
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 21 September 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.698732
Background: KEYNOTE-181, ATTRACTION-3, and ESCORT trials have opened the era of programmed death 1 (PD-1) inhibitors in the second-line therapy for esophageal squamous cell carcinoma (ESCC). There is no head-to-head comparison of pembrolizumab vs. nivolumab vs. camrelizumab in the second-line setting for ESCC. We performed an indirect comparison to explore the optimal choice of immune checkpoint inhibitor (ICI) for advanced ESCC.
Methods: Patients in ATTRACTION-3 and ESCORT were all squamous carcinoma, while KEYNOTE-181 enrolled both adenocarcinoma and squamous carcinoma patients. We only extract information of patients with squamous carcinoma from KEYNOTE 181 study and all the patients from ATTRACTION-3 and ESCORT. The main clinical outcomes for this study were overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events (TRAEs).
Results: Indirect analysis showed similar survival benefit among three PD-1 inhibitors. Nivolumab was comparable with pembrolizumab in most subgroups except that nivolumab was slightly better for patients with performance status (PS) score of 1 [HRnivo/pembro: 0.68 (95% confidence interval (CI): 0.45–1.02], p = 0.07). Compared with nivolumab indirectly, pembrolizumab and camrelizumab had better PFS [HRpembro/nivo: 0.85 (95% CI: 0.63–1.14), p = 0.29; HRcam/nivo: 0.64 (95% CI: 0.47–0.87), p = 0.004] and significantly higher ORR [RRpembro/nivo: 2.51 (95% CI: 1.22–5.15), p = 0.01; RRcam/nivo: 3.52 (95% CI: 1.73–7.18), p = 0.001]. Compared with camrelizumab indirectly, pembrolizumab had slightly worse PFS [HRpembro/cam: 1.33 (95% CI: 0.99–1.79), p = 0.057] and comparable ORR [RRpembro/cam: 0.71 (95% CI: 0.32–1.60; p = 0.41)]. Camrelizumab had a significantly higher rate of all grade TRAEs than both pembrolizumab and nivolumab.
Conclusions: Combining the safety and potential survival benefit, we recommend nivolumab for ESCC patients with PS score of 1 and pembrolizumab or camrelizumab for patients with better PS and seeking for higher efficacy or longer PFS.
Metastatic esophageal cancer has poor prognosis, with a 5-year overall survival rate of ≤8% (1). Esophageal squamous cell carcinoma (ESCC) is the dominant histological subtype for esophageal cancer (2). Current chemotherapy options for second-line ESCC including taxol or irinotecan, offer poor survival and are associated with toxicity (3). There is an urgent need for new effective therapy for patients with ESCC.
Programmed death 1 (PD-1) inhibitors showed promising antitumor activity in patients with advanced esophageal carcinoma who were refractory to or intolerant of standard chemotherapies (4–6). Recently, the results of three randomized phase 3 studies in second-line setting for esophageal carcinoma were released. Compared with chemotherapy, pembrolizumab improved survival in PD-L1 combined positive score (CPS) ≥10 subgroups in KEYNOTE-181 study (7). Meanwhile, ATTRACTION-3, a randomized phase III study, showed a statistically significant and clinically meaningful improvement in overall survival (OS) of nivolumab versus chemotherapy in patients with advanced ESCC (8). ESCORT study found camrelizumab significantly improved OS in patients with advanced or metastatic ESCC compared with chemotherapy (9).
However, there is no head-to-head comparison of pembrolizumab vs. nivolumab vs. camrelizumab in the second-line setting for ESCC. Therefore, we performed an indirect comparison of KEYNOTE-181, ATTRACTION-3, and ESCORT studies to explore the optimal choice of anti-PD1 treatment for previously treated advanced ESCC. Furthermore, subgroup-immune checkpoint inhibitors (ICI) interaction test was performed in order to identify subsets of patients who would benefit most in terms of survival from different PD-1 inhibitors.
Patients in ATTRACTION-3 and ESCORT were all squamous carcinoma, while KEYNOTE-181 enrolled both adenocarcinoma and squamous carcinoma patients. In the present study, we only extract information of patients with squamous carcinoma from KEYNOTE 181 study.
The main clinical outcomes for this study were OS, progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events (TRAEs). Hazard ratio (HR) and its 95% confidence interval (CI) were extracted for OS and PFS, while risk ratio (RR) was extracted for ORR and TRAEs. Data were retrieved from the main outcomes of the three clinical trials by two independent investigators (YZ and PC).
We performed indirect analyses to compare the three PD-1 inhibitors using the frequentist methods (10), which indirectly compared arm A versus arm B (pembrolizumab vs. nivolumab, pembrolizumab vs. camrelizumab, and camrelizumab vs. nivolumab, respectively), linked by arm chemotherapy (C) with the formula: log HRAB = log HRAC-log HRBC, and [standard error (SE)]. The same formula was used for RR.
Moreover, subgroup meta-analyses were applied to investigate the subgroup-ICI interaction for OS (11). Five subgroups were available for the analyses, including gender (male vs. female), CPS of PD-L1 expression (≥10% vs. <10%), performance status (PS) (0 vs. 1), region (Asia vs. extra-Asia), and age (<65 vs. ≥65 years old). In each subgroup of each study, subgroup-ICI interaction was calculated using the indirect analysis mentioned above. We then conducted direct analyses (inverse-variance-weighted method) to calculate the pooled estimates with subgroup data from the three studies. Either fixed-effect or random-effect model was applied according to the heterogeneity.
The statistical analyses were performed using Stata (version 15.0, Stata Corporation, College Station, TX, USA), with a statistical significance setting at P <.05 (two-sided).
A total of 1,178 ESCC patients (329 patients from ATTRACTION-3, 401 patients from KEYNOTE-181, and 448 patients from ESCORT study) were enrolled in the analyses for OS, PFS, and ORR, and 1,475 patients were included in the analysis for TRAEs. Table 1 presents the main features and outcomes of these three studies.
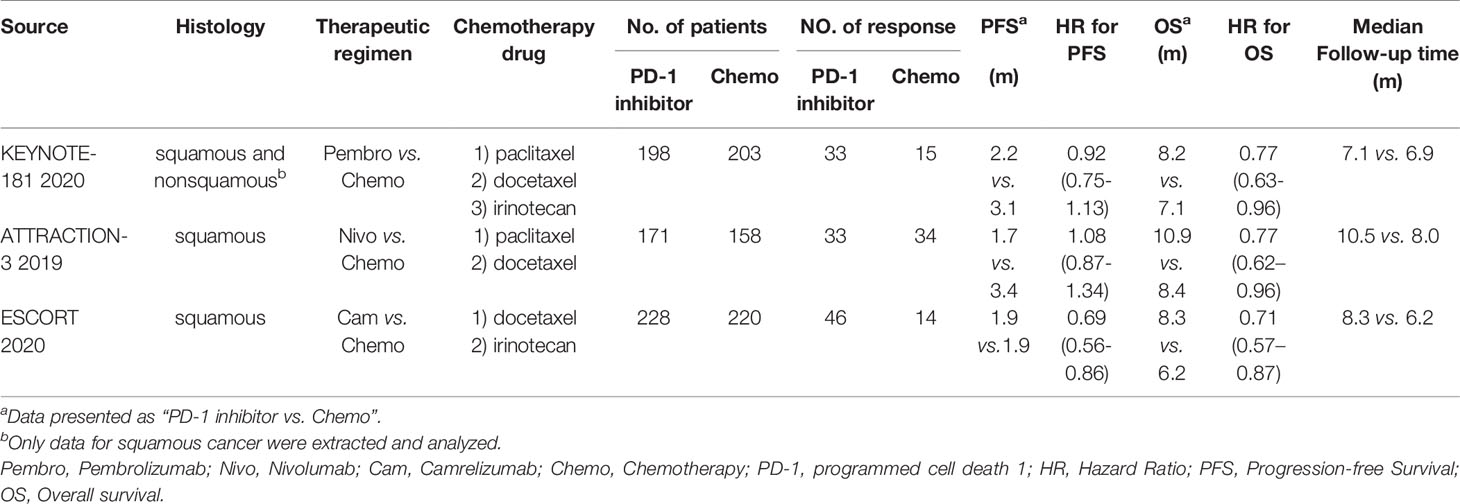
Table 1 Principal features and outcomes in included trials comparing PD-1 inhibitor with chemotherapy.
Indirect analysis showed similar survival benefit among these three PD-1 inhibitors: HRpembro/nivo: 1.00 (95% CI: 0.74–1.35), p = 1.000; HRpembro/cam: 1.08 (95% CI: 0.80–1.46), p = 0.594; Hcan/nivo: 0.92 (95% CI: 0.68–1.25), p = 0.601 (Figure 1). In subgroup survival analysis compared with chemotherapy, nivolumab improved survival in patients who were younger [HR: 0.65 (95% CI: 0.47–0.89)], male [HR: 0.79 (95% CI: 0.63–0.99)], PS score of 1 [HR: 0.61 (95% CI: 0.45–0.82)], and Asian [HR: 0.78 (95% CI: 0.62–0.97)]; pembrolizumab improved survival in those with PS score of 0 [HR: 0.64 (95% CI: 0.45–0.91)], PD-L1 CPS ≥10 [HR: 0.63 (95% CI: 0.46–0.90)], male [HR: 0.78 (95% CI: 0.63–0.95)], and Asian [HR: 0.63 (95% CI: 0.50–0.80)]; and camrelizumab improved survival in patients with PS score of 1, PD-L1 CPS <10. Indirect analysis showed that these three PD-1 inhibitors were comparable in most subgroups except that nivolumab was slightly better than pembrolizumab for patients with PS score of 1 [HRnivo/pembro: 0.68 (95% CI: 0.45–1.02), p = 0.07] (Table 2).
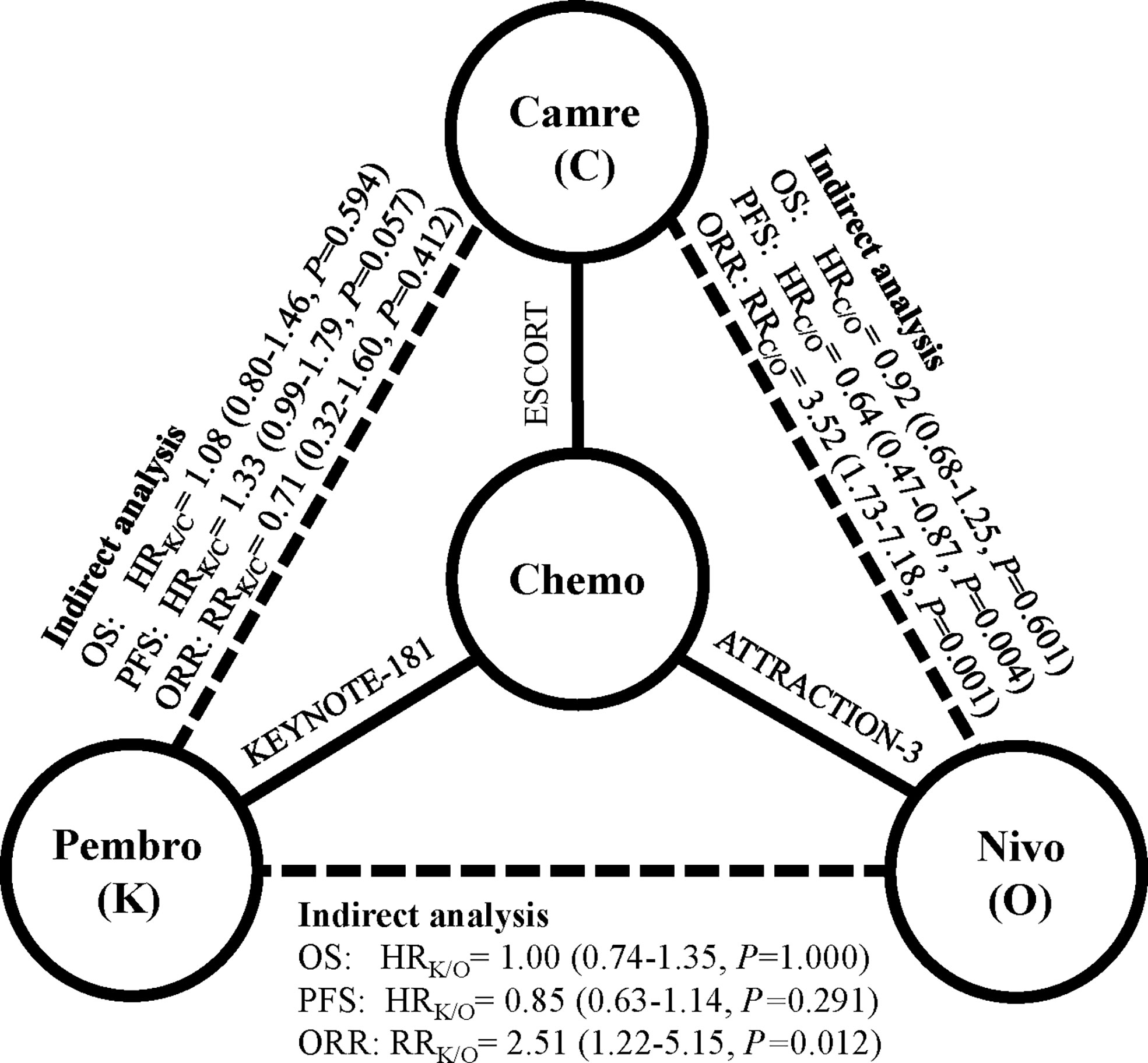
Figure 1 Indirect comparison among pembrolizumab, nivolumab, and camrelizumab in terms of overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). Solid lines represent the existence of direct comparisons between treatment regimens, and dashed line represents the indirect comparison among the three PD-1 inhibitors. All statistical tests were two sided. Abbreviations: Pembro, pembrolizumab; Nivo, nivolumab; Cam, camrelizumab; PD-1, programmed cell death 1; HR, hazard ratio; RR, risk ratio.
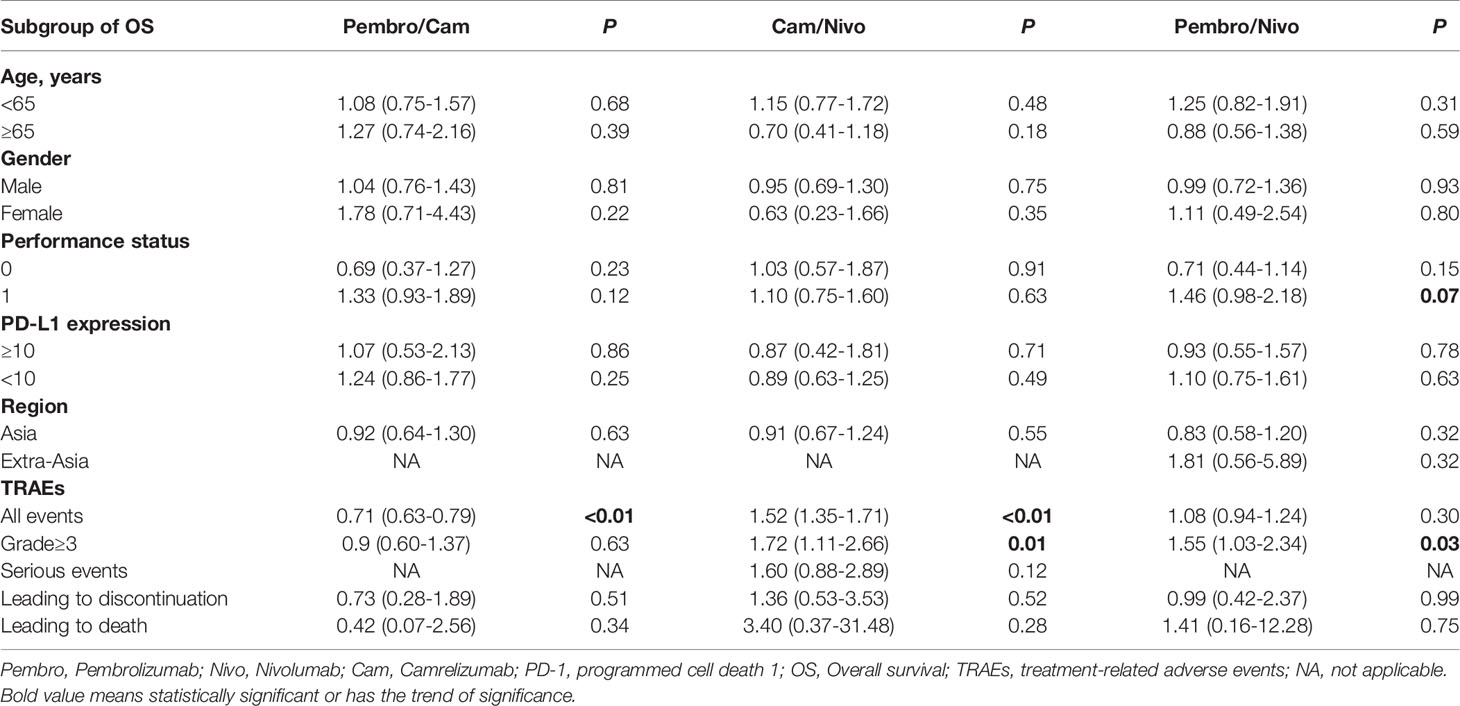
Table 2 Indirect comparisons among pembrolizumab, nivolumab, and camrelizumab in terms of predefined subgroup of OS and treatment-related adverse events.
Nivolumab had no clear PFS or ORR benefit towards chemotherapy [PFS—HRnivo/chemo: 1.08 (95% CI: 0.87–1.34); ORR—RRnivo/chemo: 0.90 (95% CI: 0.58–1.37)], while pembrolizumab had a significantly higher ORR than chemotherapy [RRpembro/chemo: 2.26 (95% CI: 1.27–4.02)]. Camrelizumab had comparable PFS and higher ORR than chemotherapy [PFS—HRcam/chemo: 0.69 (95% CI: 0.56–0.86); ORR—RRcam/chemo: 3.17 (95% CI: 1.80–5.60)] (Table 1). Compared with nivolumab indirectly, pembrolizumab had numerically better PFS [HRpembro/nivo: 0.85 (95% CI: 0.63–1.14), p = 0.29] and significantly higher ORR [RRpembro/nivo: 2.51 (95% CI: 1.22–5.15); p = 0.01]. Compared with camrelizumab indirectly, pembrolizumab had slightly worse PFS [HRpembro/cam: 1.33 (95% CI: 0.99–1.79), p = 0.057] and comparable ORR [RRpembro/cam: 0.71 (95% CI: 0.32–1.60); p = 0.41]. Compared with nivolumab indirectly, camrelizumab had significantly better PFS [HRcam/nivo: 0.64 (95% CI: 0.47–0.87), p = 0.004] and higher ORR [RRpembro/nivo: 3.52 (95% CI: 1.73–7.18), p = 0.001].
Analyses of TRAEs suggested that nivolumab was associated with significantly lower rate of ≥ grade 3 TRAEs [RRpembro/nivo: 1.55 (95% CI: 1.03–2.34), p = 0.03; RRcam/nivo: 1.72 (95% CI: 1.11–2.66), p = 0.01]. Camrelizumab had a significantly higher rate of all grade TRAEs than both pembrolizumab and nivolumab [RRpembro/cam: 0.71 (95% CI: 0.63–0.79), p < 0.01; RRcam/nivo: 1.52 (95% CI: 1.35–1.71), p < 0.01] (Table 2). The rates of serious TRAEs and TRAEs leading to discontinuation or death were similar among these three PD-1 inhibitors.
In the subgroup analyses (Figure 2), no significant difference was found in subgroups including PD-L1 status, PS, age, gender, or region, but PD-L1 expression and region demonstrated a numerical difference of interaction with ICI in terms of OS. Overall HR for ICI vs. chemotherapy (reference) was 0.65 (95% CI: 0.51–0.83, p < 0.001) and 0.78 (95% CI: 0.67–0.90, p = 0.001) for PD-L1 CPS ≥10 and PD-L1 CPS <10 subgroups, respectively. The HR for ICI/PD-L1 expression interaction was 0.80 (95% CI: 0.60–1.07, p = 0.129) (PD-1 inhibitor efficacy in PD-L1 CPS ≥10 patients vs. PD-L1 CPS <10 patients). The second most relevant interaction was ICI and region. Overall HR for PD-1 antibody vs. chemotherapy (reference) was 0.72 (95% CI: 0.63–0.83, p < 0.001) and 0.92 (95% CI: 0.68–1.25, p = 0.591) for Asia and extra-Asia subgroups, respectively. The HR for ICI/region interaction was 0.75 (95% CI: 0.50–1.12, p = 0.155) (PD-1 inhibitor efficacy in Asia patients vs. extra-Asia patients), but this was not statistically significant.
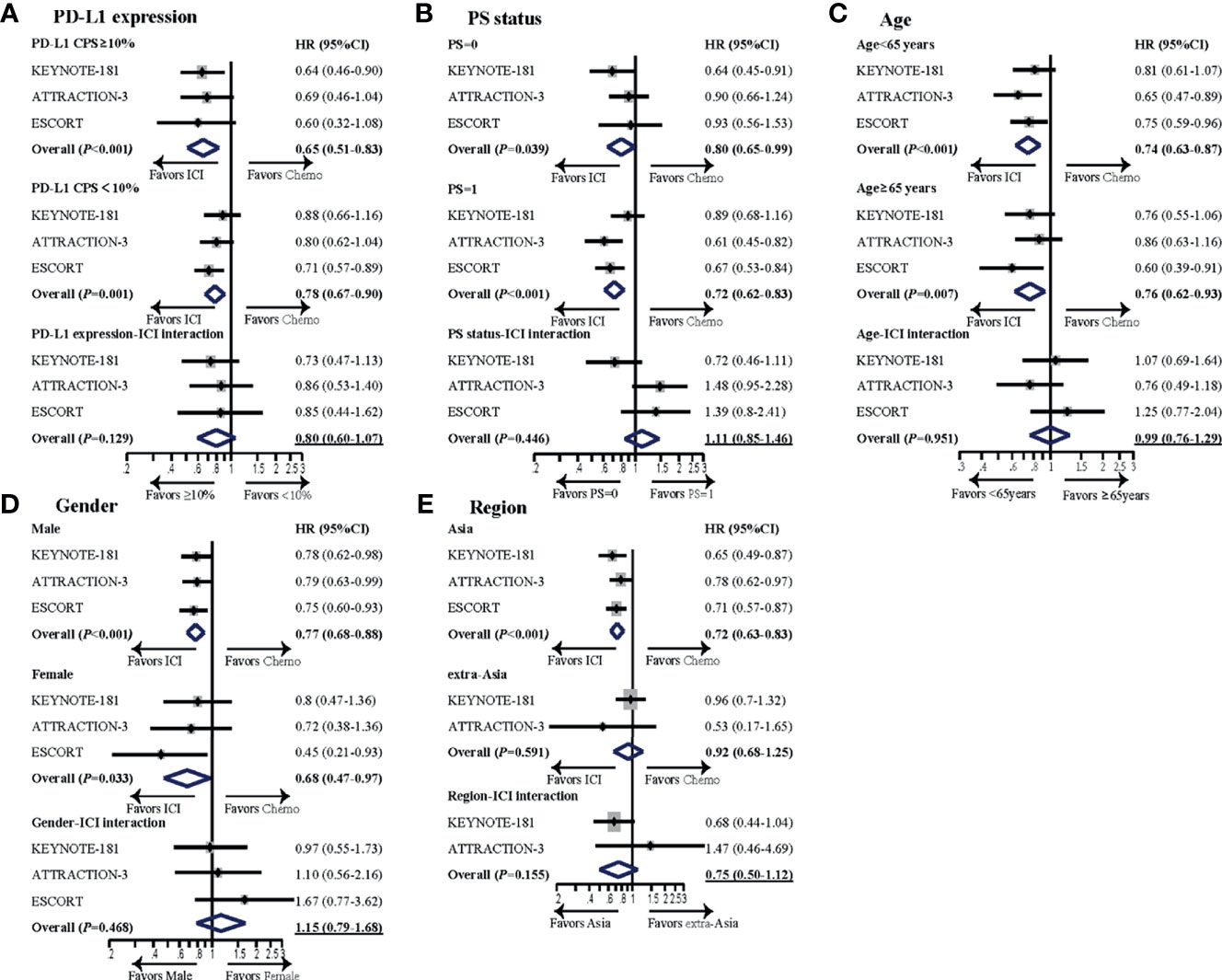
Figure 2 Subgroup meta-analyses investigating survival benefit from PD-1 inhibitors in different subsets of patients. Subgroups analyzed included (A) PD-L1 expression, (B) PS status, (C) age, (D) gender, and (E) region. For each subgroup, forest plot of hazard ratio (HR) was performed for OS. The horizontal line crossing the dot represents the 95% confidence interval (CI). The dot represented the estimated overall effect, based on the analysis. In each study, subgroup-ICI interaction was calculated using the indirect analysis. Then, meta-analyses were performed for the pooled estimates with all the enrolled studies. The final pooled results of subgroup-ICI interaction were shown by the data with underline. All statistical tests were two sided. PD-L1, programmed cell death ligand-1; CPS, combined positive score; HR, hazard ratio; ICI, immune checkpoint inhibitor; Chemo, chemotherapy; PS, performance status.
About 64% of patients in KEYNOTE-181 were squamous carcinoma. All the patients in ATTRACTION-3 and ESCORT were squamous carcinoma, so we only compared squamous carcinoma in these three studies. The three PD-1 inhibitors showed similar survival benefit in ESCC patients. In the subgroup analysis for survival, nivolumab seemed to be better than pembrolizumab in patients whose PS score is 1. This may be partially explained by the better safety profile of nivolumab. Based on the indirect analysis of AEs, camrelizumab had a significantly higher rate of all AE events than both pembrolizumab and nivolumab, and pembrolizumab had a numerically higher rate of all events than nivolumab. Similar results were found in ≥ grade 3 TRAEs. Therefore, the sequence of safety for these three PD-1 inhibitors was nivolumab followed by pembrolizumab and then camrelizumab. A recent systematic review and network meta-analysis for safety of ICIs in cancer also showed that nivolumab had the best safety profile (12). Camrelizumab had a high incidence rate of reactive cutaneous capillary endothelial proliferation (RCCEP) (13), which is 79% in the ESCORT study, but the incidence rate of grade 3 events was only 0.4% (9). Combining the safety and potential survival benefit, we recommend nivolumab for ESCC patients whose PS score is 1.
According to this indirect comparison, both pembrolizumab and camrelizumab had significantly higher ORR than nivolumab; moreover, camrelizumab had numerically higher ORR than pembrolizumab. Therefore, the sequence of ORR for these three PD-1 inhibitors was camrelizumab followed by pembrolizumab and then nivolumab. Moreover, camrelizumab had a significantly better PFS than nivolumab (p < 0.01), and camrelizumab has a tendency for longer PFS than pembrolizumab, and pembrolizumab also had better PFS than nivolumab, though these two differences were not significant, p = 0.06 and 0.29. In terms of PFS, camrelizumab is better than pembrolizumab followed by nivolumab. We would recommend camrelizumab or pembrolizumab for patients with better PS score and seeking for higher efficacy.
In the subgroup analyses, we found that PD-1 inhibitors were more effective in patients with PD-L1 CPS ≥10 than CPS <10 (HR interaction: 0.80), though it was not statistically significant. PD-L1 expression has been found as a predictor to PD-1 inhibitors in several diseases, such as gastric cancer (14, 15) and lung cancer (16, 17). However, the cutoff value for PD-L1 expression was controversial, and some studies used tumor-positive score (TPS) (16–18), while other studies use CPS (11, 12). In ESCC, PD-L1 CPS ≥10 is more commonly used (5, 7). Moreover, it seems that the result of pembrolizumab relies on the expression of PD-L1, while other PD-1 inhibitors, such as nivolumab and camrelizumab are independent of PD-L1 status. Based on our analysis, though the difference was not significant, we found that patients with PD-L1 CPS ≥10 benefit more from PD-1 inhibitors than those with CPS <10.
Subgroup analysis from clinical trials of PD-1 inhibitors has reported the impact of ethnic differences on outcomes (19, 20). In a meta-analysis to compare the therapeutic efficacy of PD-1/PD-L1 inhibitors in Asian and non-Asian patients, the author showed that Asian patients benefited significantly more than non-Asian patients in terms of OS (21). In our present analysis, we also found Asian ESCC patients benefit more from PD-1 antibody than non-Asia patients, though the difference was not significant. The exact mechanisms for different response to PD-1 inhibitors between Asian and extra-Asian populations are unclear, and potential explanations include difference in pharmacokinetic and genetic mutation profile (22, 23). Though both Japan and China belong to Asia, patients from different countries may benefit differently from PD-1 inhibitors (24). However, in the present study, we do not have enough data to compare the different efficacies between Japanese and Chinese patients.
The major limitation of this study was the indirect comparison analysis, which might compromise the evidence level. Secondly, expression of PD-L1 was measured with different antibodies, 28-8 pharmDx assay in ATTRACTION-3, 22C3 assay in KEYNOTE-181, and 6E8 assay in ESCORT, which might have influence on the evaluation of PD-L1expression. Thirdly, the chemotherapy used in the control group was not exactly the same among these three studies. In ATTRACTION-3, only paclitaxel and docetaxel were allowed, while in KEYNOTE-181 and ESCORT, except for paclitaxel, irinotecan was also one option. Paclitaxel and irinotecan have been confirmed to have no significant difference for OS in second-line treatment for advanced gastric cancer (25). Moreover, we only made the comparison among squamous carcinoma patients.
Our study firstly compared nivolumab, pembrolizumab, and camrelizumab for advanced ESCC in second line and found the three PD-1 inhibitors provided comparable OS benefit. Noteworthy, nivolumab might further improve OS in patients whose PS score is 1. Based on the present analysis, the sequence for ORR or PFS of these three PD-1 inhibitors was camrelizumab followed by pembrolizumab and then nivolumab, while the sequence for safety was nivolumab followed by pembrolizumab and then camrelizumab. Therefore, we recommend nivolumab for ESCC patients with PS score of 1 and pembrolizumab or camrelizumab for patients with better PS and seeking for higher efficacy or longer PFS. Additional studies are warranted to confirm this finding.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Conception and design of this study were carried out by M-ZQ, Y-XZ, and BZ. Y-XZ, M-ZQ, PC, and Y-TS extracted the data and statistical analysis. All authors wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (NSFC: 82073377, 81903176, 81772587), Natural Science Foundation of Guangdong (2021A1515012439, 2019A1515011596), Science and Technology Program of Guangdong (2019B020227002); Guangdong Esophageal Cancer Institute Science and Technology Program (grant number M201809), CSCO-HengRui Oncology Research Fund (grant number Y-HR2018-184), the Third Outstanding Young Talents Training Plan, and Medical Scientist Program of Sun Yat-Sen University Cancer Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, et al. Cancer Incidence and Survival Trends by Subtype Using Data From the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev (2017) 26:632–41. doi: 10.1158/1055-9965.EPI-16-0520
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
3. Ilson DH, van Hillegersberg R. Management of Patients With Adenocarcinoma or Squamous Cancer of the Esophagus. Gastroenterology (2018) 154:437–51. doi: 10.1053/j.gastro.2017.09.048
4. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab Treatment for Oesophageal Squamous-Cell Carcinoma: An Open-Label, Multicentre, Phase 2 Trial. Lancet Oncol (2017) 18:631–9. doi: 10.1016/S1470-2045(17)30181-X
5. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol (2019) 5:546–50. doi: 10.1001/jamaoncol.2018.5441
6. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients With Advanced Esophageal Carcinoma. Clin Cancer Res (2018) 24:1296–304. doi: 10.1158/1078-0432.CCR-17-2439
7. Kojima T, Shah MA. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
8. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Previous Chemotherapy (ATTRACTION-3): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
9. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab Versus Investigator's Choice of Chemotherapy as Second-Line Therapy for Advanced or Metastatic Oesophageal Squamous Cell Carcinoma (ESCORT): A Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2020) 21:832–42. doi: 10.1016/S1470-2045(20)30110-8
10. Zhou Y, Lin Z, Zhang X, Chen C, Zhao H, Hong S, et al. First-Line Treatment for Patients With Advanced Non-Small Cell Lung Carcinoma and High PD-L1 Expression: Pembrolizumab or Pembrolizumab Plus Chemotherapy. J Immunother Cancer (2019) 7:120. doi: 10.1186/s40425-019-0600-6
11. Formica V, Morelli C, Patrikidou A, Shiu KK, Nardecchia A, Lucchetti J, et al. A Systematic Review and Meta-Analysis of PD-1/PD-L1 Inhibitors in Specific Patient Subgroups With Advanced Gastro-Oesophageal Junction and Gastric Adenocarcinoma. Crit Rev Oncol Hematol (2021) 157:103173. doi: 10.1016/j.critrevonc.2020.103173
12. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. Bmj (2018) 363:k4226. doi: 10.1136/bmj.k4226
13. Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen Z, et al. Reactive Cutaneous Capillary Endothelial Proliferation in Advanced Hepatocellular Carcinoma Patients Treated With Camrelizumab: Data Derived From a Multicenter Phase 2 Trial. J Hematol Oncol (2020) 13:47. doi: 10.1186/s13045-020-00886-2
14. Tabernero J, Van Cutsem E, Bang Y, Fuchs C, Wyrwicz L, Lee K, et al. Pembrolizumab With or Without Chemotherapy Versus Chemotherapy for First-Line Treatment of Advanced Gastric or Gastroesophageal Junction (G/GEJ) Adenocarcinoma: The Phase 3 KEYNOTE-062 Study. Ann Oncol (2019) 30(Suppl 4):iv152–3. doi: 10.1093/annonc/mdz183.001
15. Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, et al. Clinicopathological and Molecular Features of Responders to Nivolumab for Patients With Advanced Gastric Cancer. J Immunother Cancer (2019) 7:24. doi: 10.1186/s40425-019-0514-3
16. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
17. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
18. Li C, Liu J, Xie Z, Zhu F, Cheng B, Liang H, et al. PD-L1 Expression With Respect to Driver Mutations in Non-Small Cell Lung Cancer in an Asian Population: A Large Study of 1370 Cases in China. Ther Adv Med Oncol (2020) 12:1758835920965840. doi: 10.1177/1758835920965840
19. Peng L, Wu YL. Immunotherapy in the Asiatic Population: Any Differences From Caucasian Population? J Thorac Dis (2018) 10:S1482–93. doi: 10.21037/jtd.2018.05.106
20. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
21. Peng L, Qin BD, Xiao K, Xu S, Yang JS, Zang YS, et al. A Meta-Analysis Comparing Responses of Asian Versus Non-Asian Cancer Patients to PD-1 and PD-L1 Inhibitor-Based Therapy. Oncoimmunology (2020) 9:1781333. doi: 10.1080/2162402X.2020.1781333
22. Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discovery (2018) 8:822–35. doi: 10.1158/2159-8290.CD-18-0099
23. Schabath MB, Welsh EA, Fulp WJ, Chen L, Teer JK, Thompson ZJ, et al. Differential Association of STK11 and TP53 With KRAS Mutation-Associated Gene Expression, Proliferation and Immune Surveillance in Lung Adenocarcinoma. Oncogene (2016) 35:3209–16. doi: 10.1038/onc.2015.375
24. Ohyama C, Kojima T, Kondo T, Naya Y, Inoue T, Tomita Y, et al. Nivolumab in Patients With Unresectable Locally Advanced or Metastatic Urothelial Carcinoma: CheckMate 275 2-Year Global and Japanese Patient Population Analyses. Int J Clin Oncol (2019) 24:1089–98. doi: 10.1007/s10147-019-01450-w
25. Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, Open-Label, Phase III Study Comparing Irinotecan With Paclitaxel in Patients With Advanced Gastric Cancer Without Severe Peritoneal Metastasis After Failure of Prior Combination Chemotherapy Using Fluoropyrimidine Plus Platinum: WJOG 4007 Trial. J Clin Oncol (2013) 31:4438–44. doi: 10.1200/JCO.2012.48.5805
Keywords: esophageal squamous cell carcinoma, PD-1 inhibitor, second line therapy, camrelizumab, nivolumab, pembrolizumab
Citation: Zhou Y-X, Chen P, Sun Y-T, Zhang B and Qiu M-Z (2021) Comparison of PD-1 Inhibitors in Patients With Advanced Esophageal Squamous Cell Carcinoma in the Second-Line Setting. Front. Oncol. 11:698732. doi: 10.3389/fonc.2021.698732
Received: 10 June 2021; Accepted: 30 August 2021;
Published: 21 September 2021.
Edited by:
Makoto Sakai, Gunma University, JapanReviewed by:
Xiuying Xiao, Shanghai JiaoTong University, ChinaCopyright © 2021 Zhou, Chen, Sun, Zhang and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bei Zhang, emhhbmdiZWlAc3lzdWNjLm9yZy5jbg==; Miao-Zhen Qiu, cWl1bXpoQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.