- 1State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
- 2Department of Respiratory and Critical Care Medicine, Jinling Hospital, Nanjing, China
Background: Checkpoint inhibitor-related pneumonitis (CIP) is a potentially fatal immune-related adverse event that occurs during treatment with immune checkpoint inhibitors (ICIs). However, the roles played by peripheral blood parameters in CIP development remain unclear. Here, we aimed to identify which blood biomarkers correlated with the development and prognosis of CIP in patients with lung cancer.
Methods: We conducted a retrospective analysis of 87 patients with CIP (CIP group) and 87 patients without CIP (control group). Cytokines, blood routine, lactate dehydrogenase (LDH) and albumin (ALB) were collected at baseline (before ICIs), at onset of pneumonitis (in the CIP group), and before the last dose of ICI (in the control group). We compared the baseline values and changes over time in various blood parameters between the CIP and control groups. The CIP outcomes were collected and compared according to the median values of these parameters.
Results: Squamous carcinoma (odds ratio [OR]: 3.02; p = 0.004) and ICI monotherapy (OR: 6.56; p = 0.004) correlated with a high risk of CIP. In the CIP group, interleukin (IL)-6 and platelet-to-lymphocyte ratio (PLR) at CIP were significantly increased relative to baseline. By contrast, IL-6 and PLR reduced over time in the control group. Significant decrease in absolute lymphocyte count (ALC) and increases in IL-10, neutrophil to lymphocyte ratio (NLR), and LDH levels were observed from baseline to CIP. No significant change in these parameters was observed in the control group relative to baseline. ALB decreased in both groups, but the decrease in the CIP group was greater (9.21% vs. 2.44%; p = 0.020). High IL-6 levels (OR: 5.23, 95% confidence interval [CI]: 1.15–23.86; p = 0.033), and low levels of ALB (OR: 0.16, 95% CI: 0.04–0.64; p = 0.009) measured at the time of CIP symptom onset were associated with severe pneumonitis. Low concentration of IL-6 (hazard ratio [HR]: 0.17, 95% CI: 0.03–0.95; p = 0.044) and high ALB levels (HR: 0.28, 95% CI: 0.08–0.94; p = 0.040) were correlated with favorable overall survival in CIP.
Conclusions: Increase in IL-6, IL-10, NLR, PLR, and LDH levels or reduced ALC and ALB levels were associated with the occurrence of CIP in lung cancer patients. High IL-6 and low ALB levels at onset of CIP were related to severe grade and poor prognosis of CIP.
Introduction
Immune checkpoint inhibitors (ICIs) provide enhanced survival benefits to patients with malignant tumors, including lung cancer (1, 2); however, ICIs sometimes cause a series of unique adverse events, known as immune-related adverse events (irAEs) (3). A review of 20 randomized controlled studies suggested that the incidence of fatal irAEs associated with programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors was 0.43%, among which checkpoint inhibitor-related pneumonitis (CIP) was the most common (4). A meta-analysis showed that lung cancer was more likely than other cancers to result in all-grade or high-grade CIP (5). CIP lacks typical clinical symptoms, and 1/3 of patients with CIP are asymptomatic at the time of onset (6). The delayed treatment of CIP patients may lead to disease aggravation. The overall survival (OS) of patients with CIP who do not recover or whose symptoms worsen is significantly shortened compared with those who recovered from CIP (7). Therefore, determining the risk factors associated with CIP and early CIP identification is crucial. Previous studies showed that age, smoking status, pre-existing lung diseases, and chest radiotherapy history might be related to CIP occurrence (8–10). However, the sample sizes of CIP patients in these studies are small, and whether other risk factors may exist is also worthy of further study.
Blood-based biomarkers have the advantages of minimally invasive, easy to collect, and reproducible. Studies have shown that C-reactive protein (CRP), interleukin (IL)-6, blood cell counts, and cytokine levels are associated with irAEs (11). Recent data suggest that the neutrophil to lymphocyte ratio (NLR) may be related to irAE onset, severity, and subsequent prognosis (12). Similarly, increased NLR values may contribute to the diagnosis of ICI-associated myocarditis (13). A recent study indicated that elevated IL-6, IL-10, and eosinophil levels might be indicators of skin-related irAEs (14). However, a few reports have examined the association between peripheral blood biomarkers and CIP occurrence. Previous reports have shown that an increased anti-CD74 autoantibody was correlated with CIP occurrence (15). However, these biomarkers are not included in routine clinical tests, and their determination requires special equipment.
Previous studies have suggested that the OS of patients with irAEs was significantly longer than that of patients without irAEs. However, in the subgroup analysis, CIP was not significantly associated with ICI efficacy (16, 17). Conversely, a study by Fukihara et al. suggested that OS was significantly shorter among patients with CIP than among those without CIP (18). Another study showed that grades 1–2 CIP was associated with favorable OS, whereas grades 3–4 CIP was not (19). The survival time for CIP patients varies greatly. Therefore, determining whether peripheral blood markers can be used to predict OS in patients with CIP remains necessary.
This study was designed to identify the potential risk factors in baseline clinical characteristics associated with the occurrence of CIP and to investigate the association between clinically accessible biomarkers in peripheral blood and the development or prognosis of CIP.
Materials and Methods
Patients
This retrospective, observational study was conducted at the First Affiliated Hospital of Guangzhou Medical University. Records for patients with unresectable stage III or IV primary lung cancer [according to the 2015 World Health Organization Classification of Lung Tumors (20)] treated with at least one dose of ICI between January 2016 and January 2021 were reviewed. Patients who developed CIP (CIP group) and randomly selected corresponding patients without CIP (control group) were included at a ratio of 1:1. Prior tuberculosis and bacterial and fungal infections in the lungs before immunotherapy were excluded. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University.
Diagnosis of CIP
CIP was diagnosed by two experienced pulmonologists and one chest radiologist, based on the guidelines of the National Comprehensive Cancer Network, the American Society for Clinical Oncology, and the European Society for Medical Oncology (21–23). We defined CIP as new-onset infiltrates on thoracic imaging and/or clinical symptoms of cough, shortness of breath, or wheezing that is likely to be caused by ICIs, and excluded other etiologies. For patients considering the diagnosis of CIP, several examinations were performed in order to exclude other lung diseases (e.g. pulmonary infections and tumor progression), such as bronchoalveolar lavage culture, sputum cultures and laboratory tests (routine blood test, procalcitonin, tumor markers, arterial gas analysis, serous D-dimer and brain natriuretic peptide, etcetera). In addition, when patients with pulmonary infection had been a poor response to anti-infection treatment, CIP with pulmonary infection may be diagnosed. We compared pneumonitis extent and previous radiation field to exclude radiation-induced pneumonitis. If the diagnosis was not clear and the patient’s physical condition allowed, a lung biopsy would be performed.
Data Collection and Outcome Assessment
The following information was retrospectively collected from each patient’s medical records: patient demographics, pre-existing lung disease, tumor histology, Eastern Cooperative Oncology Group Performance Status (ECOG PS), radiation therapy administrations, treatment data, and driver gene status. The ECOG PS was evaluated prior to ICI treatment. Driver gene status was tested before any anti-tumor treatments were applied. In the CIP group, we also collected the time course of CIP, maximum CIP grade, and CIP outcomes. The severity of CIP was graded according to the Common Toxicity Criteria for Adverse Events (CTCAE version 4.0). In the CIP group, OS was calculated from the date of CIP diagnosis until death or the last follow-up date (April 1, 2021).
Among patients with CIP, we collected peripheral blood parameters at two time points: baseline (prior to ICI treatment), and at the time of CIP diagnosis. In the control group, we recorded these parameters at two time points: baseline before starting ICI treatment and before the last dose of ICI. Peripheral blood parameters included IL-2, IL-4, IL-6, IL-10, interferon-gamma (IFN-γ), tumor necrosis factor-α (TNF-α), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute eosinophil count (AEC), platelet count (PLT), lactate dehydrogenase (LDH), and albumin (ALB). The NLR was calculated as ANC divided by ALC. The platelet-to-lymphocyte ratio (PLR) was calculated by dividing PLT by ALC.
Statistical Analysis
Continuous variables were summarized as the median and interquartile range (IQR). Categorical data were summarized as the frequency (percentage). Differences in continuous variables at baseline were assessed using either an independent-samples t-test or the Mann–Whitney U test. Chi-square (χ2) or Fisher’s exact test was used to analyze categorical variables.
Logistic univariate analysis was used to determine which factors were associated with CIP. Multivariate logistic regression analysis was used to analyze those variables with p-value <0.1 in the univariate analysis to determine potential CIP risk factors. Changes in peripheral blood parameters over time were evaluated using a paired t-test or the Wilcoxon signed-rank test. The calculation of percentage change was performed as follows: (difference from baseline/baseline value) × 100. The Mann–Whitney U test was used to compare changes in blood parameters between the CIP and control groups. For those blood parameters with significant changes over time, the median value at the time of CIP diagnosis was used to perform logistic univariate and multivariate analyses to identify potential biomarkers associated with severe-grade CIP in the CIP group.
Finally, the Kaplan–Meier method was used to evaluate OS, with 95% confidence intervals (CIs), and the log-rank test was used to determine the significance of differences between two or more subgroups in CIP patients. A Cox proportional hazards model was used to identify prognostic factors associated with OS in the CIP group using multivariable survival analysis, including those variables with p-values <0.01 in the univariate analysis. Univariate and multivariate hazard ratios (HRs), with 95% CI values, were calculated.
Statistical analyses were conducted using IBM SPSS Statistics (Armonk, NY), version 25. All p-values were based on the two-sided hypothesis test, and a p-value <0.05 was considered significant.
Results
Participants
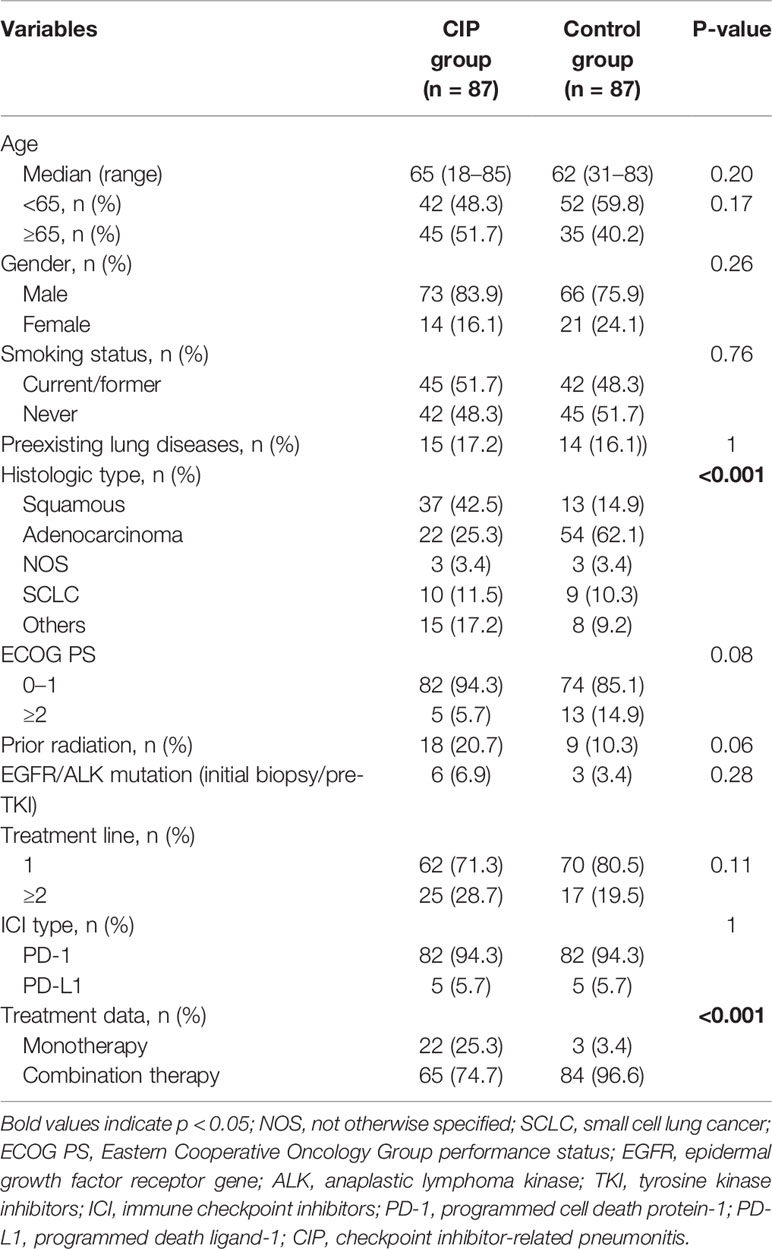
A total of 848 patients with advanced lung cancer who were treated with ICIs at our institution were deemed eligible for potential study inclusion. Finally, 87 patients (10.3%) who developed CIP (CIP group) and 87 randomly selected patients without CIP (control group) were included in the analysis (Figure 1). All patients were treated with PD-1 or PD-L1 inhibitors, with PD-1 inhibitors being more commonly used. The demographic characteristics were similar between the CIP and control groups (Table 1). However, the distributions of tumor types and treatment data among cases and controls were significantly different. Squamous cell carcinoma was the most common histologic type among the CIP group (42.5%), whereas adenocarcinoma (62.1%) was the most common type in the control group. In addition, combination therapy (including ICI + chemotherapy and ICI + chemotherapy + antiangiogenic drugs) was the predominant treatment type for both groups, but ICI monotherapy comprised a larger proportion (25.3%) of treatment types in the CIP group (p <0.001). Compared with the control group, the CIP group had a higher frequency of prior radiation (10.3% vs. 20.7%; p = 0.006).
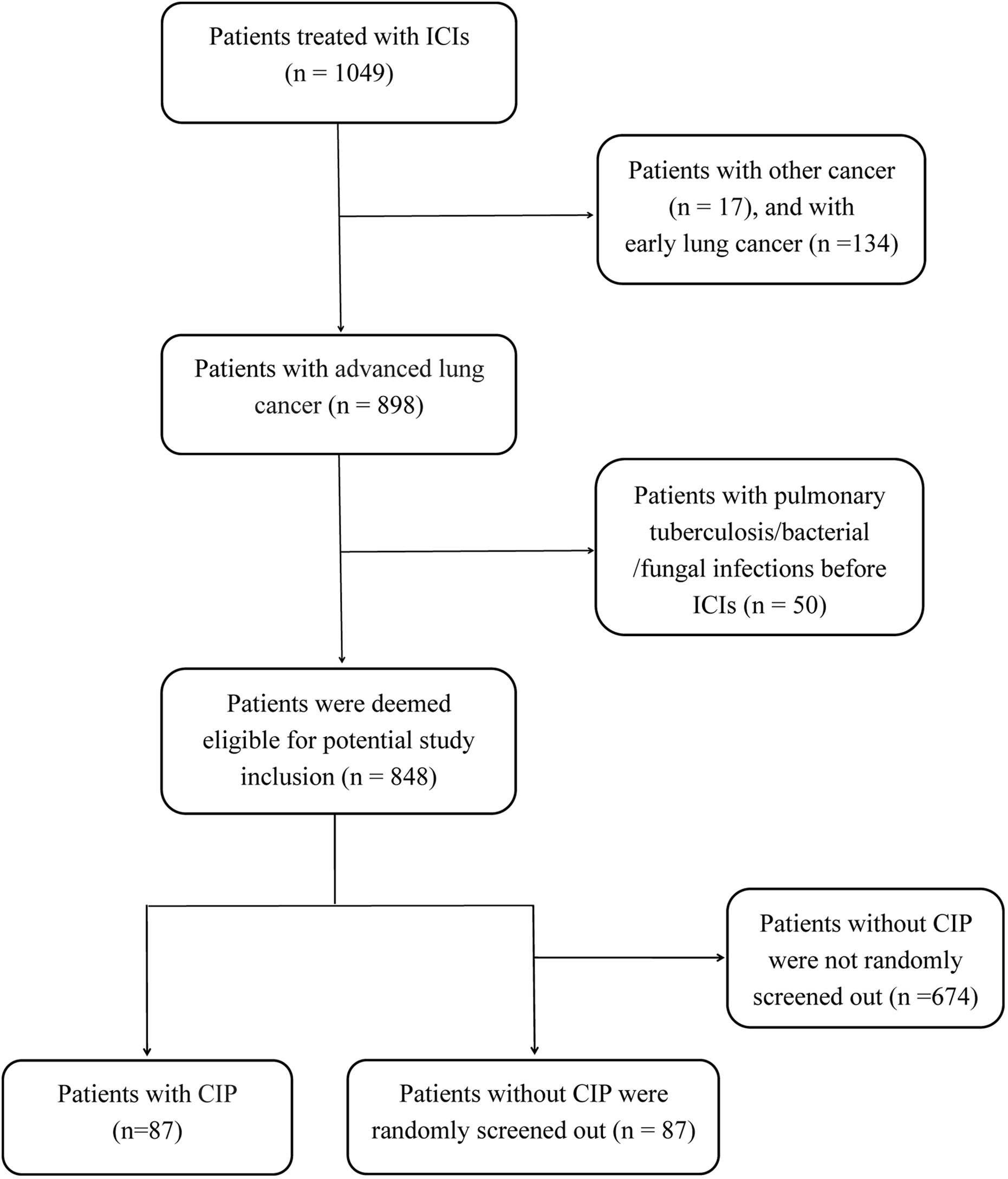
Figure 1 The flow chart of study design and patients inclusion. ICIs, immune checkpoint inhibitors; CIP, checkpoint inhibitor-related pneumonitis.
Among the 87 patients with CIP, the median age was 65 years (range: 18–85 years), and 83.9% were men. The median time from the initial administration of ICIs to the development of CIP was 3.8 months (range: 0.2–20.7 months). Among the CIP patients, 38 patients (43.7%) had severe (grades 3–5) CIP. The baseline TNF-α level of patients with CIP tended to be lower than that among those without CIP, but no significant difference was observed (p = 0.06; Supplementary Table 1).
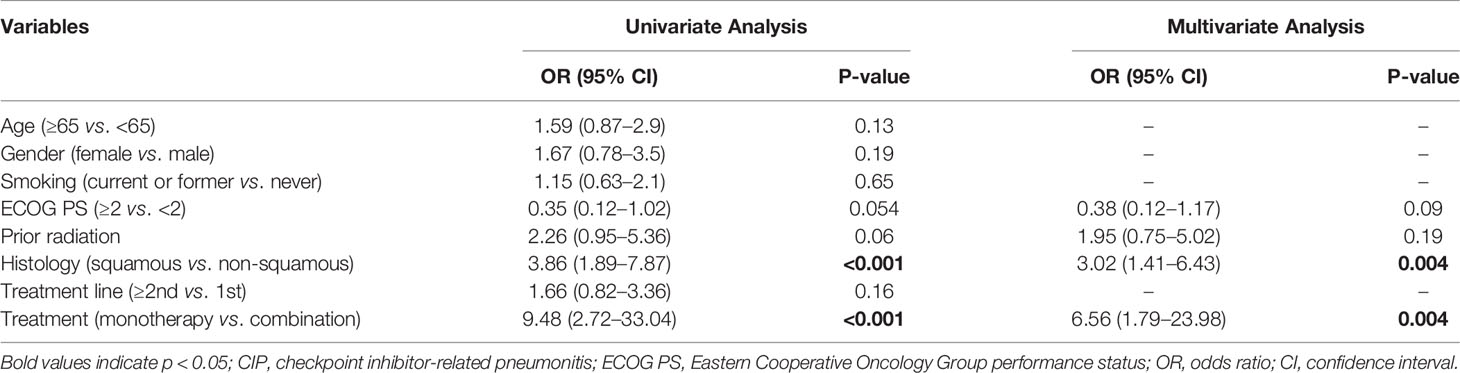
In the univariate and multivariate analysis (Table 2), squamous carcinoma (odds ratio [OR]: 3.02, 95% CI: 1.41–6.43; p = 0.004) and ICI monotherapy (OR: 6.56, 95% CI: 1.79–23.98; p = 0.004) correlated independently and significantly with the occurrence of CIP.
Correlation of Biomarkers With CIP
IL-6 increased significantly from baseline to CIP [7.62 pg/ml (IQR: 5.42–17.46) to 11.81 pg/ml (IQR: 5.10–63.34); p = 0.001] in the CIP group. By contrast, a significant decrease in IL-6 levels was observed over time [6.66 pg/ml (IQR: 4.24–19.38) to 6.45 pg/ml (IQR: 3.92–12.79); p = 0.030] in the control group (Figure 2A). Similarly, the median levels of IL-10 at baseline and CIP were 2.41 and 3.79 pg/ml (p = 0.025), respectively in the CIP group, and no change in the IL-10 over time was observed among controls (p = 0.94; Figure 2B).
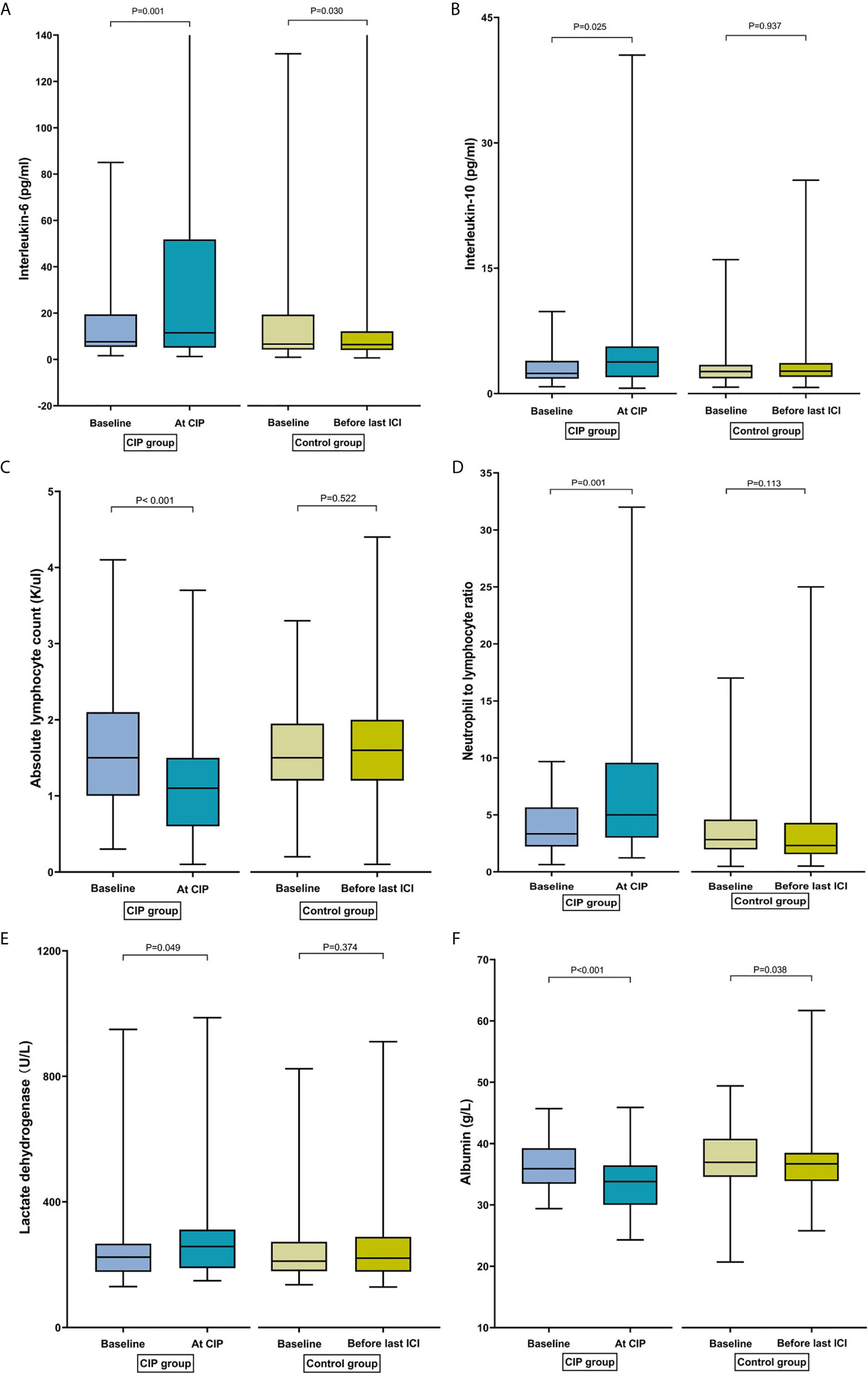
Figure 2 Bar plots of peripheral blood parameters in patients with checkpoint inhibitor-related pneumonitis (CIP) and controls at different time points. (A) Interleukin-6. (B) Interleukin-10. (C) Absolute lymphocyte count. (D) Neutrophil-to-lymphocyte ratio. (E) Lactate dehydrogenase. (F) Albumin.
In the CIP group, ALC decreased significantly from baseline to CIP presentation [1.50 K/µl (IQR: 1.00–2.08) to 1.15 K/µl (IQR: 0.63–1.50); p <0.001]. However, ALC did not change over time in the control group [1.50 K/µl (IQR: 1.20–2.10) to 1.60 K/µl (IQR: 1.20–2.00); p = 0.52] (Figure 2C). Among CIP cases, a significant increase in NLR was observed from baseline to CIP presentation [3.58 (IQR: 2.44–6.79) to 5.38 (IQR: 3.07–10.32); p = 0.001]. However, no change in NLR over time was observed in the control group [2.82 (IQR: 1.97–4.58) to 2.31 (IQR: 1.55–4.29); p = 0.11] (Figure 2D). Similarly, an increase in the PLR was observed from baseline to CIP development [179.70 (IQR: 123.09–331.75) to 263.76 (IQR: 152.65–432.77); p = 0.008]. By contrast, the PLR decreased significantly from baseline to before the last ICI dose [161.11 (IQR: 121.05–231.58); p = 0.042] in the control group.
LDH of patients with CIP increased significantly from baseline to CIP [223.80 U/L (IQR, 177.03–398.93) to 257.85 U/L (IQR, 189.03–311.83); p = 0.049]. Nevertheless, there was no change in the LDH over time among patients without CIP (p = 0.37; Figure 2E). There was a significant decrease in the ALB from baseline to CIP [35.85 g/L (IQR, 33.45–39.25) to 33.80 g/L (IQR, 30.00–36.45); p <0.001]. Median ALB concentration was also comparable over time (36.95 vs. 36.70 g/L; p = 0.038) at baseline among cases. However, the decrease of ALB was higher in the CIP group than in the control group (9.21% vs. 2.44%; p = 0.020) (Figure 2F).
In the CIP group, IL-2, IL-4, IFN-γ, TNF-α, ANC, AEC, and PLT had no significant changes from baseline to presentation with CIP (Supplementary Table 2). No matter in the experimental group or the control group, in the subgroup analysis, changes in IL-6, IL-10, ALC, NLR, PLR, LDH and ALB over time were not statistically significant between the squamous carcinoma and non-squamous carcinoma groups, or between the combination therapy and monotherapy groups.
Correlation of Biomarkers and Severe CIP
During follow-up, severe CIP occurred in 38 cases (43.7%). In the logistic univariate analysis, high IL-6, NLR, and PLR levels were associated with severe pneumonitis (grade 3 or higher) in the CIP group. By contrast, high concentrations of ALC and ALB were negatively correlated with severe pneumonitis. Multivariate regression analysis showed that high levels of IL-6 (OR: 5.23, 95% CI: 1.15–23.86; p = 0.033) and low levels of ALB (OR: 0.16, 95% CI: 0.04–0.64; p = 0.009) were significantly associated with CIP (Table 3).
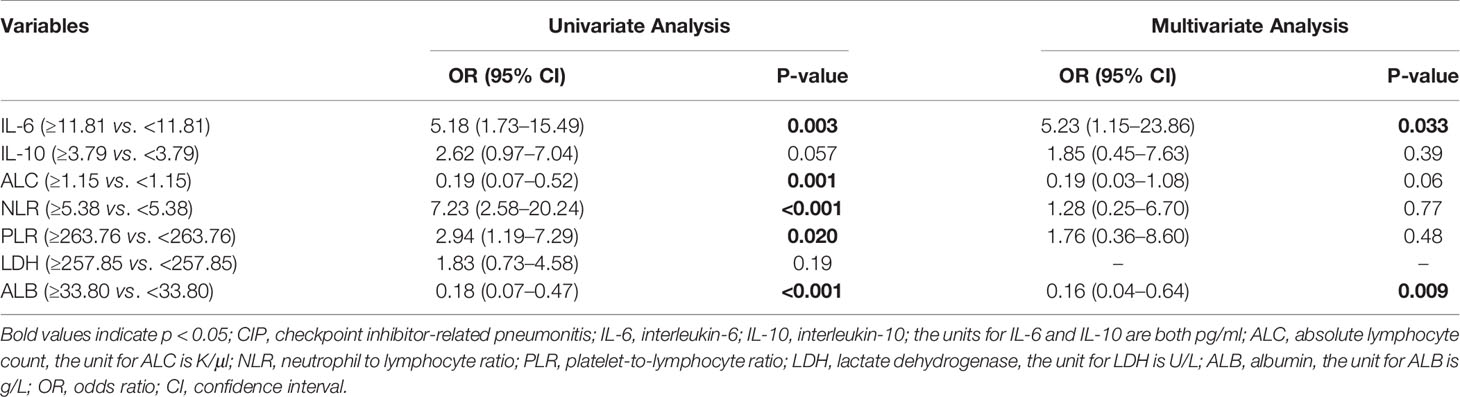
Table 3 Univariate and multivariate logistic regression analysis for the risk factors of grades 3–4 CIP in the CIP group.
Correlation of Biomarkers and Overall Survival
Among all patients with CIP, the median OS was 11.1 months (95% CI: 4.4–17.8 months), and the one-year survival rate was 46.5%. We generated a univariate Cox proportional hazards regression model of variables measured at the time of pneumonitis diagnosis. The results showed that CIP grade, and IL-6, ALC, NLR, and ALB levels were significantly correlated with OS (Table 4 and Figure 3). The median OS was significantly different according to treatment line (1st vs ≥2nd: 18.6 vs 5.5 months; HR: 0.37, 95% CI: 0.18–0.78; p = 0.009), CIP grade (1–2 vs. ≥3: 22.1 vs 3.7 months; HR: 0.11, 95.0% CI: 0.05–0.27; p <0.001), IL-6 (<11.81 vs. ≥11.81: 22.1 vs 6.1 months; HR: 0.07, 95.0% CI: 0.02–0.34; p = 0.001) (Figure 4A), ALC (≥1.15 vs. <1.15: 10.9 vs. 5.5 months; HR: 0.42, 95% CI: 0.20–0.92; p = 0.029), NLR (<5.38 vs. ≥5.38: 22.1 vs. 9.1 months; HR: 0.33, 95.0% CI: 0.15–0.74; p = 0.007), and ALB (≥33.80 vs. <33.80: 18.6 vs. 8.1 months; HR: 0.32, 95% CI: 0.14–0.73; p = 0.007) (Figure 4B).
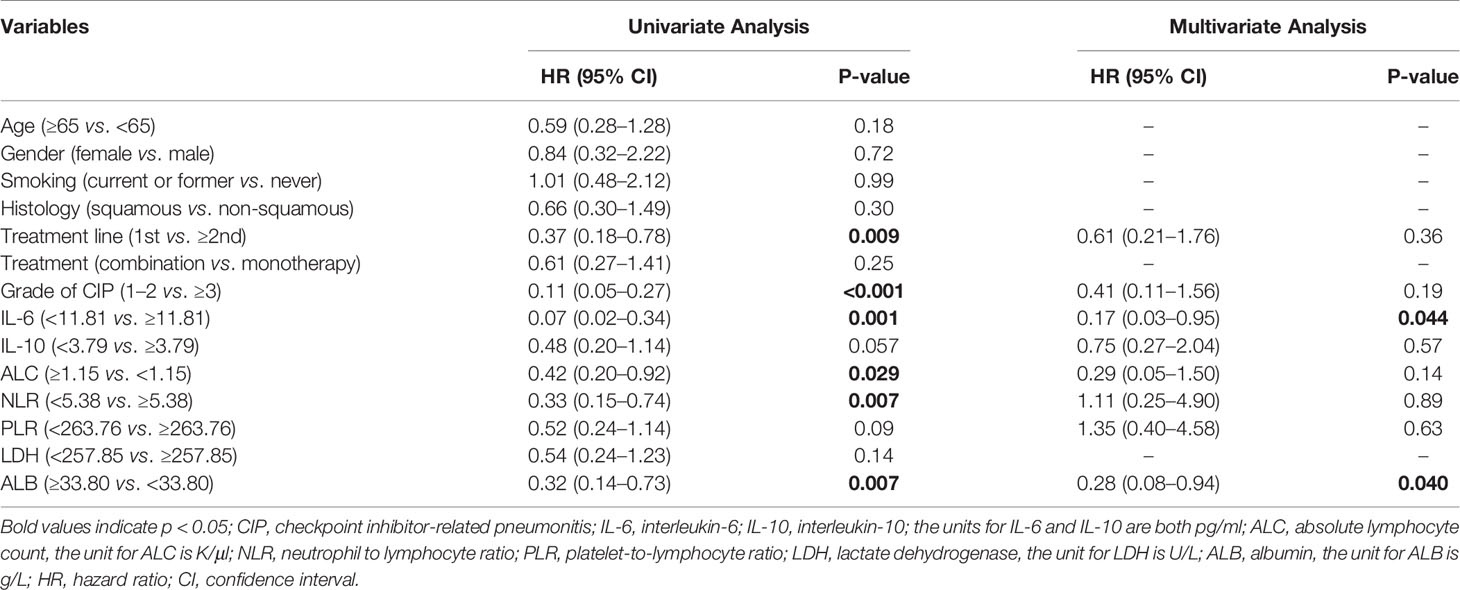
Table 4 Cox proportional hazards regression analysis of clinical factors associated with overall survival of CIP patients.
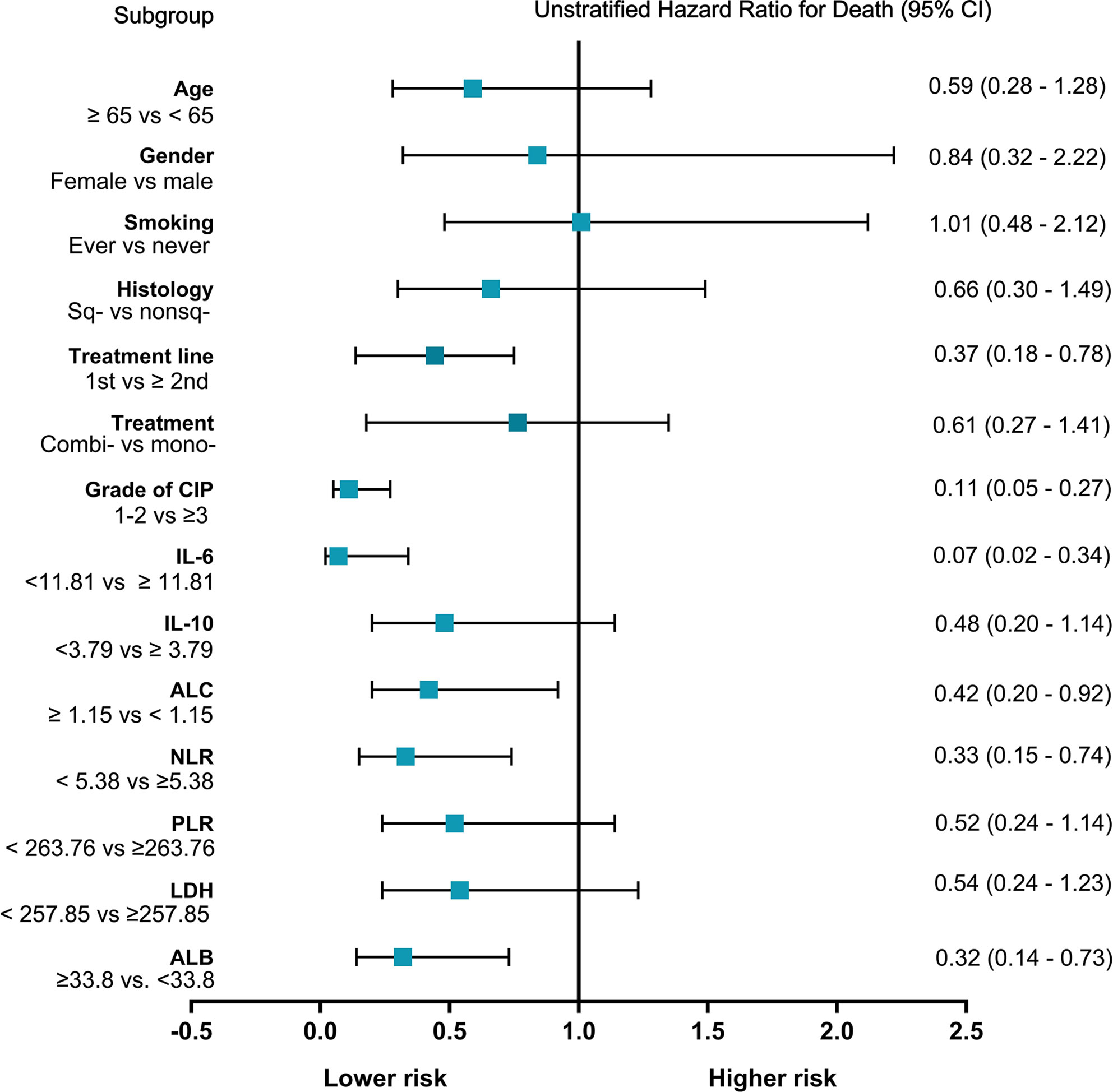
Figure 3 Forest plot of subgroup analyses of prognostic factors for overall survival of checkpoint inhibitor pneumonitis (CIP). Sq-, squamous; nonsq-, nonsquamous; combi-, combination; mono-, monotherapy; IL-6, interleukin-6; IL-10, interleukin-10; the units for IL-6 and IL-10 are both pg/ml; ALC, absolute lymphocyte count, the unit for ALC is K/μl; NLR, neutrophil to lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LDH, lactate dehydrogenase, the unit for LDH is U/L; ALB, albumin, the unit for ALB is g/L; CI, confidence interval.
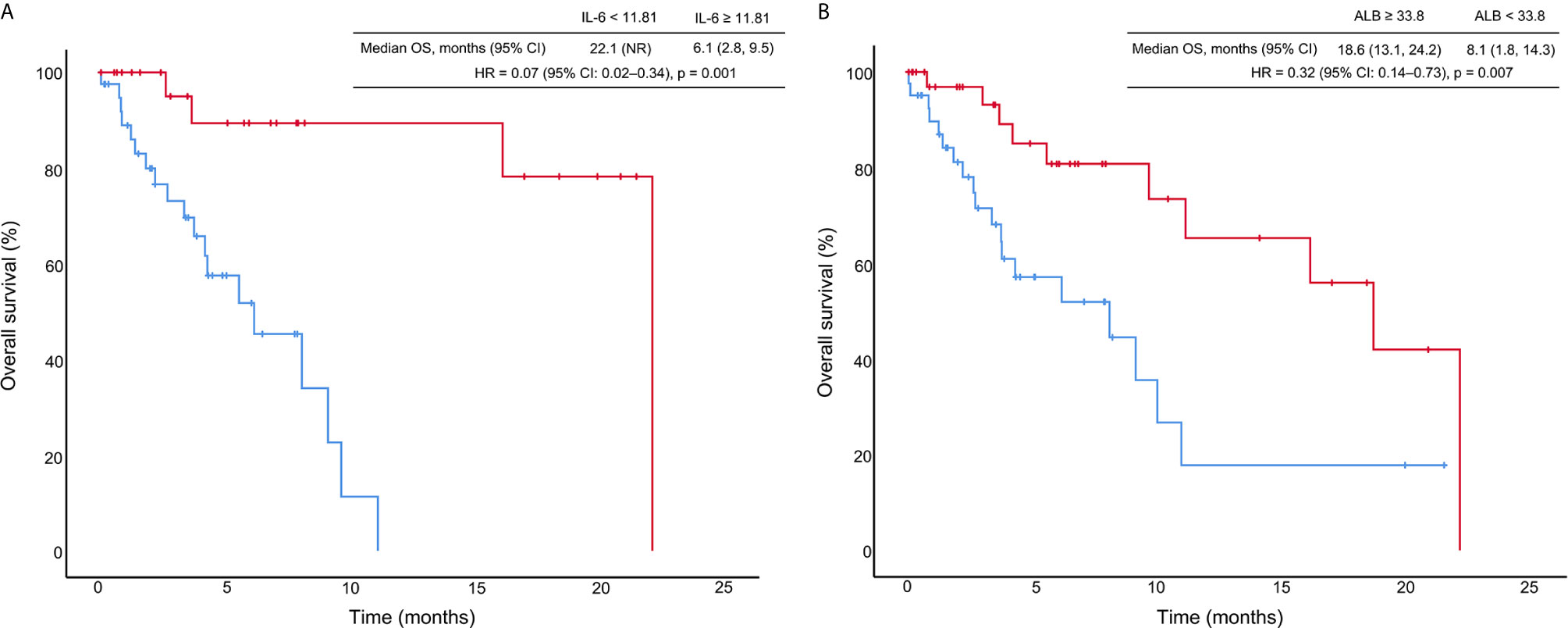
Figure 4 Kaplan–Meier curves of overall survival (OS) stratified by interleukin-6 (IL-6) levels (A) and albumin (ALB) concentration (B). HR, hazard ratio; CI, confidence interval; the unit for IL-6 is pg/ml; the unit for ALB is g/L.
In the multivariate Cox proportional hazards regression model, only IL-6 (<11.81 vs. ≥11.81: HR = 0.17, 95% CI: 0.03–0.95; p = 0.044) and ALB (≥33.80 vs. <33.80: HR = 0.28, 95% CI: 0.08–0.94; p = 0.040) were significantly and independently correlated with OS in patients with CIP (Table 4).
Discussion
This real-world, retrospective, observational study suggested that the histologic cancer type and ICI monotherapy may be risk factors of CIP occurrence. We found that IL-6, IL-10, ALC, NLR, PLR, LDH, and ALB levels changed significantly over time in patients with CIP. In addition, IL-6 and ALB levels at the time of CIP diagnosis were significantly correlated with severity and OS in CIP patients.
In our study, the overall CIP incidence was estimated at 10.3%, and 4.5% of patients developed grade 3 or higher CIP, which were larger proportions than those reported in previous clinical trials (24) but were consistent with a previous real-world study (10). The incidence of prior radiation was higher in CIP group than those in control group (20.7% vs. 10.3%; p = 0.06). In univariate logistic regression analysis, prior radiation tended to be associated with CIP (OR: 2.26, 95% CI: 0.95–5.36; p = 0.06). Multiple studies have shown that the history of prior radiotherapy could increase the risk of developing pneumonitis (8, 25). Our logistic regression analyses suggested that squamous carcinoma was associated with a high incidence of CIP. A previous study also reported that squamous carcinoma might be a risk factor for pneumonitis (26). One study showed that obstructive pneumonia may increase the risk of CIP (27). Most squamous cell carcinomas are central lung cancer, and obstructive pneumonia occurs more frequently, which may explain the increased incidence of pneumonitis in patients with squamous carcinoma. The association between pathological cancer types and CIP occurrence is worthy of further study. Our finding of a higher (OR: 6.56, 95% CI: 1.79–23.98; p = 0.004) CIP incidence among patients treated with ICI monotherapy was consistent with the findings of a recent meta-analysis (28), which showed that ICI monotherapy was associated with a higher risk of CIP (OR: 2.14, 95% CI: 1.12–4.80), compared with ICIs plus chemotherapy. This may be partly explained by cytotoxic chemotherapy drugs that can cause immunosuppression, and possibly the use of glucocorticoids as a pretreatment of chemotherapy, which may suppress the immune system as well as treat certain underlying lung diseases (e.g. asthma and chronic obstructive pulmonary disease) (28). In addition, antiangiogenic drugs (e.g. bevacizumab) could reduce vascular permeability and pulmonary exudation, which may contribute to the recovery of early pneumonitis (29). A case report showed that the addition of nintedanib to immunotherapy may prevent CIP (30).
With the development of irAEs, increased serum IL-6 and IL-10 levels have been demonstrated in case reports and retrospective studies with small samples (31–36). However, changes in the levels of these cytokines have only been reported in individual CIP cases. A case study showed a significant increase in IL-6 at the onset of CIP (37). Our study represents the first retrospective study to explore the relationship between cytokines and CIP development. We found that IL-6 and IL-10 levels increased significantly at CIP onset compared with those at baseline. However, the IL-10 levels remained unchanged, and the IL-6 levels decreased in patients without CIP over time. Elevated IL-6 was an independent biomarker for CIP severity and was an independent predictor for early death. In addition, high levels of IL-10 tended to be associated with severe CIP (p = 0.057). A study showed that the lymphocytes in the alveolar lavage fluid (BAL) of patients with CIP increased, predominantly CD4+ T helper (Th) lymphocytes (38). Th2 cells, an important subset of CD4 + cells, can produce cytokines (such as IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13), which in turn leads to excessive inflammation (39). These data supported the hypothesis that the excessive activation and proliferation of T cells cause an excessive cascade of cytokine release, which, in turn, causes an excessive immune response, leading to the occurrence of CIP. A previous case report showed that a patient developed severe cytokine release syndrome (CRS) after treatment with a PD-1 inhibitor (40). Thus, severe CIP may be related to CRS, which is a systemic inflammatory response caused by the release of inflammatory cytokines after the activation of monocytes, macrophages, and other lymphocyte populations, and elevated IL-6 plays a key role in this process (41). Stroud et al. reported that 27/34 patients with irAEs had improved clinical symptoms after receiving tocilizumab (IL-6 inhibitors) (42). Thus, IL-6 inhibitors may be an option for individualized treatment of CIP patients.
We observed that peripheral blood ALC values decreased from baseline to CIP, whereas no change was observed in the control group. A previous study suggested that a higher baseline ALC level (>2000 cells/mL) was a risk factor for irAE (43). In univariate analysis, low ALC levels were correlated with severe pneumonitis. Fujisawa et al. reported that a decrease in ALC values was associated with the incidence of grades 3–4 CIP in melanoma patients treated with nivolumab (44). This phenomenon may be caused by the large number of lymphocytes transported from the blood that infiltrate the focus of pneumonitis, resulting in a reduction of ALCs in the circulating pool, especially in severe patients, which is manifested as reduced peripheral blood ALC values (45). CIP should be distinguished from pulmonary infections, especially bacterial pneumonia. Bacterial pneumonia is primarily characterized by increased neutrophils; however, in our study, CIP patients did not present with increased neutrophils, and changes in the NLR appeared to primarily be due to a decrease in lymphocytes. Therefore, decreased ALC values may represent an indicator that can be used to differentiate CIP from bacterial pneumonia.
In our study, NLR and PLR increased significantly in CIP compared with baseline values. In univariate analysis, the observed increases in these two biomarkers at the time of CIP symptom onset were associated with CIP severity. No previous data have examined the role of PLR in CIP detection. A recent report (12) by Matsukane et al. analyzed NLR fluctuations in solid tumors and found that increased NLR was significantly associated with the occurrence of irAEs, especially in pneumonitis. They also indicated that elevated NLR levels at the time of CIP diagnosis were correlated with the occurrence of high-grade CIP. Conversely, a study showed that NLR and PLR were not associated with irAEs but were associated with the response to ICI treatment (31). However, this study only included patients treated with cytotoxic T-lymphocyte-associated protein 4 (CTL-4) inhibitors and did not analyze specific organs.
Multiple studies have shown that NLR and PLR are associated with OS in lung cancer patients treated with ICIs (46–48). However, the relationship between these indicators and the OS of patients with CIP is rarely reported. The univariate analysis showed that elevated NLR and low ALC levels at the time of initial CIP symptom onset were associated with shorter OS in patients with CIP. In a previously published study, compared with patients with a rapid decrease in elevated NLR, those patients who maintain elevated NLR had a poorer OS (12).
Studies have reported that damaged lung tissue cells release LDH, leading to increased serum LDH levels and suggesting that elevated LDH may serve as an indicator of acute lung injury (49–52). However, whether LDH is elevated in CIP has not yet been reported. Our study found that LDH was significantly higher in CIP than at baseline. A previous study suggested that patients with LDH levels greater than twice the upper limit of the normal tended to have a reduced risk of severe irAEs than patients with normal LDH levels (53). However, our study found no correlation between baseline LDH and the occurrence of CIP. Additionally, no correlation was observed between LDH and the severity of CIP.
In the current study, decreased ALB levels were associated with CIP development. A previous study showed that low ALB level was a risk factor for CIP. CIP may result in the release of both proinflammatory and inflammatory cytokines, which increase capillary permeability and promote the entry of cell and plasma solutes (such as ALB) into lesion tissue, increasing the interstitial volume and changing the distribution of ALB, which manifest as a decrease in serum ALB (54). In the multivariate analysis, high ALB levels were negatively correlated with severe pneumonitis (OR: 0.16, 95% CI: 0.04–0.64). In addition, low ALB level was a predictor of poor OS. Consistent with a previous study, these results suggested that low serum ALB may serve as a biomarker of inflammation severity and was associated with reduced quality of life and longevity (54).
These data indicate that the measurement of these indicators could be performed when CIP is clinically suspected, particularly when other measurement methods, such as chest CT or chest X-ray, are not available or are inconclusive. In addition, these indicators may help identify patients who are at risk of severe CIP and may be used to predict CIP prognosis. However, this study has some limitations. First, this study is a real-world retrospective study. Second, we did not monitor all changes in these blood parameters from the beginning of ICI to the onset of CIP. Third, CIP was diagnosed by symptoms and radiology, and only 19.5% of patients were confirmed by histopathology.
In conclusion, squamous carcinoma and ICI monotherapy may represent risk factors for CIP development. Increases in IL-6, IL-10, NLR, PLR, and LDH levels or reductions in ALC and ALB levels during ICI treatment may also serve as biomarkers for early diagnosis of CIP. High levels of IL-6 and low concentrations of ALB at the time of initial onset of CIP symptoms were predictive of severe pneumonitis. Importantly, high IL-6 or low ALB levels could be applied to improve risk stratification in pneumonitis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (Guangzhou, Guangdong, China). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XL, HD, and CZ designed the study. HD, YY, JW, GQ, and SL collected the patients’ data. XL, HD, XX, ML, ZX, and YQ analyzed the data. XL, HD, YY, JW, and CZ drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Fundamental and Applied Fundamental Research Project of City-School (Institute) Joint Funding Project, Guangzhou Science and Technology Bureau (202102010345, 202102010357), and Zhongnanshan Medical Foundation of Guangdong Province (ZNSA-2020003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank the participating patients and all medical staff members.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.698832/full#supplementary-material
References
1. Tan PS, Aguiar P Jr., Haaland B, Lopes G. Comparative Effectiveness of Immune-Checkpoint Inhibitors for Previously Treated Advanced Non-Small Cell Lung Cancer - A Systematic Review and Network Meta-Analysis of 3024 Participants. Lung Cancer (Amsterdam Netherlands) (2018) 115:84–8. doi: 10.1016/j.lungcan.2017.11.017
2. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative Analysis of Immune Checkpoint Inhibitors and Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Medicine (2018) 97(33):e11936. doi: 10.1097/md.0000000000011936
3. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review. Eur J Cancer (Oxford England: 1990) (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
4. Zhao B, Zhao H, Zhao J. Fatal Adverse Events Associated With Programmed Cell Death Protein 1 or Programmed Cell Death-Ligand 1 Monotherapy in Cancer. Ther Adv Med Oncol (2020) 12:1758835919895753. doi: 10.1177/1758835919895753
5. Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The Relative Risk and Incidence of Immune Checkpoint Inhibitors Related Pneumonitis in Patients With Advanced Cancer: A Meta-Analysis. Front Pharmacol (2018) 9:1430. doi: 10.3389/fphar.2018.01430
6. Chuzi S, Tavora F, Cruz M, Costa R, Chae YK, Carneiro BA, et al. Clinical Features, Diagnostic Challenges, and Management Strategies in Checkpoint Inhibitor-Related Pneumonitis. Cancer Manage Res (2017) 9:207–13. doi: 10.2147/cmar.S136818
7. Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of Checkpoint Inhibitor Pneumonitis on Survival in NSCLC Patients Receiving Immune Checkpoint Immunotherapy. J Thoracic Oncol: Off Publ Int Assoc Study Lung Cancer (2019) 14(3):494–502. doi: 10.1016/j.jtho.2018.11.016
8. Shibaki R, Murakami S, Matsumoto Y, Yoshida T, Goto Y, Kanda S, et al. Association of Immune-Related Pneumonitis With the Presence of Preexisting Interstitial Lung Disease in Patients With Non-Small Lung Cancer Receiving Anti-Programmed Cell Death 1 Antibody. Cancer Immunol Immunother (2020) 69(1):15–22. doi: 10.1007/s00262-019-02431-8
9. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous Radiotherapy and the Clinical Activity and Toxicity of Pembrolizumab in the Treatment of Non-Small-Cell Lung Cancer: A Secondary Analysis of the KEYNOTE-001 Phase 1 Trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/s1470-2045(17)30380-7
10. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, Incidence, and Risk Factors of Immune Checkpoint Inhibitor-Related Pneumonitis in Patients With Non-Small Cell Lung Cancer. Lung Cancer (2018) 125:150–6. doi: 10.1016/j.lungcan.2018.09.015
11. Jia XH, Geng LY, Jiang PP, Xu H, Nan KJ, Yao Y, et al. The Biomarkers Related to Immune Related Adverse Events Caused by Immune Checkpoint Inhibitors. J Exp Clin Cancer Res: CR (2020) 39(1):284. doi: 10.1186/s13046-020-01749-x
12. Matsukane R, Watanabe H, Minami H, Hata K, Suetsugu K, Tsuji T, et al. Continuous Monitoring of Neutrophils to Lymphocytes Ratio for Estimating the Onset, Severity, and Subsequent Prognosis of Immune Related Adverse Events. Sci Rep (2021) 11(1):1324. doi: 10.1038/s41598-020-79397-6
13. Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, Lee C, et al. Decreased Absolute Lymphocyte Count and Increased Neutrophil/Lymphocyte Ratio With Immune Checkpoint Inhibitor-Associated Myocarditis. J Am Heart Assoc (2020) 9(23):e018306. doi: 10.1161/jaha.120.018306
14. Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A, et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. J Clin Oncol: Off J Am Soc Clin Oncol (2019) 37(30):2746–58. doi: 10.1200/jco.18.02141
15. Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune Antibodies Correlate With Immune Checkpoint Therapy-Induced Toxicities. Proc Natl Acad Sci USA (2019) 116(44):22246–51. doi: 10.1073/pnas.1908079116
16. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association Between Immune-Related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Clin Lung Cancer (2019) 20(3):201–7. doi: 10.1016/j.cllc.2018.10.002
17. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations Between the Immune-Related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients. Clin Lung Cancer (2019) 20(4):237–47.e1. doi: 10.1016/j.cllc.2019.02.006
18. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer (2019) 20(6):442–50.e4. doi: 10.1016/j.cllc.2019.07.006
19. Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High Mortality and Poor Treatment Efficacy of Immune Checkpoint Inhibitors in Patients With Severe Grade Checkpoint Inhibitor Pneumonitis in Non-Small Cell Lung Cancer. Thorac Cancer (2019) 10(10):2006–12. doi: 10.1111/1759-7714.13187
20. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thoracic Oncol: Off Publ Int Assoc Study Lung Cancer (2015) 10(9):1243–60. doi: 10.1097/jto.0000000000000630
21. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing Toxicities Associated With Immune Checkpoint Inhibitors: Consensus Recommendations From the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
22. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol: Off J Am Soc Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/jco.2017.77.6385
23. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of Toxicities From Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol: Off J Eur Soc Med Oncol (2017) 28(suppl_4):iv119–42. doi: 10.1093/annonc/mdx225
24. Suresh K, Naidoo J, CT L, Danoff S. Immune Checkpoint Immunotherapy for Non-Small Cell Lung Cancer: Benefits and Pulmonary Toxicities. Chest (2018) 154(6):1416–23. doi: 10.1016/j.chest.2018.08.1048
25. Kanai O, Kim YH, Demura Y, Kanai M, Ito T, Fujita K, et al. Efficacy and Safety of Nivolumab in Non-Small Cell Lung Cancer With Preexisting Interstitial Lung Disease. Thoracic Cancer (2018) 9(7):847–55. doi: 10.1111/1759-7714.12759
26. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thoracic Oncol: Off Publ Int Assoc Study Lung Cancer (2018) 13(12):1930–9. doi: 10.1016/j.jtho.2018.08.2035
27. Atchley WT, Alvarez C, Saxena-Beem S, Schwartz TA, Ishizawar RC, Patel KP, et al. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health-Care Centers in North Carolina. Chest (2021) S0012-3692(21):00340–8. doi: 10.1016/j.chest.2021.02.032
28. Chen X, Zhang Z, Hou X, Zhang Y, Zhou T, Liu J, et al. Immune-Related Pneumonitis Associated With Immune Checkpoint Inhibitors in Lung Cancer: A Network Meta-Analysis. J Immunother Cancer (2020) 8(2):e001170. doi: 10.1136/jitc-2020-001170
29. Barratt SL, Flower VA, Pauling JD, Millar AB. VEGF (Vascular Endothelial Growth Factor) and Fibrotic Lung Disease. Int J Mol Sci (2018) 19(5):1269. doi: 10.3390/ijms19051269
30. Yamakawa H, Oba T, Ohta H, Tsukahara Y, Kida G, Tsumiyama E, et al. Nintedanib Allows Retreatment With Atezolizumab of Combined Non-Small Cell Lung Cancer/Idiopathic Pulmonary Fibrosis After Atezolizumab-Induced Pneumonitis: A Case Report. BMC Pulm Med (2019) 19(1):156. doi: 10.1186/s12890-019-0920-9
31. Khoja L, Atenafu EG, Templeton A, Qye Y, Chappell MA, Saibil S, et al. The Full Blood Count as a Biomarker of Outcome and Toxicity in Ipilimumab-Treated Cutaneous Metastatic Melanoma. Cancer Med (2016) 5(10):2792–9. doi: 10.1002/cam4.878
32. Husain B, Kirchberger MC, Erdmann M, Schüpferling S, Abolhassani AR, Fröhlich W, et al. Inflammatory Markers in Autoimmunity Induced by Checkpoint Inhibitors. J Cancer Res Clin Oncol (2021) 147(6):1623–30. doi: 10.1007/s00432-021-03550-5
33. Okiyama N, Tanaka R. Varied Immuno-Related Adverse Events Induced by Immune-Check Point Inhibitors - Nivolumab-Associated Psoriasiform Dermatitis Related With Increased Serum Level of Interleukin-6. Nihon Rinsho Men’eki Gakkai kaishi = Jap J Clin Immunol (2017) 40(2):95–101. doi: 10.2177/jsci.40.95
34. Saibil SD, Bonilla L, Majeed H, Sotov V, Hogg D, Chappell MA, et al. Fatal Myocarditis and Rhabdomyositis in a Patient With Stage IV Melanoma Treated With Combined Ipilimumab and Nivolumab. Curr Oncol (Toronto Ont) (2019) 26(3):e418–21. doi: 10.3747/co.26.4381
35. Yoshino K, Nakayama T, Ito A, Sato E, Kitano S. Severe Colitis After PD-1 Blockade With Nivolumab in Advanced Melanoma Patients: Potential Role of Th1-Dominant Immune Response in Immune-Related Adverse Events: Two Case Reports. BMC Cancer (2019) 19(1):1019. doi: 10.1186/s12885-019-6138-7
36. Khan S, Khan SA, Luo X, Fattah FJ, Saltarski J, Gloria-McCutchen Y, et al. Immune Dysregulation in Cancer Patients Developing Immune-Related Adverse Events. Br J Cancer (2019) 120(1):63–8. doi: 10.1038/s41416-018-0155-1
37. Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR. Interleukin-6 as One of the Potential Mediators of Immune-Related Adverse Events in Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Blockade: Evidence From a Case Report. Acta Oncol (Stockholm Sweden) (2018) 57(5):705–8. doi: 10.1080/0284186x.2017.1406668
38. Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, et al. The Alveolar Immune Cell Landscape Is Dysregulated in Checkpoint Inhibitor Pneumonitis. J Clin Invest (2019) 129(10):4305–15. doi: 10.1172/JCI128654
39. Romagnani S. T-Cell Subsets (Th1 Versus Th2). Ann Allergy Asthma Immunol (2000) 85(1):9–18. doi: 10.1016/S1081-1206(10)62426-X
40. Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe Cytokine Release Syndrome in a Patient Receiving PD-1-Directed Therapy. Pediatr Blood Cancer (2017) 64(12):10. doi: 10.1002/pbc.26642
41. Kroschinsky F, Stölzel F, von Bonin S, Beutel G, Kochanek M, Kiehl M, et al. New Drugs, New Toxicities: Severe Side Effects of Modern Targeted and Immunotherapy of Cancer and Their Management. Crit Care (London England) (2017) 21(1):89. doi: 10.1186/s13054-017-1678-1
42. Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the Management of Immune Mediated Adverse Events Secondary to PD-1 Blockade. J Oncol Pharm Pract (2019) 25(3):551–7. doi: 10.1177/1078155217745144
43. Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships Between Lymphocyte Counts and Treatment-Related Toxicities and Clinical Responses in Patients With Solid Tumors Treated With PD-1 Checkpoint Inhibitors. Oncotarget (2017) 8(69):114268–80. doi: 10.18632/oncotarget.23217
44. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Fluctuations in Routine Blood Count Might Signal Severe Immune-Related Adverse Events in Melanoma Patients Treated With Nivolumab. J Dermatol Sci (2017) 88(2):225–31. doi: 10.1016/j.jdermsci.2017.07.007
45. Farmer JR. Testing Immune-Related Adverse Events in Cancer Immunotherapy. Clinics Lab Med (2019) 39(4):669–83. doi: 10.1016/j.cll.2019.07.012
46. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-To-Lymphocyte Ratio (NLR) and Platelet-To-Lymphocyte Ratio (PLR) as Prognostic Markers in Patients With Non-Small Cell Lung Cancer (NSCLC) Treated With Nivolumab. Lung Cancer (Amsterdam Netherlands) (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
47. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic Biomarkers in Stage IV Non-Small Cell Lung Cancer (NSCLC): Neutrophil to Lymphocyte Ratio (NLR), Lymphocyte to Monocyte Ratio (LMR), Platelet to Lymphocyte Ratio (PLR) and Advanced Lung Cancer Inflammation Index (ALI). Trans Lung Cancer Res (2019) 8(6):886–94. doi: 10.21037/tlcr.2019.11.16
48. Ksienski D, Wai ES, Alex D, Croteau NS, Freeman AT, Chan A, et al. Prognostic Significance of the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Advanced Non-Small Cell Lung Cancer Patients With High PD-L1 Tumor Expression Receiving Pembrolizumab. Trans Lung Cancer Res (2021) 10(1):355–67. doi: 10.21037/tlcr-20-541
49. Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of Lactate Dehydrogenase and Its Isoenzymes as Indicators of Lung Damage or Inflammation. Eur Respir J (1996) 9(8):1736–42. doi: 10.1183/09031936.96.09081736
50. Hagadorn JE, Bloor CM, Yang MS. Elevated Plasma Activity of Lactate Dehydrogenase Isoenzyme-3 (LDH 3) in Experimentally Induced Immunologic Lung Injury. Am J Pathol (1971) 64(3):575–81.
51. Nagy O, Tóthová C, Seidel H, Paulíková I, Kovác G. The Effect of Respiratory Diseases on Serum Lactate Dehydrogenase and Its Isoenzyme Patterns in Calves. Polish J Veter Sci (2013) 16(2):211–8. doi: 10.2478/pjvs-2013-0030
52. Klein R, Nagy O, Tóthová C, Chovanová F. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Veter Med Int (2020) 2020:5346483. doi: 10.1155/2020/5346483
53. Verheijden RJ, May AM, Blank CU, van der Veldt AAM, Boers-Sonderen MJ, Aarts MJB, et al. Lower Risk of Severe Checkpoint Inhibitor Toxicity in More Advanced Disease. ESMO Open (2020) 5(6):e000945. doi: 10.1136/esmoopen-2020-000945
Keywords: checkpoint inhibitor-related pneumonitis, immune checkpoint inhibitor, interleukin-6, lymphocyte, albumin, lung cancer
Citation: Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S, Xie X, Liu M, Xie Z, Qin Y, Song Y and Zhou C (2021) Peripheral Blood Biomarkers for Early Diagnosis, Severity, and Prognosis of Checkpoint Inhibitor-Related Pneumonitis in Patients With Lung Cancer. Front. Oncol. 11:698832. doi: 10.3389/fonc.2021.698832
Received: 22 April 2021; Accepted: 24 June 2021;
Published: 13 July 2021.
Edited by:
Shengxiang Ren, Tongji University, ChinaReviewed by:
Pierpaolo Correale, Azienda ospedaliera ‘Bianchi-Melacrino-Morelli’, ItalyJun Wang, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, China
De-Hua Wu, Southern Medical University, China
Copyright © 2021 Lin, Deng, Yang, Wu, Qiu, Li, Xie, Liu, Xie, Qin, Song and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengzhi Zhou, ZG9jdG9yemN6QDE2My5jb20=
†These authors have contributed equally to this work
 Xinqing Lin
Xinqing Lin Haiyi Deng
Haiyi Deng Yilin Yang1†
Yilin Yang1† Jianhui Wu
Jianhui Wu Zhanhong Xie
Zhanhong Xie Yinyin Qin
Yinyin Qin Yong Song
Yong Song Chengzhi Zhou
Chengzhi Zhou