus Open Surgery for Rectal Cancer. N Engl J Med (2015) 373(2):194. doi: 10.1056/NEJMc1505367
17. Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open Versus Laparoscopic Surgery for Mid-Rectal or Low-Rectal Cancer After Neoadjuvant Chemoradiotherapy (COREAN Trial): Survival Outcomes of an Open-Label, Non-Inferiority, Randomised Controlled Trial. Lancet Oncol (2014) 15(7):767–74. doi: 10.1016/s1470-2045(14)70205-0
18. Lin J, Peng J, Qdaisat A, Li L, Chen G, Lu Z, et al. Severe Weight Loss During Preoperative Chemoradiotherapy Compromises Survival Outcome for Patients With Locally Advanced Rectal Cancer. J Cancer Res Clin Oncol (2016) 142(12):2551–60. doi: 10.1007/s00432-016-2225-1
19. Gao YH, Lin JZ, An X, Luo JL, Cai MY, Cai PQ, et al. Neoadjuvant Sandwich Treatment With Oxaliplatin and Capecitabine Administered Prior to, Concurrently With, and Following Radiation Therapy in Locally Advanced Rectal Cancer: A Prospective Phase 2 Trial. Int J Radiat Oncol Biol Phys (2014) 90(5):1153–60. doi: 10.1016/j.ijrobp.2014.07.021
20. Short MN, Aloia TA, Ho V. The Influence of Complications on the Costs of Complex Cancer Surgery. Cancer (2014) 120(7):1035–41. doi: 10.1002/cncr.28527
21. Tevis SE, Kohlnhofer BM, Stringfield S, Foley EF, Harms BA, Heise CP, et al. Postoperative Complications in Patients With Rectal Cancer Are Associated With Delays in Chemotherapy That Lead to Worse Disease-Free and Overall Survival. Dis Colon Rectum (2013) 56(12):1339–48. doi: 10.1097/DCR.0b013e3182a857eb
22. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J Clin Oncol Off J Am Soc Clin Oncol (2012) 30(16):1926–33. doi: 10.1200/jco.2011.40.1836
23. Limaiem F, Azzabi S, Sassi A, Mzabi S, Bouraoui S. Colorectal Cancer in Young Adults: A Retrospective Study of 32 Tunisian Patients. Pan Afr Med J (2018) 31(62):1–9. doi: 10.11604/pamj.2018.31.62.11043
24. Chiang JM, Chen MC, Changchien CR, Chen JS, Tang R, Wang JY, et al. Favorable Influence of Age on Tumor Characteristics of Sporadic Colorectal Adenocarcinoma: Patients 30 Years of Age or Younger may be a Distinct Patient Group. Dis colon Rectum (2003) 46(7):904–10. doi: 10.1007/s10350-004-6683-1
25. Cusack JC, Giacco GG, Cleary K, Davidson BS, Izzo F, Skibber J, et al. Survival Factors in 186 Patients Younger Than 40 Years Old With Colorectal Adenocarcinoma. J Am Coll Surgeons (1996) 183(2):105–12.
26. Liang JT, Huang KC, Cheng AL, Jeng YM, Wu MS, Wang SM. Clinicopathological and Molecular Biological Features of Colorectal Cancer in Patients Less Than 40 Years of Age. Br J Surg (2003) 90(2):205–14. doi: 10.1002/bjs.4015
27. Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing Incidence of Rectal Cancer in Patients Aged Younger Than 40 Years: An Analysis of the Surveillance, Epidemiology, and End Results Database. Cancer (2010) 116(18):4354–9. doi: 10.1002/cncr.25432
28. You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-Onset Colorectal Cancer: Is It Time to Pay Attention? Arch Internal Med (2012) 172(3):287–9. doi: 10.1001/archinternmed.2011.602
29. Mäkelä J, Kiviniemi H, Laitinen S. Prognostic Factors After Surgery in Patients Younger Than 50 Years Old With Colorectal Adenocarcinoma. Hepato-Gastroenterology (2002) 49(46):971–5.
30. Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P, et al. Clinicopathologic and Molecular Features of Sporadic Early-Onset Colorectal Adenocarcinoma: An Adenocarcinoma With Frequent Signet Ring Cell Differentiation, Rectal and Sigmoid Involvement, and Adverse Morphologic Features. Modern Pathol an Off J United States Can Acad Pathol Inc (2012) 25(8):1128–39. doi: 10.1038/modpathol.2012.61
31. O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Ko CY. Are Survival Rates Different for Young and Older Patients With Rectal Cancer? Dis colon rectum (2004) 47(12):2064–9. doi: 10.1007/s10350-004-0738-1
32. Brenner H, Hoffmeister M, Arndt V, Haug U. Gender Differences in Colorectal Cancer: Implications for Age at Initiation of Screening. Br J Cancer (2007) 96(5):828–31. doi: 10.1038/sj.bjc.6603628
33. DeCosse JJ, Ngoi SS, Jacobson JS, Cennerazzo WJ. Gender and Colorectal Cancer. Eur J Cancer Prev Off J Eur Cancer Prev Organ (ECP) (1993) 2(2):105–15. doi: 10.1097/00008469-199303000-00003
34. Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the Colon: Potential Mechanisms for Cancer Prevention. Lancet Oncol (2008) 9(4):385–91. doi: 10.1016/s1470-2045(08)70100-1
35. Yang Y, Wang G, He J, Ren S, Wu F, Zhang J, et al. Gender Differences in Colorectal Cancer Survival: A Meta-Analysis. Int J Cancer (2017) 141(10):1942–9. doi: 10.1002/ijc.30827
36. Pulte D, Jansen L, Brenner H. Social Disparities in Survival After Diagnosis With Colorectal Cancer: Contribution of Race and Insurance Status. Cancer Epidemiol (2017) 48:41–7. doi: 10.1016/j.canep.2017.03.004
37. Johansen C, Schou G, Soll-Johanning H, Mellemgaard A, Lynge E. Influence of Marital Status on Survival From Colon and Rectal Cancer in Denmark. Br J Cancer (1996) 74(6):985–8. doi: 10.1038/bjc.1996.470
38. Villingshøj M, Ross L, Thomsen BL, Johansen C. Does Marital Status and Altered Contact With the Social Network Predict Colorectal Cancer Survival? Eur J Cancer (Oxford Engl 1990) (2006) 42(17):3022–7. doi: 10.1016/j.ejca.2006.04.027
39. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital Status and Survival in Patients With Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2013) 31(31):3869–76. doi: 10.1200/jco.2013.49.6489
40. Nozoe T, Rikimaru T, Mori E, Okuyama T, Takahashi I. Increase in Both CEA and CA19-9 in Sera Is an Independent Prognostic Indicator in Colorectal Carcinoma. J Surg Oncol (2006) 94(2):132–7. doi: 10.1002/jso.20577
41. Duffy MJ. Carcinoembryonic Antigen as a Marker for Colorectal Cancer: Is It Clinically Useful? Clin Chem (2001) 47(4):624–30. doi: 10.1093/clinchem/47.4.624
42. Zhong W, Yu Z, Zhan J, Yu T, Lin Y, Xia ZS, et al. Association of Serum Levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and Disease Characteristics in Colorectal Cancer. Pathol Oncol Res POR (2015) 21(1):83–95. doi: 10.1007/s12253-014-9791-9
43. Cai D, Huang ZH, Yu HC, Wang XL, Bai LL, Tang GN, et al. Prognostic Value of Preoperative Carcinoembryonic Antigen/Tumor Size in Rectal Cancer. World J Gastroenterol (2019) 25(33):4945–58. doi: 10.3748/wjg.v25.i33.4945
44. Araujo SE, Bernardo WM, Habr-Gama A, Kiss DR, Cecconello I. DNA Ploidy Status and Prognosis in Colorectal Cancer: A Meta-Analysis of Published Data. Dis colon rectum (2007) 50(11):1800–10. doi: 10.1007/s10350-007-9013-6
45. Sobin LH, Greene FL. TNM Classification: Clarification of Number of Regional Lymph Nodes for pNo. Cancer (2001) 92(2):452. doi: 10.1002/1097-0142(20010715)92:2<452::aid-cncr1342>3.0.co;2-b
46. National Comprehensive Cancer Network: Rectal Cancer (Version 5.2020). Available at: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
47. Tepper JE, O’Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB 3rd, et al. Impact of Number of Nodes Retrieved on Outcome in Patients With Rectal Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2001) 19(1):157–63. doi: 10.1200/jco.2001.19.1.157
48. Norwood MG, Sutton AJ, West K, Sharpe DP, Hemingway D, Kelly MJ. Lymph Node Retrieval in Colorectal Cancer Resection Specimens: National Standards Are Achievable, and Low Numbers Are Associated With Reduced Survival. Colorectal Dis Off J Assoc Coloproctol Great Britain Ireland (2010) 12(4):304–9. doi: 10.1111/j.1463-1318.2009.01788.x
49. Lee S, Hofmann LJ, Davis KG, Waddell BE. Lymph Node Evaluation of Colon Cancer and Its Association With Improved Staging and Survival in the Department of Defense Health Care System. Ann Surg Oncol (2009) 16(11):3080–6. doi: 10.1245/s10434-009-0620-4
50. Stocchi L, Fazio VW, Lavery I, Hammel J. Individual Surgeon, Pathologist, and Other Factors Affecting Lymph Node Harvest in Stage II Colon Carcinoma. Is a Minimum of 12 Examined Lymph Nodes Sufficient? Ann Surg Oncol (2011) 18(2):405–12. doi: 10.1245/s10434-010-1308-5
51. Xu Z, Berho ME, Becerra AZ, Aquina CT, Hensley BJ, Arsalanizadeh R, et al. Lymph Node Yield Is an Independent Predictor of Survival in Rectal Cancer Regardless of Receipt of Neoadjuvant Therapy. J Clin Pathol (2017) 70(7):584–92. doi: 10.1136/jclinpath-2016-203995
52. Zhou Y, Zhang J, Cao S, Li Y. The Evaluation of Metastatic Lymph Node Ratio Staging System in Gastric Cancer. Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2013) 16(3):309–17. doi: 10.1007/s10120-012-0190-1
53. Wang J, Hassett JM, Dayton MT, Kulaylat MN. The Prognostic Superiority of Log Odds of Positive Lymph Nodes in Stage III Colon Cancer. J Gastrointestinal Surg Off J Soc Surg Aliment Tract (2008) 12(10):1790–6. doi: 10.1007/s11605-008-0651-3
54. Schwarz RE, Smith DD. Clinical Impact of Lymphadenectomy Extent in Resectable Gastric Cancer of Advanced Stage. Ann Surg Oncol (2007) 14(2):317–28. doi: 10.1245/s10434-006-9218-2
55. Lee CM, Cho JM, Jang YJ, Park SS, Park SH, Kim SJ, et al. Should Lymph Node Micrometastasis be Considered in Node Staging for Gastric Cancer?: The Significance of Lymph Node Micrometastasis in Gastric Cancer. Ann Surg Oncol (2015) 22(3):765–71. doi: 10.1245/s10434-014-4073-z
56. Tavares A, Monteiro-Soares M, Viveiros F, Maciel Barbosa J, Dinis-Ribeiro M. Occult Tumor Cells in Lymph Nodes of Patients With Gastric Cancer: A Systematic Review on Their Prevalence and Predictive Role. Oncology (2015) 89(5):245–54. doi: 10.1159/000433543
Keywords: T3N0 rectal cancer, nomogram, prognosis, adjuvant therapy, TME
Citation: Zhang C, Zhao S and Wang X (2021) A Prognostic Nomogram for T3N0 Rectal Cancer After Total Mesorectal Excision to Help Select Patients for Adjuvant Therapy. Front. Oncol. 11:698866. doi: 10.3389/fonc.2021.698866
Received: 22 April 2021; Accepted: 03 November 2021;
Published: 25 November 2021.
Edited by:
Valentina Giaccaglia, Mohammed Bin Rashid University of Medicine and Health Sciences, United Arab EmiratesReviewed by:
Vladimir Cuk, Clinical Hospital Center Zvezdara, SerbiaCopyright © 2021 Zhang, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Wang, d2FuZ3h1ZEBqbHUuZWR1LmNu; Shutao Zhao, emhhb3NodXRhbzAwMUBqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.